Abstract
Sequencing avian infectious bronchitis virus spike genes re-isolated from vaccinated chicks revealed that many sequence changes are found on the S1 spike gene. In the ArkDPI strain, Y43H and ∆344 are the two most common changes observed. This study aims to examine the roles of Y43H and ∆344 in selection in vivo. Using recombinant ArkDPI S1 proteins, we conducted binding assays on chicken tracheas and embryonic chorioallantoic membrane (CAM). Protein histochemistry showed that the Y43H change allows for enhanced binding to trachea, whereas the ArkDPI S1 spike with H43 alone was able to bind CAM. Using Western blot under denaturing conditions, ArkDPI serotype-specific sera did not bind to S1 proteins with ∆344, suggesting that ∆344 alters antigenicity of S1. These findings are important because they propose that specific changes in S1 enhances virus fitness by more effective binding to host tissues (Y43H) and by evading a vaccine-induced antibody response (∆344).
Keywords: Infectious bronchitis virus, Arkansas-type vaccine, Spike glycoprotein, S1 spike, Coronavirus spike
Graphical abstract
Highlights
-
•
Binding behaviors of recombinant IBV ArkDPI vaccine S1 proteins are examined.
-
•
Mutation Y43H enhances binding to mature tracheal tissue but not embryonic tissue.
-
•
A ∆344 mutation alters epitopes on S1 preventing antibody binding.
-
•
These two mutations enhance the vaccine viruses' overall fitness in chickens.
1. Introduction
Infectious bronchitis virus (IBV) is a member of the Coronavirus family, genus Gammacoronavirus. IBV is a highly contagious respiratory pathogen in chickens though some IBV strains may be nephropathogenic and can cause lesions in the kidney. IBV has a narrow host range; chickens are the only species known to be naturally infected. Outbreaks of IBV can cause significant economic loss, particularly for commercial egg producers (Cavanagh, 2007, Jackwood and de Wit, 2013).
Like many members of Coronaviridae, IBV exhibits high genetic diversity. Many serotypes of IBV are known and cross-protection between serotypes is poor (Darbyshire, 1980, Hopkins, 1974, Sjaak de Wit et al., 2011). For this reason, poultry growers must match their vaccination program with circulating IBV strains in their area. In the United States, the most common serotype isolated in the field is the Arkansas (Ark)-type IBV (Jackwood et al., 2005). Currently, only ArkDPI (Arkansas Delmarva Poultry Industry) vaccine is commercially available against Ark-type IBV. The ArkDPI vaccine was originally attenuated by passage of the virus in embryonated eggs 100+ times (Gelb and Cloud, 1983). Experimental and field trials using ArkDPI vaccine have shown that ArkDPI does not provide adequate protection (Jackwood et al., 2009, Roh et al., 2013). One reason may be because ArkDPI vaccine virus does not replicate well in chicks when applied by hatchery spray cabinet (Roh et al., 2014). Moreover, it has been shown that the ArkDPI vaccine persists in the flock (Jackwood et al., 2009) causing a rolling reaction, wherein transmission of the vaccine virus continuously occurs between birds (McElreath et al., 2013).
Re-isolation of ArkDPI virus from vaccinated birds has shown certain sequence changes are frequently observed (McKinley et al., 2008, Nix et al., 2000, Toro et al., 2012b). Certain nucleotide changes are attributed to particular manufacturers but some are common to most ArkDPI re-isolated from vaccinated birds. The existence of these nucleotide changes support the concept that ArkDPI contains viral subpopulations that differ in capacities to infect and replicate in birds (Toro et al., 2012a). Most of the nucleotide changes observed in re-isolated ArkDPI vaccine virus is found on the S1 spike protein (Ammayappan et al., 2009, McKinley et al., 2008, Ndegwa et al., 2012, Nix et al., 2000, Toro et al., 2012b, van Santen and Toro, 2008).
The spike protein of coronaviruses is responsible for attachment and entry into host cells. It has two subunits; S1 and S2. The S1 subunit is primarily responsible for attachment to receptors on host cells (Casais et al., 2003, Cavanagh and Davis, 1986) while the S2 subunit is responsible for the fusion of virus and host membranes (Bosch et al., 2003).
The S1 spike protein contains the receptor-binding domain (RBD) (Promkuntod et al., 2014) and is primarily responsible for host and tissue tropism (Wickramasinghe et al., 2015, Wickramasinghe et al., 2011). Epitopes of neutralizing antibodies are also primarily found in S1 (Cavanagh et al., 1988). Thus, the S1 spike also has significant influence in protective immune responses against IBV. Examination of the S1 spike protein from various serotypes demonstrated that the amino terminal region of S1 exhibits the highest sequence diversity across the genome (Koch et al., 1990, Niesters et al., 1986, Wang and Huang, 2000). Rapid mutation in the S1 spike gene, together with selective pressure, allows the virus to exist as viral subpopulations (Holland et al., 1982).
Two amino acid changes in the S1 spike protein are commonly found in most of the studies that looked at the spike gene sequences of ArkDPI vaccine virus re-isolated from chickens. These are a change from tyrosine to histidine at position 43 (Y43H) and a deletion of an asparagine residue at position 344 (∆344) (McKinley et al., 2008, Ndegwa et al., 2012, Toro et al., 2012b, van Santen and Toro, 2008). We hypothesize that these amino acid changes provide the virus a selective advantage in the chicken host. We chose to study the Y43H and ∆344 changes using recombinant S1 proteins, but it should be recognized that other amino acid changes in the S1 spike protein (eg. residue 213) found in many of the subpopulations from vaccinated chickens (van Santen and Toro, 2008), could also provide a selective advantage in the chicken host. Although the Y43H change and ∆344 are both found in birds up to 13 days post-vaccination and ∆344 can be found in contact exposed birds (one transmission event) up to 13 days post-exposure (McKinley et al., 2008), it is not clear if one or both are found after many transmission events from one bird to the next.
It is difficult to determine the role and interactions of all the changes observed in S1; However, controlled experiments where mutations can be made in recombinant proteins allows us to examine the effect of one or more specific changes in binding to the host cell. In this study, recombinant S1 spike proteins that contained Y43H and/or ∆344 changes were used in protein histochemistry to examine binding to host tissues. Recombinant S1 spike protein with the Y43H change demonstrated differential binding to mature chicken tracheas and to embryonic chorioallantoic membrane tissue. Additionally, S1 spike protein with ∆344 was unable to bind ArkDPI serotype-specific sera, demonstrating that ∆344 alters antigenicity. Taken together, these results help to explain the selection of these polymorphisms in re-isolated ArkDPI vaccine viruses. It appears that the Y43H and ∆344 changes offer selective advantages over the ArkDPI vaccine virus by enhancing binding to host tissue, thereby increasing infectivity (Y43H); and by evading the immune response by altering antigenicity of the spike (∆344).
2. Results and discussion
Many studies have demonstrated that certain polymorphisms in the spike gene can often be found in viruses isolated from chickens that are vaccinated with the ArkDPI vaccine (Ammayappan et al., 2009, McKinley et al., 2008, Ndegwa et al., 2012, Nix et al., 2000, Toro et al., 2012b, van Santen and Toro, 2008). Two of the most common polymorphisms are in the S1 region of the spike gene, specifically at amino acid position 43 where a tyrosine to histidine is changed (Y43H) and at amino acid position 344 where an asparagine residue is deleted (∆344).
The goal of this study was to investigate the role of those two polymorphisms found in the spike gene of re-isolated ArkDPI vaccine in binding to host tissues and in reactions with serotype-specific antibodies. To this end, four recombinant ArkDPI S1 spike proteins were cloned, expressed, and purified. These are (1) primary ArkDPI vaccine spike (Y43/N344), (2) ArkDPI spike with the Y43H change, (3) ArkDPI spike with the ∆344 change, and (4) ArkDPI spike with both the Y43H and ∆344 changes. As a control, a Strep-tagged GFP protein was also produced to function as a control for transfection efficiency as well as a control for protein histochemistry.
2.1. Expression and purification of S1 proteins
Codon-optimized S1 genes were cloned into a mammalian expression vector and transfected into HEK 293 cells. The S1 fusion proteins were purified using StrepTactin affinity chromatography and visualized by Western blot analysis using StrepTactin ( Fig. 1). Also shown in Fig. 1 are recombinant proteins that were digested with PNGase F. As expected, de-glycosylated S1 fusion proteins digested with PNGase F were observed to be approximately 60 kDa in size. Fully N-glycosylated S1 fusion protein trimers were observed to be about 260 kDa, whereas S1 fusion protein monomers were approximately 100 kDa, as expected.
Fig. 1.
S1 fusion proteins were successfully expressed and purified from HEK 293 cells (A) S1 spike protein monomers migrated to a band about 100 kDa, as expected. (B) PNGase digestion was performed to remove N-glycosylation from the recombinant proteins. As expected, unglycosylated S1 fusion proteins migrated to a band 60 kDa in size. Also shown in (B) are fully glycosylated trimeric S1 spike proteins that are about 260 kDa in size.
2.2. Protein histochemistry with recombinant S1 proteins on mature trachea
Binding of the spike onto formalin-fixed tracheal and chorioallantoic membrane (CAM) tissues was examined using protein histochemistry. To detect binding, recombinant spike proteins were pre-complexed with a horseradish peroxidase-conjugated StrepTactin. After which, the complexes were applied onto slides containing sections of mature chicken trachea ( Fig. 2A, B) or embryonic CAM ( Fig. 3A, B).
Fig. 2.
The amino acid position 43 is critical for spike binding onto tracheal tissue. Binding of recombinant S1 spike proteins on tissue sections of mature tracheal tissue was examined using protein histochemistry (A). Four S1 spike proteins were used for the binding experiments namely, the primary ArkDPI S1 spike (DPI), the ArkDPI spike with the Y43H change (Y43H), the ArkDPI spike with a deletion at position 344 (∆344), and finally, the ArkDPI spike with both Y43H and ∆344 changes. StrepTactin-tagged GFP protein (GFP-Strep) was used as a negative control. To detect binding, we pre-complexed the S1 spike proteins with horseradish peroxidase-conjugated StrepTactin and developed a brown signal using DAB as a substrate. Quantification of the signals was performed by densitometry using the ImageJ software (B). Highest binding to ciliated epithelium of the trachea was observed for Y43H spike. Statistical analysis was done in GraphPad Prism software.*Indicates p <0.05 when compared to other groups.
Fig. 3.
The primary ArkDPI spike exhibits highest binding to embryonic tissue. Protein histochemistry on embryonic tissue chorioallantoic membrane (CAM) was performed using purified recombinant S1 spike proteins (A). As with the protein histochemistry with tracheal tissues, the four S1 proteins were used: the primary ArkDPI S1 spike (DPI), the ArkDPI spike with the Y43H change (Y43H), the ArkDPI spike with a deletion at position 344 (∆344), and the ArkDPI spike with both Y43H and ∆344 changes. As a negative control, StrepTactin-tagged GFP protein (GFP-Strep) was also used in protein histochemistry. S1 spike proteins were pre-complexed with horseradish peroxidase-conjugated StrepTactin prior to addition onto CAM tissue sections. Binding signal was developed using DAB as a substrate, thereby producing a brown color. Quantification of the brown signal was performed by densitometry using the ImageJ software (B). Substantial binding to CAM was only observed for the primary ArkDPI vaccine spike protein. Densitometry was performed using the ImageJ software. Statistical analysis was done in GraphPad Prism software.*Indicates p <0.05 when compared to other groups.+Indicates very low level of signal detected.
Protein histochemistry on mature tracheas showed that the S1 spike binds primarily on the ciliated cells as expected, since IBV has a tropism for ciliated epithelial cells. The highest binding to trachea was observed for the spike containing the Y43H mutation. The primary ArkDPI vaccine spike (Y43/N344), the spike with ∆344, and the spike with both sequence changes (Y43H and ∆344) have considerably less binding to the mature tracheal tissue. It is interesting that the S1 spike protein containing both Y43H and ∆344 had poor binding to trachea. This shows that while Y43H enhances binding to trachea, it cannot enhance binding in the presence of ∆344 perhaps due to a conformational change associated with the deletion. This result also highlights the importance of testing all of the changes associated with a virus subpopulation as different changes can have either a positive, negative, or neutral effect on tissue binding.
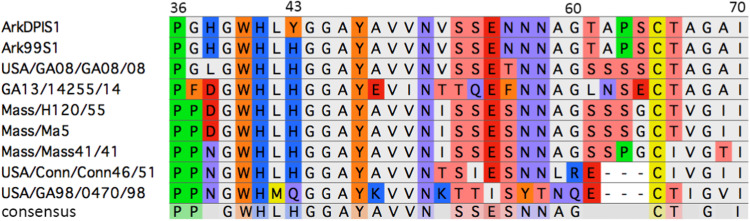
The results of protein histochemistry assays on mature tracheas are consistent with the hypothesis that viral subpopulations containing the Y43H change have a spike that has an enhanced ability to bind to chicken tissues i.e. trachea. In this manner, viral subpopulations with Y43H are able to infect the host better than the primary ArkDPI vaccine virus (Y43/N344). Amino acid position 43 has previously been described as one of the four amino acid positions that are critical for binding of the Massachusetts-type IBV S1 spike onto chicken tracheal tissues (Promkuntod et al., 2014). Additionally, Fig. 4 shows a multiple sequence alignment in the region where the four critical amino acids reside. The histidine at position 43 is conserved among many of the common IBV serotypes, highlighting its importance in spike. Indeed, H43 is also conserved in the Ark99 strain, which belongs to the same serotype as ArkDPI.
Fig. 4.
Multiple sequence alignment of the partial S1 spike protein. The sequence above shows amino acid positions 35–70. Previous studies have shown there are four amino acid positions (38, 43, 63, and 69) that are important for binding of the spike on tracheal tissue (Becker et al., 1967, Promkuntod et al., 2014). Multiple sequence alignment was performed using ClustalW and eBioX software.
2.3. Protein histochemistry with recombinant S1 proteins on CAM
Passage of virus in embryonated eggs is a common strategy to attenuate IBV for vaccine production. Moreover, commercial IBV vaccine is produced by propagating the vaccine virus in embryonated chicken eggs. The ArkDPI vaccine was developed and is produced in this same fashion. A virulent ArkDPI strain was passaged in embryonated eggs about 100 times before it sold as a commercial vaccine. Like many other poultry viruses, IBV has been shown to replicate in the chorioallantoic membrane (CAM) of embryonated eggs (Becker et al., 1967, Domermuth and Edwards, 1957). For this reason, binding of S1 spike on CAM was also examined.
Protein histochemistry on CAM using the recombinant S1 spike proteins showed that only the primary ArkDPI vaccine S1 spike (Y43/N344) was able to significantly bind onto CAM (Fig. 3A, B). This result is consistent with the hypothesis that the spike protein of the ArkDPI vaccine virus is well-adapted to bind and infect embryonic tissues.
2.4. Antigenicity of recombinant S1 proteins
The S1 spike protein is the main target of neutralizing antibodies against IBV (Cavanagh et al., 1988, Koch et al., 1990, Mockett et al., 1984). Epitope mapping of the S1 spike has shown that there are five antigenic sites targeted by neutralizing monoclonal antibodies (Kant et al., 1992). About three quarters of the monoclonal antibodies tested had epitopes found in the region spanning amino acids 290-400 of the S1 spike protein.
As mentioned above, the ∆344 polymorphism is often observed in ArkDPI sequences re-isolated from vaccinated chickens. We postulate that, since amino acid position 344 sits in a prominent antigenic region, the ∆344 change could alter antigenicity of the ArkDPI S1 spike protein. To test this hypothesis, immunoblots on the recombinant S1 spike proteins were performed using serotype-specific antisera against virulent ArkDPI (S1 spike contains N344) under native and denaturing conditions. Under native conditions, there appears to be no difference in the extent of binding between the recombinant S1 spike proteins ( Fig. 5A). In contrast, western blot of S1 spike proteins electrophoresed under denaturing conditions revealed that S1 spike proteins with ∆344 alone or together with Y43H are unable to bind ArkDPI serotype-specific antisera (Fig. 5B). The primary ArkDPI S1 vaccine spike (Y43/N344) and the spike with Y43H were still able to bind ArkDPI serotype-specific antisera under denaturing conditions.
Fig. 5.
Immunoblot analysis of recombinant S1 proteins. ArkDPI serotype-specific antiserum was used to examine antigenicity of recombinant S1 spike proteins. (A) Dot blot was performed under native conditions. An equal amount of protein (350 ng) was spotted onto a nitrocellulose membrane strip and subsequently probed with ArkDPI serotype-specific sera and StrepTactin, as a control. No differences were observed in the amount of antibody binding between recombinant spike proteins. (B) Conventional Western blot under denaturing conditions. We observed that denatured S1 spike proteins with ∆344 singly or in combination with Y43H were unable to bind to ArkDPI-serotype specific antisera. Densitometry analysis was done using ImageJ software. NTC = non-transfected control, nAF = normal, non-infected allantoic fluid.
Neutralizing antibodies found in serotype specific antisera against spike generally recognize conformationally-dependent epitopes. However, denaturation of the spike protein associated with the vaccine virus grown in embryonated eggs still bound to serotype specific antisera indicating that at least one linear epitope was recognized. The ∆344 change in S1 abolished this binding. It has yet to be determined if antibodies that bind to a linear epitope/s associated with ∆344 are neutralizing.
The ArkDPI serotype-specific antisera used for the immunoblots was taken from birds infected with virulent ArkDPI virus grown in embryonated eggs, whose S1 spike gene sequence contains an asparagine at position 344. The lack of binding to denatured S1 proteins with ∆344 suggests the deletion of asparagine could offer a selective advantage to viral subpopulations with ∆344 by helping to evade antibodies that bind to spike.
Inactivated vaccines are frequently given to breeder birds after priming with a live-attenuated vaccine. Since the predominant virus in the inactivated vaccine preparation has N344, breeder birds would likely have antibodies that can recognize S1 spike with N344. It has long been known that maternal antibodies can protect chicks from challenge (Mockett et al., 1987), thus, it appears that maternal antibodies present in chicks provides a selection pressure against viruses with N344, allowing for the emergence of viruses with ∆344. Additionally, we would predict that birds vaccinated with live attenuated ArkDPI with the ∆344 mutation would not generate antibodies against virulent ArkDPI virus, which does have an asparagine at position 344.
2.5. Summary and conclusions
In this study, the roles of Y43H and ∆344 polymorphisms in the S1 spike on binding to the host tissues and antigenicity were examined. Binding experiments on host tissues showed that Y43H enhances the ability of the spike protein to bind to mature tracheal tissue but not to embryonic CAM. On the other hand, the primary ArkDPI vaccine S1 spike protein (Y43/N344) has an inverse binding behavior, wherein it binds robustly to CAM but not to mature trachea.
The second set of experiments looking at the antigenicity of S1 spike proteins demonstrated that ∆344 alters linear epitopes on spike, thereby abolishing spike's interaction to antibodies present in ArkDPI serotype-specific antisera. Taking the results altogether, we postulate that turnabout of viral populations upon vaccination of chicks can be explained, at least in part, by the selective advantage offered by amino acid changes found in the spike protein. These polymorphisms enable a virus population either to bind robustly onto different host tissues, in the case of Y43H; or to evade neutralization by antibodies from the host, in the case of ∆344. In this manner, these amino acid changes enhance the virus population's overall fitness in vaccinated chicks. Furthermore, the primary ArkDPI vaccine population is maintained in the vaccine preparation because its spike is well suited to infection of embryonic tissues. Since residue 43 is located in the S1 RBD and was one of the residues found to be critical for binding by the Mass type of IBV, a change at that site could directly affect binding through its interaction with the host cell receptor and/or by altering tertiary structure; whereas the 344 deletion which is located outside of the S1 RBD could indirectly affect binding by altering the tertiary structure of the protein.
3. Materials and methods
3.1. Construction of plasmids with recombinant S1 fusion protein gene
The S1 spike glycoprotein gene from an Arkansas Delmarva Poultry Industry (ArkDPI) vaccine isolate (accession number ADP06471.2) was obtained from the database of the National Center for Bioinformatics (NCBI). Plasmids (pUC57-Amp) containing codon-optimized ArkDPI S1 genes with one or two amino acid changes (Y43H and/or ∆344) were obtained from Genewiz (New Jersey, USA). The amino acids 19–540 of the ArkDPI S1 spike gene were fused to the GCN4 trimerization domain (Harbury et al., 1993) in frame to allow for oligomerization (designated as S1-GCN4). The sequence of the GCN4 trimerization domain is RMKQIEDKIEEIESKQKKIENEIARIKKLVPRGSLE. NheI and BsaI restrictions sites were appended at the 5′ and 3′ ends of S1-GCN4, respectively. The plasmid pEXPR-IBA42 (IBA Life Sciences; Goettingen, Germany) was used as the plasmid vector since it allows for the production and secretion of Strep-tagged proteins. In order to digest with the methylation-sensitive restriction enzyme BsaI, pEXPR-IBA42 and pUC57-Amp plasmids containing the S1 genes were maintained in SCS110 cells (Agilent; California, USA).
The vector pEXPR-IBA42 and pUC57-Amp plasmids containing the S1-GCN4 fusion protein genes were digested with NheI at 37 °C for one hour. Digested DNAs were then purified using the GeneJet PCR Purification kit (Thermo Scientific; Massachusetts, USA). After which, purified DNA fragments were digested with BsaI at 37 °C for 1 h. Digested DNAs were then electrophoresed in an agarose gel. Appropriate bands were excised and digested DNAs were purified using the GeneJet Gel Purification kit (Thermo Scientific). To ligate S1-GCN4 fusion genes into pEXPR-IBA42 vector, digested fragments were combined at a 1:3 (vector: insert) ratio and added to a ligation reaction mix containing T4 ligase (Thermo Scientific). Ligation products were incubated at 4 °C overnight and then transformed into chemically competent JM109 E. coli cells (Promega; Wisconsin, USA) using the heat shock method described by the manufacturer. The transformation reaction mix was plated on Luria-Bertani agar plates supplemented with 100 μg/mL carbenicillin. Overnight cultures were prepared from several bacterial colonies obtained and plasmid extraction was performed using the GeneJet plasmid miniprep kit (Thermo Scientific). To identify positive transformants, plasmid minipreps from each bacterial colony were subjected to digestion with NheI and subsequently, sequencing by Sanger method at the Georgia Genomics Facility (University of Georgia). A large batch of plasmids to be used for transformation of HEK 293 cells was prepared using the GeneJet Maxiprep kit (Thermo Scientific) following the manufacturer's recommended protocol.
3.2. Expression and purification of recombinant S1 fusion proteins
HEK 293 cells were transfected with plasmids coding for ArkDPI S1 fusion protein using Turbofect (Thermo Scientific) according to the manufacturer's recommended protocol. The DNA:OptiMEM:Turbofect ratio used was 6 μg:0.8 mL:10 μL for every 106 cells. Culture media was harvested 6 days after transfection and centrifuged at 3000×g for 15 min at 4 °C to pellet any carried over cells. Supernatants were subjected to affinity chromatography with a StrepTactin-Sepharose column (IBA Life Sciences) using the manufacturer's recommended protocol. Presence of S1 fusion proteins was confirmed by performing Western blot using StrepTactin-HRP (IBA Life Sciences) following the manufacturer's recommended protocol. Samples for SDS-PAGE were prepared by adding purified S1 fusion proteins to urea-SDS Buffer (200 mM Tris, 8 M urea, 1.1 mM EDTA, 5% SDS, 0.03% bromophenol blue, 0.1 M DTT) at a 1:1 volume to volume ratio. Samples were then heated to 100 °C for 10 min before SDS-PAGE. Spike proteins were digested with PNGase F (New England Biolabs; Massachusetts, USA) to remove N-glycosylations and to accurately measure molecular weight in Western blot. Briefly, we denatured S1 fusion proteins in 1× Glycoprotein denaturation buffer (New England Biolabs) at 100 °C for 10 min. Subsequently, PNGase F digestion was performed using 1 U PNGase F enzyme for a 20-μL reaction for 1 h at 37 °C. Samples were then analyzed by SDS-PAGE and Western blot. Purified S1 fusion proteins were quantified using a Nanodrop spectrophotometer (Thermo Scientific) ( Table 1).
Table 1.
Summary of binding protein histochemistry results.
| DPI | Y43H | ∆344 | ∆Y43H & ∆344 | |
|---|---|---|---|---|
| Trachea, | + | +++ | + | + |
| CAM | + | ± | ± | ± |
+++ highly intense staining, + moderate staining, ± very mild staining.
3.3. Protein histochemistry
Protein histochemistry was performed as previously described (Promkuntod et al., 2014, Wickramasinghe et al., 2011). A trachea was obtained from a 6-week old commercial broiler chicken and chorioallantoic membrane (CAM) was obtained from 18-d old commercial chicken embryos. All tissues were fixed in 10% formalin for 48–72 h, embedded in paraffin, and cut into 4-μm thick sections. Slides were de-paraffinized and antigen retrieval was performed in citrate buffer (10 mM citrate buffer, pH 6.0) for 45 min, with the use of a steamer. After antigen retrieval, the slides were blocked with 10% normal goat serum in PBS for 30 min at room temperature. Endogenous peroxidase activity was also quenched using BLOXALL blocking solution (Vector Labs; California, USA) for 10 min at room temperature. The recombinant S1 fusion proteins were pre-complexed with the StrepTactin-HRP at a 1:200 ratio (StrepTactin: S1 spike) for 30 min on ice. A concentration of 70 μg/mL of recombinant S1 spike proteins was used for each slide. Slides were incubated with the recombinant S1 spike proteins overnight at 4 °C in a humidified chamber. The slides were then washed thrice with PBS and signal was developed using the Vectastain ABC kit (Vector Labs). After 10–15 min, the substrate was washed off with distilled water three times. Slides were counterstained with hematoxylin for 2 min and examined by light microscopy.
Images of stained slides were taken with an Olympus BX41 microscope (New Jersey). Five tissue sections were placed on each slide and a representative image was taken for each tissue section at a 40× magnification. Quantification of the signal was performed on these images using the Image J software (Schneider et al., 2012).
3.4. Production of serotype-specific sera and western blot
Specific pathogen free chickens at 3–8 weeks old were used to generate serotype specific sera. Each bird was intratracheally inoculated with 1×105 50% embryo infectious dose (EID50) of the ArkDPI challenge strain. After two weeks, birds were boosted by intravenous inoculation with 1×105 EID50 of the ArkDPI challenge strain. Terminal bleeds were then performed at 3 weeks after boosting. Subsequently, the sera were pooled and inactivated at 56 °C for 30 min. Virus neutralization tests were performed to confirm that the sera only reacted to ArkDPI. For Western blotting of our S1 fusion proteins, we used the ArkDPI serotype specific serum at a 1:1000 dilution as a primary antibody and a horseradish peroxidase-conjugated goat anti-chicken IgG (Sigma) at a 1:4000 dilution.
Experimental strategies described in this work were adapted from previously published papers (Cornelissen et al., 2010, Promkuntod et al., 2014, Wickramasinghe et al., 2011).
Funding source
This project was funded by the US Poultry and Egg Association from grant number F062. US Poultry and Egg Association did not have any involvement in the study design, data collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Acknowledgments
We would like to thank Lisa Stabler and Debbie Hilt for their technical assistance.
Contributor Information
Christina Leyson, Email: cmleyson@uga.edu.
Monique França, Email: mfranca@uga.edu.
Mark Jackwood, Email: mjackwoo@uga.edu.
Brian Jordan, Email: brian89@uga.edu.
References
- Ammayappan A., Upadhyay C., Gelb J., Jr, Vakharia V.N. Identification of sequence changes responsible for the attenuation of avian infectious bronchitis virus strain Arkansas DPI. Arch. Virol. 2009;154:495–499. doi: 10.1007/s00705-009-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W.B., McIntosh K., Dees J.H., Chanock R.M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E) J. Virol. 1967;1:1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J. Gen. Virol. 1986;67:1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Mockett A.P. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res. 1988;11:141–150. doi: 10.1016/0168-1702(88)90039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen L.A., de Vries R.P., de Boer-Luijtze E.A., Rigter A., Rottier P.J., de Haan C.A. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS One. 2010;5:e10645. doi: 10.1371/journal.pone.0010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyshire J.H. Assessment of cross-immunity dm chickens to strains of avian infectious bronchitis virus using tracheal organ cultures. Avian Pathol. 1980;9:179–184. doi: 10.1080/03079458008418401. [DOI] [PubMed] [Google Scholar]
- Domermuth C.H., Edwards O.F. An electron microscope study of chorioallantoic membrane infected with the virus of avian infectious bronchitis. J. Infect. Dis. 1957;100:74–81. doi: 10.1093/infdis/100.1.74. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Cloud S.S. Effect of serial embryo passage of an Arkansas-type avian infectious bronchitis virus isolate on clinical response, virus recovery, and immunity. Avian Dis. 1983;27:679–687. [PubMed] [Google Scholar]
- Harbury P.B., Zhang T., Kim P.S., Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982;215:1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hopkins S.R. Serological comparisons of strains of infectious bronchitis virus using plaque-purified isolants. Avian Dis. 1974;18:231–239. [PubMed] [Google Scholar]
- Jackwood M.W., de Wit S. Infectious bronchitis. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V., editors. Diseases of Poultry. 13 ed. John Wiley and Sons, Inc.; Ames, Iowa: 2013. pp. 139–159. [Google Scholar]
- Jackwood M.W., Hilt D.A., McCall A.W., Polizzi C.N., McKinley E.T., Williams S.M. Infectious bronchitis virus field vaccination coverage and persistence of Arkansas type viruses in commercial broilers. Avian Dis. 2009;53:175–183. doi: 10.1637/8465-090308-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Lee C.-W., Kwon H.M., Callison S.A., Moore K.M., Moscoso H., Sellers H., Thayer S. Data from 11 years of Molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- Kant A., Koch G., van Roozelaar D.J., Kusters J.G., Poelwijk F.A., van der Zeijst B.A. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J. Gen. Virol. 1992;73:591–596. doi: 10.1099/0022-1317-73-3-591. [DOI] [PubMed] [Google Scholar]
- Koch G., Hartog L., Kant A., van Roozelaar D.J. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J. Gen. Virol. 1990;71:1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- McElreath, J.S., Jordan, B.J., Hilt, D., Jackwood, M.W., 2013. Arkansas vaccine virus transmitted to SPF chickens from vaccinated broilers does not provide protection from challenge. In: Southern Conference on Avian Diseases, Atlanta, GA.
- McKinley E.T., Hilt D.A., Jackwood M.W. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008;26:1274–1284. doi: 10.1016/j.vaccine.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett A., Cavanagh D., Brown T. Monoclonal antibodies to the S1 spike and membrane proteins of avian infectious bronchitis coronavirus strain massachusetts M41. J. Gen. Virol. 1984;65:2281–2286. doi: 10.1099/0022-1317-65-12-2281. [DOI] [PubMed] [Google Scholar]
- Mockett A.P., Cook J.K., Huggins M.B. Maternally-derived antibody to infectious bronchitis virus: Its detection in chick trachea and serum and its role in protection. Avian Pathol. 1987;16:407–416. doi: 10.1080/03079458708436391. [DOI] [PubMed] [Google Scholar]
- Ndegwa E.N., Joiner K.S., Toro H., van Ginkel F.W., van Santen V.L. The proportion of specific viral subpopulations in attenuated Arkansas Delmarva poultry industry infectious bronchitis vaccines influences vaccination outcome. Avian Dis. 2012;56:642–653. doi: 10.1637/10108-022912-Reg.1. [DOI] [PubMed] [Google Scholar]
- Niesters H., Lenstra J.A., Spaan W., Zijderveld A.J., Bleumink P.N., Hong F., van S.G., Horzinek M.C., Van, Der, Zeijst Bam. The peplomer protein sequence of the M41 strain of coronavirus IBV and its comparison with Beaudette strains. Virus Res. 1986;5:253–263. doi: 10.1016/0168-1702(86)90022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix W.A., Troeber D.S., Kingham B.F., Keeler C.L., Jr., Gelb J., Jr. Emergence of subtype strains of the Arkansas serotype of infectious bronchitis virus in Delmarva broiler chickens. Avian Dis. 2000;44:568–581. [PubMed] [Google Scholar]
- Promkuntod N., van Eijndhoven R.E., de Vrieze G., Grone A., Verheije M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology. 2014;448:26–32. doi: 10.1016/j.virol.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh H.J., Hilt D.A., Jackwood M.W. Detection of infectious bronchitis virus with the use of real-time quantitative reverse transcriptase-PCR and correlation with virus detection in embryonated eggs. Avian Dis. 2014;58:398–403. doi: 10.1637/10764-010914-Reg.1. [DOI] [PubMed] [Google Scholar]
- Roh H.J., Hilt D., Williams S.M., Jackwood M.W. Evaluation of infectious bronchitis virus Arkansas-type vaccine failure in commercial broilers. Avian Dis. 2013;57:248–259. doi: 10.1637/10459-112812-Reg.1. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaak de Wit J.J., Cook J.K., van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H., van Santen V.L., Jackwood M.W. Genetic diversity and selection regulates evolution of infectious bronchitis virus. Avian Dis. 2012;56:449–455. doi: 10.1637/10072-020212-Review.1. [DOI] [PubMed] [Google Scholar]
- Toro H., Pennington D., Gallardo R.A., van Santen V.L., van Ginkel F.W., Zhang J., Joiner K.S. Infectious bronchitis virus subpopulations in vaccinated chickens after challenge. Avian Dis. 2012;56:501–508. doi: 10.1637/9982-110811-Reg.1. [DOI] [PubMed] [Google Scholar]
- van Santen V.L., Toro H. Rapid selection in chickens of subpopulations within ArkDPI-derived infectious bronchitis virus vaccines. Avian Pathol. 2008;37:293–306. doi: 10.1080/03079450802043783. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Huang Y.C. Relationship between serotypes and genotypes based on the hypervariable region of the S1 gene of infectious bronchitis virus. Arch. Virol. 2000;145:291–300. doi: 10.1007/s007050050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe A.I.N., de Vries R.P., Weerts E.A., van Beurden S.J., Peng W., McBride R., Ducatez M., Guy J., Brown P., Eterradossi N., Grone A., Paulson J.C., Verheije M.H. Novel receptor specificity of avian gammacoronaviruses that cause enteritis. J. Virol. 2015;89:8783–8792. doi: 10.1128/JVI.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe I.N., de Vries R.P., Grone A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]