Graphical abstract
(a) The Yin and Yang symbol: The philosophical basis of some of the assumed properties and research on Cordyceps (see text)? (b) Cordyceps spp. parasitizing a dead Campanotus insect and (c) cordyheptapeptide A, a natural product from the fungus.

Keywords: Fungus, Cordyceps sinensis, Cordyceps militaris, Lepidopteron, Traditional Chinese medicine, Cancer, Diabetes, Apoptosis
Abstract
Traditional Chinese medicines (TCM) are growing in popularity. However, are they effective? Cordyceps is not studied as systematically for bioactivity as another TCM, Ganoderma. Cordyceps is fascinating per se, especially because of the pathogenic lifestyle on Lepidopteron insects. The combination of the fungus and dead insect has been used as a TCM for centuries. However, the natural fungus has been harvested to the extent that it is an endangered species. The effectiveness has been attributed to the Chinese philosophical concept of Yin and Yang and can this be compatible with scientific philosophy? A vast literature exists, some of which is scientific, although others are popular myth, and even hype. Cordyceps sinensis is the most explored species followed by Cordyceps militaris. However, taxonomic concepts were confused until a recent revision, with undefined material being used that cannot be verified. Holomorphism is relevant and contamination might account for some of the activity. The role of the insect has been ignored. Some of the analytical methodologies are poor. Data on the “old” compound cordycepin are still being published: ergosterol and related compounds are reported despite being universal to fungi. There is too much work on crude extracts rather than pure compounds with water and methanol solvents being over-represented in this respect (although methanol is an effective solvent). Excessive speculation exists as to the curative properties. However, there are some excellent pharmacological data and relating to apoptosis. For example, some preparations are active against cancers or diabetes which should be fully investigated. Polysaccharides and secondary metabolites are of particular interest. The use of genuine anamorphic forms in bioreactors is encouraged.
1. Introduction
Too much about Cordyceps is unsubstantiated. This literature is written to sell so-called medicines to potentially vulnerable people with serious diseases. On the other hand, there is convincing scientific information that indicates significant pharmacological properties which are worth assessing (see Table 1 ). The present large review undertakes this task and deals with papers that use the name Cordyceps sometimes in its most general sense, especially when the revision of Sung et al. (2007) is considered. There follows an extended introduction to the topic.
Table 1.
Medically related purported effects of various Cordyceps taxa (or preparations) as described by various authors
| Taxon | Purported effect |
|---|---|
| Cordyceps | Negative for its many biological activities (sic); tonic to restore vital functions (Shin et al., 2001) |
| Reputed for broad biological activities; tonic to replenish vital function (Shim et al., 2000) | |
| Prevents disease and onset of senility (Leung et al., 2005) | |
| Replenishment of body health (Li et al., 2002) | |
| Maintain balance of Yin and Yang (sic) (Leung et al., 2005) | |
| C. sinensis | Immunomodulatory; increases survival of lupus mice (Chen et al., 1999) |
| Eternal youth (Fujita et al., 1990) | |
| Treats a wide range of disorders; used for centuries; cultivated has same properties as natural (Yang et al., 2005) | |
| Precious tonic and medicine since ancient times (Zhang et al., 2005) | |
| Treatment for wide range of diseases; anti-oxidant/anti-apoptotic properties (Buenz et al., 2004) | |
| Wide range of diseases (Hui et al., 2006) | |
| Treats asthma, and bronchial and lung inflammation (Kuo et al., 2001) | |
| Replenishment of body health (Li et al., 2001a, Li et al., 2001b, Li et al., 2001c) | |
| One of most valued herbs in TCM (Yamaguchi et al., 2000a, Yamaguchi et al., 2000b) | |
| Treats general debility after sickness and for old persons (Chiou et al., 2000) | |
| CTM for nephritis (Lin et al., 1999) | |
| Prized traditional medical materials (Ng and Wang, 2005) | |
| Immunomodulator (Kuo et al., 2005) | |
| Highly valued for properties (Park et al., 2005) | |
| Well known for effect on immune system (Chiu, 1998) | |
| Benefits to the kidneys (Wojcikowski et al., 2004) | |
| C. militaris | Tonic (Young et al., 2001) |
| Wide range of diseases (Hui et al., 2006) | |
| Prized traditional medical materials (Ng and Wang, 2005) | |
| Cancer (in oriental medicine) (Park et al., 2005) | |
| Popular nutraceutical and TCM (Jung et al., 2007) | |
| C. pruinosa | Prized traditional medical material (Ng and Wang, 2005) |
| C. ophioglossoides | Prized traditional medical material (Ng and Wang, 2005) |
The title relates to another in this journal concerning the fungal traditional Chinese medicine (FTCM), Ganoderma (Paterson, 2006). In that case, the fungus was indeed a biofactory in the sense that numerous compounds have been reported from the fungus. What is the situation with another FTCM, Cordyceps? The immediate answer is that the state of the art is considerably less developed (see Table 2 for a list of secondary metabolites). There is a general impression that this fungus is being used in a modern context, before the benefits, and even what is being used, have been determined scientifically. Cordyceps is one of a growing number of FTCM being considered as cures for modern human diseases. Many commercial products are available in the market (e.g. Didanosine from Cordyceps militaris). These nutraceuticals are considered to relieve the “stress for humans of living in technologically developed societies” by stimulating basic and secondary responses of the immune system (Lakhanpal and Rana, 2005).
Table 2.
Example of the range of species and some of the low molecular-weight secondary metabolites from Cordyceps
| Species | Secondary metabolites |
|---|---|
| C. sinensis | Cyclic peptides, H1-A |
| C. militaris | Cyclic peptides, cordycepin, 10-membered macrolides, cepharosporolides C, E and F, pyridine-2,6-dicarboxylic acid and 2-carboxymethyl-4-(30-hydroxybutyl) furan, dipicolinic acid |
| C. pseudomilitaris | Bioxanthracenes |
| C. brunnearubra | Cordyformamide |
| C. sinclairii | (2S,3S,4R)-(E)-2-Amino-3,4-dihydroxy-2-hydroxymethyl-14-oxoeicos-6-enoic acid |
| C. cicadae | Ergosterol peroxide |
| C. nipponica | Cordypyridones A and B |
| C. ophioglossoides | Ophiocordin, glycoprotein containing N-acetylgalactosamine |
| C. heteropoda | Cicadapeptins I and II, myriocin |
The number of compounds is small compared to that for Ganoderma (Paterson, 2006).
The fungus represents a genus of perithecial ascomycetes (Phylum Ascomycota) classified in the Clavicipitaceae, a monophyletic group included in the order Hypocreales. The genus contains over 400 species and the anamorphs of most are unknown. Paecilomyces is considered traditionally to host the anamorphs but this has been disputed. Sung et al. (2007) should be consulted for an up-to-date revision (and see later). Cordyceps are parasites of insects or fungi, often exhibiting a high degree of host specificity (Fig. 1 ). However, the Cordyceps species associated with Lepidopteran hosts do not represent a monophyletic group. There is even a high degree of genetic variation within Cordyceps sinensis which creates difficulties in verifying samples. A taxonomic review of the fungus is now available (see later section) a similar review is required for Ganoderma (e.g. see Paterson, 2006, Paterson, 2007). Larval infection via meiotic and/or mitotic spores/conidia and multiplication within the insect is from yeast-like budding. However, the fungus grows through the insect by hyphae. The accumulation of the biomass eventually kills the host (and/or a toxin(s) may be involved). It would be interesting to determine the biochemical parameters that cause these changes but this is not reported in the literature. The fungus ruptures the host body following over wintering and forms the sexual perithecial stroma that are connected to the dead larva below ground which grow upward to emerge above the soil surface (Fig. 1). The complete insect/fungus combination is used traditionally, but not exclusively, for medicinal purposes. The present reviewer has seen no reports of the insect per se being given as a treatment.
Fig. 1.
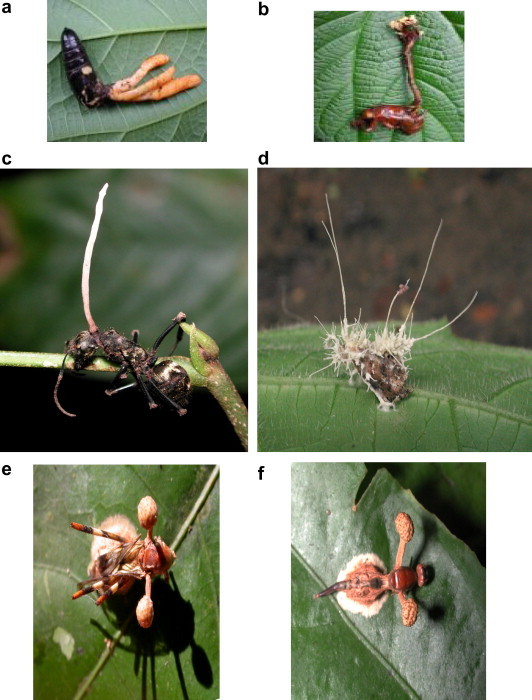
(a) Cordyceps militaris on dead insect; (b) Cordyceps polycephala on dead insect host; (c) Cordyceps spp. on Campanotus; (d) Cordyceps spp. on an unidentified moth; (e) Cordyceps spp. on a micropezid fly; (f) Cordyceps spp. on another micropezid fly.
C. sinensis (Berk.) Sacc., is one of the most famous traditional Chinese medicines (TCM) and health foods. The fungus parasitises larvae of moths (Lepidoptera), especially Hepialus armoricanus (and Thitarodes), and converts each larva into a sclerotium, from which the stroma and fruit-body grows. The complex (including the larva body) has been used as a health food and traditional medicine to “invigorate the lung and nourish the kidney” in China for hundreds of years, and at least from the 17th century (Dong and Yao, 2007, Kuo et al., 1994). Although what these preparations actually represented is impossible to determine given the difficulties in taxonomy of even modern times (Burnett, 2003, Korf, 2005; and see Sung et al., 2007). Understandably, conservation and sustainable harvest are important issues. There is need for (a) research on biological screening, (b) a better understanding of the status in natural habitats, and (c) artificial cultivation of the fungus.
Cordyceps and products are available in “Western” countries as over-the-counter medicine/tonics which advertise them as Chinese herbs with anti-aging, “pro-sexual”, anti-cancer and immune boosting effects, although with poor supporting scientific evidence. The believe is that C. sinensis (CS) has various beneficial effects on humans, including those of a psychological nature. The FTCM, is also called Dong Chong Xia Cao in Chinese (=winter worm summer grass) (Li et al., 2006a). Primarily it is prescribed as a tonic for body strengthening after serious disease. More recently other treatments have been claimed such as for (a) respiratory, renal, liver, nervous system and cardiovascular diseases, and (b) tumours, aging, hyposexuality and hyperlipidemia (Kuo et al., 2006, Chen et al., 2006, Wang and Shiao, 2000). It has been officially classified as a drug in the Chinese Pharmacopoeia since 1964. Furthermore, the outbreak of the Severe Acute Respiratory Syndrome (SARS) in China in 2003 has increased use considerably. This would have been an excellent opportunity to have determined how effective it was. However, this does not appear to have been undertaken. The market demand for CS is growing sharply in many countries (Dong and Yao, 2007). They would surely be hailed as medical breakthroughs if the efficacy of any of these treatments were confirmed. Nevertheless, the identities of active components have not been determined (in all cases) (Li et al., 2006b). Research has shown that at least some of the traditional uses “may” relate to pharmacological activities (Zhu et al., 1998a, Zhu et al., 1998b) (if not pharmacological activities then what?). Herbs have been used throughout history to enhance physical performance, but scientific scrutiny with controlled clinical trials has only recently been used to study such effects (Bucci, 2000). The authors mention that Cordyceps remain untested which is surprising given the interest in the fungus.
The fungus is endemic to the alpine habitats of the Tibetan Plateau above 3000 m in south-western China, and there has been large-scale harvesting of the wild material from Nepal and India more recently. It is agreed generally to have been over-harvested. Furthermore, the price of natural products of CS is over US$ 12,000 kg−1 (2006 prices) for only “average quality” (how this is determined is not clear) in the market and increasing (Sharma, 2004). So one can understand the pressures on supply. The socioeconomic implications of the FTCM are highly significant to the regions where it is harvested. The fungus has officially been classified as an endangered species by CITES Management Authority of China and China Customers and this scarcity is of considerable concern to all. Consequently, living strains have been isolated from natural CS and cultivated in large quantity by bioreactor technology which is a promising method to meet the needs of human consumption and to reduce the pressure on natural resources of the species (Dong and Yao, 2007).
In vitro culture of the fungus has been employed increasingly and Yang et al. (2005) state, “It is generally accepted that its cultivated CS fungi possess the same functions as CS natural “herbs” (sic)”. Some other issues that require addressing are that natural C. militaris is not readily available and is costly. Thus, a growing number of so-called Cordyceps products that derive from mycelial cultures of the asexual forms of these fungi have become commercially available (Hamburger, 2007). Mycelia cultivation has resulted in establishing a number of cultures derived from the holomorphic CS. These are referred to by the anamorphic names Paecilomyces hepiali and Cephalosporium sinensis, although the anamorph of CS appears to be Hirsutella sinensis (and see later). However, the situation is confused with some taxonomist using outdated names. To paraphrase Buenz et al., 2004, Buenz et al., 2005: while these strains undoubtedly support ecologically sustainable use of CS, the actual similarities between the wild fungus and the cultures are not clear.
The consumption of complimentary medicine has increased dramatically, with over 42% of people in the United States of America reported as “users”. Sales were US$ 3.3 billion in 1999 (Buenz et al., 2005). An important factor was the passage of the Dietary Supplement Health and Education Act in 1994 in the USA which opened the market for TCM (Cooper and Chang, 2001). One can appreciate how journals advocating these have increased concomitantly. Why have they not been developed by big pharmaceutical companies and made available to the public in pure compound form? No doubt there could be many reasons why this has not happened (e.g. not enough profit, “sticky” intellectual property rights issues (see Paterson, 2008), difficulty in mass production or synthesis, etc.) – apart from the possibility that they simply may not be effective. Buenz et al. mention that “one of the most interesting supplements is the not yet well-characterized C. sinensis (Berk.) Sacc.”.
CS has attracted much research interest for anti-oxidant activity and there is considerable evidence of this from the fungus as a treatment of a wide range of diseases. However, unauthenticated material has been used in some cases. For example, a polysaccharide was isolated which can protect PC12 cells against hydrogen peroxide-induced neuronal cell toxicity, but the Cordyceps mycelia used was from the Wan Fong Pharmaceutical Factory (Zhejiang, China) and derived from Ce. sinensis Chen sp. nov. This is a nomenclaturally illegitimate fungal name, which raised doubts as to its relationship to CS. In fact, it was later proved to be a different species (Dong and Yao, 2007) and this is a specific example of a general problem in the field. An example of another problem is CordyMax Cs-4, a commercial mycelial fermentation product that lowered fasting plasma levels of glucose and insulin, improved oral glucose tolerance and increased the glucose–insulin index, which measures insulin sensitivity, in rats (Zhao et al., 2002). However, the following statement is given on the web site of the product, “These (health-related) statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease”.
Extracts from artificially cultivated fruit-bodies of CS from the Xinhui Xinhan Artificial Cordyceps Factory (Guangdong, China) could scavenge ROS by inhibiting malondialdehyde formation by the peroxynitrite generator SIN-1. These results have been since referred to uncritically (e.g. Buenz et al., 2004, Buenz et al., 2005, Li et al., 2003). However, the fungal material may have been unauthentic, because reports exist that cultivation of fruit-bodies of this fungus was not repeatable and that the manufacturer is actually selling C. militaris. Furthermore, Li et al., 2001b, Li et al., 2001a, Li et al., 2001c compared the anti-oxidant activities of natural CS and cultured Cordyceps mycelia from different sources and were able to show the similar effects of the cultured mycelia to the natural products. However, the cultured material used was derived from a wide range of strains, and not from a valid CS. Some of these were products from the Chinese Medicine Factory of Jiangxi and Hebei Boding Pharmaceutical Factory. In addition, the FTCM are boiled in water or soaked in alcohol to drink for medications/health foods. Obviously, the various solvents and temperatures may have resulted in different compounds (Dong and Yao, 2007). Nevertheless, Guo et al. (2007) state that the ongoing exploration of CS has shown that the species can produce “many” bioactive compounds, and the medicinal benefits of CS have been demonstrated extensively.
In addition, there is (unbelievably for scientific journals) the use of words such as “Yin” and “Yang” as a basis for undertaking scientific research on material activities. Canney (2006) discusses kidney “yang” and one needs to ask what this is from a scientific perspective. Why include this word when it has no scientific currency? The fungus is also referred to as a herb and indeed the title of the piece asks, “C. sinensis animal, vegetable or both?”, whereas it is neither.
Various bioactive constituents from Cordyceps species have been reported. These include cordycepin and other anti-bacterial and anti-tumour adenosine derivatives, ophicordin, an anti-fungal agent, and l-tryptophan. Recent reports have indicated that CS contains polysaccharides exhibiting anti-oxidant activity and nucleosides that inhibit platelet aggregation (Wu et al., 2005). The bioactive compounds involved in the activities claimed include polysaccharides, modified nucleosides, and cyclosporin-like metabolites which are produced by this fungus and related species. The beneficial effects on (a) renal and hepatic function and (b) immunomodulation-related anti-tumour activities are most promising and deserve great attention. An increasing number of studies have used cultured mycelia in investigations. More mechanism-based, disease-oriented pharmacological studies are required to ensure clinical efficacy for particular diseases. However, Pang et al. (2002) state revealingly that studies have demonstrated repeatedly that many natural products marketed as nutraceuticals or health food do not deliver the health benefit as claimed and are inconsistent from batch to batch.
In the popular mind, CS first gained worldwide attention when it was revealed that several Chinese runners who broke world records in 1993 had included this fungus in their diet as part of their training program. Although scientist need to desist from quoting such reports as they are unsubstantiated and far-fetched. Purported and unsubstantiated effects of the fungus include use as an aphrodisiac, analgesic, immune modulator, and free radical scavenger. A review of the literature uncovers the predictable collection of general papers concerning medical mushrooms some with a distinctly “alternative” flavour. I have no concerns about being alternative but are they scientific? These overviews often are written in breathless, overblown and unscientific terms; others are well balanced. DaSilva (2005) talks about “novel mushroom-based healthcare products and therapeutics licensed for medical use can contribute to the good health status and feeling of the poverty-stricken strata of urban societies and populations. Indeed, mushroom cultural practices and medicines are being widely accepted as the integral skeins in the fabric of the human society of tomorrow”.
A valid response to this situation of a wide range in the quality of papers would be to only review those papers that have a high impact factor (or any impact factor). Temporarily putting the debate about the value of impact factors to one side, the reader may find it beneficial to concentrate only on those journals reviewed herein that do indeed have such ratings. Of course, the disadvantage is that valuable information may be missed. It may be worth mentioning that, Asian nations on the world stage have realised that biomedicine offers a unique chance to develop new industries and markets. Universities in Singapore, Korea, Hong Kong and China are appearing concomitantly in world tables for the best and citations data suggest they are producing well-regarded papers (Ince, 2007).
The following review concentrates on the biological activity of various preparations of Cordyceps spp. (the name as used in the essentially non-taxonomic papers) because of the quantity of data and importance of medicinal claims, at the expense of taxonomic and quality control issues.
2. Preparations
The various pure compounds, extracts, whole fungus and other preparations as they relate to pharmacological activities are discussed next, as these divisions are considered to be most relevant to a biochemical/phytochemical perspectives of the topic.
2.1. Pure compounds
Data from pure compounds are the most revealing in terms of determining effects of the fungus/insect. It is noted that these reports are scarce. Some compounds from Cordyceps (as defined here) are not particularly unusual.
Ng and Wang (2005) review the chemical constituents and pharmacological properties. The chemical constituents include (a) cordycepin (3′-deoxyadenosine) and its derivatives, (b) ergosterol, (c) polysaccharides, (d) a glycoprotein and (e) peptides containing α-aminoisobutyric acid. The activities ascribed to the fungus are anti-tumour, anti-metastatic, immunomodulatory, anti-oxidant, anti-inflammatory, insecticidal, anti-microbial, hypolipidaemic, hypoglycaemic, anti-aging, neuroprotective and renoprotective effects: So a vast a range of properties from a narrow spread of compounds.
Polysaccharides account for the anti-inflammatory, anti-oxidant, anti-tumour, anti-metastatic, immunomodulatory, hypoglycaemic, steroidogenic and hypolipidaemic effects. Cordycepin contributes to the anti-tumour, insecticidal and anti-bacterial activity. Ergosterol (a universal fungal compound) exhibits anti-tumour and immunomodulatory activity. Finally, a DNase has been characterized. These are not particularly novel compounds and one wonders why there are so many reports of the effects of crude extracts rather than much more work on the effects of novel pure compounds.
Cordycepin (Fig. 2 ), 3-deoxyadenosine, is a derivative of the nucleoside adenosine differing from the latter by the absence of oxygen in the 3′ position of its ribose entity. As such is may be quite common. Initially, it was extracted from Cordyceps; however, it is now produced synthetically. Some enzymes do not discriminate between adenosine and so it can participate in certain reactions. For example, it can be incorporated into RNA molecules causing premature termination of its synthesis. It is classified as an anti-cancer compound.
Fig. 2.
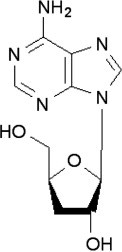
Chemical structure of cordycepin.
Cordycepin inhibited the growth of Clostridium paraputrificum and Clostridium perfringens, but had no effect on Bifidobacterium spp. and Lactobacillus spp. (Ahn et al., 2000). In addition, larvicidal activity against Plutella xylostella after 2–4 days of treatment was observed (Kim et al., 2002). It is interesting that cordycepin, a compound originally isolated from C. militaris as much as 60 years ago (Cunningham et al., 1950), is known to exert cytotoxic effects through nucleic acid methylation (Kredich, 1980), with possible implications for the PCR of these fungi (see Paterson, 2007, Paterson, 2008). If it is a truly useful compound it is surprising that it is not a well-known pharmaceutical by now.
The presence of cordycepin in CS has been difficult to confirm, although it has been confirmed by NMR (Chen and Chu, 1996). However, other groups have not been able to detect this compound (Shiao et al., 1994). It is clearly important to confirm the presence of the compound in CS in terms of determining the active components of the fungus and ultimately for chemotaxonomic purposes. Cho et al. (2007) state that cordycepin is isolated from C. militaris and is (claimed to be) an ingredient in TCM which is prescribed for various diseases, such as cancer and chronic inflammation (again note how vague this verbatim statement is). In this study, the novel effect of cordycepin inhibiting collagen-induced platelet aggregation was reported. The data suggests that the inhibitory effect of cordycepin might be associated with the down-regulation of [Ca2+]i and the elevation of cAMP/cGMP production. This result has obvious significance for prevention of thrombus formation. Finally, cordycepin inhibited the growth of B16 melanoma cells inoculated subcutaneously into right murine footpads (Yoshikawa et al., 2004).
Cordyheptapeptide A (Fig. 3 ), a novel cycloheptapeptide, was isolated from a strain of Cordyceps together with four known bioxanthracenes. There were only two previous reports on the isolation of cyclic peptides from this genus and these were from C. militaris and CS. The metabolite exhibited anti-malarial activity against Plasmodium falciparum and cytotoxicity to Vero cell lines. Also, the anti-malarial and cytotoxic activities of the bioxanthracenes were reported (Rukachaisirikul et al., 2006).
Fig. 3.
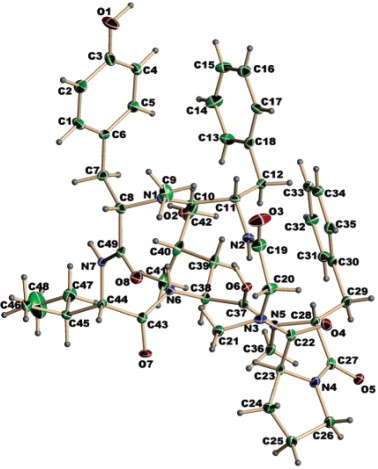
ORTEP view of cordyheptapeptide A (Rukachaisirikul et al., 2006).
In an extensive and impressive report, compounds designated as ES-242s, were isolated from a Verticillium strain and identified as bioxanthracenes (Isaka et al., 2007a). These compounds were known to exhibit potent activity as N-methyl-d-aspartate (NMDA) receptor antagonists. In addition, five novel ES-242 analogues were isolated with nine known compounds from a Cordyceps strain. A closely related strain provided cordyheptapeptide A, cordyheptapeptide B, and known ES-242s. The structures of the novel bioxanthracenes were 6′-O-desmethyl analogues of the compounds described. Furthermore, cordyheptapeptide B has an N-methyl-l-phenylalanine residue in place of the N-methyl-l-tyrosine. The isolation, structure elucidation, and anti-malarial activity of ES-242s and their analogues from the insect pathogenic fungus Cordyceps pseudomilitaris (from a Lepidoptera larva) were reported previously. Cycloheptapeptide, cordyheptapeptide A, and some known ES-242s were isolated from a Cordyceps strain from an elaterid larva.
In a continuing search for bioactive compounds from insect pathogenic fungi it was noticed that culture extracts of six Cordyceps strains, collected in the same location (from Coleoptera larvae, at Doi Innthanon National Park, Chiang Mai Province, Thailand), showed similar 1H NMR spectra. This suggested the presence of bioxanthracenes (ES-242s) and cordyheptapeptide A as major constituents. Two of these strains were subjected to mass fermentation (15 L) and chemical investigation. As a result, five new ES-242 analogues and nine known compounds and cordyheptapeptide A were isolated from an undefined strain. Cordyheptapeptide B, was isolated, together with other known compounds. Some of these were tested for activity against P. falciparum and cytotoxicity to KB cells (oral human epidermoid carcinoma), BC cells (human breast cancer), NCI-H187 cells (human small cell lung cancer), and noncancerous Vero cells (African green monkey kidney fibroblasts). Cordyheptapeptide A exhibited anti-malarial activity, while cordyheptapeptide B was inactive and both cyclic peptides showed moderate cytotoxicity. Furthermore, cordyformamide is a plausible biogenetic precursor of xanthocillin Y, and was isolated from a culture broth of Cordyceps brunnearubra BCC 1395. Cordyformamide was found to exhibit activity against P. falciparum, whereas it showed weak or no cytotoxicity (Isaka et al., 2007b).
Production of the nonribosomal peptides cicapeptins I and II (Fig. 4 ) were reported by Krasnoff et al. (2005) which was the first report from fungi of consecutive Hyp or Pro residues in a nonribosomal linear peptide. The compounds exhibited anti-bacterial and anti-fungal activity.
Fig. 4.
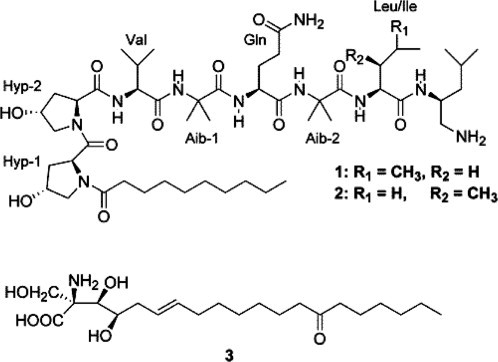
(1 and 2) Cicapeptins I and II; (3) myriocin from Cordyceps heteropoda (Krasnoff et al., 2005).
A novel immunosuppressant was isolated from the culture broth of Isaria sinclairii, the anamorph of C. sinclairii, and characterized as (2S,3S,4R)-(E)-2-amino-3,4-dihydroxy-2-hydroxymethyl-14-oxoeicos-6-enoic acid, which was identical to anti-fungal substances, myriocin and thermozymocidin (Fujita et al., 1990). The suppressive activity was found to be equal to, or higher than cyclosporin A which is used clinically. The activities of the 10 derivatives were also examined, indicating the following relationships between structure and activity – the: (a) lactone formation between the carboxy group at C-1 and the hydroxy group at C-4, and the reduction of the carbonyl group at C-14 to the hydroxy group do not affect the suppressive activity; (b) hydrogenation of the double bond at C-6 resulted in decreased activity; and (c) acetylation of the amino group and the thioketalization on the carbonyl group at C-14 “drastically” reduced the suppressive activity. Also, the compound suppressed the production of anti-bodies to sheep red blood cells and induction of cytotoxic lymphocyte T cells more strongly than cyclosporin A. Obviously, this is an important lead compound and hence one of the more satisfactory papers.
Chen et al. (1999) isolated a pure compound (H1-A) from CS and investigated whether autoimmune disease progression in mice was affected by administration of the metabolite. The authors are vague as to what the compound is and state that, “it is a kind of ergosterol and looks like testosterone”. The authors also provide a chemical structure which confirms that it is a common sterol and a systematic name could have been provided. Their results demonstrated that mice treated daily exhibited a progressive reduction in anti-ds-DNA production. In clinical presentation, the treated group had a reduction in lymphadenopathy, a delayed progression of proteinuria, and an improvement in kidney function. Histological analysis of kidney tissue indicated that H1-A inhibited mesangial proliferation that was evident in lupus nephritis. However, there was no change in immune complex deposition. H1-A “may be” useful for treating systemic lupus erythematosus in human patients. However, more work is required. H1-A was claimed to be effective in the treatment of autoimmune disorders (Yang et al., 2003). Results demonstrated inhibition of cell proliferation and promotion of apoptosis of activated human mesangial cells in vitro: the activities were not a result of cytotoxicity. In addition, H1-A inhibited tyrosine phosphorylation of human mesangial proteins. These findings suggest that H1-A modulated some (unspecific) subcellular signal-transduction pathways and changed the balance between proliferation and apoptosis of mesangial cells in vitro and in vivo. The conclusions were that H1-A may be effective in the management of autoimmune disorders, and the modulation of the signal-transduction proteins may represent a target for future pharmacologic interventions. More correctly, they probably do represent a target, and such vague statements should be avoided. In an older report, HI-A alleviated immunoglobulin A nephropathy (Berger’s disease) with histological and clinical improvement (Lin et al., 1999). HI-A inhibited the proliferation of human mesangial cells and promoted apoptosis by suppressing tyrosine phosphorylation of Bcl-2 and Bcl-XL (Yang et al., 2003) and reduced anti-ds-DNA production and lymphadenopathy, delayed progression of proteinuria, improved kidney function and inhibited mesangial proliferation (Yang et al., 1999).
Moving on to more interesting compounds, cordypyridones A and B were detected from the uncommon species, C. nipponica. These are atropisomers, and demonstrated potent anti-malarial activity in vitro (Isaka et al., 2001b). Shin et al. (2001) state that, “in an effort to evaluate the pharmacological effects, including the anti-aging effect” of the fruiting bodies of the cultivated Paecilomyces japonica fungus, “a new type” of Cordyceps sp. was investigated. Two pure compounds were isolated as active principles from low molecular-weight fractions, and a protein-bound polysaccharide that showed a marked increase in the liver enzyme activities, and a significant inhibition of lipid peroxidation was found. Boros et al. (1994) reported that ophiocordin, an anti-fungal antibiotic from Cordyceps ophioglossoides (Kneifel et al., 1977) and balanol from Verticillium balanoides are structurally identical. This may indicate a particularly close taxonomic relationship between the two taxa. The structure of ophiocordin was falsely assigned and balanol was the compound of interest. Balanol was under development as an anti-cancer agent as it established to be a selective inhibitor of protein kinase C (see Paterson, 2008).
It is more common for pure compounds to be tested in the fields of anti-bacterial, anti-fungal, anti-malarial and insecticidal activity which is to be recommended more generally. Ophiocordin is an anti-fungal antibiotic isolated from submerged cultures of C. ophioglossoides. However, it is devoid of anti-bacterial activity (Kneifel et al., 1977). Bioxanthracenes (see also previously) were isolated from C. pseudomilitaris (Isaka et al., 2001a, Jaturapat et al., 2001) and appear to be anti-malarial. Ten-membered macrolides, cepharosporolides C, E and F, cordycepin, pyridine-2,6-dicarboxylic acid and 2-carboxymethyl-4-(30-hydroxybutyl) furan were reported from C. militaris by Rukachaisirikul et al. (2004). However, only cordycepin was anti-malarial. Krasnoff et al. (2005) reported cicadapeptins I and II (nonribosomal peptides containing aminoisobutyric acid), which were anti-bacterial and antifungal, and myriocin (anti-fungal) from C. heteropoda isolated from an Australian cicada. Finally, a glycoprotein containing N-acetylgalactosamine was isolated from C. ophioglossoides but activity data are not available (Kawaguchi et al., 1986).
An inhibitor of the prophenoloxidase activation was isolated from a culture filtrate of C. militaris and identified as dipicolinic acid (DPA). The production of DPA in a range of Clavicipitaceae fungi was examined. Entomogenous fungi that produce DPA were integrated into one group by a phylogenetic analysis based on 18S rDNA. Interestingly, it was suggested that the group acquired an ability to produce DPA during its evolution from plant pathogenic fungi to entomogenous fungi (Watanabe et al., 2006).
In a useful comparison of crude extracts and pure compound, the anti-diabetic effect of various fractions of C. militaris, CCCA (crude cordycepin containing adenosine), CMESS (ethanol soluble supernatant), and cordycepin were evaluated in diabetic mice (Yun et al., 2003). CMESS showed a potent inhibitory activity of 34.7% in starch-loaded mice: CMESS reduced blood glucose level by 35.5%. However, CCCA, and cordycepin showed no difference. After 7 days administrations of these drugs, CMESS, and cordycepin dramatically reduced blood glucose level. CCCA with a high concentration of cordycepin did not reduce blood glucose level. Proliferation of T-lymphocyte was significantly decreased; while NO production was increased more than two-fold in the cordycepin-administered group. The proliferation of macrophages and NO production were significantly decreased in the CMESS administered group. CMESS and cordycepin may be (a) useful tools in the control of blood glucose level in diabetes and (b) promising new drugs as an anti-hyperglycemic agent without the defects of lowered immune responses and other side effects, the authors suggest.
Furthermore, cordycepin, 3′-amino-3′-deoxyadenosine, homocitrullyl aminoadenosine, adenine, cordycepic acid and d-mannitol have been reported from Cordyceps spp. (Cunningham et al., 1950, Chatterjee et al., 1957, Kredich and Guarino, 1961, Guarino and Kredich, 1963, Kaczka et al., 1964, Liu et al., 1989). Ergosterol peroxide isolated from C. cicadae inhibited phytohaemagglutinin-induced T cell proliferation, and arrested the progression of activated T cells from G1 to S phase of the cell cycle. Early gene transcripts, in particular those of cyclin E, interferon, and interleukins were suppressed (Kuo et al., 2003). The glycosylated form of ergosterol peroxide from CS was more potent than the aglycone in inhibiting proliferation of tumour cells (Bok et al., 1999). However, ergosterol peroxide is widespread in fungi and Cordyceps does not offer any particular advantage in its preparation.
It is worth noting that eight different Cordyceps species (CS. C. militaris, C. cicadae, C. ophioglossoides, C. heteropoda, C. pseudomilitaris, C. nipponica, C. sinclairii) are listed in the above paragraph indicating the extent of the possible diversity involved in the biology and activity (Table 1, Table 2). Although whether they are distinct species is open to question.
2.2. Polysaccharides and fractions
Water-soluble crude polysaccharides were obtained from the fruiting bodies of cultured C. militaris by hot-water extraction followed by ethanol precipitation. The polysaccharides were successively purified by chromatography giving three polysaccharide fractions. In the in vitro anti-oxidant assay, P70-1 was found to possess hydroxyl radical scavenging activity. The polysaccharide is a heteropolysaccharide and is occasionally branched. The fundamental information obtained from this work is beneficial to the interpretation in the relationship of polysaccharide structure and its biological functions. This provides the “experimental evidence and scientific explanation for the folkloric uses of C. militaris as a substitute for CS” (Yu et al., 2007a).
The effect of an exopolysaccharide fraction (EPSF) from anamorphic strains of CS on the immunocyte activity of tumour-bearing mice was investigated. EPSF significantly inhibited the H22 tumour growth, and elevated the activity of immunocytes. It enhanced the phagocytosis capacity of peritoneal macrophages and proliferation ability of spleen lymphocytes. EPSF promoted (a) TNF-α expression of macrophages, (b) the cytotoxicity of spleen lymphocytes, and (c) TNF-α and IFN-γ mRNA expression of splenic lymphocytes (Zhang et al., 2007a).
CS possesses anti-tumour, anti-oxidation and stimulation of the immune system activities (Chen et al., 1997). However, the identity of active component(s) has not been determined (Li et al., 2003). Towards this end, a polysaccharide was isolated from cultured Cordyceps mycelia which had strong anti-oxidation activity, and which contained glucose, mannose and galactose. The pre-treatment of the isolated polysaccharide on cultured rat pheochromocytoma cells demonstrated strong protective effect against hydrogen peroxide (H2O2)-induced insult. Treatment prior to H2O2 exposure significantly elevated the survival of PC12 cells in culture. This was the first report that identified a polysaccharide from Cordyceps, which protected against the free radical-induced neuronal cell toxicity. A water-soluble polysaccharide fraction, a poorly water-soluble polysaccharide, and a protein fraction stimulated steroidogenesis (Huang et al., 2001b). Interestingly, galactomannans isolated from the insect portion of C. cicadae demonstrate potent hypoglycaemic activity in mice (Kiho et al., 1990).
In an investigation into a polysaccharide from CS mycelium hypocholesterolaemic and hypotriglyceridaemic activity in mice was exhibited (Kiho et al., 1996). Chen et al. (1997) studied a polysaccharide fraction from CS as to its effect on the proliferation and differentiation of human leukaemia cells using an in vitro culture system. The conditioned medium had an activity that significantly inhibited proliferation. Differentiated cells also possessed phagocytosis functions and supported superoxide production. Antibody neutralization studies further revealed that the tumouricidal and differentiating effects of the compounds were mainly derived from the elevated cytokine concentrations. Finally, galactosaminoglycan from C. ophioglossoides reacted with sera from patients with certain collagen diseases and its use as an index of serological activity is thus of diagnostic value (Ikeda et al., 1993).
An aqueous extracted polysaccharide from cultured C. militaris demonstrated general anti-inflammatory activity (Yu et al., 2004a) as did ethanolic extracts of cultured fruiting bodies and mycelia of C. militaris applied topically in the croton oil-induced ear oedema test in mice. The fact that in vitro fruiting bodies were employed rather than in vivo is interesting as most papers report using fruiting bodies in vivo and/or in vitro biomass. However, the paper is flawed as the details of the cultivation of the fruiting bodies were not provided.
Antioxidant activity in the xanthine oxidase, haemolysis and lipid peroxidation assay systems was demonstrated by Li et al. (2001a) from water extracts, and a polysaccharide fraction, of cultured CS mycelia. Interestingly, the fruiting body and the caterpillar parts of CS are claimed to be similar in chemical composition and hence anti-oxidant activity, because the fungus had presumable replaced the insect constituents with fungal (Li et al., 2002). It would be interesting to determine (a) how this occurs in terms of insect substrate utilisation and optimisation of yields of bioactive fungal components, (b) when the preparation is at the correct stage for use as a medicinal treatment, and (c) if these data could be extrapolated to in vitro culture.
An ambiguous statement is made by Shin et al. (2001), “Cordyceps is negative for its many biological activities and a tonic for restoring vital functions in traditional Chinese medicine”. It proceeds to state that P. japonica is a new type of Cordyceps species, which is incomprehensible. It also mentions that P. japonica is (or produces) mushrooms. Paecilomyces is considered to be an anamorph of Cordyceps and so this appears to be incorrect and, what appear to be mushrooms, may be synnemata (compacted conidiophores). However, it is unwise to speculate what the material actually is as descriptions are vague. A protein-bound polysaccharide that inhibited lipid peroxidation and increased the activity of anti-oxidant was described from the fungus. Finally, phaeochromocytoma cells were protected against H2O2-induced injury by a 210-kDa polysaccharide from CS mycelium (Li et al., 2003).
Yamada et al. (1984) reported that a water-insoluble extracellular glucan isolated from the culture filtrate of C. ophioglossoides suppressed potently the growth of sarcoma 180 solid-type tumours. Remarkably, a protein-bound polysaccharide fraction from C. ophioglossoides extended the life of mice bearing Ehrlich carcinoma or a syngeneic tumour (Ohmori et al., 1986). Also, Ohmori et al., 1989a, Ohmori et al., 1989b isolated a galactosaminoglycan that inhibited the proliferation of sarcoma 180 cells and the growth of a syngeneic solid tumour in vivo: it exhibited cytotoxicity against cancer cells in vitro.
Chen et al. (1997) observed that medium from blood mononuclear cells stimulated with the polysaccharide fraction from CS inhibited the proliferation of human leukaemic cells, and induced approximately 50% to differentiate into mature monocytes/macrophages expressing non-specific esterase activity and certain surface antigens. The anti-proliferation and differentiating effects were demonstrated to be caused by an elevated production of cytokines, i.e. a tumour necrosis factor and an interferon. The exopolysaccharide fraction of CS inhibited metastasis of melanoma cells and down-regulated concomitantly the levels of Bcl-2 protein into the lungs and the liver (Zhang et al., 2005).
The exopolysaccharide fraction of cultivated Cordyceps stimulated peritoneal macrophages to take up neutral red and splenic lymphocytes to proliferate (Zhang et al., 2005). Crude and neutral polysaccharides of CS exerted hypoglycaemic activity in normal mice. However, the polysaccharide did not affect the circulating insulin level in normal mice (Kiho et al., 1993). The compound lowered the plasma glucose level in diabetic mice (Kiho et al., 1996). Another unspecific polysaccharide from a hot-water extract of mycelia also lowered the plasma glucose level in normal, adrenaline-induced hyperglycaemic and diabetic mice (Kiho et al., 1999).
Some Cordyceps-like strains have been isolated from the fruiting bodies of wild CS that have been reported to show the same properties as the natural product. However, care in interpretation is required as these could conceivably be contaminants (see later). An exopolysaccharide fraction was prepared from cultivated CS (Zhang et al., 2005). The results showed that it enhanced significantly the Neutral Red uptake capacity of peritoneal macrophages and spleen lymphocyte proliferation in melanoma-bearing mice. The metastasis of B16 melanoma cells to lungs and livers was significantly inhibited. Moreover, the levels of Bcl-2 in the lungs and livers were decreased. The results suggest that the polysaccharide has an immunomodulatory function and anti-tumour activity.
However, Yang et al. (2005) state that although certain polysaccharides from CS are bioactive, the anti-tumour effect has not been confirmed. The authors investigated the effects of the exopolysaccharide fraction of cultivated CS fungus on c-Myc, c-Fos, and vascular endothelial growth factor (VEGF) expression of tumour-bearing mice. The expression in the lungs and livers of treated mice were found to be significantly lower than those of untreated mice. The authors suggest that the fraction had inhibited tumour growth in the lungs and livers of mice, and that it is an adjuvant in cancer therapy.
In addition, Yu et al., 2004a, Yu et al., 2004b isolated four polysaccharides from C. militaris, CPS-1 was shown to possess a significant anti-inflammatory activity and suppressed the humoral immunity in mice but had no significant effects on cellular immunity and non-specific immunity. In a previous study using anti-oxidant activity-guided fractionation CSP-1 from cultured Cordyceps, mycelium was isolated. The hypoglycemic effect of CSP-1 on mice and rats was demonstrated. CSP-1 increased circulating insulin level in diabetic animals, which suggests that the compound(s) may stimulate pancreatic release of insulin and/or reduce insulin metabolism. Chen et al. (2006) undertook further work on the biological activity of the isolate: the polysaccharide from fungus and its anti-oxidant activity on H22-tumour bearing mice was investigated. The H22 tumour growth was inhibited and SOD activity of liver, brain and serum and GSH-Px activity of liver and brain in tumour-bearing mice were enhanced. In general, beneficial effects were observed in the liver and brain of tumour-bearing mice.
Finally, four exopolysaccharides with different molecular masses ranging from 50 to 2260 kDa were reported from C. militaris by Kim et al., 2003b, Kim et al., 2003c as part of yield optimisation studies. An extracellular polysaccharide extracted from the mycelia of CS with hot-water indicated that this d-glucan consisted of a backbone composed of (1 → 3)-β-d-glucosyl residues and carried a single (1 → 4)-β-linked d-glucosyl residue: sugar residues were linked with β-glycosidic bonds (Wu et al., 2005).
2.3. Lectin
A lectin from C. militaris exhibited hemagglutination activity in mouse and rat erythrocytes, but not in human ABO erythrocytes (Jung et al., 2007). However, the N-terminal amino acid sequence differed greatly from those of other lectins. It exhibited mitogenic activity against mouse splenocytes.
2.4. Extracts
The following section concerns solvent extraction of the fungi. In effect, this is often how the preparations will be consumed as a TCM. The significance of tests on extracts is much reduced compared to those of pure compounds. There is a great deal of data. In general, this type of work needs to be deemphasised in favour of that of pure compounds.
2.4.1. Water extract
Reports on the metal chelating and reducing power from CS are not available in the scientific literature. Therefore, there is a demand to obtain an overall measure of the anti-oxidant activity of extracts using reliable fungal material because of increasing interest in the relationship between anti-oxidants and diseases. The anti-oxidant activities from natural and cultured mycelia of CS were investigated in vitro. Optimal effects were demonstrated on the inhibition of linoleic peroxidation. The results suggested that the cultured and natural mycelia have direct and potent activities and that the cultured mycelia could be used for the anti-oxidant activity which would tend to reduce the pressures on the natural fungus, which is, after all, an endangered species (Dong and Yao, 2007).
The anti-oxidant efficiency of C. militantis extract (CME) and CS extract (CSE) in protecting lipid, protein, and low-density lipoprotein (LDL) against oxidative damage was reported (Hui et al., 2006). However, this study provoked a strong response from Hamburger (2007) which was subsequently rebutted by one of the original authors (Duh, 2007). This is something of an unexpected bonus to a reviewer of the literature such as myself as another opinion is obtained. The questions are, what biological material is being worked with, and can other scientists obtain it to repeat the experiment? The current author has encountered this before (Paterson, 2005) where commercial interests are involved. The Hamburger response can be applied in a general sense to some of the other work cited in the present review. CME and CSE showed weakly inhibitory effect on liposome oxidation. The inhibitory effect of CME on protein oxidation was inferior to that of CSE. CME and CSE showed inhibition of LDL oxidation. The contents of the bioactive ingredients cordycepin and adenosine in CME were higher than those of CSE; however, cordycepin and adenosine showed no significant anti-oxidant activity. In addition, a polysaccharide present in CME and CSE displayed anti-oxidant activity. The authors concluded that the protective effects of CME and CSE against oxidative damage of biomolecules are a result of their free radical scavenging abilities. However, the experimental data and some of the conclusions need a critical comment (Hamburger, 2007). The author criticised the report on the bases of poor taxonomy, biochemistry and extrapolation of data to imply possible cures of diseases. In particular, the authors’ claims of a potential treatment for human disease on the basis of in vitro data are called into question. The rebuttal by Duh simply confirms that appropriate information about the strains was not provided; the comments on the inadequate analytical procedures are largely accepted. And there is no doubt that the conclusions could have been rewritten to indicate that the results were preliminary. Hamburger (personal communication, 2007) stated that the rebuttal was evasive, an assessment with which I agree.
Kuo et al. (2005) describe the effects of CS against Group A streptococcus infection in mice. The preparation protected by decreasing bacterial growth “and dissemination”, thereby increasing mouse survival rate. IL-12 and IFN-gamma expression and macrophage phagocytic activity also increased. Kuo et al. (2007) claim to demonstrate that CS increased phagocytosis in human monocytic cells and abrogated inhibition of phagocytosis by causing cytokine production. These two reports are sound and in good journals. However, the fungus used was from a company called Simpson Biotech and very few or no details are supplied about how the material was collected, identified, maintained, and grown. Of course, this is unsatisfactory.
Shahed et al. (2001) refer somewhat unusually to CS as a “black blade” fungus. Their results showed that CS improved renal function and reduced the expression of inflammatory and apoptotic genes in rats. The authors make the very conditional statement that, CS extract may play a potential therapeutic role in renal transplantation”; on the other hand it may not play an actual role. After all, precision in what is written in such important areas of medicinal research is crucial. The authenticity of the fungal material can be questioned: it was obtained from a company from the United States of America and there is little indication about the standards of collecting, purity, identities and maintenance of the materials. Basically, what level of accreditation applies to such organizations?
A C. militaris inhibited the growth of human umbilical vein endothelial cells (HUVEC) and HT 1080 cells. It down-regulated, in dose- and time-dependent manners, bFGF gene expression in HUVEC cells and MMP-9 gene expression in HT 1080 cells. The growth of melanoma cells in mice was suppressed. In addition, anti-angiogenic activity was manifested (Yoo et al., 2004). It is gratifying that adequate details of the fungal material are provided in this Chinese journal, which acts as a model for others generally.
Chiou et al. (2000) observed a hypotensive effect of CS in anaesthetized rats and a vaso-relaxant effect in isolated aorta. The fungus counteracted arrhythmia in rats and increased the dosage of ouabain required to produce arrhythmia in guinea-pigs. In addition, the heart rate in anaesthetized rats and the contractility of isolated papillary muscle or atria in guinea-pigs were decreased (Mei et al., 1989).
Cultured fruiting bodies of CS prevented deposition of cholesterol in the aorta of atherosclerotic mice by inhibiting free radical-mediated LDL oxidation in an investigation into hypolipidaemic activity (Yamaguchi et al., 2000a). A hot-water extract of mycelia (a) lowered the total cholesterol concentration, (b) reduced the concentration of cholesterol carried by LDL and very-low-density lipoprotein, and (c) elevated the high density lipoprotein (HDL)-cholesterol concentration in the serum of mice fed a cholesterol enriched diet (Koh et al., 2003a). Water extracts of CS: increased survival time of mice inoculated with carcinoma cells or syngeneic fibrosarcoma cells (Yoshida et al., 1989); inhibited spontaneously liver metastasis of carcinoma cells and melanoma in syngeneic mice and results suggested that the activity was not attributable to cordycepin (Nakamura et al., 1999a); prolonged the survival period of mice inoculated with B16 melanoma cells when coadministered with methotrexate (Nakamura et al., 2003) and caused apoptosis of melanoma cells (Nakamura et al., 1999b).
An orally administered CS was considered to be “quite” safe based on body weight gain, and liver/kidney weights of mice (Nakamura et al., 1999a, Nakamura et al., 1999b). The authors concluded the extract could inhibit aortic cholesterol deposition in atherosclerotic mice by scavenging free radicals in vivo. These extracts “may have” beneficial effects on the process of atherogenesis and aging with few side effects (Yamaguchi et al., 2000a). This is good news but surely more evidence is required. Towards these ends, Tsai et al. (2001) also demonstrated the hydroxyl radical scavenging activity of CS. Whereas reduced lipid peroxidation in rats was demonstrated by Shen and Chen (2001). Antioxidant activity in the xanthine oxidase, haemolysis and lipid peroxidation assay systems was reported from water extracts, and a polysaccharide fraction, of cultured CS mycelia (Li et al., 2001a). As mentioned previously, the fruiting body and the caterpillar parts of CS are claimed to be similar in chemical composition and hence anti-oxidant activity (Li et al., 2002). It would be interesting to determine (a) how this occurs in terms of insect substrate utilisation by the fungus with a view to optimising yields of bioactive fungal components, (b) when the preparation is at the correct stage for use as a medicinal treatment, and (c) if these data could be extrapolated to in vitro culture. Cho et al., 2003, Wang et al., 2005 also reported radical scavenging activity with Wang et al. reporting activity against colorectal tumour cells.
The influence of CS on the immunoactivity of macrophages was determined to probe the mechanism of its alleged tonic effect. The phagocytosis of macrophages were enhanced significantly (Jia and Lau, 1997) and “maybe” the tonic effect of CS is accomplished by an enhancing effect on the immune system. Some chemical fractions had insulin like and insulin release promoting activity and “could be developed” as an anti-diabetic agent (Young et al., 2001). Cordyceps possessed a strong anti-oxidation activity in all assays tested by Li et al. (2001a). The cultured Cordyceps mycelia had equally strong anti-oxidation activity compared to in vivo Cordyceps. Further, the anti-oxidation activities were increased ×10–30 in the partially purified polysaccharide fractions. In an intriguing report, Liu et al. (2006) mentions that identification of an effective non-toxic biological radioactivity protector is a “matter of some urgency”. Orally administered CS protected mice from bone marrow and intestinal injuries after total-body irradiation. The levels of free radical species within cells are suggested to be a likely mechanism for the purported effects. The biochemical mechanisms of anti-proliferative effects of C. militaris in human leukemia cells were investigated in a convincing study involving cancer treatment (Park et al., 2005). It was found that they inhibited cell growth in a dose-dependent manner, which was associated with morphological change and apoptotic cell death such as formation of apoptotic bodies and DNA fragmentation. Furthermore, the treatment caused a dose-dependent inhibition of cyclooxygenase-2 (see Paterson, 2008) and prostaglandin E2 accumulation. Taken together, these results indicated that the anti-proliferative effects were associated with the induction of apoptotic cell death through regulation of several major growth regulatory gene products. The extracts “may have therapeutic potential” in human leukemia treatment. In addition, corticosterone output by cultured rat adrenocortical cells was increased without increasing the intracellular cAMP level. The steroidogenic effect was abolished by the protein kinase C inhibitor calphostin C, indicating that its action may involve stimulation of protein kinase C (Wang et al., 1998). C. militaris reduced the fasting serum glucose level and enhances glucose utilisation in skeletal muscles in rats (Choi et al., 2004). The fungus demonstrated cytotoxic activities on the three kinds of human cancer cell lines, stomachic adenocarcinoma, colorectal adenocarcinoma, and hepatocellular carcinoma (Lim et al., 2004). Cytotoxic activity-guided isolation and identification of active fractions afforded the well-known, cordycepin as an active component (see above).
Koh et al. (2003b) reported that CS mycelia prolonged swimming endurance capacity and produced an anti-fatigue action in mice. CS reduced the hepatic content of malondialdehyde and the serum concentrations of transaminases and alkaline phosphatase in rats with hepatic fibrosis. Treatment with the extracellular biopolymers resulted in a reduction in hepatic hydroxyproline content and normalization of morphological characteristics of the liver, indicating an anti-fibrotic action (Nan et al., 2001). Finally, crystals of the fungus stimulated proliferation of erythroid progenitor cells in mouse bone marrow (Li et al., 1993).
2.4.2. Methanol
In a very interesting report, the insect-body part (of the TCM) inhibited proliferation of enhanced human mononuclear cells (HMNC). Any differences between the fungus and insect components are well worth further investigation. The production of interleukin-2 and interferon was stimulated by the aqueous methanolic extracts and inhibited by the methanolic extracts (Weng et al., 2002). Treatment of patients with condyloma acuminata brought about an increase in interleukin-2 and a decrease in interleukin-10, indicating a recovery in the balance of Th1/Th2 cytokines. Recurrence was also diminished (Gao et al., 2000). Interestingly, the ergosterol esters concentrations were much higher in the (dead) caterpillar than the fruiting bodies (Yuan et al., 2007) although ergosterol was similar. Although why these compounds are of particular interest is “mystifying” as they are universal in fungi.
In a surprising paper, the following may be an example of the mystical nature of some of the reports (see Paterson, 2006). It represents a bizarre rational for undertaking the work and it is surprising that it was published from a scientific perspective. The rational for the work is that CS is a popular Chinese tonifying herb, and was/is revered for being, what is referred to as, ‘Yin-nourishing’ and ‘Yang-invigorating’ in Chinese medicine (Siu et al., 2004). In order to establish the pharmacological basis for the ‘Yin-nourishing’ and ‘Yang-invigorating’ action of Cordyceps, the effects of wild and cultured Cordyceps on concanavalin A stimulated splenocytes, an in vitro bioassay for ‘Yin-nourishment’, and myocardial ATP generation capacity, an ex vivo bioassay for ‘Yang-invigoration’, were investigated in mice. The results indicated that wild and cultured Cordyceps enhanced the Con A-stimulated splenocyte proliferation in vitro and myocardial mitochondrial ATP generation ex vivo in mice, with no significant difference in potency of action between the two types of Cordyceps. While the immunopotentiating effect was associated with the increase in interleukin-2 production, the stimulation of myocardial ATP generation was paralleled by an enhancement in mitochondrial electron transport. When compared with typical ‘Yin’ and ‘Yang’ tonifying Chinese herbs, Cordyceps was found to possess both ‘Yin-nourishing’ and ‘Yang-invigorating’ activities, with a lower potency in both modes of action. It is impossible to take reports such as these seriously from a scientific standpoint and is given considerable space here to indicate a general problem which may have motivated some of the published reports reviewed herein. This is without attempting to detract from the philosophical aspects of the concepts of Yin and Yang in terms of two mutually correlated opposites in a general sense. A comprehensive definition of the corresponding philosophy of science is inappropriate: It is enough to state that it is vitally important for science that the information about the surrounding world and the objects of study be as accurate and as reliable as possible.
To continue, CS fruiting bodies inhibited various tumour cell lines (Kuo et al., 1994): two fractions were particularly potent. Growth inhibitors other than cordycepin and polysaccharides may have been involved; two fractions significantly inhibited (a) the blastogenesis response, (b) NK cell activity and (c) IL-2 production (Kuo et al., 1996). Neither fraction was cytotoxic, and immunosuppressive ingredients were found to be intracellular. Fruiting bodies inhibited human mesangial cells (HMC) activation by IL-1 plus IL-6 (Lin et al., 1999) and liver toxicity or mutagenicity were not observed. The fraction was purified to obtain purified compound H1-A (see above). The authors claim a novel treatment for human Berger’s disease “in the future”. A fraction (a) dose dependently suppressed bronchoalveolar lavage fluids (BALF) cells proliferation, (b) reduced interleukin production in LPS activated BALF cell cultures and (c) affected interleukin mRNAs in various significant manners (Kuo et al., 2001). The purported therapeutic activity may be related to modulation of cells functions in bronchial airways. Furthermore, the molecular mechanism of Cordyceps pruinosa pharmacological and biochemical actions of macrophages in inflammation has not been clearly elucidated (Kim et al., 2003a). The authors suggest that an extract suppresses inflammation through suppression of NF-κB-dependent inflammatory gene expression, and hence may be beneficial for treatment of endotoxin shock or sepsis.
Shim et al. (2000) wrote that they attempted to develop “a new type Cordyceps”. To obtain this, they investigated the effects of the fruiting bodies of the cultivated fungus of P. japonica grown on silkworm larvae on hyperglycemia in rats and mice and on immunological functions in mice. As mentioned previously, it is not at all clear whether Paecilomyces (even) contains anamorphs of Cordyceps. Therefore, this report is a cause of some concern. Immunostimulating activity and a significant anti-fatigue effect in mice were observed. Kuo et al. (1994) reported unidentified substances which inhibited tumour cells, but which were not cordycepin or polysaccharides, in the methanolic extract of CS – this report requires further investigation.
C. cicadae ascocarps enhanced HMNC proliferation (Weng et al., 2002). In contrast, the insect-body portion suppressed HMNC proliferation. This is a most interesting result in the current author’s opinion because of the different effects of the two components. The action mechanisms of the fractions may involve the regulation of interleukin and interferon production in HMNC. Overall, the results demonstrated that C. cicadae contained growth modulators for HMNC. Unfortunately, the compounds responsible were not characterised. Koh et al. (2002) demonstrated that a hot-water extract modulated interleukin-6 production by activation of macrophages and augmented the secretion of haematopoietic growth factors. Whereas aqueous methanolic extracts of the ascocarp stimulated proliferation of phytohaemagglutinin-induced proliferation of HMNC. Of course, such differences indicate that different compounds are involved or the same compounds are at different concentrations.
C. ophioglossoides mycelia prevented cell death in neuronal cells and memory deficits in rats (Jin et al., 2004). Two fractions from fruiting bodies inhibited (a) the blastogenesis response, (b) natural killer cell activity, (c) interleukin-2 production and (d) tumour necrosis factor production in phytohaemagglutinin-stimulated human mononuclear cells (Kuo et al., 1996). The levels of interferon, interleukin-1 and tumour necrosis factor produced by cultured rat Kupffer cells were increased by the fungus (Liu et al., 1996a). Proliferation of cells in BALF was inhibited which also reduced tumour necrosis factor (Kuo et al., 2001).
In an effort to evaluate the pharmacological effects, including the anti-aging, of the fruiting bodies of the cultivated P. japonica fungus, a new type of Cordyceps sp. was investigated (Shin et al., 2001). This statement by the authors is incomprehensible (see above). Types of fungi are the typical specimens often held in culture collections. It is not proposed to discuss the described effects herein. Hot-water extracts of Cordyceps scarabaecola stromata exhibited potent intestinal immune system-modulating activity, while the methanol-soluble fraction manifested intermediate activity (Yu et al., 2003).
2.4.3. Ethanol
Xu et al. (1992) detected inhibition in melanoma colony formation in murine lungs by CS. The fruiting bodies of “P. japonica” reduced tumour weight and volume and lengthened the life span of mice inoculated with sarcoma 180 cells (Shin et al., 2003) but what this fungus represents in unclear. Water and ethanol extracts of CS possessed a potent anti-oxidant activity (Yamaguchi et al., 2000b). Anti-lipid peroxidation activities also were detected and accumulation of cholesteryl ester in macrophages was inhibited via suppression of LDL oxidation. Hot-water extracts were particularly effective. “P. japonica” exhibited immunostimulating activity. Its ethanolic extract stimulated phagocyteosis and macrophage acid phosphatase activity (Shin et al., 2001, Shin et al., 2003).
C. militaris demonstrated general anti-inflammatory activity (Yu et al., 2004a) in mice. The fact that cultured fruiting bodies were employed is interesting rather than extraction from those from the wild and most papers seen for this review either use fruiting bodies from the wild and/or in vitro biomass. However, it is disappointing that details of the cultivation of the fruiting bodies are not provided as this is required. Nitric oxide production and iNOS gene expression in LPS-stimulated RAW 264.7 cells are suppressed by ethanolic preparations (Won and Park, 2005). A unique insight into how the cultured fruiting bodies were produced in this report and should be referred to: this detail is required in future reports. CS (as supplied by the “Xinhui Xinhan Artificial Cordyceps Factory” (sic)) inhibited MDA generation via hydroxyl radicals induced by the peroxynitrite generator SIN-1 and macrophage accumulation of esterified cholesterol (Yamaguchi et al., 2000a, Yamaguchi et al., 2000b). The authors concluded that the cultured CS has anti-oxidant and anti-lipid peroxidation properties and inhibits accumulation of cholesteryl ester in macrophages via suppression of LDL oxidation. The authors conclude correctly that this cultured Chinese medicine appears to merit further investigation as an anti-atherosclerotic.
2.4.4. Ethyl acetate
In the unusual use of this solvent to create an extract from CS mycelia, apoptosis in human pre-myelocytic leukaemia HL60 cells was induced. In addition, cell proliferation was inhibited (Zhang et al., 2004a). Obviously, ethyl acetate may extract different compounds from the fungus compared to the more common water or methanol extracts and the constituents need to be determined.
2.4.5. Various
An alcoholic extract of CS inhibited abdominal aortic thrombus formation in rabbits by preventing platelet aggregation (Zhao, 1991). CS was extracted in PBS and dialyzed (Chiou et al., 2000) the resulting macromolecule fraction was assayed in anesthetized rats for hypotensive effects and in isolated aorta for vasorelaxant effects. A constituent(s) in CS relaxed vascular beds directly. The in vivo and in vitro effects and its extracted fractions on the secretion of testosterone in mice were studied (Hsu et al., 2003a). CS, water-soluble protein, and poorly water-soluble polysaccharide and protein significantly stimulated in vitro testosterone production in purified mouse Leydig cells. The authors concluded that it is “possible” that CS “might” contribute to an alternative medicine for the treatment of some reproductive problems caused by insufficient testosterone levels in human males, which is a large leap in conclusions. Increase antigen expression was found in hepatoma cells and “will” provide more effective host immune surveillance against tumour cells (Chiu, 1998) from a Cordyceps extract. In an interesting study, the beneficial effects of the “traditional Chinese medicine CS”, on mice with hypoferric anaemia were evaluated by NMR spectroscopy (Manabe et al., 2000). The extract increased hepatic energy metabolism in anaemic mice and was concluded to be due to increased hepatic blood flow. Zhang et al. (2006) concluded that “their” extract “is” effective in resisting the oxidative damage on liver mitochondria of diabetic mice.
Qiao and Jian (2007) attempted to identify the signaling pathways for the induction of HL-60 cell apoptosis by CS mycelium extract (CSME). CSME induced nuclear fragmentation and DNA degradation, two hallmark events of apoptosis, in the HL-60 cells within 12-24 h of treatment. Concomitantly, several major events in the mitochondrial signal pathway occurred, including (a) the loss of MTP, (b) cytochrome c release into the cytoplasm, (c) the decrease in Bcl-2 protein level, (d) the translocation of Bax protein from cytoplasm into mitochondria, and (e) the activation of caspase-2, -3, and -9. However, caspase-8, the initiator caspase in the death receptor pathway, was not activated. These results suggest that CSME induces apoptosis in HL-60 cell through the mitochondrial pathway rather than the death receptor pathway.
Treatment of d-galactose-induced-aged-mice with an unspecific CS extract resulted in (a) an improved learning ability and memory, (b) an increase in superoxide dismutase activity in erythrocytes, liver and brain, (c) an increase in catalase and glutathione peroxidase activity in blood, (d) reductions in malondialdehyde levels in brain and liver and (e) a reduction in monoamine oxidase activity in the brain (Wang et al., 2004). Extracts enhanced the antibody response, restored the phagocytic activity of macrophages in tumour-bearing mice, and lengthened the survival period of the mice (Yamaguchi et al., 1990). An extract down-regulated apoptotic genes in the rat kidney following ischaemia/reperfusion (Shahed et al., 2001). Manabe et al., 1996, Manabe et al., 2000 found that a mycelial extract increased hepatic energy metabolism, as demonstrated by liver ATP:Pi value, in diet-induced hypoferric anaemic mice by increasing hepatic blood flow.
The following section considers the use of the fungus as whole mycelium and/or fruit-bodies, although the preparations may have to be prepared in water or ethanol in practise.
2.5. Whole fungus
CS down-regulated inflammation-related genes in the rat kidney following ischaemia/reperfusion (Shahed et al., 2001). A similar treatment improved lung function in guinea-pigs and airway inflammation in rats, suggesting a possible asthma treatment (Lin et al., 2001). C. pruinosa inhibited (a) gene expression of an interleukin tumour necrosis factor, (b) inducible nitric oxide synthase (iNOS) and cyclooxygenase-2, and (c) nuclear transcription factor NF-κB activation in a lipopolysaccharide (LPS)-stimulated mouse macrophage cell line: This indicated a role in the treatment of endotoxin shock or sepsis (Kim et al., 2003a).
Anti-ds-DNA production was inhibited and improved survival in mice indicated that CS may be beneficial to patients with systemic lupus erythematosus, an autoimmune disease with involvement of multiple organ systems (Chen et al., 1993). The fungus inhibited lymphadenectasis, reduced proteinuria and plasma anti-ds-DNA antibody and improved renal function in MRL 1pr/1pr mice (Fu and Lin, 2001). An oral dose of 2–4 g daily for 3 years prevented the recurrence of lupus nephritis and protected renal function in lupus nephritis patients (Lu, 2002).
Carcinogenesis in the murine forestomach was suppressed by Cordyceps (Lin, 1984). Results from Liu et al., 1996b, Liu et al., 1996a indicated that the levels of IL-1 and INF, produced by cultured rat kupffer cells were increased from rats fed on CS. Studies have demonstrated that polysaccharides extracted from these natural products have anti-hyperglycemic effects (Lo et al., 2001). These authors investigated the effects of intragastrically administered Cordyceps sp. for alleviating fasting hyperglycemia in diabetic rats. Animals had significantly increased serum levels of triglyceride, cholesterol and blood urea nitrogen, and significantly decreased body weight, serum albumin levels and weights of the thymus, lungs and gastrocnemius muscle compared to animals in the control group. In addition, blood glucose was significantly increased compared to the control group; these results suggest that enterally administered Cordyceps sp. has potential anti-hyperglycemic ability. These findings reveal that the fungus “may be used as a nutraceutical to alleviate hyperglycemia in diabetes”.
Oral administration of Cordyceps alleviates fasting hyperglycemia (Lin et al., 2002). Recent evidence has shown that an extract has immunoregulatory activity. The objective was to investigate whether Cordyceps has biological activity in regulating the lymphocyte subsets in diabetes. After 2 weeks, body weight, thymus weight, thymocyte number, and the percentages of total T and T helper cells in the thymus were significantly lower in the treatment groups than in the controls. Results demonstrate that STZ-induced diabetic rats had significantly decreased numbers and subsets of T cells in the thymus. However, oral administration of Cordyceps did not improve these changes. The results suggested that oral administration had no significant effect on lymphocyte subsets in STZ-induced diabetic rats.
Hsu et al. (2003b) demonstrated in a convincing study, that CS mycelium regulates mouse cell testosterone production and may suppress stimulated testosterone production via P450 scc enzyme activity. Hsu et al. (2003a) demonstrated that the fungus and fractions from it were capable of stimulating testosterone production. The steroidogenic activity was observed in vivo in male mice after 7 days of treatment (Huang et al., 2004b). Chen et al. (2005) found that protein kinase A and protein kinase C pathways are acted upon to stimulate steroidogenesis in MA-10 mouse Leydig tumour cells. Inhibitors of protein kinase A, protein kinase C and phospholipase C and calmodulin antagonists (see Paterson, 2008) reduce Leydig cell steroidogenesis induced by the fungus.
C. militaris inhibited (a) the growth and metastasis of Lewis lung cancer cells, and (b) the growth of sarcoma S180 cells implanted in mice. In addition, the survival period of the mice was increased (Liu et al., 1997). Liu et al. (1991) showed that P. sinensis inhibited lipid peroxidation but increases the amount of glutathione peroxidase and superoxide dismutase in mouse liver. Again the name of the fungus raises similar questions as to those raised for P. japonica and consequently as to what material is being used and whether the experiments could be repeated. Finally, Cordyceps is included in a list of anti-aging TCM (Chen and Li, 1993).
CS stimulated mitochondrial electron transport and ATP production (Siu et al., 2004). The effect of the fungus on hepatic fibrogenesis induced in rats was studied by Liu and Shen (2003). It was found that it delayed cirrhotic development and improves liver function by inhibiting expression of transforming growth factor- and platelet-derived growth factor and deposition of procollagen I and III. Zhou et al. (1990) presented evidence for the beneficial effects of CS on chronic hepatitis B.
The fungus increased DNA synthesis in primary cultured rat tubular epithelial cells (Tian et al., 1991). Proximal tubular cells were protected from the toxic effects of gentamicin. The possible mechanisms include protection of sodium pump activity, reduction of lipid peroxidation and attenuation of lysosomal over-activity in tubular cells due to phagocytosis of gentamicin (Zhen et al., 1992, Li et al., 1996). Also, rat kidneys were protected from cyclosporin-induced nephrotoxicity and ameliorated glomerular and interstitial damage (Zhao and Li, 1993). Bao et al. (1994) reported that “old” patients were protected from amikacin sulphate toxicity as demonstrated by decreases in urinary nephroaminoglycosidase and microglobulin. Also, inhibition by C. militaris of LDL-induced proliferation of cultured human glomerular mesangial cells, which are involved in the development of glomerulosclerosis was observed (Zhao-Long et al., 2000). Treatment of mice with C. militaris lengthened the swimming time to exhaustion (Jung et al., 2004). An unspecified Cordyceps is one of the components of Fuzheng Huayu recipe, which is used to control the development of post-hepatic cirrhosis or to prevent complications from the disease (Liu et al., 1996b).
The macrophage-stimulating activity of natural fungus and cultured mycelia has been described by Zhang (1985). Zhang and Xia (1990) demonstrated immunosuppressant effects in the heterotropic heart allograft model in rats and determined that prolonged survival periods were possible. Also, Zhu and Yu (1990) found prolonged mouse skin allograft survival time. The number of T helper cells was increased, as were Lyt-1/Lyt-2 (T helper cells to T suppressor cells) in peripheral blood and spleen (Chen et al., 1991). A mitogenic action was demonstrated on splenic lymphocytes and interleukin-2 from spleen cells of rats with chronic renal failure was augmented (Cheng, 1992). In addition, natural killer cell activity was enhanced (Xu et al., 1992). Treatment of patients with post-hepatic cirrhosis resulted in (a) enhancement of natural killer cell function, (b) increased number and improved ratio of CD4þ and CD8þ cells, and (c) reduction in IgA and IgG levels (Zhu and Liu, 1992). Treatment of chronic hepatitis B resulted in increased CD4/CD8 ratios, and reductions in hyaluronic acid and procollagen type III. The data indicate the usefulness of the fungus in adjusting the level of T lymphocyte subsets and treating hepatic fibrosis (Gong et al., 2000). Liu et al. (1992) demonstrated increased peripheral natural killer cell activity from healthy subjects and leukaemia patients. Improved renal function and augmented cellular immune function in chronic renal failure has also been observed (Guan et al., 1992). The fruiting body portion, but not the carcass portion, of Cordyceps reduced weight loss, polydipsia and hyperglycaemia in diabetic rats (Lo et al., 2004).
Some other somewhat obscure papers are available on the effect of whole fungi (i.e. Peizhong, 1984, Chen, 1985, Zhang, 1985, Shao, 1985, Liu and Xu, 1985, Zhang, 1987, Chen, 1987, Du, 1986, Sun, 1985, Li et al., 1993).
2.6. Mycelium
Feng et al. (1987) reported the vasodilating effect of cultured mycelia of CS in an investigation of the cardiovascular system of dogs. Thirty three cases of chronic hepatitis B patients treated with cultured CS mycelia have shown that the drug (a) improves liver function, (b) promotes negative transfer HBsAg, (c) markedly helps to raise plasma albumin, (d) helps patients resist high gamma globulin and (e) adjusts body immunocompetence (Zhou et al., 1990). It is suggested that the fungus may be a medicine for chronic hepatitis B patients in adjusting protein metabolism and correcting inversion of albumin and globulin. Since inflammation has been reported to be associated with chronic diseases inhibitory dietary factors such as the fungus may be beneficial in alleviating disease (Hong and Lin, 2004).
17β-Estradiol may directly influence the quality of maturing oocytes and thus the outcome of assisted reproduction treatment. CS mycelium is “believed” to enhance libido and fertility in both sexes. However, the mechanism of its effect in women has not been determined. This is surely a rather thin basis on which to undertake research. Huang et al. (2004a) concluded that treatment of granulosa-lutein cells with CS results in increased 17β-estradiol production due, in part, to increased StAR and aromatase expression. If these data are confirmed, CS may help in the development of treatment regimens to improve the success rate of in vitro fertilization, they state.
Zhu and Liu (1992) found that cultivated Cordyceps mycelia inhibited humoral immune hyperfunction and increase the serum complement level in patients with post-hepatic cirrhosis, and improved liver function. Whole body insulin sensitivity in rats was increased (Balon et al., 2002). The fungus increased the basal plasma insulin level (Zhang et al., 2003) and inhibited hepatic fibrogenesis (Zhang et al., 2004b) in rats with CCl4-induced liver fibrosis. Previous studies by Lo et al. (2006) demonstrated that the fruiting bodies of CS attenuated diabetes-induced weight loss, polydipsia, and hyperglycemia in rats. Rats were orally administered, fruiting bodies, fermented mycelia, spent broth, or mycelia plus spent broth of the fungus. The results revealed that the mycelia and spent broth had anti-hyperglycemic activities similar to those of the fruiting bodies. The authors’ bold claim that the fermented products could be developed as potential anti-diabetic agents or functional foods for persons with a high risk of diabetes mellitus needs further confirmation. Yang et al. (2006) mention that mycelium can inhibit tumour growth and induce tumour cell apoptosis. However, the antitumour mechanisms are not fully understood. So, the molecular mechanism was determined. The authors conclude that cell apoptosis is induced by activating caspase-8-dependent and caspase-9-independent pathways and downregulating NF-κB protein expression.
Mycelia induced human granulosa-lutein cells to produce 17β-estradiol by upregulating expression of steroidogenic acute regulatory protein (StAR) and aromatase (Huang et al., 2004a). Also, steroidogenesis in mouse Leydig tumour cells (Huang et al., 2001a) was stimulated without involvement of StAR (Huang et al., 2000). Testosterone production was inhibited by human chorionic gonadotropin or dibutyryl cyclic AMP. Thus, its effect on the signal-transduction pathway for steroidogenesis may be after the production of cyclic AMP.
2.7. Commercial preparations
It is revealing to read the web sites of some of the commercial products: These are often written in unscientific terms. The best of them at least contain warnings that, “These statements have not been evaluated by the (US) Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease”.
Colson et al. (2005) undertook a revealing experiment to determine the effect on male cyclists’ performance using a combination of CS and Rhodiola rosea “herbs” – a traditional herbal medicine. Importantly, and unusually, the study followed a double blind, randomized, placebo-treatment, pre-post test design. Essentially no effect was observed. This is an example of why scientists need to circumspect about making unsubstantiated claims about the powers of these preparations even if spin does help project funding.
“In this regard, the maintenance of a balance of Yin and Yang – two opposing components involved in life activities as exemplified by the antagonistic action of the sympathetic and parasympathetic nervous systems-is essential in achieving a healthy condition” (Leung et al., 2005). Statements such as these leaves the current reviewer bewildered. Why these terms are used in a scientific publication does not appear to merit comment. Previous studies have shown that long-term treatment with a “Yang-invigorating” Chinese herbal formula (VI-28) could increase red cell CuZn-superoxide dismutase (SOD) activity in male human subjects. Yet the authors conclude, the beneficial effect of VI-28 treatment on mitochondrial functional ability and antioxidant capacity may have clinical implications in the prevention of age-related diseases.
Ka Wai Lee et al. (2006) investigated a commercial preparation of a cultivated strain of CS. They state that the immunomodulatory activities have been renowned for centuries. The report describes positively the immunomodulatory features: In vitro results demonstrated that the fungus induced the production of interleukin (IL)-1, IL-6, IL-10 and tumour necrosis factor alpha, augmented surface expression of CD25 on lymphocytes, and elevated macrophage phagocytosis and monocyte production of H2O2. The authors state that, “Our results possibly provide the biochemical basis for future clinical trials”. The current author emphasises that such over extrapolation of data needs to be discouraged.
Dai et al. (2001) found that CordyMax Cs-4 (a mycelial fermentation product of CS) “improved the bioenergy status” in the mouse liver. These findings may explain why CordyMax Cs-4 is claimed to alleviate fatigue and improve physical endurance especially in aged subjects. Reports of athletes’ performance being improved may also be supported from these types of reports. CordyMax Cs-4 lowered fasting plasma levels of glucose and insulin, improved oral glucose tolerance and increased the glucose–insulin index, which measures insulin sensitivity, in rats (Zhao et al., 2002).
An unspecified Cordyceps is reported to be a component of Fuzheng Huayu recipe, which is used to control the development of post-hepatic cirrhosis or to prevent its complications (Liu et al., 1996b). As part of the eternal search to find an excelsior of youth, many are interested in anti-aging activity but it is difficult to define what his means scientifically. Are the authors stating that the aging process is slowed for example? Do people live longer? In any case, this was published in the journal, Chinese Journal of Integrated Traditional and Western Medicine which is an admirable sentiment but difficult to do in practice. A preparation of CS referred to as Jinshuibao capsule, increased the depressed superoxide dismutase activity and reduced elevated malondialdehyde (MDA) level caused by aging when tested in senile patients. In addition, they enhanced the repair of damaged non human animal DNA (Zhang et al., 1997). Quite extraordinarily beneficial effects on senile patients are claimed and, of course, the constituents of the capsule may be difficult to verify. Zhou and Lin (1995) used “Jinshuibao”, to restore cellular immune function and “improve the quality of life” of patients with advanced cancer without affecting humoral immune function. Obviously, quality of life is a subjective notion and is difficult to define.
Remarkably, a dried powder preparation of mycelia called Bailing capsule (a) prevented rejection of renal transplants, (b) protected renal and hepatic function, (c) stimulated haematopoietic function, (d) improved hypoproteinaemia and hyperlipidaemia and (e) reduced the incidence of infections (Sun et al., 2004). However, such claims must surely carry considerable elements of doubt.
In another case of a combined treatment with as many as six other components in addition to CS, the treatment was more effective at preventing acute renal failure than chronic in rats. Obviously, it is difficult or impossible to assess the efficacy of the individual components (Ngai et al., 2005).
3. Apoptosis
Inhibition of apoptosis is a novel area of clinical investigation with great promise and so merits a separate section in this review and will be of particular interest to pharmacologists. In one of the more critical papers, Buenz et al. (2005) mention that there is a wide range of uses of CS in the literature, and those claiming altered apoptotic homeostasis are of the most intriguing. However, they emphasize problems created by the (a) difficulty of identifying the species of Cordyceps and (b) many conflicting reports of pharmacological function. In response, the authors (a) outline what is known about the ability of CS to alter apoptotic homeostasis, (b) attempt to reconcile differences in function, and (c) identify the challenges and how to progress CS research.
Many disorders (e.g. stroke, myocardial infarction and HIV) incorporate apoptosis in their aetiology and pathogenesis. The ability to inhibit apoptosis has emerged as an important potential therapy (e.g. cancer). There are reports of CS extracts inhibiting and inducing apoptosis (Shahed et al., 2001); this is not contradictory but allows a foundation to determine the molecular mechanism of activity. Indeed, Cordyceps may contain compounds that inhibit apoptosis; however, conflicting evidence has been obtained. The fungus can scavenge reactive oxygen species by inhibiting malondialdehyde formation by SIN-1, the peroxynitrite generator, which has been confirmed by xanthine oxidase, hemolysis, and lipid peroxidation assays. Furthermore, an isolated extract of CS, H1-A (this is reported as a pure compound (see above), inhibited induced apoptosis, by permeabilizing the cell membrane and upregulating nitric oxide synthase (Trubiani et al., 2003). On the other hand, extracts of CS did not inhibit hydrogen peroxide-induced apoptosis (Buenz et al., 2004), in a reactive oxygen species model (Fauconneau et al., 2002). Alternatively, CS down-regulated apoptotic genes and modulated apoptosis in a rat kidney ischemia reperfusion model.
Reported anti-apoptotic effects of CS include in (a) the mouse (anti-cytotoxic activity, anti-oxidant activity, cell proliferation inhibition), (b) cell culture (anti-proliferation activity, cell proliferation inhibition, hemolysis inhibitory activity, lipid peroxide formation inhibition, radical scavenging effect), (c) human cells (proliferation inhibition, natural killer cell inhibition, tumour necrosis factor inhibition) and (d) the rat (gene expression inhibition). Whereas the reported apoptotic effects of the fungus are in: (a) the mouse (anti-tumour activity, metastasis inhibition) and (b) cell culture (proliferation stimulation, cytotoxic activity). In reality these opposite effects are not incompatible. There are three prominent factors that may contribute to the apparent discrepancies (the first and third are fairly obvious). First, certain fungi contain different biologically active compounds. Second, extracts may contain a pro-drug; a metabolism step may be required to generate the biologically active form. Third, different constituents may be assayed as there are multiple methods of extraction utilized in the literature.
Furthermore, decreases in Fas, Fas ligand and tumour necrosis factor expression and decreased caspase-3 activity have been demonstrated (Shahed et al., 2001). CS inhibited TNF-α expression (Kuo et al., 1996). However, when apoptosis was initiated via a Fas agonist antibody (CH-11) (Alderson et al., 1994), aqueous and alcohol extracts of CS did not rescue cells induced by Fas receptor ligation (Buenz et al., 2004). Furthermore, cell cycle arrest and/or inhibition of proliferation yield cells resistant to apoptosis. Papers concerning proliferation of leukemic U937 cells (Chen et al., 1997) and glomerular mesangial cells inhibition (Zhao-Long et al., 2000, Lin et al., 1999) may be explained by conferring a apoptotic resistance to cells: the alteration may involve p53 (Fridman and Lowe, 2003) or NF-κB (Karin et al., 2004).
Overall, aqueous, and organic extracts of CS have the ability to inhibit apoptosis. Cells pre-incubated with CS extracts were equally sensitive to hydrogen peroxide and Fas-mediated apoptosis. Thus, the putative antioxidant and anti-apoptotic properties of CS were insufficient to rescue cells from apoptosis in vitro (Buenz et al., 2004). Furthermore, cancer chemotherapeutics have involved the ability to induce apoptosis. Hence the polysaccharide fraction (H1-A) induced apoptosis by inhibiting (a) phosphorylation of Bcl-2 and Bcl-xL and (b) apoptosis induced by dimethyl sulfoxide (Yang et al., 2003). These data require to be confirmed by further work (Buenz et al., 2005). Finally, direct cytotoxic activity may be a factor (Nakamura et al., 1999a, Nakamura et al., 1999b, Kuo et al., 1994, Sato, 1989, Buenz et al., 2005).
4. Compounds of fungi isolated from Cordyceps
A factor which is not often considered is whether the activities of extracts and pure compounds are from the Cordyceps of interest or from other contaminating fungi. The issues of anamorph/teleomorph associations are relevant here. For example, epicoccins A–D were isolated from cultures of a Cordyceps-colonizing isolate of Epicoccum nigrum (Zhang et al., 2007b). Gliocladinins A and B are of an isolate of Gliocladium sp. that colonized CS (Guo et al., 2007). It is essential that such activity is differentiated from that obtained from the traditional medicine or Cordyceps per se. In a series of papers, (a) Paecilomyces militaris is shown to possess militarinones A, B, C, D and (b) farinosomes and a deoxymilitarinone substance were detected from Paecilomyces farinosus (Schmidt et al., 2002, Schmidt et al., 2003, Cheng et al., 2004, Cheng et al., 2006). The authors do not make statements as to the holomophic connections to Cordyceps. Hamburger (2007) does make this link to C. militaris (teleomorph) from P. militaris (anamorph) apparently on the basis of the same pigment production. However, it is has been suggested that Paecilomyces does not contain anamophic species of Cordyceps (Chen et al., 2001). Hence, comment on these reports is avoided by the current author because the relationships between Paecilomyces and Cordyceps remain ill-defined.
5. What about the insect?
Another complicating facet is that the medicine may contain a proportion of the insect host and so what does this bring to the activity? This is an area of investigation which simply is not reported. Indeed does the healthy insect have interesting activities, and/or at what stage does the activity occur in the infected insect? Could the activity be greater at these stages? The accumulation of bioactive plant metabolites by insects is well documented (Brown and Trigo, 1994) and insects are used as medicines (Zhao et al., 2007). These aspects are almost totally ignored in the case of the medicinal Cordyceps. It appears as if entomologists have simply not been involved in the field. Does the insect have to be dead before the system works or are more active components produced by the fungus as it is in the process of killing the insect? Research into these factors could be fruitful.
Hence, it is worth reporting what is known about the differences between the insect and fungus parts: Interestingly, galactomannans isolated from the insect portion of C. cicadae demonstrate potent hypoglycaemic activity in mice (Kiho et al., 1990). The fruiting body portion, but not the carcass portion, of Cordyceps reduced weight loss, polydipsia and hyperglycaemia in diabetic rats (Lo et al., 2004). Aqueous methanol extracts of C. cicadae ascocarps enhanced human mononuclear cells (HMNC) proliferation (Weng et al., 2002). In contrast, the methanol (100%) extracts of the C. cicadae insect-body portion suppressed HMNC proliferation. Interestingly, the ergosterol esters concentrations were much higher in the (dead) caterpillar than the fruiting bodies (Yuan et al., 2007) although ergosterol was similar. Finally, the fruiting body and the caterpillar parts of CS are claimed to be similar in chemical composition and hence anti-oxidant activity because the fungus had presumable replaced the insect constituents with fungal (Li et al., 2002).
Little scientific evidence exists to support the numerous herbs used to improve diabetes-related metabolic disorders (Lo et al., 2004). The dual organism medicine (i.e. fruiting body and carcass) has been proposed to have multiple medicinal activities. In one investigation, the effects of the fruiting body and carcass of the preparation on hyperglycemia were investigated. Diabetic rats had significantly lower weight gain and higher blood glucose response in oral glucose tolerance test than the control rats; and these changes were significantly reduced by administrating the fruiting body of Cordyceps. The results revealed that the fruiting body (not the carcass) of Cordyceps attenuated the diabetes-induced weight loss, polydipsia and hyperglycemia, and these improvements suggest that fruiting body has a potential to be a functional food for diabetes.
The water extracts from the fruiting body and “worm” of natural Cordyceps were analyzed for their content of nucleosides and polysaccharides; the results showed that the worm had a chemical composition similar to the fruiting body (Li et al., 2002). In addition, both the fruiting body and worm of Cordyceps showed similar potency in their anti-oxidation activities in the xanthine oxidase assay, the induction of hemolysis assay and the lipid peroxidation assay. These results suggest that the function of the worm is to provide a growth medium for the fruiting body, and that eventually, the worm is totally invaded by mycelia. Aqueous methanol extracts of C. cicadae ascocarps enhanced human mononuclear cells HMNC proliferation (Weng et al., 2002). In contrast, the methanol (100%) extracts of the C. cicadae insect-body portion suppressed HMNC proliferation. This is a most interesting result in the current author’s opinion.
6. Profiling chemical constituents
The following are references concerning the chemical constituents as determined by analytical profiles (Guo, 1985, Xiao, 1983, Li and Li, 1991). Yu et al. (2007b) developed a method involving “biospecific” extraction and HPLC for potential immunological components in CS. The two active compounds were identified as guanosine and adenosine. Li et al., 2001b, Li et al., 2001c found that cultured CS mycelia have a much higher content of nucleosides than natural CS. Guo et al. (1998) described an HPLC method for quantitative determination of adenosine and deoxyadenosine. Li et al. (1999) determined adenosine in fermented products of Cordyceps by reversed-phase HPLC. Ergosterol in CS can be determined by HPLC (Li and Li, 1991, Li et al., 2004), and, of course, ergosterol is present in all fungi.
7. Optimisation
When there are many questions as to what are the active components, a section on optimisation may seem premature. However, Kim and Yun (2005) investigated the optimal culture conditions for the production of exopolysaccharides (EPS) and cordycepin during submerged mycelial culture of C. militaris and CS. This was not done in the paper Hamburger (2007) comments upon. Fermentations were performed in flasks and in 5-L stirred-tank bioreactors. The concentrations of mycelial biomass, EPS and cordycepin achieved in submerged culture of C. militaris were higher than those of CS. As the authors claim, comparative studies between the two fungi are not available and the paper was the first report on the optimum medium composition for submerged culture of CS. Cordyceps nutans Pat. is another entomopathogenitic Ascomycete belonging to the family Clavicipitaceae which is parasitic on hemipteran insects (Sasaki et al., 2005). Very few investigations have been made with this fungus. In this research, optimum temperature and pH for mycelial growth was determined. However, it appeared that growth remained low and commercial exploitation perhaps limited. However, it would be interesting to include this species in any future taxonomic treatments of the genus. Optimisation of culture conditions for mycelial growth and production of polysaccharides and cordycepin were described by Park et al., 2001b, Park et al., 2002, Xu et al., 2002, Kim et al., 2003b, Kim et al., 2003c, Xiao et al., 2004, Mao and Zhong, 2004, Hsieh et al., 2005, although cordycepin is usually produced synthetically at present.
8. Poisonings
Two cases of lead poisoning were reported (Wu et al., 1996). These two patients took Cordyceps herbal medicine for treatment of underlying diseases. Loss of appetite and anemic signs of lead poisoning were manifested in one patient with a high blood lead level, while the other patient was asymptomatic. The lead content in the Cordyceps powder was found to be as high as 20,000 ppm. After cessation of intake in the asymptomatic patient, and cessation of intake and treatment with chelating agents in the symptomatic patient, the blood lead levels returned to normal range. This report raises concerns about lead poisoning from unusual herbal medicine in general.
9. Taxonomic considerations
This present review is not intended to be a taxonomic paper, but is essentially a review of the papers that employ the name Cordyceps more or less loosely, to describe the fungus used in medicinally related experiments. However, in a most impressive paper, Sung et al. (2007) state that Cordyceps comprise over 400 species in their modern phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Cordyceps Fr. host range is broad, ranging from 10 orders of arthropods to the truffle-like genus Elaphomyces, although most species are restricted to a single host species or closely related host species. The main objectives of the study was to reassess the (a) morphological traits used currently, (b) taxonomic utility of the anamorphic forms and (3) classification in relation to phylogenetic relationships. It may be worth mentioning that PCRs may be subjected to inhibition, and nucleic acids may be affected by the medium in which the fungus was grown (see Paterson, 2007, Paterson, 2008). Unfortunately, Sung et al. (2007) do not provide details of how the fungi were grown for analysis and so it is difficult to conjecture. Obviously, as discussed in this review, the fungus produces numerous bioactive compounds which may be inhibitors or mutagens and the effect of metabolite production on the DNA preparations need to be determined or misleading results may be obtained.
However, to continue, it is worth mentioning that C. militaris maintains its name in the study while C. sinensis is now classified as Ophiocordyceps sinensis. The taxon was historically classified in the Clavicipitaceae, based on cylindrical asci, thickened ascus apices and filiform ascospores, which often disarticulate into part-spores. The fungus was characterized by having a pathological ecology on Elaphomyces and arthropods, with infrageneric classifications emphasizing ascospore morphology, host affiliation and arrangement of perithecia. The production of well-developed often stipitate stromata was typical. Sung et al. re-classified on the basis of phylogenetic relationships between 162 taxa by employing five to seven genetic loci. Three clavicipitaceous clades were determined which rejected the monophyly of Cordyceps and Clavicipitaceae and most diagnostic characters used in Cordyceps were not supported as being phylogenetically informative. However, the most consistent characters with the phylogeny were pigmentation, morphology and morphology of stromata. Cordycipitaceae was validated based on C. militaris, the type of Cordyceps, which included most Cordyceps species that possess bright, fleshy stromata. The new family Ophiocordycipitaceae was proposed. The majority of species here produce stromata that often possess aperithecial apices and are darkly pigmented, tough to pliant. Elaphocordyceps was proposed for a subclade of the Ophiocordycipitaceae, which includes species of Cordyceps that parasitize (a) the fungal genus Elaphomyces and (b) arthropods. The family Clavicipitaceae included the core clade of grass symbionts, and the entomopathogenic genus Hypocrella and relatives. The new genus Metacordyceps is proposed for Cordyceps species that are closely related to the grass symbionts in the Clavicipitaceae s. s. Metacordyceps includes teleomorphs linked to Metarhizium and other closely related anamorphs. Lists of accepted names for species in Cordyceps, Elaphocordyceps, Metacordyceps and Ophiocordyceps are provided. Obviously, this work needs to be consulted in future work on the fungus for medicinal properties as does Stadler et al. (2003).
10. Conclusions
Too often authors have used highly conditional statements such as; the preparation “may” have “possible” activity against, for example, cancer, which are meaningless. It is claimed that modern studies are demonstrating anti-oxidant, vascular, immune, and anti-inflammatory effects (Meletis and Barker, 2005) and the mechanisms by which these mushrooms work are being elucidated. However, the journal in which the paper is published is dedicated to complimentary and alternative medicines. This implies it is not mainstream scientific and so scientists may not give it as much weight in comparison to, for example, Phytochemistry or the Journal of Natural Products. Nevertheless, the former reports could be considered as providing leads for more scientific research.
In general, problems include too many chemically uncharacterised crude extracts, inadequate taxonomic vigour, and over extrapolation of in vitro data to imply therapeutic value. This needs to be avoided as it falsely raises hopes for cures for serious diseases. Furthermore, there are no biochemical data on how the insect are converted into fungal components, or if the insect host per se has pharmacological properties. Poor (i.e. inadequate or obsolete) biochemical procedures and assays can also be cause problems (see Hamburger, 2007). The biological material being used often is not well characterised and there are few indications of specimens being available for other workers to repeat the described work for example (i.e. no voucher specimens are available), with some notable exceptions. A rather limited range of solvents have been employed to extract the components and, for example, chloroform/methanol is particularly efficacious for fungal compounds (e.g. Paterson and Bridge, 1994). There is very little on the use of non-polar solvents. Paterson et al., 2004, Paterson, 2006, Paterson, 2008 have advocated a method which may have utility in standardising bioactive fungi. In this scheme, a “common, readily identifiable” morphological character is determined and then the fungus is analyzed for particular metabolites. In this case the steps may be:
-
1.
Produces stroma on Lepidopteron insects.
-
2.
Produces detectable concentrations of, for example, cordycepin.
The analysis for other compounds would be desirable and some potential marker compounds are provided in Table 3 . However, the phylogenic approach of Sung et al. (2007) will set the standards for decades to come and will profoundly influence future publications in all fields relating to Cordyceps.
Table 3.
Summary of markers currently used for quality control of Cordyceps and associated activities (after Li et al., 2006a)
| Compound type | Pharmacological activities | Comments |
|---|---|---|
| Nucleosides | Anti-tumour activities; Ca2+ antagonist; the release of various neurotransmitters presynaptically and anticonvulsant activity; stimulate axon growth in vitro and in the adult central nerve system | Nucleosides, especially adenosine, are usually used as markers, and the profiles can be applied for authentication of Cordyceps |
| Polysaccharides | Anti-oxidation, immunopotentiation, anti-tumour, and hypoglycemic activity; anti-inflammatory activity and suppress the humoral immunity in mice | Represents the most biological properties of Cordyceps, and less used as marker for quality control. Thus, it should be developed |
| Ergosterol and its analogs | Cytotoxic activity, anti-viral activity, and anti-arrhythmia effect; suppress the activated human mesangial cells and alleviate immunoglobulin A nephropathy (Berger’s disease) | Ergosterol can be used as marker for quality control |
| Mannitol | Diuretic, anti-tussive and anti-free radical activities | It sometimes is used as marker for quality control |
| Peptides | Anti-tumour and immunopotentiation activities | A potential marker for quality control |
Acknowledgements
R.R.M. Paterson is funded by Grant SFRH/BPD/34879/2007 from Fundação para a Ciência e a Tecnologia, Portugal. Professor Nelson Lima, Universidade do Minho is gratefully appreciated for his unstinting support, as are other colleagues therein. L.E. Gilbert, University of Texas, Austin was generous for allowing the use of his images of infected insects (Fig. 1c–f). Marc Stadler, University of Bayreuth, Germany is thanked for discussions.
Biography

R. Russell M. Paterson is working as a research scientist at the Institute for Biotechnology and Bioengineering (IBB), Centre of Bioengineering, University of Minho, Portugal. A secondment for the IOI Professorial Chair, Faculty of Agriculture, University Putra Malaysia, Malaysia, has been accepted by him recently, which will commence in early 2008. His B.Sc. in Applied Microbiology was obtained at the University of Strathclyde, Glasgow, where his nascent interest in filamentous fungi was nurtured by Professor J.E. Smith. Crucially, he undertook his Ph.D. in the Microbial Chemistry Laboratory of Professor J.D. Bu’Lock, the “inventor” of fungal secondary metabolism. Postdoctoral studies took him to The Boyce Thompson Institute at Cornell University, New York, where he worked at United States Department of Agriculture laboratories on insect pathogenic fungi under the supervision of Dr Dick Soper, which is relevant particularly to the present review. After his return to the University of Strathclyde, he was employed as a fungal natural products specialist at CABI Bioscience, Egham, where he was privileged to be employed in the era when Professor D. L. Hawksworth was the director. The secondary metabolism of fungi is his primary interest and vice versa.
References
- Ahn Y.J., Park S.J., Lee S.G., Shin S.C., Choi D.H. Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agric. Food Chem. 2000;48:2744–2748. doi: 10.1021/jf990862n. [DOI] [PubMed] [Google Scholar]
- Alderson M.R., Tough T.W., Braddy S., Davis-Smith T., Roux E., Schooley K., Miller R.E., Lynch D.H. Regulation of apoptosis and T cell activation by Fas-specific mAb. Int. Immunol. 1994;6:1799–1806. doi: 10.1093/intimm/6.11.1799. [DOI] [PubMed] [Google Scholar]
- Balon T.W., Jasman A.P., Zhu J.S. A fermentation product of Cordyceps sinensis increases whole-body insulin sensitivity in rats. J. Alternat. Complement. Med. 2002;8:315–323. doi: 10.1089/10755530260128005. [DOI] [PubMed] [Google Scholar]
- Bao Z.D., Wu Z.G., Zheng F. Amelioration of aminoglycoside nephrotoxicity by Cordyceps sinensis in old patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14:271–273. [PubMed] [Google Scholar]
- Bok J.W., Lermer L., Chilton J., Klingeman H.G., Towers G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- Boros C., Hamilton S.M., Katz B., Kulanthaivel P. Comparison of balanol from Verticillium balanoides and ophiocordin from Cordyceps ophioglossoides. J. Antibiot. (Tokyo) 1994;47:1010–1016. doi: 10.7164/antibiotics.47.1010. [DOI] [PubMed] [Google Scholar]
- Brown K.S., Jr., Trigo J.R. Multi-level complexity in the use of plant allelochemicals by aposematic insects. Chemoecology. 1994;5:119–126. [Google Scholar]
- Bucci L.R. Selected herbals human exercise performance. Am. J. Clin. Nutr. 2000;72:624S–636S. doi: 10.1093/ajcn/72.2.624S. [DOI] [PubMed] [Google Scholar]
- Buenz E.J., Bauer B.A., Osmundson T.W., Motley T.J. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J. Ethnopharmacol. 2005;96:19–29. doi: 10.1016/j.jep.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Buenz E.J., Weaver J.G., Bauer B.A., Chalpin S.D., Badley A.D. Cordyceps sinensis extracts do not prevent Fas-receptor and hydrogen peroxide-induced T-cell apoptosis. J. Ethnopharmacol. 2004;90:57–62. doi: 10.1016/j.jep.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Burnett J. Oxford University Press Inc.; New York: 2003. Fungal Populations and Species. [Google Scholar]
- Canney S. Cordyceps sinensis animal, vegetable or both? J. Chin. Med. 2006;80:43–49. [Google Scholar]
- Chatterjee R., Srinivasan K.S., Maiti P.C. Cordyceps sinesis (Berkeley) Saccardo: structure of cordycepic acid. J. Am. Pharm. Assoc.: Am. Pharm. Assoc. 1957;46:114–118. doi: 10.1002/jps.3030460211. [DOI] [PubMed] [Google Scholar]
- Chen D.M. Platelet hemopoiesis and ultrastructure observations in mice treated with natural Cordyceps sinensis and its cultured mycelia. Zhong Yao Tong Bao (Beijing, China: 1981) 1987;12:47–49. [PubMed] [Google Scholar]
- Chen D.M. The effect of natural Cordyceps sinensis and its cultured mycelia on murine immuno-organs and function of the mononuclear macrophage system. Chin. J. Mod. Dev. Trad. Med. 1985;5:42–44. [PubMed] [Google Scholar]
- Chen G.Z., Chen G.L., Sun T., Hsieh G.C., Henshall J.M. Effects of Cordyceps sinensis on murine T lymphocyte subsets. Chin. Med. J. 1991;104:4–8. [PubMed] [Google Scholar]
- Chen J.R., Yen J.H., Lin C.C., Tsai W.J., Liu W.J., Tsai J.J., Lin S.F., Liu H.W. The effects of Chinese herbs on improving survival and inhibiting anti-ds DNA antibody production in lupus mice. Am. J. Chin. Med. 1993;21:257–262. doi: 10.1142/S0192415X93000303. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang W., Lu T., Li J., Zheng Y., Kong L. Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sci. 2006;78:2742–2748. doi: 10.1016/j.lfs.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Chen K., Li C. Recent advances in studies on traditional Chinese anti-aging material medica. J. Trad. Chin. Med. 1993;13:223–226. [PubMed] [Google Scholar]
- Chen S.Z., Chu J.Z. NMR and IR studies on the characterization of cordycepin and 2′-deoxyadenosine. Zhongguo Kang Sheng Su Za Shi (Chin. J. Antibiot.) 1996;21:9–12. [Google Scholar]
- Chen Y.C., Huang Y.L., Huang B.M. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. Int. J. Biochem. Cell Biol. 2005;37:214–223. doi: 10.1016/j.biocel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Chen Y.-J., Shiao M.-S., Lee S.-S., Wang S.-Y. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997;60:2349–2359. doi: 10.1016/s0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- Chen Y.-J., Zhang Y.-P., Yang Y.-X., Yang D.-R. Genetic diversity and taxonomic implication of Cordyceps sinensis as revealed by RAPD markers. Biochem. Genet. 1999;37:201–213. doi: 10.1023/a:1018738706123. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Q., Wang N., Qu L.-H., Li T.-H., Zhang W.-M. Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA. Biochem. Syst. Ecol. 2001;29:597–607. doi: 10.1016/s0305-1978(00)00100-9. [DOI] [PubMed] [Google Scholar]
- Cheng Q. Effect of Cordyceps sinensis on cellular immunity in rats with chronic renal insufficiency. Zhonghua Yi Xue Za Zhi. 1992;72:27–29. [PubMed] [Google Scholar]
- Cheng Y., Schneider B., Riese U., Schubert B., Li Z., Hamburger M.J. Farinosones A–C, neurotrophic alkaloidal metabolites from the entomogenous Deuteromycete Paecilomyces farinosus. J. Nat. Prod. 2004;67:1854–1858. doi: 10.1021/np049761w. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Schneider B., Riese U., Schubert B., Li Z., Hamburger M. Novel tetramic acids and pyridone alkaloids, militarinones B, C, and D, from the insect pathogenic fungus Paecilomyces militaris. J. Nat. Prod. 2006;69:436–438. doi: 10.1021/np020430y. [DOI] [PubMed] [Google Scholar]
- Chiou W.F., Chang P.C., Chou C.J., Chen C.F. Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis. Life Sci. 2000;66:1369–1376. doi: 10.1016/s0024-3205(00)00445-8. [DOI] [PubMed] [Google Scholar]
- Chiu J.-H. Cordyceps sinensis increases the expression of major histocompatibility complex class II antigens on human hepatoma cell line HA22T/VGH cells. Am. J. Chin. Med. 1998;26:159–170. doi: 10.1142/S0192415X9800021X. [DOI] [PubMed] [Google Scholar]
- Cho H.-J., Cho J.Y., Rhee M.H., Park H.-J. Cordycepin (3′-deoxyadenosine) inhibits human platelet aggregation in a cyclic AMP- and cyclic GMP-dependent manner. Eur. J. Pharmacol. 2007;558:43–51. doi: 10.1016/j.ejphar.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Cho J., Kang J.S., Long P.H., Jing J., Back Y., Chung K.S. Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and Cordyceps mushroom extract. Arch. Pharmacol. Res. 2003;26:821–825. doi: 10.1007/BF02980027. [DOI] [PubMed] [Google Scholar]
- Choi S.B., Park C.H., Choi M.K., Jun D.W., Park S. Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Biosci. Biotechnol. Biochem. 2004;68:2257–2264. doi: 10.1271/bbb.68.2257. [DOI] [PubMed] [Google Scholar]
- Colson S.N., Wyatt F.B., Johnston D.L., Autrey L.D., FitzGerald Y.L., Earnest C.P. Cordyceps sinensis- and Rhodiola rosea-based supplementation in male cyclists and its effect on muscle tissue oxygen saturation. J. Strength Condition. Res. 2005;19:358–363. doi: 10.1519/R-15844.1. [DOI] [PubMed] [Google Scholar]
- Cooper R., Chang J. Asian herbals: opportunities for marketing traditional Chinese medicines in the west. J. Nutraceut. Funct. Med. Food. 2001;3:25–37. [Google Scholar]
- Cunningham K.G., Manson W., Spring F.S., Hutchinson S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- Dai G., Bao T., Xu C., Cooper R., Zhu J.S. CordyMax Cs-4 improves steady-state bioenergy status in mouse liver. J. Alternat. Complement. Med. 2001;7:231–240. doi: 10.1089/107555301300328106. [DOI] [PubMed] [Google Scholar]
- DaSilva E.J. Mushrooms in medicine and culture. Int. J. Med. Mush. 2005;7:75–78. [Google Scholar]
- Dong, C.-H., Yao, Y.-J., 2007. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. LWT: Food Sci. Technol. doi:10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed]
- Du D.J. Antitumor activity of Cordyceps sinensis and cultured Cordyceps mycelia. Zhong Yao Tong Bao (Beijing, China: 1981) 1986;11:51–54. [PubMed] [Google Scholar]
- Duh P.-D.J. Rebuttal on comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Agric. Food Chem. 2007;55:7215–7216. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- Fauconneau B., Petegnief V., Sanfeliu C., Piriou A., Planas A.M. Induction of heat shock proteins (HSPs) by sodium arsenite in cultured astrocytes and reduction of hydrogen peroxide-induced cell death. J. Neurochem. 2002;83:1338–1348. doi: 10.1046/j.1471-4159.2002.01230.x. [DOI] [PubMed] [Google Scholar]
- Feng M.G., Zhou Q.G., Feng G.H. Vasodilating effect of cultured Cordyceps sinensis (Berk) Sacc. mycelia in anesthetized dogs. Zhong Yao Tong Bao. 1987;12:41–45. [PubMed] [Google Scholar]
- Fridman J.S., Lowe S.W. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Fu T., Lin J. Effect of Cordyceps sinensis on inhibiting systemic lupus erythematosus in MRL 1pr/1pr mice. Zhong Yao Cai. 2001;24:658–659. [PubMed] [Google Scholar]
- Fujita T., Inoue K., Yamamoto S., Takeda Y., Takaishi Y., Ichihara T., Ikumoto T., Okumoto T. A novel immunosuppressant, ISP-I, of Isaria sinclairii. J. Pharmacobio: Dynam. 1990;13:s-48. [Google Scholar]
- Gao Q., Wu G., He D. Effect of Cordyceps sinensis on the Th1/Th2 cytokines in patients with condyloma acuminatum. Zhong Yao Cai. 2000;23:402–404. [PubMed] [Google Scholar]
- Gong H.Y., Wang K.Q., Tang S.G. Effects of Cordyceps sinensis on T lymphocyte subsets and hepatofibrosis in patients with chronic hepatitis B. Hunan Yi Ke Da Xue Xue Bao. 2000;25:248–250. [PubMed] [Google Scholar]
- Guan Y.J., Hu Z., Hou M. Effect of Cordyceps sinensis on T-lymphocyte subsets in chronic renal failure. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:338–339. [PubMed] [Google Scholar]
- Guarino A.J., Kredich N.M. Isolation and identification of 3′-amino-3′-deoxyadenosine from Cordyceps militaris. Biochim. Biophys. Acta. 1963;68:317–319. doi: 10.1016/0006-3002(63)90149-5. [DOI] [PubMed] [Google Scholar]
- Guo C., Zhu J., Zhang C., Zhang L. Determination of adenosine and 3′-deoxyadenosine in Cordyceps militaris (L.) Link. by HPLC. Zhongguo Zhong Yao Za Zhi. 1998;23:236–237. [PubMed] [Google Scholar]
- Guo H., Hu H., Liu S., Liu X., Zhou Y., Che Y. Bioactive p-terphenyl derivatives from a Cordyceps-colonizing isolate of Gliocladium sp. J. Nat. Prod. 2007;70:1519–1521. doi: 10.1021/np070231k. [DOI] [PubMed] [Google Scholar]
- Guo Y.W. Preliminary study of Cordyceps barnesii – comparison of the chemical constituents of Cordyceps barnesii and Cordyceps sinensis. Zhong Yao Tong Bao (Beijing, China: 1981) 1985;10:33–35. [PubMed] [Google Scholar]
- Hamburger M. Comment on comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2007;55:7213–7214. doi: 10.1021/jf070054t. [DOI] [PubMed] [Google Scholar]
- Hong Y.-H., Lin B.-F. Evaluation of the anti-inflammation screening model of macrophages cell line by the proinflammatory mediators secretions. Nutr. Sci. J. 2004;29:159–167. [Google Scholar]
- Hsieh C., Tsai M.J., Hsu T.H., Chang D.M., Lo C.T. Medium optimization for polysaccharide production of Cordyceps sinensis. Appl. Biochem. Biotechnol. 2005;120:145–158. doi: 10.1385/abab:120:2:145. [DOI] [PubMed] [Google Scholar]
- Hsu C.-C., Huang Y.-L., Tsai S.-J., Sheu C.-C., Huang B.-M. In vivo and in vitro stimulatory effects of Cordyceps sinensis on testosterone production in mouse Leydig cells. Life Sci. 2003;73:2127–2136. doi: 10.1016/s0024-3205(03)00595-2. [DOI] [PubMed] [Google Scholar]
- Hsu C.C., Tsai S.J., Huang Y.L., Huang B.M. Regulatory mechanism of Cordyceps sinensis mycelium on mouse Leydig cell steroidogenesis. FEBS Lett. 2003;543:140–143. doi: 10.1016/s0014-5793(03)00427-7. [DOI] [PubMed] [Google Scholar]
- Huang B.M., Chuang Y.M., Chen C.F., Leu S.F. Effects of extracted Cordyceps sinensis on steroidogenesis in MA-10 mouse Leydig tumor cells. Biol. Pharm. Bull. 2000;23:1532–1535. doi: 10.1248/bpb.23.1532. [DOI] [PubMed] [Google Scholar]
- Huang B.-M., Hsiao K.-Y., Chuang P.-C., Wu M.-H., Pan H.-A., Tsai S.-J. Upregulation of steroidogenic enzymes and ovarian 17β-estradiol in human granulosa-lutein cells by Cordyceps sinensis mycelium. Biol. Reprod. 2004;70:1358–1364. doi: 10.1095/biolreprod.103.022855. [DOI] [PubMed] [Google Scholar]
- Huang B.M., Hsu C.C., Tsai S.J., Sheu C.C., Leu S.F. Effects of Cordyceps sinensis on testosterone production in normal mouse Leydig cells. Life Sci. 2001;69:2593–2602. doi: 10.1016/s0024-3205(01)01339-x. [DOI] [PubMed] [Google Scholar]
- Huang B.M., Ju S.Y., Wu C.S., Chuang W.J., Sheu C.C., Leu S.F. Cordyceps sinensis and its fractions stimulate MA-10 mouse Leydig tumor cell steroidogenesis. J. Androl. 2001;22:831–837. [PubMed] [Google Scholar]
- Huang Y.L., Leu S.F., Liu B.C., Sheu C.C., Huang B.M. In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sci. 2004;75:1051–1062. doi: 10.1016/j.lfs.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hui M.Y., Wang B.-S., Shiow C.H., Duh P.-D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006;54:3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Tsuru S., Ohmori T., Kitahara S., Inouye T., Healy G.B. CO–N reaction—a new serological activity index—on Wegener’s granulomatosis. J. Laryngol. Otolaryngol. 1993;107:607–610. doi: 10.1017/s0022215100123849. [DOI] [PubMed] [Google Scholar]
- Ince, M., 2007. World University Rankings. The Times Higher Education Supplement. p. 8.
- Isaka M., Boonkhao B., Rachtawee P., Auncharoen P. A xanthocillin-like alkaloid from the insect pathogenic fungus Cordyceps brunnearubra BCC 1395. J. Nat. Prod. 2007;70:656–658. doi: 10.1021/np060509t. [DOI] [PubMed] [Google Scholar]
- Isaka M., Kongsaeree P., Thebtaranonth Y. Bioxanthracenes from the insect pathogenic fungus Cordyceps pseudomilitaris BCC 1620. II. Structure elucidation. J. Antibiot. (Tokyo) 2001;54:36–43. doi: 10.7164/antibiotics.54.36. [DOI] [PubMed] [Google Scholar]
- Isaka M., Srisanoh U., Lartpornmatulee N., Boonruangprapa T. ES-242 derivatives and cycloheptapeptides from Cordycepssp. strains BCC 16173 and BCC 16176. J. Nat. Prod. 2007;70:1601–1604. doi: 10.1021/np070357h. [DOI] [PubMed] [Google Scholar]
- Isaka M., Tanticharoen M., Kongsaeree P., Thebtaranonth Y. Structures of cordypyridones A–D, antimalarial N-hydroxy- and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J. Org. Chem. 2001;66:4803–4808. doi: 10.1021/jo0100906. [DOI] [PubMed] [Google Scholar]
- Jaturapat A., Isaka M., Hywel-Jones N.L., Lertwerawat Y., Kamchonwongpaisan S., Kirtikara K., Tanticharoen M., Thebtaranonth Y. Bioxanthracenes from the insect pathogenic fungus Cordyceps pseudomilitaris BCC 1620. I. Taxonomy, fermentation, isolation and antimalarial activity. J. Antibiot. (Tokyo) 2001;54:29–35. doi: 10.7164/antibiotics.54.29. [DOI] [PubMed] [Google Scholar]
- Jia T., Lau B.H.S. The immuno-enhancing effect of Chinese herbal medicine Cordyceps sinensis on macrophage J774. Chin. Pharm. J. 1997;32:142–144. [Google Scholar]
- Jin D.Q., Park B.C., Lee J.S., Choi H.D., Lee Y.S., Yang J.H., Kim J.A. Mycelial extract of Cordyceps ophioglossoides prevents neuronal cell death and ameliorates beta-amyloid peptide-induced memory deficits in rats. Biol. Pharm. Bull. 2004;27:1126–1129. doi: 10.1248/bpb.27.1126. [DOI] [PubMed] [Google Scholar]
- Jung E.C., Kim K.D., Bae C.H., Kim J.C., Kim D.K., Kim H.H. A mushroom lectin from ascomycete Cordyceps militaris. Biochim. Biophys. Acta Gen. Subj. 2007;1770:833–838. doi: 10.1016/j.bbagen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Jung K., Kim I.H., Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J. Ethnopharmacol. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Ka Wai Lee S., Kwok Wong C., Kai Kong S., Nam Leung K., Wai Kei Lam C. Immunomodulatory activities of HERBSnSENSES™ Cordyceps – in vitro and in vivo studies. Immunopharmacol. Immunotoxicol. 2006;28:341–360. doi: 10.1080/08923970600809470. [DOI] [PubMed] [Google Scholar]
- Kaczka E.A., Trenner N.R., Arison B., Walker R.W., Folkers K. Identification of cordycepin, a metabolite of Cordyceps militaris, as 3′-deoxyadenosine. Biochem. Biophys. Res. Commun. 1964;14:456–457. doi: 10.1016/0006-291x(64)90086-5. [DOI] [PubMed] [Google Scholar]
- Karin M., Yamamoto Y., Wang Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N., Ohmori T., Takeshita Y., Kawanishi G., Katayama S., Yamada H. Occurrence of Gal beta (1–3) GalNAc-Ser/Thr in the linkage region of polygalactosamine containing fungal glycoprotein from Cordyceps ophioglossoides. Biochem. Biophys. Res. Commun. 1986;140:350–356. doi: 10.1016/0006-291x(86)91097-1. [DOI] [PubMed] [Google Scholar]
- Kiho T., Hui J., Yamane A., Ukai S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1993;16:1291–1293. doi: 10.1248/bpb.16.1291. [DOI] [PubMed] [Google Scholar]
- Kiho T., Nagai K., Miyamoto I., Watanabe T., Ukai S. Polysaccharides in fungi. XXV. Biological activities of two galactomannans from the insect-body portion of Chan hua (fungus: Cordyceps cicadae) Yakugaku Zasshi. 1990;110:286–288. doi: 10.1248/yakushi1947.110.4_286. [DOI] [PubMed] [Google Scholar]
- Kiho T., Ookubo K., Usui S., Ukai S., Hirano K. Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1999;22:966–970. doi: 10.1248/bpb.22.966. [DOI] [PubMed] [Google Scholar]
- Kiho T., Yamane A., Hui J., Usui S., Ukai S. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol. Pharm. Bull. 1996;19:294–296. doi: 10.1248/bpb.19.294. [DOI] [PubMed] [Google Scholar]
- Kim H.O., Yun J.W. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures J. Appl. Microbiol. 2005;99:728–738. doi: 10.1111/j.1365-2672.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- Kim J.R., Yeon S.H., Kim H.S., Ahn Y.J. Larvicidal activity against Plutella xylostella of cordycepin from the fruiting body of Cordyceps militaris. Pest Manage. Sci. 2002;58:713–717. doi: 10.1002/ps.508. [DOI] [PubMed] [Google Scholar]
- Kim K.-M., Kwon Y.-G., Chung H.-T., Yun Y.-G., Pae H.-O., Han J.-A., Ha K.-S., Kim Y.-M. Methanol extract of Cordyceps pruinosa inhibits in vitro and in vivo inflammatory mediators by suppressing NF-κB activation. Toxicol. Appl. Pharmacol. 2003;190:1–8. doi: 10.1016/s0041-008x(03)00152-2. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Hwang H.J., Xu C.P., Sung J.M., Choi J.W., Yun J.W. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J. Appl. Microbiol. 2003;94:120–126. doi: 10.1046/j.1365-2672.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Xu C.P., Hwang H.J., Choi J.W., Kim C.W., Yun J.W. Production and characterization of exopolysaccharides from an enthomopathogenic fungus Cordyceps militaris NG3. Biotechnol. Prog. 2003;19:428–435. doi: 10.1021/bp025644k. [DOI] [PubMed] [Google Scholar]
- Kneifel H., Konig W.A., Loeffler W., Muller R. Ophiocordin, an antifungal antibiotic of Cordyceps ophioglossoides. Arch. Microbiol. 1977;113:121–130. doi: 10.1007/BF00428591. [DOI] [PubMed] [Google Scholar]
- Koh J.H., Kim J.M., Chang U.J., Suh H.J. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis. Biol. Pharm. Bull. 2003;26:84–87. doi: 10.1248/bpb.26.84. [DOI] [PubMed] [Google Scholar]
- Koh J.H., Kim K.M., Kim J.M., Song J.C., Suh H.J. Antifatigue and antistress effect of the hot-water fraction from mycelia of Cordyceps sinensis. Biol. Pharm. Bull. 2003;26:691–694. doi: 10.1248/bpb.26.691. [DOI] [PubMed] [Google Scholar]
- Koh J.H., Yu K.W., Suh H.J., Choi Y.M., Ahn T.S. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci. Biotechnol. Biochem. 2002;66:407–411. doi: 10.1271/bbb.66.407. [DOI] [PubMed] [Google Scholar]
- Korf R.P. Reinventing taxonomy: a curmudgeon’s view of 250 years of fungal taxonomy, the crises in biodiversity, and the pitfalls of the phylogenetic age. Mycotaxon. 2005;93:407–415. [Google Scholar]
- Krasnoff S.B., Reategui R.F., Wagenaar M.M., Gloer J.B., Gibson D.M. Cicadapeptins I and II: new Aib-containing peptides from the entomopathogenic fungus Cordyceps heteropoda. J. Nat. Prod. 2005;68:50–55. doi: 10.1021/np0497189. [DOI] [PubMed] [Google Scholar]
- Kredich N.M., Guarino A.J. Homocitrullylaminoadenosine, a nucleoside isolated from Cordyceps militaris. J. Biol. Chem. 1961;236:3300–3302. [PubMed] [Google Scholar]
- Kredich N.M. Inhibition of nucleic acid methylation by cordycepin. In vivo synthesis of S-3-deoxyadenosylmethionine by WIL2 human lymphoblasts. J. Biol. Chem. 1980;255:7380–7385. [PubMed] [Google Scholar]
- Kuo C.-F., Chen C.-C., Lin C.-F., Jan M.-S., Huang R.Y., Luo Y.-H., Chuang W.-J., Lin Y.-S. Abrogation of streptococcal pyrogenic exotoxin B-mediated suppression of phagocytosis in U937 cells by Cordyceps sinensis mycelium via production of cytokines. Food Chem. Toxicol. 2007;45:278–285. doi: 10.1016/j.fct.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Kuo C.-F., Chen C.-C., Luo Y.-H., Huang R.Y., Chuang W.-J., Sheu C.-C., Lin Y.-S. Cordyceps sinensis mycelium protects mice from group A streptococcal infection. J. Med. Microbiol. 2005;54:795–802. doi: 10.1099/jmm.0.45704-0. [DOI] [PubMed] [Google Scholar]
- Kuo H.-C., Su Y.-L., Yang H.-L., Huang I.-C., Chen T.-Y. Differentiation of Cordyceps sinensis by a PCR-single-stranded conformation polymorphism-based method and characterization of the fermented products in Taiwan. Food Biotechnol. 2006;20:161–170. [Google Scholar]
- Kuo Y.C., Lin C.Y., Tsai W.J., Wu C.L., Chen C.F., Shiao M.S. Growth inhibitors against tumor cells in Cordyceps sinensis other than corydcepin and polysaccharides. Cancer Invest. 1994;12:611–615. doi: 10.3109/07357909409023046. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Tsai W.J., Shiao M.S., Chen C.F., Lin C.Y. Cordyceps sinensis as an immunomodulatory agent. Am. J. Chin. Med. 1996;24:111–125. doi: 10.1142/S0192415X96000165. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Tsai W.J., Wang J.Y., Chang S.C., Lin C.Y., Shiao M.S. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001;68:1067–1082. doi: 10.1016/s0024-3205(00)01011-0. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Weng S.C., Chou C.J., Chang T.T., Tsai W.J. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br. J. Pharmacol. 2003;140:895–906. doi: 10.1038/sj.bjp.0705500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhanpal T.N., Rana M. Medicinal and nutraceutical genetic resources of mushrooms. Plant Genet. Resour.: Charact. Util. 2005;3:288–303. [Google Scholar]
- Leung H.Y., Chiu P.Y., Poon M.K.T., Ko K.M. A Yang-invigorating Chinese herbal formula enhances mitochondrial functional ability and antioxidant capacity in various tissues of male and female rats. Rejuvenation Res. 2005;8:238–247. doi: 10.1089/rej.2005.8.238. [DOI] [PubMed] [Google Scholar]
- Li L.S., Zheng F., Liu Z.H. Experimental study on effect of Cordyceps sinensis in ameliorating aminoglycoside induced nephrotoxicity. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:733–737. [PubMed] [Google Scholar]
- Li S.P., Li P., Dong T.T., Tsim K.W. Determination of nucleosides in natural Cordyceps sinensis and cultured Cordyceps mycelia by capillary electrophoresis. Electrophoresis. 2001;22:144–150. doi: 10.1002/1522-2683(200101)22:1<144::AID-ELPS144>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Li S.P., Li P., Dong T.T.X., Tsim K.W.K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine. 2001;8:207–212. doi: 10.1078/0944-7113-00030. [DOI] [PubMed] [Google Scholar]
- Li S.P., Li P., Ji H., Zhang P., Dong T.T., Tsim K.W. The contents and their change of nucleosides from natural Cordyceps sinensis and cultured Cordyceps mycelia. Yao Xue Xue Bao. 2001;36:436–439. [PubMed] [Google Scholar]
- Li S.P., Li P., Lai C.M., Gong Y.X., Kan K.K., Dong T.T., Tsim K.W., Wang Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A. 2004;1036:239–243. doi: 10.1016/j.chroma.2004.02.080. [DOI] [PubMed] [Google Scholar]
- Li S.P., Su Z.R., Dong T.T., Tsim K.W. The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine. 2002;9:319–324. doi: 10.1078/0944-7113-00134. [DOI] [PubMed] [Google Scholar]
- Li S.P., Yang F.Q., Tsim K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Li S.P., Zhang G.H., Zeng Q., Huang Z.G., Wang Y.T., Dong T.T.X., Tsim K.W.K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine. 2006;13:428–433. doi: 10.1016/j.phymed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Li S.P., Zhao K.J., Ji Z.N., Song Z.H., Dong T.T.X., Lo C.K., Cheung J.K.H., Tsim K.W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- Li X., Wang Y., Bao T. RP-HPLC determination of adenosine in fermented Cordyceps. Zhongguo Zhong Yao Za Zhi. 1999;24:12–14. [PubMed] [Google Scholar]
- Li Y., Chen G.Z., Jiang D.Z. Effect of Cordyceps sinensis on erythropoiesis in mouse bone marrow. Chin. Med. J. (Engl.) 1993;106:313–316. [PubMed] [Google Scholar]
- Li Y.H., Li X.L. Determination of ergosterol in Cordyceps sinensis and Cordyceps black-bone chicken capsules by HPLC. Yao Xue Xue Bao. 1991;26:768–771. [PubMed] [Google Scholar]
- Lim H.W., Kwon Y.M., Cho S.M., Kim J.H., Yoon G.H., Lee S.J., Kim H.W., Lee M.W. Antitumor activity of Cordyceps militaris on human cancer cell line. Korean J. Pharmacognosy. 2004;35:364–367. [Google Scholar]
- Lin C.Y., Ku F.M., Kuo Y.C., Chen C.F., Chen W.P., Chen A., Shiao M.S. Inhibition of activated human mesangial cell proliferation by the natural product of Cordyceps sinensis (H1-1): an implication for treatment of IGA mesangial nephropathy. J. Lab. Clin. Med. 1999;133:55–63. doi: 10.1053/lc.1999.v133.a94239. [DOI] [PubMed] [Google Scholar]
- Lin P.Z. Inhibitory effect of Cordyceps on carcinogenesis of the forestomach in mice. Zhonghua Zhong Liu Za Zhi. 1984;6:335–337. [PubMed] [Google Scholar]
- Lin S.-C., Lo H.-C., Lin C.-S. Oral administration of Cordyceps does not affect lymphocyte subsets in STZ-induced diabetic rats. Nutr. Sci. J. 2002;27:77–83. [Google Scholar]
- Lin X.X., Xie Q.M., Shen W.H., Chen Y. Effects of fermented Cordyceps powder on pulmonary function in sensitized guinea pigs and airway inflammation in sensitized rats. Zhongguo Zhong Yao Za Zhi. 2001;26:622–625. [PubMed] [Google Scholar]
- Liu C., Lu S., Ji M.R. Effects of Cordyceps sinensis (CS) on in vitro natural killer cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:267–269. [PubMed] [Google Scholar]
- Liu G.T., Xu R.L. Immuno-pharmacologic activity of Cordyceps sinensis (Berk) Sacc. Zhong Xi Yi Jie He Za Zhi/Chin. J. Mod. Dev. Trad. Med. 1985;5:622–624. [PubMed] [Google Scholar]
- Liu J., Yang S., Yang X., Chen Z., Li J. Anticarcinogenic effect and hormonal effect of Cordyceps militaris Link. Zhongguo Zhong Yao Za Zhi. 1997;22:111–113. [PubMed] [Google Scholar]
- Liu J.M., Zhong Y.R., Yang Z., Cui S.L., Wang F.H. Chemical constituents of Cordyceps militaris (L.) Link. Zhongguo Zhong Yao Za Zhi. 1989;14:608–609. [PubMed] [Google Scholar]
- Liu P., Liu C., Hu Y.Y. Effect of fuzheng huayu recipe in treating posthepatitic cirrhosis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:459–462. [PubMed] [Google Scholar]
- Liu, P., Zhu, J., Huang, Y., Liu, C., 1996a. Influence of Cordyceps sinensis (Berk.) Sacc. and rat serum containing same medicine on IL-1, IFN and TNF produced by rat Kupffer cells. China J. Chin. Mater. Med. 21, 367–369. [PubMed]
- Liu W.-C., Wang S.-C., Tsai M.-L., Chen M.-C., Wang Y.-C., Hong J.-H., McBride W.H., Chiang C.-S. Protection against radiation-induced bone marrow and intestinal injuries by Cordyceps sinensis, a Chinese herbal medicine. Radiat. Res. 2006;166:900–907. doi: 10.1667/RR0670.1. [DOI] [PubMed] [Google Scholar]
- Liu Y.K., Shen W. Inhibitive effect of Cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World J. Gastroenterol. 2003;9:529–533. doi: 10.3748/wjg.v9.i3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wu C., Li C. Anti-oxidation of Paecilomyces sinensis (S. Pnov.) Zhongguo Zhong Yao Za Zhi. 1991;16:240–242. [PubMed] [Google Scholar]
- Lo H.-C., Hsu T.-H., Tsai F.-A., Lin S.-C. Intragastrically administered chinese herbal medicine Cordyceps alleviates fasting hyperglycemia in diabetic rats. Nutr. Sci. J. 2001;26:11–21. [Google Scholar]
- Lo H.-C., Hsu T.-H., Tu S.-T., Lin K.-C. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. Am. J. Chin. Med. 2006;34:819–832. doi: 10.1142/S0192415X06004314. [DOI] [PubMed] [Google Scholar]
- Lo H.-C., Tu S.-T., Lin K.-C., Lin S.-C. The anti-hyperglycemic activity of the fruiting body of Cordyceps in diabetic rats induced by nicotinamide and streptozotocin. Life Sci. 2004;74:2897–2908. doi: 10.1016/j.lfs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Lu L. Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:169–171. [PubMed] [Google Scholar]
- Manabe N., Azuma Y., Sugimoto M., Uchio K., Miyamoto M., Taketomo N., Tsuchita H., Miyamoto H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. Br. J. Nutr. 2000;83:197–204. doi: 10.1017/s0007114500000258. [DOI] [PubMed] [Google Scholar]
- Manabe N., Sugimoto M., Azuma Y., Taketomo N., Yamashita A., Tsuboi H., Tsunoo A., Kinjo N., Nian-Lai H., Miyamoto H. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn. J. Pharmacol. 1996;70:85–88. doi: 10.1254/jjp.70.85. [DOI] [PubMed] [Google Scholar]
- Mao X.B., Zhong J.J. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol. Prog. 2004;20:1408–1413. doi: 10.1021/bp049765r. [DOI] [PubMed] [Google Scholar]
- Mei Q.B., Tao J.Y., Gao S.B., Xu G.C., Chen L.M., Su J.K. Antiarrhythmic effects of Cordyceps sinensis (Berk.) Sacc. Zhongguo Zhong Yao Za Zhi. 1989;14:616–618. [PubMed] [Google Scholar]
- Meletis C.D., Barker J.E. Medicinal mushrooms: a selective overview. Altern. Complement. Ther. 2005;11:141–145. [Google Scholar]
- Nakamura K., Konoha K., Yamaguchi Y., Kagota S., Shinozuka K., Kunitomo M. Combined effects of Cordyceps sinensis and methotrexate on hematogenic lung metastasis in mice. Recept. Chan. 2003;9:329–334. doi: 10.3109/713745176. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Yamaguchi Y., Kagota S., Kwon Y.M., Shinzuka K., Kunitomo M. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn. J. Pharmacol. 1999;79:335–341. doi: 10.1254/jjp.79.335. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Yamaguchi Y., Kagota S., Shinozuka K., Kunitomo M. Activation of in vivo Kupffer cell function by oral administration of Cordyceps sinensis in rats. Jpn. J. Pharmacol. 1999;79:505–508. doi: 10.1254/jjp.79.505. [DOI] [PubMed] [Google Scholar]
- Nan J.X., Park E.J., Yang B.K., Song C.H., Ko G., Sohn D.H. Antifibrotic effect of extracellular biopolymer from submerged mycelial cultures of Cordyceps militaris on liver fibrosis induced by bile duct ligation and scission in rats. Arch. Pharmacol. Res. 2001;24:327–332. doi: 10.1007/BF02975101. [DOI] [PubMed] [Google Scholar]
- Ng T.B., Wang H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- Ngai H.H.Y., Sit W.-H., Wan J.M.F. The nephroprotective effects of the herbal medicine preparation, WH30+, on the chemical-induced acute and chronic renal failure in rats. Am. J. Chin. Med. 2005;33:491–500. doi: 10.1142/S0192415X05003089. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Tamura K., Fukui K., Kawanishi G., Mitsuyama M., Nomoto K., Miyazaki T. Isolation of galactosaminoglycan moiety (CO–N) from protein-bound polysaccharide of Cordyceps ophioglossoides and its effects against murine tumors. Chem. Pharm. Bull. (Tokyo) 1989;37:1019–1022. doi: 10.1248/cpb.37.1019. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Tamura K., Ohgane N., Nakamura T., Kawanishi G., Yamada H., Nomoto K. The correlation between molecular weight and antitumor activity of galactosaminoglycan (CO–N) from Cordyceps ophioglossoides. Chem. Pharm. Bull. (Tokyo) 1989;37:1337–1340. doi: 10.1248/cpb.37.1337. [DOI] [PubMed] [Google Scholar]
- Ohmori T., Tamura K., Tsuru S., Nomoto K. Antitumor activity of protein-bound polysaccharide from Cordyceps ophioglossoides in mice. Jpn. J. Cancer Res. 1986;77:1256–1263. [PubMed] [Google Scholar]
- Pang P.K.T., Benishin C., Lewanczuk R., Shan J. Problems in the use of herbal and natural substances, with a specific example concerning the cardiovascular system. Clin. Exp. Pharmacol. Physiol. 2002;29:731–734. doi: 10.1046/j.1440-1681.2002.03709.x. [DOI] [PubMed] [Google Scholar]
- Park C., Hong S.H., Lee J.Y., Kim G.Y., Choi B.T., Lee Y.T., Park D.I., Choi Y.H. Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis. Oncol. Rep. 2005;13:1211–1216. [PubMed] [Google Scholar]
- Park J.P., Kim S.W., Hwang H.J., Yun J.W. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett. Appl. Microbiol. 2001;33:76–81. doi: 10.1046/j.1472-765x.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- Park J.P., Kim Y.M., Kim S.W., Hwang H.J., Cho Y.J., Lee Y.S., Song C.H., Yun J.W. Effect of agitation intensity on the exo-biopolymer production and mycelial morphology in Cordyceps militaris. Lett. Appl. Microbiol. 2002;34:433–438. doi: 10.1046/j.1472-765x.2002.01126.x. [DOI] [PubMed] [Google Scholar]
- Paterson R.R.M. The coronamycin producer: a case of mistaken identity? Mycol. Res. 2005;109:850–851. [Google Scholar]
- Paterson R.R.M. Ganoderma – a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Paterson R.R.M. Ganoderma disease of oil palm – a white rot perspective necessary for integrated control. Crop Prot. 2007;26:1369–1376. [Google Scholar]
- Paterson R.R.M. Internal amplification controls have not been employed in diagnostic fungal PCR hence potential false negative results. J. Appl. Microbiol. 2007;102:1–10. doi: 10.1111/j.1365-2672.2006.03220.x. [DOI] [PubMed] [Google Scholar]
- Paterson R.R.M. Fungal enzyme inhibitors as pharmaceuticals, toxins, and scourge of PCR. Curr. Enzyme Inhib. 2008;4:46–59. [Google Scholar]
- Paterson, R.R.M., Bridge, P.D., 1994. Biochemical techniques for filamentous fungi. IMI Techniques Series No. 1. CAB International, Wallingford.
- Paterson R.R.M., Venâncio A., Lima N. Solutions to Penicillium taxonomy crucial to mycotoxin research and health. Res. Microbiol. 2004;155:507–513. doi: 10.1016/j.resmic.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Peizhong L. Inhibitory effect of Cordyceps on carcinogenesis of the forestomach in mice. Chin. J. Oncol. 1984;6:335–337. [PubMed] [Google Scholar]
- Qiao X.Z., Jian Y.W. Cordyceps sinensis mycelium extract induces human premyelocytic leukemia cell apoptosis through mitochondrion pathway. Exp. Biol. Med. 2007;232:52–57. [PubMed] [Google Scholar]
- Rukachaisirikul V., Chantaruk S., Tansakul C., Saithong S., Chaicharernwimonkoon L., Pakawatchai C., Isaka M., Intereya K. A cyclopeptide from the insect pathogenic fungus Cordycepssp. BCC 1788. J. Nat. Prod. 2006;69:305–307. doi: 10.1021/np050433l. [DOI] [PubMed] [Google Scholar]
- Rukachaisirikul V., Pramjit S., Pakawatchai C., Isaka M., Supothina S. 10-membered macrolides from the insect pathogenic fungus Cordyceps militaris BCC 2816. J. Nat. Prod. 2004;67:1953–1955. doi: 10.1021/np0401415. [DOI] [PubMed] [Google Scholar]
- Sasaki F., Miyamoto T., Tamai Y., Yajima T. Optimum temperature and pH for mycelial growth of Cordyceps nutans Pat. (Ascomycetes) Int. J. Med. Mush. 2005;7:301–304. [Google Scholar]
- Sato A. Studies on anti-tumor activity of crude drugs. I. The effects of aqueous extracts of some crude drugs in shortterm screening test. J. Pharm. Soc. Jpn. 1989;109:407–423. doi: 10.1248/yakushi1947.109.6_407. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Gunther W., Stoyanova S., Schubert B., Li Z., Hamburger M. Militarinone A, a neurotrophic pyridone alkaloid from Paecilomyces militaris. Org. Lett. 2002;4(2):197–199. doi: 10.1021/ol016920j. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Riese U., Li Z., Hamburger M.J. (+)-N-Deoxymilitarinone A, a neuritogenic pyridone alkaloid from the insect pathogenic fungus Paecilomyces farinosus. Nat. Prod. 2003;66(3):378–383. doi: 10.1021/np050418g. [DOI] [PubMed] [Google Scholar]
- Shahed A.R., Kim S.I., Shoskes D.A. Down-regulation of apoptotic and inflammatory genes by Cordyceps sinensis extract in rat kidney following ischemia/reperfusion. Transplant. Proc. 2001;33:2986–2987. doi: 10.1016/s0041-1345(01)02282-5. [DOI] [PubMed] [Google Scholar]
- Shao G. Treatment of hyperlipidemia with cultivated Cordyceps – a double-blind, randomized placebo control trial. Chin. J. Mod. Dev. Trad. Med. 1985;5:652–654. [PubMed] [Google Scholar]
- Sharma S. Trade of Cordyceps sinensis from high altitudes of the Indian Himalaya: conservation and biotechnological priorities. Curr. Sci. 2004;86:1614–1619. [Google Scholar]
- Shen Q., Chen S. Effect of Cordyceps militaris on the damage of rats induced by n-hexane. Zhong Yao Cai. 2001;24:112–116. [PubMed] [Google Scholar]
- Shiao M.-S., Wang Z.-N., Lin L.-J., Lien J.-Y., Wang J.-J. Profiles of nucleosides and nitrogen bases in Chinese medicinal fungus Cordyceps sinensis and related species. Bot. Bull. Acad. Sin. New Ser. 1994;35:261–267. [Google Scholar]
- Shim J.Y., Lee Y.S., Lim S.S., Shin K.H., Hyun J.E., Kim S.Y., Lee E.B. Pharmacological activities of Paecilomyces japonica, a new type Cordyceps sp. Korean J. Pharmacognosy. 2000;31:163–167. [Google Scholar]
- Shin K.H., Lim S.S., Lee S., Lee Y.S., Jung S.H., Cho S.Y. Anti-tumour and immuno-stimulating activities of the fruiting bodies of Paecilomyces japonica, a new type of Cordyceps spp. Phytother. Res. 2003;17:830–833. doi: 10.1002/ptr.1253. [DOI] [PubMed] [Google Scholar]
- Shin K.H., Lim S.S., Lee S.H., Lee Y.S., Cho S.Y. Antioxidant and immunostimulating activities of the fruiting bodies of Paecilomyces japonica, a new type of Cordyceps sp. Ann. N. Y. Acad. Sci. 2001;928:261–273. doi: 10.1111/j.1749-6632.2001.tb05655.x. [DOI] [PubMed] [Google Scholar]
- Siu K.M., Mak D.H.F., Chiu P.Y., Poon M.K.T., Du Y., Ko K.M. Pharmacological basis of ‘Yin-nourishing’ and ‘Yang-invigorating’ actions of Cordyceps, a Chinese tonifying herb. Life Sci. 2004;76:385–395. doi: 10.1016/j.lfs.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Stadler M., Tichy H.-V., Katsiou E., Hellwig V. Chemotaxonomy of Pochonia and other conidial fungi with Verticillium like anamorphs. Mycol. Prog. 2003;2:95–122. [Google Scholar]
- Sun M., Yang Y.R., Lu Y.P., Gao R., Wang L., Wang J., Tang K. Clinical study on application of bailing capsule after renal transplantation. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:808–810. [PubMed] [Google Scholar]
- Sun Y.H. Cordyceps sinensis and cultured mycelia. Zhong Yao Tong Bao (Beijing, China: 1981) 1985;10:3–5. [PubMed] [Google Scholar]
- Sung G.-H., Hywel-Jones N.L., Sung J.-M., Luangsaard J.J., Shrestha B., Spatafora J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Chen X.M., Li L.S. Effects of Cordyceps sinensis, rhubarb and serum renotropin on tubular epithelial cell growth. Zhong Xi Yi Jie He Za Zhi. 1991;11:547–549. [PubMed] [Google Scholar]
- Trubiani O., Salvolini E., Staffolani R., Di Primio R., Mazzanti L. DMSO modifies structural and functional properties of RPMI-8402 cells by promoting programmed cell death. Int. J. Immunopathol. Pharmacol. 2003;16:253–259. doi: 10.1177/039463200301600311. [DOI] [PubMed] [Google Scholar]
- Tsai C.H., Stern A., Chiou J.F., Chern C.L., Liu T.Z. Rapid and specific detection of hydroxyl radical using an ultraweak chemiluminescence analyzer and a low-level chemiluminescence emitter: application to hydroxyl radicalscavenging ability of aqueous extracts of food constituents. J. Agric. Food. Chem. 2001;49:2137–2141. doi: 10.1021/jf001071k. [DOI] [PubMed] [Google Scholar]
- Wang B.J., Won S.J., Yu Z.R., Su C.L. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem. Toxicol. 2005;43:543–552. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Wang S.M., Lee L.J., Lin W.W., Chang C.M. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J. Cell Biochem. 1998;69:483–489. doi: 10.1002/(sici)1097-4644(19980615)69:4<483::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang S.-Y., Shiao M.-S. Pharmacological functions of Chinese medicinal fungus Cordyceps sinensis and related species. J. Food Drug Anal. 2000;8:248–257. [Google Scholar]
- Wang Y.H., Ye J., Li C.L., Cai S.Q., Ishizaki M., Katada M. An experimental study on anti-aging action of Cordyceps extract. Zhongguo Zhong Yao Za Zhi. 2004;29:773–776. [PubMed] [Google Scholar]
- Watanabe N., Hattori M., Yokoyama E., Isomura S., Ujita M., Hara A. Entomogenous fungi that produce 2,6-pyridine dicarboxylic acid (dipicolinic acid) J. Biosci. Bioeng. 2006;102:365–368. doi: 10.1263/jbb.102.365. [DOI] [PubMed] [Google Scholar]
- Weng S.-C., Chou C.-J., Lin L.-C., Tsai W.-J., Kuo Y.-C. Immunomodulatory functions of extracts from the Chinese medicinal fungus Cordyceps cicadae. J. Ethnopharmacol. 2002;83:79–85. doi: 10.1016/s0378-8741(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Won S.Y., Park E.H. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J. Ethnopharmacol. 2005;96:555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wu T.-N., Yang K.-C., Wang C.-M., Lai J.-S., Ko K.-N., Chang P.-Y., Liou S.-H. Lead poisoning caused by contaminated Cordyceps, a Chinese herbal medicine: two case reports. Sci. Total Environ. 1996;182:193–195. doi: 10.1016/0048-9697(96)05054-1. [DOI] [PubMed] [Google Scholar]
- Wu Y., Sun C., Pan Y. Structural analysis of a neutral (1 → 3),(1 → 4)-β-d-glucan from the mycelia of Cordyceps sinensis. J. Nat. Prod. 2005;68:812–814. doi: 10.1021/np0496035. [DOI] [PubMed] [Google Scholar]
- Wojcikowski K., Johnson D.W., Gobé G. Medicinal herbal extracts – renal friend or foe? Part two: Herbal extracts with potential renal benefits. Nephrology. 2004;9:400–405. doi: 10.1111/j.1440-1797.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- Xiao J.H., Chen D.X., Liu J.W., Liu Z.L., Wan W.H., Fang N., Xiao Y., Qi Y., Liang Z.Q. Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. J. Appl. Microbiol. 2004;96:1105–1116. doi: 10.1111/j.1365-2672.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y.Q. Studies on chemical constituents of Cordyceps sinensis I. Zhong Yao Tong Bao (Beijing, China: 1981) 1983;8:32–33. [PubMed] [Google Scholar]
- Xu C.P., Kim S.W., Hwang H.J., Yun J.W. Application of statistically based experimental designs for the optimization of exo-polysaccharide production by Cordyceps militaris NG3. Biotechnol. Appl. Biochem. 2002;36:127–131. doi: 10.1042/ba20020032. [DOI] [PubMed] [Google Scholar]
- Xu R., Peng X.E., Chen G.Z., Chen G.L. Effects of Cordyceps sinensis on natural killer activity and colony formation of B16 melanoma. Chin. Med. J. 1992;105:97–101. [PubMed] [Google Scholar]
- Yamada H., Kawaguchi N., Ohmori T., Takeshita Y., Taneya S., Miyazaki T. Structure and antitumor activity of an alkali-soluble polysaccharide from Cordyceps ophioglossoides. Carbohydr. Res. 1984;125:107–115. doi: 10.1016/0008-6215(84)85146-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Yoshida J., Ren L.J., Chen H., Miyazawa Y., Fujii Y., Huang Y.X., Takamura S., Suzuki S., Koshimura S. Augmentation of various immune reactivities of tumor-bearing hosts with an extract of Cordyceps sinensis. Biotherapy. 1990;2:199–205. doi: 10.1007/BF02173520. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Kagota S., Nakamura K., Shinozuka K., Kunitomo M. Inhibitory effects of water extracts from fruiting bodies of cultured Cordyceps sinensis on raised serum lipid peroxide levels and aortic cholesterol deposition in atherosclerotic mice. Phytother. Res. 2000;14:650–652. doi: 10.1002/1099-1573(200012)14:8<650::aid-ptr675>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Kagota S., Nakamura K., Shinozuka K., Kunitomo M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother. Res. 2000;14:647–649. doi: 10.1002/1099-1573(200012)14:8<647::aid-ptr670>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Yang H.-Y., Leu S.-F., Wang Y.-K., Wu C.-S., Huang B.-M. Cordyceps sinensis mycelium induces MA-10 mouse leydig tumor cell apoptosis by activating the caspase-8 pathway and suppressing the NF-κB pathway. Arch. Androl. 2006;52:103–110. doi: 10.1080/01485010500315818. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang W., Shi P., Chen J., Han X., Wang Y. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathol. Res. Pract. 2005;201:745–750. doi: 10.1016/j.prp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Yang L.Y., Chen A., Kuo Y.C., Lin C.Y. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J. Lab. Clin. Med. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- Yang L.Y., Huang W.J., Hsieh H.G., Lin C.Y. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J. Lab. Clin. Med. 2003;141:74–83. doi: 10.1067/mlc.2003.6. [DOI] [PubMed] [Google Scholar]
- Yoo H.S., Shin J.W., Cho J.H., Son C.G., Lee Y.W., Park S.Y., Cho C.K. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol. Sin. 2004;25:657–665. [PubMed] [Google Scholar]
- Yoshida J., Takamura S., Yamaguchi N., Ren L.J., Chen H., Koshimura S., Suzuki S. Antitumor activity of an extract of Cordyceps sinensis (Berk.) Sacc. against murine tumor cell lines. Jpn. J. Exp. Med. 1989;59:157–161. [PubMed] [Google Scholar]
- Yoshikawa N., Nakamura K., Yamaguchi Y., Kagota S., Shinozuka K., Kunitomo M. Antitumour activity of cordycepin in mice. Clin. Exp. Pharmacol. Physiol. 2004;31:S51–S53. doi: 10.1111/j.1440-1681.2004.04108.x. [DOI] [PubMed] [Google Scholar]
- Young M.K., Su M.C., Jee H.K., Jae H.L., Yeon A.L., Seung J.L., Min W.L. Hypoglycemic effect of Cordyceps militaris. Korean J. Pharmacognosy. 2001;32:327–329. [Google Scholar]
- Yu K.W., Kim K.M., Suh H.J. Pharmacological activities of stromata of Cordyceps scarabaecola. Phytother. Res. 2003;17:244–249. doi: 10.1002/ptr.1119. [DOI] [PubMed] [Google Scholar]
- Yu R., Song L., Zhao Y., Bin W., Wang L., Zhang H., Wu Y., Ye W., Yao X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia. 2004;75:465–472. doi: 10.1016/j.fitote.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Yu R., Wang L., Zhang H., Zhou C., Zhao Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia. 2004;75:662–666. doi: 10.1016/j.fitote.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Yu R., Yang W., Song L., Yan C., Zhang Z., Zhao Y. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris. Carbohydr. Polym. 2007;70:430–436. [Google Scholar]
- Yu L., Zhao J., Zhu Q., Li S.P. Macrophage biospecific extraction and high performance liquid chromatography for hypothesis of immunological active components in Cordyceps sinensis. J. Pharm. Biomed. Anal. 2007;44:439–443. doi: 10.1016/j.jpba.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Yuan J-P., Wang J.-H., Liu X., Kuang H.-C., Zhao S.-Y. Simultaneous determination of free ergosterol and ergosteryl esters in Cordyceps sinensis by HPLC. Food Chem. 2007;105:1755–1759. [Google Scholar]
- Yun Y., Han S., Lee S., Ko S.K., Lee C.-K., Ha N.-J., Kim K. Anti-diabetic effects of CCCA, CMESS, and Cordycepin from Cordyceps militaris and the immune responses in streptozotocin-induced diabetic mice. Nat. Prod. Sci. 2003;9:291–298. [Google Scholar]
- Zhang L., Chen S.-Z., Liu S.-S. Prosecutable function of Cordyceps sinensis extracts for hepatic mitochondrial oxidative injuries in diabetic mice. Chin. J. Clin. Rehab. 2006;10:132–134. [Google Scholar]
- Zhang Q., Wu J., Hu Z., Li D. Induction of HL-60 apoptosis by ethyl acetate extract of Cordyceps sinensis fungal mycelium. Life Sci. 2004;75:2911–2919. doi: 10.1016/j.lfs.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Zhang S.L. Activation of murine peritoneal macrophages by natural Cordyceps sinensis and its cultured mycelia. Zhong Xi Yi Jie He Za Zhi. 1985;5:45–47. [PubMed] [Google Scholar]
- Zhang S.L. Lewis lung cancer of mice treated with Cordyceps sinensis and its artificial cultured mycelia. Zhong Yao Tong Bao (Beijing, China: 1981) 1987;12:53–54. [PubMed] [Google Scholar]
- Zhang, W., Li, J., Qiu, S., Chen, J., Zheng, Y., 2007a. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia. doi:10.1016/j.fitote.2007.09.001. [DOI] [PubMed]
- Zhang W., Yang J., Chen J., Hou Y., Han X. Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumour-bearing mice. Biotechnol. Appl. Biochem. 2005;42:9–15. doi: 10.1042/BA20040183. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu Y.K., Shen W., Shen D.M. Dynamical influence of Cordyceps sinensis on the activity of hepatic insulinase of experimental liver cirrhosis. Hepatob. Pancreat. Dis. Int. 2004;3:99–101. [PubMed] [Google Scholar]
- Zhang X., Liu Y.K., Zheng Q., Shen W., Shen D.M. Influence of Cordyceps sinensis on pancreatic islet beta cells in rats with experimental liver fibrogenesis. Zhonghua Gan Zang Bing Za Zhi. 2003;11:93–94. [PubMed] [Google Scholar]
- Zhang Y., Liu S., Che Y., Liu X. Epicoccins A–D, pipolythiodioxopiperazines from a Cordyceps-colonizing isolate of Epicoccum nigrum. J. Nat. Prod. 2007;70:1522–1525. doi: 10.1021/np070239u. [DOI] [PubMed] [Google Scholar]
- Zhang Z.J., Luo H.L., Li J.S. Clinical and experimental studies on elimination of oxygen free radical of jinshuibao capsule in treating senile deficiency syndrome and its deoxyribonucleic acid damage repairing effects. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:35–38. [PubMed] [Google Scholar]
- Zhang Z., Xia S.S. Cordyceps sinensis –I as an immunosuppressant in heterotopic heart allograft model in rats. J. Tongji Med. Chem. 1990;10:100–103. doi: 10.1007/BF02887870. [DOI] [PubMed] [Google Scholar]
- Zhao C.S., Yin W.T., Wang J.Y., Zhang Y., Yu H., Cooper R., Smidt C., Zhu J.S. CordyMax Cs-4 improves glucose metabolism and increases insulin sensitivity in normal rats. J. Altern. Complement. Med. 2002;8:309–314. doi: 10.1089/10755530260127998. [DOI] [PubMed] [Google Scholar]
- Zhao-Long W., Xiao-Xia W., Wei-Ying C. Inhibitory effect of Cordyceps sinensis and Cordyceps militaris on human glomerular mesangial cell proliferation induced by native LDL. Cell Biochem. Funct. 2000;18:93–97. doi: 10.1002/(SICI)1099-0844(200006)18:2<93::AID-CBF854>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhao M., Chen X.-M., Sun L., Feng Y., Ye S.-D. Investigation on distribution and habitat of Blaps rynchopetera fairmaire (Coleoptera: Tenebrionidae) in Yunnan. Forest Res. 2007;20:356–362. [Google Scholar]
- Zhao X., Li L. Cordyceps sinensis in protection of the kidney from cyclosporine A nephrotoxicity. Zhonghua Yi Xue Za Zhi. 1993;73:410–412. [PubMed] [Google Scholar]
- Zhao Y. Inhibitory effects of alcoholic extract of Cordyceps sinensis on abdominal aortic thrombus formation in rabbits. Zhonghua Yi Xue Za Zhi. 1991;71:612–615. [PubMed] [Google Scholar]
- Zhen F., Tian J., Li L.S. Mechanisms and therapeutic effect of Cordyceps sinensis (CS) on aminoglycoside induced acute renal failure (ARF) in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:288–291. [PubMed] [Google Scholar]
- Zhou D.H., Lin L.Z. Effect of Jinshuibao capsule on the immunological function of 36 patients with advanced cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:476–478. [PubMed] [Google Scholar]
- Zhou L., Yang W., Xu Y., Zhu Q., Ma Z., Zhu T., Ge X., Gao J. Short-term curative effect of cultured Cordyceps sinensis (Berk.) Sacc. Mycelia in chronic hepatitis B. China J. Chin. Mater. Med. 1990;15:53–55. [PubMed] [Google Scholar]
- Zhu J.L., Liu C. Modulating effects of extractum semen Persicae and cultivated Cordyceps hyphae on immuno-dysfunction of inpatients with posthepatitic cirrhosis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:207–209. [PubMed] [Google Scholar]
- Zhu J.-S., Halpern G.M., Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis Part II. J. Altern. Complement. Med. 1998;4:429–457. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- Zhu J.-S., Halpern G.M., Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis Part I. J. Altern. Complement. Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- Zhu X.Y., Yu H.Y. Immunosuppressive effect of cultured Cordyceps sinensis on cellular immune response. Zhong Xi Yi Jie He Za Zhi. 1990;10:485–487. [PubMed] [Google Scholar]


