Abstract
Objective
To review the severe acute respiratory syndrome (SARS) epidemic in Beijing using basic epidemiological principles omitted from the original analysis.
Study design
Analysis of Prospective surveillance data for Beijing collected during the outbreak.
Methods
Surveillance data were reclassified according to World Health Organization criteria. Cases previously excluded without date of onset of illness were included in the epidemic curve from estimates using the average time between date of onset and date of hospitalization for cases with both dates. Cases who failed to give a contact history were now included; 7% () of cases during the import phase and 61% () during the peak phase. Previously excluded cases were included for plotting on an epidemic curve, and basic spot mapping for distribution of cases was used from attack rates recalculated for age, gender, occupation, residential location, date of onset of illness and demographics.
Results
The spot map effectively illustrated clusters by residency, with the inner-city sustaining the highest attack rate (33.42 per 100,000), followed by an easterly distribution 5–30 km away (21.62 per 10,000), and lowest in districts 60–160 km away (9.21 per 100,000). The new epidemic curve shows the outbreak commencing 10 days earlier than initially reported, with a three-fold greater increase in cases during the escalation phase than previously estimated.
Conclusion
In hindsight, the investigation of the Beijing SARS would have benefited from the use of spot maping as an essential outbreak tool for early identification of specific geographical area(s) for quarantining. If a spot map of incidence density rates was used during the early phase of the outbreak, the inner city might have been identified as a major risk factor requiring rapid quarantining. Contact history became uncommon as the outbreak progressed, suggesting that hospitals were over-burdened or pathogenesis and environment risk factors changed, strengthening the usefulness of early spot mapping and the need to modify risk factors included as contact history as the epidemic progresses.
Keywords: SARS outbreak, Epidemic curve, Spot mapping
Introduction
The origin of the novel coronavirus (SARS CoV)1 responsible for severe acute respiratory syndrome (SARS) infection was traced to the Guangdong province of mainland China.2 It spread within China and from Guangdong to the Hong Kong Special Administration Region of China (Hong Kong-SAR) in February 2003 and on to five continents, infecting more than 8000 residents from more than 30 countries.3 Not surprisingly, the densely populated mainland of the People's Republic of China sustained the largest burden of SARS cases, an estimated 5327 cases, followed by China's Hong Kong-SAR, with 1755 confirmed cases.3 Within the mainland, the case burden was the greatest in its capital, Beijing, with 2521 probable cases, representing 31% of cases globally and 25% of all deaths. Retrospective examination of reports from the three regions in China most affected, Beijing,4 Guangdong2 and Hong Kong-SAR,3 suggested regional deficiencies in epidemiological data. Post-epidemic analysis using spatial diffusion process provides a powerful understanding of the geographic relationship between populations and their environment.5 However, it does not provide preliminary assistance that would identify potential hot spots of exposure as the outbreak unfolds. We carried out simple re-analysis of the Beijing data to determine which data could have provided information that would have resulting in early containment.
Methods
The original epidemic curve excluded those cases without a history for date of onset of illness, whereas re-analysis included these cases and plotted cases on an epidemic curve substituting the date of presentation to hospital for date of onset of illness. During the early phase (import and escalation phases) of the outbreak, case definition was in accordance with the Ministry of Health criteria6 in the absence of World Health Organization (WHO) criteria and a serology test. Re-analysis, therefore, also included cases initially excluded if they did not fulfil the Ministry of Health diagnostic criteria of SARS6 requiring cases with contact history of either direct case contact or contact with an endemic area. These cases were now re-classified according to WHO criteria,7 with cases classified as ‘probable’ cases only where their signs and symptoms were in accordance with criteria for one of three WHO definitions for ‘probable’ SARS. All 5327 cases available from the SARS epidemic database, collated by the Disease Control and Prevention Centre, Beijing,6 were now included in the epidemic curve. Cases were categorized into 15 occupational groups, with restaurants combined with hospitality services and the military and police services combined.8 Re-analysis included an epidemic curve for ‘probable’ SARS cases only and calculations of the Beijing population-based rate, stratified by age and sex, using the Fifth General Census of China (version 2000). The original analysis did not include a spot map. Re-analysis included cases plotted for 18 geographic areas with rates calculated per 100,000 residents and then regrouped into three geographic areas according to distance from the city centre: inner city, 5–10 km, and more than 10 km from the inner city. Denominator data were not available to calculate specific exposure rates, such as number of hospitalized patients for a healthcare-acquired infection rate, number of healthcare workers for an occupationally acquired rate or healthcare worker hours for occupationally acquired rate per hours of exposure. The epidemiologic curves were developed using Microsoft Excel 2000, and SPSS version 10 was used to calculate case fatality rates (CFR), odds ratios, 95% confidence intervals for proportions, rates, odds and rates, and tests for significance.
Results
Re-classification of case data
Of the 5327 cases on mainland China,3 4844 cases originated in Beijing between 8 March and 28 May 2003. Using the Ministry of Health's clinical criteria, 52% (2521/4844) were originally correctly classified as ‘probable’ SARS, whereas the remainder were ‘possible’ or ‘non-SARS’ cases. The case fatality rate for ‘probable’ cases was 7.6% (192/2521). Date of onset of illness for ‘probable’ cases was available for 74.0% (1867/2521), gender for 99.9% (2518/2521), age for 99.9% (2518/2521), 97.1% (2447/2521) residential area, 93.4% (2356/2521) occupation and 65.1% (125/192) time of death. New data for symptoms and radiological data enabled 96.9% (2443/2521) of previously classified cases to remain classified as ‘probable’, with 3.1% (78/2521) reclassified to ‘possible’ owing to undefined or unclear dates of onset of symptoms. Of the 2443 cases who fulfilled the ‘probable’ criteria, 576 failed to have date of onset of illness documented, but most of such cases (99.6%, 574/576) had date of admission, and all 574 were admitted early in the epidemic, during either March or April 2003. Analysis of the dataset for 1876 cases with documented dates for onset of illness and hospitalization identified time between onset of symptoms and hospitalization as on average 4.1 days. This average was used for 574 cases without date of onset of illness but with date of hospitalization, enabling a total of 2443 ‘probable’ cases to be included on a new epidemic curve. A total of 2323 who did not fulfil criteria for either ‘probable’ or ‘possible’ SARS were reclassified as non-SARS and excluded from all analysis.
Epidemic curve for cases and deaths
After adjusting for onset of illness for 574 cases admitted early in the epidemic, the commencement of the epidemic was actually 10 days earlier (1 March) than the unadjusted curve (Fig. 1 ). The import phase continued until 24 March, with an average daily incidence of three, and contributed 3.1% of all cases. The corrected index case in the Beijing epidemic was a visitor from Shanxi province to Beijing hospitalized on the 1 March 2003 and transferred for treatment 2 days later to a second hospital. Nineteen secondary cases were subsequently traced to this index case; five relatives, 10 patients with healthcare-acquired SARS and four healthcare workers with occupationally acquired SARS.
Figure 1.
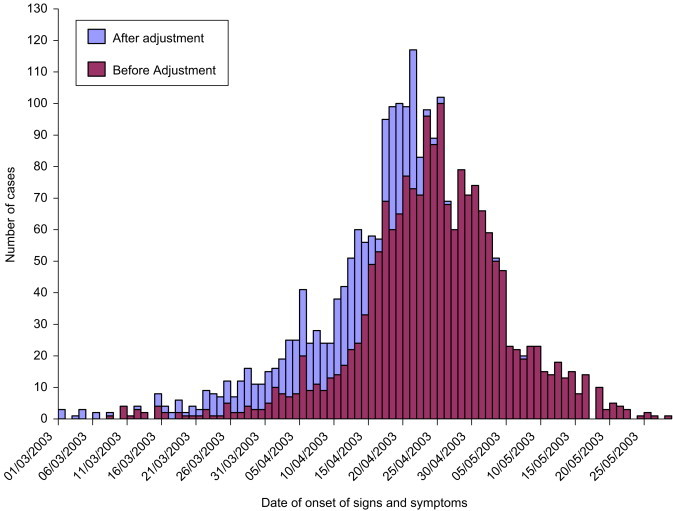
Epidemic curve before and after adjustment for date of onset of illness.
After adjustment, the escalation phase commenced earlier (on 25 March), during which time the average daily incidence was 29 cases, and this phase contributed just over one-quarter (27.5%, 672/2443) of all cases. The escalation phase continued, with a daily incidence escalating to 10 times that for phase 1 until 16 April. The peak occurred 6 days earlier than previously identified (on 17 April), continuing for 9 days until 25 April 2003, averaging 98 cases per day and contributing to 36.1% (882/2443) of all cases. The adjustment of data did not affect the declining and terminating phases. The decline began on 26 April, with the daily incidence falling to 69 from 102 the previous day, and continued until 18 May when case reports returned to single digits for more than 1 day. Termination was rapid (19–28 May), contributing to only 1.1% (20/2443) of cases.
Less than half, 41.2%, of all probable cases gave a contact with SARS history (Table 1 ). During the import phase with few cases, 75, it was most common (88.0%) for a contact history to be given. Reporting contact history significantly changed with each phase (, P<0.0001), finally dropping to 10% for cases presenting during the termination phase. During the crisis phases of the epidemic (import and peak), cases were 24.6 times (95% CI 17.7–34.2) more likely to give a contact history compared with cases during the period when there was no growth in the epidemic (the decline and termination phases).
Table 1.
The distribution of cases and contact history in different stages of the epidemic.
| Epidemic phase | Date of onset of illness after (before) adjustment | Incidence after (before) adjustment |
Known history of contact |
Death with documented time and date |
||||
|---|---|---|---|---|---|---|---|---|
| N | % | Cumulative % | N/Incidence after adjustment | % | N | % | ||
| Phase 1 | 01.03–24.03 | 75 | 3.1 | 3.1 | 66/75 | 88.0 | 0.8 | |
| Import | (10.03–31.03) | (51) | (2.7) | (2.7) | 1 | |||
| Phase 2 | 25.03–16.04 | 672 | 27.5 | 30.6 | 243/672 | 36.2 | 6 | 4.8 |
| Escalation | (01.04–22.04) | (722) | (38.7) | (41.4) | ||||
| Phase 3 | 17.04 –25.04 | 882 | 36.1 | 66.7 | 652/882 | 73.9 | 27 | 21.6 |
| Peak | (23.04–25.04) | (283) | (15.2) | (56.6) | ||||
| Phase 4 | 26.04–18.05 | 794 | 32.5 | 99.2 | 43/794 | 5.4 | 53 | 42.4 |
| Decline | (26.04–18.05) | (791) | (42.4) | (99.0) | ||||
| Phase 5 | 19.05–28.05 | 20 | 0.8 | 100 | 2/20 | 10.0 | 38 | 30.4 |
| Termination | (19.05–28.05) | (20) | (1.0) | (100) | ||||
| Total | 2443 | 100 | 1006/2443 | 41.2 | 125 | 100 | ||
| (1867) | (100) | |||||||
Between 20 March and 24 June, 125 of the 192 total deaths attributed to ‘probable’ SARS had a recorded date and time of death (Table 1). As deaths lagged the epidemic curve, most (72.8%) occurred during the decline phase. The average daily death rate was less than one from the import phase until 30 April when there were, on average, five deaths daily; thereafter, the daily rate dropped to 1.2.
Distribution of cases and deaths by age and sex
The incidence rate of SARS in Beijing was 18.6 per 100,000 residents and sustained a mortality rate of 1.4 per 100,000. The revised CFR was 7.6 per 100 clinically diagnosed ‘probable’ SARS, not 8.4% as originally reported.4 As the original and adjusted epidemic curves shifted by 10 days, the revised CFR at the beginning of the termination phase had reached 3.6%. The revised CFR identified that just over one-quarter (27.2%) occurred between the import phase and end of the peak (between 1 March and 25 April), when the CFR was 1.4%. By 20 April, one death occurred on average per day. The daily average number of deaths accelerated between 21 April and 30 April to five and, by 2 May, 50% of fatalities occurred; thereafter, during the decline and termination phase, the daily average for deaths fell to 1.2.
The age-specific mortality rate was greatest (4.5 per 100,000) in older residents aged between 50 and 80 years and older, compared with 0.03 and 0.80 per 100,000 for young and younger adult groupings aged 0–19 years and 20–49 years of age (P<0.00001).
Although the male to female ratio of cases was close (1.09 : 1.00), the population-based attack rate was significantly higher for females (19.1 per 100,000) compared with males (18.0 per 100,000) () (Table 2 ). The age of cases ranged from 1 to 93 years, with a median age 37.1 years (SD 15.8), 4 years older than the original estimate. Regardless of gender, the population-based attack rate was significantly (P<0.0001) higher for those aged 20–29 years, 30.8 per 100,000, whereas the lowest attack rate was sustained by the youngest, 2.5 cases per 100,000. Most infections, 72.3% (1822/2518), were sustained by people aged between 20 and 49 years, with significantly () more female cases, 26.3 per 100,000, than male, 21.9 per 100,000, within this grouping. Within this age group, the mortality rates for females, 0.82 per 100,000, did not differ significantly compared with males 0.79 per 100,000 (). When the attack rates were examined by three age groupings (young, 0–19 years; younger adult, 20–49 years; older, ), adults and elderly people constituted higher rates of SARS cases (6.1, 24.0 and 17.5, respectively) (P<0.00001).
Table 2.
Attack rate in Beijing of severe acute respiratory syndrome by age and sex.
| Age group | Number of cases |
Number of deaths |
Fifth General Beijing Population Census 2000 |
Attack rate (mortality rate) by sex and age/100,000 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total (%) | Male | Female | Total (%) | Male | Female | Total | Male | Female | Total | |
| 0–14 | 24 | 23 | 47 | 0 | 0 | 0 | 958,876 | 884,892 | 1,843,768 | 2.5 | 2.6 | 2.5 |
| (1.9) | (0.0) | (0.0) | (0.0) | (0.0) | ||||||||
| 15–19 | 95 | 47 | 142 | 0 | 1 | 1 | 651,212 | 582,961 | 1,234,173 | 14.6 | 8.1 | 11.5 |
| (5.6) | (0.5) | (0.0) | (0.2) | (0.1) | ||||||||
| 20–29 | 409 | 372 | 781 | 5 | 5 | 10 | 1,388,248 | 1,142,667 | 2,530,915 | 29.5 | 32.6 | 30.8 |
| (31.0) | (5.2) | (0.4) | (0.4) | (0.4) | ||||||||
| 30–39 | 277 | 290 | 567 | 11 | 8 | 19 | 1,469,672 | 1,262,088 | 2,731,760 | 18.8 | 23.0 | 20.7 |
| (22.5) | (9.9) | (0.7) | (0.6) | (0.7) | ||||||||
| 40–49 | 205 | 269 | 474 | 16 | 16 | 32 | 1,200,006 | 1,131,265 | 2,331,271 | 17.1 | 23.8 | 20.3 |
| (18.8) | (16.7) | (1.3) | (1.4) | (1.4) | ||||||||
| 50–59 | 115 | 113 | 228 | 19 | 19 | 38 | 586,027 | 609,770 | 1,195,797 | 19.6 | 18.5 | 19.1 |
| (9.1) | (19.8) | (3.2) | (3.1) | (3.2) | ||||||||
| 60–69 | 78 | 71 | 149 | 20 | 17 | 37 | 513,228 | 541,092 | 1,054,320 | 15.2 | 13.1 | 14.1 |
| (5.9) | (19.3) | (3.9) | (3.1) | (3.5) | ||||||||
| 70–79 | 58 | 48 | 106 | 30 | 16 | 46 | 251,195 | 263,067 | 514,262 | 23.1 | 18.2 | 20.6 |
| (4.2) | (24.0) | (11.9) | (6.1) | (8.9) | ||||||||
| ⩾80 | 16 | 8 | 24 | 5 | 4 | 9 | 56,054 | 76,874 | 132,928 | 28.5 | 10.4 | 18.0 |
| (1.0) | (4.7) | (8.9) | (5.2) | (6.8) | ||||||||
| Total | 1277a | 1241 | 2518 | 106 | 86 | 192 | 7,074,518 | 6,494,676 | 13,569,194 | 18.0 | 19.1 | 18.6 |
| (100) | (100) | (1.5) | (1.3) | (1.4) | ||||||||
Missing data for three cases.
Distribution of cases and deaths by occupation
Just over half (51.3%) of all cases were contributed by four occupation groups: healthcare workers (17.3%, revised from 16.2%);4 government officials (12.9%); retired people (11.4%); and factory workers (9.7%). These four occupational groups contributed more than half (66.3%) of all deaths, with retired people having sustained the highest death rate (24.5%), followed by factory worker cases (8.8%), government officials (5.3%), and healthcare workers (2.5%). Those whose socio-economic status would seems to be low (e.g. retired people, farmers and unemployed people), sustained the greatest proportion of deaths (24.5%, 11.7% and 10.6%, respectively).
Distribution of cases and deaths by residency
For the 2447 cases with place of residence, a plot of 18 Beijing districts identified significant increases in rates associated with geographic location (P<0.00001), with the epicentre located in the inner-city where the incidence rates ranged from 9.8 to 44.1, averaging 33.4 per 100,000 (Fig. 2 ) (Table 3 ). Districts 5–10 km away from the inner-city sustained rates between 16.7 and 23.5, and averaged 21.6 per 100,000. Moving 11 or more kilometres from the inner-city rates were lower still, ranging from 0.5 to 35.3 and averaging 9.2 per 100,000. Of specific interest was the Tongzhou district (60–160 km from the city), with a rate as high as that for the inner-city, at 35.3 per 100,000. The CFR increased from 6.2% for cases located further from the city to 9.2% in the inner-city (χ 2 for slope 4.09, , ).
Figure 2.
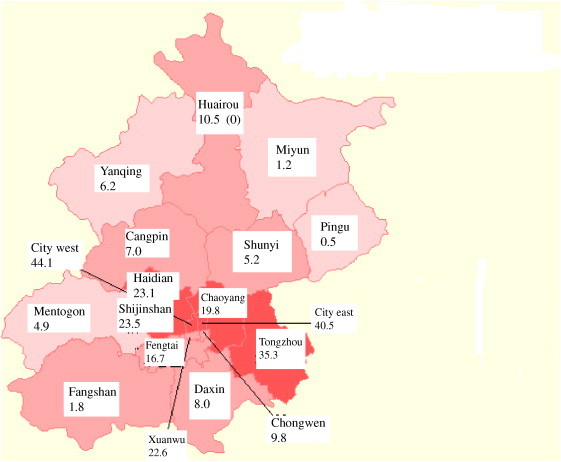
Distribution of severe acute respiratory syndrome cases in Beijing as a rate per 100,000 population.
Table 3.
Rate of severe acute respiratory syndrome per 100,000 Beijing population and approximate kilometres from city centre of Beijing.
| City centre | SARS rate/100, 000 (682 cases/ 2, 040,630 residents) | Case fatality rate/ 100,000 (63/682) [95%CI] |
|---|---|---|
| City east | 40.5 | |
| City west | 44.1 | |
| Xuanwu | 22.6 | |
| Chongwen | 9.8 | |
| Total | 33.42 | 9.2 [7.2–11.7] |
| 5–30 km from the city centre | SARS rate/100, 000 (1314/ 6,076,452 residents) | Case fatality rate/ 100,000 (92/1314) [95%CI] |
| Chaoyang | 19.8 | |
| Haidian | 23.1 | |
| Shijingshan | 23.5 | |
| Fengtai | 16.7 | |
| Total | 21.62 | 7.0 [5.7–8.5] |
| 60–160 km from city centre | SARS rate /100,000 (451/5,896,490 residents) | Case fatality rate/ 100,000 (28/451) [95%CI] |
| Tongzhou | 35.3 | |
| Daxing | 8.0 | |
| Changping | 7.0 | |
| Shunyi | 5.2 | |
| Mentougou | 4.9 | |
| Fangshan | 1.8 | |
| Pinggu | 0.5 | |
| Yanqing | 6.2 | |
| Huaiyou | 10.5 | |
| Miyun | 1.2 | |
| Total | 9.21 | 6.2 [4.2–88] |
Measures taken to control the epidemic based on unadjusted phases
Contact history
The import phase of the Beijing epidemic occurred rapidly, between 1 and 10 March, with 14 cases admitted with an acute pneumonia of unknown cause without history taken for exposure to a case of respiratory illness or environmental contact. Although the first case was admitted on 1 March, this case was transferred to a second hospital and was not to be recognized as SARS until contact tracing identified this case as the index case for five family members, four healthcare workers and 10 patients at two hospitals. On re-analysis of the import phase, 88% of patients had a known contact history (Table 1). By 16 April, most patients fulfilled the clinical requirements of a case, but a known contact history could not be elicited in more than 36.2% of cases.
Discussion
The spot map developed by John Snow9 remains a rudimentary epidemiological tool in outbreak investigation. Our analysis for spot mapping clearly illustrates that the highest attack rate was sustained by residents living in a most densely populated area, within 30 km of the city centre, concurring with the post-epidemic sophisticated analysis of spatial diffusion process.5 During the 89-day epidemic, similar durations were experienced elsewhere in China;10, 11 although our database could not identify community-acquired from occupationally acquired risk, an early spot map would have identified clustering of cases, indicating a likely community exposure. The decline in contact history should have raised suspicion that the definition of ‘contact’ in the initial phase could have benefited from a re-definition throughout the epidemic. Contact history during an outbreak involving progressive transmission should list exposure to animals, environmental contact (i.e. a hospital or geographic contact by virtue of residential location), as potential contacts. Such re-defining, along with spot maps for place of residency or occupation, would have still revealed the heavy case loads in the inner city areas. Although this is not always suggestive of a causative agent, it would be strongly supportive of the usefulness of geographical quarantining.
Even though more contacts acquired SARS from spouses (15.4%), the proportion was greater with non-household contact (11.6%)12 than household contact (8.8%), or friend (10.0%), suggesting that environmental exposure is a causal factor and supported by the clustering on our spot map and the high attack rate in those of working age. Daily commuting to work across densely populated cities assists the dissemination of airborne or droplet spread diseases even when infectivity may be low.13 Excellent control measures were instigated during the early phase, including quarantining contacts and closing public social and education venues.12 However, basic spot mapping could support a policy for rapid and severe restriction of population movement within 30 km circumference from the city centre.
The WHO diagnostic criteria was not available during the initial phase of the outbreak. As a result, during the first 24 days into the epidemic, the magnitude was under-estimated by close to one-third by excluding cases without contact history or date of onset of illness. This resulted in a delay of 10 days in which an epidemic could have been recognized for containment. With any outbreak of unknown origin, there will be a paucity of crucial epidemiological data, including the significance of asymptomatic infection, an accurate incubation period, the infectivity and likelihood of person-to-person spread and changes in clinical presentation. The rapid decline in reports of contact history should strongly suggest that strict application of a current case definition could under-estimate the magnitude of the epidemic and efficacy of interventions. To fulfil the criteria of ‘contact history’, such contact would have had to occur between 1 and 16 days previously.14 The dramatic decline in contact history may be caused by: (1) failure by healthcare workers to elicit contact history; (2) burden of recall of a protracted incubation period (up to 2 weeks); (3) infectivity of the agent improving enabling spread via asymptomatic cases; and (4) spread through casual environmental contamination.
In China, 32% of our 1.3 billion population live in cities, with movement of both susceptible and infected people within and outside of China inevitably making public health hygiene of paramount importance. For management of future possible airborne outbreaks, we would recommend the development of a colour-coded public warning system, similar to the three tiered numerical typhoon warning system. ‘Yellow’ warning would be given to the public to heed specific hygiene practices, reduce public gathering (cinema, dining out etc.), or both, with the understanding that this level could be a false-alarm or upgraded to ‘orange’, indicating further cases fulfilling a firmer case definition, finishing with ‘red’ to indicate a definite outbreak. All levels could be down-graded or up-graded with continuous analysis of data. Containment of an outbreak requires interpretation of data analysis using the three public health tenets, ‘time, place and person’. These analyses carried out early may be flawed; however epidemiologists must be able to issue early warning with support from both government and non-government bodies and withstand pressure from economic lobby groups when warnings are false alarms or require geographic quarantining.
Post-epidemic population movement analysis is crucial to our understanding of disease dynamics, which identified that population density was more significant than population size for the spread of SARS.5 However, although continuous basic outbreak analysis is more primitive than statistical modelling, it would have immediately illustrated the effect of the population density. Although the use of a consistent definition is sound practice, periodic analysis, including cases that do not strictly fit a given criterion (close contact or date of illness) common during our peak phase, may have illustrated a different curve, enabling earlier recognition of a potential control opportunity: quarantining large areas of a city.
References
- 1.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. SARS Working Group. A novel Coronavirus associated with severe respiratory syndrome. N Engl J Med 2003;348:1953–66. [DOI] [PubMed]
- 2.Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003; Available from: http://www.who.int/csr/sars/country/table2003_09_23/en (last accessed 15 December 2006).
- 4.Liang W, Zhu Z, Guo J, Liu Z, He X, Zhou W, et al. Schuchat A for the Beijing Joint SARS Expert Group. Severe acute respiratory syndrome, Beijing, 2003. Emerg Infect Dis 2004 January [serial online]. Available from: http://www.cdc.gov/ncidod/EID/vol10no1/03-0553.htm (last accessed 15 December 2006). [DOI] [PMC free article] [PubMed]
- 5.Meng B., Wang J., Liu J., Wu J., Zhong E. Understanding the spatial diffusion process of severe acute respiratory syndrome in Beijing. Public Health. 2005;119:1080–1187. doi: 10.1016/j.puhe.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health 2003, no. 40 document and supplement. SARS and Occupation Statistics.
- 7.WHO case definition for SARS. Available from: http://www.who.intl/csr/sars/casedefinition/en/.
- 8.Ministry of Health 2003, no. 57 document. SARS and Occupation Statistics.
- 9.Snow J. The cholera near Golden-square, and at Deptford. Med Times Gazette. 1854;9:321–322. [Google Scholar]
- 10.Ming W., Du L., Hua-rei Z. A preliminary study on SARS epidemics, and evaluation of its prevention and control in Guangzhou city. Chinese J Epidemiol. 2003;24:353–357. [Google Scholar]
- 11.Riley S., Fraser C., Donnelly C.A., Ghani A.C., Abu-Raddad L.J., Hedley A.J. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 12.Pang X., Zhu Z., Xu F., Guo J., Gong X., Liu D. Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in Beijing, 2003. JAMA. 2003;290:3215–3221. doi: 10.1001/jama.290.24.3215. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitch M., Cohen T., Cooper B., Robins J.M., Ma S., James L. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly C.A., Ghani A.C., Leung G.M., Hedley A.J., Fraser C., Riley S. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


