Abstract
To establish a characteristic host response to predict the pathogenicity and tissue tropism of infectious bronchitis viruses (IBV), we investigated innate immune responses (IIR) and apoptosis in chicken embryo kidney cells (CEKC) and tracheal organ cultures (TOC) infected with three IBV strains. Results showed nephropathogenic IBV strains 885 and QX induced greater apoptosis in CEKC than M41, which induced greater apoptosis in TOCs compared to 885 and QX. Elevated IIR is associated with tissue tropism of different IBV strains. Compared to M41, 885 and QX caused greater induction of toll like receptor 3 (TLR3), melanoma differentiation associated protein 5 (MDA5) and interferon beta (IFN-β) in CEKC. In contrast, M41 infection caused greater expression of these genes than 885 or QX in TOCs. In summary, greater levels of apoptosis and elevated levels of TLR3, MDA5 and IFN-β expression are associated with increased pathogenicity of IBV strains in renal and tracheal tissues.
Keywords: Infectious bronchitis virus, Pathogenicity, Tissue tropism, Apoptosis, Innate immune responses, Chicken embryo kidney cells, Tracheal organ cultures
Highlights
-
•
Innate immune responses and apoptosis in CEK cells and TOCs infected with 3 IBV strains.
-
•
885 and QX caused greater induction of TLR3, MDA5 and IFN-β and apoptosis in CEK cells.
-
•
M41 caused greater induction of TLR3, MDA5 and IFN-β and apoptosis in TOCs.
-
•
Correlated with increased pathogenicity of IBV strains in renal and tracheal tissues.
Introduction
Infectious bronchitis (IB) is an acute and highly contagious disease caused by a gamma coronavirus that affects chickens of all ages and is characterized by lesions in respiratory and urogenital organs (Cavanagh, 2007, Dolz et al., 2006). Avian infectious bronchitis virus (IBV) continues to cause serious economic losses to global chicken production. Along with highly pathogenic avian influenza (HPAI) and velogenic Newcastle disease (ND), IB is the most economically important viral respiratory disease affecting poultry industry worldwide (Cook et al., 2012). Vaccination has been considered as the most reliable approach for IBV control (Meeusen et al., 2007) however current vaccines have proved to be inadequate due to constant emergence of new variant viruses (De Wit, 2000, de Wit et al., 2011). Concurrent circulation of both classic and variant IBVs has been identified in most parts of the world, raising major challenges to the current IBV prevention and control strategies.
Current IBV isolates present high antigenic diversity (Hofstad, 1975), and emergent strains that differ in antigenic properties, tissue tropism and pathogenicity are continuously being reported across the world (Gelb et al., 1991, Jackwood, 2012, Shaw et al., 1996, Zanella et al., 2000). While all IBV strains appear to initially infect chickens via the respiratory tract, vireamia enables spread to secondary sites for further replication and persistence. An example is IBV strain M41, which replicates primarily in the respiratory tract and subsequently spreads and replicates in a range of other tissues. In contrast, strains of IBV such as strain QX and IS/885/00 are primarily nephropathogenic (Benyeda et al., 2009, Meir et al., 2004). IS/885/00, referred to as a nephropathogenic infectious bronchitis virus (NIBV), was first isolated from a severe outbreak of renal disease in several broiler farms in Israel (Meir et al., 2004) and has been detected in many other Middle East countries (Abdel-Moneim et al., 2012, Awad et al., 2014, Mahmood et al., 2011). IBV QX was first isolated in China from chickens with proventriculitis (YuDong et al., 1998) but was later found to cause renal, (Ganapathy et al., 2012, Liu and Kong, 2004, Terregino et al., 2008, Worthington et al., 2008), respiratory and reproductive lesions (Ducatez et al., 2009, Terregino et al., 2008) in chicken flocks in Europe, Asia, Africa and Middle East (Beato et al., 2005, Domanska-Blicharz et al., 2006, Gough et al., 2008, Jackwood, 2012).
Differences in tissue tropism and thus differences in the pathogenicity of IBV strains have been hypothesized to be associated with differences in the binding properties of their spike proteins (Casais et al., 2003, Wickramasinghe et al., 2011). While the ability to bind to susceptible host cells is the first step in the viral life cycle, host innate immune responses could also be a major contributing factor to the pathological outcome of IBV infection. Variant IBVs continually emerge and the host determinants of IBV pathogenicity are not yet fully understood. Early cellular and innate immune responses of virus infected cells in vitro could act as useful indicators for predicting the pathological outcome of viral infection in vivo. For example, the three different genotypes of Newcastle disease viruses (NDV) produce distinct host response patterns in chicken spleenocytes, which is useful to differentiate the NDV genotypes (Hu et al., 2012). In order to establish the characteristic host response to predict the tissue tropism and pathogenicity of IBVs, we investigated apoptosis and innate immune responses in chicken embryo kidney (CEK) cells and tracheal organ cultures (TOCs) following infection with IS/885/00-like, QX-like and M41 IBV strains.
Results
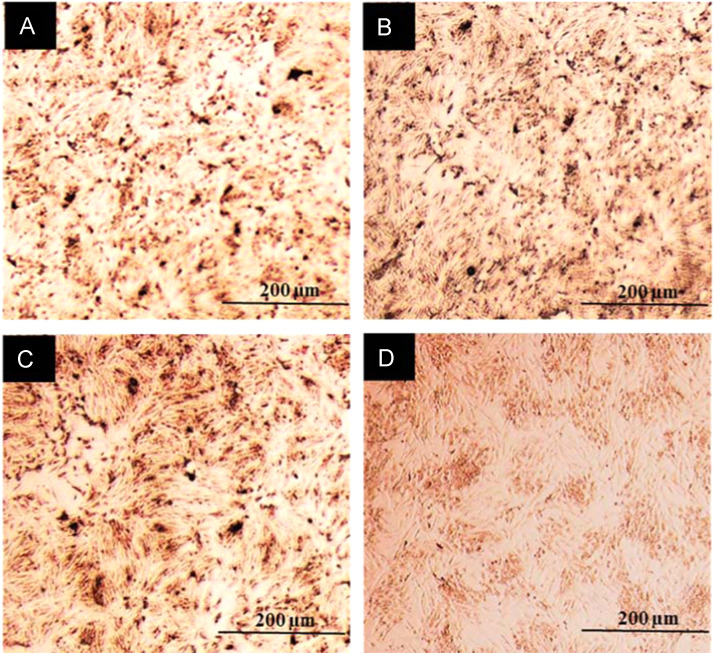
CEK cells infected with IBV strains 885, QX or M41 were subjected to immuno-cytochemical staining of viral nucleoprotein (NP) at 6 h post-infection (hpi). The dose (MOI 1.0) used resulted in a similar level of infection in CEK cells across all the three virus strains ( Fig. 1).
Fig. 1.
Chicken embryo kidney (CEK) cells infected with (A) 885, (B) QX (C) M41 or (D) mock-infected and immuno-cytochemical staining of viral NP at 6 hpi (hours post-infection), revealing similar levels of infection of all the three viruses.
IBV infections resulted in significant increase in apoptosis of CEK cells and TOCs
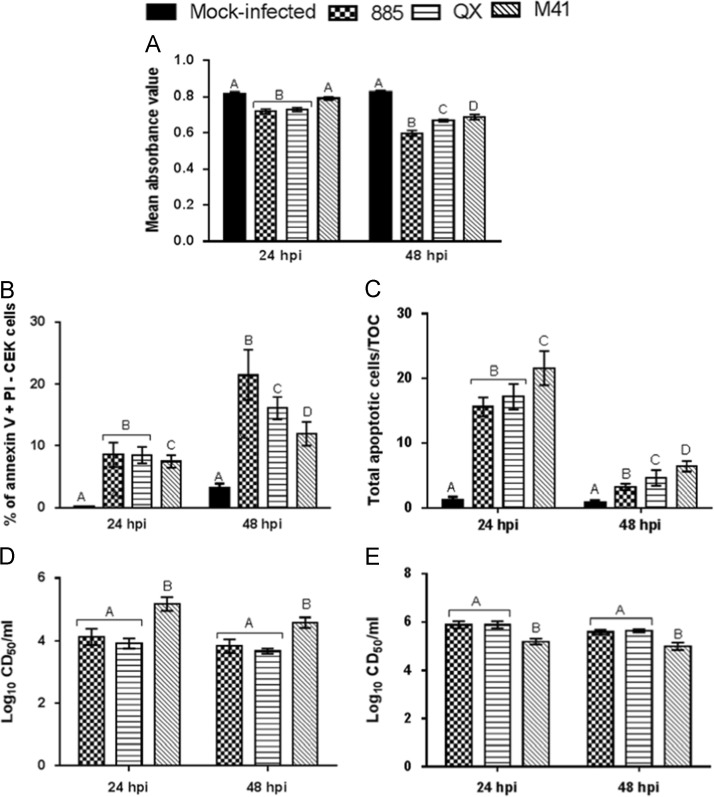
CEK cells and TOCs were infected with IBV strains 885, QX, M41 or mock infected, cell metabolic activity and percentage of apoptotic cells were evaluated at 24 and 48 hpi by MTT assay and Annexin V binding assay, respectively. A significant (p<0.05) reduction in cell metabolic activity was found in IBV infected CEK cells, compared with mock infected cells, both at 24 and 48 hpi ( Fig. 2A). A significantly (p<0.05) greater level of apoptosis was also found in IBV infected CEK cells at 24 and 48 h after virus infection (Fig. 2B). Total apoptotic cells in IBV or mock infected TOCs were evaluated by TUNEL assay ( Fig. 3). It was found that IBV infection resulted in significant increase in total apoptotic cells in TOCs when compared with mock infected controls at 24 and 48 hpi (Fig. 3C). Notably, infection of CEK cells with IBV strains 885 and QX resulted in significantly (p<0.05) greater level of cell death, as shown by reduced metabolic activity (Fig. 2A) and increased apoptosis (Fig. 2B) when compared to M41 infected cells both at 24 and 48 hpi. In contrast, significantly higher levels of apoptosis were observed in M41 infected TOCs compared with those infected with 885 or QX (Fig. 2C). Infection with IBV strains 885 or QX resulted in significantly (p<0.05) lower infectious virus production from CEK cells at 24 and 48 hpi when compared with M41 infected cells (Fig. 2D). In sharp contrast, significantly (p<0.05) lower infectious virus production was observed from M41 infected TOCs compared with 885 or QX infected TOCs (Fig. 2E).
Fig. 2.
IBV infections resulted in significant increase in apoptosis of chicken embryo kidney (CEK) cells and chicken embryo tracheal organ cultures (TOCs). CEK cells and TOCs were infected with IBV strains 885, QX, M41 or mock infected. At 24 and 48 hpi, cell metabolic activity of CEK cells was evaluated by (A) MTT assay and percent of apoptosis by (B) Annexin V binding, respectively. Total apoptotic cells in TOCs were evaluated by (C) TUNEL assay. Virus production in the (D) CEK cells and (E) TOC supernatants were determined in chicken embryo TOCs at designated time points after infection. Data represents mean of triplicate wells, with error bar showing standard error. Different letters indicate that the differences between groups at that time point are significant (p<0.05).
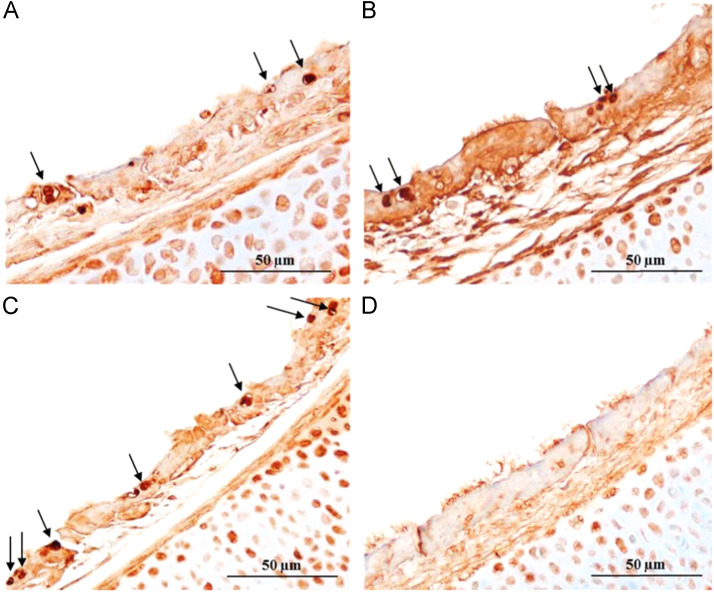
Fig. 3.
In situ terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining of chicken embryo tracheal organ cultures (TOCs) infected with (A) 885, (B) QX (C) M41 or (D) mock infected at 24 hpi (hours post-infection). Apoptosis TUNEL staining of TOCs infected with IBVs showing multiple apoptotic cells with degeneration and loss of cilia. Arrows indicate apoptotic cells.
Infection of CEK cells with IBVs resulted in significantly higher up-regulation of TLR3 and MDA5
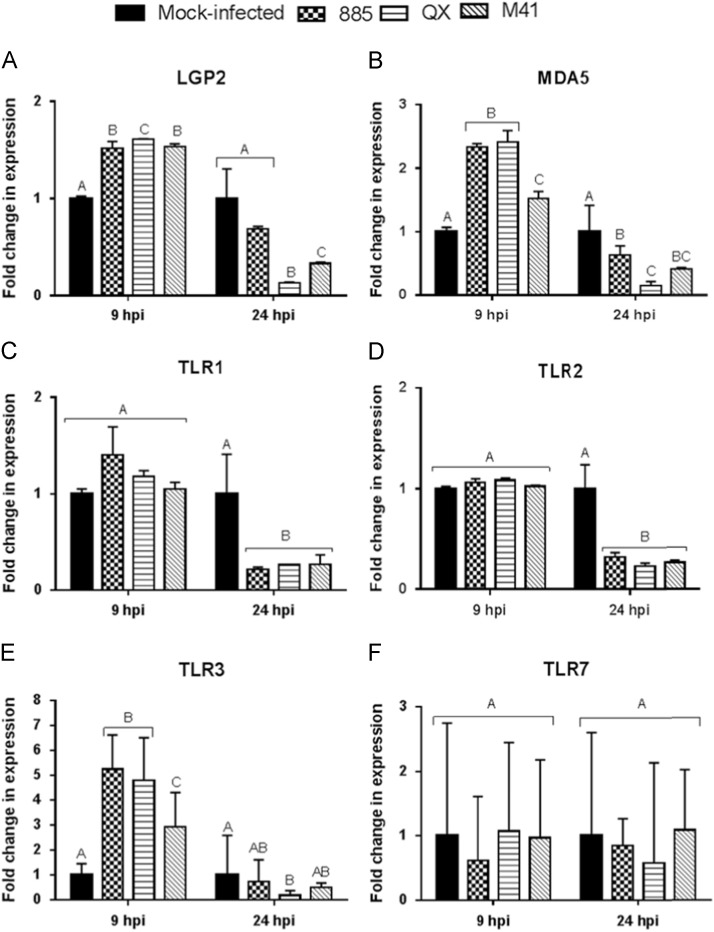
CEK cells were infected with IBV strains 885, QX, M41 or mock infected at a MOI of 1.0, and expression of LGP2, MDA, TLR1, TLR2, TLR3 and TLR7 were analysed at 9 and 24 hpi. It was observed that expression of LGP2 ( Fig. 4A) and MDA5 (Fig. 4B) was significantly up-regulated at 9 hpi but down-regulated at 24 hpi in IBV infected CEK cells compared with mock infected cells (p<0.05). However, at 9 hpi, infection with IBV strains 885 and QX resulted in significantly (p<0.05) greater MDA5 expression than M41 in CEK cells (Fig. 4B). There was no significant change in expression level of TLR1 (Fig. 4C). TLR2 (Fig. 4D) was observed at 9 hpi (p<0.05), but the expression of these genes was significantly (p<0.05) down-regulated at 24 hpi in IBV infected CEK cells, compared to mock infected cells (Fig. 4C and D). A significant (p<0.05) up-regulation of TLR3 expression was detected at 9 hpi but compared with mock infected cells, was either unchanged or significantly (p<0.05) down-regulated at 24 hpi in IBV infected CEK cells (Fig. 4E). Interestingly, IBV strains 885 and QX infection resulted in significantly greater TLR3 expression than M41 infection in CEK cells at 9 hpi (Fig. 4E). In summary, infection of CEK cells with IBV strains 885 and QX, compared with M41, resulted in significantly greater TLR3 and MDA5 expression in the early stage of infection (9 hpi).
Fig. 4.
Transcriptional regulation of innate viral sensing molecules in chicken embryo kidney (CEK) cells at 9 and 24 hpi (hours post-infection). Relative mRNA expression of (A) LGP2, (B) MDA5, (C) TLR1, (D) TLR2, (E) TLR3, (F) TLR7 in CEK cells infected with IBV strains 885, QX, or M41 at MOI 1.0 and gene expression was analysed at 9 and 24 hpi. Relative mRNA expression was determined by real-time PCR, normalised to 18S rRNA. Graph values are the mean of three biological replicates with error bars as standard error and are expressed as fold change relative to the mocked-infected group. Different letters indicate that the differences between groups at that time point are significant (p<0.05).
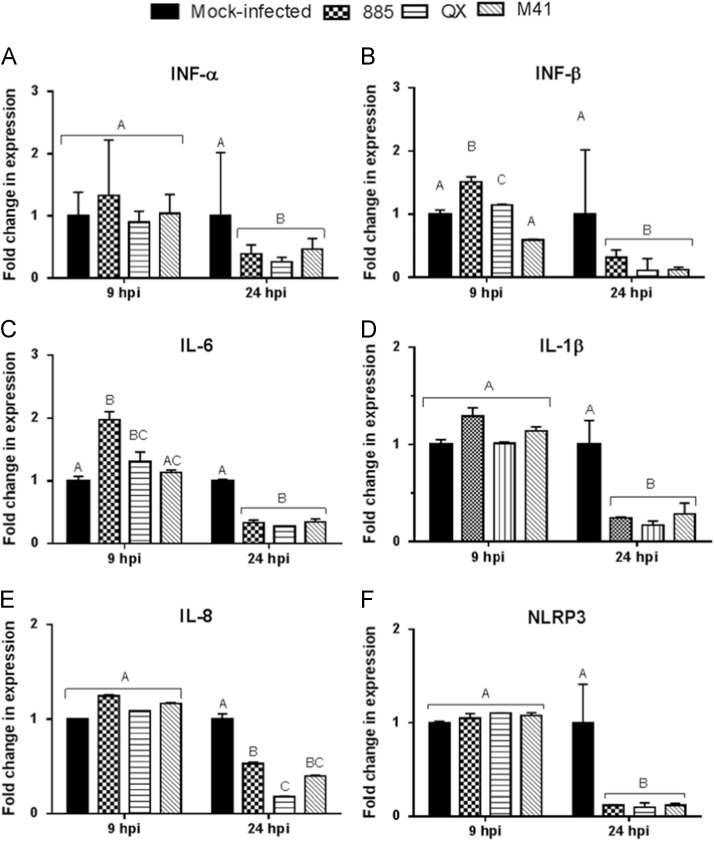
Higher induction of IFN-β and IL-6 in IBV infected CEK cells
Notably, infection of CEK cells with IBVs 885 and QX resulted in significantly (p<0.05) greater IFN-β expression than M41 at 9 hpi ( Fig. 5B). However, IFN-β expression was significantly (p<0.05) down-regulated at 24 hpi in all IBV infected CEK cells compared with mock infected cells (Fig. 5B). The significant (p<0.05) up-regulation of IL-6 (Fig. 5C) expression was observed at 9 hpi in 885 and QX infected CEK cells, but was not significantly (p<0.05) affected in M41 infected cells. There was no significant (p<0.05) change at 9 hpi but a significant (p<0.05) down-regulation at 24 hpi in the levels of IFN-α (Fig. 5A), IL-1β (Fig. 5D), IL-8 (Fig. 5E) and NLRP3 (Fig. 5F) expression was noticed in IBV infected CEK cells, compared with mock infected cells.
Fig. 5.
Type I interferon (IFN) and pro-inflammatory cytokineresponse in IBV infected chicken embryo kidney (CEK) cells. Relative mRNA expression of (A) INF-α, (B) INF-β, (C) IL-6, (D) IL-1β, (E) IL-8, (F) NLRP3 in CEK cells infected with IBV strains 885, QX, or M41 at MOI 1.0 was analysed for gene expression at 9 and 24 hpi (hours post-infection). Relative mRNA expression was determined by real-time PCR normalised to 18S rRNA. Graph values are the mean of three biological replicates with error bars as standard error, and are expressed as fold change relative to the mocked-infected group. Different letters indicate that the differences between groups at that time point are significant (p< 0.05).
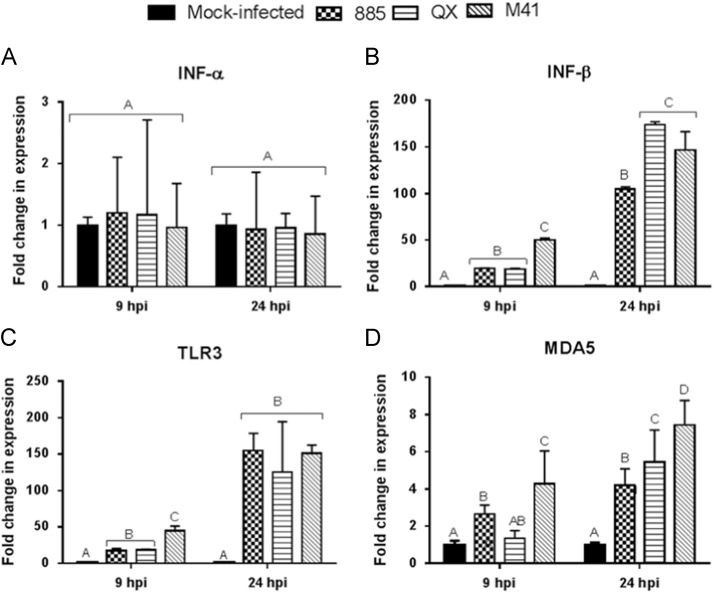
Regulation of Type I IFN, TLR3 and MDA5 genes in IBVs infected chicken TOCs
Transcriptional regulation of Type I IFN, TLR3 and MDA5 in TOCs infected with IBV strains 885, QX, M41 or mock infection was analysed at 9 and 24 hpi. INF-α ( Fig. 6A) expression was not significantly (p<0.05) changed both at 9 and 24 hpi in IBV infected TOCs compared with mock infected controls. There was a significant (p<0.05) up-regulation in the expression levels of IFN-β (Fig. 6B), TLR3 (Fig. 6C) and MDA5 (Fig. 6D) at 9 and 24 hpi in virus infected TOCs compared to controls. It was found that M41 infection resulted in significantly higher expression of IFN-β, TLR3 and MDA5 compared to 885 or QX infection in TOCs at 9 hpi.
Fig. 6.
Type I interferon and innate viral sensing gene response in IBV infected chicken embryo tracheal organ cultures (TOCs). Relative mRNA expression of (A) INF-α, (B) INF-β, (C) TLR3, (D) TLR7 in TOCs infected with IBV strains 885, QX, or M41 at a dose of 4log10 CD50 per individual ring or mock-infected and gene expression was analysed at 9 and 24 hpi (hours post-infection). Relative mRNA expression was determined by real-time PCR normalised to 18S rRNA. Graphed values are the mean of three biological replicates with error bars as standard error and are expressed as fold change relative to the mocked-infected group. Different letters indicate that the differences between groups at that time point are significant (p<0.05).
Discussion
We investigated apoptosis and innate immune responses in CEK cells and TOCs following infection with IBV strains 885, QX and M41. Infection of CEK cells with IBV strains 885 and QX that predominantly cause renal lesions in chickens resulted in significantly greater levels of apoptosis when compared to the M41 strain that causes mainly respiratory lesions. Similarly, infection of TOCs with M41 resulted in greater apoptosis than with the other two nephropathogenic strains.
A variety of viruses have been shown to induce apoptosis in infected host cells (Clarke and Tyler, 2009, Shen and Shenk, 1995, Teodoro and Branton, 1997). IBVs are known to induce apoptosis in infected cells and B-cell lymphoma 2 (Bcl-2) family proteins modulate IBV-induced apoptosis (Li et al., 2007, Liu et al., 2001, Zhong et al., 2012a, Zhong et al., 2012b). The modulation of Bcl-2 family proteins during IBV infection has also been postulated to be under the regulation of signalling pathways such as endoplasmic reticulum (ER) stress and Mitogen-Activated Protein Kinase/Extracellular signal-Regulated Kinase (MAPK/ERK) pathways (Fung and Liu, 2014, Zhong et al., 2012a). Furthermore, IBV infection results in a growth-inhibitory effect by inducing cell cycle arrest at S and G2/M phases (Li et al., 2007), both of which results in apoptosis. Our findings suggest a strong association between apoptosis in CEK cells or TOCs to the ability of IBV strains to cause renal or respiratory lesions respectively in chickens.
Nephropathogenic IBV strains 885 and QX resulted in significantly greater up-regulation of innate immune sensing genes namely TLR3 and MDA5 along with greater IFN-β mRNA levels in CEK cells at 9 h of infection when compared to M41 infection. In contrast, these genes were significantly up-regulated in M41 infected TOCs than those infected with 885 or QX at 9 hpi. Concurrent with our findings, a previous study observed a significant increase of TLR3 mRNA expression in both the tracheas and lungs of IBV infected chickens, when compared to uninfected controls (Kameka et al., 2014). We did not identify any particular change in regulation pattern of other innate immune genes, namely TLR1 and TLR2.
TLR3 and TLR7 are well known for recognition of RNA virus encoded pathogen associated molecular patterns (PAMPs) (Akira, 2001). TLR7 was not significantly affected by IBV infection in CEK cells. The end products of the TLR3 signalling pathway are the production of anti-viral type I interferon (IFN)-α and -β, whereas TLR7 activation results in the production of pro-inflammatory cytokines (Guillot et al., 2005). Concurrent with TLR3 activation, we found a significant up-regulation of IFNβ, but not IFNα mRNA expression, at 9 h after IBV infection in CEK cells. Likewise, the lack of TLR7 induction correlated with no change in the expression of pro-inflammatory cytokines, namely IL-1β, IL-8 and NLRP3 in IBV infected CEK cells. In contrast to our findings, a recent study found that IFN-β transcription was not found in CEK cells until 12 h after infection with IBV M41 at MOI of 0.1 (Kint et al., 2015). This difference may have been due to variation in the MOI used and origin of cells.
Notably, expressions of all the innate immune genes tested were down-regulated at 24 h after IBV infection in CEK cells. This could be a consequence of host shut-off induced by IBV virus replication. Corona viruses (CoVs) are known to down-regulate host gene expression and increased host mRNA degradation in order to suppress host innate immune responses (Kamitani et al., 2006, Tohya et al., 2009). Corona virus nucelocapsid (N) protein has been shown to interfere with the 2′,5′-oligoadenylate synthetase/RNaseL (2′–5′ OAS) activation, which is responsible for Type I IFN induction and can also inhibit the production of various pro-inflammatory cytokines and chemokines via global translational shutdown (Ye et al., 2007, Zhong et al., 2012b). However, MDA5 and IFN-β expression was also up-regulated in TOCs at 24 h after IBV infection suggesting a sustained immune response in TOCs compared with CEK cells. We found a similar virus replication pattern in CEKs and TOCs with a comparable virus output.
Higher levels of apoptosis and IFN-β expression is also associated with significantly lower infectious virus particle production from both CEK cells and TOCs. This could be due to increased antiviral state induced by IFN-β and/or increased apoptosis. Apoptosis of cultured cells have previously been shown to be associated with reduced production of infectious influenza virus in duck cells (Kuchipudi et al., 2012a).
Several in vitro and ex vivo studies have suggested a key role of TLR3 in viral detection (Alexopoulou et al., 2001, Bowie and Haga, 2005, Finberg and Kurt-Jones, 2004, Tabeta et al., 2004). However, recent evidence suggests TLR3 contributes to pathogenic effects of viral infections. TLR3 mediates West Nile virus entry into the brain, causing lethal encephalitis (Wang et al., 2004) and contributes to a detrimental inflammatory response to influenza virus infection in mice, resulting in acute pneumonia (Le Goffic et al., 2006). A recent study has shown that MDA5, but not TLR3, is involved in the sensing of IBV (Kint et al., 2015). However, higher mRNA levels of MDA5 and TLR3 correlated with the in vivo pathogenicity of the IBV strains used in this study, such that the nephropathogenic and tracheotropic IBVs induced much higher levels in CEK cells and TOCs, respectively. It is possible that MDA5 activation is a protective antiviral host response against the virus whereas TLR3 contributes to some detrimental effects from viral infection. The precise role of MDA5 and TLR3 in host defence against IBV infection and/or disease pathogenesis is not yet fully understood and hence, warrants further in-depth studies.
We found that LGP2 expression was significantly increased at 9 h but down-regulated at 24 h after IBV infection in CEK cells. LGP2 functions as a negative regulator by interfering with the recognition of viral RNA by RIG-I and MDA5 (Yoneyama et al., 2005). MDA5 is IFN-inducible (Kang et al., 2004), so once an IFN response is triggered, this innate antiviral loop initiates auto-amplification of MDA5 until the natural inhibitor LGP2 is induced (Yoneyama et al., 2005). However, we found that the expression of LGP2, MDA5 and IFN-β mRNA levels were down-regulated at 24 h after IBV infection, possibly due to the virus induced host gene shutoff. In agreement with our findings, a recent study also reported a down-regulation of TLR3, IL-1β and IFN-γ expression in IBV infected chicken tracheas at 12 hpi (Kameka et al., 2014). In this study, we infected cells at MOI of 1.0; however, infection at a lower MOI could be helpful to profile the effect of LGP2 on MDA5 and IFN-β regulation in IBV infected cells.
There have been conflicting reports on the difference in virulence among IBV strains using this in vitro system. Cook et al. (1976) did not find marked differences when compared three strains of IBV on the basis of their effect on tracheal cilia. Raj and Jones (1996) also reported little difference among several IBV strains using measurement of ciliary activity as a criterion for damage to the tracheal epithelium. However, Abd El Rahman et al. (2009) has revealed that the strains differed in their efficiency to infect the tracheal epithelium. Infection by Beaudette and QX resulted in a larger number of infected cells compared to Itlay-02 and 4/91 strains, when tracheal organ cultures (TOCs) were infected with the same amount of virus (Abd El Rahman et al., 2009). In summary, our study found that greater levels of apoptosis and elevated expression of TLR3, MDA5 and IFN-β is associated with increased pathogenicity of IBVs in kidney (CEK cells) and respiratory (TOCs) tissues. The findings of this study using CEK cells and TOCs raise a strong possibility that host innate immune response could aid generation of a predictive prognosis for the tissue tropism of novel IBV strains. However, further studies are needed to confirm the association between pathogenicity and host response in vivo and to establish the mechanisms underlying such responses.
Materials and methods
Cell cultures
Monolayers of primary chicken kidney (CEK) cells were prepared from kidneys of specific-pathogen-free (SPF) chicken embryos after 18 day incubation (Lohmann, Germany). Kidneys removed aseptically were collected in Minimum Essential Medium (MEM), supplemented with 0.5% fetal bovine serum (Sigma) and 1% antibiotics (penicillin and streptomycin). The kidneys were minced into small pieces using sterile scissors, followed by trypsinization (0.25% trypsin) for 30 min at 37 °C and filtered through a 100 µm mesh to remove tissue debris. After centrifugation at 1500 g for 10 min, cell pellet was re-suspended in the MEM medium and seeded at 1×105 cells/cm2 in cell culture plates.
Viruses
We used a traditional IBV strain M41, and two variant IBV strains namely IS/885/00-like (885) (Awad et al., 2013) and QX-like strain KG3P (Ganapathy et al., 2012) were used. IS/885/00 causes mortality, poor weight gain and severe renal damage, while QX causes renal and reproductive problems.
Allantoic fluid containing these IBV strains were propagated in TOCs prepared from 19 to 20-day old SPF chicken embryos (Cook et al., 1976). For the CEK cell infection study, all IBV strains were titrated on CEK cells using an immuno-cytochemical focus assay described previously (Kuchipudi et al., 2012a).
In brief, serial 2-fold dilutions of a known volume of TOC supernatants were used to infect CEK cells in 96-well culture plates (CELLSTAR, Grenier bio-one, UK). Cells were washed after a 2 h incubation with virus, followed by a further 4 h incubation, and then fixed in 1:1 acetone:methanol for 10 min. Cells were subjected to viral nucleoprotein detection by anti-IBV nucleoprotein monoclonal antibody (Prionics, UK) followed by visualization with Envision+system-HRP (DAB; Dako, Ely, UK). Cells expressing viral nucleoprotein (NP) were counted, and the mean number of positive cells in five fields was used to calculate focus-forming units of virus per microlitre of inoculum.
For TOC infection study viruses were titrated in chicken embryo TOCs and expressed in median ciliostatic doses (CD50)/ml (Cook et al., 1976). In brief, for each dilution of virus, three TOC rings with 100% of the cilia beating were used. TOCs were inoculated with viruses (0.1 ml) in serial dilutions before being incubated at 37.0 °C in a rotating incubator (Lab Thermal Equipment, Greenfield, NR OLDHAM) at 8 rpm. The TOCs were observed for ciliary activity post-infection, a reduction in at least 5% mean activity compared with mock infected were considered as positive for IBV infection (Cook et al., 1976) and further confirmed by RT-PCR followed by sequencing (Worthington et al., 2008).
Virus infection of CEK cells
CEK cells were seeded on cell culture plates (CELLSTAR, Grenier bio-one, UK) at a density of 1×105 cells/cm2. After incubation at 37 °C for 48 h, 80–90% confluent monolayers were washed three times with phosphate-buffered saline (PBS), and then either infected with different IBV strains at multiplicity of infection (MOI) 1.0 or mock infected with cell culture medium. Triplicate wells were used for each virus or mock infection for each time point. Cells were analyzed at 24 and 48 h post-infection (hpi) to determine the effect of virus infection on cell metabolic activity and induction of apoptosis. Cell culture supernatant was titrated for infectious virus in TOC expressed as CD50/ml (Cook et al., 1976). At 9 and 24 hpi cell pellets were collected in RLT buffer (Qiagen, UK) for total RNA extraction and cell metabolic activity was analysed at 24 and 48 hpi using CellTiter 96, a non-radioactive cell proliferation assay (Promega, Madison, WI, USA), according to the manufacturer׳s instructions.
Virus infection of TOCs
TOCs were infected 5 days after preparation to allow the early inflammatory responses of the tissue to subside (Reemers et al., 2009). TOCs were infected with IBV strains using a dose of 4 log10 CD50 per individual ring, or mock-infected with TOC medium without virus. Three rings were used for each virus or mock infection for each time point. The infection dose of 4 log10 CD50 was chosen based on a preliminary study wherein this dose caused 100% cilliostasis in TOC by day 3 after infection. Samples of TOC supernatant or complete rings were collected at 9, 24 and 48 hpi. TOC rings were analysed for apoptotic cells using in situ Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay, and supernatants were titrated for quantification of infectious virus at 24 and 48 hpi. Total RNA extracted from TOC rings collected at 9 and 24 hpi were used for the quantification of host gene expression analysis.
Analysis of apoptosis
At 24 and 48 h after virus or mock infection, CEK cells in 24-well tissue culture plates were analysed to quantify the percent of apoptotic cells. Briefly, cells were washed in PBS and removed from the tissue culture plate by trypsinization, followed by inactivation with growth medium containing serum. The cells were then washed, resuspended in binding buffer (Clontec, CA), and subsequently analysed by staining with Annexin V-FITC and PI according to the manufacturer׳s instructions (ApoAlert®, Annexin V-FITC apoptosis kit, Clontech, CA) using flow cytometer (BD Accuri C6, San Jose, CA). Fluorescence was quantified using CFlow software (BD Accuri). The single-dye positive controls were prepared by staining representative cells with FITC and PI dye individually and used for estimation of proper colour compensation. Unstained cell samples were used as a negative control to adjust the threshold.
Apoptotic cells were detected and quantified by the TUNEL assay using the peroxidase (POD) in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer׳s instructions. A total of five sections were analyzed for each time point. Briefly, cells were fixed in paraformaldehyde and permeabilized with 0.1% Triton X-100 at room temperature. After equilibration, specimens were overlaid with 50 μl TUNEL reaction mixture or 50 μl label solution of the no-enzyme control. After initial incubation for 1 h at 37 °C in a dark humidified atmosphere, reactions were then developed using peroxidase substrate kit DAB (Vector Laboratories, Burlingame, USA). Sections were counterstained with haematoxylin (Merck, Germany) and mounted with DPX (dibutyl phthalate, polystyrene granules and xylene) aqueous mounting medium (VWR International, Leuvan). For each sample, the total number of positive cells/400×microscopic field was calculated.
Quantification of infectious virus
Supernatants from virus infected CEK cells and TOCs were titrated for infectious virus and expressed as CD50/ml (Cook et al., 1976). In brief, TOCs were inoculated with 0.1 ml of supernatant in serial dilutions, and three rings with 100% of the cilia beating were used for each dilution and incubated at 37.0 °C in a rotating incubator (Lab Thermal Equipment, Greenfield, NR OLDHAM) at lowest rotation speed. The TOCs were observed for ciliary activity; those exhibiting less than 5% mean activity were recorded as IBV positive.
Host gene expression analysis
Total RNA from cell pellets in RLT buffer (Qiagen, UK) and TOC ring was extracted using RNeasy Plus Mini-QIAshredder Kit (Qiagen, UK) following the manufacturer׳s instructions. RNA concentration was quantified by NanoDrop® ND-1000 (NanoDrop, Wilmington, DE/ NanoDrop ND-1000; Peqlab). The cDNA was generated from 1 µg of RNA using the Superscript III First-strand cDNA synthesis system (Life Technologies) with random primers as per the manufacturer׳s recommendations. The generated cDNA samples further diluted to 1:50 in nuclease-free water were used in quantitative reverse transcription PCR (qRT-PCR) analysis.
QRT-PCR was performed on the LightCycler® 480 (Roche, UK), using LightCycler 480 SYBR Green I Master mix (Roche, UK). Primers were designed with Primer Express version 2.0 (Applied Biosystems), based on previously reported sequences ( Table 1). All primers were provided by Eurofins Genomics (Edersberg, Germany). The cycling conditions were 10 min at 95 °C, 45 cycles of 10 s at 95 °C, 10 s at 60 °C annealing temperature and 10 s at 72 °C, finally followed by a melting curve analysis to ensure the specificity of the SYBR green PCR. QRT-PCR data was normalised using a relative standard curve method to 18S ribosomal RNA (18SrRNA) expression (Kuchipudi et al., 2012b) and the data were presented as fold difference in gene expression of virus versus mock infected samples.
Table 1.
Primers used in the study.
| Gene name | Primer sequences: sense (S) and anti-sense (AS) |
|---|---|
| 18S rRNA (18S ribosomal RNA ) (Kuchipudi et al., 2012b) | (S) TGTGCCGCTAGAGGTGAAATT |
| (AS) TGGCAAATGCTTTCGCTTT | |
| LGP2 (Laboratory of genetics and physiology 2) | (S) AGCCCACGAAGCAGTACGA |
| (AS) CGGCAACTCGGGCATCT | |
| MDA5 (Melanoma differentiation-associated protein 5) | (S) AGGAGGACGACCACGATCTCT |
| (AS) CCACCTGTCTGGTCTGCATGT | |
| TLR1 (Toll like receptor 1) | (S) GGGAAACCGCTCTGCAGTT |
| (AS) CATTCTTCACCCACAGGGAATC | |
| TLR2 (Toll like receptor 2) | (S) CCACCGGTCCCTCCTAGTGT |
| (AS) ACCCAACGACCACCAGGAT | |
| TLR3 (Toll like receptor 3) | (S) GCAATTTCTCCTTCACCTTTTCA |
| (AS) CCTTTATGTTTGCTATGTTGTTATTGCT | |
| TLR7 (Toll like receptor 7) | (S) GAATTCAAGAGGTTCAGGAACATGA |
| (AS) TTAGGGCAGGGAGTACAAGGATAT | |
| IFNα (Interferon alpha) (Kuchipudi et al., 2014) | (S) CTTCCTCCAAGACAACGATTACAG |
| (AS) AGGAACCAGGCACGAGCTT | |
| IFNβ (Interferon beta) | (S) TCCAACACCTCTTCAACATGCT |
| (AS) TGGCGTGTGCGGTCAAT | |
| IL1β (Interleukin 1 beta) | (S) TGCTGGTTTCCATCTCGTATGT |
| (AS) CCCAGAGCGGCTATTCCA | |
| IL-6 (Interleukin 6) (Kuchipudi et al., 2014) | (S) CACGATCCGGCAGATGGT |
| (AS) TGGGCGGCCGAGTCT | |
| NLRP3 (NLR family, pyrin domain containing 3) | (S) TGTGTGTCATCCCTGTCATGAG |
| (AS) TGGTCTTAGAGCACGCAAGGA | |
| IL-8 (Interleukin 8) (Kuchipudi et al., 2014) | (S) CCCTCGCCACAGAACCAA |
| (AS) CAGCCTTGCCCATCATCTTT |
Statistical analysis
The data were analysed using one-way ANOVA, followed by the post-hoc LSD multiple comparison test using GraphPad Prism version 6 software. Differences between groups at that time point were considered significant at p<0.05.
Author contributions
G.K., S.V.K, and R.C conceived and designed the study. G.K supervised R.C who carried out all the experiments. S.V.K and R.C analyzed the data and wrote the manuscript with editorial contributions from all other authors.
Acknowledgments
Rajesh Chhabra is a Commonwealth Scholar, funded by the UK Government (INCS-2012-180).
Contributor Information
Rajesh Chhabra, Email: Rajesh.Chhabra@liverpool.ac.uk, rajesh.chhabra@luvas.edu.in.
Suresh V Kuchipudi, Email: skuchipudi@psu.edu.
Julian Chantrey, Email: Chantrey@liverpool.ac.uk.
Kannan Ganapathy, Email: K.Ganapathy@liverpool.ac.uk.
References
- Abd El Rahman S., El-Kenawy A.A., Neumann U., Herrler G., Winter C. Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol. 2009;38:41–45. doi: 10.1080/03079450802632049. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A., Afifi M., El-Kady M. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch. Virol. 2012;157:1–5. doi: 10.1007/s00705-012-1445-1. [DOI] [PubMed] [Google Scholar]
- Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Awad F., Baylis M., Ganapathy K. Detection of variant infectious bronchitis viruses in broiler flocks in Libya. IJVSM. 2014;2:78–82. [Google Scholar]
- Awad, F., Forrester, A., Jones, R., Capua, I., Baylis, M., Chhabra, R., Ganapathy, K., 2013. Immunopathogenesis of infectious bronchitis virus related to IS/1494/06 and IS/885 in specific pathogen-free chicks, XVIIIth Congress WVPA, Nantes, France, p. 385.
- Beato M.S., De Battisti C., Terregino C., Drago A., Capua I., Ortali G. Evidence of circulation of a Chinese strain of infectious bronchitis virus (QXIBV) in Italy. Vet. Rec. 2005;156:720. doi: 10.1136/vr.156.22.720. [DOI] [PubMed] [Google Scholar]
- Benyeda Z., Mató T., Süveges T., Szabó É., Kardi V., Abonyi-Tóth Z., Rusvai M., Palya V. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathol. 2009;38:449–456. doi: 10.1080/03079450903349196. [DOI] [PubMed] [Google Scholar]
- Bowie A.G., Haga I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Clarke P., Tyler K.L. Apoptosis in animal models of virus-induced disease. Nat. Rev. Microbiol. 2009;7:144–155. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Darbyshire J.H., Peters R.W. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch. Virol. 1976;50:109–118. doi: 10.1007/BF01318005. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- De Wit J.J. Detection of infectious bronchitis virus. Avian Pathol. 2000;29:71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Nieuwenhuisen-van Wilgen J., Hoogkamer A., van de Sande H., Zuidam G.J., Fabri T.H.F. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- Dolz R., Pujols J., Ordonez G., Porta R., Majo N. Antigenic and molecular characterization of isolates of the Italy 02 infectious bronchitis virus genotype. Avian Pathol. 2006;35:77–85. doi: 10.1080/03079450600597295. [DOI] [PubMed] [Google Scholar]
- Domanska-Blicharz K., Minta Z., Smietanka K., Porwan T. New variant of IBV in Poland. Vet. Rec. 2006;158:808. doi: 10.1136/vr.158.23.808-c. [DOI] [PubMed] [Google Scholar]
- Ducatez M.F., Martin A.M., Owoade A.A., Olatoye I.O., Alkali B.R., Maikano I., Snoeck C.J., Sausy A., Cordioli P., Muller C.P. Characterization of a new genotype and serotype of infectious bronchitis virus in Western Africa. J. Gen. Virol. 2009;90:2679–2685. doi: 10.1099/vir.0.012476-0. [DOI] [PubMed] [Google Scholar]
- Finberg R.W., Kurt-Jones E.A. Viruses and toll-like receptors. Microbes Infect. 2004;6:1356–1360. doi: 10.1016/j.micinf.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy K., Wilkins M., Forrester A., Lemiere S., Cserep T., McMullin P., Jones R.C. QX-like infectious bronchitis virus isolated from cases of proventriculitis in commercial broilers in England. Vet. Rec. 2012;171:597. doi: 10.1136/vr.101005. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Lunt R.L., Metz A.L., Fries P.A. Attenuation of avian infectious bronchitis virus by cold-adaptation. Avian Dis. 1991;35:847–853. [PubMed] [Google Scholar]
- Gough R.E., Cox W.J., Welchman Dd.B., Worthington K.J., Jones R.C. Chinese QX strain of infectious bronchitis virus isolated in the UK. Vet. Rec. 2008;162:99–100. doi: 10.1136/vr.162.3.99. [DOI] [PubMed] [Google Scholar]
- Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M., Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Hofstad M.S. Immune response to infectious bronchitis virus. Am. J. Vet. Res. 1975;36:520–521. [PubMed] [Google Scholar]
- Hu Z., Hu J., Hu S., Liu X., Wang X., Zhu J., Liu X. Strong innate immune response and cell death in chicken splenocytes infected with genotype VIId Newcastle disease virus. Virol. J. 2012;9:208. doi: 10.1186/1743-422X-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Kameka A.M., Haddadi S., Kim D.S., Cork S.C., Abdul-Careem M.F. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology. 2014;450–451:114–121. doi: 10.1016/j.virol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani W., Narayanan K., Huang C., Lokugamage K., Ikegami T., Ito N., Kubo H., Makino S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA. 2006;103:12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.C., Gopalkrishnan R.V., Lin L., Randolph A., Valerie K., Pestka S., Fisher P.B. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–1800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- Kint J., Fernandez-Gutierrez M., Maier H.J., Britton P., Langereis M.A., Koumans J., Wiegertjes G.F., Forlenza M. Activation of the chicken type I interferon response by infectious bronchitis coronavirus. J. Virol. 2015;89:1156–1167. doi: 10.1128/JVI.02671-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi S.V., Dunham S.P., Nelli R., White G.A., Coward V.J., Slomka M.J., Brown I.H., Chang K.C. Rapid death of duck cells infected with influenza: a potential mechanism for host resistance to H5N1. Immunol. Cell Biol. 2012;90:116–123. doi: 10.1038/icb.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi S.V., Tellabati M., Nelli R.K., White G.A., Perez B.B., Sebastian S., Slomka M.J., Brookes S.M., Brown I.H., Dunham S.P., Chang K.C. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol. J. 2012;9:230. doi: 10.1186/1743-422X-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi S.V., Tellabati M., Sebastian S., Londt B.Z., Jansen C., Vervelde L., Brookes S.M., Brown I.H., Dunham S.P., Chang K.C. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet. Res. 2014;45:118. doi: 10.1186/s13567-014-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. Detrimental contribution of the toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.Q., Tam J.P., Liu D.X. Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53. Virology. 2007;365:435–445. doi: 10.1016/j.virol.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Xu H.Y., Liu D.X. Induction of caspase-dependent apoptosis in cultured cells by the avian coronavirus infectious bronchitis virus. J. Virol. 2001;75:6402–6409. doi: 10.1128/JVI.75.14.6402-6409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood Z.H., Sleman R.R., Uthman A.U. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet. Micro. 2011;150:21–27. doi: 10.1016/j.vetmic.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen E.N., Walker J., Peters A., Pastoret P.P., Jungersen G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir R., Rosenblut E., Perl S., Kass N., Ayali G., Perk S., Hemsani E. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48:635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. An in vitro comparison of the virulence of seven strains of infectious bronchitis virus using tracheal and oviduct organ cultures. Avian Pathol. 1996;25:649–662. doi: 10.1080/03079459608419172. [DOI] [PubMed] [Google Scholar]
- Reemers S.S., Groot Koerkamp M.J., Holstege F.C., van Eden W., Vervelde L. Cellular host transcriptional responses to influenza A virus in chicken tracheal organ cultures differ from responses in in vivo infected trachea. Vet. Immunol. Immunopathol. 2009;132:91–100. doi: 10.1016/j.vetimm.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Shaw K., Britton P., Cavanagh D. Sequence of the spike protein of the Belgian B164S isolate of nephropathogenic infectious bronchitis virus. Avian Pathol. 1996;25:607–611. doi: 10.1080/03079459608419165. [DOI] [PubMed] [Google Scholar]
- Shen Y., Shenk T.E. Viruses and apoptosis. Curr. Opin. Genet. Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Tabeta K., Georgel P., Janssen E., Du X., Hoebe K., Crozat K., Mudd S., Shamel L., Sovath S., Goode J., Alexopoulou L., Flavell R.A., Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro J.G., Branton P.E. Regulation of apoptosis by viral gene products. J. Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terregino C., Toffan A., Beato M.S., De Nardi R., Vascellari M., Meini A., Ortali G., Mancin M., Capua I. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- Tohya Y., Narayanan K., Kamitani W., Huang C., Lokugamage K., Makino S. Suppression of host gene expression by nsp1 proteins of group 2 bat coronaviruses. J. Virol. 2009;83:5282–5288. doi: 10.1128/JVI.02485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe I.N., de Vries R.P., Grone A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Ye Y., Hauns K., Langland J.O., Jacobs B.L., Hogue B.G. Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist. J. Virol. 2007;81:2554–2563. doi: 10.1128/JVI.01634-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- YuDong W., YongLin W., ZiChun Z., GenChe F., YiHai J., Xiang E.L. Isolation and identification of glandular stomach type IBV (QX IBV) in chickens. Chin. J. Anim. Quar. 1998;15:1–3. [Google Scholar]
- Zanella A., Coaro R., Fabris G., Marchi R., Lavazza A. Avian infectious bronchitis virus: isolation of an apparently new variant in Italy. Vet. Rec. 2000;146:191–193. doi: 10.1136/vr.146.7.191. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Liao Y., Fang S., Tam J.P., Liu D.X. Up-regulation of Mcl-1 and Bak by coronavirus infection of human, avian and animal cells modulates apoptosis and viral replication. PLoS One. 2012;7:e30191. doi: 10.1371/journal.pone.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Tan Y.W., Liu D.X. Recent progress in studies of arterivirus- and coronavirus-host interactions. Viruses. 2012;4:980–1010. doi: 10.3390/v4060980. [DOI] [PMC free article] [PubMed] [Google Scholar]