Abstract
Porcine deltacoronavirus (PDCoV) was first identified in Hong Kong in 2009–2010 and reported in United States swine for the first time in February 2014. However, diagnostic tools other than polymerase chain reaction for PDCoV detection were lacking and Koch׳s postulates had not been fulfilled to confirm the pathogenic potential of PDCoV. In the present study, PDCoV peptide-specific rabbit antisera were developed and used in immunofluorescence and immunohistochemistry assays to assist PDCoV diagnostics. The pathogenicity and pathogenesis of PDCoV was investigated following orogastric inoculation of 5-day-old piglets with a plaque-purified PDCoV cell culture isolate (3×104 TCID50 per pig). The PDCoV-inoculated piglets developed mild to moderate diarrhea, shed increasing amount of virus in rectal swabs from 2 to 7 days post inoculation, and developed macroscopic and microscopic lesions in small intestines with viral antigen confirmed by immunohistochemistry staining. This study experimentally confirmed PDCoV pathogenicity and characterized PDCoV pathogenesis in neonatal piglets.
Keywords: PDCoV, Coronavirus, Atrophic enteritis, Peptide-specific antisera, Immunohistochemistry
Introduction
Coronaviruses (CoV) are enveloped, positive-sense, single-stranded RNA viruses in the family Coronaviridae of the order Nidovirales and have the largest RNA genomes among the recognized RNA viruses thus far (Woo et al., 2010). The traditional group 1, 2, and 3 coronaviruses have been replaced with three genera designated Alphacoronavirus, Betacoronavirus, and Gammacoronavirus, respectively. In recent years, a group of novel coronaviruses was identified in Asian leopard cats and some avian species (Dong et al., 2007, Woo et al., 2009) and they were proposed to represent a new coronavirus genus, i.e. the fourth genus Deltacoronavirus (Woo et al., 2010). During a molecular surveillance study conducted by a Hong Kong group, additional deltacoronaviruses were identified in avian and mammalian species including two porcine CoVs (HKU-15-44 and HKU-15-155) detected in pig samples collected in 2009 (Woo et al., 2012). Hereby, five coronaviruses have been identified in pigs: porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and porcine respiratory coronavirus (PRCV) in the genus Alphacoronavirus; porcine hemagglutinating encephalomyelitis virus (PHEV) in the genus Betacoronavirus; and porcine deltacoronavirus (PDCoV) in the genus Deltacoronavirus.
PDCoV was first detected in US swine in the state of Ohio in February 2014 from pigs with diarrhea (Wang et al., 2014a) and has been confirmed in 17 US states as of December 2014 (www.aasv.org). PDCoV has also recently been detected in South Korea (Lee and Lee, 2014). It remains unclear when PDCoV was introduced into the US, but a recent PCR-based retrospective evaluation of diagnostic samples revealed that PDCoV could be detected as early as August 2013 in pig samples from the US (Sinha et al., unpublished data). The US PDCoV sequences determined thus far share high nucleotide identity (≥99.8%), with 98.9–99.2% nucleotide identity to the Hong Kong strains, and 99.6–99.8% nucleotide identity to the Korean strain, at the whole genome level (Lee and Lee, 2014, Li et al., 2014, Marthaler et al., 2014a, Marthaler et al., 2014b, Wang et al., 2014a, Wang et al., 2014b, Woo et al., 2012). The genome organization and arrangement of PDCoV are in the order of: 5′ untranslated region (UTR), open reading frame 1a/1b (ORF1a/1b), spike (S), envelope (E), membrane (M), nonstructural protein 6 (NS 6), nucleocapsid (N), nonstructural protein 7 (NS 7), and 3′ UTR (Li et al., 2014, Woo et al., 2012).
Thus far, all peer-reviewed literature related to PDCoV has focused on polymerase chain reaction (PCR) detection and genetic analyses. There are no other published assays for diagnosing PDCoV infection worldwide. Although PDCoV has been detected in swine samples, its clinical significance as an etiological pathogen has not been experimentally confirmed in pigs. In this paper, the development of PDCoV peptide-specific rabbit antisera and the use of these antisera in immunofluorescence and immunohistochemistry assays for PDCoV detection are described. Additionally, 5-day-old piglets were experimentally inoculated with a plaque-purified US PDCoV cell culture isolate to characterize the pathogenicity and pathogenesis of this virus.
Results
PDCoV propagation
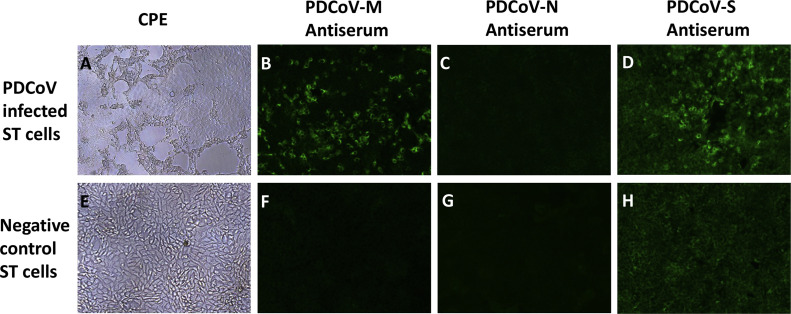
Swine testicle (ST) cells infected with the plaque-purified PDCoV cell culture isolate USA/IL/2014 at a multiplication of infection (MOI) of 1 developed >80% cytopathic effects (CPE) at 24 h post infection (hpi), characterized by syncytia formation and cell detachment ( Fig. 1A). Virus harvested at 24 hpi had a titer of 1.8×107 TCID50/ml and was stored at −80 °C until being diluted to 3×103 TCID50/ml for pig inoculation.
Fig. 1.
Cytopathic effect (CPE) and IFA staining on PDCoV-infected ST cells or negative control ST cells (100× magnifications). (A) PDCoV CPE on ST cells at 24 h post infection; (E) negative control ST cells; (B and F) IFA staining with PDCoV M-peptide rabbit antiserum; (C and G) IFA staining with PDCoV N-peptide rabbit antiserum; (D and H) IFA staining with PDCoV S-peptide rabbit antiserum.
PDCoV peptide-specific rabbit antisera
Rabbit antisera against the PDCoV M-peptide, N-peptide, and S-peptide collected at Day 0, 28, 56, 72 and 90 post immunization were diluted (1:100, 1:200, 1:500, and 1:1000) and evaluated by IFA on PDCoV-infected ST cells. Sera collected at Day 28, 56, 72 and 90 from one rabbit (#8005) immunized with the PDCoV M-peptide yielded positive IFA staining at 1:100, 1:200 or 1:500 dilutions. There were no marked staining differences for sera collected at Day 28, 56, 72 or 90. However, 1:200 dilutions gave best IFA staining signals with less background staining. One representative image is shown in Fig. 1B. Sera at Day 0 were IFA negative. Similarly, sera collected at Day 28, 56, 72 and 90 from one rabbit (#8049) immunized with the PDCoV S-peptide were IFA positive (Fig. 1D). However, even after optimization of serum dilutions, the PDCoV S-peptide rabbit antisera still produced stronger background staining in the negative control ST cells (Fig. 1H) compared to the negative control ST cells tested by the PDCoV M-peptide rabbit antisera (Fig. 1F). Sera collected from the second rabbit immunized with the PDCoV M-peptide, from the second rabbit immunized with the PDCoV S-peptide, and from two rabbits immunized with the PDCoV N peptide, were always IFA negative regardless of collection date or dilutions. Thus, the PDCoV M-peptide rabbit antisera (#8005) were selected for subsequent evaluation and assay development.
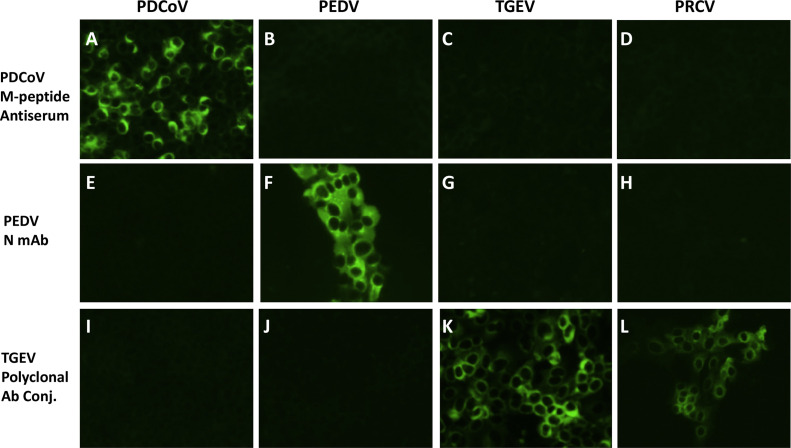
We further evaluated potential cross staining of PDCoV M-peptide rabbit antisera, PEDV monoclonal antibody conjugate SD6-29, and a TGEV polyclonal antibody conjugate on PDCoV-, PEDV-, TGEV- and PRCV-infected cells. As shown in Fig. 2A, B, C and D, PDCoV M-peptide rabbit antisera specifically stained PDCoV-infected ST cells but did not stain PEDV-infected Vero cells or TGEV- or PRCV-infected ST cells. PEDV N-based monoclonal antibody specifically stained PEDV-infected cells but not in PDCoV-, TGEV-, or PRCV-infected cells (Fig. 2E, F, G and H). The TGEV polyclonal antibody conjugate stained TGEV- and PRCV-infected cells but not in PDCoV- or PEDV-infected cells (Fig. 2I, J, K and L).
Fig. 2.
Evaluation of cross staining of PDCoV M-peptide rabbit antiserum, PEDV nucleocapsid monoclonal antibody conjugate, and TGEV polyclonal antibody conjugate on PDCoV-, PEDV-, TGEV-, and PRCV-infected cells.
Clinical assessment
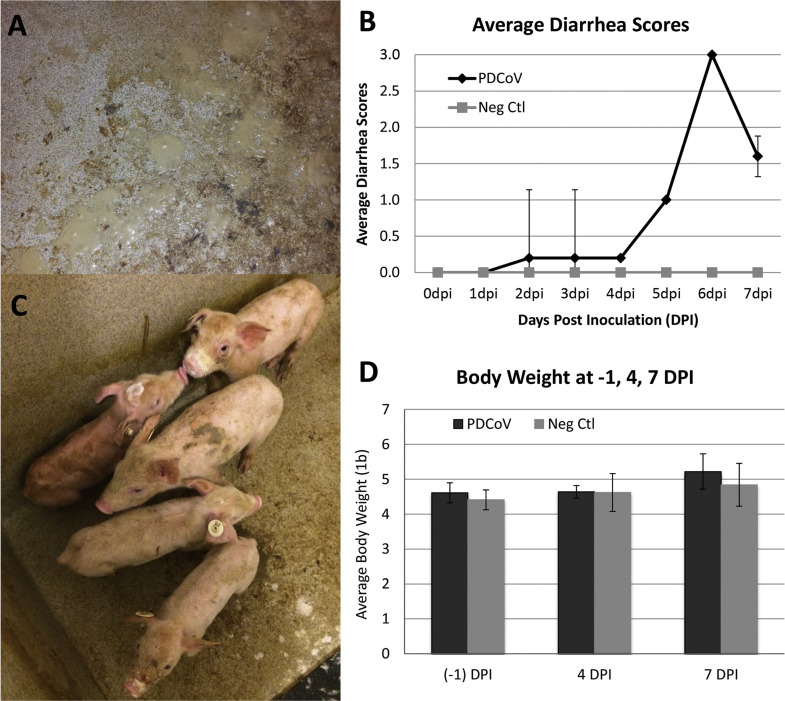
In PDCoV-inoculated pigs, 2/10 pigs had soft feces during 2-4 DPI, 5/5 pigs developed mild diarrhea at 5 DPI, 5/5 pigs had profuse watery diarrhea at 6 DPI, and 3 of 5 pigs recovered to mild diarrhea at 7 DPI ( Fig. 3A and B). Inoculated pigs remained active but had fecal staining on the skin (Fig. 3C). No vomiting, dehydration, severe loss in body condition, lethargy, loss of appetite, or mortality was observed in PDCoV-inoculated pigs in spite of the presence of diarrhea. The negative control pigs were active and fleshy throughout the study period with no observed clinical signs.
Fig. 3.
Clinical assessment of PDCoV-inoculated pigs. (A) Watery diarrhea observed at 6 days post inoculation (DPI). (B) Average diarrhea scores. (C) Pigs at 5 DPI. (D) Average body weight.
There were no significant differences in average body weights between the PDCoV-inoculated and the negative control pigs at (−1) DPI (P-value=0.62), 4 DPI (P-value=0.74), or 7 DPI (P-value=0.65) (Fig. 3D). Average daily gain was lower in the PDCoV-inoculated compared to the negative control pigs between (−1) and 4 DPI but the differences were not statistically significant (P-value=0.06). Average daily gain between (−1) and 7 DPI of the two groups was also not significantly different (P-value=0.22).
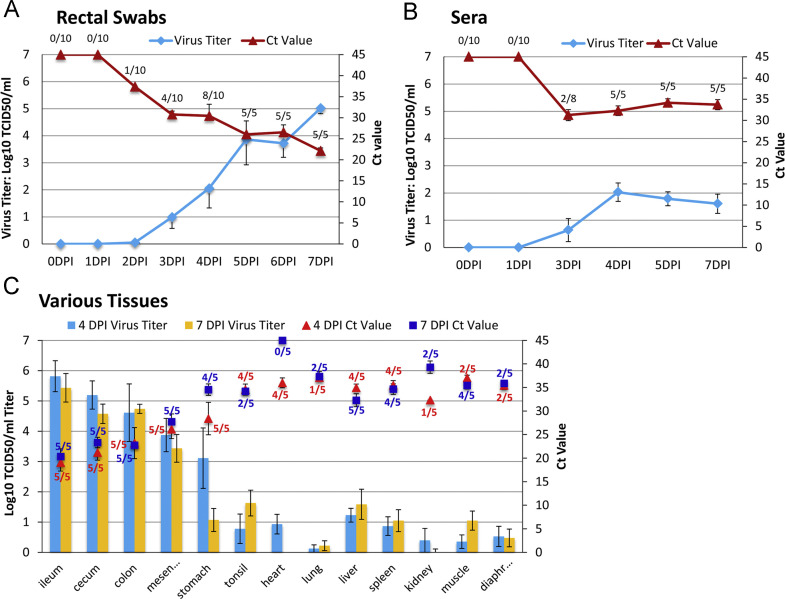
Virus shedding and distribution
Fecal virus shedding of the PDCoV-inoculated pigs is summarized in Table 2 and Fig. 4A. PDCoV RNA was detected in rectal swab samples from 1/10 pigs at 2 DPI with a Ct value of 37.4 (equivalent to 3 TCID50/ml), from 4/10 pigs at 3 DPI with Ct ranges of 28.99–32.23 (102–103 TCID50/ml), and from 8/10 pigs at 4 DPI with wide Ct ranges of 19.05–37.53 (100.5–105 TCID50/ml). All of the 5 remaining pigs after the first necropsy were PDCoV PCR positive on their rectal swabs and shed virus of 104–105 TCID50/ml with Ct ranges of 20.6–24.2 at 7 DPI.
Table 2.
Real-time RT-PCR Ct values on rectal swabs of PDCoV-inoculated pigs.
| Treatment group | Pig ID | PDCoV rRT-PCR Ct values on rectal swabs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 DPI | 1 DPI | 2 DPI | 3 DPI | 4 DPI | 5 DPI | 6 DPI | 7 DPI | ||
| PDCoV | 1 | >45 | >45 | >45 | >45 | 35.51 | X | X | X |
| PDCoV | 13 | >45 | >45 | >45 | 32.23 | 19.40 | X | X | X |
| PDCoV | 20 | >45 | >45 | 37.40 | 28.99 | 27.01 | X | X | X |
| PDCoV | 21 | >45 | >45 | >45 | 32.10 | 19.05 | X | X | X |
| PDCoV | 39 | >45 | >45 | >45 | 29.78 | 31.32 | X | X | X |
| PDCoV | 16 | >45 | >45 | >45 | >45 | 37.53 | 21.05 | 21.86 | 21.09 |
| PDCoV | 32 | >45 | >45 | >45 | >45 | >45 | 25.10 | 29.33 | 21.49 |
| PDCoV | 47 | >45 | >45 | >45 | >45 | 37.47 | 34.41 | 24.98 | 23.20 |
| PDCoV | 50 | >45 | >45 | >45 | >45 | 36.23 | 17.61 | 24.72 | 24.25 |
| PDCoV | 56 | >45 | >45 | >45 | >45 | >45 | 32.07 | 31.74 | 20.61 |
Note: Piglets #1, 13, 20, 21 and 39 were euthanized and necropsied at 4 DPI.
Fig. 4.
Virus shedding in rectal swabs (A), sera (B) and various tissues (C) of PDCoV-inoculated pigs. Data labels (fractions) represent the number of PDCoV PCR positive pigs out of all pigs examined at that time point. The Ct values depicted in each figure were mean Ct values of PCR-positive pigs. The virus titers (Log10TCID50/ml) were mean virus titers of all pigs (both PCR-positive and negative pigs). Standard error bars are shown in each figure. At 3 DPI, sera were successfully collected from 8 out of 10 pigs.
As shown in Fig. 4B, viral RNA was detected in sera of 2/8 PDCoV-inoculated pigs at 3 DPI with Ct values of 29.96–32.57 (102.2–102.9 TCID50/ml). Viral RNA was detected from sera of all pigs from 4 to 7 DPI with Ct ranges of 29.06–37.39 (100.5–103 TCID50/ml); average Ct values were 32.27–34.15 in sera of 4–7 DPI.
Virus distributions in various tissues were examined at 4 DPI and 7 DPI necropsies using PDCoV-specific PCR (Fig. 4C). Virus was detected from all (100%) ileums, ceca, and colons at both 4 and 7 DPI with average Ct values of 19.03–23.32 (average titers 104.6–105.8 TCID50/ml). Virus was detected from all mesenteric lymph nodes at both 4 and 7 DPI with average Ct values of 26.13–27.74 (average titers 103.4–103.9 TCID50/ml), from 5/5 stomachs at 4 DPI with an average Ct of 28.40 (average titer 103.1 TCID50/ml) and from 4/5 stomachs at 7 DPI with an average Ct of 34.54 (average titer 101.1 TCID50/ml). Virus could be detected in low quantities in tonsil, lung, heart, liver, spleen, kidney, muscle from rear leg, and diaphragm (Fig. 4C).
All fecal, sera, and tissue samples from the negative control pigs were negative by PDCoV PCR.
Gross pathology
Small intestines, ceca and colons of PDCoV-inoculated pigs often contained yellow, soft to watery contents at 4 and 7 DPI. Thin-walled and/or gas-distended small intestines, and gas-distended ceca and colons were observed in most PDCoV-inoculated pigs at 4 and 7 DPI ( Fig. 5A). No lesions were observed in other examined tissues including mesenteric lymph nodes, stomach, tonsil, lung, heart, liver, spleen, kidney, muscle from rear leg, and diaphragm, regardless of inoculation status.
Fig. 5.
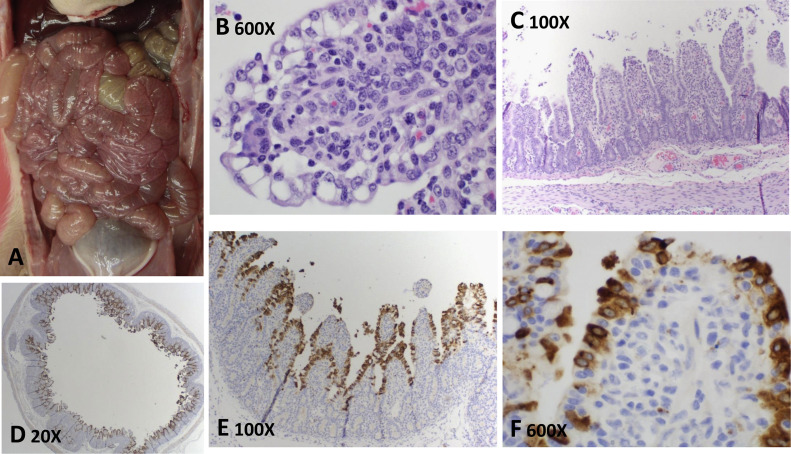
Macroscopic and microscopic lesions and IHC staining. Thin-walled small intestines from a PDCoV-inoculated pig at 4 DPI (A). Hematoxylin and eosin-stained tissue sections of small intestine (middle jejunum) from a PDCoV-inoculated pig at 4 DPI (B, 600× magnification and C, 100× magnification). IHC-stained tissue sections of small intestine (middle jejunum) from a PDCoV-inoculated pig at 4 DPI (D, E, F with 20×, 100×, and 600× magnifications, respectively).
Histopathology
Mild to severe villous atrophy consistent with viral infection was observed in 4/5 pigs at 4 DPI necropsy and 4/5 pigs at 7 DPI necropsy in the PDCoV-inoculated group. Lesions varied across all pigs and were primarily observed in the middle and distal jejunum and ileum and were either unapparent or minimal in duodenum and proximal jejunum. Lesions consisted of multifocal to diffuse villous enterocyte swelling and vacuolation (Fig. 5B) or moderate to severe villous blunting and atrophy in some pigs at 4 DPI (Fig. 5C). Villous enterocytes were mildly attenuated or severely flattened and necrotic with sloughing of degenerate enterocytes into the lumen. In addition, low numbers of lymphocytes and neutrophils infiltrated a moderately contracted lamina propria that occasionally demonstrated apoptotic debris and congested blood vessels. Similar lesions were observed at 7 DPI that included mild to moderate enterocyte attenuation and necrosis with sloughing enterocytes. Villous atrophy and occasional fusion of villi were apparent at 7 DPI with low numbers of neutrophils and lymphocytes in the lamina propria. Crypt hyperplasia and elongation was mild throughout all sections. Enterocyte syncytia were not observed. Microscopic lesions were not apparent in sections of cecum and colon at any time point.
Villus height, crypt depth, and villus-height-to-crypt-depth ratio were measured and compared on small intestines of PDCoV-inoculated and negative control pigs ( Table 3). At 4 DPI, the PDCoV-inoculated pigs had significantly decreased average villus heights, increased average crypt depths, and lower average villus/crypt ratios in middle and distal jejunums and ileum than the negative control pigs but the average villus heights, crypt depths and villus/crypt ratios were not significantly different in duodenum and proximal jejunum between the two groups of pigs. At 7 DPI, significant differences were observed in average villus heights of middle and distal jejunum and ileum, average crypt depths of distal jejunum, and average villus/crypt ratios of distal jejunum and ileum between the PDCoV-inoculated and negative control pigs.
Table 3.
Mean villus height (µm), crypt depth (µm), and villus/crypt ratio in various intestinal segments from PDCoV-inoculated and negative control pigs at 4 and 7 days post inoculation (DPI).
| DPIa | Parameterb | Duodenum (Mean±SE)c |
Proximal jejunum (Mean±SE) |
Middle jejunum (Mean±SE) |
Distal jejunum (Mean±SE) |
Ileum (Mean±SE) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PDCoV | P | Control | PDCoV | P | Control | PDCoV | P | Control | PDCoV | P | Control | PDCoV | P | ||
| 4 | Villus height | 657±35 | 693±32 | 0.467 | 518±14 | 523±22 | 0.838 | 608±32 | 358±46 | <0.001 | 570±20 | 327±13 | <0.001 | 504±35 | 321±12 | <0.001 |
| 4 | Crypt depth | 109±5.7 | 112±5.9 | 0.697 | 103±7.2 | 119±4.4 | 0.073 | 106±4.3 | 127±8.1 | 0.028 | 119±6.3 | 150±12 | 0.025 | 115±4.5 | 155±7.9 | <0.001 |
| 4 | Villus/Crypt ratio | 6.1±0.7 | 6.3±0.8 | 0.860 | 5.1±0.3 | 4.4±0.5 | 0.296 | 5.8±0.6 | 2.9±0.7 | 0.020 | 4.9±0.4 | 2.4±0.5 | 0.008 | 4.4±0.5 | 2.1±0.3 | 0.006 |
| 7 | Villus height | 598±32 | 634±20 | 0.348 | 500±22 | 438±25 | 0.075 | 496±24 | 401±22 | 0.008 | 458±23 | 346±23 | 0.002 | 453±15 | 319±15 | <0.001 |
| 7 | Crypt depth | 118±6.5 | 115±7.7 | 0.748 | 113±7.9 | 109±8.3 | 0.710 | 124±5.9 | 130±12 | 0.683 | 114±7.7 | 139±8.6 | 0.038 | 101±5.3 | 121±11 | 0.117 |
| 7 | Villus/Crypt ratio | 5.0±0.1 | 5.7±0.4 | 0.160 | 4.4±0.2 | 4.4±0.8 | 0.980 | 4.0±0.3 | 3.7±1.0 | 0.790 | 4.0±0.2 | 2.6±0.4 | 0.017 | 4.6±0.2 | 2.9±0.5 | 0.012 |
At either 4 or 7 DPI, 5 PDCoV-inoculated pigs and 5 negative control pigs were necropsied.
For each intestinal segment of each pig, villus height and crypt depth were measured on 3 sections.
The mean villus heights, crypt depths, and villus/crypt ratios and the standard errors are shown.
No significant microscopic lesions were observed in cecum, colon, and other non-intestinal tissues for both PDCoV and negative control pigs at either 4 or 7 DPI necropsy.
Immunohistochemistry
PDCoV antigen was detected in the cytoplasm of villous enterocytes of PDCoV-inoculated pigs at both 4 and 7 DPI and examples of positive staining are presented in Fig. 5D, E and F. Specifically, at 4 DPI, 0/5, 1/5, 5/5, 4/5, and 4/5 PDCoV-inoculated pigs were IHC positive, and at 7 DPI, 2/5, 3/5, 4/5, 4/5, and 4/5 PDCoV-inoculated pigs were IHC positive, in duodenum, proximal jejunum, middle jejunum, distal jejunum and ileum, respectively ( Table 4). The IHC scores varied among small intestine segments and pigs. Overall, the middle and distal jejunum and ileum had increased number of immunoreactive enterocytes compared to the duodenum and proximal jejunum (Table 4). PDCoV IHC staining was not observed in examined sections of cecum, colon, lung, liver, spleen, kidney, heart, tonsil, diaphragm, muscle, stomach and mesenteric lymph node of the PDCoV-inoculated pigs. PDCoV IHC staining was negative on all of the examined tissues from the negative control pigs.
Table 4.
PDCoV PCR on feces and IHC staining on various small intestine tissues of PDCoV-inoculated and negative control pigs at 4 and 7 days post inoculation (DPI).
| Group | Necropsy | Pig number | PCR on fecesa |
IHC positive pigs (Average IHC score) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| PCR positive | Mean Ct | Duodenum | Proximal jejunum | Middle jejunum | Distal Jejunum | Ileum | |||
| PDCoV | 4 DPI | N=5 | 5/5 | 26.4 | 0/5 (N/A) | 1/5 (0.80) | 5/5 (2.35) | 4/5 (1.90) | 4/5 (2.05) |
| Control | 4 DPI | N=5 | 0/5 | >45 | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) |
| PDCoV | 7 DPI | N=5 | 5/5 | 22.1 | 2/5 (0.50) | 3/5 (1.10) | 4/5 (1.75) | 4/5 (1.75) | 4/5 (1.55) |
| Control | 7 DPI | N=5 | 0/5 | >45 | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) | 0/5 (N/A) |
N/A: Not applicable.
Feces collected on necropsy day.
Testing for other enteric viral and bacterial pathogens
Fecal swabs collected at 4 and 7 DPI from both PDCoV-inoculated and negative control groups immediately before necropsy were negative for PEDV, TGEV, and porcine rotaviruses (groups A, B and C) by virus-specific PCRs and were negative for hemolytic Escherichia coli and Salmonella by routine bacterial culture.
Discussion
While deltacoronavirus has been detected previously in pigs, many questions regarding the significance of this detection remained unanswered given the lack of available direct detection assays. The objectives of this study were to develop PDCoV-specific immunofluorescence and IHC assays to assist with disease diagnosis and also to investigate the pathogenecity and pathogenesis of PDCoV in experimentally infected pigs.
There are different approaches to generate antibody reagents against viruses, such as (1) generation of virus-specific monoclonal antibody, (2) generation of polyclonal antibody by cloning, expressing and purifying recombinant virus proteins and then immunizing animals, (3) generation of virus peptide-specific polyclonal antisera. The first two approaches could take longer time to obtain the desired antibody reagents. In contrast, it is easier to synthesize peptides and generate peptide-specific antibody in less time. In this study, we took the latter approach to generate PDCoV M-, N-, and S-peptide-specific antisera in rabbits. As early as 28 days post immunization, PDCoV M-peptide-specific and S-peptide-specific rabbit antisera with high antibody titers were obtained and the antibody titers remained through 90 days post primary immunization. However, the sera generated from one of the two rabbits immunized with the PDCoV M-peptide or the PDCoV S-peptide, and the sera from two rabbits immunized with the PDCoV N peptide were negative by IFA in PDCoV-infected cells. Interestingly, all of the rabbit antisera contained high concentrations of antibodies against the corresponding target peptides when evaluated in an ELISA using the synthetic peptides as coating antigens (data not shown). This suggests that anti-peptide antibodies generated in some rabbits may not recognize and react with the natively expressed virus proteins. The PDCoV M-peptide rabbit antiserum was used for further evaluation and it specifically reacted to PDCoV but not to other porcine coronaviruses such as PEDV, TGEV, and PRCV. The PDCoV-specific immunofluorescence assay and IHC assay developed in this study provide additional tools to confirm virus isolation results, to determine virus distribution and cellular localization in tissues, and to facilitate study on PDCoV pathogenesis.
Although PDCoV has been detected from naturally-infected pigs with diarrhea on farms, experimental infections under controlled conditions are needed to definitely prove PDCoV as an etiological pathogen especially considering that co-infections of PDCoV with other enteric viruses such as PEDV and rotavirus were common in natural infections (Marthaler et al., 2014b). Clinical materials positive for PDCoV by PCR are not ideal to evaluate viral pathogenicity because, although the clinical materials can be proved negative for the common enteric pathogens such as PEDV, TGEV, rotavirus etc., there is no absolute certainty that they do not contain other unknown pathogens. In contrast, plaque-purified cell culture isolate of PDCoV can be utilized to more precisely investigate PDCoV pathogenicity and clinical outcomes. Ideally, the pathogenicity and pathogenesis of PDCoV should be examined in pigs at different production stages (e.g. gilts, sows, weaned pigs, and neonatal pigs etc.). In the present study, 5-day-old neonatal pigs were chosen as the first step of these investigations because it is generally recognized that neonatal pigs are more sensitive than pigs at other production stages to infection with the swine enteric coronaviruses PEDV and TGEV.
In our current study, 5-day-old pigs orally inoculated with a plaque-purified PDCoV cell culture isolate (USA/IL/2014, 3×104 TCID50/pig) did not experience apparent loss of appetite, lethargy, dehydration or mortality. Average weight gain was also not significantly affected by PDCoV infection during the study period. Diarrhea was transient in PDCoV-inoculated pigs. Virus shedding in feces was not detected by PCR for 48 h post-inoculation and was then detected in 10%, 40%, 80% and 100% of rectal swabs at 2, 3, 4, 5–7 DPI, respectively, with average virus titers gradually increasing from 2 to 7 DPI, indicating that PDCoV infection progressed slowly under the conditions of this report. This study was terminated at 7 DPI in efforts to adequately capture gross and microscopic lesions caused by PDCoV infection; however, it would be interesting to investigate virus shedding dynamics in future studies by extending the duration of the study. Results from this study may help guide future PDCoV experimental designs as the clinical diarrhea and virus shedding patterns following PDCoV infection in the present study were distinctly different from published studies on PEDV and TGEV infections. While not a direct comparison to the pigs used in the present study, caesarian-derived-colostrum-deprived (CDCD) piglets at 1–3 days old inoculated with PEDV CV777 strain (2 ml of 104 pig infectious doses), Korean PEDV isolate SNUVR971496 (2×106.5 TCID50), or US/Iowa/18984/2013 PEDV isolate (103 plaque forming units), developed diarrhea at 12–24 hours post inoculation (hpi) (Debouck et al., 1981, Kim and Chae, 2003, Madson et al., 2015) and all CDCD pigs inoculated with a US virulent PEDV shed virus in rectal swabs as early as 24 hpi (Madson et al., 2015). One-day old CDCD piglets inoculated with a Korean strain or two American strains of TGEV (2×106.5 TCID50) developed diarrhea at 24–36 hpi (Kim and Chae, 2002). Even in 5-day-old naïve neonatal piglets, the same pig model as used in the present study, three US virulent PEDV isolates caused severe diarrhea after 24 hpi and 100% of piglets shed virus in rectal swabs at 24 hpi (Chen et al., unpublished data).
Gross lesions consistent with virus infection were observed in the small intestines, ceca and colons of some PDCoV-inoculated pigs at both 4 and 7 DPI; however, histopathological lesions were only observed in small intestines. Pigs necropsied at 4 and 7 DPI tested negative for other enteric viral pathogens such as PEDV, TGEV, and porcine rotaviruses (groups A, B and C) and negative for enteric bacterial pathogens hemolytic E. coli and Salmonella. Although PRRSV and PCV2 are mainly respiratory rather than enteric pathogens, they can cause lethargy and occasional diarrhea. Pigs necropsied at 4 and 7 DPI were tested for PRRSV and PCV2 by virus-specific PCRs and were negative. These data indicate that the observed gross lesions and histopathological lesions in PDCoV-inoculated pigs were not due to concurrent infections with other known pathogens. Histopathological lesions and IHC staining were mainly observed in the middle and distal jejunum and ileum of the PDCoV-inoculated pigs, suggesting that middle and distal jejunum and ileum are better intestinal segments than duodenum and proximal jejunum for microscopic and IHC evaluations. This information is useful for choosing appropriate tissues for PDCoV diagnostic investigations. In this study, PDCoV viral antigen was detected by IHC in the villous epithelial cells of small intestines but was not detected in cecum, colon or other examined tissues. In contrast, PEDV viral antigen could be detected in mesenteric lymph node and some percentage of colon and spleen tissues (Jung et al., 2014, Madson et al., 2015).
PDCoV RNA was detected in sera of 100% of the challenged pigs at 4, 5 and 7 DPI. Previous studies also showed that PEDV viremia can occur in the acute stage of infection (Jung et al., 2014, Madson et al., 2015). However, it remains unclear if the PDCoV and PEDV detected in serum are present as free virus or cell-associated. Relatively high levels PDCoV RNA were detected in cecum, colon, mesenteric lymph node and stomach. However, the significance of these findings is unclear as no microscopic lesions or IHC staining was observed in these tissues. It is well known that IHC has lower analytical sensitivity than PCR and this may be one potential explanation for this difference in detection. Another possibility is that PDCoV RNA was present in gastric or fecal content that was included into the tissue homogenate during tissue processing for PCR but was not present within the actual tissue. Low levels of PDCoV RNA were detected in other tissues including tonsil, lung, heart, liver, spleen, kidney, skeletal muscle, and diaphragm, yet the significance of these findings is unknown and it is unsure if this was due to effect of viremia.
In conclusion, PDCoV peptide-specific rabbit antisera were generated for the development of PDCoV-specific immunofluorescence and IHC assays. These direct detection assays will be a valuable tool not only for future research purpose but also for confirming PDCoV-associated disease in field cases submitted to veterinary diagnostic laboratories. The pathogenic potential of PDCoV was confirmed as naive pigs developed mild to moderate diarrhea, shed virus in rectal swabs, and developed macroscopic and microscopic lesions in small intestines with viral antigen confirmed within lesions by IHC staining. However, under the condition of this report, the outcomes of PDCoV infection appear to be less severe than those caused by virulent PEDV and TGEV infections. The susceptibility of pigs at different stages of production to PDCoV infection, effect of virus administration dose, the duration of virus shedding, and the immune responses induced by PDCoV infection warrant further investigations.
Materials and methods
Virus and cells
A US PDCoV cell culture isolate (USA/IL/2014 strain, Lot # 026PDV1402) was obtained from the USDA National Veterinary Services Laboratories (NVSL). The virus was isolated on swine testicle (ST) cells (ATCC® CRL-1746™) and plaque purified twice. The PDCoV-infected ST cells were negative by indirect immunofluorescence assays using antisera against TGEV, PRCV, PHEV, porcine rotavirus, porcine reovirus, swine influenza viruses, porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus 2 (PCV-2), pseudorabies virus, porcine adenovirus, porcine teschoviruses 1–7, porcine sapelovirus, porcine parvovirus, Seneca Valley virus, swine pox virus, and porcine rubulavirus. The whole genome sequence of the PDCoV isolate USA/IL/2014 (GenBank accession number KP981395) was >99% identical to other reported US PDCoV sequences. The PDCoV isolate obtained from NVSL (passage 10) was propagated one additional passage in ST cells for more volume prior to use in this study. The propagated PDCoV culture was confirmed negative for PEDV, TGEV, porcine rotaviruses (groups A, B, and C), PCV-2, and PRRSV by virus-specific PCRs at the Iowa State University Veterinary Diagnostic Laboratory. The minimum essential medium supplemented with tryptose phosphate broth (0.3%), yeast extract (0.02%), and trypsin 250 (5 µg/ml) was used for virus propagation and titration following similar procedures previously described for PEDV (Chen et al., 2014).
Generation and evaluation of PDCoV peptide-specific rabbit antisera
The PDCoV S, M and N protein sequences were analyzed using a proprietary antigen software from the Thermo Fisher Scientific and one predicted antigenic peptide was selected for each protein: PDCoV-S-276:294 (EVEDGFYSDPKSAVRARQR), PDCoV-M-167:184 (DTFHYTFKKPVESNNDPE), and PDCoV-N-294:311 (KPTKDKKPDKQDQSAKPK). These antigen peptides were synthesized, purified, and injected into rabbits to generate antisera by a commercial laboratory following their established routine procedures (Thermo Fisher Scientific, Rockford, IL). Briefly, each peptide was conjugated with a carrier protein keyhole limpet hemocyanin (KLH). Two specific pathogen-free, six-month-old, New Zealand White rabbits were immunized with each peptide. At Day 0, each rabbit was subcutaneously injected with 0.25 mg KLH-conjugated antigen peptide that was emulsified (1:1 ratio) with complete Freund׳s adjuvant; at Day 14, 42 and 56, the rabbits were boosted with the corresponding antigen peptide, each time with 0.10 mg KLH-conjugated antigen peptide emulsified with incomplete Freund׳s adjuvant. Serum samples were collected at Day 0, 28, 56, 72 and 90 (terminal bleeding) post the initial administration of the antigen peptide for evaluation.
The rabbit antisera against each PDCoV peptide were evaluated by indirect immunofluorescence assay (IFA) on PDCoV-infected ST cells. Briefly, PDCoV-infected cells in 96-well plates at 24 hour post infection were fixed with 80% ice-cold acetone for 10 min and then air-dried. Rabbit antisera at different dilutions (1:100, 1:200, 1:500, and 1:1000) were incubated with fixed cells for 40 min at 37 °C. The cells were washed three times and then incubated with 100× dilutions of FITC-labeled goat anti-rabbit IgG antibodies (Jackson ImmunoResearch Inc., West Grove, PA) for 40 min at 37 °C. Cell staining was examined under a fluorescent microscope. The specificity of PDCoV M-peptide antisera was evaluated on PDCoV-infected ST cells, PEDV-infected VERO cells (ATCC® CCL-81™), TGEV-infected ST cells, and PRCV-infected ST cells by IFA. FITC-labeled PEDV monoclonal antibody SD6-29 targeting the nucleocapsid protein (Medgene, Brookings, SD) and FITC-labeled TGEV polyclonal antisera (CJ-F-TGE-10 ML, VMRD Inc., Pullman, WA) were evaluated by IFA on PDCoV, PEDV, TGEV, and PRCV-infected cells for cross-staining examination.
Experimental design of the pig study
The animal study protocol was approved by the Iowa State University Institutional Animal Care and Use Committee. Twenty 5-day-old piglets purchased from a conventional breeding farm were confirmed negative for PDCoV, PEDV, TGEV, and porcine rotaviruses (groups A, B, and C) by virus-specific PCRs on rectal swabs and negative for PEDV and PDCoV by virus-specific indirect fluorescent antibody assays. Upon arrival at the Iowa State University Laboratory Animal Resources (LAR) facilities, all pigs were administered an intramuscular injection of Excede® (Zoetis, Florham Park, NJ) per label instructions to prevent or alleviate subsequent bacterial infections. Pigs were randomized by weight into two groups of 10 each and housed in two separated rooms on a solid floor. Pigs were fed a mixture of Esbilac liquid milk replacer and yogurt, and had free access to water. After 1 day acclimation, one group was inoculated with the PDCoV cell culture isolate with the titer of 3×103 TCID50/ml (10 ml per pig), via orogastric gavage using an 8 gauge French catheter. The other group was orogastrically inoculated with volume-matched virus-negative culture medium and served as negative control group (Table 1).
Table 1.
Study design of five-day-old piglets orogastrically inoculated with PDCoV isolate US/IL/2014.
| Group | Piglet | Inoculum | Necropsy 4 DPI | Necropsy 7 DPI |
|---|---|---|---|---|
| PDCoV | N=10 | PDCoV 3×103 TCID50/ml; 10 ml | N=5 | N=5 |
| Neg control | N=10 | Virus-negative culture medium; 10 ml | N=5 | N=5 |
Piglets were evaluated daily for clinical signs of vomiting, diarrhea, lethargy, and body condition. Diarrhea severity was scored with the following criteria: 0=normal, 1=soft (cowpie), 2=liquid with some solid content, 3=watery with no solid content. Body condition was recorded as: 0=normal, 1=mild loss (flat flank), 2=moderate (flank tucked in), 3=severe (backbone/ribs prominent).
Body weights were recorded prior to inoculation and then at 4 and 7 days post inoculation (DPI). Rectal swabs were collected daily from each pig from 0 DPI to necropsy and were submerged into 1 ml PBS immediately after collection. Five pigs from each group were randomly selected for necropsy at 4 DPI, and the remaining pigs were necropsied at 7 DPI. Serum samples were collected at 0, 1, 3, and 4 DPI from pigs necropsied at 4 DPI and at 0, 1, 3, 5, and 7 DPI from pigs necropsied at 7 DPI. At 3 DPI, sera were successfully collected from 8 out of 10 PDCoV-inoculated pigs.
Fresh and formalin-fixed samples were collected at necropsy in the following order to minimize potential carry-over contamination: tonsil, heart, lung, diaphragm, liver, spleen, kidney, muscle from rear leg, stomach, mesenteric lymph node, duodenum, proximal jejunum, middle jejunum, distal jejunum, ileum, cecum, and colon.
At necropsy, the small intestine, cecum and colon were examined for gross lesions (normal, thin-walled, and/or gas distended).
PDCoV-specific real-time RT-PCR
Rectal swabs, serum and various tissues were tested by a PDCoV M-gene based real-time RT-PCR including viral standards with known infectivity titers for quantification. Briefly, viral RNA was extracted from rectal swabs, serum, and 10% tissue homogenates as previously described (Chen et al., 2014). Five µl of each template was used in PCR setup in a 25 µl total reaction using Path-ID™ Multiplex One-Step RT-PCR Kit (Life Technologies, Carlsbad, CA) and primers (forward primer 5′-CGACCACATGGCTCCAATTC-3′, reverse primer 5′-CAGCTCTTGCCCATGTAGCTT-3′) and probe (5′-CACACCAGTCGTTAAGCATGGCAAG C-3′). The probe was labeled using the FAM/ZEN/3′Iowa Black detector (Integrated DNA Technologies, Coralville, IA). The RT-PCR was run on an ABI 7500 Fast instrument (Life technologies, Carlsbad, CA) with the following conditions: 1 cycle of 48 °C for 10 min, 1 cycle of 95 °C for 10 min, and 45 cycles of 95 °C for 15 s and 60 °C for 45 s. A PDCoV isolate with known infectivity titer was 10-fold serially diluted for generating a standard curve in each PCR plate. Virus concentration (TCID50/ml) in tested samples was calculated based on the standard curve. The mean cycle threshold (Ct) values were calculated based on PCR positive samples, and the mean virus titers were calculated based on all pigs within the group.
Histopathology
Lung, liver, spleen, kidney, heart, tonsil, diaphragm, muscle, stomach, mesenteric lymph node, duodenum, proximal jejunum, middle jejunum, distal jejunum, ileum, cecum, and colon tissues were routinely fixed in 10% formalin, embedded, sectioned, and stained with hematoxylin and eosin (H&E). Non-enteric tissues were evaluated for evidence of inflammation. Three representative villi and crypts with integrated longitudinal sections were randomly selected from each H&E stained duodenum, proximal jejunum, middle jejunum, distal jejunum, and ileum; villus heights and crypt depths were measured blindly by a veterinary pathologist using a computerized image system as previously described for PEDV (Madson et al., 2014). Villus-heights-to-crypt-depth ratio of each tissue was the quotient of the average villus length divided by the average crypt depth.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tissue sections were mounted on positively charged glass slides and air dried for at least 30 min. Slides were further dried at 60 °C for 10 min prior to loading them to the Leica Bond III autostainer (Leica Biosystems, Buffalo Grove, IL). Slides were deparaffinized using the Leica Bond Deparaffinization Solution (AR9222, Leica Biosystems), rinsed and treated with the Leica Bond Epitope Retrieval Solution High pH (AR9640, Leica Biosystems) at 97 °C for 20 min. Approximately 150 µl of PDCoV M-peptide rabbit antiserum primary antibody, diluted 1:700, was applied to each slide for 15 min. Slides were rinsed with the Leica Bond Wash Solution (AR9590, Leica Biosystems) prior to applying 150 µl of 3.0% hydrogen peroxide for 10 min. Slides were then rinsed with the Leica Bond Wash Solution and the Leica Bond Polymer Refine Detection System (DS9800, Leica Biosystems) was applied according to the Leica protocol. Slides were rinsed with the Leica Bond Wash Solution, distilled water, and treated with diaminobenzidine (DAB) for approximately 10 min. Finally, slides were rinsed with distilled water, Leica Bond Wash Solution prior to hematoxylin counterstain for approximately 7 min. Slides were then dehydrated, cleared with xylene and cover slipped. The IHC antigen detection was semi-quantitatively scored based on the percentage of villous enterocytes within the section showing positive staining signal with the following criteria: 0=no staining; 1=approximately 1–10% enterocytes with positive staining; 2=approximately 10–25% enterocytes with positive staining; 3=approximately 25–50% enterocytes with positive staining; 4=approximately 50–100% enterocytes with positive staining. IHC scoring was performed by a single veterinary pathologist blinded to the treatment groups.
Statistics
Generalized linear mixed (GLIMMIX) model was used for statistical comparisons with Statistical Analysis System (SAS) version 9.3 (SAS institute, Cary, NC) on weight, intestine contents and gross lesion scores, villus heights, crypt depths and villus/crypt ratios. Statistical analyses on IHC data were performed using Fisher׳s exact test. P-value <0.05 was defined as statistically significant.
Acknowledgment
This study was supported by Dr. Jianqiang Zhang׳s start-up fund. We are grateful to the Iowa State University Veterinary Diagnostic Laboratory faculty and staff for assistance with some testing. We also thank the Iowa State University LAR staff for animal care.
References
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014;52(1):234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck P., Pensaert M., Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV777. Vet. Microbiol. 1981;6:157–165. [Google Scholar]
- Dong B.Q., Liu W., Fan X.H., Vijaykrishna D., Tang X.C., Gao F., Li L.F., Li G.J., Zhang J.X., Yang L.Q., Poon L.L., Zhang S.Y., Peiris J.S., Smith G.J., Chen H., Guan Y. Detection of a novel and highly divergent coronavirus from asian leopard cats and Chinese ferret badgers in Southern China. J. Virol. 2007;81(13):6920–6926. doi: 10.1128/JVI.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20(4):662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Chae C. Experimental infection of piglets with transmissible gastroenteritis virus: a comparison of three strains (Korean, Purdue and Miller) J. Comp. Pathol. 2002;126(1):30–37. doi: 10.1053/jcpa.2001.0517. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. Experimental infection of piglets with a korean strain of porcine epidemic diarrhoea virus. J. Comp. Pathol. 2003;129(1):55–60. doi: 10.1016/S0021-9975(02)00170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee C. Complete genome characterization of Korean porcine deltacoronavirus Strain KOR/KNU14-04/2014. Genome Announc. 2014;2:6. doi: 10.1128/genomeA.01191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen Q., Harmon K.M., Yoon K.J., Schwartz K.J., Hoogland M.J., Gauger P.C., Main R.G., Zhang J. Full-length genome sequence of porcine deltacoronavirus strain USA/IA/2014/8734. Genome Announc. 2014;2(2) doi: 10.1128/genomeA.00278-14. e00278-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson D., Arruda P., Magstadt D., Burrough E., Hoang H., Sun D., Bower L., Bhandari M., Gauger P., Stevenson G., Wilberts B., Wang C., Zhang J., Yoon K.J. Characterization of porcine epidemic diarrhea virus isolate US/Iowa/18984/2013 infection in one-day-old caesarian derived colostrum deprived (CDCD) piglets. Vet. Pathol. 2015 doi: 10.1177/0300985815591080. (in press) [DOI] [PubMed] [Google Scholar]
- Madson D.M., Magstadt D.R., Arruda P.H., Hoang H., Sun D., Bower L.P., Bhandari M., Burrough E.R., Gauger P.C., Pillatzki A.E., Stevenson G.W., Wilberts B.L., Brodie J., Harmon K.M., Wang C., Main R.G., Zhang J., Yoon K.J. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014;174(1–2):60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Marthaler D., Jiang Y., Collins J., Rossow K. Complete Genome Sequence of Strain SDCV/USA/Illinois121/2014, a Porcine Deltacoronavirus from the United States. Genome Announc. 2014;2 doi: 10.1128/genomeA.00218-14. e00218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014;20(8):1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20(7):1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Porcine coronavirus HKU15 detected in 9 US states, 2014. Emerg. Infect. Dis. 2014;20(9):1594–1595. doi: 10.3201/eid2009.140756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lai K.K., Huang Y., Lee P., Luk G.S., Dyrting K.C., Chan K.H., Yuen K.Y. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. J. Virol. 2009;83(2):908–917. doi: 10.1128/JVI.01977-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Huang Y., Lau S.K., Yuen K.Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]