Abstract
Avian infectious bronchitis is a serious and highly contagious disease caused by infectious bronchitis virus (IBV). We isolated a highly virulent IBV strain (CK/CH/JS/TAHY) from kidneys of diseased chickens. Phylogenetic analysis based on the S1 gene revealed that CK/CH/JS/TAHY clustered with the QX-like type. The S1 gene has 1,620 nucleotides and encoded a polypeptide of 540 amino acids with typical coronavirus cleavage recognition sites of HRRR. About 1-day-old specific pathogen-free White Leghorn chickens inoculated with CK/CH/JS/TAHY at 105.5 EID50 exhibited clinical signs including coughing, sneezing, nasal discharge, and tracheal vocalization accompanied by depression with 84% mortality and 100% morbidity. The kidneys of dead birds were swollen and pale and exhibited severe urate deposition. Histopathological examination revealed kidney hemorrhages, multifocal necrosis of the renal tubules and trachea with cilia loss, sloughing of epithelial cells, and edema of the lamina propria. IBV-specific antibodies appeared at 10 D post-infection. Chickens vaccinated with a CK/CH/JS/TAHY oil-emulsion vaccine showed 26.7% morbidity and 3% mortality indicating a protective effect. In conclusion, the IBV strain is a virulent avian IBV and that exhibited severe pathogenicity in chickens and is a vaccine candidate to prevent infection by Chinese QX-like nephropathogenic IBV strains.
Key words: avian infectious bronchitis, QX-like type, pathogenicity
INTRODUCTION
Avian infectious bronchitis caused by the infectious bronchitis virus (IBV) is a major and highly contagious, acute disease with severe economic losses in the global poultry industry (Cavanagh, 2005; Cavanagh, 2007). IBV is a member of the Gammacoronavirus genus that contains an unsegmented, single-stranded, positive-sense RNA genome of 27.6 kb. The genome encodes four structural proteins: envelope (E), nucleocapsid (N), membrane glycoprotein (M), and the spike glycoprotein (S) (Boursnell et al., 1987). The S protein is a multimeric coiled-coil protein that is post-translationally cleaved into S1 and S2 subunits by cellular proteases (Stern and Sefton, 1982; Cavanagh et al., 1992). S1 contains virus-neutralizing epitopes and is anchored to the viral membrane by the S2 subunit and is necessary for attachment to host cells (Koch et al., 1990). Genetic analysis of the S1 gene has been widely used to differentiate between IBV genotypes and serotypes (Ignjatovic and Galli, 1994; Kingham et al., 2000; Ladman et al., 2006; Cavanagh, 2007).
IBV replicates and survives in upper respiratory tract (URT) epithelial cells causing typical URT symptoms in chickens such as nasal discharge and rales. However, its host range is not limited to the URT and the virus can infect the proventriculus, kidney, gut, and oviduct. This spread results in decreased fertility and egg production as well as a decline in the overall health of laying chickens (Sjaak de Wit et al., 2011; Cook et al., 2012; Jackwood, 2012). There are no effective drugs to treat IBV infections and vaccination shows the most promise for IBV control (Meeusen et al., 2007). The live attenuated vaccines and inactivated oil-emulsion vaccines derived from Massachusetts (Mass) serotype strains such as H120 and H52 have been widely and extensively used in China (Liu et al., 2006; Liu et al., 2009a). However, vaccinated flocks develop new variant infectious strains due to the poor cross-protection between different serotypes and genotypes (Li et al., 2013).
The QX-like type IBV is a member of the LX4 group and is one of the most important IBV genotypes globally. The QX-like IBV types have been the predominant IBV type in China since 1999 (Zhao et al., 2017; Jiang et al., 2018). IBV isolates from southern China between 2009 and 2012 were mostly clustered in the group of QX-like strains (Li et al., 2013) and 50 to 70% of Chinese IBV isolates during this period were of the QX-like type (Liu et al., 2009b; Han et al., 2011; Ji et al., 2011). In the present study, an IBV strain CK/CH/JS/TAHY belonging to the QX-like type was isolated from the kidneys of a broiler chicken in 2017 in Jiangsu province, China. We characterized the genetic organization, pathogenicity and antigenicity of this strain to provide information for vaccine development to prevent and reduce the spread of IBV in poultry.
METHODS AND MATERIALS
Ethics Statement
Our study was approved by Animal Care and Use Committee of Guangdong Province, China. All animal procedures were performed according to guidelines developed by the China Council on Animal Care and protocols approved by Animal Care and Use Committee of Guangdong, Province, China.
Virus Propagation
The IBV CK/CH/JS/TAHY strain was isolated from the kidneys of broiler chicken in 2017 on a farm in Taizhou City, Jiangsu province, China. The stock virus was propagated by passage through the allantoic cavities of 9-day-old specific pathogen-free (SPF) chicken embryos (Guangdong Wens Dahuanong Biotechnology, Guangdong, China) incubated at 37°C. Allantoic fluids were harvested at 48 h post-inoculation. The presence of IBV was identified and verified by reverse transcription-polymerase chain reaction (RT-PCR) specific for the IBV M gene and 3′UTR as previously designed (M gene 5′-CCTAAGAACGGTTGGAAT-3, 5′-TACTCTACA CACACAC-3, 3′UTR 5′-GGAAGATAGGCATGTAGC TT-3, 5′-CTAACTCTATACTAGCCTAT -3,).
RNA, DNA, and PCR Manipulations
PCR primers for amplifying the entire S1 gene were designed using Primer Premier 5.0 software (Version 5.0, Premier Biosoft, Palo Alto, CA, USA) and the NCBI reference genome. The primers 5′-AAGACTGAACAAAAGACCGACCT-3, 5′-CAAAACCTGCCATAACTAACATA-3′ were synthesized by Sangon Biotechnology (Shanghai, China). Viral RNA was extracted from IBV-infected allantoic fluid using Trizol reagent (Takara, Dalian, China) according to the manufacturer's instructions. The S1 gene was amplified using the PrimeScript One Step RT-PCR Kit Ver. 2 (Takara) in a 50 µL reaction volume containing 2 µL of PrimeScript One Step Enzyme Mix, 25 µL of 2 × one step buffer, 2 µL of extracted viral RNA and 1 µL of each primer (0.4 µM). The PCR conditions were as follows: 50°C for 30 min and then 94°C for 3 min, followed by 32 cycles at 94°C for 30 s, 52.5°C for 30 s, 72°C for 2 min, and a final extension at 72°C for 10 min. The products were detected by electrophoresis on a 1.0% agarose gel and then observed using an ultraviolet transilluminator. PCR amplicons were purified from agarose gels using an AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, USA) according to the manufacturer's instructions. Purified PCR products were inserted into vector pMD18-T (Takara) and transformed into DH5α Escherichia coli competent cells and plasmid inserts were sequenced as described above.
Nucleotide sequences were aligned using the Editseq program in the Lasergene package and analyzed for homology with reference IBV sequences using MegAlign (Dnastar, Madison, WI, USA). Phylogenetic trees were constructed using MEGA (Version 7) with neighbor-joining method with 1,000 bootstrap replicates (https://www.megasoftware.net). The sequence of this IBV isolate was deposited in GenBank with the accession number MK061428.
Pathogenicity Assays in SPF Chickens
Specific pathogen-free 1-day-old White Leghorn chickens were randomly divided into 2 groups of 25 birds each. One group of birds was inoculated with sterile PBS (negative controls). The second group was infected with IBV strain CK/CH/JS/TAHY intranasally and via the eye drop inoculation route using 200 µL of inoculum containing 105.5 EID50 of virus. All birds were observed twice daily for clinical signs including tracheal rales, nasal discharge, coughing, eye irritation, and watery diarrhea. Morbidity and mortality were recorded daily.
Four chickens from each group were euthanized at 3, 7, 10, and 14 D post-infection (dpi) and their kidneys, lungs, and trachea were processed for histopathologic examination and blood samples were collected for serology. Some birds died between the intervals and tissue samples these were also collected for histopathologic examination. Virus recovery from chickens was via nasopharyngeal and cloacal swabs that were placed into separate tubes containing 0.6 mL PBS containing 2000 U/mL penicillin G, 200 g/mL gentamicin sulfate, and 4 g/mL amphotericin B (Sigma-Aldrich, St Louis, MO, USA) and stored at −70°C until virus recovery. Tissue samples were fixed in 10% formalin for 48 h at room temperature and processed for haematoxylin and eosin (HE) staining. Serum samples were tested for the presence of IBV-specific antibodies using a commercial ELISA kit (Idexx, Westbrook, ME, USA).
Inactivated IBV Vaccine and Testing
Virus strain CK/CH/JS/TAHY that was propagated in allantoic cavities (see above) was used for vaccine production and challenge. Viral stocks were inactivated with the addition of formaldehyde to 0.2%. The formaldehyde inactivated antigen solution was emulsified with oil adjuvant at a ratio of 25:75 (w/w). Chickens were immunized with the oil-emulsion vaccine using 105.5 EID50 in 0.2 mL per chicken. Vaccination and challenge studies used 7-day-old SPF chickens in 3 groups of 30 birds each. Group 1 was vaccinated with 200 µL IBV CK/CH/JS/TAHY oil-emulsion vaccine containing 105.5 EID50 antigens per chicken intramuscularly. Group 2 was inoculated with sterile PBS and used as infection control that was unvaccinated but challenged. Group 3 was inoculated with sterile PBS and used as negative controls (unvaccinated and unchallenged). Booster vaccinations were carried out at 14 dpi and after 3 wk birds in groups 1 and 2 were challenged with 0.2 mL IBV CK/CH/JS/TAHY containing 105.5 EID50 virus as described above. All birds were observed twice daily for clinical signs as described above. Serum samples were obtained at 10 time points: 0, 7, and 14 D after the first immunization and 7, 14, and 21 D after the second immunization and 5, 10, 14, and 21 D after challenge. Serum was tested for the presence of IBV-specific antibodies as described above.
Statistical Analysis
SPSS 19.00 software (SPSS, Chicago, IL, USA) was used to analyze the result of antibody response. Result was represented as the means ± standard error of mean. Statistically significant differences were analyzed by one-way analysis of variance followed by Duncan's post hoc test. Statistical significance was set as P < 0.05.
RESULTS
Phylogenetic Analysis of S1 Gene
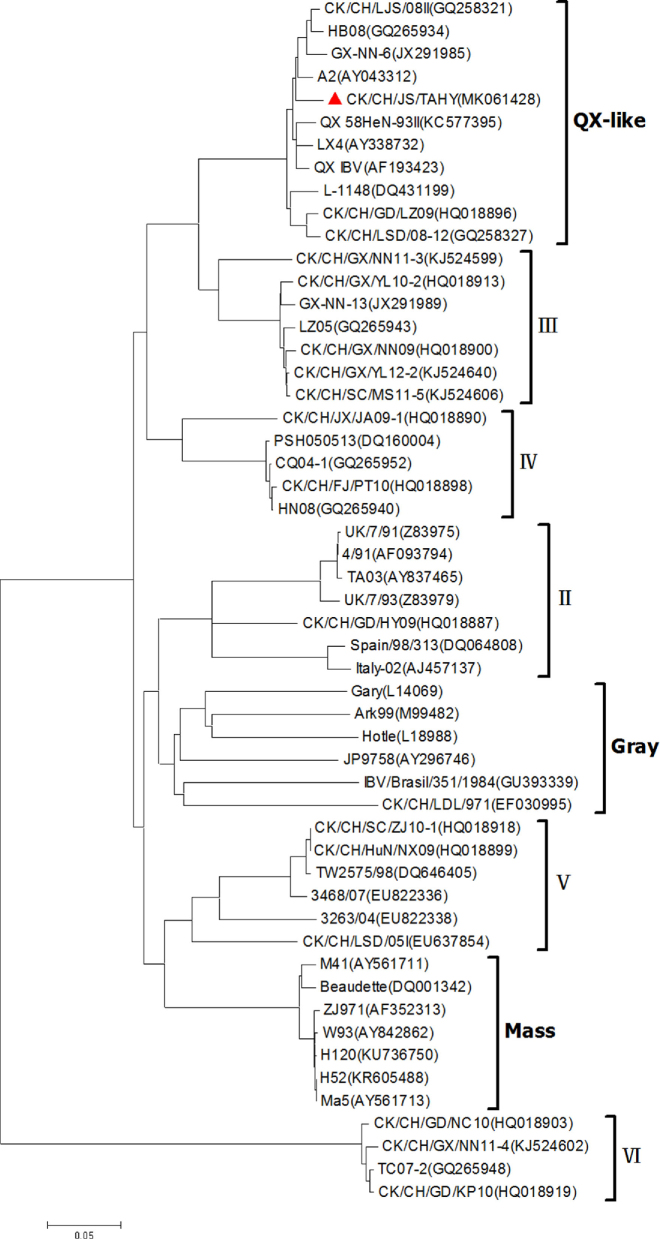
We cloned and sequenced the S1 gene of IBV isolate CK/CH/JS/TAHY that was 1,620 nucleotides in length with a predicted open reading frame encoding 540 amino acids (GenBank accession number: MK061428). The predicted cleavage recognition sites were HRRRR. A phylogenetic tree using the S1 sequence displayed 8 distinct genetic groups. The isolate CK/CH/JS/TAHY was a member of the QX-like group with nucleotide sequence identities of 94.6 to 98.6% with other members of this group. The Mass genotype is evolutionarily distant from the QX-like genotypes and CK/CH/JS/TAHY had only low levels of homology with the conventional vaccine strains H120 and H52 at 77.7 and 77.8% nucleotide and 76.4 and 76.5% amino acid identity, respectively (Figure 1).
Figure 1.
Phylogenetic tree based on the S1 nucleotide sequences from the ATG start codon to the cleavage site of the spike protein. Phylogeny of CK/CH/JS/TAHY was tested with 52 IBV reference strains representing eight genotypes. The bar represents a genetic distance of 0.05. The IBV strains used in this study are indicated as red triangles in bold.
Pathogenicity
All chickens inoculated with the virus isolate CK/CH/JS/TAHY at 105.5 EID50 exhibited respiratory signs 1–3 D after inoculation. These included coughing, sneezing, nasal discharge, and tracheal vocalization accompanied by depression and watery diarrhea. A total of 3 chickens died 5 D post-inoculation and more chickens died continuously thereafter with a mortality rate of 84% and morbidity rate of 100%. Necropsies showed kidneys that were pale, mottled, and swollen with renal tubules and ureters that were distended with excess urate (Figure 2A). Lesions were completely absent in the negative control group (Figure 2B). In addition, slight hemorrhaging with serous catarrhal exudates were observed in the tracheas of infected chickens (Figure 2C) and control chickens did not show this pathology (Figure 2D).
Figure 2.
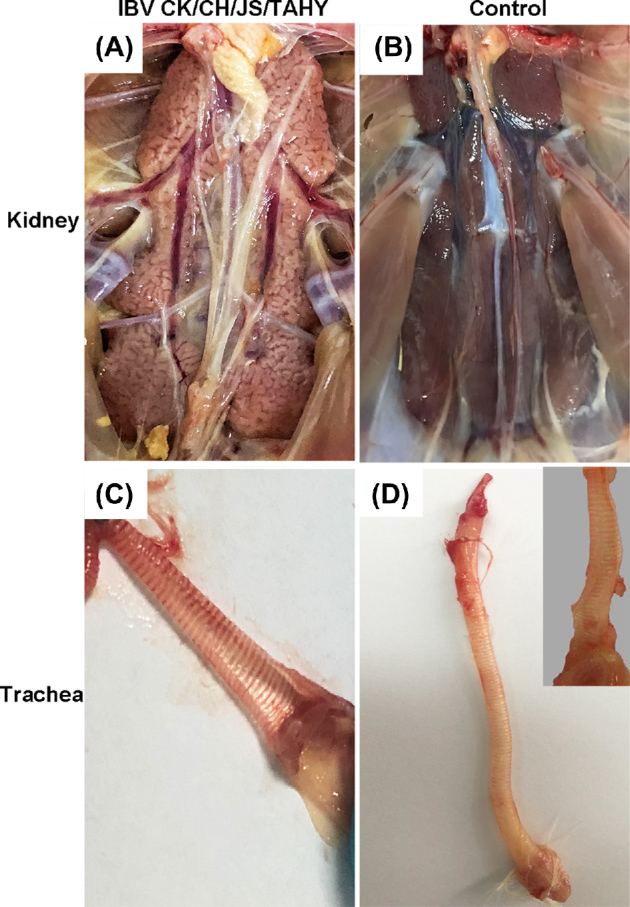
Gross lesions in kidney and trachea from dead chickens. (A) Swollen and pale kidney after challenging with strain CK/CH/JS/TAHY. (B) Negative control kidney. (C) Trachea illustrating hyperemia and serious catarrhal exudates after challenge with strain CK/CH/JS/TAHY. (D) Control trachea. Inset shows trachea's mucosal surface.
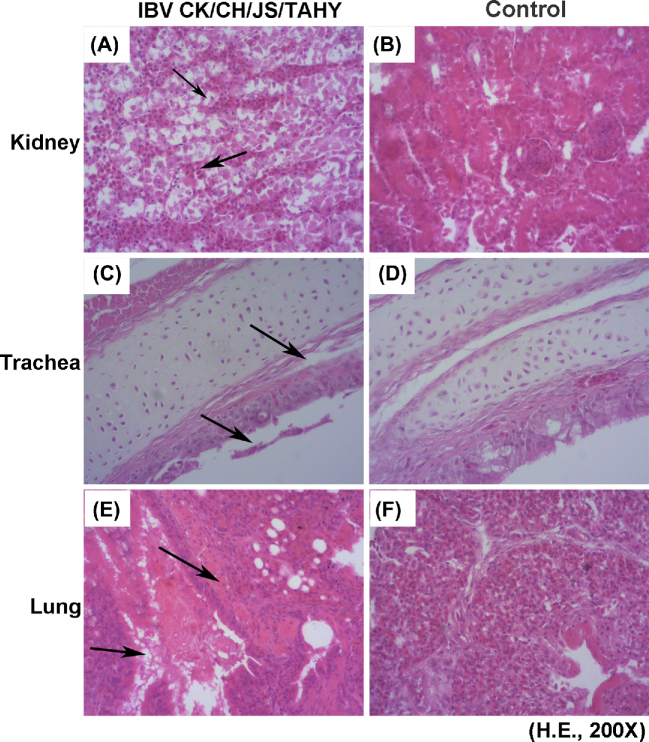
The most characteristic histological lesions in the kidney were hemorrhages, multifocal necrosis of the renal tubules, and infiltration of inflammatory cells (Figure 3A). Histopathological examination revealed that the tracheas had lost cilia and showed sloughing of epithelial cells as well as edema of the lamina propria (Figure 3C). Lung lesions included hemorrhages, congestion, and lymphocytic infiltration in the alveolar lumen (Figure 3E). No lesions were observed in the corresponding tissues of chickens in the control groups (Figures 3B, 3D, and 3F).
Figure 3.
Histopathologic analysis of tissues from 1-day-old chickens infected with infectious bronchitis virus CK/CH/JS/TAHY strain. (A) Kidney tissues with severe renal lesions including hemorrhages, degeneration, multifocal necrosis of the renal tubules and inflammatory cell infiltration. Solid arrows indicate degeneration and inflammatory cell infiltration. (C) Trachea tissues illustrating loss of cilia, sloughing of epithelial cells, and edema of lamina propria. Solid arrows indicate sloughing of epithelial. (E) Lung tissues with hemorrhages, congestion, and lymphocytic infiltration in the alveolar lumen. Solid arrows indicate hemorrhages and congestion. Panels (B), (D), and (F) correspond to negative control tissues. Hematoxylin and eosin staining.
Viral shedding from the respiratory and digestive tracts of infected chickens was determined by virus recovery from nasopharyngeal and cloacal swabs. Virus was recovered from the nasopharyngeal swabs of all birds at 3 dpi but this number declined by 14 dpi. Based on the cloacal swabs, viral shedding from birds lasted until 14 dpi (Table 1). Antibody responses in birds that survived infection were measured at 5 different times post-infection. IBV-specific antibodies appeared at 10 dpi and the levels had increased by 14 dpi. Differences between inoculated and negative control groups were statistically significant (P < 0.05) at 7, 10, and 14 dpi. The negative control group was negative for the presence of IBV-specific Abs (Figure 4).
Table 1.
Recovery of IBV strains CK/CH/JS/TAHY from chickens infected when 1 D old.
| Day post-infection |
||||||
|---|---|---|---|---|---|---|
| Group | Viral shedding | 3 d | 5 d | 7 d | 10 d | 14 d |
| CK/CH/JS/TAHY | Nasopharyngeal swabs | 5/5 | 4/5 | 3/5 | 1/5 | 0/5 |
| Cloacal swabs | 4/5 | 5/5 | 3/5 | 3/5 | 3/5 | |
| Control | Nasopharyngeal swabs | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Cloacal swabs | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
Number of chickens yielding virus/number of chickens tested.
Figure 4.
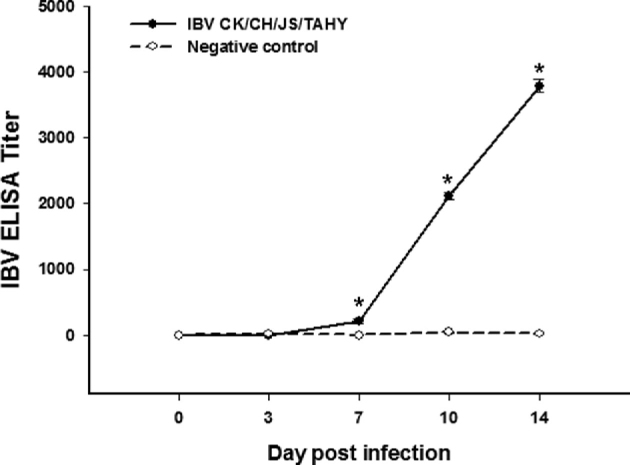
Antibody response of chickens after infection with strain CK/CH/JS/TAHY. The error bars indicate means ± standard error of mean. Asterisks (*) mark the days in which the antibody titers were significantly different between inoculated with CK/CH/JS/TAHY and negative control groups (P < 0.05).
Vaccination Test and Challenge Protection
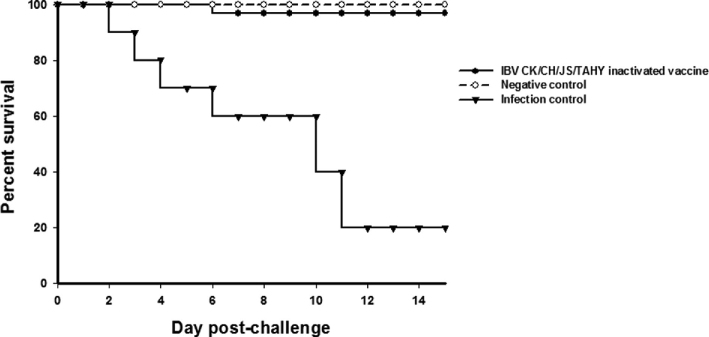
Chickens vaccinated with CK/CH/JS/TAHY oil-emulsion vaccine showed 73.3% protection with 26.7% (8/30) morbidity and 3% (1/30) mortality. The unvaccinated but challenged group showed 100% morbidity and 80% mortality with typical IBV clinical signs as described above. No clinical signs were observed in the animals from the negative control group (Figure 5). After vaccination with the inactivated CK/CH/JS/TAHY strain, antibody titers rose slowly after the primary immunization and kept increasing during the observation period after the second immunization. At day 5 post-challenge, the antibody titers declined and were maximal at 10 D post-challenge and began to decrease at 21 D post-challenge. Differences between inoculated with CK/CH/JS/TAHY inactivated vaccine and infection control groups were statistically significant (P < 0.05) at 7, 14, 21, 28, 35, 40, 45, 49, and 56 D post-primary immunization (Figure 6).
Figure 5.
Survival curves in vaccinated SPF chickens following challenge with IBV CK/CH/JS/TAHY strain.
Figure 6.
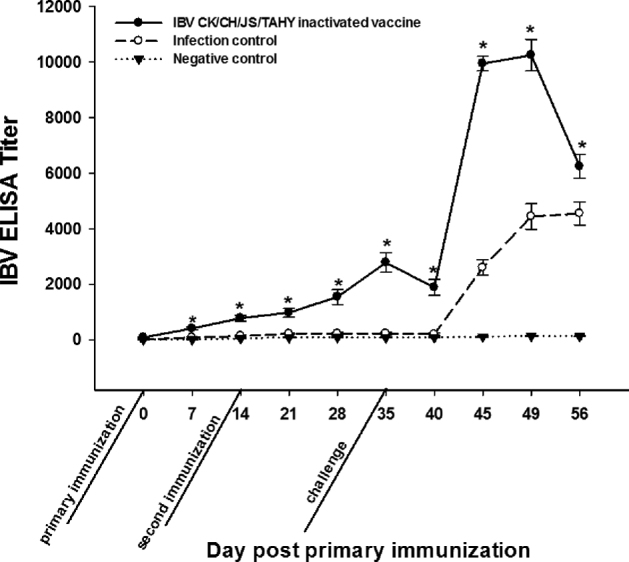
Antibody response of chickens after immunization with CK/CH/JS/TAHY oil-emulsion vaccine. The primary immunization was carried out at 7 D of age (0 D post-primary immunization). The second immunization was carried out at 14 D post-primary immunization. The third immunization was carried out 3 wk after the second immunization challenge (35 D post-primary immunization). The error bars indicate means ± standard error of mean. Asterisks (*) mark the days in which the antibody titers were significantly different between inoculated with CK/CH/JS/TAHY inactivated vaccine and infection control groups (P < 0.05).
DISCUSSION
IBV infection leads to severe economic losses in the poultry industry due to the condemnation of infected chickens. Vaccines are an effective measure to control IBV and in China, commercial vaccines are derived from Mass serotype strains including H120, H52, Ma5, W93, and M41. The H120 vaccines are the most frequently used on chicken farms. However, immune failure still occurs in China (Han et al., 2011; Ji et al., 2011; Li et al., 2013).
In this study, a CK/CH/JS/TAHY strain was isolated from a vaccinated chicken flock. Genotyping results based on the S1 gene sequence of CK/CH/JS/TAHY strain indicated that the strain belonged to the QX-like type. There are currently 8 IBV genotypes circulating in China. The predominant genotype is a QX-like IBV that has spread widely in Asia, Europe, Africa, and the Middle East (Liu and Kong, 2004; Cook et al., 2012; Knoetze et al., 2014; Shokri et al., 2018). Interestingly, the QX-like IBV strains have undergone divergent evolutionary paths resulting in different variants in Europe and China (Worthington et al., 2008; Zhao et al., 2017). To date, several QX-like vaccine candidates were reported recently in China but there is no official approval for their use (Zhao et al., 2015; Huo et al., 2016; Xia et al., 2018; Yan et al., 2018). Therefore, there is a shortage of a vaccine against endemic strains of IBV in China and IBV infections remain a problem in the Chinese poultry industry.
In the current study, the chickens inoculated with CK/CH/JS/TAHY oil-emulsion vaccine showed 26.7% (8/30) morbidity and 3% (1/30) mortality. An attenuated vaccine (Sczy3C100 attenuated) produced via passage in chicken embryo kidney (CEK) cells 100 times was reported to reduce the morbidity, mortality, mean lesion scores when challenged by QX-like type virus (Xia et al., 2018). Other candidate vaccine strains were prepared via attenuation in embryonated eggs were also reported. These also efficiently prevent infection of Chinese QX-like nephropathogenic IBV (Zhao et al., 2015; Huo et al., 2016; Yan et al., 2018). Since the phylogenetic and antigenic features of IBV types vary with time and region, those candidate vaccines should be carefully examined before their field application.
Clinical signs, gross pathologic lesions, histopathological changes, and tissue tropism were used to evaluate the pathogenicity of IBV. In this study, our analyses revealed that the CK/CH/JS/TAHY strain induced severe pathogenicity in 1-day-old SPF chickens with severe kidney and tracheal lesions. Our results confirmed the ability of CK/CH/JS/TAHY strain to induce both respiratory and renal disease which was consistent with QX-like characteristic symptoms (Zou et al., 2010; Bande et al., 2017). As reported, IBV replicated in many respiratory tissues such as trachea, lungs, and air sacs and some urogenital tissues including kidney and even in liver and brain (Cavanagh, 2003; Feng et al., 2012). In our study, the CK/CH/JS/TAHY strain was detected in the trachea, lung, and kidney by RT-PCR (data not shown).
The European QX-like IBV strains have been reported that to cause respiratory signs in 86% of infected animals, litter or enteric problems in 22% but only 2% had swollen kidneys (Worthington et al., 2008). The QX-like IBV strains that were recently isolated in China caused 10 to 50% mortality in infected chickens (Liu et al., 2009a; Shi et al., 2011; Sun et al., 2011; Feng et al., 2015). Other predominant strains in China include YN belonging to the CK/CH/LSC/99I group (Feng et al., 2012) and the tl/CH/LDT3/03 strain belonging to the tl/CH/LDT3/03 group (Han et al., 2016). These caused 65 and 70% mortality in chickens, respectively. In the present study, the CK/CH/JS/TAHY strain induced severe pathogenicity in 1-day-old SPF chickens with 84% mortality and 100% morbidity. This result indicated that CK/CH/JS/TAHY is a virulent avian IBV and highly pathogenic in chickens. A more detailed biologic and molecular analysis is needed for CK/CH/JS/TAHY to determine the reason for this high level of virulence.
In conclusion, the CK/CH/JS/TAHY strain is a virulent avian IBV that exhibited severe pathogenicity in chickens. This strain is a potential vaccine candidate to prevent infection by Chinese QX-like nephropathogenic IBV strains.
ACKNOWLEDGEMENTS
This work was supported by Guangdong Wens Dahuanong Biotechnology Co., Ltd.
REFERENCES
- Bande F., Arshad S.S., Omar A.R., Hair-Bejo M., Mahmuda A., Nair V. Global distributions and strain diversity of avian infectious bronchitis virus: a review. Anim. Health. Res. Rev. 2017;18:70–83. doi: 10.1017/S1466252317000044. [DOI] [PubMed] [Google Scholar]
- Boursnell M.E., Brown T.D., Foulds I.J., Green P.F., Tomley F.M., Binns M.M. The complete nucleotide sequence of avian infectious bronchitis virus: analysis of the polymerase-coding region. Adv. Exp. Med. Biol. 1987;218:15–29. doi: 10.1007/978-1-4684-1280-2_3. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Feng J., Hu Y., Ma Z., Yu Q., Zhao J., Liu X., Zhang G. Virulent avian infectious bronchitis virus, People's Republic of China. Emerg. Infect. Dis. 2012;18:1994–2001. doi: 10.3201/eid1812.120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K., Xue Y., Wang J., Chen W., Chen F., Bi Y., Xie Q. Development and efficacy of a novel live-attenuated QX-like nephropathogenic infectious bronchitis virus vaccine in China. Vaccine. 2015;33:1113–1120. doi: 10.1016/j.vaccine.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C., Zhang Q., Ma Y., Shao Y., Liu Q., Kong X., Liu S. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect. Genet. Evol. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Zhang T., Xu Q., Gao M., Chen Y., Wang Q., Zhao Y., Shao Y., Li H., Kong X., Liu S. Altered pathogenicity of a tl/CH/LDT3/03 genotype infectious bronchitis coronavirus due to natural recombination in the 5′- 17 kb region of the genome. Virus Res. 2016;213:140–148. doi: 10.1016/j.virusres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y.F., Huang Q.H., Lu M., Wu J.Q., Lin S.Q., Zhu F., Zhang X.M., Huang Y.Y., Yang S.H., Xu C.T. Attenuation mechanism of virulent infectious bronchitis virus strain with QX genotype by continuous passage in chicken embryos. Vaccine. 2016;34:83–89. doi: 10.1016/j.vaccine.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Ji J., Xie J., Chen F., Shu D., Zuo K., Xue C., Qin J., Li H., Bi Y., Ma J., Xie Q. Phylogenetic distribution and predominant genotype of the avian infectious bronchitis virus in China during 2008–2009. Virol J. 2011;8:184. doi: 10.1186/1743-422X-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Han Z., Chen Y., Zhao W., Sun J., Zhao Y., Liu S. Characterization of the complete genome, antigenicity, pathogenicity, tissue tropism, and shedding of a recombinant avian infectious bronchitis virus with a ck/CH/LJL/140901-like backbone and an S2 fragment from a 4/91-like virus. Virus Res. 2018;244:99–109. doi: 10.1016/j.virusres.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham B.F., Keeler C.L., Jr., Nix W.A., Ladman B.S., Gelb J., Jr. Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S-1 gene. Avian Dis. 2000;44:325–335. [PubMed] [Google Scholar]
- Knoetze A.D., Moodley N., Abolnik C. Two genotypes of infectious bronchitis virus are responsible for serological variation in KwaZulu-Natal poultry flocks prior to 2012. Onderstepoort J. Vet. Res. 2014;81 doi: 10.4102/ojvr.v81i1.769. [DOI] [PubMed] [Google Scholar]
- Koch G., Hartog L., Kant A., van Roozelaar D.J. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J. Gen. Virol. 1990;71:1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- Ladman B.S., Loupos A.B., Gelb J., Jr Infectious bronchitis virus S1 gene sequence comparison is a better predictor of challenge of immunity in chickens than serotyping by virus neutralization. Avian Pathol. 2006;35:127–133. doi: 10.1080/03079450600597865. [DOI] [PubMed] [Google Scholar]
- Li M., Mo M.L., Huang B.C., Fan W.S., Wei Z.J., Wei T.C., Li K.R., Wei P. Continuous evolution of avian infectious bronchitis virus resulting in different variants co-circulating in Southern China. Arch. Virol. 2013;158:1783–1786. doi: 10.1007/s00705-013-1656-0. [DOI] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang X., Wang Y., Li C., Han Z., Shao Y., Li H., Kong X. Molecular characterization and pathogenicity of infectious bronchitis coronaviruses: complicated evolution and epidemiology in china caused by cocirculation of multiple types of infectious bronchitis coronaviruses. Intervirology. 2009;52:223–234. doi: 10.1159/000227134. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Su J.L., Zhao J.X., Zhang G.Z. Complete genome sequence analysis of a predominant infectious bronchitis virus (IBV) strain in China. Virus Genes. 2009;38:56–65. doi: 10.1007/s11262-008-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.W., Zhang Q.X., Chen J.D., Han Z.X., Liu X., Feng L., Shao Y.H., Rong J.G., Kong X.G., Tong G.Z. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Arch. Virol. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen E.N., Walker J., Peters A., Pastoret P.P., Jungersen G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.M., Zhao Y., Gao H.B., Jing Z., Wang M., Cui H.Y., Tong G.Z., Wang Y.F. Evaluation of recombinant fowlpox virus expressing infectious bronchitis virus S1 gene and chicken interferon-gamma gene for immune protection against heterologous strains. Vaccine. 2011;29:1576–1582. doi: 10.1016/j.vaccine.2010.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri S., Karimi V., Langeroudi A.G., Marandi M.V., Hashamzadeh M., Zabihipetroudi T., Najafi H., Tehrani F. Seroprevalence and genotyping of avian infectious bronchitis virus detected from Iranian unvaccinated backyard chickens. Iran. J. Microbiol. 2018;10:65–71. [PMC free article] [PubMed] [Google Scholar]
- Sjaak de Wit J.J., Cook J.K., van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D.F., Sefton B.M. Coronavirus proteins: biogenesis of avian infectious bronchitis virus Virion proteins. J. Virol. 1982;44:794–803. doi: 10.1128/jvi.44.3.794-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Han Z., Ma H., Zhang Q., Yan B., Shao Y., Xu J., Kong X., Liu S. Phylogenetic analysis of infectious bronchitis coronaviruses newly isolated in China, and pathogenicity and evaluation of protection induced by Massachusetts serotype H120 vaccine against QX-like strains. Avian Pathol. 2011;40:43–54. doi: 10.1080/03079457.2010.538037. [DOI] [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Xia J., He X., Du L.J., Liu Y.Y., You G.J., Li S.Y., Liu P., Cao S.J., Han X.F., Huang Y. Preparation and protective efficacy of a chicken embryo kidney cell-attenuation GI-19/QX-like avian infectious bronchitis virus vaccine. Vaccine. 2018;36:4087–4094. doi: 10.1016/j.vaccine.2018.05.094. [DOI] [PubMed] [Google Scholar]
- Yan S., Zhao J., Xie D., Huang X., Cheng J., Guo Y., Liu C., Ma Z., Yang H., Zhang G. Attenuation, safety, and efficacy of a QX-like infectious bronchitis virus serotype vaccine. Vaccine. 2018;36:1880–1886. doi: 10.1016/j.vaccine.2018.02.053. [DOI] [PubMed] [Google Scholar]
- Zhao W., Gao M., Xu Q., Xu Y., Zhao Y., Chen Y., Zhang T., Wang Q., Han Z., Li H., Chen L., Liang S., Shao Y., Liu S. Origin and evolution of LX4 genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2017;198:9–16. doi: 10.1016/j.vetmic.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Cheng J.L., Liu X.Y., Zhao J., Hu Y.X., Zhang G.Z. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 2015;180:49–58. doi: 10.1016/j.vetmic.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou N.L., Zhao F.F., Wang Y.P., Liu P., Cao S.J., Wen X.T., Huang Y. Genetic analysis revealed LX4 genotype strains of avian infectious bronchitis virus became predominant in recent years in Sichuan area, China. Virus Genes. 2010;41:202–209. doi: 10.1007/s11262-010-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]