Graphical abstract
Five Ib-type cyclopeptide alkaloids, jubanines F–J (1–5) were isolated from the roots of Ziziphus jujuba. Compound 2, 3 showed potent inhibitory effects on porcine epidemic diarrhea virus replication.
Keywords: Ziziphus jujuba; Rhamnaceae, Cyclopeptide alkaloids; Jubanines; Antiviral
Highlights
-
•
Five cyclopeptide alkaloids were separated from the roots of Ziziphus jujuba.
-
•
Their structures were fully characterized by spectroscopic analyses in combination with chemical derivatizations.
-
•
Antiviral activities of plant-derived cyclopeptide alkaloids are reported.
Abstract
Five Ib-type cyclopeptide alkaloids, jubanines F–J (1–5), and three known compounds, nummularine B (6), daechuine-S3 (7), and mucronine K (8) were isolated from the roots of Ziziphus jujuba. Their structures were fully characterized by spectroscopic analyses in combination with chemical derivatization. Compounds 1–3, and 6 were evaluated for their antiviral activity against the porcine epidemic diarrhea virus (PEDV). Compounds 2, 3, and 6 showed potent inhibitory effects on PEDV replication.
1. Introduction
Cyclopeptide alkaloids are one of the chemotaxonomic specific constituents in Rhamnaceae plants, especially for Ziziphus species. They usually contain a p- or m-ansa 13-, 14-, or 15-membered ring structure that consists of a styrylamine moiety and two or three α-amino acid residues (Tan and Zhou, 2006). In some cases, they possess one or two N-methyl or N,N-dimethyl amino acids. Cyclopeptide alkaloids of Rhamnaceae plants are divided into three types: Ia, Ib, or Ic, according to ring structure size. The Ib-type alkaloids have 13-membered macrocyclic structures composed of a m-oxystyrylamino moiety, a ring bond amino acid, and a β-oxyproline moiety as an intermediate amino acid. Sedative, antibacterial, antifungal, antiplasmodial, antimycobacterial, and antimalarial effects have been reported for some cyclopeptide alkaloids (Gournelis et al., 1998, Panseeta et al., 2011, Tan and Zhou, 2006).
Ziziphus jujuba Mill. is a common species in the genus Ziziphus and is widely cultivated in southern Europe and Asia, including Russia, India, the Middle East, and China (Outlaw et al., 2002). Although the fruit is the most widely used part of the plant for food, cyclopeptide alkaloids have been separated from other plant parts, especially from the stem bark. Four Ia-type (frangufoline, scutianine D, jubanine C, and mauritine A) and eight Ib-type cyclopeptide alkaloids (amphibine H, jubanine A, jubanine B, jubanine E, mucronine D, nummularine A, nummularine B, and zizyphine A) have been isolated and reported from the Z. jujuba stem bark (Devi et al., 1987, Pandey et al., 2008, Tripathi et al., 2001, Tschesche et al., 1976). However, the chemical constituents of Z. jujuba roots have been rarely reported. To the best of our knowledge, only one publication has reported cyclopeptide alkaloids from the Z. jujuba root. These alkaloids are mauritine A and seven Ib-type cyclopeptide alkaloids: mucronine D, amphibine H, jubanine A, jubanine B, jubanine D, nummularine A, and nummularine B (Khokhar et al., 1994).
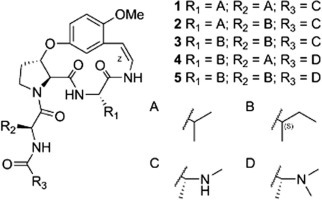
In this paper, the isolation and structural elucidation are described of five new Ib-type cyclopeptide alkaloids (1–5) with three known cyclopeptide alkaloids (6–8) (Fig. 1 ). Antiviral evaluations against PEDV are also reported.
Fig. 1.
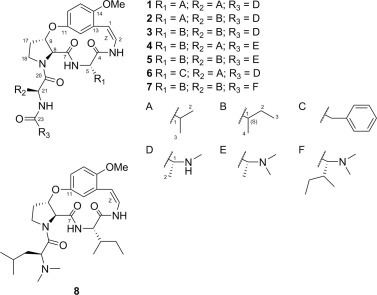
Chemical structures of compounds 1–8.
2. Results and discussion
Air-dried pulverized roots of Z. jujuba were macerated with MeOH before preparing the alkaloid fraction with an acid-base method from the MeOH extract. This alkaloid fraction was subjected to silica gel column chromatography eluted with increasingly polar mixtures of CHCl3/MeOH. Further purification was performed using Sephadex LH-20 and preparative HPLC. As a result, eight cyclopeptide alkaloids (1–8) were isolated and chemically characterized. The UV spectra of 1–8 showed absorption bands at around 270 and 320 nm, which were caused by the characteristic styrylamine chromophore (Panseeta et al., 2011). All eight compounds, therefore, could be 13-membered cyclopeptide alkaloids. This is because these absorption bands are not observed with 14-membered rings due to the strain of the ring system, except when there is a tryptophan moiety (Gournelis et al., 1998). Three aromatic protons of the cyclopeptides exhibited an ABX spin system, e.g. δ H 6.70 (1H, d, J = 3.2 Hz, H-12), δ H 6.87 (1H, d, J = 9.2 Hz, H-15), and δ H 6.80 (1H, dd, J = 3.2, 9.2 Hz, H-16) for 1 (Table 2). This suggests that compounds 1–8 had a m-oxystyrylamino moiety, which then suggests that they are 13-membered cyclopeptide alkaloids (Lin et al., 2000). In addition, based on 1H–1H COSY and 1H–13C HMBC spectroscopic data analyses and comparison with previously reported chemical shift analogs, the presence of a β-oxyproline moiety is possible for compounds 1–8 (Tan and Zhou, 2006). Thus, compounds 1–8 were confirmed as Ib-type cyclopeptide alkaloids. The olefinic protons (H-1 and H-2) of 1–8 showed coupling constants (J) near 9.0 Hz, and a double bond geometry in styrylamino moieties were indicated to be in Z configurations.
Table 2.
1H NMR spectroscopic data (δ (J in Hz)) for compounds 1–5.
| 1c,d | 2b,d | 3a,d | 4a,e | 5a,e | |
|---|---|---|---|---|---|
| 1 | 5.92 d (9.2) | 5.92 d (9.0) | 5.94 d (9.1) | 5.84 d (8.9) | 5.85 d (8.7) |
| 2 | 6.93 dd (9.2, 11.5) | 6.91 dd (9.0, 11.2) | 6.93 dd (9.2, 11.5) | 6.81 dd (8.9, 3.0) | 6.82 dd (8.7, 3.2) |
| 3-NH | 8.42 d (11.5) | 8.51 d (11.2) | 8.44 d (11.5) | 9.11 d (10.1) | 9.09 d (10.1) |
| 5 | 4.21 dd (4.6, 4.6) | 4.23 dd (5.3, 5.3) | 4.27 dd (4.6, 5.0) | 4.46 dd (8.8, 8.8) | 4.46 dd (8.3, 8.3) |
| 6-NH | 7.24f | 7.5 br s | 7.19 d (5.0) | 8.16 d (8.2) | 7.83 d (8.7) |
| 8 | 4.50 d (3.2) | 4.49 d (3.2) | 4.49 d (3.2) | 4.52 d (1.8) | 4.51 d (2.3) |
| 9 | 5.52 td (3.2, 7.3) | 5.43 td (3.2, 5.0) | 5.53 td (3.2, 7.4) | 5.16 m | 5.15 m |
| 12 | 6.70 d (3.2) | 6.71 d (2.8) | 6.69 d (2.8) | 6.77 d (3.0) | 6.73f |
| 15 | 6.87 d (9.2) | 6.85 d (9.0) | 6.87 d (8.7) | 7.01 d (9.1) | 7.02 d (9.1) |
| 16 | 6.80 dd (3.2, 9.2) | 6.80 dd (2.9, 9.0) | 6.80 dd (2.8, 8.7) | 6.81 dd (3.0, 9.0) | 6.76f |
| 17 | 2.29, 2.59 m | 2.32, 2.58 m | 2.32, 2.59 m | 2.13, 2.54 m | 2.15, 2.53 m |
| 18 | 3.56, 4.23 m | 3.60, 4.30 m | 3.55, 4.31 m | 3.59, 4.25 m | 3.60, 4.12 m |
| 21 | 4.56 dd (7.8, 9.2) | 4.60 dd (8.1, 8.1) | 4.60 dd (7.3, 9.1) | 4.03 m | 4.06 dd (6.9, 8.7) |
| 22-NH | 7.64 d (9.2) | 7.87 d (7.1) | 7.60 d (9.1) | 8.25 d (8.7) | 8.26 d (8.7) |
| R1 (ringbound a.a) | Val | Val | Ile | Ile | Ile |
| 1′ | 2.33 m | 2.30 m | 2.09 m | 1.75 m | 1.72 m |
| 2′ | 0.99 d (6.9) | 0.97 d (7.0) | 1.11, 1.38 m | 1.06, 1.42 m | 1.08, 1.43 m |
| 3′ | 0.88 d (7.3) | 0.86f | 0.87f | 0.76f | 0.79 d (7.3) |
| 4′ | 0.96 d (6.9) | 0.67 d (6.6) | 0.79 d (7.3) | ||
| R2 (intermediate a.a) | Val | Ile | Ile | Val | Ile |
| 1″ | 1.97 m | 1.76 m | 1.77 m | 1.85 m | 1.85 m |
| 2″ | 0.87 d (7.8) | 1.09, 1.42 m | 1.10, 1.44 m | 0.80f | 1.18, 1.34 m |
| 3″ | 0.85 d (6.9) | 0.81f | 0.84f | 0.86f | 0.80 d (7.3) |
| 4″ | 0.85f | 0.83f | 0.86 d (6.9) | ||
| R3 (terminal a.a.) | N-Me Ala | N-Me Ala | N-Me Ala | N,N-diMe Ala | N,N-diMe Ala |
| 1‴ | 3.11 q (6.8) | 3.58f | 3.10 q (6.8) | 2.77 q (10.4) | 2.99 q (6.9) |
| 2‴ | 1.30 d (6.8) | 1.30 d (6.8) | 1.30 d (6.8) | 1.75f | 1.07 d (6.9) |
| OMe | 3.78 s | 3.77 s | 3.78 s | 3.16 s | 3.74 s |
| NMe | 2.37 s | 2.43 s | 2.37 s | 2.17 s | 2.17 s |
Recorded at 600 MHz.
Recorded at 500 MHz.
Recorded at 400 MHz.
Recorded in CDCl3.
Recorded in DMSO-d6.
Overlapped.
Compound 1 was obtained as a white amorphous powder with molecular formula C28H41N5O6, as indicated by ESI-qTOF-MS. From the analysis of 1H, 13C, 1H–1H COSY, and 1H–13C HMBC NMR spectroscopic data (Fig. 2 ), two valines and one terminal N-methylalanine moiety were suggested in 1 (Table 1 ). The HMBC correlations between H-3 to C-4, H-6 to C-7, and H-9 to C-11 indicated linkages between a m-oxystyrylamino moiety and a valine, the valine and a β-oxyproline, and the β-oxyproline and the m-oxystyrylamino moiety, respectively. A NOE relationship between H-18 and H-21 was observed, which agreed with the β-oxyproline connection with another valine moiety. Thus, structure 1 was established as shown in Fig. 1. The CD spectrum of 1 exhibited two negative Cotton effects around 265 and 321 nm, suggesting 5S,8S,9S-configurations (Schmidt et al., 1983). No significant NOE enhancements were observed between H-5/H-8 and H-8/H-9 in the NOESY spectrum. This supported the 5S,8S,9S-configuration. A weak vicinal coupling between H-8 and H-9 (J = 3.2 Hz) suggests a trans orientation (Suksamrarn et al., 2005). To confirm the configuration, acid hydrolysis of 1 was performed. The hydrolysate of 1 was treated with the d-FDLA (Nα-(5-fluoro-2,4-dinitrophenyl)-d-leucinamide) or l-FDLA (Nα-(5-fluoro-2,4-dinitrophenyl)-l-leucinamide) and the reaction products were analyzed by LC–MS (Fujii et al., 1997). d-FDLA derivatives are retained longer than the l-FDLA derivatives on C-18 reverse phased HPLC, exhibiting amino acid residues of 1 as l-Val and l-N-Me-Ala, respectively. As a result, the configuration of compound 1 is shown in Fig. 1. Compound 1 was named jubanine F after its plant origin.
Fig. 2.
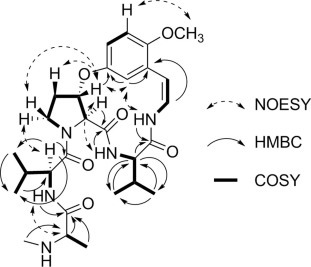
Selected COSY, HMBC, and NOESY interactions for compound 1.
Table 1.
13C NMR spectroscopic data (δ) for compounds 1–5.
| 1c,d | 2b,d | 3a,d | 4a,e | 5a,e | |
|---|---|---|---|---|---|
| 1 | 106.8 | 107.0 | 106.7 | 107.9 | 107.8 |
| 2 | 121.5 | 121.5 | 121.5 | 122.0 | 116.9 |
| 4 | 167.1 | 167.4 | 167.1 | 168.5 | 169.8 |
| 5 | 60.7 | 60.7 | 60.3 | 53.8 | 53.4 |
| 7 | 170.4 | 170.4 | 170.4 | 170.4 | 172.2 |
| 8 | 64.5 | 64.7 | 64.5 | 64.8 | 64.8 |
| 9 | 77.2 | 77.2 | 77.3 | 77.8 | 77.2 |
| 11 | 151.1 | 150.9 | 151.1 | 150.6 | 150.5 |
| 12 | 111.3 | 111.2 | 111.2 | 111.9 | 111.3 |
| 13 | 124.3 | 124.2 | 124.3 | 124.1 | 124.1 |
| 14 | 151.5 | 151.0 | 151.5 | 150.9 | 150.9 |
| 15 | 113.8 | 113.7 | 113.8 | 113.6 | 113.7 |
| 16 | 117.8 | 117.6 | 117.8 | 116.9 | 116.9 |
| 17 | 32.6 | 32.6 | 32.6 | 32.6 | 32.3 |
| 18 | 46.7 | 46.8 | 46.8 | 46.2 | 46.1 |
| 20 | 171.6 | 171.5 | 171.7 | 168.5 | 170.3 |
| 21 | 54.6 | 54.5 | 53.8 | 60.4 | 59.0 |
| 23 | 175.0 | 172.6 | 175.0 | 169.8 | 172.5 |
| R1 (ringbound a.a) | Val | Val | Ile | Ile | Ile |
| 1′ | 28.3 | 28.6 | 35.2 | 36.5 | 36.5 |
| 2′ | 19.7 | 19.6 | 24.5 | 24.3 | 24.1 |
| 3′ | 17.3 | 17.4 | 11.7 | 11.1 | 10.7 |
| 4′ | 16.2 | 15.6 | 14.8 | ||
| R2 (intermediate a.a) | Val | Ile | Ile | Val | Ile |
| 1″ | 31.3 | 37.4 | 37.6 | 34.8 | 34.8 |
| 2″ | 19.2 | 24.5 | 24.5 | 18.2 | 24.5 |
| 3″ | 17.6 | 10.8 | 11.0 | 19.4 | 10.5 |
| 4″ | 15.1 | 15.4 | 15.6 | ||
| R3 (terminal a.a.) | N-Me Ala | N-Me Ala | N-Me Ala | N,N-diMe Ala | N,N-diMe Ala |
| 1‴ | 60.2 | 58.4 | 60.2 | 70.4 | 63.1 |
| 2‴ | 19.5 | 17.8 | 19.4 | 32.4 | 13.2 |
| OMe | 56.1 | 56.0 | 56.1 | 55.9 | 56.0 |
| NMe | 34.9 | 32.9 | 35.0 | 41.0 | 41.7 |
Recorded at 150 MHz.
Recorded at 125 MHz.
Recorded at 100 MHz.
Recorded in CDCl3.
Recorded in DMSO-d6.
The molecular formula of compound 2 was determined to be C29H43N5O6 by ESI-qTOF-MS. Compared to the 1H and 13C NMR spectra of 1, 2 differed in only one amino acid residue. Instead of a valine, two methyl (δ C 10.8 and 15.1), one methylene (δ C 24.5), and one methine (δ C 37.4) signals were observed. 1H–1H COSY analysis indicated that these comprised an isoleucine residue. The HMBC cross-peaks of H-3 to C-4, H-6 to C-7, and H-22 to C-23 indicated connections between a m-oxystyrylamino moiety with a valine, the valine with a β-oxyproline, and an isoleucine with a N-methylalanine, respectively. The absolute configurations of C-5, C-8, C-9, C-21, and C-1‴ were identified as S, S, S, S, and S by CD and advanced Marfey’s method. However, 2 contained an isoleucine residue, and the absolute configuration at C-1″ was determined by chromatographic analysis on GITC (2,3,4,6-tetra-O-acetyl-β-d-glucopyranosylisothiocyanate) derivatives (Hess et al., 2004). The GITC derivatives of l-Ile and l-allo-Ile eluted very closely and co-injection experiments proved that the configuration of the Ile residue was l-Ile (21S, 1″S) instead of l-allo-Ile (21S, 1″R). Compound 2 was named jubanine G.
The molecular formula of compound 3 was determined as C30H45N5O6 by ESI-qTOF-MS. Since it is similar to compounds 1 and 2, it was determined that 3 has two isoleucine moieties as the ring bound amino acid and the intermediate amino acid residues, respectively. The absolute configurations of 5S, 8S, 9S, 21S, 1′S, 1″S, and 1‴S were identified by the same methods for 1 and 2. Compound 3 was named jubanine H.
The molecular formula of compound 4 was determined to be C30H45N5O6 using ESI-qTOF-MS. Different from compounds 1–3, compound 4 has N-methyl signals (δ H 2.17, s) equivalent to six protons. Thus, 4 was deduced to have a terminal N,N-dimethylalanine instead of a mono-methyl substituted one. Additionally, the presence of a valine and an isoleucine residue was inferred from 1H, 13C, 1H–1H COSY, and HMBC NMR spectroscopic data. The links between a m-oxystyrylamino moiety with an isoleucine, the isoleucine with a β-oxyproline, and a valine with a N,N-dimethylalanine were respectively confirmed through HMBC interactions of H-3 to C-4, H-6 to C-7, and H-22 to C-23. The absolute configurations of 5S, 8S, 9S, 21S, 1′S, and 1″S were identified by the same methods as used for the previous compounds 1–3. The absolute configuration of N,N-dimethylalanine was confirmed by chromatographic analyses of the N,N-dimethylalanine with phenylglycine methylester hydrochloride (PGME) amide coupling products (Um et al., 2013). The PGME derivatives of the synthetic N,N-dimethylalanine were analyzed by LC–MS and the synthetic amides of l- and d-N,N-dimethylalanine had retention times of 8.83 min and 10.24 min, respectively. The N,N-dimethylalanine-PGME amide derived from compound 4 was detected at 8.79 min. Thus, the absolute configuration of N,N-dimethylalanine in compound 4 was identified as l (S) and compound 4 was named jubanine I.
The molecular formula of compound 5 was determined to be for C31H47N5O6 utilizing ESI-qTOF-MS. Similar to compound 4, compound 5 was deduced to have a terminal N,N-dimethylalanine residue, which was confirmed by amino acid hydrolysate analysis. Two isoleucine residues and their spin systems with styrylamine, β-oxyproline, and terminal N,N-dimethylalanine moieties were inferred by COSY and HMBC correlations. The absolute configuration of the two isoleucine residues and a N,N-dimethylalanine residue in compound 5 were also determined by the chromatographic analysis on GITC and PGME derivatives. The absolute configurations of C-5, C-8, C-9, C-21, C-1′, C-1″ and C-1‴ of 5 were all identified as S. Compound 5 was named jubanine J.
After comparing NMR spectra with previously reports, known compounds 6 and 7 were identified as nummularine B (Panseeta et al., 2011) and daechuine-S3 (Lee et al., 2001), respectively. Compound 8 was identified as a compound previously isolated and reported, but not given a trivial name (Barboni et al., 1994). For convenience, compound 8 is named mucronine K, after the plant name in which this compound was first isolated.
Pocrine epidemic diarrhea virus (PEDV) infection in pigs causes epidemic diarrhea, dehydration, and vomiting. Most newborn piglets infected by PEDV would be dying with almost 100% mortality and pigs of all ages are also affected. Infection with this virus has been become a serious economic loss in the swine industry and recent outbreaks led to serious economic losses in many swine producing countries. Only compounds 1–3, and 6 were screened for their antiviral potential against PEDV, due to the scarce amounts of other compounds that were obtained. Compounds 2, 3, and 6 showed potent inhibitory effects on PEDV infected Vero cells, as shown in Table 3 . Compounds 3 and 6 showed EC50 values at the micromolar range (EC50 4.49 ± 0.67 and 6.17 ± 0.50 μM, respectively), which was potent as compared to the positive control 6-azauridine (5.58 ± 0.53 μM). However, both compounds showed much lower cytotoxicity (CC50 211.26 ± 29.64 and 165.30 ± 16.49 μM, respectively) compared to the positive control (44.47 ± 6.11 μM), so they had higher selective index (SI) values (47.11 ± 0.49 and 26.75 ± 0.54, respectively) than 6-azauridine (7.98 ± 0.37). Compound 2 exhibited lower potency than 3 and 6 (EC50 13.41 ± 1.13 μM), but 2 also demonstrated high SI values due to the very low cytotoxicity (CC50 > 400 μM).
Table 3.
Inhibitory effects on PEDV replication of compounds 1–3, and 6.
| Compound | CC50 (μM) | EC50 (μM) | SI |
|---|---|---|---|
| 1 | >400 | NA | |
| 2 | >400 | 13.41 ± 1.13 | >30.04 ± 2.74 |
| 3 | 211.26 ± 29.64 | 4.49 ± 0.67 | 47.11 ± 0.49 |
| 6 | 165.30 ± 16.49 | 6.17 ± 0.50 | 26.75 ± 0.54 |
| 6-Azauridine | 44.47 ± 6.11 | 5.58 ± 0.53 | 7.98 ± 0.37 |
3. Conclusion
In the phytochemical investigation herein of the Z. jujuba roots, five new Ib-type cyclopeptide alkaloids were isolated with three known cyclopeptide alkaloids. Among the isolated compounds, three new and one known alkaloids were subjected to in vitro antiviral assays. Three compounds showed potent inhibitory activities against a swine-infecting coronavirus, PEDV. To our knowledge, this is the first report on the antiviral activities of plant-derived cyclopeptide alkaloids.
4. Experimental
4.1. General experimental procedures
Column chromatography (CC) was carried out with Kieselgel 60 silica gel (40–60 μm, 230–400 mesh, Merck, Darmstadt, Germany) and Sephadex LH-20 (25–100 μm, Pharmacia, Piscataway, NJ, USA). TLC was performed on the Kieselgel 50 F254 coated normal silica gel (Merck). The preparative HPLC system consisted of a G-321 pump (Gilson, Middleton, WI, USA), a G-151 UV detector (Gilson), and a XBridge Prep C18 column (250 mm × 10 mm i.d.; 5 μm, Waters, Milford, MA, USA). All solvents (analytical grade) were purchased from Daejung Chemicals & Metals Co. Ltd. (Si-heung, Korea). NMR spectra were recorded on Bruker AMX 500 and 600 spectrometers (Bruker, Billerica, MA, USA). High resolution ESI-qTOF-MS were obtained by a Waters Xevo G2 qTOF mass spectrometer (Waters MS Technologies, Manchester, UK). UV and CD spectra were obtained using a Chirascan and CD spectrometer (Applied photophysics, Surrey, UK). Optical rotations were taken on a JASCO P-2000 polarimeter (JASCO, Easton, MD, USA). IR spectra were recorded on JASCO FT/IR-4200 spectrometer. Advanced Marfey’s reagent (d-FDLA and l-FDLA) and GITC were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). The (S)-(+)-Phenylglycine methyl ester hydrochloride [(S)-(+)-PGME] reagent was purchased from Sigma–Aldrich (St. Louis, MO, USA). The absolute configuration of amino acids was analyzed using Agilent 6120 quadruple MSD consisting of the 1260 Infinity pump, 1260 Infinity autosampler, 1260 Infinity DAD detector (Agilent Technologies, Santa Clara, CA, USA), a Phenomenex column (Luna 5μ C18 (2) 100 Å New Column, 4.6 mm × 100 mm, i.d.; 5 μm, Sungmoon Systech Co. Ltd., Seoul, Korea), and an Openlab ChemStation (Agilent) for data acquisition and processing.
4.2. Plant materials
Z. jujuba roots were collected in JinJu, Korea, in 2012. The plant was identified by Prof. Eun Ju Jeong (Gyeongnam National University of Science and Technology, JinJu, Republic of Korea). A voucher specimen (SUPH-1204-01) is deposited in the Herbarium in the Medicinal Plant Garden, College of Pharmacy at Seoul National University in Korea.
4.3. Extraction and isolation
Powdered dried roots of Z. jujuba (14.5 kg) were extracted through maceration with MeOH (2 × 60 L, for one week each) at rt. A crude extract (0.5 kg) resulted from extraction solvent removal. The extract was suspended in H2O and acidified with 1 N HCl to pH 3–4. The acidic solution was firstly extracted with EtOAc to yield the EtOAc fraction (186.2 g). The aqueous residue was basified with NaOH to pH 9 and extracted with CHCl3 to provide the alkaloid fraction (1.7 g), with the latter subjected to silica CC eluted with increasingly polar CHCl3–MeOH combinations (30:1, 10:1, 5:1, 3:1 and 1:1) to yield four subfractions, A1–4. Subfraction A3 (348.5 mg) was separated by Sephadex LH-20 CC eluted with CH2Cl2–MeOH (3:1) to give five fractions, A3a–A3e. A3c was purified by a preparative HPLC (0.1% NH4Ac in H2O–MeCN, 7:3, 4 mL/min) to yield 1 (7.8 mg), 2 (53.1 mg), 3 (6.9 mg) and 6 (13.5 mg). A2 (207.8 mg) was subjected to Sephadex LH-20 CC eluted with CH2Cl2–MeOH (3:1) to give four subfractions, A2a–A2d. A2b was separated by preparative HPLC (0.1% NH4Ac in H2O–MeCN, 3:2 to 1:4, 4 mL/min) to yield 4 (2.7 mg), 5 (2.0 mg), 7 (1.6 mg), and 8 (2.0 mg).
4.3.1. Jubanine F (1)
White amorphous powder; UV (MeOH) λmax (log ε) 276 (3.47), 323 (3.87) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−2.2), 294 (−0.9), 265 (−4.4), 234 (−1.8), 213 (−5.9) nm; [α]D 20 = −309.8 (MeOH); IR ν max 2966, 2352, 2317, 1674, 1645, 1514, 1566, 1222 cm−1; See Table 1, Table 2 for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 544.3112 [M+H]+ (calcd. for C28H42N5O6, 544.3135).
4.3.2. Jubanine G (2)
White amorphous powder; UV (MeOH) λmax (log ε) 273 (3.22), 323 (3.06) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−2.5), 294 (−0.9), 264 (−4.9), 231 (−0.5), 215 (−3.7) nm; [α]D 20 = −247.8 (MeOH); IR ν max 3332, 2964, 1643, 1513, 1446, 1222, 1186, 1038, 1027 cm−1; See Table 1, Table 2 for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 558.3296 [M+H]+ (calcd. for C29H44N5O6, 558.3292).
4.3.3. Jubanine H (3)
White amorphous powder; UV (MeOH) λmax (log ε) 271 (3.65), 323 (3.50) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−4.2), 295 (−1.7), 262 (−8.9), 231 (−0.8), 215 (−6.2) nm; [α]D 20 = −280.9 (MeOH); IR ν max 3337, 2966, 1678, 1643, 1513, 1432, 1222, 1031, 1006 cm−1; See Table 1, Table 2 for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 572.3451 [M+H]+ (calcd. for C30H46N5O6, 572.3448).
4.3.4. Jubanine I (4)
White amorphous powder; UV (MeOH) λmax (log ε) 271 (3.15), 322 (3.27) nm; CD (c 0.02, MeOH) λmax (Δε) 322 (−0.8), 293 (0.0), 264 (−1.6), 236 (−0.7), 212 (−2.5) nm; [α]D 20 = −64.3 (MeOH); IR ν max 2966, 2936, 2350, 1681, 1516, 1391, 1342, 1033, 1011 cm−1; See Table 1, Table 2 for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 572.3451 [M+H]+ (calcd. for C30H46N5O6, 572.3448).
4.3.5. Jubanine J (5)
White amorphous powder; UV (MeOH) λmax (log ε) 271 (3.13), 323 (2.82) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−2.0), 296 (−0.8), 262 (−4.3), 232 (−0.4), 215 (−3.2) nm; [α]D 20 = −172.8 (MeOH); IR ν max 3707, 2972, 2873, 2350, 1643, 1513, 1220, 1054, 1033, 1013 cm−1; See Table 1, Table 2 for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 586.3597 [M+H]+ (calcd. for C31H48N5O6, 586.3605).
4.3.6. Nummularine B (6)
White amorphous powder; UV (MeOH) λmax (log ε) 270 (3.84), 320 (3.68) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−6.6), 295 (−3.1), 264 (−12.5), 231 (+0.3), 217 (−9.6) nm; See Supplementary data for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 592.3107 [M+H]+ (calcd. for C32H42N5O6, 592.3125).
4.3.7. Daechuine-S3 (7)
White amorphous powder; UV (MeOH) λmax (log ε) 272 (3.95), 320 (3.79) nm; CD (c 0.40, MeOH) λmax (Δε) 321 (−22.7), 296 (−9.6), 263 (−49.1), 232 (−12.7), 216 (−45.0) nm; See Supplementary data for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 628.4074 [M+H]+ (calcd. for C34H54N5O6, 628.4059).
4.3.8. Mucronine K (8)
White amorphous powder; UV (MeOH) λmax (log ε) 270 (3.76), 323 (3.87) nm; CD (c 0.10, MeOH) λmax (Δε) 321 (−6.3), 295 (−2.6), 264 (−12.7), 232 (+0.9), 217 (−7.1) nm; See Supplementary data for 1H and 13C NMR spectroscopic data; ESI-qTOF-MS (positive ion mode) m/z 515.3245 [M+H]+ (calcd. for C28H44N4O5, 515.3245).
4.4. Acid hydrolysis of compounds
Approximately 0.2 mg of 1–3 and 0.1 mg of 4–5 were hydrolyzed with 6 N HCl (100 μL) at 110 °C for 30 min with stirring. The hydrolysates were evaporated to dryness and then the dried hydrolysates were resuspended in H2O (100 μL). The solutions were concd under reduced pressure.
4.4.1. Determining absolute configurations of the amino acids in 1–5 by the advanced Marfey’s method using LC–MS
Each hydrolysate (30 μg) was added to 1 M NaHCO3 (200 μL) and 1% d- or l-FDLA in acetone (25 μL). The reaction vials were incubated and stirred for 30 min at 50 °C. The reactions were then quenched with 2 N HCl (100 μL). MeOH (100 μL) was added to prepare LC–MS samples. The reaction products were analyzed by HPLC–MS with a positive ion detection mode. H2O–MeCN containing 0.05% HCO2H was used as eluents with MeCN containing 0.05% HCO2H increasing from 5% to 100% over 19 min at a flow rate of 0.7 mL/min. Authentic standards (200 μg) were also prepared and analyzed using the same procedure. The retention times of the hydrolysates and amino acid standard d and l-FDLA-derivatives were as follows: l-Val-d-FDLA (t R 13.51 min, m/z 412 [M+H]+), l-Val-l-FDLA (t R 12.04 min, m/z 412 [M+H]+), l-N-Me-Ala-l-FDLA (t R 11.92 min, m/z 398 [M+H]+) at 2, l-N-Me-Ala-d-FDLA (t R 12.05 min, m/z 398 [M+H]+), l-Ile/l-allo-Ile-l-FDLA (t R 12.62 min, m/z 426 [M+H]+) at 2, and l-Ile/l-allo-Ile-d-FDLA (t R 14.04 min, m/z 426 [M+H]+).
4.4.2. Determining absolute configurations of the amino acids in 2–5 by GITC analysis using LC–MS
Each hydrolysate (30 μg) was treated with 6% trimethylamine (40 μL) and 1% GITC reagent (40 μL). After 30 min of incubation at rt, 5% AcOH (40 μL) was added to a reaction vial to quench the reaction. MeOH (50 μL) was added to the reaction residues to prepare LC–MS samples. An authentic standard was also prepared from the same procedure. For the GITC product analysis of natural hydrolysates and standard amino acids, the following chromatographic method was used: solvent A was H2O:MeCN with 0.1% HCO2H (95:5, v/v), solvent B was MeCN with 0.1% HCO2H. The LC–MS program for detecting the GITC-derivatives was set as 5% solvent B (0–7 min), 5–27% solvent B (7–12 min), 27–28.5% solvent B (12–45 min), 28.5–100% solvent B (45–46 min), and 100–100% solvent B (46–51 min) at a flow rate of 1 mL/min. The reaction products were analyzed on a positive mode with a LC–MS system. The co-injection experiments of the GITC-derivatized hydrolysates with authentic amino acid derivatives (l-Ile and l-allo-Ile) were established so that Ile residues in 2–5 are all l-Ile (Retention time: l-Ile-GITC (33.67 min)/l-allo-Ile-GITC (33.31 min)).
4.4.3. Preparing l- and d-N,N-dimethylalanine (Choi et al., 2012)
The l and d-alanine (8 mg) standards in H2O were individually treated with HCHO (27 μL) and 10% Pd/C (10.4 mg). The mixtures were subjected to H2 for 16 h and each reaction mixture was boiled and then dried under reduced pressure.
4.4.4. Determining absolute configurations of N,N-dimethylalanine in 4 and 5 by PGME derivatization and LC/MS analyses
Each dried l- and d-N,N-dimethylalanine was dissolved in tetrahydrofuran (THF, 500 μL). After adding 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC, 8.8 μL) to each vial, each mixture was stored at rt and stirred for 5 min. (S)-(+)-PGME (10.5 mg) was added to each vial and each mixture was stirred for 6 h at rt. The reaction products were dried under N2 gas and extracted with CH2Cl2. TheCH2Cl2 soluble products were analyzed by LC–MS [same column but 150 mm length instead of 100 mm; solvent A was H2O:MeCN with 0.05% HCO2H (95:5, v/v), solvent B was MeCN with 0.05% HCO2H with gradient solvent system as follows: 5% solvent B (0–2 min), 5–10% solvent B (2–12.5 min), 10–30% solvent B (12.5–15 min), flow rate 0.7 mL/min]. The authentic amides, the (S)-(+)-PGME product of l- and d-N,N-dimethylalanine (m/z 265 [M+H]+), were eluted at 8.83 and 10.24 min, respectively. The (S)-(+)-PGME product of N,N-dimethylalanine in 4 and 5 hydrolysates were observed at 8.79 and 8.73 min, respectively, retention time for LC–MS analyses. The absolute configurations of N,N-dimethylalanine in 4 and 5 were identified as both l forms (S configuration).
4.5. Cell culture and virus stock
Vero cells (African green monkey kidney cell line; ATCC CCR-81) were provided by the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 100 U/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum (FBS). PEDV was obtained from Choong Ang Vaccine Laboratory, Korea. The virus stock was kept at −80 °C before use.
4.5.1. Cytotoxicity assay
The cell viability was calculated using a MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay. Vero cells were adhered at 1 × 105 cells per well in 96-well plates and grown for 24 h before treatment. The cells were treated with various concentrations of compounds. To avoid solvent toxicity, the final DMSO concentration was maintained under 0.05% (v/v) in the culture medium. After incubating further for 48 h, MTT solution (2 mg/mL, 20 μL) was added to each well and kept for 4 h. After removing the supernatant, DMSO (100 μL) was added to solubilize formazan crystals. Consequently, the absorbance was measured at 550 nm. The percentage cell viability is the absorbance in the experiment well compared to that in the control wells and compound toxicity is the percentage cell viability. Regression analysis was used to calculate 50% cytotoxic concentration (CC50).
4.5.2. Cytopathic effect (CPE) inhibition assay
Vero cells were seeded onto 96-well plates at 1 × 105 cells per well. The medium was removed a day later and washed with phosphate buffered saline (PBS). PEDV at 0.01 MOI was inoculated onto near-confluent Vero cell monolayers for 2 h. The media was replaced by DMEM with various concentrations of compounds. After incubating for 72 h at 37 °C under 5% CO2 atmosphere, cells were replaced with DMEM and MTT (2 mg/mL, 20 μL) to each well and incubated for 4 h at 37 °C. The 50% effective concentration (EC50) was calculated using regression analysis, and the formula SI = CC50/EC50 determined the selective index (SI).
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A2A2A01016296). We would like to thank Mr. Bong Geun Jeong and Prof. Eun Ju Jeong (Gyeongnam National University of Science and Technology, JinJu, Republic of Korea) for kindly providing the plant materials.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.phytochem.2015.09.001.
Appendix A. Supplementary data
This document contains supplementary information.
References
- Barboni L., Gariboldi P., Torregiani E., Verotta L. Cyclopeptide alkaloids from Ziziphus mucronata. Phytochemistry. 1994;35:1579–1582. [Google Scholar]
- Choi H., Mevers E., Byrum T., Valeriote F.A., Gerwick W.H. Lyngbyabellins K–N from two palmyra atoll collections of the marine cyanobacterium Moorea bouillonii. Eur. J. Org. Chem. 2012;27:5141–5150. doi: 10.1002/ejoc.201200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S., Pandey V.B., Singh J.P., Shah A.H. Peptide alkaloids from Zizyphus species. Phytochemistry. 1987;26:3374–3375. [Google Scholar]
- Fujii K., Ikai Y., Oka H., Suzuki M., Harada K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997;69:5146–5151. [Google Scholar]
- Gournelis D.C., Laskaris G.G., Verpoorte R. Cyclopeptide alkaloids. In: Herz W., Falk H., Kirby G.W., Moore R.E., Tamm C.H., editors. vol. 75. Springer-Verlag/Wein; New York: 1998. pp. 1–179. (Progress in the Chemistry of Organic Natural Products). [DOI] [PubMed] [Google Scholar]
- Hess S., Gustafson K.R., Milanowski D.J., Alvira E., Lipton M.A., Pannell L.K. Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A. 2004;1035:211–219. doi: 10.1016/j.chroma.2004.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar I., Ahmed A., Kashmiri M. Alkaloidal studies of medicinal plants of Pakistan from the root bark of Zizyphus jujuba mill. J. Nat. Sci. Math. 1994;34:159–163. [Google Scholar]
- Lee S.S., Su W.C., Liu K.C.S.C. Cyclopeptide alkaloids from stems of Paliurus ramossisimus. Phytochemistry. 2001;58:1271–1276. doi: 10.1016/s0031-9422(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Chen C.H., You B.J., Liu K.C., Lee S.S. Cyclopeptide alkaloids from Paliurus ramossisimus. J. Nat. Prod. 2000;63:1338–1343. doi: 10.1021/np000136a. [DOI] [PubMed] [Google Scholar]
- Outlaw W.H., Zhang S.Q., Riddle K.A., Womble A.K., Anderson L.C., Outlaw W.M., Outlaw N.N., Outlaw E.C., Thistle A.B. The jujube (Ziziphus jujuba Mill.), a multipurpose plant. Econ. Bot. 2002;56:198–200. [Google Scholar]
- Pandey M.B., Singh A.K., Singh J.P., Singh V.P., Pandey V.B. Three new cyclopeptide alkaloids from Zizyphus species. J. Asian Nat. Prod. Res. 2008;10:709–713. doi: 10.1080/10286020802016321. [DOI] [PubMed] [Google Scholar]
- Panseeta P., Lomchoey K., Prabpai S., Kongsaeree P., Suksamrarn A., Ruchirawat S., Suksamrarn S. Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry. 2011;72:909–915. doi: 10.1016/j.phytochem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Lieberknecht A., Bokens H., Griesser H. Total synthesis of Zizyphine A. Synthesis of peptide alkaloids. 8. Amino acids and peptides. 40. J. Org. Chem. 1983;48:2680–2685. [Google Scholar]
- Suksamrarn S., Suwannapoch N., Aunchai N., Kuno M., Ratananukul P., Haritakun R., Jansakul C., Ruchirawat S. Ziziphine N, O, P and Q, new antiplasmodial cyclopeptide alkaloids from Ziziphus oenoplia var. brunoniana. Tetrahedron. 2005;61:1175–1180. [Google Scholar]
- Tan N.H., Zhou J. Plant cyclopeptides. Chem. Rev. 2006;106:840–895. doi: 10.1021/cr040699h. [DOI] [PubMed] [Google Scholar]
- Tripathi M., Pandey M.B., Jha R.N., Pandey V.B., Tripathi P.N., Singh J.P. Cyclopeptide alkaloids from Zizyphus jujuba. Fitoterapia. 2001;72:507–510. doi: 10.1016/s0367-326x(01)00278-7. [DOI] [PubMed] [Google Scholar]
- Tschesche R., Khokhar I., Wilhelm H., Eckhardt G. Jubanine-A and Jubanine-B, new cyclopeptide alkaloids from Ziziphus jujuba. Phytochemistry. 1976;15:541–542. [Google Scholar]
- Um S., Choi T.J., Kim H., Kim B.Y., Kim S.H., Lee S.K., Oh K.B., Shin J., Oh D.C. Ohmyungsamycins A and B: cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J. Org. Chem. 2013;78:12321–12329. doi: 10.1021/jo401974g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains supplementary information.


