Abstract
Community-acquired pneumonia (CAP) occurs more often in early childhood than at almost any other age. Many microorganisms are associated with pneumonia, but individual pathogens are difficult to identify, which poses problems in antibiotic management. This article reviews the common as well as new, emerging pathogens, as well as the guidelines for management of pediatric CAP. Current guidelines for pediatric CAP continue to recommend the use of high-dose amoxicillin for bacterial CAP and azithromycin for suspected atypical CAP (usually caused by Mycoplasma pneumoniae) in children.
Keywords: Community-acquired pneumonia, Pediatric pneumonia, Children, CAP, Cause, Management
Key points
-
•
The diagnosis of community-acquired pneumonia (CAP) in children is not very sensitive or specific.
-
•
Causative pathogens are challenging to isolate, and age is the best predictor of cause.
-
•
A substantial proportion of CAP is mixed bacterial and viral infections; overdiagnosis of mild to moderate CAP can lead to overtreatment.
-
•
The difficulty in differentiating between bacterial and viral pneumonia often leads to unnecessary antibiotic use.
-
•
Current guidelines by the Pediatrics Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) recommend amoxicillin (90 mg/kg/d orally in 2 doses) as the primary therapy for presumed bacterial pneumonia and azithromycin (10 mg/kg on day 1, followed by 5 mg/kg once daily on days 2–5) as the primary therapy for presumed atypical pneumonia in children younger than 5 years or aged 5 years or older.
-
•
For children aged 5 years or older with presumed bacterial CAP who do not have clinical, laboratory, or radiographic evidence that distinguish bacterial CAP from atypical CAP, PIDS/IDSA guidelines note that a macrolide can be added to the β-lactam antibiotic.
-
•
Much of the pediatric CAP guidelines is based on evidence of poor to moderate quality, and there many gaps in the evidence remain.
Introduction
Community-acquired pneumonia (CAP) is fundamentally different in children and in adults. The evaluation, diagnosis, and treatment that distinguish the approach to management in children from that of adult disease is based on the differing causes of childhood pneumonia, and this microbial spectrum has changed over the past 3 decades with the introduction of conjugate vaccines and improved molecular diagnostic assays.
There are 2 main challenges in the diagnosis of CAP: the first is the definition of CAP, particularly in young children, in whom bacterial and viral infections can occur with similar frequencies, and in whom overdiagnosis of mild symptoms and signs may lead to unnecessary antibiotic use; the second is the identification of a causative pathogen, which is frequently impractical and inadequate in children, and in whom the failure to isolate an organism can result in unnecessary antibiotic use. These problems affect the management of CAP, and lead to emergence of resistance as a result of overtreatment. Management decisions are complicated because there are few randomized controlled trials in children that evaluate different antibiotic therapies and treatment duration. Although guidelines exist, they are often based on poor-quality evidence.
The challenge for the general pediatrician is to recognize lower respiratory tract illness, to refer for hospitalization when it is severe, and to appropriately treat with antibiotics if a bacterial pneumonia is suspected. This review focuses on practical issues of clinical relevance for the general pediatrician, including what to elicit from the patient history and examination, keeping in mind not only the infections that are commonly seen but also emerging infectious diseases and those that are less frequently seen but that are severe when they occur.
Cause and epidemiology
The first key clinical issue is to diagnose CAP in children, and then determine which pathogen is responsible. There are discrepant definitions of CAP depending on whether the consideration is epidemiologic, which has more sensitive criteria, or regulatory, which is more specific (Table 1 ). Causative agents in children have been difficult to identify; however, clues in the history may help point toward certain infectious pathogens (Table 2 ). The cause of pediatric pneumonia is usually based on age, because that is the best predictor available (Table 3 ).
Table 1.
Definitions of CAP in children
| World Health Organization79, 80 |
|
| British Thoracic Society43 | Persistent or repetitive fever >38.5°C together with chest recession and an increased respiratory rate |
| Infectious Diseases Society of America33 | Presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital |
Table 2.
Clues to the cause of pneumonia from history and physical examination
| Factor | Possible Agent(s) |
|---|---|
| Host factor | |
| Sickle cell disease | Streptococcus pneumoniae |
| Human immunodeficiency virus infection and CD4+ lymphocyte count of <200/μL | Streptococcus pneumoniae, Haemophilus influenzae, Cryptococcus neoformans, Mycobacterium tuberculosis |
| Structural lung disease (bronchiectasis) | Pseudomonas aeruginosa |
| Travel | |
| Travel to southeast Asia | Burkholderia pseudomallei, Mycobacterium tuberculosis |
| Travel to China, Taiwan, Toronto, Canada, Middle East | Coronavirus causing SARS |
| Travel to tuberculosis-endemic countries | Mycobacterium tuberculosis |
| Travel to desert regions of southwestern United States, and Central and South America | Coccidioides immitis |
| Travel to Ohio and St Lawrence River valleys | Histoplasma capsulatum |
| Travel to Peru | Sporothrix schenckii |
| Travel to Vancouver Island, Canada, and Pacific Northwest (camping, residence) | Cryptococcus gattii |
| Other environmental factors | |
| Pneumonia outbreak in a homeless shelter | Streptococcus pneumoniae, Mycobacterium tuberculosis |
| Lawn mowing in an endemic area, including southcentral and western states and Martha’s Vineyard | Francisella tularensis |
| Exposure to parturient cats, sheep, goats, and cattle in an endemic area, including western and plains states where ranching and rearing of cattle are common | Coxiella burnetii |
| Sleeping in a rose garden, playing on bales of hay | Sporothrix schenckii |
| Exposure to windstorm in an endemic area | Coccidioides immitis, Coxiella burnetii |
| Exposure to bats, excavation or residence in an endemic area | Histoplasma capsulatum |
| Camping, cutting down trees in an endemic area, including the Mississippi River and Ohio River valley basins and around the Great Lakes | Blastomyces dermatitidis |
| Exposure to mouse droppings in an endemic area, including the Four Corners and Yosemite National Park | Hantavirus |
| Immunosuppressed and exposure to hot tub; grocery store mist machine; recent stay in a hotel; visit to or recent stay in a hospital with Legionellaceae-contaminated drinking water | Legionella pneumophila, other Legionellaceae |
Table 3.
Microbial causes of CAP in childhood, according to age in descending order of frequency, and associated testing
| Age Grouping, Cause | Associated Testing33, 81, 82 |
|---|---|
| Birth–20 d | |
| Group B streptococci | Blood cultures should not be routinely performed in fully immunized, nontoxic children in outpatient setting, but should be obtained if children fail to improve or have progressive symptoms or clinically deteriorate after starting antibiotic therapy; blood and pleural fluid culture are insensitive, but there are no established alternatives in children |
| Gram-negative enteric bacteria | |
| Listeria monocytogenes | |
| 3 wk–3 mo | |
| Chlamydia trachomatis | Quadrupling of acute and convalescent serology, NP culture or NP PCR; although IDSA does not recommend diagnostic testing, because reliable and readily available diagnostic tests do not exist |
| RSV | NP swab for PCR or immunofluorescence, viral culture and DFA staining, acute and convalescent serology. Whereas influenza and RSV have winter-spring seasonality, PIV3 is present year-round |
| PIV 3 | |
| Streptococcus pneumoniae | Blood culture (yield <10%), urinary antigen (low specificity, many false-positives because of NP carriage), pneumolysin-based PCR of blood, pleural fluid and secretions |
| Bordetella pertussis | Culture, immunofluorescence assay, PCR assay of NP secretions |
| Staphylococcus aureus | Blood culture, pleural fluid culture |
| 4 mo–4 y | |
| RSV, parainfluenza viruses, influenza virus, adenovirus, rhinovirus | NP swab for PCR or immunofluorescence, viral culture and DFA staining, acute and convalescent serology |
| Streptococcus pneumoniae | Blood culture (yield <10%), urinary antigen (low specificity, many false-positive results because of NP carriage), pneumolysin-based PCR of blood, pleural fluid and secretions |
| Haemophilus influenzae | Blood culture, pleural fluid culture |
| Mycoplasma pneumoniae | Quadrupling of acute and convalescent serology is diagnostic, IgM antibody in acute or early convalescent serum is helpful, throat or NP swab PCR (high specificity and positive predictive value) |
| Mycobacterium tuberculosis | Identify bacteria in culture of sputum or gastric aspirates, with positive tuberculin skin test or interferon γ release assay |
| 5–15 y | |
| Mycoplasma pneumoniae | Quadrupling of acute and convalescent serology is diagnostic, IgM antibody in acute or early convalescent serum is helpful, throat or NP swab PCR (high specificity and positive predictive value) |
| Chlamydia pneumoniae | Quadrupling of acute and convalescent serology, NP culture or NP PCR; although IDSA does not recommend diagnostic testing, because reliable and readily available diagnostic tests do not exist |
| Streptococcus pneumoniae | Blood culture (yield <10%), urinary antigen (low specificity, many false-positive results because of NP carriage), pneumolysin-based PCR of blood, pleural fluid, and secretions |
| Influenza A or B, adenovirus | NP swab for PCR or immunofluorescence, viral culture and DFA staining, acute and convalescent serology |
| Nontypeable Haemophilus influenzae | Blood culture, pleural fluid culture |
| Mycobacterium tuberculosis | Identify bacteria in culture of sputum or gastric aspirates, with positive tuberculin skin test or interferon γ release assay |
| All ages, severe pneumonia requiring admission to intensive care unit: | |
| Streptococcus pneumoniae, Staphylococcus aureus, group A streptococci, Haemophilus influenzae type b, Mycoplasma pneumoniae, adenovirus | |
| Uncommon causes of pediatric CAP: | |
| Viruses: varicella zoster virus, coronaviruses, enteroviruses (coxsackievirus and echovirus), cytomegalovirus, Epstein-Barr virus, mumps virus, herpes simplex virus (in newborns), bocaviruses, polyomaviruses, measles virus, hantavirus | Varicella zoster virus: quadrupling of acute and convalescent serology, immunofluorescent assay of skin secretions; cytomegalovirus, Epstein-Barr virus: quadrupling of acute and convalescent serology, IgM antibody in acute serum; measles virus: quadrupling of acute and convalescent serology, immunofluorescent assay of NP secretions; hantavirus: quadrupling of acute and convalescent serology, IgM antibody in acute serum NP secretions or antibody in serum; sufficiently uncommon that IgM or IgG antibody in serum is essentially diagnostic |
| Chlamydia: Chlamydia psittaci | Quadrupling of acute and convalescent serology is diagnostic |
| Coxiella: Coxiella burnetii | Quadrupling of acute and convalescent serology is diagnostic |
| Bacteria: Streptococcus pyogenes, anaerobic mouth flora (Streptococcus milleri, Peptostreptococcus), nontype B (but typeable) Haemophilus influenzae, Bordetella pertussis, Klebsiella pneumoniae, Escherichia coli, Listeria monocytogenes, Neisseria meningitides (often group Y), Legionella, Burkholderia pseudomallei, Francisella tularensis, Brucella abortus, Leptospira | Francisella tularensis: quadrupling of acute and convalescent serology is diagnostic (blood or sputum culture requires special medium and may pose danger of infection to laboratory workers and they should be notified before handling specimen); Legionella pneumophila and other Legionella species: sputum or tracheal aspirate culture, urinary antigen (urinary antigen tests detect only Legionella pneumophila antigen); Brucella abortus: blood culture, quadrupling of acute and convalescent serology |
| Fungi: Coccidioides immitis, Histoplasma capsulatum, Blastomyces dermatitidis | Urinary Histoplasma or Blastomyces antigen, stain or culture of respiratory tract secretions, serum IgM antibody; quadrupling of acute and convalescent serology |
Abbreviations: DFA, direct fluorescent antibody; IDSA, Infectious Diseases Society of America; NP, nasopharyngeal; PCR, polymerase chain reaction.
Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) have been characterized as 2 bacteria predominately responsible for cases of fatal pneumonia in children.1 However, widespread introduction of Hib and pneumococcal conjugate vaccines has led to significant declines, especially of Hib, although Streptococcus pneumoniae is still the predominant bacteria isolated from bacterial CAP in children. There is increasing recognition of the prevalence of mixed bacterial and viral infections, which have been documented in 23% to 33% of cases of pneumonia.2, 3, 4
Atypical organisms (eg, Mycoplasma pneumoniae, Chlamydia pneumoniae) account for up to a third of cases.5, 6 A new Chlamydia-like organism, Simkania negevensis, has been associated with bronchiolitis and pneumonia in children,7 and has also been found in healthy, asymptomatic individuals and may be an opportunistic organism rather than a true pathogen.8
Viral pathogens are responsible for most clinical disease in younger children, accounting for 77% of clinical pneumonia in children younger than 1 year compared with 59% in those older than 2 years.4, 9 Viruses account for 30% to 67% of pediatric CAP, with influenza A, respiratory syncytial virus (RSV), and parainfluenza virus (PIV) 1, 2, and 3 most commonly identified.4 A study by Singleton and colleagues10 recovered respiratory viruses from 90% of Alaskan children younger than 3 years hospitalized with respiratory infections. In that case-control study of 865 children, RSV, PIV, human metapneumovirus (hMPV), and influenza were significantly more common in hospitalized cases than control children, but rhinovirus, adenovirus, and coronavirus were not.
The high occurrence of asymptomatic carriage, such as in up to a third of asymptomatic children harboring rhinovirus, complicates the frequent detection of these respiratory viruses10, 11, 12; the causative nature of many respiratory viruses in pneumonia, particularly ones identified by sensitive new molecular diagnostics, remain unclear. However, rhinovirus identification has been associated with bronchiolitis, asthma, and wheezing.13, 14 hMPV, isolated in 2001,15 has been recovered in 3.8% to 8.3% of isolates of nasopharyngeal and throat specimens of hospitalized children or children with CAP.16, 17 Human bocavirus, first described in 2005, has been detected in up to 19% of clinical samples, mainly in infants and young children18; its detection in serum and stool suggests that the virus may cause systemic disease.18 In 2007, WU and KI polyomaviruses were detected in the respiratory tract samples of adults and children,19, 20 and subsequently shown to be present in respiratory secretions in patients with acute respiratory illness.21, 22
Severe acquired respiratory syndrome (SARS)-associated coronavirus was the first novel coronavirus to be characterized among a succession of other novel coronaviruses that decade.23, 24, 25 Using sensitive reverse transcription polymerase chain reaction (PCR) assays, Dominguez and colleagues26 detected coronavirus RNAs in 5% of pediatric respiratory specimens, and 41% of coronavirus-positive patients had evidence of a lower respiratory tract infection. Twenty-six percent of that group presented with vomiting or diarrhea, and 8% with meningoencephalitis or seizures, which suggests that coronavirus may have more systemic involvement than previously believed.
Fungal pathogens such as Histoplasma, Coccidioides, Blastomyces, and Cryptococcus can cause pneumonia the immunocompromised patient population and may cause clinical illness in immunocompetent hosts, but usually not with the presentation of CAP. Blastomyces can cause prolonged fever and respiratory symptoms that mimic a prolonged CAP; however. Cryptococcus gattii, which can infect immunocompetent hosts,27 has caused an increasing number of infections in the US Pacific Northwest since 2004 and has been detected in the southeastern United States.28 The pathogen can cause cryptococcomas in the lungs, and in 1 report, 54% of infected patients had documented pneumonia.27 Mycobacterium tuberculosis and nontuberculous mycobacteria can likewise cause CAP, but tend to be limited to people with high-risk exposures. Similarly, other high-risk exposures suggest specific causes, as outlined in Table 2.
Patient history
Symptoms (fever, chills, cough) of pneumonia can overlap significantly with a spectrum of conditions, such as bacterial sepsis or severe anemia, making differentiation between these diagnoses challenging. Signs such as crackles and egophony are more specific, but are often absent in clinical pneumonia in children. Chest radiography is considered the gold standard for confirmation of pneumonia,29 but is also of questionable benefit in children, as discussed later. The limitation in diagnosis is reflected in the various definitions of pediatric CAP that exist (see Table 1), none of which is considered sufficiently sensitive or specific, and few of which have been validated in children.30 For example, the World Health Organization (WHO) criteria for mild to moderate CAP are based on cough or breathing difficulties and age-adjusted tachypnea; such a definition places CAP with all other types of lower respiratory tract disease.
Patient examination
Clinical features in children with CAP vary with the age of the child, although none of them is very specific. Cevey-Macherel and colleagues2 showed that more than a fifth of patients diagnosed with CAP as defined by WHO guidelines had completely normal breath sounds on admission, and auscultation was ruled to be poorly sensitive and specific in diagnosing WHO guideline-defined CAP in young children. However, WHO guidelines rely solely on respiratory rate and, using other criteria for a diagnosis of pneumonia or lower respiratory tract infection, respiratory rate has been shown to be less sensitive and specific in the first 3 days of illness.31 In a study that defined pneumonia/lower respiratory tract infection as the presence of crepitations (crackles), wheeze, bronchial breathing, or chest radiograph (CXR) abnormalities, respiratory rates of less than 40 breaths/min were seen in 55% of children older than 35 months with a diagnosis of pneumonia.32 Canadian guidelines regard oxygenation as a good indication of the severity of disease,29 and oxygen saturation is recommended by the Infectious Diseases Society of America (IDSA) as a guide for referral of care and further diagnostic testing.33
Clinical features do not reliably distinguish between viral, bacterial, and atypical pneumonias.34, 35 Pneumococcal pneumonia has been associated with a history of fever and breathlessness and signs of tachypnea, indrawing, and toxic appearance, but these features can be indistinguishable from staphylococcal pneumonia at the beginning of illness. Similarly, mycoplasma pneumonia has been associated with cough, chest pain, and wheezing, symptoms being worse than the signs, as well as nonrespiratory symptoms such as arthralgia and headache, but studies have not successfully distinguished viral and bacterial CAP or between types of CAP by clinical signs alone.36, 37 In addition, many children with CAP have mixed bacterial and viral infections. Mycoplasma pneumonia is more common in children aged 5 years or younger, and is typically characterized by slow progression, sore throat, low-grade fever and cough developing over 3 to 5 days. Although some children have more abrupt onset of symptoms or higher fevers, this pattern may help to distinguish it from pneumococcal or staphylococcal pneumonia, and influence treatment decisions, because there are no rapid laboratory tests that allow clear distinction between classic bacterial and atypical pneumonia.
Diagnostic evaluation
Neither IDSA nor the British Thoracic Society (BTS) recommends CXRs for confirmation of suspected CAP in patients well enough to be treated in an outpatient setting. CXRs in children, unlike in adults with pneumonia, may not show any abnormalities, especially if taken at the onset of illness.38 Beyond providing radiographic evidence of opacification, the use of a CXR is limited and has not been shown to correlate with clinical signs39 or to help differentiate bacterial from viral pneumonia.40 A prospective study in Switzerland enrolling 99 patients found that only 79% of patients had radiographic consolidation, with poor correlation between radiographic findings and diminished breath sounds, and no association with severity or cause of pneumonia.2 A prospective study in the United Kingdom by Clark and colleagues41 showed that lobar CXR changes were not associated with severity. In a study from Brazil, upper lobe involvement was shown to have 84% specificity and 65% positive predictive value for severity in children aged 1 year or younger hospitalized with CAP.42
Whereas IDSA guidelines support a posteroanterior (PA) and lateral CXR in patients with suspected or documented hypoxemia or significant respiratory distress, and in those who have failed an initial course of antibiotic therapy, BTS guidelines do not recommend that a lateral CXR should be performed routinely.43 This recommendation was based on a retrospective study from the United States that indicated that a frontal CXR was 100% sensitive and specific for lobar consolidation but would underdiagnose nonlobar infiltrates in 15% of cases. The investigators considered the clinical implications to be unclear. However, patients hospitalized for management of CAP (Box 1 ) should have both PA and lateral CXRs, to ensure that all infiltrates are detected and to assess for pleural effusion. Follow-up CXRs are unnecessary in children who recover uneventfully from an episode of CAP, but should be obtained in children who do not improve clinically and in those with progressive symptoms or clinical deterioration after 48 to 72 hours of antibiotic therapy.33, 43
Box 1. Severe pediatric CAP and criteria for hospital admission.
Indications of severe pediatric CAP43:
-
1.
Temperature greater than 38.5°C
-
2.
Respiratory rate: in infants, more than 70 breaths/min; in older children, more than 50 breaths/min
-
3.
Moderate to severe recession
-
4.
Nasal flaring
-
5.
Cyanosis
-
6.
Grunting respiration
-
7.
In infants, intermittent apnea and not feeding
-
8.
Tachycardia
-
9.
Signs of dehydration
-
10.
Capillary refill time 2 seconds or more
Criteria for hospital admission of a child with CAP33, 43:
-
1.
Hypoxemia with O2 less than 90% at sea level
-
2.
Infants younger than 3 to 6 months
-
3.
Suspected or documented CAP caused by a pathogen with increased virulence, such as community-associated (CA) methicillin-resistant Staphylococcus aureus (MRSA)
-
4.
Concern about careful observation at home; those who are unable to comply with therapy; those unable to be followed up
Transfer to intensive care should be considered if33, 43:
-
1.
Oxygen saturation is less than 92% on inspired oxygen of 50% or greater
-
2.
Severe respiratory distress
-
3.
Sustained tachycardia, inadequate blood pressure, or need for pharmacologic support of blood pressure or perfusion
-
4.
Exhaustion
-
5.
Apnea
-
6.
Slow breathing
-
7.
Altered mental status
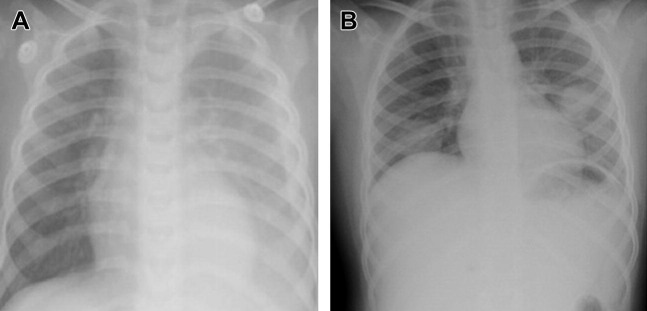
Most patients with bacterial pneumonia present with a lobar infiltrate (Fig. 1 ). Interstitial infiltrates can be found in bacterial as well as viral and atypical pneumonia.35 Mycoplasma pneumoniae pneumonia can have diffuse infiltrate radiologically out of proportion with clinical findings; lobar consolidation, atelectasis, nodular infiltration, and hilar adenopathy have also been described. Streptococcus pneumoniae pneumonia can present with round infiltrates (see Fig. 1B), as can also be seen with other bacteria.
Fig. 1.
CXRs of CAP in: (A) a 15-month-old patient with group A Streptococcus and (B) a 10-year-old patient with ulcerative colitis on immunosuppression, with Legionella pneumophila. Other causes of round pneumonia include Streptococcus pneumoniae, Klebsiella pneumoniae, Haemophilus influenzae, Coxiella burnetii, and Mycobacterium tuberculosis. Fungal infections, hydatid cysts, and lung abscesses may have a similar appearance.
Microbiologic recovery sampled directly from the infected region of lung is the gold standard, although less invasive sampling methods are usually used to achieve a diagnosis (see Table 3). For a child with suspected CAP in the community, there are no indications for any general investigations. However, if a child with CAP is hospitalized (see Box 1), testing for potential pathogens as well as obtaining acute phase reactants (white blood count [WBC], C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], procalcitonin [PCT]) may be helpful for clinical management. No studies have found this to be definitively useful in differentiating the cause or severity of CAP.44, 45 Don and colleagues46 studied 101 children and showed that an increase of 4 serum nonspecific inflammatory markers (WBC, CRP, ESR, PCT) was significantly associated with radiographic evidence of CAP; however, the role of these studies in the screening for bacterial and viral CAP was limited.
A study by Lahti and colleagues47 involving children 6 months and older showed that inducing sputum production by inhalation of 5% hypertonic saline for 5 to 10 minutes and then aspirating or expectorating a sputum sample provided good-quality sputum specimens with 90% microbiological yield. However, the practicality of this relatively labor-intensive approach in a busy outpatient clinic is debatable. New molecular diagnostic tests have become available, particularly rapid antigen detection for respiratory viruses. Despite this development, one-quarter to one-third of patients still fail to yield an obvious cause.48
Streptococcus pneumoniae is the most common bacterial pathogen in most studies but remains challenging to identify, because less than 10% of children with pneumonia are bacteremic and there are no definitive, noninvasive, and accurate tests.3, 49 Urinary antigen tests for Streptococcus pneumoniae are not recommended by IDSA for the diagnosis of pneumococcal pneumonia in children, because false-positive tests are common and likely attributable to nasopharyngeal carriage.50 Diagnosis of Mycoplasma pneumoniae infection can be made by testing for IgM to Mycoplasma pneumoniae in serum or plasma by enzyme-linked immunosorbent assay (ELISA), or by PCR testing of nasal, throat, or sputum specimens. PCR seems to detect more Mycoplasma infections than IgM ELISA testing, but a single standardized test has not been used across studies, so definitive data are difficult to obtain. Rapid IgM testing kits are being assessed, and may be a promising way to more rapidly detect Mycoplasma infection, but none has been validated as highly sensitive and specific in multiple studies.33
Treatment
The main issue for a general pediatrician in management of suspected pediatric CAP is whether or not to treat with antibiotics. This decision is complicated because it is difficult to distinguish bacterial from viral pneumonia, and hence to decide whether antibiotics may be warranted. There are also few randomized controlled trials to guide antibiotic choice and duration. Because age is the best predictor of cause of pediatric pneumonia, guidelines for treatment are typically categorized by age and suspected pathogens.33 BTS guidelines recommend that children with a clear clinical diagnosis of pneumonia should receive antibiotics, given that bacterial and viral pneumonia cannot be reliably differentiated. However, because viral pathogens are responsible for most of clinical disease in preschool-aged children with CAP, IDSA does not routinely recommend antimicrobial therapy for that group.
Streptococcus pneumoniae is the most prominent bacterial pathogen across all age groups, hence amoxicillin is recommended as first-line therapy for previously healthy, appropriately immunized patients.33 Because the Hib vaccine offers no protection against nontypeable Haemophilus influenzae, there have not been alterations to choice of empiric antibiotic therapy for vaccinated children with suspected bacterial pneumonia. Atypical bacterial pathogens such as Mycoplasma pneumoniae are more prominent in school-aged children and older patients, and macrolide therapy is recommended for those with compatible findings, generally based on clinical findings. However, there are few high-quality data on the effectiveness of macrolide antibiotics in the treatment of children with Mycoplasma pneumonia, so the recommendation of treatment with a macrolide for this condition is listed as a weak recommendation in the PIDS/IDSA guidelines.33 Bacterial-viral coinfections have been well documented to occur with influenza and Streptococcus pneumoniae, Staphylococcus aureus, and group A Streptococcus.51 Hence, children with serious viral lower respiratory tract infections may still benefit from empiric therapy for bacterial agents. Adjunctive treatment with corticosteroids has been studied but has not shown proven benefit on outcomes.52, 53
The summary PIDS/IDSA guidelines for CAP in children continue to recommend amoxicillin (90 mg/kg/d orally in 2 doses) as the primary therapy for presumed bacterial pneumonia and azithromycin (10 mg/kg on day 1, followed by 5 mg/kg once daily on days 2–5) as the primary therapy for presumed atypical pneumonia in children younger than 5 years or aged 5 years or older. For children aged 5 years or older with presumed bacterial CAP who do not have clinical, laboratory, or radiographic evidence to distinguish bacterial CAP from atypical CAP, PIDS/IDSA guidelines note that a macrolide can be added to the β-lactam antibiotic.33 As noted earlier, there are no definitive clinical, laboratory, or radiographic findings that distinguish bacterial from atypical CAP, so the decision to use or add a macrolide remains based on an overall indication from the combined findings, rather than from a clearly defined set of criteria based on these findings.
Aside from influenza, there are no data from prospective, controlled studies for antiviral therapy against viruses associated with pediatric CAP. Adamantanes and neuraminidase inhibitors are effective against susceptible strains of influenza A, and neuraminidase inhibitors are effective for susceptible strains of influenza B. Because substantial genetic variation can occur in influenza from year to year, influenza virus strains can become resistant to either class of antiviral agents; most strains of influenza A isolated since the 2005 to 2006 season have been adamantine-resistant.54 The recommended doses of antiviral agents for seasonal influenza were developed for fully susceptible strains33 and evaluated in trials in which treatment was provided early, usually within 48 hours of onset of symptoms.55 The degree of benefit provided by treatment after 48 hours of symptoms has not been defined.
Few studies have looked at the appropriate duration of antimicrobial therapy for CAP in children. Treatment courses of 10 days for β-lactams and 5 days for azithromycin have been best studied, although shorter courses have been studied showing no difference in acute cure or relapse rates between the groups.56, 57, 58 Shorter courses are acknowledged to be just as effective, particularly for milder cases, although prolonged courses may be required for some pathogens, such as CA-MRSA.59
Similarly, there are no randomized controlled studies evaluating the optimum time to switch from parenteral to oral therapy; hence IDSA and BTS guidelines do not provide a statement recommendation, although Canadian guidelines recommend switching from parenteral to oral therapy after 2 to 4 days if the patients are afebrile without complications.29
Treatment resistance
Antimicrobial-resistant Streptococcus pneumoniae has been a recognized problem since the 1990s.60 The hope that the heptavalent pneumococcal conjugate vaccine (PCV7), which contains the 5 serotypes accounting for 89% of penicillin-resistant pneumococcal isolates in the United States,61 would decrease antimicrobial-resistant pneumococci in the community has seen marginal success. One longitudinal surveillance program showed penicillin-resistant isolates decreasing from 21.5% to 14.6%, but intermediate penicillin resistance increasing from 2.7% to 17.9%, after introduction of PCV7.62
The effect of antibiotic resistance on clinical outcomes in children is less evident. Pneumococcal pneumonias that were penicillin-resistant or penicillin-sensitive were not associated with differences in outcome, hospital course, or complications.63 No association between antimicrobial resistance and treatment failure has yet been shown in children. To minimize antimicrobial resistance, IDSA recommends limiting antibiotic exposure, using antibiotics with narrow spectrum activity and for shortest effective duration.33 However none of these recommendations is based on strong-quality evidence.
Fluoroquinolones are generally avoided in children younger than 18 years because of concerns for arthropathy, but experience with its use to treat children for a variety of serious infectious diseases has led to consensus statements that fluoroquinolones can treat specific infections in children safely and effectively, and may be considered as an alternative therapy in serious infectious diseases.64 Bradley and colleagues65 enrolled 738 children from 6 months to 16 years with CAP in a noninferiority trial, which showed similar cure rates with levofloxacin as with a comparator drug. Fifty-two percent of children treated with levofloxacin experienced 1 or more adverse events, compared with 53% in the comparator-treated children; musculoskeletal adverse events (arthralgia, myalgia) were experienced in 4% versus 3% in the comparator group who received current standard of care antibiotics.65 None of these differences was statistically significant. In an era of emerging drug resistance, fluoroquinolones may be an effective alternative therapy in treating children with CAP.
Evaluation of outcome and prevention
Children with CAP who are on adequate therapy should clinically improve within 48 to 72 hours. If there is no improvement, possible complications to be considered include pleural effusions and empyema, necrotizing pneumonia, septicemia and metastatic infection (such as Staphylococcus aureus causing osteomyelitis or septic arthritis), and hemolytic-uremic syndrome. All of these complications require further investigation and possible hospitalization. Children with severe pneumonia, empyema, and lung abscess should be followed up after discharge until complete resolution clinically and on CXR.43
Vaccines play a crucial part in prevention of pneumonia. Pertussis causes pneumonia in about 5% of all reported cases, which doubles to 11.8% of reported cases for those younger than 6 months.66 Pneumonia complicating measles occurs in up to 27% to 77%, depending if the study was based in the community or hospital setting.67 Half of measles-related pneumonias are caused by bacterial superinfection, and it contributes to 56% to 86% of all deaths in measles.67
Influenza vaccines can prevent 87% of influenza-associated pneumonia hospitalizations,68 including pneumococcal and CA-MRSA superinfection.69, 70 Pneumococcal conjugate vaccines have decreased radiologically confirmed pneumonia by 20% to 37% and 67% to 87% of vaccine-serotype bacteremic pneumonia.71, 72 The distribution of the 13-valent pneumococcal conjugate vaccine (PCV13) in 2010 may address the most common replacement serotypes that have been increasing since introduction of the 7-valent pneumococcal conjugate vaccine in the United States in 2000.73 Particularly, PCV13 contains the additional serotypes associated with empyema and necrotizing pneumonia,74, 75 and thus it is hoped that the number of these complications will decrease. For high-risk infants, RSV-specific monoclonal antibody can decrease the risk of severe pneumonia and hospitalization.
Summary
Pneumonia occurs more often in early childhood than at any other age, with the exception of adults older than 75 years, and kills more children than any other disease worldwide.76 The central clinical issue of CAP in children is that the diagnosis is typically not through microbiologic isolation, but rather through inference and deductions from clinical symptoms and signs, and supported by radiography and serum laboratory tests. This approach requires that the general pediatrician has a high level of suspicion and attempts to discern the cause as bacterial or viral, because this has implications for antimicrobial management.
CAP is changing, both in cause and in management approaches as more molecular diagnostics become available and well-performed trials are conducted. Despite this finding, although guidelines are available for patients with severe pediatric CAP, recommendations for mild to moderate cases, which constitute most cases seen by the general pediatrician, are based on evidence of poor to moderate quality.77 This situation can lead to overtreatment41 and, given the imprecise diagnostic methods available, unnecessary antibiotic treatment. There remain substantial gaps in the evidence for optimum management of pediatric CAP, from appropriate criteria for hospitalization to reference standards for identifying cases that need antibiotics, to what constitutes optimal antibiotic therapy.78
Footnotes
Disclosures: No potential conflicts of interest.
References
- 1.Levine O.S., O'Brien K.L., Deloria-Knoll M. The pneumonia etiology research for child health project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(Suppl 2):S93–S101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cevey-Macherel M., Galetto-Lacour A., Gervaix A. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168(12):1429–1436. doi: 10.1007/s00431-009-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juven T., Mertsola J., Waris M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Michelow I.C., Olsen K., Lozano J. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 5.Baer G., Engelcke G., Abele-Horn M. Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community-acquired pneumonia in hospitalised children and adolescents. Eur J Clin Microbiol Infect Dis. 2003;22(12):742–745. doi: 10.1007/s10096-003-1037-9. [DOI] [PubMed] [Google Scholar]
- 6.Kurz H., Gopfrich H., Wabnegger L. Role of Chlamydophila pneumoniae in children hospitalized for community-acquired pneumonia in Vienna, Austria. Pediatr Pulmonol. 2009;44(9):873–876. doi: 10.1002/ppul.21059. [DOI] [PubMed] [Google Scholar]
- 7.Heiskanen-Kosma T., Paldanius M., Korppi M. Simkania negevensis may be a true cause of community acquired pneumonia in children. Scand J Infect Dis. 2008;40(2):127–130. doi: 10.1080/00365540701558680. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg D., Banerji A., Friedman M.G. High rate of Simkania negevensis among Canadian Inuit infants hospitalized with lower respiratory tract infections. Scand J Infect Dis. 2003;35(8):506–508. doi: 10.1080/00365540310014648. [DOI] [PubMed] [Google Scholar]
- 9.Cilla G., Onate E., Perez-Yarza E.G. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80(10):1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton R.J., Bulkow L.R., Miernyk K. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen R.R., Wieringa J., Koekkoek S.M. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49(7):2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltola V., Waris M., Osterback R. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197(3):382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 13.Iwane M.K., Prill M.M., Lu X. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204(11):1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 14.Miller E.K., Bugna J., Libster R. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129(1):e60–e67. doi: 10.1542/peds.2011-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams J.V., Edwards K.M., Weinberg G.A. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201(12):1890–1898. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf D.G., Greenberg D., Shemer-Avni Y. Association of human metapneumovirus with radiologically diagnosed community-acquired alveolar pneumonia in young children. J Pediatr. 2010;156(1):115–120. doi: 10.1016/j.jpeds.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schildgen O., Muller A., Allander T. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304. doi: 10.1128/CMR.00030-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allander T., Andreasson K., Gupta S. Identification of a third human polyomavirus. J Virol. 2007;81(8):4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaynor A.M., Nissen M.D., Whiley D.M. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3(5):e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin F., Zheng M., Li H. WU polyomavirus in children with acute lower respiratory tract infections, China. J Clin Virol. 2008;42(1):94–102. doi: 10.1016/j.jcv.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan X.H., Jin Y., Xie Z.P. Prevalence of human KI and WU polyomaviruses in children with acute respiratory tract infection in China. J Clin Microbiol. 2008;46(10):3522–3525. doi: 10.1128/JCM.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 24.Kuiken T., Fouchier R.A., Schutten M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rota P.A., Oberste M.S., Monroe S.S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez S.R., Robinson C.C., Holmes K.V. Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol. 2009;81(9):1597–1604. doi: 10.1002/jmv.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris J.R., Lockhart S.R., Debess E. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis. 2011;53(12):1188–1195. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 28.Sellers B., Hall P., Cine-Gowdie S. Cryptococcus gattii: an emerging fungal pathogen in the southeastern United States. Am J Med Sci. 2012;343(6):510–511. doi: 10.1097/MAJ.0b013e3182464bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadavji T., Law B., Lebel M.H. A practical guide for the diagnosis and treatment of pediatric pneumonia. CMAJ. 1997;156(5):S703–S711. [PMC free article] [PubMed] [Google Scholar]
- 30.Langley J.M., Bradley J.S. Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med. 2005;6(Suppl 3):S9–S13. doi: 10.1097/01.PCC.0000161932.73262.D7. [DOI] [PubMed] [Google Scholar]
- 31.Palafox M., Guiscafre H., Reyes H. Diagnostic value of tachypnoea in pneumonia defined radiologically. Arch Dis Child. 2000;82(1):41–45. doi: 10.1136/adc.82.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherian T., John T.J., Simoes E. Evaluation of simple clinical signs for the diagnosis of acute lower respiratory tract infection. Lancet. 1988;2(8603):125–128. doi: 10.1016/s0140-6736(88)90683-6. [DOI] [PubMed] [Google Scholar]
- 33.Bradley J.S., Byington C.L., Shah S.S. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klig J.E. Office pediatrics: current perspectives on the outpatient evaluation and management of lower respiratory infections in children. Curr Opin Pediatr. 2006;18(1):71–76. doi: 10.1097/01.mpo.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 35.Korppi M., Don M., Valent F. The value of clinical features in differentiating between viral, pneumococcal and atypical bacterial pneumonia in children. Acta Paediatr. 2008;97(7):943–947. doi: 10.1111/j.1651-2227.2008.00789.x. [DOI] [PubMed] [Google Scholar]
- 36.Bettenay F.A., de Campo J.F., McCrossin D.B. Differentiating bacterial from viral pneumonias in children. Pediatr Radiol. 1988;18(6):453–454. doi: 10.1007/BF00974077. [DOI] [PubMed] [Google Scholar]
- 37.Isaacs D. Problems in determining the etiology of community-acquired childhood pneumonia. Pediatr Infect Dis J. 1989;8(3):143–148. [PubMed] [Google Scholar]
- 38.Coote N., McKenzie S. Diagnosis and investigation of bacterial pneumonias. Paediatr Respir Rev. 2000;1(1):8–13. doi: 10.1053/prrv.2000.0002. [DOI] [PubMed] [Google Scholar]
- 39.Hazir T., Fox L.M., Nisar Y.B. Ambulatory short-course high-dose oral amoxicillin for treatment of severe pneumonia in children: a randomised equivalency trial. Lancet. 2008;371(9606):49–56. doi: 10.1016/S0140-6736(08)60071-9. [DOI] [PubMed] [Google Scholar]
- 40.Virkki R., Juven T., Rikalainen H. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57(5):438–441. doi: 10.1136/thorax.57.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark J.E., Hammal D., Spencer D. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92(5):394–398. doi: 10.1136/adc.2006.097402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kin Key N., Araujo-Neto C.A., Nascimento-Carvalho C.M. Severity of childhood community-acquired pneumonia and chest radiographic findings. Pediatr Pulmonol. 2009;44(3):249–252. doi: 10.1002/ppul.20988. [DOI] [PubMed] [Google Scholar]
- 43.Harris M., Clark J., Coote N. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 44.Korppi M. Non-specific host response markers in the differentiation between pneumococcal and viral pneumonia: what is the most accurate combination? Pediatr Int. 2004;46(5):545–550. doi: 10.1111/j.1442-200x.2004.01947.x. [DOI] [PubMed] [Google Scholar]
- 45.Korppi M., Remes S., Heiskanen-Kosma T. Serum procalcitonin concentrations in bacterial pneumonia in children: a negative result in primary healthcare settings. Pediatr Pulmonol. 2003;35(1):56–61. doi: 10.1002/ppul.10201. [DOI] [PubMed] [Google Scholar]
- 46.Don M., Valent F., Korppi M. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int. 2009;51(1):91–96. doi: 10.1111/j.1442-200X.2008.02678.x. [DOI] [PubMed] [Google Scholar]
- 47.Lahti E., Peltola V., Waris M. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64(3):252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 48.Scott J.A., Brooks W.A., Peiris J.S. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118(4):1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vuori E., Peltola H., Kallio M.J. Etiology of pneumonia and other common childhood infections requiring hospitalization and parenteral antimicrobial therapy. SE-TU Study Group. Clin Infect Dis. 1998;27(3):566–572. doi: 10.1086/514697. [DOI] [PubMed] [Google Scholar]
- 50.Dowell S.F., Garman R.L., Liu G. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin Infect Dis. 2001;32(5):824–825. doi: 10.1086/319205. [DOI] [PubMed] [Google Scholar]
- 51.Madhi S.A., Klugman K.P. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorman S.K., Slavik R.S., Marin J. Corticosteroid treatment of severe community-acquired pneumonia. Ann Pharmacother. 2007;41(7):1233–1237. doi: 10.1345/aph.1H660. [DOI] [PubMed] [Google Scholar]
- 53.Weiss A.K., Hall M., Lee G.E. Adjunct corticosteroids in children hospitalized with community-acquired pneumonia. Pediatrics. 2011;127(2):e255–e263. doi: 10.1542/peds.2010-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bright R.A., Shay D.K., Shu B. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA. 2006;295(8):891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- 55.Heinonen S., Silvennoinen H., Lehtinen P. Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51(8):887–894. doi: 10.1086/656408. [DOI] [PubMed] [Google Scholar]
- 56.Pakistan Multicentre Amoxycillin Short Course Therapy (MASCOT) pneumonia study group Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double-blind trial. Lancet. 2002;360(9336):835–841. doi: 10.1016/S0140-6736(02)09994-4. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal G., Awasthi S., Kabra S.K. Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ. 2004;328(7443):791. doi: 10.1136/bmj.38049.490255.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haider B.A., Saeed M.A., Bhutta Z.A. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD005976.pub2. CD005976. [DOI] [PubMed] [Google Scholar]
- 59.Blaschke A.J., Heyrend C., Byington C.L. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289–294. doi: 10.1097/INF.0b013e3182002d14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breiman R.F., Butler J.C., Tenover F.C. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271(23):1831–1835. [PubMed] [Google Scholar]
- 61.Richter S.S., Heilmann K.P., Coffman S.L. The molecular epidemiology of penicillin-resistant Streptococcus pneumoniae in the United States, 1994-2000. Clin Infect Dis. 2002;34(3):330–339. doi: 10.1086/338065. [DOI] [PubMed] [Google Scholar]
- 62.Richter S.S., Heilmann K.P., Dohrn C.L. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004-2005. Clin Infect Dis. 2009;48(3):e23–e33. doi: 10.1086/595857. [DOI] [PubMed] [Google Scholar]
- 63.Tan T.Q., Mason E.O., Jr., Barson W.J. Clinical characteristics and outcome of children with pneumonia attributable to penicillin-susceptible and penicillin-nonsusceptible Streptococcus pneumoniae. Pediatrics. 1998;102(6):1369–1375. doi: 10.1542/peds.102.6.1369. [DOI] [PubMed] [Google Scholar]
- 64.Committee on Infectious Diseases The use of systemic fluoroquinolones. Pediatrics. 2006;118(3):1287–1292. doi: 10.1542/peds.2006-1722. [DOI] [PubMed] [Google Scholar]
- 65.Bradley J.S., Arguedas A., Blumer J.L. Comparative study of levofloxacin in the treatment of children with community-acquired pneumonia. Pediatr Infect Dis J. 2007;26(10):868–878. doi: 10.1097/INF.0b013e3180cbd2c7. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention (CDC) Pertussis–United States, 1997-2000. MMWR Morb Mortal Wkly Rep. 2002;51(4):73–76. [PubMed] [Google Scholar]
- 67.Duke T., Mgone C.S. Measles: not just another viral exanthem. Lancet. 2003;361(9359):763–773. doi: 10.1016/S0140-6736(03)12661-X. [DOI] [PubMed] [Google Scholar]
- 68.Allison M.A., Daley M.F., Crane L.A. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003-2004 season. J Pediatr. 2006;149(6):755–762. doi: 10.1016/j.jpeds.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 69.Ampofo K., Bender J., Sheng X. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122(2):229–237. doi: 10.1542/peds.2007-3192. [DOI] [PubMed] [Google Scholar]
- 70.Finelli L., Fiore A., Dhara R. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122(4):805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 71.Cutts F.T., Zaman S.M., Enwere G. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 72.Klugman K.P., Madhi S.A., Huebner R.E. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 73.Pilishvili T., Lexau C., Farley M.M. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 74.Kalaskar A.S., Heresi G.P., Wanger A. Severe necrotizing pneumonia in children, Houston, Texas, USA. Emerg Infect Dis. 2009;15(10):1696–1698. doi: 10.3201/eid1510.090589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Obando I., Munoz-Almagro C., Arroyo L.A. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis. 2008;14(9):1390–1397. doi: 10.3201/eid1409.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black R.E., Cousens S., Johnson H.L. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 77.Woodhead M. Community-acquired pneumonia guidelines: much guidance, but not much evidence. Eur Respir J. 2002;20(1):1–3. doi: 10.1183/09031936.02.01042002. [DOI] [PubMed] [Google Scholar]
- 78.Esposito S., Principi N. Unsolved problems in the approach to pediatric community-acquired pneumonia. Curr Opin Infect Dis. 2012;25(3):286–291. doi: 10.1097/QCO.0b013e328352b60c. [DOI] [PubMed] [Google Scholar]
- 79.WHO guidelines on detecting pneumonia in children. Lancet. 1991;338(8780):1453–1454. [PubMed] [Google Scholar]
- 80.World Health Organization . WHO; Geneva (Switzerland): 1991. Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. [Google Scholar]
- 81.Kumar P., McKean M.C. Evidence based paediatrics: review of BTS guidelines for the management of community acquired pneumonia in children. J Infect. 2004;48(2):134–138. doi: 10.1016/j.jinf.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 82.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346(6):429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]