Abstract
Effective prevention, diagnosis, and treatment of infectious diseases after transplantation are key factors contributing to the success of organ transplantation. Most transplant patients experience different kinds of infections during the first year after transplantation. Children are at particular risk of developing some types of infections by virtue of lack of immunity although they may be at risk for other types due the effect of immunosuppressive regimens necessary to prevent rejection. Direct consequences of infections result in syndromes such as mononucleosis, pneumonia, gastroenteritis, hepatitis, among other entities. Indirect consequences are mediated through cytokines, chemokines, and growth factors elaborated by the transplant recipient in response to microbial replication and invasion, which contribute to the net state of immunosuppression among other effects. This review summarizes the major infections that occur after pediatric organ transplantation, highlighting the current treatment and prevention strategies, based on the available data and/or consensus.
Keywords: Transplantation, Pediatric, Infections, Posttransplant, Prophylaxis
Organ transplantation is the most practical means of rehabilitating patients with a variety of forms of end organ dysfunction. This procedure is arguably the outstanding clinical biomedical accomplishment of the last 3 decades. Potent immunosuppressive drugs have dramatically reduced the incidence of rejection of transplanted organs, but have also increased the susceptibility of patients to opportunistic infections.1 Thus, the success of organ transplantation is dependent in part on effective prevention, diagnosis, and treatment of infectious diseases after transplantation. To this end, emphasis is increasingly being placed on prevention. Most transplant patients will have evidence of microbial invasion in the first year after transplant. The effects of this microbial invasion are diverse, resulting in direct and indirect consequences. The direct consequences result in a variety of clinical infectious disease syndromes such as mononucleosis, pneumonia, gastroenteritis, hepatitis, among other entities. The indirect consequences are mediated through cytokines, chemokines, and growth factors elaborated by the transplant recipient in response to microbial replication and invasion, which contribute to the net state of immunosuppression, the pathogenesis of acute and chronic allograft injury, and in some cases, the development of lymphoproliferative or malignant disorders.
General principles and risk factors for infection
The risk of infection in the solid organ transplant patient is largely determined by the interaction of 3 factors: technical/anatomic factors that involve the transplant procedure itself, and the perioperative aspects of care such as the management of vascular access, drains, and the endotracheal tube; environmental exposures (Box 1 ); and the patient's net state of immunosuppression (Box 2 ). In the case of technical/anatomic mishaps, the best way to prevent infection is to correct the anatomic abnormality under coverage of appropriate antimicrobial therapy as antimicrobial treatment alone will not eliminate the risk of developing recurrent infections related to the uncorrected problem. As a consequence, the transplant recipient remains at high risk of subsequent infections with an increased risk of developing antimicrobial resistance until successful correction of the underlying abnormality.1, 2
Box 1. Epidemiologic exposures of importance for the organ transplant recipient.
-
A.In the community
-
1.Mycobacterium tuberculosis
-
2.Geographically restricted systemic mycoses
- Blastomycosis, coccidioidomycosis, histoplasmosis
-
3.Strongyloides stercoralis
-
4.Respiratory viruses
- Influenza
- Parainfluenza
- Respiratory syncytial virus
- Adenoviruses
-
5.Infections acquired by the ingestion of contaminated food/water
- Salmonella species
- Campylobacter jejuni
- Listeria monocytogenes
- Giardia lamblia
-
6.Environmental fungi (Aspergillus species and others)
-
7.Vector-borne (eg, West Nile virus)
-
1.
-
B.In the hospital
-
1.From the contaminated air
- Aspergillus species
- Pseudomonas aeruginosa and other gram-negative bacilli
-
2.From contaminated potable water
- Legionella pneumophila
- Other Legionella species
-
3.Unwashed hands of medical personnel
- Candida species (including azole resistant)
- Methicillin-resistant Staphylococcus aureus
- Vancomycin-resistant enterococci
- Highly resistant gram-negative bacilli
-
1.
-
C.Global travel (selected examples only)
-
1.Gastrointestinal bacterial and viral pathogens
- Salmonella, Shigella, Campylobacter, Vibrio
- Escherichia coli (multiple types)
- Viral gastroenteritis (eg, on cruise ships)
-
2.Parasitic infections
- Malaria
- Strongyloidiasis and other intestinal parasitic diseases
- Leishmaniasis
-
3.Respiratory infections
- SARS coronavirus
-
4.Viral hepatitis
- Hepatitis A, E or hepatitis B for long-term travel or residence
-
1.
Box 2. Factors contributing to the net state of immunosuppression in the organ transplant recipient.
-
1.
Dose, duration, and temporal sequence of immunosuppressive therapy
-
2.
Neutropenia, lymphocytopenia
-
3.Metabolic abnormalities
- Protein-calorie malnutrition
- Uremia
- Hyperglycemia
-
4.Infection with immunomodulating viruses
- Cytomegalovirus
- Epstein-Barr virus
- Human herpes virus 6
- Hepatitis B virus
- Hepatitis C virus
- Human immunodeficiency virus
When one is considering therapy in the transplant patient, the concept of the therapeutic prescription package is useful. This package has 2 major components: an immunosuppressive component to prevent and treat rejection and an antimicrobial component to make it safe. Thus, the nature of the antimicrobial program being administered must be closely linked to the nature and intensity of the immunosuppressive program required and the resulting net state of immunosuppression.1, 2
There are 3 modes in which antimicrobial agents can be administered to the transplant recipient: a therapeutic mode, in which antimicrobial agents are administered in the treatment of established clinical infection; a prophylactic mode, in which antimicrobial agents are administered to an entire population before an event to prevent the occurrence of an infection important enough to justify this intervention: and a preemptive mode, in which antimicrobial agents are administered to a subpopulation noted to be at particular risk of clinically important infection based on clinical, epidemiologic, or laboratory markers. This review focus on preventive strategies (prophylactic and preemptive) and on the diagnosis and management of established infection.
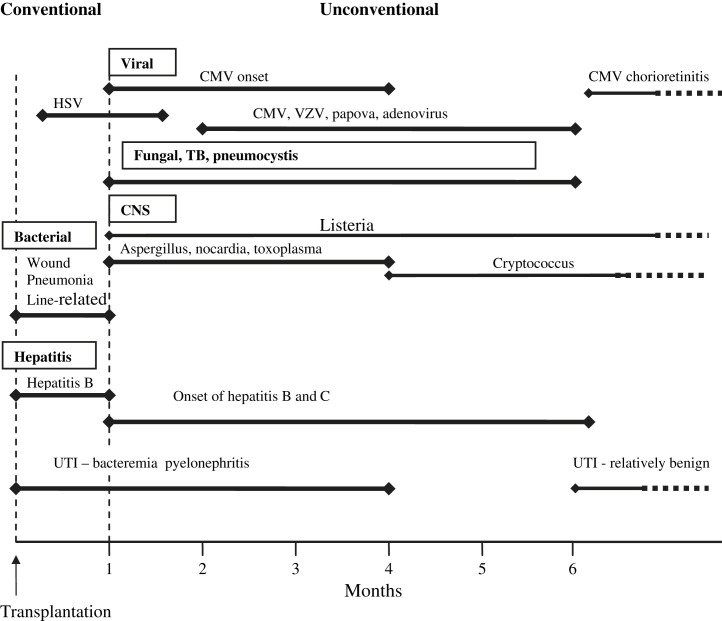
Infection in the posttransplant period has a stereotyped temporal pattern, a timetable. Although some clinical syndromes, such as pneumonia, can occur at any time point after transplant, the causes may be very different at different time points. Fig. 1 delineates the timetable for the onset of infections after organ transplantation in the absence of effective preventative strategies. When preventative antimicrobial therapy fails to completely protect the patient, a common clinical effect is to extend the time period in which the infectious complication will likely appear. For example, in the case of cytomegalovirus (CMV) infection, in the absence of prophylaxis CMV-induced clinical disease is most common 1 to 3 months after transplantation. When prophylaxis is used, but fails, it is common for the disease to occur 4 to 8 months after transplantation (depending on the nature and duration of the prophylaxis and the immunosuppressive regimen).2, 3
Fig. 1.
Timetable of infection following organ transplantation. (Adapted from Rubin RH, Wolfson JS, Cosimi AB, et al. Infection in the renal transplant recipient. Am J Med 1981;70:405–11; with permission.)
Like all patients, the transplant recipient is at risk of acquiring infections in the health care and community settings. Such infections are not necessarily transplant specific.
Time line of infections after transplantation
Fig. 1 is a graphical representation of the timing of infections during the posttransplant period.2 In general, 3 time periods are recognized, each with differing forms of infection: 1, 2, 3
First Month After Transplantation
In the first month, there are 3 major causes of infection: (1) infection that was present in the recipient before transplant, with its effects now increased as a result of surgery, anesthesia, and immunosuppressive therapy; (2) infection conveyed with a contaminated allograft; and (3) the same bacterial and candidal infections of the wound, lungs, drainage catheters, and vascular access devices that are seen in nonimmunosuppressed patients undergoing comparable surgery. Most (more than 95%) of the infections occurring in the first month after transplant fall into this last category; the main factor determining the incidence of such infections is the technical aspects of surgery as well as specific aspects of perioperative and postoperative care.
One to 6 Months After Transplantation
This second time period is when the effect of immune suppression is most notable on the risk of infection. During this period, 2 major classes of infection predominate. The first of these is attributable to a group of viral pathogens that are associated with latent and/or chronic infections. Examples include CMV, Epstein-Barr virus (EBV), human herpes virus 6 (HHV-6), and the hepatitis viruses (B and C); all of which may cause disease through acquisition of primary infection (typically from the donor) or secondary infection within the recipient under the pressure of immune suppression (secondary infection includes reactivation of latent pathogens and reinfection with a new strain). The second set of pathogens observed in this time period cause so-called opportunistic infections and include organisms such as Listeria monocytogenes, Aspergillus fumigatus, and Pneumocystis jiroveci. Development of infection with these opportunistic pathogens is attributable to the combination of sustained immunosuppression, which is often combined with the immunomodulating effects of viral infection creating a net state of immunosuppression great enough that these opportunistic infections can occur without an especially intensive environmental exposure.
More Than 6 Months After Transplantation
Information describing infections occurring in children more than 6 months after transplant is limited because transplant recipients commonly return to their homes, which are often far from their transplant centers. Accordingly, details regarding infectious complications occurring in this time period may be biased to include more significant infections resulting in hospitalization. Despite this limitation, experience supports dividing individuals with infections during this last time period into 2 main categories: (1) most patients with a good result from transplantation (maintenance immunosuppression, good allograft function) are at greatest risk from typical community-acquired infections (such as influenza, parainfluenza, and respiratory syncytial virus); (2) a smaller group of patients with poorer outcomes from transplantation (excessive acute and chronic immunosuppression, poor allograft function, and, often, chronic viral infection). These patients remain at high risk for recurrent infections related to uncorrected mechanical problems as well as opportunistic infections attributable to organisms like Pneumocystis jiroveci, Listeria monocytogenes, Cryptococcus neoformans, and Nocardia asteroides.
Summary of specific infections in the posttransplant period
The time line of infections after transplantation outlines the wide spectrum of infections that occur after transplantation. Among these infections, the major burden is represented by bacteria, Candida species, CMV, EBV, adenovirus, varicella zoster virus, and community-acquired respiratory viruses. In addition, certain infections represent challenges for specific organ groups (eg, BK virus infection in renal transplant recipients and toxoplasma infection in heart/heart-lung transplant recipients). Selected aspects of these infections are summarized later.
Bacterial Infections
As indicated earlier, bacterial infections are most commonly seen during the early posttransplant period. However, bacterial infections can occur at any time after transplantation. Risk factors include the presence of indwelling catheter devices, including endotracheal tubes, Foley catheters and central venous catheters. In this regard, hospital-acquired gram-negative organisms, coagulase-negative staphylococci and Staphylococcus aureus are often encountered. The nature of these infections and the specific pathogens involved vary according to the organ transplanted, sites of infection, the microbiologic flora of the institution, and the pretransplant status of the patient.
In general, the most common site of bacterial infection is at or near the site of transplantation. Urinary tract infection, notably pyelonephritis, has been recognized as the most common infectious complication among renal transplant recipients.4 Among liver transplant recipients, the most frequent site of bacterial infection is within the intraabdominal space, often accompanied by bacteremia.5, 6 Intraabdominal and wound infections are also commonly seen in intestinal transplant recipients. Bacteremia, which can be partly explained by disruption of the mucosal barrier associated with harvest injury or rejection, is commonly seen.7, 8 Infection of the lower respiratory tract (including pneumonia and lung abscess) is the most common site of infection reported in most, but not all, series of pediatric heart transplant recipients.9, 10, 11, 12 Mediastinitis is another important infection after thoracic transplantation, particularly if re-exploration of the chest is required. Pathogens associated with mediastinitis include S aureus and gram-negative enteric bacilli. Children undergoing lung transplantation because of cystic fibrosis experience a high rate of infectious complications as they often have preexisting colonization with resistant organisms, including Pseudomonas species, Burkholderia species and other bacterial pathogens.13, 14, 15, 16 Given the importance and difficulty in treating these often resistant organisms, transplant centers usually recommend a thorough microbiologic evaluation of heart-lung or lung transplant candidates before transplantation.
The transplant patient is also at risk of developing infection as a result of community-acquired bacterial pathogens, the most important of which is Streptococcus pneumoniae (pneumococcus). Transplant recipients are known to be at increased risk of pneumococcal sepsis.17 Among these patients, heart recipients who have been transplanted at a young age seem to be at an increased risk compared with other pediatric organ recipients.17
Fungal Infections
The frequency of fungal infections varies according to the type of organ transplanted.18, 19, 20 For example, invasive fungal infections are uncommon after renal transplantation. For these patients, the most frequently encountered entity is Candida urinary tract infection. Similarly for liver, heart, and intestinal transplant recipients, the major fungal infections are also caused by Candida species. For all of these patients, invasive aspergillosis and other mycoses occur uncommonly. The consequences of invasive aspergillosis and other noncandidal mycoses associated with invasive infections are frequently devastating. Lung transplant recipients are unique in that they experience proportionately more infections with Aspergillus species compared with other organ recipients. These infections are often seen in children undergoing transplantation as treatment of cystic fibrosis and reflect infection with Aspergillus that was present in the recipient before transplantation. However, Aspergillus is also frequently recovered from the lungs of transplant recipients with obliterative bronchiolitis (chronic rejection of the lung) regardless of the cause of their original lung disease leading to transplantation.21
CMV
CMV infection and disease remain important causes of mortality and morbidity among pediatric organ transplant recipients.22 Data on the precise burden in pediatric organ transplant recipients are limited, however, by wide differences in data collection and reporting. In addition, nonuniform approaches to the laboratory diagnosis and definition of CMV disease applied in retrospective studies affects the ability to interpret available data. In 5 centers in the United States, 10% to 20% of liver transplant patients experienced CMV disease within 2 years after transplantation.23 A review of first-time pediatric lung transplant patients indicated that among at-risk subjects, the incidence of CMV viremia was 29% to 32%, whereas the incidence of CMV pneumonitis was 20% during the first year after transplantation.24, 25
CMV disease is often associated with fever and hematologic abnormalities, including leucopenia, atypical lymphocytosis, and thrombocytopenia. Visceral sites affected may include the gastrointestinal tract, lungs, and liver. Central nervous system involvement, including chorioretinitis, is rare in organ transplant recipients.
The diagnosis of CMV infection and disease in organ transplant recipients can be affected by the variable lack of sensitivity and/or specificity of different diagnostic tests. Serology has no role in the diagnosis of active CMV disease after transplantation as it does not differentiate between prior infection and active disease. The interpretation of serologic results is further confused by the potential presence of passive antibody from blood products provided during or after the transplant procedure. In addition, the altered immune responses after transplantation might impair the patient's ability to mount predictable humoral responses. Viral culture of blood for CMV has limited clinical usefulness for diagnosis of disease caused by poor sensitivity. There is no role for CMV urine culture in the diagnosis of disease caused by poor specificity.26 A positive culture from bronchoalveolar lavage specimens may not correlate with disease.27, 28 The presence of a positive measurement of CMV load in the peripheral blood (measured by either nucleic acid amplification techniques (NAT) or pp65 antigenemia assay) in a patient with a compatible CMV clinical syndrome is strongly suggestive of CMV disease. However, the CMV load may be positive before the onset or in the absence of clinical disease and may be seen in the presence of disease from other causes. Further, the CMV load in the peripheral blood may be negative in some patients with tissue invasive disease, especially CMV involving the gastrointestinal tract. Given the variable usefulness of these tests, histopathologic examination of involved organs is essential to confirm the presence of CMV when the diagnosis of invasive CMV disease is being considered.
Intravenous ganciclovir (10 mg/kg/d, given twice daily) remains the preferred drug for the treatment of CMV disease in pediatric transplant recipients. Reduction of immunosuppression is desirable unless concurrent evidence of rejection precludes this. Ganciclovir therapy is sometimes accompanied by CMV hyperimmune globulin therapy in some centers. Typically, a clinical response to treatments is expected in 5 to 7 days after treatment has been initiated. Foscarnet and cidofovir may be considered in the setting of ganciclovir resistance. The optimal length of treatment should be determined by monitoring viral loads weekly.22 Treatment is typically continued until 2 consecutive negative samples are obtained. In cases of serious disease and in tissue invasive disease without viremia, longer treatment periods with clinical monitoring of the specific disease manifestation are recommended.
Data are emerging on the use of valganciclovir in the prevention and treatment of CMV infection/disease among adult transplant recipients.29, 30 Considerably less data are available for children.31 A summary of the approach to prophylaxis is outlined in Table 1 , including the roles of ganciclovir with or without immune globulin and suggestions on duration of their use, where indicated.
Table 1.
Regimens and targets for prophylaxis in the posttransplant period
| Infections | Target Groups | Prophylaxis Regimens/Comments | Suggested Duration of Prophylaxis |
|---|---|---|---|
| Bacterial infection (postoperative wound infection and sepsis) | All recipients | Perioperative antimicrobials regimens vary depending on organ, nature of surgery, and recipient factors (eg, selected regimens for cystic fibrosis) | 48–72 h |
| Herpes simplex | Seropositive recipients | Acyclovir | 3 months |
| CMV | Stratification of risk based on CMV donor/recipient serostatus | Intravenous ganciclovir (with/without intravenous immune globulin in some centers) Emerging data for valganciclovir in low- to intermediate-risk older children |
Typically 3 months; some centers use prophylactics for shorter periods (2 weeks) or longer (6 months) |
| EBV | High-risk patients are D+R− patients | No established regimens; preemptive reduction in immune suppression in response to rising EBV load in peripheral blood in use by growing number of centers; ganciclovir with/without immune globulin used in some centers | Duration variable if antivirals with/without immune globulin used |
| Candida species | All recipients | Fluconazole selectively; lipid amphoterin B products selectively; nystatin often used | Up to 4 weeks depending on risk factors |
| Aspergillus | Lung/heart-lung recipients | Voriconazole; intraconazole; amphotericin B products | Duration variable; up to 4–6 months depending on risk |
| Pneumocystis jiroveci | All recipients | Trimethoprim-sulfamethoxazole | Typically 6–12 months; for lung and small bowel transplant recipients, as well as any transplant patient with a history of prior PCP infection or chronic CMV disease, lifelong prophylaxis may be indicated |
| Toxoplasma gondii | Heart/heart-lung recipients | Pyrimethamine/sulfa for D+R− patients Trimethoprim/sulfa of some value for R+ patients |
6 months |
EBV
Although the most feared EBV-associated disease after transplantation is posttransplant lymphoproliferative disorder (PTLD), patients may experience a broad range of clinical symptoms that do not meet the definitions of PTLD. These might include the manifestations of infectious mononucleosis (fever, malaise, exudative pharyngitis, lymphadenopathy, hepatosplenomegaly, and atypical lymphocytosis), specific organ diseases such as hepatitis, pneumonitis, gastrointestinal symptoms, and hematological manifestations such as leucopenia, thrombocytopenia, hemolytic anemia, and haemophagocytosis.32 EBV-associated leiomyosarcoma has also been described.33 EBV disease is seen most frequently in patients experiencing primary EBV infections following transplantation. Rates of EBV disease and PTLD vary according to the organ transplanted with recipients of intestines and lungs being at the highest risk and those receiving liver, kidney, and heart at lower risk.
As for CMV disease, serology is not useful for diagnosis in the posttransplant period. The presence of increased EBV viral load in the peripheral blood as determined by quantitative polymerase chain reaction (PCR) is widely accepted as an assay to predict or indicate the likely presence of PTLD. However, these assays are limited in specificity and may remain persistently elevated in asymptomatic patients. The definitive diagnosis of EBV diseases, including PTLD requires histopathologic examination of biopsy material. The use of EBV-specific assays (eg, EBV encoded RNA [EBER] staining) enhances the sensitivity and specificity of histologic examination in these patients.
The approach to the treatment of EBV disease and PTLD remains somewhat controversial. Reduction of immune suppression is widely accepted as critical in the management of patients with these complications. The role of the antiviral agents acyclovir and ganciclovir are unproven, although many transplant clinicians use them in the treatment of EBV infection.34, 35 Treatment approaches are often modified from regimens used to treat CMV disease. Currently, when antiviral agents are used to treat EBV, the agent of choice is ganciclovir, as in vitro it is 10 times more active against EBV compared with acyclovir. The controversy on the use of these agents for EBV/PTLD arises because although these agents can suppress EBV lytic infection, they seem to be of limited value in treatment nonlytic EBV proliferation, which is believed to be the dominant component of EBV-related PTLD. Increasing evidence (albeit anecdotal) supports the use of the anti-CD20 monoclonal rituximab in the treatment of EBV disease and PTLD. However, the optimal timing and treatment strategy for this agent remain to be defined. Additional alternative strategies such as the use of chemotherapy require collaborative input from oncologists familiar with the management of EBV-related disease in organ transplant recipients.
The prevention of posttransplant EBV diseases, including PTLD remains controversial. Antiviral regimens have been modeled from the CMV scenario. To date, preemptive reduction in immunosuppression in the setting of increasing viral load may have the most supportive data and is increasingly being used (see Table 1).
Adenovirus
Adenovirus infection may be acquired by exogenous means or endogenously as a result of reactivation of latent infection. The clinical spectrum of infection and disease in pediatric transplant recipients is variable.36 There are more than 51 serotypes that generally show some fidelity as this relates to the types of organs affected and the resultant syndromes.37 Among liver transplant patients, disease manifestations include self-limited fever, gastroenteritis, cystitis, hepatitis, and pneumonitis. These manifestations may occur in other transplant recipients, depending on the level of immunosuppression. Adenovirus DNA can be detected in the peripheral blood using qualitative or quantitative PCR techniques. In the appropriate clinical setting, the presence of adenovirus DNA in the blood provides presumptive evidence of infection, with examination of tissue by histopathology providing more definitive evidence of infection. The management of adenovirus infection poses challenges because of limited effective treatment options. Cidofovir is currently accepted as the drug of choice. However, this conclusion is primarily based on a retrospective review of historical experience and the agent is not approved for this indication by the US Food and Drug Administration or similar agencies. Nonetheless, ongoing experience continues to support a role for the treatment of adenoviral infections with this agent. Before the advent of cidofovir, intravenous ribavirin was used with anecdotal reports of successes and failures.38
BK Virus
Although the major burden of BK virus infection is among adult renal transplant patients, the role of this virus in pediatric organ transplantation is becoming more clearly defined. Most infections are as a result of reactivation in adults. Primary infection may occur, notably among pediatric transplant recipients. The major clinical manifestation in the renal transplant recipient is tubulointerstitial nephritis. Renal biopsy is required for definitive diagnosis. Noninvasive testing modalities include screening of blood and urine for BK DNA using PCR.39 There is no firm consensus on the preferred approach to the management of BK nephropathy. Early detection is a desired goal. To that end, quantitative PCR monitoring for BK DNA is performed in some centers. This often provides opportunities to modulate immunosuppression. In situations where antiviral therapy is used, the agent most often used is cidofovir, for which there are reports of success.40 However, at present no consensus exists supporting the therapeutic efficacy of this agent.
Varicella Zoster Virus
Varicella zoster virus (VZV) is a major threat to pediatric transplant patients and many individuals enter transplantation without immunity to this virus.41 Immunosuppressed individuals are at risk of severe outcomes from VZV infection. Visceral involvement may accompany severe infection and clinicians should be reminded that disseminated disease can rarely occur in the absence of typical cutaneous vesicles.42 Pretransplant vaccination has been shown to provide sustained humoral immunity for at least 2 years after transplantation.43 It is strongly recommended that transplant candidates be vaccinated before transplantation. Given that this is a live vaccine, the minimum interval between vaccination and transplantation is recommended to be 4 to 6 weeks. Although some centers have selectively considered the use of VZV vaccine in susceptible children after transplantation, this approach cannot be recommended at this time because of the lack of safety data, given the known risk of live vaccines in immunosuppressed individuals.
Families of transplant patients should be educated to be alert to potential exposures in settings such as schools and should report them promptly to health care providers to allow for postexposure prophylaxis. Varicella-susceptible transplant recipients should receive varicella zoster immune globulin within 96 hours after a varicella exposure.44 If this window has passed or if varicella zoster immune globulin is not available, there is the option for the use of postexposure chemoprophylaxis with acyclovir (80 mg/kg/d, given 4 times daily for 7 days; maximum dose 800 mg, 4 times daily) starting at day 7 to 10 after exposure.44 In the absence of profound immunosuppression, no prophylaxis is usually necessary for exposed organ recipients who are immune to VZV as a result of prior infection or vaccination before transplantation.
Treatment of the transplant patient with VZV infection is usually initiated with intravenous acyclovir until there is evidence of clinical improvement (fever abates, no new lesions, lesions starting to crust, no visceral disease). Outpatient treatment with oral acyclovir or valacyclovir has been used in children with mild infection, low levels of immunosuppression, and when there are no concerns regarding the adequacy of follow-up. Famciclovir and valacyclovir are approved for use in adults. Famciclovir is the prodrug of penciclovir, which has an extended half-life in infected cells. Valayclovir is the prodrug of acyclovir and produces fourfold greater serum levels than those produced by acyclovir. Pediatric formulations are current not available.
Community-acquired Respiratory Viruses
Most children who have undergone organ transplantation experience community-acquired viral infections and have no significant problems. However, it is well recognized that children who are significantly immunocompromised can have severe disease caused by these viruses, including respiratory syncytial virus infection, parainfluenza, and influenza viruses.45, 46 For pediatric transplant recipients, the likelihood of more severe outcomes is greater during the early months after transplantation or during periods of peak immunosuppression.
In 2009, the advent of a pandemic strain of influenza A (pandemic H1N1 2009) has been cause for concern.47, 48 In general, the principles that govern the prevention and treatment of pandemic H1N1 in pediatric transplant patients are similar to those for seasonal influenza. Transplant patients are among those who are known to be at increased risk of severe outcomes from pandemic H1N1. They are candidates for treatment with oseltamivir or zanamivir (where appropriate) if they have acute respiratory illness that is suspected or confirmed to be caused by H1N1.49 Like other immunocompromised patients, they are at an increased risk of having prolonged shedding of virus and the harboring of drug-resistant strains of influenza A, including pandemic H1N1. Pediatric transplant patients are candidates for vaccination against this virus (as they are for seasonal influenza A) if they are greater than 6 months of age. Most experts currently delay vaccination until after the first months following organ transplantation.49
Selected Opportunistic Pathogens
Pneumocystis jiroveci pneumonia
Pneumocystis pneumonia (PCP) is a recognized threat in the posttransplant period.50, 51 The risk is greatest during the first 6 to 12 months after transplantation, with the time of onset being usually after the first month. Trimethoprim-sulfamethoxazole remains the prophylactic agent of choice.52 This agent is also preferred for initiation of therapy in individuals who develop PCP. Although the optimal duration of PCP prophylaxis remains unclear, most experts provide PCP prophylaxis for a minimum of 6 to 12 months, with some recommending indefinite use, especially for solid organ transplant recipients requiring more prolonged periods of higher levels of immunosuppression.
Intravenous pentamidine is an alternative for treatment of PCP for patients who are intolerant of trimethoprim-sulfamethoxazole or whose disease has not responded to this agent after 5 to 7 days.52 However, pentamidine is associated with a relatively high incidence of adverse events, including pancreatitis, renal dysfunction, hypoglycemia, and hyperglycemia. Atovaquone may be used to treat milder forms of PCP among adults; however, pediatric data are limited.
Alternatives to trimethoprim-sulfamethoxazole for prophylaxis include oral atovaquone or dapsone. Aerosolized pentamidine is recommended if children cannot tolerate these oral agents. Another alternative is intravenous pentamidine, albeit at the risk of greater toxicity.52
Toxoplasma gondii
Toxoplasma gondii infection is of greatest concern among heart transplant patients, but infection can occur in other categories of transplant recipients, including kidney and liver recipients.53, 54 Toxoplasma organisms can remain encysted within muscle tissue, such as cardiac muscle. Thus, infection is acquired as a result of the reactivation of cysts that remain dormant in the donor hearts of toxoplasma seronegative children. Clinical manifestations can occur as early as 2 weeks after transplantation. Manifestations include pneumonia, fever syndrome, myocarditis, chorioretinitis, and central nervous system disease. Current prophylaxis includes pyrimethamine/sulfadiazine for D+R− patients. Trimethoprim-sulfamethoxazole is typically used in R+ patients. However, some experts also recommend trimethoprim-sulfamethoxazole for D+R− patients. The duration of prophylaxis is usually 6 months.
Strongyloides sterocoralis
Infection with this parasitic worm is of relevance to individuals who previously acquired infection following a period of residence in endemic regions.55, 56 Donor-associated transmission of Stronygloides has also occurred. Asymptomatic immunocompromised persons, including transplant recipients are at risk of strongyloides hyperinfection, which results from dissemination of larvae via the systemic circulation, resulting in abdominal pain, diffuse pulmonary infiltrates, and septicemia or meningitis from enteric gram-negative bacilli. Serologic screening is recommended for individuals from endemic regions (Table 2 ). Ivermectin treatment is indicated for screen-positive individuals.
Table 2.
Screening tests for transplant candidates
| Tests | Comments/Action Required for Abnormal Results |
|---|---|
| HIV-1 and 2 | HIV-related management as indicated |
| HTLV-1 and 2 | Counselling as indicated |
| Hepatitis A | IgG and IgM serology |
| Hepatitis B | Obtain full panel of hepatitis B serology, including surface antigen and anti-core antibody |
| Hepatitis C | |
| Hepatitis D | Obtain if hepatitis B seropositive |
| CMV | Obtain IgG; urine culture for seropositive infants <2 years |
| EBV | Viral capsid antigen and EBNA |
| Herpes simplex virus | |
| Varicella zoster virus | Vaccinate seronegative candidates at least 6 weeks before transplantation |
| Toxoplasma gondii | Obtain heart, heart-lung candidates |
| Measles | Immunize if ≥3 months before expected transplantation |
| Mumps | Immunize if ≥3 months before expected transplantation |
| Rubella | Immunize if ≥3 months before expected transplantation |
| Mycobacterium tuberculosis | Mantoux test; IGRA being evaluated; intervention for latent TB may be required |
| Strongyloides sterocoralis | Positive serology requires intervention; ivermectin |
| Respiratory tract pathogens | Sputum cultures on patients with cystic fibrosis and other heart-lung transplant candidates; Aspergillus colonization indication for suppressive therapy |
| Radiographic imaging | These tests are as clinically indicated |
Abbreviations: EBNA, Epstein-Barr nuclear antigen; HTLV, human T-lymphotrophic virus; IGRA, interferon-gamma release assays.
Mycobacterium tuberculosis
Tuberculosis (TB) is always a concern for immunocompromised hosts.57, 58, 59, 60 Incidence rates are low in most transplant centers in the developed world, but outcomes of TB can be devastating in organ transplant recipients. Before transplantation a careful history for TB exposure or infection, Mantoux test screening, and a chest radiograph can assists in establishing the diagnosis of latent tuberculosis infection. The interferon-gamma release assays are currently being evaluated to define their role in settings where the TB skin test has poor utility.61, 62 The use of antituberculous agents in transplant patients poses challenges because of the interaction between isoniazid and rifampin with immunosuppressive medications. However, this should not be seen as a contraindication to the use of antituberculous agents, which have to be used when warranted by the clinical situation.
Strategies for prevention of posttransplant infections
Pretransplant Evaluation
The pretransplant phase is arguably the most important phase of transplantation. A detailed history and physical examination are necessary to identify conditions that influence the risk or management of infections after transplantation. This assessment allows for the identification of preexisting conditions that require treatment or prophylaxis in the period before or after transplantation. Table 2 summarizes screening tests that should be performed in the pretransplant period.
Immunizations
Immunizations represent an important strategy for preventing infections in the transplant patient.63, 64, 65, 66, 67, 68, 69, 70, 71, 72 Wherever possible, vaccines should be administered in the pretransplant period to improve the chances of optimal immunologic take. A guideline on vaccinations for the transplant candidate/patient has recently been published.73 In some situations, accelerated vaccination schedules may be used for selected vaccines. Given differences in childhood vaccination schedules in different jurisdictions, clinicians should acquaint themselves with the appropriate schedules and the circumstances under which accelerated schedules could be used. When using vaccines after transplantation, one needs to be concerned about safety as well as efficacy. In general, all live virus vaccines should be avoided in the transplant recipient. The oral polio, yellow fever, and oral typhoid vaccines are live and are contraindicated in immunosuppressed patients. The live attenuated intranasal influenza vaccine is also contraindicated. Measles, mumps, and rubella vaccines are somewhat contraindicated and their use should be limited to outbreak scenarios. The varicella vaccine is also somewhat contraindicated and is not approved for use in transplant patents. Although limited published data support the potential use of this vaccine in transplant recipients,72 most experts continue to advise against this practice. In the cases of the nonlive vaccines, the major concern is not safety, but efficacy. Thus, in general, it is advisable to give nonlive vaccines at times when the level of immunosuppression would allow for immunogenicity. Table 3 summarizes the vaccines that are indicated and contraindicated in transplant recipients. Given the relative burden and importance of invasive pneumococcal disease in pediatric transplant recipients, the importance of pneumococcal vaccination should not be underestimated.17, 63, 69
Table 3.
Vaccines that are recommended and contraindicated in transplant recipients
| Vaccine | Inactivated/Live Attentuated (I/LA) | Recommended Before Transplantation | Recommended After Transplantation |
|---|---|---|---|
| Influenza | I | Yes | Yes |
| LA | No | No | |
| Hepatitis B | I | Yes | Yes |
| Hepatitis A | I | Yes | Yes |
| Pertussis | I | Yes | Yes |
| Diphtheria | I | Yes | Yes |
| Tetanus | I | Yes | Yes |
| Inactivated polio vaccine | I | Yes | Yes |
| Haemophilus influenzae | I | Yes | Yes |
| Streptococcus pneumoniae (conjugate/23-valent polysaccharide) | I/I | Yes | Yes |
| Neisseria meningitidis (conjugate and polysaccharide) | I | Yes | Yes |
| Human papillomavirus | I | Yes | Yes |
| Rabies | I | Yes | Yes |
| Varicella | LA | Yes | No |
| Rotavirus | LA | Yes | No |
| Measles | LA | Yes | No |
| Mumps | LA | Yes | No |
| Rubella | LA | Yes | No |
| BCGa | LA | Yes | No |
| Smallpox | LA | No | No |
| Anthrax | I | No | No |
Where appropriate.
Donor Organ Screening
The organ donor is a frequent source of exposure to pathogens in the organ transplant recipient. Accordingly, screening of the donor organ is a crucial aspect of the preventive strategies aimed at minimizing adverse outcomes from infections in the posttransplant period. Despite a long-standing recognition of the importance of donor-derived infections, increased concern about this problem has emerged because of recent donor-related transmission of human immunodeficiency virus (HIV). This case, as well as concerns about the lack of sensitivity of serologic testing and the relatively long time period until seroconversion against HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV), have led to interest in the use of NAT-based testing for the pathogens HIV, HCV, and HBV. Although arguments exist for and against the use of NAT testing, a final international consensus addressing if and when to use these tests is only beginning to emerge.74 Decisions relating to the use of such tests must consider not only the reliability of this technology but also the feasibility of universal implementation of these testing procedures for all procurement organizations. Recent cases of donor-associated transmission of lymphocytic choriomeningitis virus and West Nile virus have also raised questions on whether the panel of routine tests performed on potential donors should be expanded to include these and other potential donor-derived pathogens. To date, a consensus has not been reached on whether or not screening against these pathogens should be routinely included in donor testing panels. It is to be hoped that the implementation of working groups and committees focusing on the problem of donor-derived infections in North America and Europe will lead to improved data to better inform subsequent recommendations regarding donor testing. Current requirements for screening of nonliving donors are shown in Table 4 . At present, no specific requirements have been implemented for screening of live donors. In general, testing strategies that are in place for deceased donors are applied to the use of these organs. The importance of documenting the presence of potential donor-transmissible pathogens is imperative not only to inform decision making regarding the use of potential donor organs but also because results of donor testing can inform specific preventative strategies even when donor-associated exposure to pathogens is unavoidable.
Table 4.
Screening tests for donor organs
| Tests | Comments/Action Required for Abnormal Results |
|---|---|
| HIV-1 and 2 | Positive test contraindicates organ use |
| HTLV-1 and 2 | Positive test contraindicates organ usea |
| Hepatitis A virus | Positive IgM contraindicates organ use |
| Hepatitis B virus | Obtain full panel of hepatitis B serology, including surface antigen and anti-core antibody; positive HBsAg contraindicates organ use |
| Hepatitis C virus | Some centers use positive organ only for positive candidates |
| CMV | Obtain IgG; urine culture for seropositive infants <2 years |
| EBV | Viral capsid antigen and EBNA |
| Toxoplasma gondii | Obtain on heart, heart-lung donor |
| Treponema pallidum | Positive confirmatory test contraindicates organ use |
Abbreviation: HBsAg, hepatitis B surface antigen.
This is currently being reexamined in some jurisdictions given lack of availability of testing platforms.
Specific Preventive Strategies
Various prophylaxis regimens are used in the posttransplant period. Although there are common basic principles, the specific regimens vary across centers and by the type of organ transplanted. For most patients, the major targets of prophylaxis are bacterial pathogens, herpes group viruses, and fungal pathogens, including pneumocystis. Perioperative antibiotics are typically used for 48 to 72 hours to provide prophylaxis against surgical contamination. The burden of CMV infection in transplant patients is such that it represents the major focus of prevention in the posttransplant period, when intravenous ganciclovir is usually used with or without CMV hyperimmune globulin in selected patient groups. Table 1 summarizes various pathogens and the regimens that are often used for prevention of infection in the posttransplant period.
Evaluation of the febrile transplant patient
In the evaluation of the febrile transplant patient, clinicians should consider if the child's fever is related to common childhood infections or infections that are unique to the immunosuppressed transplant recipient. To this end, the timing of infections after transplantation (see Fig. 1) provides guidance regarding the most likely pathogens. For example, as discussed earlier, the most likely causes of infection within the first month after transplantation are often bacterial or candidal and are largely similar to what is seen in nonimmunosuppressed patients who have undergone comparable surgery. The nature of the evaluation will depend on the clinical status of the patient and whether or not a source of infection has been identified.
Examination abnormal, focus of infection defined. Admission to hospital may be indicated depending on the clinical status of the patient and the site of the infection. The diagnostic evaluation varies, but should include a minimum of a complete blood count and differential, blood, and urine cultures. Additional investigations depend on the clinical assessment and the timing of presentation after transplantation.
Examination normal, no focus of infection defined. Patients who are clinically unwell typically require admission for evaluation and treatment. The diagnostic evaluation should consider the likely differential diagnoses. Consultation with infectious diseases is recommended.
Patients who are well may not necessarily require admission. However, this depends on several factors, including the adequacy of follow-up, the degree of immune suppression and the suspected diagnoses. The diagnostic evaluation should include a minimum of a complete blood count and differential, blood, and urine cultures.
In all of these situations, clinicians need to be aware of the spectrum of viral infections that are associated with febrile syndromes without necessarily having a readily apparent organ focus of infection (eg, CMV virus).
References
- 1.Fishman J.A., Rubin R.H. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 2.Rubin R.H., Wolfson J.S., Cosimi A.B. Infection in the renal transplant patient. Am J Med. 1981;70:405–411. doi: 10.1016/0002-9343(81)90780-4. [DOI] [PubMed] [Google Scholar]
- 3.Rubin R., Ikonen T., Gummert J. The therapeutic prescription for the organ transplant recipient: the linkage of immunosuppression and antimicrobial strategies. Transpl Infect Dis. 1999;1(1):29–39. doi: 10.1034/j.1399-3062.1999.10104.x. [DOI] [PubMed] [Google Scholar]
- 4.Krieger J.N., Brem A.S., Kaplan M.R. Urinary tract infection in pediatric renal transplantation. Urology. 1980;15(4):362–369. doi: 10.1016/0090-4295(80)90471-9. [DOI] [PubMed] [Google Scholar]
- 5.Zitelli B.J., Gartner J.C., Malatack J.J. Pediatric liver transplantation: patient evaluation and selection, infectious complications, and life-style after transplantation. Transplant Proc. 1987;19(4):3309–3316. [PubMed] [Google Scholar]
- 6.Colonna J.O., Winston D.J., Brill J.E. Infectious complications in liver transplantation. Arch Surg. 1988;123(3):360–364. doi: 10.1001/archsurg.1988.01400270094015. [DOI] [PubMed] [Google Scholar]
- 7.Sigurdsson L., Reyes J., Kocoshis S.A. Bacteremia after intestinal transplantation in children correlates temporally with rejection or gastrointestinal lymphoproliferative disease. Transplantation. 2000;70(2):302–305. doi: 10.1097/00007890-200007270-00011. [DOI] [PubMed] [Google Scholar]
- 8.Green M., Bueno J., Sigurdsson L. Unique aspects of the infectious complications of intestinal transplantation. Curr Opin Organ Transplant. 1999;4(4):361–367. [Google Scholar]
- 9.Green M., Wald E.R., Fricker F.J. Infections in pediatric orthotopic heart transplant recipients. Pediatr Infect Dis J. 1989;8(2):87–93. [PubMed] [Google Scholar]
- 10.Baum D., Bernstein D., Starnes V.A. Pediatric heart transplantation at Stanford: results of a 15-year experience. Pediatrics. 1991;88(2):203–214. [PubMed] [Google Scholar]
- 11.Bailey L.L., Wood M., Razzouk A. Heart transplantation during the first 12 years of life. Loma Linda University Pediatric Heart Transplant Group. Arch Surg. 1989;124(10):1221–1225. doi: 10.1001/archsurg.1989.01410100127022. [DOI] [PubMed] [Google Scholar]
- 12.Braunlin E.A., Canter C.E., Olivari M.T. Rejection and infection after pediatric cardiac transplantation. Ann Thorac Surg. 1990;49(3):385–390. doi: 10.1016/0003-4975(90)90241-w. [DOI] [PubMed] [Google Scholar]
- 13.Noyes B.E., Michaels M.G., Kurland G. Pseudomonas cepacia empyema necessitatis after lung transplantation in two patients with cystic fibrosis. Chest. 1994;105(6):1888–1891. doi: 10.1378/chest.105.6.1888. [DOI] [PubMed] [Google Scholar]
- 14.De Soyza A., McDowell A., Archer L. Burkholderia cepacia complex genomovars and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet. 2001;358(9295):1780–1781. doi: 10.1016/S0140-6736(01)06808-8. [DOI] [PubMed] [Google Scholar]
- 15.Aris R.M., Routh J.C., LiPuma J.J. Lung transplantation for cystic fibrosis, patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am J Respir Crit Care Med. 2001;164(11):2102–2106. doi: 10.1164/ajrccm.164.11.2107022. [DOI] [PubMed] [Google Scholar]
- 16.LiPuma J.J. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med. 2005;11(6):528–533. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 17.Tran L., Hébert D., Dipchand A. Invasive pneumococcal disease in pediatric organ transplant recipients: a high-risk population. Pediatr Transplant. 2005;9(2):183–186. doi: 10.1111/j.1399-3046.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 18.Verma A., Wade J.J., Cheeseman P. Risk factors for fungal infection in paediatric liver transplant recipients. Pediatr Transplant. 2005;9(2):220–225. doi: 10.1111/j.1399-3046.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 19.Schaenman J.M., Rosso F., Austin J.M. Trends in invasive disease due to Candida species following heart and lung transplantation. Transpl Infect Dis. 2009;11(2):112–121. doi: 10.1111/j.1399-3062.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 20.van Hal S.J., Marriott D.J., Chen S.C. Australian Candidemia Study. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis. 2009;11(2):122–127. doi: 10.1111/j.1399-3062.2009.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigt S.S., Elashoff R.M., Huang C. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9(8):1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotton C., Kumar D., Caliendo A. On behalf of The Transplantation Society International CMV Consensus Group. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Am J Transplant. 2010;10(1):18–25. [Google Scholar]
- 23.Green M., Michaels M.G., Katz B.Z. CMV-IVIG for prevention of Epstein Barr virus disease and posttransplant lymphoproliferative disease in pediatric liver transplant recipients. Am J Transplant. 2006;6(8):1906–1912. doi: 10.1111/j.1600-6143.2006.01394.x. [DOI] [PubMed] [Google Scholar]
- 24.Danziger-Isakov L.A., DelaMorena M., Hayashi R.J. Cytomegalovirus viremia associated with death or retransplantation in pediatric lung-transplant recipients. Transplantation. 2003;75(9):1538–1543. doi: 10.1097/01.TP.0000061607.07985.BD. [DOI] [PubMed] [Google Scholar]
- 25.Danziger-Isakov LA, Worley S, Michaels MG, et al. The risk, prevention & outcome of cytomegalovirus after pediatric lung transplantation. Transplantation, in press. [DOI] [PMC free article] [PubMed]
- 26.Pillay D., Ali A.A., Liu S.F. The prognostic significance of positive CMV cultures during surveillance of renal transplant recipients. Transplantation. 1993;56(1):103–108. doi: 10.1097/00007890-199307000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Storch G.A., Ettinger N.A., Ockner D. Quantitative cultures of the cell fraction and supernatant of bronchoalveolar lavage fluid for the diagnosis of cytomegalovirus pneumonitis in lung transplant recipients. J Infect Dis. 1993;168(6):1502–1506. doi: 10.1093/infdis/168.6.1502. [DOI] [PubMed] [Google Scholar]
- 28.Buffone G.J., Frost A., Samo T. The diagnosis of CMV pneumonitis in lung and heart/lung transplant patients by PCR compared with traditional laboratory criteria. Transplantation. 1993;56(2):342–347. doi: 10.1097/00007890-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Asberg A., Humar A., Jardine A.G. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009;9(5):1205–1213. doi: 10.1111/j.1600-6143.2009.02617.x. [DOI] [PubMed] [Google Scholar]
- 30.Monforte V., Lopez C., Santos F. A multicenter study of valganciclovir prophylaxis up to day 120 in CMV-seropositive lung transplant recipients. Am J Transplant. 2009;9(5):1134–1141. doi: 10.1111/j.1600-6143.2009.02574.x. [DOI] [PubMed] [Google Scholar]
- 31.Vaudry W., Ettenger R., Jara P. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am J Transplant. 2009;9(3):636–643. doi: 10.1111/j.1600-6143.2008.02528.x. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Pediatricas . Epstein-Barr virus infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book. 2009 report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 289–292. [Google Scholar]
- 33.Nur S., Rosenblum W.D., Katta U.D. Epstein-Barr virus-associated multifocal leiomyosarcomas arising in a cardiac transplant recipient: autopsy case report and review of the literature. J Heart Lung Transplant. 2007;26(9):944–952. doi: 10.1016/j.healun.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Green M. Management of Epstein-Barr virus-induced posttransplant lymphoproliferative disease in recipients of solid organ transplantation. Am J Transplant. 2001;1(2):103–108. [PubMed] [Google Scholar]
- 35.Preiksaitis J.K. New developments in the diagnosis and management of posttransplantation lympholiferative disorders in solid organ transplant recipients. Clin Infect Dis. 2004;39(7):1016–1023. doi: 10.1086/424447. [DOI] [PubMed] [Google Scholar]
- 36.de Mezerville M.H., Tellier R., Richardson S. Adenoviral infections in pediatric transplant recipients: a hospital-based study. Pediatr Infect Dis J. 2006;25(9):815–818. doi: 10.1097/01.inf.0000233542.48267.fd. [DOI] [PubMed] [Google Scholar]
- 37.American Academy of Pediatricas . Adenovirus infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book. 2009 report of the Committee on Infectious Diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 204–206. [Google Scholar]
- 38.Hoffman J.A., Shah A.J., Ross L.A. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(7):388–394. doi: 10.1053/bbmt.2001.v7.pm11529489. [DOI] [PubMed] [Google Scholar]
- 39.Babel N., Fendt J., Karaivanov S. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88(1):89–95. doi: 10.1097/TP.0b013e3181aa8f62. [DOI] [PubMed] [Google Scholar]
- 40.Vats A., Shapiro R., Singh Randhawa P. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation. 2003;75(1):105–112. doi: 10.1097/00007890-200301150-00020. [DOI] [PubMed] [Google Scholar]
- 41.Pandya A., Wasfy S., Hébert D. Varicella Zoster infection in solid organ transplant recipients: a hospital-based retrospective study. Pediatr Transplant. 2001;5(3):1–9. doi: 10.1034/j.1399-3046.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- 42.Whitley R.J. Varicella zoster virus. In: Mandell G.C., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. 5th edition. Churchill Livingstone; Philadelphia: 2000. pp. 1580–1585. [Google Scholar]
- 43.Barton M., Wasfy S., Melbourne T. Sustainability of humoral responses to varicella vaccine in pediatric transplant recipients following a pre-transplantation immunization strategy. Pediatr Transplant. 2008;13(8):1007–1013. doi: 10.1111/j.1399-3046.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 44.American Academy of Pediatricas . Varicella-zoster infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book. 2009 report of the Committee on Infectious Diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 714–727. [Google Scholar]
- 45.Pohl C., Green M., Wald E.R. Respiratory syncytial virus infections in pediatric liver transplant recipients. J Infect Dis. 1992;165(1):166–169. doi: 10.1093/infdis/165.1.166. [DOI] [PubMed] [Google Scholar]
- 46.Apalsch A.M., Green M., Ledesma-Medina J. Parainfluenza and influenza virus infections in pediatric organ transplant recipients. Clin Infect Dis. 1995;20(2):394–399. doi: 10.1093/clinids/20.2.394. [DOI] [PubMed] [Google Scholar]
- 47.Scalera N.M., Mossad S.B. The first pandemic of the 21st century: a review of the 2009 pandemic variant influenza A (H1N1) virus. Postgrad Med. 2009;121(5):43–47. doi: 10.3810/pgm.2009.09.2051. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention Outbreak of swine-origin influenza A (H1N1) virus infection – Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1–3. [PubMed] [Google Scholar]
- 49.Kumar D., Morris M.I., Kotton C.N. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010;10(1):18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 50.Schafers H.J., Cremer J., Wahlers T. Pneumocystis carinii pneumonia following heart transplantation. Eur J Cardiothorac Surg. 1987;1(1):49–52. doi: 10.1016/s1010-7940(87)80014-3. [DOI] [PubMed] [Google Scholar]
- 51.Gryzan S., Paradis I.L., Zeevi A. Unexpectedly high incidence of Pneumocystis carinii infection after lung-heart transplantation. Implications for lung defense and allograft survival. Am Rev Respir Dis. 1988;137(6):1268–1274. doi: 10.1164/ajrccm/137.6.1268. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatricas . Pneumocystis jirovecii infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book. 2009 report of the Committee on Infectious Diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 536–540. [Google Scholar]
- 53.Luft B.J., Naot Y., Araujo F.G. Primary and reactivated toxoplasma infection in patients with cardiac transplants. Clinical spectrum and problems in diagnosis in a defined population. Ann Intern Med. 1983;99(1):27–31. doi: 10.7326/0003-4819-99-1-27. [DOI] [PubMed] [Google Scholar]
- 54.Michaels M.G., Wald E.R., Fricker F.J. Toxoplasmosis in pediatric recipients of heart transplants. Clin Infect Dis. 1992;14(4):847–851. doi: 10.1093/clinids/14.4.847. [DOI] [PubMed] [Google Scholar]
- 55.Scoggin C.H., Call N.B. Acute respiratory failure due to disseminated strongyloidiasis in a renal transplant recipient. Ann Intern Med. 1977;87(4):456–458. doi: 10.7326/0003-4819-87-4-456. [DOI] [PubMed] [Google Scholar]
- 56.Nolan T.J., Schad G.A. Tacrolimus allows autoinfective development of the parasitic nematode Strongyloides stercoralis. Transplantation. 1996;62(7):1038. doi: 10.1097/00007890-199610150-00029. [DOI] [PubMed] [Google Scholar]
- 57.Sternecik M., Ferrell S., Asher N. Mycobacterial infection after liver transplantation: a report of three cases and review of the literature. Clin Transplant. 1992;6:55–61. [Google Scholar]
- 58.Higgins R., Kusne S., Reyes J. Mycobacterium tuberculosis after liver transplantation: management and guidelines for prevention. Clin Transplant. 1992;6:81–90. [Google Scholar]
- 59.Singh N., Paterson D.L. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27(5):1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 60.American Society of Transplantation Infectious Diseases Community of Practice Mycobacterium tuberculosis. Am J Transplant. 2004;10(Suppl 4):37–41. [Google Scholar]
- 61.Detjen A.K., Keil T., Roll S. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45(3):322–328. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 62.Okada K., Mao T.E., Mori T. Performance of an interferon-gamma release assay for diagnosing latent tuberculosis infection in children. Epidemiol Infect. 2008;136(9):1179–1187. doi: 10.1017/S0950268807009831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barton M., Wasfy S., Dipchand A. Seven-valent pneumococcal conjugate vaccine in pediatric solid organ transplant recipients: a prospective study of safety and immunogenicity. Pediatr Infect Dis J. 2009;28(8):688–692. doi: 10.1097/INF.0b013e31819d97be. [DOI] [PubMed] [Google Scholar]
- 64.Madan R.P., Tan M., Fernandez-Sesma A. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis. 2008;46(5):712–718. doi: 10.1086/527391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arslan M., Wiesner R.H., Sievers C. Double-dose accelerated hepatitis B vaccine in patients with end-stage liver disease. Liver Transpl. 2001;7(4):314–320. doi: 10.1053/jlts.2001.23069. [DOI] [PubMed] [Google Scholar]
- 66.Carey W., Pimentel R., Westveer M.K. Failure of hepatitis B immunization in liver transplant recipients: results of a prospective trial. Am J Gastroenterol. 1990;85(12):1590–1592. [PubMed] [Google Scholar]
- 67.Stark K., Gunther M., Neuhaus R. Immunogenicity and safety of hepatitis A vaccine in liver and renal transplant recipients. J Infect Dis. 1999;180(6):2014–2017. doi: 10.1086/315125. [DOI] [PubMed] [Google Scholar]
- 68.Balloni A., Assael B.M., Ghio L. Immunity to poliomyelitis, diphtheria and tetanus in pediatric patients before and after renal or liver transplantation. Vaccine. 1999;17(20–21):2507–2511. doi: 10.1016/s0264-410x(99)00064-x. [DOI] [PubMed] [Google Scholar]
- 69.Lin P.L., Michaels M.G., Green M. Safety and immunogenicity of the American Academy of Pediatrics–recommended sequential pneumococcal conjugate and polysaccharide vaccine schedule in pediatric solid organ transplant recipients. Pediatrics. 2005;116(1):160–167. doi: 10.1542/peds.2004-2312. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2–10 years with quadrivalent meningococcal conjugate vaccine (MCV4) MMWR Morb Mortal Wkly Rep. 2008;57(17):462–465. [PubMed] [Google Scholar]
- 71.Olson A.D., Shope T.C., Flynn J.T. Pretransplant varicella vaccination is cost-effective in pediatric renal transplantation. Pediatr Transplant. 2001;5(1):44–50. doi: 10.1034/j.1399-3046.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 72.Danziger-Isakov L., Kumar D., AST Infectious Diseases Community of Practice Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9(Suppl 4):S258–S262. doi: 10.1111/j.1600-6143.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 73.Weinberg A., Horslen S.P., Kaufman S.S. Safety and immunogenicity of varicella-zoster virus vaccine in pediatric liver and intestine transplant recipients. Am J Transplant. 2006;6(3):565–568. doi: 10.1111/j.1600-6143.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- 74.Humar A., Morris M., Blumberg E. Special report. Nucleic acid testing (NAT) of organ donors: is the “best” test the right test? Am J Transplant. 2010 doi: 10.1111/j.1600-6143.2009.02992.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]