Graphical abstract
Drought stress increased the amount of glycyrrhizin in the stolons of Glycyrrhiza glabra. The expression of sequalene synthase (SQS) and β-amyrin synthase (bAS) genes were increased under drought stress, whereas expression of cycloartenol synthase (CAS) was not changed.

Keywords: Glycyrrhiza glabra, Triterpenoid, Gene expression, Drought stress, Glycyrrhizin
Highlights
-
•
We applied various drought stresses in liquorice.
-
•
Drought stress increased the expression of SQS and bAS, whereas expression of CAS was not changed.
-
•
Drought stress increased the amount of glycyrrhizin in the stolons of G. glabra.
-
•
Applying special irrigation regimes can principally improve secondary metabolite production.
Abstract
Glycyrrhiza glabra is an important medicinal plant throughout the world. Glycyrrhizin is a triterpenoid that is among the most important secondary metabolites produced by liquorice. Drought stress is proposed to enhance the levels of secondary metabolites. In this study, the effect of drought stress on the expression of important genes involved in the glycyrrhizin biosynthetic pathway was examined. Drought stress at the seedling stage was applied to 8-day-old plants using polyethylene glycol. Subsequently, the samples were collected 0, 4, 8 or 24 h post-treatment. At the adult plant stage, 10-month-old plants were subjected to drought stress by discontinuing irrigation. Subsequently, samples were collected at 2, 16 and 28 days after drought imposition (S2d, S16d and S28d, respectively). We performed semi-quantitative RT-PCR assays to evaluate the gene expression levels of sequalene synthase (SQS), β-amyrin synthase (bAS), lupeol synthase (LUS) and cycloartenol synthase (CAS) during stress. Finally, the glycyrrhizin content of stolons was determined via HPLC. The results revealed that due to osmotic stress, the gene expression levels of SQS and bAS were increased, whereas those of CAS were relatively unchanged at the seedling stage. At the adult plant stage, the expression levels of SQS and bAS were increased under drought stress conditions, whereas the gene expression level of CAS remained relatively constant. The glycyrrhizin content in stolons was increased only under severe drought stress conditions (S28d). Our results indicate that application of controlled drought stress up-regulates the expression of key genes involved in the biosynthesis of triterpenoid saponins and directly enhances the production of secondary metabolites, including glycyrrhizin, in liquorice plants.
Introduction
The roots and stolons of Glycyrrhiza plants (G. glabra L.) are among the most important sources of crude herbal drugs worldwide (Gibson, 1978). Triterpene saponins are high molecular weight glycosides that are composed of a sugar moiety linked to triterpene or a steroid aglycone. Precursors of saponins typically undergo various modifications prior to the addition of sugar molecules. The types and extent of the sugar molecules that comprise triterpene saponins differ greatly among saponin-containing plants (Hayashi et al., 1990, Hayashi et al., 1992, Vincken et al., 2007). The root of liquorice contains a large amount (up to 15%) of glycyrrhizin and oleanane-type triterpene saponins, which are known to have various food, industrial, cosmetic and pharmaceutical applications (Armanini et al., 2005). Saponins are commercially used in food and industrial settings as foaming, detergent, wetting, emulsifying and sweetening agents (Hostettmann and Marston, 2005, Shibata, 2000). They are also utilised as ingredients in the cosmetics, cleansing and personal care sectors, such as shower gels, shampoos, hair conditioners, lotions, liquid soaps, baby care products, mouthwashes, and toothpastes. The pharmacological properties of glycyrrhizin and structural elucidation of triterpenes and saponins have been widely studied, revealing that these compounds have important pharmaceutical activities aside from their involvement in plant defence responses (Agrell et al., 2003, Mahato et al., 1992, Mahato et al., 1988, Osbourn, 1996). These important properties include anti-inflammatory, anti-bacterial, molluscicidal, antiulcer, insecticidal, and anti-allergic activities, as well as involvement in immune system activation (De Leo et al., 2006, Gopalsamy et al., 1990, He et al., 2001, Kuzina et al., 2009, Matsui et al., 2004, Park et al., 2004, Takahara et al., 1994). Glycyrrhizin also displays antivirus activity against several viruses, such as HIV (Ito et al., 1987, Ito et al., 1988) and severe acute respiratory syndrome (SARS) caused by Coronavirus (Cinatl et al., 2003).
A number of sequential enzymatic reactions are required for the biosynthesis of triterpene saponins in plants. Squalene is the immediate biological precursor of all triterpenoids. Squalene synthase (SQS) catalyses the formation of squalene from farnesyl diphosphate (Hayashi, 2009). In the triterpene biosynthesis pathway, a common intermediate of triterpenoids, saponins and phytosterols is 2,3-oxidosqualene, which is cyclised via oxidosqualene cyclases (OSCs) (Abe et al., 1993, Haralampidis et al., 2002, Xu et al., 2004). In G. glabra, there are three OSCs: β-amyrin synthase (bAS), lupeol synthase (LUS) and cycloartenol synthase (CAS). These three enzymes are involved in the cyclisation of 2,3-oxidosqualene to generate oleanane-type triterpene saponins (glycyrrhizin and soyasaponins), lupane-type triterpene (betulinic acid) and phytosterols, respectively (Mangas et al., 2006). In previous studies, cDNA from the genes encoding the oxidosqualene cyclases was cloned and characterised in G. glabra (Hayashi et al., 2000, Hayashi et al., 1999, Hayashi et al., 2001, Hayashi et al., 2004). The availability of the sequences of these genes essentially facilitates the identification of the conditions under which their expression levels are enhanced.
One critical abiotic stress that affects plant physiology and development is water deficiency caused by drought (Gueta-Dahan et al., 1997). The production of plant secondary metabolites is strongly associated with the growth conditions, and stress conditions in particular exert a strong impact on the corresponding metabolic pathways. Plants that are exposed to drought stress generally produce higher levels of secondary metabolites, including triterpenoids (Selmar and Kleinwächter, 2013). Successful and efficient use of deliberate drought stress can directly enhance secondary metabolite production. This enhancement can be achieved by applying special irrigation regimes that are both simple and inexpensive, but this approach requires extensive examination to optimise metabolite production (Selmar and Kleinwächter, 2013). Drought stress generally results in oxidative stress (Lei et al., 2006), which causes the formation of reactive oxygen species (ROS) in chloroplasts and mitochondria (Liu et al., 2011). ROS, such as superoxide (O2 −), hydrogen peroxide (H2O2), hydroxyl radical (HO−), and singlet oxygen (1O2), disturb natural metabolism by inducing oxidative damage to lipids, proteins, nucleic acids, and photosynthesis pigments and enzymes (Ozkur et al., 2009, Smirnoff, 1993). To prevent oxidative stress, plants employ enzymatic and non-enzymatic antioxidant defence mechanisms to scavenge ROS (Smirnoff, 1993). There is evidence from various plant systems indicating that environmental stress, particularly drought and salt stresses, alters the quantities, activities and steady-state mRNA levels of enzymes involved in oxygen radical scavenging (Gueta-Dahan et al., 1997). It has been found that triterpenoids display antioxidant activity (Okubo and Yoshiki, 2000). It has been demonstrated that glycyrrhizin and its hydrolysed metabolite 18β-glycyrrhetinic acid from G. glabra play an important role in ROS scavenging, resulting in a significant reduction in oxidative damage (Kim and Lee, 2008).
As mentioned above, not only are glycyrrhizin and its derivatives involved in plant defence responses, but they also display important pharmaceutical activities. Thus, the identification of key genes involved in glycyrrhizin production, as well as the conditions under which gene expression reaches to a maximal level, is of interest to the biomedical industry. To determine the optimal conditions under which glycyrrhizin production is maximally increased, we applied various drought stresses and assessed the expression levels of key genes, including SQS, bAS, LUS and CAS, that are involved in triterpenoid saponin production. In addition, we assessed the enhancement of glycyrrhizin production in liquorice stolons treated with different wa tering regimes at various stages.
Results
Relative water content (RWC)
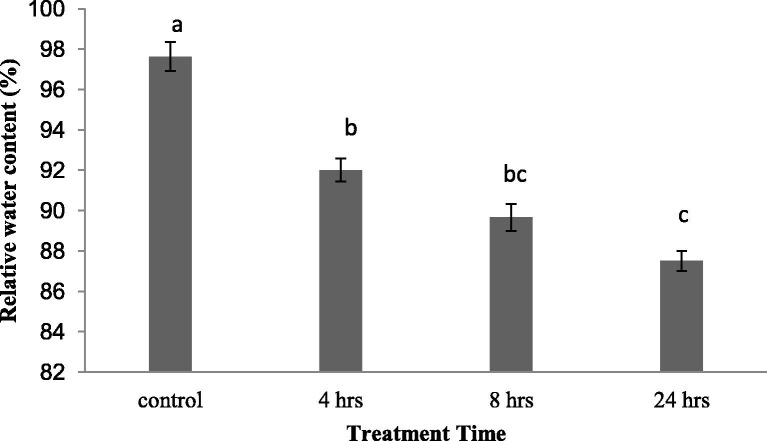
At the seedling stage, the RWC of the entire seedlings was significantly decreased (P < 0.01) throughout the experimental period (4, 8 and 24 h) under the water-stressed conditions achieved using 15% (w/v) PEG6000, reaching a minimum value at 24 h (Fig. 1 ).
Fig. 1.
Relative water content of liquorice seedlings subjected to osmotic stress. The control plants were maintained under normal water irrigation conditions. The values are expressed as the means of 3 replicates. Shared letters refer to groups that are not significantly different (P > 0.01) based on Duncan’s multiple range test.
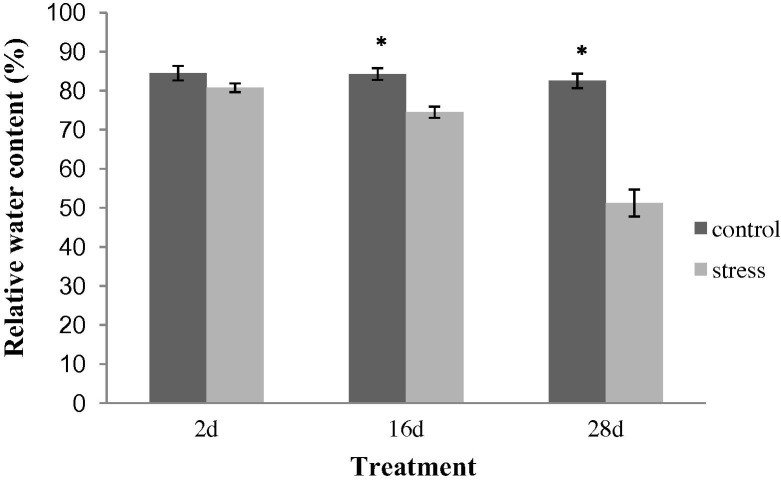
At the adult plant stage, 10-month-old plants were selected for drought stress treatment, in which irrigation was discontinued thereafter. The stolons of these plants were collected after 2, 16 and 28 days of water stress, designated as S2d, S16d, and S28d, respectively. The control plants (C2d, C16d and C28d) were maintained under normal irrigation conditions. No significant difference was detected in the RWC between the S2d and C2d samples, indicating that S2d was not significantly stressed. However, the RWC was significantly reduced (P < 0.01) in the S16d and S28d samples compared to their corresponding controls (C16d and C28d, respectively). S16d was considered as moderate stress, whereas S28d displayed more severe stress. There was no significant difference in the RWC between the control samples from C2d, C16d and C28d (data not shown) (Fig. 2 ).
Fig. 2.
The RWC of liquorice stolons subjected to drought stress. The control plants (C2d, C16d and C28d) were maintained under normal irrigation conditions. For the water-stressed plants (S2d, S16d and S28d), irrigation was discontinued, and the samples were collected after 2, 16 and 28 days after withdrawing irrigation, respectively. The values are expressed as the means of 3 replicates. The mean values of each sample were analysed using the t-test to compare the corresponding control and stress treatments. The differences were considered to be significant if P < 0.01, as indicated by an asterisk (∗).
Effects of drought and osmotic stress on the gene expression levels of SQS, bAS, CAS and LUS
We assessed the gene expression levels of SQS, bAS, CAS and LUS at the seedling stage under drought stress conditions for four durations (0, 4, 8 and 24 h) and compared these expression levels to those of the control plants via semi-quantitative PCR using gene-specific primers. The data in Fig. 3 illustrate the levels of SQS expression in liquorice seedlings over time. The expression level of SQS under stress conditions was increased shortly after stress treatment (4 h) compared to the control conditions and remained unchanged afterwards. Similarly, the expression level of bAS gene under stress conditions (at 4, 8 and 24 h after stress treatment) was higher than that of the control samples. In contrast, the gene expression level of CAS was unchanged throughout the duration of osmotic stress compared to that of the control conditions. The level of LUS gene expression was not detectable in this experiment (data not shown).
Fig. 3.
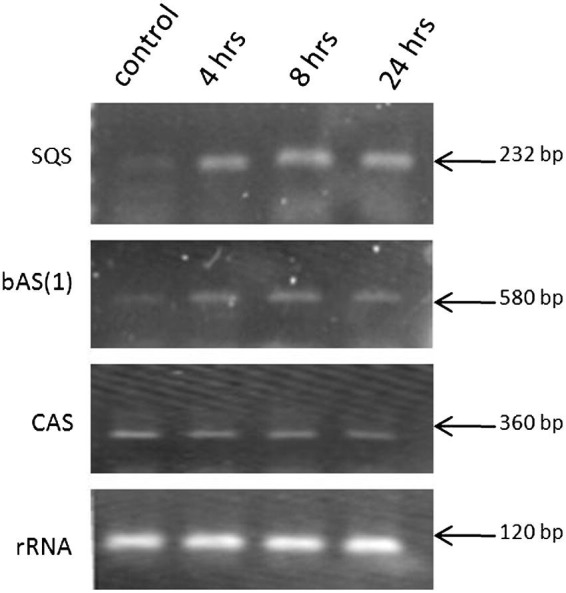
The expression levels of sequalene synthase (SQS), β-amyrin synthase (bAS) and cycloartenol synthase (CAS) at the seedling stage of G. glabra plants under control or water-stressed conditions (4, 8 and 24 h).
The gene expression of bAS, SQS, CAS and LUS in stolons was also analysed semi-quantitatively for two durations of stress treatment (C16d, S16d C28d, and S28d). The results revealed that the gene expression levels of SQS in the S16d and S28d samples were higher than those of the control samples (C16d and C28d, respectively). The highest gene expression levels of SQS were detected in the S16d sample (Fig. 4 ). The expression levels of the bAS gene at S16d and S28d were significantly higher than those of the respective C16d and C28d controls. The mRNA expression level of bAS was higher in the S16d sample than the S28d sample (Fig. 4). On the other hand, the gene expression level of CAS was relatively constant throughout the drought stress (Fig. 4). The gene expression level of LUS was not detectable in this experiment (data not shown).
Fig. 4.
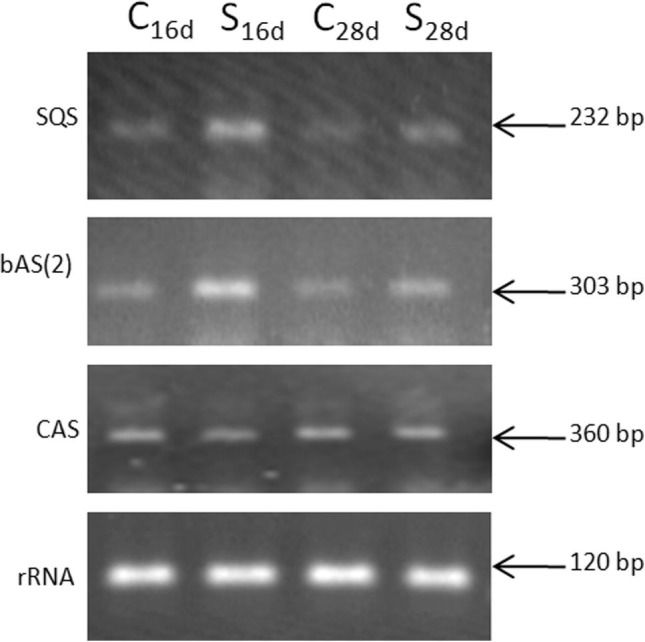
The expression levels of SQS, bAS and CAS in stolons of G. glabra in water-stressed samples (S16d and S28d refer to the samples subjected to 16 or 28 days after withdrawal of irrigation, respectively) and control samples (C16d and C28d refer to control samples that remained under optimal irrigation conditions).
Effects of drought stress on the production of glycyrrhizin in stolons of liquorice
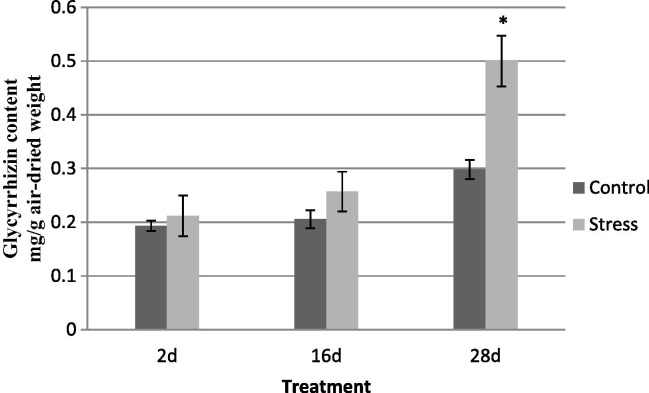
HPLC analysis was performed to compare the glycyrrhizin content in the stolons of water-stressed samples (S2d, S16d and S28d) and their respective controls (C2d, C16d and C28d). The results revealed that the glycyrrhizin content in the S28d sample was the highest of all of the samples, up to 1.7-fold greater than that of the control plants (P < 0.01), indicating that under severe stress conditions, glycyrrhizin production can be enhanced in the stolons of liquorice plants. The glycyrrhizin content in other samples, including C2d, S2d, S16d, C16d, S16d and C28d, was not significantly different (Fig. 5 ).
Fig. 5.
The effects of drought stress on the glycyrrhizin content of stolons. C2d and C28d refer to the samples cultivated under optimal irrigation conditions as controls. S2d, S16d and S28d refer to water-stressed samples cultivated for 2, 16 and 28 days after withdrawal of irrigation, respectively. ∗, Significant differences compared to control values, which were calculated using the independent sample t-test (P < 0.01).
Discussion
The enzymes SQS, bAS CAS and LUS are known to be involved in triterpenoid synthesis (Hayashi, 2009). SQS converts squalene to 2,3-oxidosqualene, which serves as the substrate for the synthesis of glycyrrhizin and other triterpenoids. CAS, bAS, and LUS encode OSCs, which catalyse the cyclisation of 2,3-oxidosqualene, a precursor of glycyrrhizin, betulinic acid and sitosterol (Abe et al., 1993, Haralampidis et al., 2002). Glycyrrhizin, soyasaponins and betulinic acid are located in different regions of intact liquorice plants, and the biosynthetic regulation of these constituents is also specific. It was previously demonstrated that the expression level of OSCs was the most influential regulator of glycyrrhizin biosynthesis (Hayashi, 2009). In addition, gene expression analysis revealed that the transcriptional expression level of bAS was higher in cell culture and thickened main roots and root nodules of liquorice plants (Hayashi, 2009). Choi et al. (2005) demonstrated that the gene expression of SQS was up-regulated in hairy root cultures of Panax ginseng by treatment with methyl jasmonate (MeJa). In another study, up-regulation of the SQS and bAS genes via MeJa and salicylic acid (SA) was reported (Chen et al., 2007, Suzuki et al., 2005). In contrast, application of gibberellin A3 resulted in down-regulation of bAS, but the gene expression levels of LUS and CAS were unchanged. Likewise, the gene expression levels of SQS and bAS were down-regulated in G. glabra cells after addition of yeast extract to the cell culture. The mRNA level of LUS was down-regulated in G. glabra cells treated with jasmonate acid (JA) or MeJA, whereas under the same conditions, the mRNA level of the CAS gene was not altered (Hayashi et al., 2004). In addition, the CAS mRNA levels in the thickened main roots of G. glabra remained relatively constant throughout the seasons, indicating that the CAS gene displays housekeeping gene-like expression in G. glabra (Hayashi et al., 2004). In agreement with these findings, our results also revealed that the gene expression of CAS remained constant in plants grown under drought stress or control conditions. Hayashi et al. (2004) reported that the level of LUS mRNA was not detectable under various cultivation conditions, which is similar to our finding that the expression of the LUS gene was not detectable (data not shown). Chen et al. (2007) found that the transcriptional expression level of bAS in the roots of Bupleurum kaoi was doubled due to treatment with MeJA. Additionally, it was reported that the transcriptional expression level of bAS was significantly increased in liquorice and Medicago truncatula plants due to treatment with salicylic acid (Suzuki et al., 2005).
Pan et al. (2006) found that when Glycyrrhiza uralensis seedlings were exposed to drought stress, the antioxidant enzymes were hyper-activated. Studies using various plant systems demonstrated that environmental stress, including drought or salt stress, enhances the transcriptional expression levels and the activities of enzymes involved in oxygen radical scavenging (Gueta-Dahan et al., 1997). Drought stress generally stimulates oxidative stress (Lei et al., 2006) resulting in the formation of ROS in organelles, including chloroplasts and mitochondria (Fu and Huang, 2001). Subsequently, as a feed-back regulation mechanism, triterpenoids are produced as an antioxidant to scavenge ROS (Okubo and Yoshiki, 2000). There are many pieces of evidence indicating that glycyrrhizin and its hydrolysed metabolite 18β-glycyrrhetinic acid exert antioxidant effects (Kim and Lee, 2008). In Bupleurum spp., an incremental effect of drought stress on the levels of triterpenoids was also reported (Zhu et al., 2009). Triterpenes and α- and β-amyrin metabolites extracted from Jatropha gaumeri leaves displayed antioxidant activity (Can-Aké et al., 2004). In Hypericum brasiliense, the content of various betulinic acids was greatly higher in plants grown under drought stress than control conditions (Nacif de Abreu and Mazzafera, 2005). Nacif de Abreu and Mazzafera (2005) also reported that the total level of secondary metabolites in H. brasiliense was increased under drought stress compared to normal conditions.
It has been reported that the three OSC genes are differentially regulated. For example, the mRNA level of bAS is up-regulated by MeJA, whereas that of LUS is down-regulated by MeJA (Hayashi et al., 2004). Furthermore, the mRNA level of bAS is down-regulated by GA3, whereas that of LUS transcript is unchanged by GA3 (Hayashi et al., 2004). In contrast, the level of CAS mRNA is not altered when either MeJA or GA3 is applied (Hayashi, 2009). Similarly, our results demonstrated that the expression of the SQS and bAS genes is differentially regulated under drought stress. Based on our results, drought stress increased the expression level of SQS and bAS, whereas that of CAS was unchanged.
Under drought stress, the concentration of enzymatic and non-enzymatic antioxidants tended to increase (Pan et al., 2006). Increasing evidence indicates that glycyrrhizin, a triterpenoid saponin found in G. glabra, exerts an anti-oxidant effect, reducing oxidative damage (Kim and Lee, 2008).
Drought stress increased the levels of glycyrrhizin in the stolons of G. glabra. Based on our results, the level of glycyrrhizin was increased under the severe stress condition (S28d). In contrast, there was no increase in the concentration of glycyrrhizin in samples that were subjected to moderate stress (S16d). Our results indicated that the glycyrrhizin content was only influenced by intense drought stress.
Glycyrrhizin is extracted from the liquorice of wild or cultivated Glycyrrhiza plants. The recent over-utilisation of wild Glycyrrhiza plants has resulted in a reduction in its natural reserves. Cultivated Glycyrrhiza is grown in some countries, but the glycyrrhizin content extracted from these plants is often low (Hayashi and Sudo, 2009). Thus, identifying the conditions under which glycyrrhizin production is maximised is of interest. In this study, we found that drought stress increased the expression of important genes involved in the glycyrrhizin biosynthetic pathway. This result indicates that intense drought stress, as well as periodic drought stress, may help to increase the glycyrrhizin content in liquorice plants. Therefore, we propose applying special irrigation regimes to directly enhance secondary metabolite production. This is technique a simple and inexpensive, but further investigation is required to optimise metabolite production.
Materials and methods
Plant material and cultivation conditions
Seeds of liquorice plants (G. glabra) were provided by Pakan-Bazr Seed Production Company (Isfahan, Iran). The seeds were disinfected using H2SO4 (98%) for 40 min and washed three times with sterile distilled water. The seeds were then allowed to germinate on sterile filter paper moistened with sterile distilled water in Petri dishes. Three-day-old seedlings were transferred to Petri dishes padded with sterilised filter paper soaked with 1 × Hoagland solution and incubated at 25 ± 2 °C for a photoperiod of 16 h of light. The drought stress experiment at seedling stage was performed by selecting 8-day-old seedlings displaying uniform growth. The seedlings were moved to filter paper soaked with distilled water containing 15% (w/v) PEG6000 to induce drought stress conditions. The entire seedlings were harvested for further analysis after 0, 4, 8 or 24 h of treatment.
The drought stress experiment at the adult plant stage was performed as follows. First, stolons of liquorice plants were obtained from Rishmac Company in Shiraz, Iran, and were grown in pots in a greenhouse. Each experiment was performed as a randomised complete block design (RCBD) with four replications. Water stress treatment was performed on 10-month-old plants by discontinuing irrigation. Subsequently, sampling was performed at 2, 16 and 28 days after drought imposition, designated as S2d, S16d and S28d, respectively, while control plants (designated as C2d, C16d and C28d, respectively) were maintained under optimal irrigation conditions. The samples of stolons were collected, immediately frozen in liquid N2 and stored at −70 °C for further analysis.
Determination of the RWC
The relative water content (RWC) of leaves and seedlings was calculated using the formula [(fresh weight–dry weight)/(saturated weight–dry weight)] * 100, as described previously (Slatyer, 1967). Fresh leaves from the treated and control plants were weighed immediately after harvesting. The saturated weight was measured after placing the leaves in vials containing distilled water at 4 °C for 24 h and then blotting the leaves on dry filter paper. The samples were dried in an oven for 72 h at 70 °C, and the dry weight of samples was measured. Analysis of variance was performed on the data using SPSS software (version 15), and significant differences compared to the control values were determined using Duncan’s multiple range test and the independent sample t-test.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from stolons of G. glabra using RNX plus solution (CinnaGen, Iran) according to the manufacturer’s instructions. To remove genomic DNA contamination, total RNA was treated with RNase-free DNase. The concentration of RNA was estimated via spectrophotometry. Approximately 6 μg of total RNA from each sample was subsequently subjected to first strand cDNA synthesis using random hexamer primers and a M-MuLV Reverse Transcriptase Kit (CinnaGen, Iran) according to the manufacturer’s instructions.
Semi-quantitative RT-PCR
PCR was performed on aliquots of the cDNA templates to determine the gene expression levels of SQS, bAS, CAS and LUS using a thermal cycler (Eppendorf) under the following parameters: 94 °C for 2 min, followed by 35 cycles of denaturing at 94 °C for 30 s, annealing at 49 °C (SQS), 57 °C (bAS(1)), 56 °C (bAS(2)) or 50 °C (CAS) for 30 s and extension at 72 °C for 30 s, and a final extension step at 72 °C for 7 min. The PCR primers used in this study were synthesised by Bioneer (Seoul, Korea). The primers were designed using Primer3 software (developed by Steve Rozen, Helen J. Skaletsky, 1996, 1997) available on-line at http://www-genome.wi.mit.edu. The SQS, bAS, CAS and LUS genes were amplified using the specific primers listed in Table 1 . 18S ribosomal RNA (Accession No. X02623) was used as an internal control (Shabani et al., 2010). The PCR products (8 μl) were electrophoresced on 1% agarose gels in TBE buffer and visually quantified.
Table 1.
List of specific primers used in this study. SQS, sequalene synthase; bAS, β-amyrin synthase; CAS, cycloartenol synthase; and LUS, lupeol synthase.
| Primer | Accession No. | Sequence | References |
|---|---|---|---|
| SQS Forward | D86410 and D86409 | 5′-CCGCTGCTGAAACTGAAAA-3′ | Shabani et al. (2010) |
| SQS Reverse | 5′-RGGCWCCTTGACATCWGT-3′ | ||
| bAS(1) Forward | AB037203 | 5′-GTTGATGCCGCTCGTCTCCA-3′ | – |
| bAS(1) Reverse | 5′-CTTCCGCACCAATCAAATACACC-3′ | ||
| bAS(2) Forward | AB037203 | 5′-GGGAGGCAGACATGGGAGT-3′ | Shabani et al. (2010) |
| bAS(2) Reverse | 5′-CAGAGGACCTGCAATTTGAG-3′ | ||
| CAS Forward | AB025968 | 5′-GGGAAAGATGTGGCTTTCAG-3′ | – |
| CAS Reverse | 5′-GGCCAATGCATCAAAATAGG-3′ | ||
| LUS(1) Forward | AB116228 | 5′-ATCCGGTGACTTCAACGCAATGTA-3′ | – |
| LUS(1) Reverse | 5′-TAGTCCCTCCACGGCAAACCA-3′ | ||
| LUS(2) Forward | AB116228 | 5′-AAGAAGTTCGTGGGCCCTAT-3′ | – |
| LUS(2) Reverse | 5′-CCCAAAGCTCTGGATTTTCA-3′ | ||
| 18s rRNA Forward | X02623 | 5′-CTCAACACGGGGAAACTTAC-3′ | Shabani et al. (2010) |
| 18s rRNA Reverse | 5′-AGACAAATCGCTCCACCAAC-3′ |
Preparation of the stolon extracts
Stolons were air-dried at room temperature for 4 days. To measure the glycyrrhizin content of each sample, 40 mg of dried stolon was lyophilised and subjected to glycyrrhizin extraction using 1 ml of 80% (v/v) methanol at 60 °C for 6 h. The samples then were centrifuged at 4000 rpm for 15 min at room temperature. The supernatant was transferred to a new tube and then evaporated to dryness using nitrogen (Hayashi et al., 1998). The residue extracts were used for high-performance liquid chromatography (HPLC).
HPLC analysis
A glycyrrhizin standard (glycyrrhizic acid ammonium salt) was purchased from Fluka (Switzerland). Preceding HPLC analysis, the residual extract of each sample was dissolved in water and filtered using a 0.4 μm filter. A 20 μl aliquot of each sample extract was analysed via HPLC at 25 °C. The HPLC system consisted of a Waters HPLC 510 pump and a Waters 2478 detector. The separation of glycyrrhizin was performed according to the method described previously (Hurst et al., 1983) as an isocratic elution using methanol–water–acetic acid (60: 34:6) at a flow rate of 1 ml/min through a RP column (3.9 × 150 mm), followed by measurement of UV absorbance at 254 nm. Analysis of variance was performed on the data using SPSS software (version 15), and significant differences compared to the control values were determined using Duncan’s multiple range test.
References
- Abe I., Rohmer M., Prestwich G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993;93:2189–2206. [Google Scholar]
- Agrell J., Oleszek W., Stochmal A., Olsen M., Anderson P. Herbivore-induced responses in alfalfa (Medicago sativa) J. Chem. Ecol. 2003;29:303–320. doi: 10.1023/a:1022625810395. [DOI] [PubMed] [Google Scholar]
- Armanini D., Fiore C., Bielenberg J., Ragazzi E. Licorice (Glycyrrhiza glabra) In: Coates P.M., editor. Encyclopedia of Dietary Supplements. Marcel Dekker; 2005. [Google Scholar]
- Can-Aké R., Erosa-Rejon G., May-Pat F., Peña-Rodríguez L.M., Peraza-Sánchez S.R. Bioactive terpenoids from roots and leaves of Jatropha gaumeri. Rev. Soc. Quim. Mex. 2004;48:11–14. [Google Scholar]
- Chen L.-R., Chen Y.-J., Lee C.-Y., Lin T.-Y. MeJA-induced transcriptional changes in adventitious roots of Bupleurum kaoi. Plant Sci. 2007;173:12–24. [Google Scholar]
- Choi D.W., Jung J., Ha Y.I., Park H.W., In D.S., Chung H.J., Liu J.R. Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep. 2005;23:557–566. doi: 10.1007/s00299-004-0845-4. [DOI] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo M., De Tommasi N., Sanogo R., D’Angelo V., Germano M.P., Bisignano G., Braca A. Triterpenoid saponins from Pteleopsis suberosa stem bark. Phytochemistry. 2006;67:2623–2629. doi: 10.1016/j.phytochem.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Fu J., Huang B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001;45:105–114. doi: 10.1016/s0098-8472(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Gibson M.R. Glycyrrhiza in old and new perspectives. Lloydia. 1978;41:348–354. [PubMed] [Google Scholar]
- Gopalsamy N., Gueho J., Julien H.R., Owadally A.W., Hostettmann K. Molluscicidal saponins of Polyscias dichroostachya. Phytochemistry. 1990;29:793–795. [Google Scholar]
- Gueta-Dahan Y., Yaniv Z., Zilinskas B.A., Ben-Hayyim G. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta. 1997;203:460–469. doi: 10.1007/s004250050215. [DOI] [PubMed] [Google Scholar]
- Haralampidis K., Trojanowska M., Osbourn A.E. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002;75:31–49. doi: 10.1007/3-540-44604-4_2. [DOI] [PubMed] [Google Scholar]
- Hayashi H. Springer; USA: 2009. Molecular Biology of Secondary Metabolism: Case Study for Glycyrrhiza Plants. Recent Advances in Plant Biotechnology. pp. 89–103. [Google Scholar]
- Hayashi H., Hiraoka N., Ikeshiro Y., Kushiro T., Morita M., Shibuya M., Ebizuka Y. Molecular cloning and characterization of a cDNA for Glycyrrhiza glabra cycloartenol synthase. Biol. Pharm. Bull. 2000;23:231–234. doi: 10.1248/bpb.23.231. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Hiraoka N., Ikeshiro Y., Yamamoto H., Yoshikawa T. Seasonal variation of glycyrrhizin and isoliquiritigenin glycosides in the root of Glycyrrhiza glabra L. Biol. Pharm. Bull. 1998;21:987–989. doi: 10.1248/bpb.21.987. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Hirota A., Hiraoka N., Ikeshiro Y. Molecular cloning and characterization of two cDNAs for Glycyrrhiza glabra squalene synthase. Biol. Pharm. Bull. 1999;22:947–950. doi: 10.1248/bpb.22.947. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Huang P., Kirakosyan A., Inoue K., Hiraoka N., Ikeshiro Y., Kushiro T., Shibuya M., Ebizuka Y. Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in liquorice. Biol. Pharm. Bull. 2001;24:912–916. doi: 10.1248/bpb.24.912. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Huang P., Takada S., Obinata M., Inoue K., Shibuya M., Ebizuka Y. Differential expression of three oxidosqualene cyclase mRNAs in Glycyrrhiza glabra. Biol. Pharm. Bull. 2004;27:1086–1092. doi: 10.1248/bpb.27.1086. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Sakai T., Fukui H., Tabata M. Formation of soyasaponins in liquorice cell suspension cultures. Phytochemistry. 1990;29:3127–3129. doi: 10.1016/0031-9422(90)83026-w. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Sudo H. Economic importance of liquorice. Plant Biotechnol. 2009;26:101–104. [Google Scholar]
- Hayashi H., Yamada K., Fukui H., Tabata M. Metabolism of exogenous 18β-glycyrrhetinic acid in cultured cells of Glycyrrhiza glabra. Phytochemistry. 1992;31:2724–2733. [Google Scholar]
- He J.X., Akao T., Nishino T., Tani T. The influence of commonly prescribed synthetic drugs for peptic ulcer on the pharmacokinetic fate of glycyrrhizin from Shaoyao-Gancao-tang. Biol. Pharm. Bull. 2001;24:1395–1399. doi: 10.1248/bpb.24.1395. [DOI] [PubMed] [Google Scholar]
- Hostettmann K., Marston A. Cambridge University Press; 2005. Saponins. [Google Scholar]
- Hurst W.J., McKim J.M., Martin R.A. High-performance liquid chromatographic determination of glycyrrhizin in liquorice products. J. Agric. Food Chem. 1983;31:387–389. [Google Scholar]
- Ito M., Nakashima H., Baba M., Pauwels R., De Clercq E., Shigeta S., Yamamoto N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)] Antiviral Res. 1987;7:127–137. doi: 10.1016/0166-3542(87)90001-5. [DOI] [PubMed] [Google Scholar]
- Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., De Clercq E., Nakashima H., Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Res. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Lee C.S. Glycyrrhizin attenuates MPTP neurotoxicity in mouse and MPP-induced cell death in PC12 cells. Korean J. Physiol. Pharmacol. 2008;12:65–71. doi: 10.4196/kjpp.2008.12.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzina V., Ekstrom C.T., Andersen S.B., Nielsen J.K., Olsen C.E., Bak S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009;151:1977–1990. doi: 10.1104/pp.109.136952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Yin C., Li C. Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006;127:182–191. [Google Scholar]
- Liu J., Wang C., Wang Z., Zhang C., Lu S., Liu J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011;126:261–269. [Google Scholar]
- Mahato S.B., Nandy A.K., Roy G. Triterpenoids. Phytochemistry. 1992;31:2199–2205. doi: 10.1016/0031-9422(92)83257-y. [DOI] [PubMed] [Google Scholar]
- Mahato S.B., Sarkar S.K., Poddar G. Triterpenoid saponins. Phytochemistry. 1988;27:3037–3067. doi: 10.1016/0031-9422(95)00782-2. [DOI] [PubMed] [Google Scholar]
- Mangas S., Bonfill M., Osuna L., Moyano E., Tortoriello J., Cusido R.M., Teresa Piñol M., Palazón J. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry. 2006;67:2041–2049. doi: 10.1016/j.phytochem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Matsui S., Matsumoto H., Sonoda Y., Ando K., Aizu-Yokota E., Sato T., Kasahara T. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int. Immunopharmacol. 2004;4:1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacif de Abreu I., Mazzafera P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005;43:241–248. doi: 10.1016/j.plaphy.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Okubo K., Yoshiki Y. The role of triterpenoid on reactive oxygen scavenging system: approach from the new chemiluminescence system (XYZ system) BioFactors. 2000;13:219–223. doi: 10.1002/biof.5520130134. [DOI] [PubMed] [Google Scholar]
- Osbourn A. Saponins and plant defence — a soap story. Trends Plant Sci. 1996;1:4–9. [Google Scholar]
- Ozkur O., Ozdemir F., Bor M., Turkan I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ. Exp. Bot. 2009;66:487–492. [Google Scholar]
- Pan Y., Wu L., Yu Z. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch) Plant Growth Regul. 2006;49:157–165. [Google Scholar]
- Park H.Y., Park S.H., Yoon H.K., Han M.J., Kim D.H. Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-d-glucuronide. Arch. Pharm. Res. 2004;27:57–60. doi: 10.1007/BF02980047. [DOI] [PubMed] [Google Scholar]
- Selmar D., Kleinwächter M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013;42:558–566. [Google Scholar]
- Shabani L., Ehsanpour A.A., Esmaeili A. Assessment of squalene synthase and beta-amyrin synthase gene expression in liquorice roots treated with methyl jasmonate and salicylic acid using real-time qPCR. Russ. J. Plant Physiol. 2010;57:480–484. [Google Scholar]
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of liquorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- Slatyer R.O. Academic Press; 1967. Plant–Water Relationships. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Reddy M.S., Naoumkina M., Aziz N., May G.D., Huhman D.V., Sumner L.W., Blount J.W., Mendes P., Dixon R.A. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:696–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. J. Hepatol. 1994;21:601–609. doi: 10.1016/s0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Vincken J.P., Heng L., de Groot A., Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Xu R., Fazio G.C., Matsuda S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry. 2004;65:261–291. doi: 10.1016/j.phytochem.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Liang Z., Han R. Saikosaponin accumulation and antioxidative protection in drought-stressed Bupleurum chinense DC. plants. Environ. Exp. Bot. 2009;66:326–333. [Google Scholar]