Abstract
Mannose binding lectin (MBL) is a collagenous C-type lectin, which plays an important role in innate immunity. It can bind to carbohydrates on the surface of a wide range of pathogens, including viruses. Here we studied the antiviral effect of recombinant chicken (rc)MBL against Infectious Bronchitis Virus (IBV), a highly contagious coronavirus of chicken. rcMBL inhibited in a dose-dependent manner the infection of BHK-21 cells by IBV-Beaudette, as detected by immunofluorescence staining of viral proteins and qPCR. ELISA and negative staining electron microscopy showed that rcMBL bound directly to IBV, resulting in the aggregation of viral particles. Furthermore, we demonstrated that MBL bound specifically to the spike S1 protein of IBV which mediates viral attachment. This subsequently blocked the attachment of S1 to IBV-susceptible cells in chicken tracheal tissues as shown in protein histochemistry. Taken together, rcMBL exhibits antiviral activity against IBV, based on a direct interaction with IBV virions.
Keywords: Mannose binding lectin, Infectious bronchitis virus, Antiviral, Chicken, Innate immunity
Highlights
-
•
Recombinant chicken MBL (rcMBL) has antiviral activity against IBV-Beaudette in cell culture.
-
•
rcMBL aggregates IBV virus-particles.
-
•
rcMBL binds through its carbohydrate recognition domain to the S1 spike protein of IBV.
-
•
rcMBL can prevent binding of IBV-S1 to chicken tracheal tissues.
1. Introduction
Avian infectious bronchitis (IB) is a highly contagious disease caused by infectious bronchitis virus (IBV), a coronavirus of domestic fowl. IB is a major problem in the poultry industry, causing worldwide economic losses through decreased egg production and quality. Although the primary target of IBV is the epithelial surface of the respiratory tract, the virus also replicates in other organs, including the kidney, gastro-intestinal, urinary and reproductive tracts depending on the viral strain (Cook et al., 2012). The most common symptoms of IB are related to the respiratory tract, including gasping, coughing, sneezing, tracheal rales and nasal discharge (Jackwood and de Wit, 2013).
IBV is an enveloped virus containing a single stranded positive sense RNA genome that encodes four major structural proteins: the spike (S) protein, the envelope (E) protein, the membrane (M) protein and the nucleocapsid (N). The S protein is the major viral attachment protein and is essential for both binding to the receptor on the target cell, and for subsequent virus-cell fusion that results in release of the viral genome into the cell. It is the largest structural protein of coronaviruses, forming club-shaped projections on the surface of the virus membrane (Masters and Perlman, 2013), and is heavily N-glycosylated with approximately 30 potential glycosylation sites (Binns et al., 1985). It is post-translationally cleaved into S1 and S2 subunits of approximately 500 and 600 amino acids, respectively (Cavanagh et al., 1986). The role of the spike in determining the virus’ tropism and pathogenesis has recently been summarized (Wickramasinghe et al., 2014).
Mannose binding lectin (MBL) is a key pattern-recognition molecule in innate immunity belonging to the family of collagenous C-type lectins, the so-called 'collectins'. MBL is synthesized in the liver, is mainly present in blood serum and it plays an important role in the first line of host defense by binding to carbohydrates on the surface of a wide range of pathogens, such as bacteria, viruses, fungi and protozoa. This interaction is mediated through the C-terminal carbohydrate recognition domain (CRD) of MBL, which depends on the presence of calcium ions that facilitate interaction with the hydroxyl groups of the glycan ligand in the binding pocket of the CRDs of MBL (Veldhuizen et al., 2011). Binding of pathogens leads to direct neutralization of the microorganism or activation of the complement system through MBL-associated serine proteases (MASPs) (Dommett et al., 2006, Ip et al., 2009). MBL deficiency increases the susceptibility to various infectious diseases and autoimmune, metabolic and cardiovascular disorders (Eisen, 2010, Turner and Hamvas, 2000), underlining the multifunctional role of the protein.
Recent evidence showed that chicken mannose binding lectin (cMBL) plays a role in immunity against both viral and bacterial pathogens in chickens (Kjaerup et al., 2014b, Norup et al., 2009, Ulrich-Lynge et al., 2015, Ulrich-Lynge et al., 2016). Chicken MBL seems to be important for shaping both the innate and the adaptive immune response for IBV (Hamzic et al., 2016, Juul-Madsen et al., 2011, Kjaerup et al., 2014b). Chickens with relatively high concentrations of cMBL appeared to clear IBV quicker and showed reduced viral titers in trachea compared to chickens with low cMBL levels (Juul-Madsen et al., 2011, Kjaerup et al., 2014a).
This study demonstrates that cMBL has direct antiviral activity towards IBV. Previously, we have generated recombinant chicken MBL (rcMBL) with similar biochemical properties as those of native chicken MBL (Zhang et al., 2016b). Using rcMBL we show that cMBL binds to IBV through its CRD in a Ca2+-dependent manner, leading to viral aggregation and reduction of virus infection. Furthermore, we demonstrate that this binding is mediated by the interaction between cMBL and the S1 spike protein of IBV, thereby reducing the binding of the major viral attachment protein to tissues.
2. Materials and methods
2.1. Cell lines
Human Embryonic Kidney (HEK) 293 T cells, Baby Hamster Kidney fibroblasts (BHK) 21 cells (ATCC CCL-10), and Human Cervical adenocarcinoma (HeLa) R19 cells were maintained in Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% Fetal Calf Serum (FCS) (Bodinco BV, The Netherlands), containing penicillin (100 units/ml) and streptomycin (100 µg/ml) (Gibco). All cells were grown at 37 °C in 5% CO2.
2.2. Propagation and purification of IBV
IBV-Beaudette and IBV-M41 were kindly provided by the Animal Health Service, Deventer, the Netherlands. IBV-Beaudette was grown and titrated in BHK-21 cells. IBV-M41 was propagated in 10-day-old specific pathogen free embryonated chicken eggs (Animal Health Service, Deventer, the Netherlands) for 48 h at 37 °C, after which the allantoic fluid containing IBV-M41 was collected. IBV-Beaudette and M41 were purified on a 20% (w/v) sucrose cushion by centrifugation at 75.000×g for 2.5 h. The viral pellet was resuspended in PBS, the concentration of viral proteins was measured by the BCA protein assay (Thermo Scientific, USA) and aliquots were stored at −80 °C.
2.3. Genes and expression vectors
The ORF encoding cMBL (GenBank Accession No. AF231714) was cloned into the expression vector pFRT (Thermo Scientific, USA) (Zhang et al., 2016b). The S1-coding domain of IBV-M41 (GenBank Accession No. AY851295) in the pCD5 expression vector as described previously (Wickramasinghe et al., 2011).
2.4. Production of recombinant proteins
rcMBL was expressed and purified as previously described (Zhang et al., 2016b). In brief, the pFRT vector containing the cMBL gene was transfected into HeLa-R19 cells and the culture supernatant was harvested at 5 days post-transfection. rcMBL was purified by affinity chromatography using mannan-coated beads, followed by gel filtration using an ÄKTA purifier10 system (GE Healthcare Bio Sciences, Sweden), that was equipped with a Hiload 16/60 Superdex 200 PREP GRADE column. Recombinant S1 protein (M41 S1) was expressed as described previously (Wickramasinghe et al., 2011).
2.5. Inhibition of the infectivity of IBV-beaudette by MBL
BHK-21 cells were seeded in 24-well plates at 200,000 cells per well. The next day, the cells were washed twice with PBS and subsequently inoculated with IBV-Beaudette at a multiplicity of infection (MOI) of 0.1 in the presence of various concentrations of rcMBL (ranging from 0.001 µg/ml to 10 µg/ml) in DMEM. The supernatant was removed at 2 h post-inoculation, cells were washed twice with PBS and fresh culture medium was added. At 8 h, the cells were washed twice with PBS and lysed for RNA isolation or fixed for immunofluorescent staining. For studies using M41 S1 as an inhibitor of rcMBL's activity, 10 µg/ml rcMBL was preincubated with 50 µg/ml M41 S1 for 45 min before addition to BHK-21 cells.
2.6. Cytotoxicity
Cytotoxicity was determined using the WST-1 assay which measures cell viability based on glycolytic production of NAD(P)H. WST-1 reagent was obtained from Roche (Basel, Switzerland). BHK-21 cells were incubated with rcMBL for 8 h, after which media was removed and replaced with 10% WST-1 reagent in culture medium. After 20 min, absorbance was measured at 450 nm with a FLUOstar Omega microplate reader (BMG Labtech GmbH, Ortenberg, Germany) and was corrected for absorbance at 630 nm. Non-treated control cells were defined as 100% mitochondrial activity.
2.7. Real-time PCR for quantification of viral RNA synthesis
Total RNA was extracted from IBV-infected cells using the High Pure RNA Tissue kit (Roche, Germany). Subsequently, cDNA was generated using the iScript cDNA Synthesis Kit (BIO-RAD, USA) and quantification of viral gene expression was performed on the BIO-RAD CFX Connect system, using the iQ SYBR Green Supermix kit (BIO-RAD). For detection of genomic IBV-Beaudette, primers targeting ORF1a/b were used: forward: 5’- CATGCAGTTTGTTGGAGATCCT-3’ and reverse 5’-GTGACCTGGTTTTACCGTTTGA-3’ (van Beurden et al., 2017). GAPDH gene expression was used for normalization purposes and detected using the specific primers: forward: 5’- CCATGGAGAAGGCTGGGG-3’ and reverse 5’-CAAAGTTGTCATGGATGACC-3’. The qPCR reaction was performed as follows: 3 min at 95 °C, 40 cycles of 30 s at 95 °C and 30 s at 60 °C. For each sample, reactions were performed in duplicate. Data were collected from three independent experiments. The relative quantitation of IBV gene expression was determined by comparison to GAPDH.
2.8. Immunofluorescent staining
BHK-21 cells were cultured on 12 mm coverslips in 24-well plates and inoculation with IBV and rcMBL was performed as described above. At 8 h, the cells were fixed with 4% paraformaldehyde in PBS for 20 min and permeabilized with 0.1% Triton X-100 for 5 min. The cells were blocked with 5% normal goat serum for 30 min at room temperature, followed by incubation with primary antibody mAb 48.4 anti-nucleocapsid (1:100, for 1 h) (Prionics #7500891) and secondary antibody Alexa-488 anti-mouse IgG (1: 100, for 45 min) (Thermo Scientific). DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, Sigma-Aldrich) was used to stain the cell nuclei at a concentration of 300 nM in PBS. Coverslips were mounted on a microscope slide and viewed with an Olympus BX60 fluorescent microscope at a magnification of 100 x. Representative images were obtained and processed using the Leica LAS-AF software.
2.9. Binding of IBVs and spike protein S1 to rcMBL
Ninety-six well flat bottom polystyrene plates (Nunc maxisorp, Denmark) were coated with 1 µg of total viral proteins of IBV-Beaudette or IBV-M41, or purified recombinant M41-S1 in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6). Wells coated with 3% (w/v) bovine serum albumin (BSA, Sigma-Aldrich, USA) were used as controls. After overnight coating at 4 °C, the plates were washed three times with 200 µl TBS/Tween buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.05% (v/v) Tween 20) and blocked for 1 h at RT with 100 µl 3% BSA in TBS/Tween buffer. The plates were washed three times with TBS/Tween and incubated with various concentrations of rcMBL for 2 h in TBS (from 10 µg/ml in two-fold dilutions, 100 µl/well) in the presence of 5 mM Ca2+. Next, the plates were washed with TBS/Tween containing 5 mM Ca2+ and incubated with primary antibody for 2 h (mouse anti-chicken MBL monoclonal antibody, 1: 4000 dilution, 6B11, Thermo scientific) and secondary antibody for 45 min (goat anti-mouse IgG-Peroxidase antibody, 1: 50,000 dilution, Sigma-Aldrich) in TBS/Tween containing 1% BSA. Bound antibody was detected by use of 100 µl tetramethylbenzidine (TMB) substrate solution (Invitrogen, USA), the reaction was terminated with 50 µl 2 N H2SO4 and the absorbance was measured at 450 nm. As a control, rcMBL was incubated in the presence of either EDTA (10 mM) or mannan (500 µg/ml) (from Saccharomyces cerevisiae, Sigma-Aldrich, USA).
2.10. Electron microscopy
Fifty microliter of IBV-Beaudette suspension (800 µg/ml) was incubated with various concentrations of rcMBL in PBS with Ca2+ and Mg2+ for 1 h at RT. The sample was then fixed by adding an equal volume of fixing buffer (4% glutaraldehyde (Polyscience), 5 mM CaCl2, 10 mM MgCl2, in 0.1 M Na-cacodylate buffer (Sigma-Aldrich)) and incubated at 4 °C overnight. Next, fixed samples were adhered to carbon coated grids and stained with 1% ammonium molybdate (pH=6), washed and dried for 1 h. Imaging was done using a FEI Tecnai 12 electron microscope at 80 kV. Representative images were taken after observation of more than 30 fields per sample. The size of aggregates was measured using Image J/Fiji software, analyzing over 30 fields per condition.
2.11. Spike histochemistry
Binding of IBV-S1 protein to chicken tracheal epithelium and the effect of rcMBL on IBV-S1 binding was determined by spike histochemistry as published previously (Wickramasinghe et al., 2011). Representative pictures were captured using a CCD camera Olympus BX41 microscope with Cell^B imaging software (Soft Imaging Solution GmbH).
2.12. Statistics
Data were analyzed using SPSS version 16.0 software (SPSS inc., Chicago, IL) with one-way analysis of variance (ANOVA) and Dunnett post hoc tests. Significant differences were defined as p < 0.05.
3. Results
3.1. rcMBL inhibits the infectivity of IBV-Beaudette
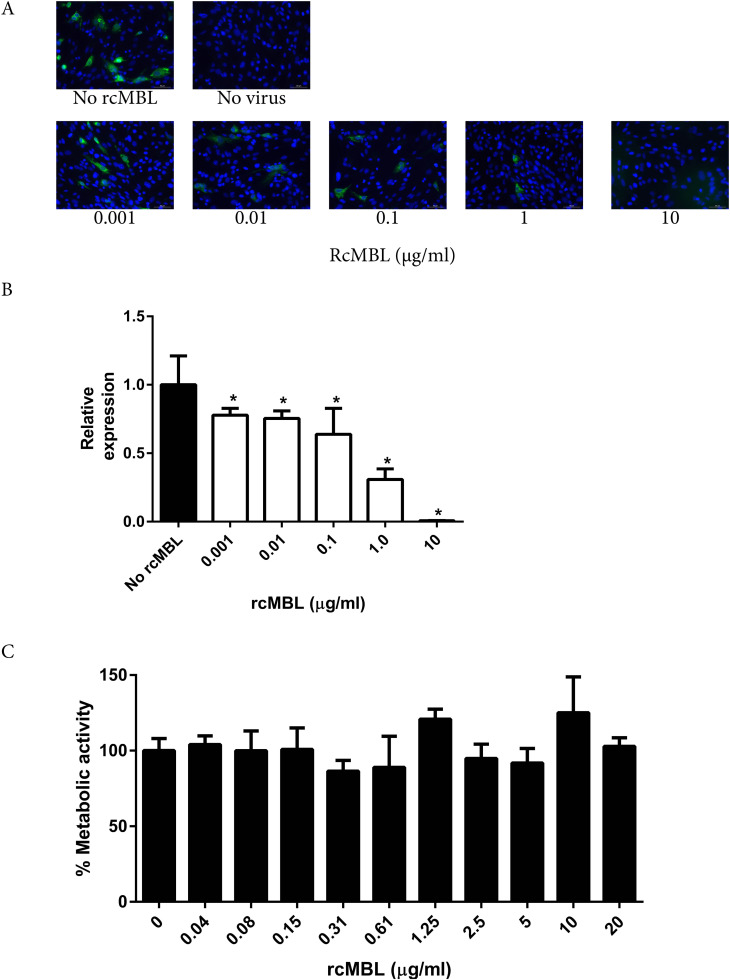
To study whether rcMBL can block the infection of IBV in vitro, BHK-21 cells were inoculated with the cell culture adapted IBV strain Beaudette in the presence of various concentrations of rcMBL. At 8 h post-inoculation, corresponding to one infection cycle of the virus, the infected cells were used for qualitative and quantitative determination of infection. Immunofluorescence staining of the IBV nucleocapsid protein was performed to visualize infected cells, as shown in Fig. 1A. Approximately 10% of the cells was infected when no rcMBL was added, corresponding to the MOI used. Increasing amounts of rcMBL resulted in decreased numbers of IBV-positive BHK cells, indicating that rcMBL reduces IBV infectivity. Addition of heat inactivated rcMBL (20 min, 65 °C) had no effect on infectivity (data not shown). In parallel, the infectivity of IBV was quantitatively determined by use of real-time PCR. The results showed that IBV infectivity was blocked by rcMBL in a concentration-dependent manner (Fig. 1B). Significant reduction was already observed at low rcMBL concentrations, while 10 µg/ml rcMBL almost completely prevented infection by IBV-Beaudette. Incubation of BHK-21 cells with only rcMBL did not result in cytotoxic effects as determined by WST-1 assay (Fig. 1C).
Fig. 1.
rcMBL inhibits IBV-Beaudette infection of BHK 21 cells in a concentration dependent manner. BHK-21 cells were inoculated after pre-incubation of IBV-Beaudette (MOI of 0.1) with various concentrations of rcMBL. A) Infection was determined by immunofluorescence using an antibody directed against the IBV nucleocapsid protein at 8 h post-infection. Bar: 50 µm. B) Infection was determined by quantitative PCR: the IBV viral genome expression relative to GAPDH gene expression was determined and expressed relative to the no-rcMBL control. C) The toxic effects of rcMBL on BHK-21 cells was determined by the WST-1 assay. Asterisks indicate statistical significant difference compared to the no rcMBL control. Shown are mean ± SEM of three independent experiments.
3.2. Binding of rcMBL to IBV
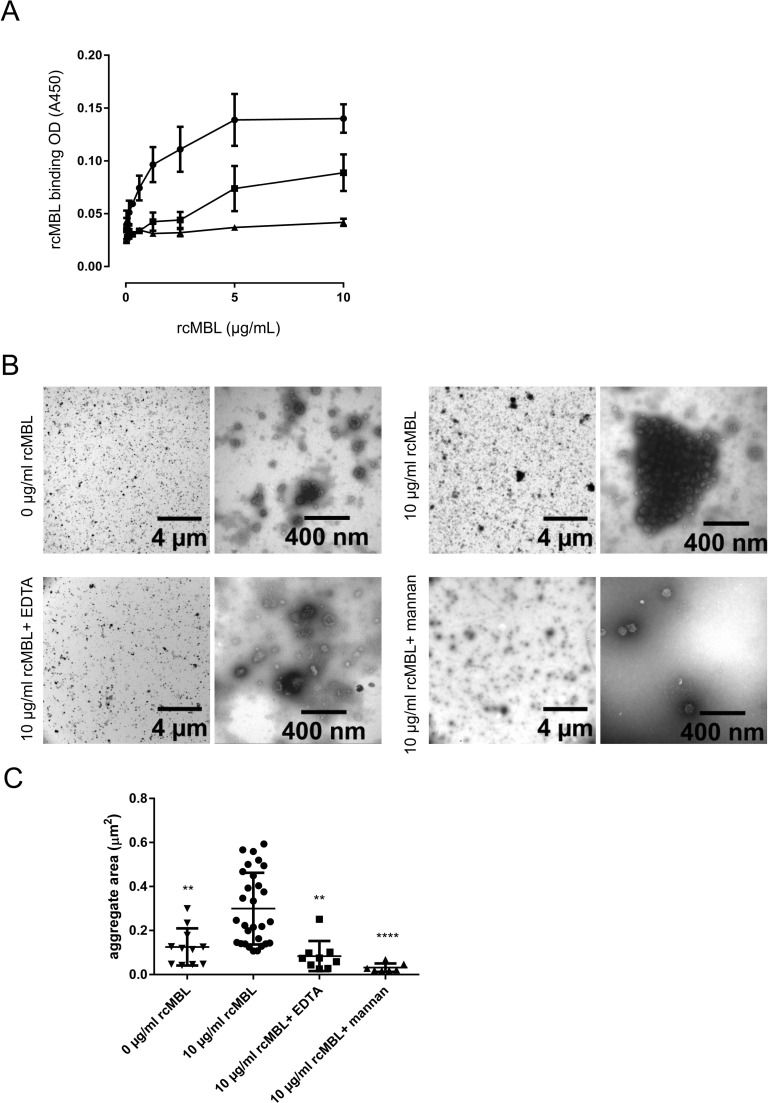
As lectins are known to bind to carbohydrates on the surface of pathogens, we studied whether there was a direct interaction between IBV and rcMBL. To this end, we developed an enzyme-linked immunosorbent assay (ELISA) in which IBV-Beaudette was coated and incubated with rcMBL in the presence of 5 mM Ca2+. As shown in Fig. 2A, rcMBL bound to viral antigens in a concentration dependent manner. The binding was completely lost in the presence of EDTA, indicating that cMBL binds IBV through its Ca2+-dependent CRD domain. Mannan, as a competitive inhibitor for MBL binding, reduced the binding of rcMBL to IBV significantly, confirming that rcMBL-binding to IBV is mediated through the CRD.
Fig. 2.
Binding of rcMBL to IBV. A) Binding of rcMBL to IBV-Beaudette was determined using ELISA. Virus particles were coated on a 96-wells plate and incubated with rcMBL. Bound rcMBL was detected using specific anti-cMBL antibodies. Shown are mean ± SEM of three independent experiments. Circles: binding in the presence of Ca2+. Squares: binding in the presence of Ca2+and 100 μg mannan. Triangles: binding in the presence of Ca2+ and EDTA. B) Negative staining electron microscopy of IBV-Beaudette aggregates upon incubation with rcMBL in the presence of Ca2+ ± EDTA or mannan. C) Quantification of viral aggregate size upon incubation with rcMBL (based on 30 fields per sample) in the presence or Ca2+ ± EDTA or mannan.
3.3. Aggregation of IBV by rcMBL
To further elucidate the mode of action of rcMBL, negative staining electron microscopy was performed on IBV-Beaudette after incubation with rcMBL. Large aggregates were observed in the presence of 10 µg/ml (Fig. 2B) as well as 1 µg/ml (data not shown) rcMBL. The observed aggregates had an average diameter of approximately 0.3 µm2 in the presence of rcMBL (Fig. 2C). No or significantly smaller aggregates (composed of 2–4 viral particles) could be detected in the absence of MBL, or when EDTA or mannan was added during incubation with rcMBL. The lack of large aggregates in the presence of EDTA or mannan suggests that aggregation of viral particles is mediated by the CRD of rcMBL, in line with binding data obtained with ELISA.
3.4. Binding of rcMBL to IBV-S1 protein
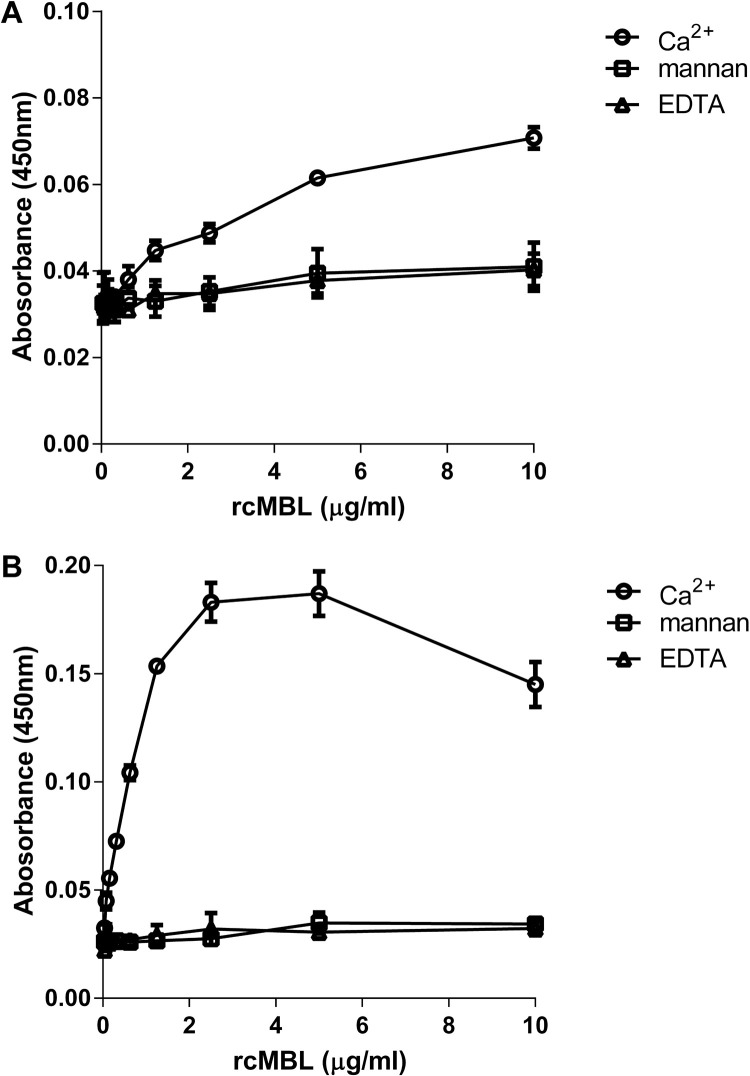
As IBV-Beaudette is a cell culture adapted strain, unable to cause disease in chickens (Geilhausen et al., 1973), we next sought to confirm the relevance of our findings using IBV-M41, a virulent prototype Massachusetts IBV field strain. ELISA, using purified total viral proteins of IBV-M41 ( Fig. 3A), showed that rcMBL also bound to IBV-M41 in a concentration-dependent manner, similar as observed for IBV-Beaudette (Fig. 2A). Binding of IBV-M41 to rcMBL could be blocked upon addition of EDTA and mannan, indicating that the binding is Ca2+-dependent and relies on the interaction of the CRD of MBL with any of the viral proteins. As the spike (S) is the major viral attachment protein of IBV, protrudes from the viral surface, and is heavily glycosylated, we tested whether MBL exhibited its mode of action through direct CRD-mediated interactions with glycans present on the spike. To this end, an ELISA was developed coating recombinantly produced M41-S1. The results show that rcMBL bound in a concentration and calcium ion dependent manner to M41-S1 (Fig. 3B).
Fig. 3.
Calcium-dependent binding of rcMBL to IBV M41 and S1 spike protein. ELISA of rcMBL binding to A) IBV M41, and B) recombinant M41 S1 protein. Shown are mean ± SEM of three independent experiments. Circles: binding in the presence of 5 mM Ca2+. Squares: binding in the presence of 5 mM Ca2+ and 100 μg mannan. Triangles: binding in the presence of 5 mM Ca2+ and 10 mM EDTA.
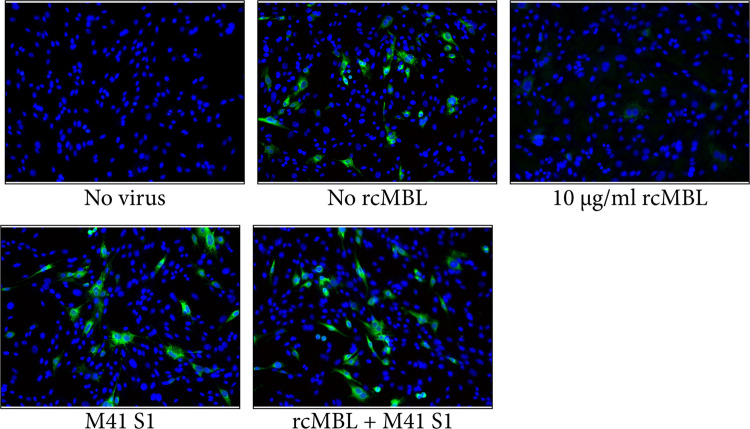
In order to determine if M41 S1 could inhibit rcMBL's antiviral activity, BHK-21 cells were infected with IBV-Beaduette in the presence of rcMBL preincubated with M41 S1. The spike protein could indeed block the activity of rcMBL leading to infection of BHK-21 cells comparable to control infected cells ( Fig. 4). The presence of M41 S1 alone had no effect on the infection indicating that this effect was not due to M41 S1 blocking of IBV receptors on BHK-21 cells.
Fig. 4.
IBV S1 inhibits rcMBLs antiviral activity. BHK-21 cells were infected with IBV Beaudette at an MOI of 0.1 in the presence or absence of 10 μg/ml rcMBL and 50 μg/ml M41 S1. Infection was determined by immunofluorescence using an antibody directed against the IBV nucleocapsid protein at 8 h post-infection.
3.5. Blocking of spike protein binding to tracheal tissue
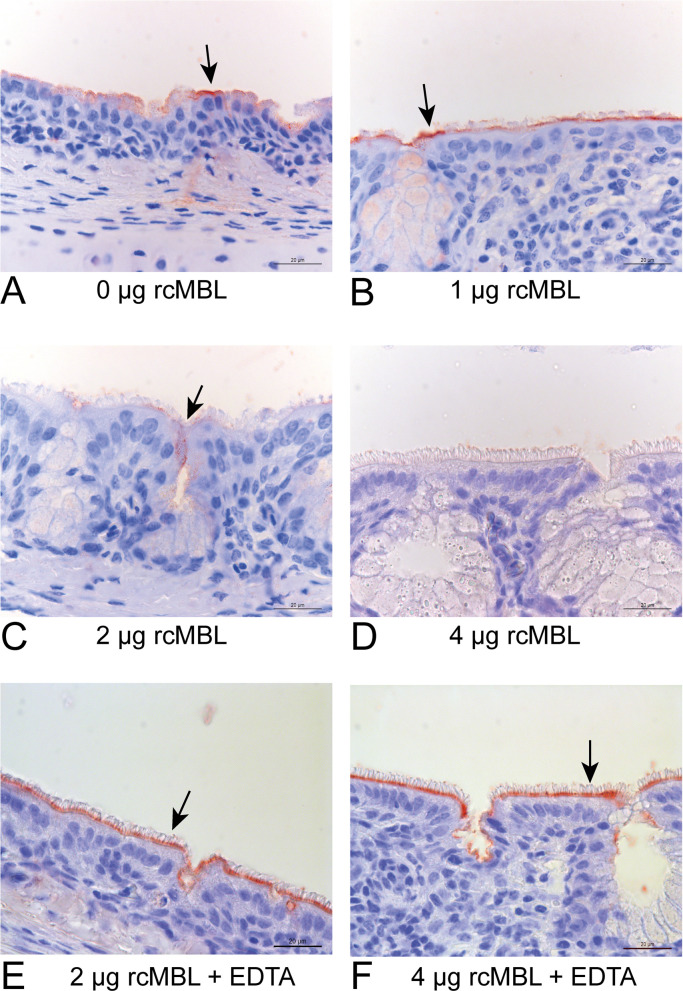
Finally, the biological relevance of the direct interaction between S1 and MBL was determined using an immunostaining technique on thin slices of avian tissue. Previously we have shown that M41 S1 specifically binds to the epithelial lining of the chicken trachea using this technique, representing the cells that are susceptible for IBV in vivo (Wickramasinghe et al., 2014). The ability of rcMBL to block binding of M41 S1 protein to chicken tracheal tissue was determined by pre-incubating recombinant S1 with various concentrations of rcMBL and subsequently adding it to the tracheal tissue. S1 showed strong binding to the epithelium layer of the chicken trachea when rcMBL was not present ( Fig. 5A). In the presence of MBL increased blocking of S1 binding to chicken trachea was observed (Fig. 5B and C) with a complete block at 4 µg rcMBL (Fig. 5D). Addition of EDTA prevented this inhibition of S1 binding to the tissue (Fig. 5E and F).
Fig. 5.
rcMBL blocks binding of IBV-S1 to chicken tracheal tissue. Binding of S1 spike protein to tracheal tissue of chicken as detected by spike immune histochemistry. Spike protein was incubated in the presence of different amounts of rcMBL, A) 0 μg, B) 1 μg, C) 2 μg, D) 4 μg; E) and F) incubation of 2 and 4 μg rcMBL in the presence of EDTA. Binding is indicated by arrow heads. Bar: 20 µm.
4. Discussion
In this study, we demonstrated that rcMBL has antiviral activity against IBV through direct interaction with viral particles, with subsequent inhibition of infection. rcMBL binds through its CRD to the S1 protein of IBV, thereby blocking the binding of the viral attachment protein to the surface of susceptible cells in the chicken trachea.
The results described in this study show the antiviral ability of chicken MBL against IBV and give insights into its mechanism of action. rcMBL acts by direct binding the viral glycoprotein spike through its CRD, thereby aggregating virus particles. For mammalian MBL, direct virus neutralization through interaction with viral glycoproteins, thereby blocking virus binding to receptors on host cells, has been described before (Brown et al., 2010, Ip et al., 2005, Ji et al., 2005, Zhou et al., 2010). Here, we observed that rcMBL binds two IBV strains, IBV-Beaudette and IBV-M41, in line with a previous observation with an unspecified IBV strain (Kjaerup et al., 2014a). As spike proteins of all other IBV strains are, while diverse in sequence, heavily glycosylated (with approximately 30 predicted N-linked glycosylation sites), it is expected that rcMBLs antiviral activity extends beyond IBV geno- or serotypes. Other lectins have also been reported to act during the initial stages of the infection of coronaviruses. Interestingly, while the red algae lectin griffithsin interacts directly with the spike protein of MERS-CoV (Millet et al., 2016), the same lectin showed post-binding antiviral activity during entry of many different coronaviruses in cell culture cells (O'Keefe et al., 2010).
IBV primarily targets to the epithelium of upper respiratory tract of chickens, while cMBL, in analogy with mammals, is mainly produced in the liver, and secreted in blood (Medzhitov and Janeway, 2000). However, gene expression of MBL has been detected in the trachea and air sacs of healthy chicken with very low amounts in the lung itself (Hogenkamp et al., 2006, Zhang et al., 2016a). In addition, no evidence is available yet that MBL protein is locally produced in the trachea of healthy chickens. Therefore, it is questionable whether MBL can exert its innate host defense activity towards IBV directly at the side of entry of the host. It might be that the production of cMBL is upregulated locally as a result of IBV infection, as has been observed for infectious laryngotracheitis virus and infectious bursal disease virus in chickens (Nielsen et al., 1998). Similar observations have been done in mice, where MBL was present in bronchoalveolar lavage fluid of influenza A virus (IAV-H1N1) infected, but not in that of healthy mice (Reading et al., 1997). Indeed, after IBV infection an increase in cMBL gene expression was measured in the trachea of chicken (Kjaerup et al., 2014b), but whether this leads to an increased cMBL production and secretion needs to be confirmed. Interestingly, serum cMBL showed an acute response during IBV infection with an approximately two-fold increase in concentration (Juul-Madsen et al., 2011, Nielsen et al., 1998, Nielsen et al., 1999). As transport of IBV from the primary site of infection to other target organs has been suggested to occur via viremia (McFerran and McNulty, 1993, Reddy et al., 2016), it might well be that MBL is able to interact directly with IBV in the blood during transport to other target organs.
In addition to the here described direct inhibition of infectivity of IBV, cMBL might also have other functions in limiting IBV disease in chickens. MBL can act as an opsonin, where binding to virions can increase phagocytosis of MBL-coated viral particles, as has been shown for several viruses, including IAV (Hartshorn et al., 1993, Hartshorn et al., 1997), but not yet for IBV. In addition, MBL might enhance viral neutralization in vivo through the activation of complement via the lectin pathway, leading to enhanced influx of innate immune cells (reviewed in (Dunkelberger and Song, 2010)). The complement activation in chickens appeared to be correlated with the cMBL concentration in serum (Juul-Madsen et al., 2003) suggesting that opsonization via complement might play a role to reduce virus titers. Indeed, reduced viral loads were observed in trachea in chickens with high MBL serum concentrations compared to those having low MBL (Kjaerup et al., 2014b). However, as many other genes are differentially expressed between the high and low MBL producing chicken breeds (Hamzic et al., 2016) the contribution of other factors cannot be ruled out.
Our observations are in line with the extensive literature available on mammalian MBL where protective effects have been described for various viral infections, such as IAV, human immunodeficiency virus (HIV), hepatitis C virus (HCV) and severe acute respiratory syndrome coronavirus (SARS-CoV) (reviewed in (Mason and Tarr, 2015)). Future studies will reveal whether other avian viruses are also subject to virus neutralization by cMBL.
Acknowledgements
The authors would like to thank Iresha Ambepitiya Wickramasinghe for help with spike histochemistry experiments; Alinda Berends and Geert de Vrieze for technical assistance in the lab, and Maarten Coorens for helping with the experimental design. This work was supported by a personal fellowship from the China Scholarship Council (CSC) to Weidong Zhang.
References
- Binns M.M., Boursnell M.E., Cavanagh D., Pappin D.J., Brown T.D. Cloning and sequencing of the gene encoding the spike protein of the coronavirus IBV. J. Gen. Virol. 1985;66:719–726. doi: 10.1099/0022-1317-66-4-719. [DOI] [PubMed] [Google Scholar]
- Brown K.S., Keogh M.J., Owsianka A.M., Adair R., Patel A.H., Arnold J.N., Ball J.K., Sim R.B., Tarr A.W., Hickling T.P. Specific interaction of hepatitis C virus glycoproteins with mannan binding lectin inhibits virus entry. Protein Cell. 2010;1:664–674. doi: 10.1007/s13238-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Pappin D.J., Binns M.M., Boursnell M.E., Brown T.D. Coronavirus IBV: partial amino terminal sequencing of spike polypeptide S2 identifies the sequence Arg-Arg-Phe-Arg-Arg at the cleavage site of the spike precursor propolypeptide of IBV strains Beaudette and M41. Virus Res. 1986;4:133–143. doi: 10.1016/0168-1702(86)90037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Dommett R.M., Klein N., Turner M.W. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Eisen D.P. Mannose-binding lectin deficiency and respiratory tract infection. J. Innate Immun. 2010;2:114–122. doi: 10.1159/000228159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilhausen H.E., Ligon F.B., Lukert P.D. The pathogenesis of virulent and avirulent avian infectious bronchitis virus. Arch. Gesamt. Virusforsch. 1973;40:285–290. doi: 10.1007/BF01242547. [DOI] [PubMed] [Google Scholar]
- Hamzic E., Kjaerup R.B., Mach N., Minozzi G., Strozzi F., Gualdi V., Williams J.L., Chen J., Wattrang E., Buitenhuis B., Juul-Madsen H.R., Dalgaard T.S. RNA sequencing-based analysis of the spleen transcriptome following infectious bronchitis virus infection of chickens selected for different mannose-binding lectin serum concentrations. BMC Genom. 2016;17:82–016-2403-1. doi: 10.1186/s12864-016-2403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K.L., Sastry K., White M.R., Anders E.M., Super M., Ezekowitz R.A., Tauber A.I. Human mannose-binding protein functions as an opsonin for influenza A viruses. J. Clin. Investig. 1993;91:1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K.L., White M.R., Shepherd V., Reid K., Jensenius J.C., Crouch E.C. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am. J. Physiol. 1997;273:L1156–L1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- Hogenkamp A., van Eijk M., van Dijk A., van Asten A.J., Veldhuizen E.J.A., Haagsman H.P. Characterization and expression sites of newly identified chicken collectins. Mol. Immunol. 2006;43:1604–1616. doi: 10.1016/j.molimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W.K., Takahashi K., Ezekowitz R.A., Stuart L.M. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., de Wit S. Infectious bronchitis. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V.L., editors. Diseases of Poultry. Wiley-Blackwell; 2013. [Google Scholar]
- Ji X., Olinger G.G., Aris S., Chen Y., Gewurz H., Spear G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Munch M., Handberg K.J., Sørensen P., Johnson A.A., Norup L.R., Jørgensen P.H. Serum levels of mannan-binding lectin in chickens prior to and during experimental infection with avian infectious bronchitis virus. Poult. Sci. 2003;82:235–241. doi: 10.1093/ps/82.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Norup L.R., Jørgensen P.H., Handberg K.J., Wattrang E., Dalgaard T.S. Crosstalk between innate and adaptive immune responses to infectious bronchitis virus after vaccination and challenge of chickens varying in serum mannose-binding lectin concentrations. Vaccine. 2011;29:9499–9507. doi: 10.1016/j.vaccine.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerup R.M., Dalgaard T.S., Norup L.R., Bergman I.M., Sørensen P., Juul-Madsen H.R. Adjuvant effects of mannose-binding lectin ligands on the immune response to infectious bronchitis vaccine in chickens with high or low serum mannose-binding lectin concentrations. Immunobiology. 2014;219:263–274. doi: 10.1016/j.imbio.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerup R.M., Dalgaard T.S., Norup L.R., Hamzic E., Sørensen P., Juul-Madsen H.R. Characterization of cellular and humoral immune responses after IBV infection in chicken lines differing in MBL serum concentration. Viral Immunol. 2014;27:529–542. doi: 10.1089/vim.2014.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C.P., Tarr A.W. Human lectins and their roles in viral infections. Molecules. 2015;20:2229–2271. doi: 10.3390/molecules20022229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S., Perlman S. Coronaviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2013. [Google Scholar]
- McFerran J., McNulty M. Elsevier; Amsterdam, The Netherlands: 1993. Virus Infections of Birds In: Anonymous. [Google Scholar]
- Medzhitov R., Janeway C., Jr Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Millet J.K., Seron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen O.L., Jørgensen P.H., Hedemand J., Jensenius J.C., Koch C., Laursen S.B. Immunohistochemical investigation of the tissue distribution of mannan-binding lectin in non-infected and virus-infected chickens. Immunology. 1998;94:122–128. doi: 10.1046/j.1365-2567.1998.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen O.L., Jensenius J.C., Jørgensen P.H., Laursen S.B. Serum levels of chicken mannan-binding lectin (MBL) during virus infections; indication that chicken MBL is an acute phase reactant. Vet. Immunol. Immunopathol. 1999;70:309–316. doi: 10.1016/s0165-2427(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Norup L.R., Dalgaard T.S., Friggens N.C., Sørensen P., Juul-Madsen H.R. Influence of chicken serum mannose-binding lectin levels on the immune response towards Escherichia coli. Poult. Sci. 2009;88:543–553. doi: 10.3382/ps.2008-00431. [DOI] [PubMed] [Google Scholar]
- O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading P.C., Morey L.S., Crouch E.C., Anders E.M. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V.R., Trus I., Desmarets L.M., Li Y., Theuns S., Nauwynck H.J. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Vet. Res. 2016;47 doi: 10.1186/s13567-016-0354-9. (70-016-0354-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.W., Hamvas R.M. Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2000;2:305–322. [PubMed] [Google Scholar]
- Ulrich-Lynge S.L., Dalgaard T.S., Norup L.R., Song X., Sørensen P., Juul-Madsen H.R. Chicken mannose-binding lectin function in relation to antibacterial activity towards Salmonella enterica. Immunobiology. 2015;220:555–563. doi: 10.1016/j.imbio.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lynge S.L., Juul-Madsen H.R., Kjaerup R.B., Okimoto R., Abrahamsen M.S., Maurischat S., Sørensen P., Dalgaard T.S. Broilers with low serum Mannose-binding Lectin show increased fecal shedding of Salmonella enterica serovar Montevideo. Poult. Sci. 2016;95:1779–1786. doi: 10.3382/ps/pew101. [DOI] [PubMed] [Google Scholar]
- van Beurden S.J., Berends A.J., Krämer-Kühl A., Spekreijse D., Chénard G., Philipp H.C., Mundt E., Rottier P.J.M., Verheije M.H. A reverse genetics system for avian coronavirus infectious bronchitis virus based on targeted RNA recombination. Virol J. 2017;14:109. doi: 10.1186/s12985-017-0775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E.J.A., van Eijk M., Haagsman H.P. The carbohydrate recognition domain of collectins. FEBS J. 2011;278:3930–3941. doi: 10.1111/j.1742-4658.2011.08206.x. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe I.N., de Vries R.P., Gröne A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe I.N., van Beurden S.J., Weerts E.A., Verheije M.H. The avian coronavirus spike protein. Virus Res. 2014;194:37–48. doi: 10.1016/j.virusres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Cuperus T., van Dijk A., Skjødt K., Hansen S., Haagsman H.P., Veldhuizen E.J. Developmental regulation of chicken surfactant protein A and its localization in lung. Dev. Comp. Immunol. 2016;61:80–87. doi: 10.1016/j.dci.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang W., van Eijk M., Guo H., van Dijk A., Bleijerveld O.B., Verheije M.H., Wang G., Haagsman H.P., Veldhuizen E.J. Expression and characterization of recombinant chicken mannose binding lectin. Immunobiology. 2016 doi: 10.1016/j.imbio.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Lu K., Pfefferle S., Bertram S., Glowacka I., Drosten C., Pöhlmann S., Simmons G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010;84:8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]