Abstract
Isatis indigotica Fortune is a popular herb in traditional Chinese medicine, and various types of metabolites are the basis for its pharmacological efficacy. The biosynthesis and accumulation of these metabolites are closely linked to nitrogen availability; the benefits of low nitrogen application on the environment and herb quality are increasingly prominent. To analyze metabolic changes in the leaves and roots of I.indigotica in nitrogen deficiency conditions, and to identify the pathways and metabolites induced by low nitrogen availability, we used untargeted liquid chromatography coupled with mass spectrometry (UHPLC-TripleTOF) to obtain metabolomics profiling of I.indigotica under two N-deficiency treatments (0 kg/hm2; 337.5 kg/hm2) and normal nitrogen treatment (675 kg/hm2). A total of 447 metabolites were annotated. Principal component analysis separated the three nitrogen treatments. A greater diversity of metabolites was observed in roots than in leaves under N-deficiency treatments, suggesting that roots have a more important function in low N tolerance. Differential metabolites were mainly enriched in purine metabolism, phenylpropanoid biosynthesis, the shikimate pathway, tryptophan metabolism, and flavonoid biosynthesis that notably induced only in leaves in low nitrogen stress. Moderate N-deficiency benefits carbohydrate accumulation, whereas accumulation of most amino acids decreases. Uniquely, L-tryptophan was maintained at a high concentration in N-deficiency conditions. Low nitrogen stress induced the accumulation of some specialized metabolites (matairesinol, dictamnine, 5-hydroxyindoleacetate (serotonin) in roots and vitexin, xanthohumol, sinapyl alcohol in leaves). N-deficiency also increased the accumulation of adenosine and quality indicators of I.indigotica (indirubin-indigo, epigoitrin and anthranilic acid) in a certain degree. Our findings showed that nitrogen deficiency modified roots and leaves conditions of I.indigotica, affecting both the primary and secondary metabolism. Moderate nitrogen reduction was beneficial to the accumulation of active ingredients. Our methods and analysis are expected to provide an insight regarding the diversity of metabolites and regulation of their synthesis in low nitrogen application, and better investigate the nitrogen deficiency effect on I.indigotica.
Keywords: Nitrogen deficiency, Metabolomics, Isatis indigotica Fortune, Secondary metabolism, Differential metabolites
Graphical abstract
Highlights
-
•
A greater diversity of metabolites was observed in roots than in leaves under N-deficiency treatments.
-
•
Differential metabolites were mainly enriched in purine metabolism, phenylpropanoid biosynthesis, the shikimate pathway, and tryptophan metabolism.
-
•
Flavonoid biosynthesis was significantly induced only in leaves.
-
•
Moderate N deficiency benefits carbohydrate and specialized metabolites accumulations, whereas decrease the most amino acid accumulation (except for L-tryptophan).
-
•
N-deficient treatments induced the synthesis and accumulation of quality indicators (indigo-indirubin, epigoitrin and anthranilic acid) in a certain degree.
1. Introduction
Isatis indigotica Fortune (Brassicaceae) has long been used as a medicinal plant for the treatment of colds, fever, and influenza (Zhou and Zhang, 2013), especially for the treatment of severe acute respiratory syndrome (SARS) and H1N1-influenza (Jie et al., 2017, Lin et al., 2005). In previous studies, I.indigotica was demonstrated to have various pharmacologically active compounds in crude herbs (Li et al., 2015, Meng et al., 2017, Yang et al., 2014). Compounds such as indican, quinazolinone, indirubin (INR), tryptanthrin, glucosinolate and their derivatives extract from I. indogotica are identified to have antiviral, anti-inflammatory and anticancer effects (He et al., 2017, Kaur et al., 2017, Shi et al., 2012). INR has a significant effect on chronic myelocytic leukemia (CML), and a possible mechanism has been investigated in animal studies (Mohan et al., 2018). Analogously, the pharmacological activities of nucleosides, organic acids, amino acids, and polysaccharide in I.indigotica have also been widely investigated (Chen et al., 2009, Zhang et al., 2016).
In plants, as a basic element, nitrogen is an important component of chlorophyll, proteins, nucleic acids, some hormones and some secondary metabolites (Frink et al., 1999, Hawkesford et al., 2012). Nitrogen affects both the final yield through the primary metabolism of plants and the quality through secondary metabolism, especially for medicinal plants, in which secondary metabolites are active components for preventing and treating human disease (Dai et al., 2010). However, the application of nitrogen fertilizer is not always efficient; plants are able to convert only 30–40% of applied nitrogen to useful products (Kant et al., 2011, Tilman et al., 2002). Residual nitrogen will pollute soil and water by volatilization, leaching, surface runoff and microbial consumption, thereby causing important ecological problems (Good and Beatty, 2011). Therefore, lowering nitrogen fertilizer input and improving the efficiency of nitrogen use are major aims in many agronomic and physiological studies (Hirel et al., 2007, Lea and Azevedo, 2006).
The effects of nitrogen-limiting condition on plant growth, photosynthesis, nitrate uptake and assimilation have been widely studied (Mae, 1997, Zhao et al., 2005). It is known that the plants regulate their morphological structure, physiological metabolism and the expression of related genes to enhance the adaptation to nitrogen deficiency, one of abiotic stresses (Cao et al., 2018, Muhammad et al., 2018). In addition, a variety of nutrient deficiencies in plants is characterized by an accumulation of specialized metabolites, and numerous studies have reported a positive correlation between low nitrogen availability and secondary metabolism in plants. Arabidopsis and tomato exhibit significant inverse relationships between nutrient availability and flavonoids accumulation, with nitrogen limitation promoting the greatest increase in flavonoids (Stewart et al., 2001). Catechin and epicatechin, which are the marker compounds in tea, show significant accumulation in low nitrogen treatment (Ruan et al., 2007). Thus, one must ask: What's the effect of low nitrogen application on I.indigotica, and how can that effect be measured?
Traditionally, indigo-indirubin and epigoitrin are the marker compounds for the quality of Isatis leaves (Daqingye) and Isatis roots (Banlangen) (Pharmacopoeia of People's Republic of China. 2015).However, with pharmacological study of the chemical compounds of I.indigotica, more and more bioactivity-based characteristics were thought to be indicators of good quality. Li et al. regarded quinazolidone alkaloids antiviral compounds as the quality indicators of Isatis roots (Li et al., 1994). Some studies show that nucleosides compounds such as hypoxanthine, adenosine, and uracil have effects on interfering virus proliferation and regulating cell's metabolism, thus they are associated with the quality formation of I.indigotica (Hu et al., 1999). Similarly, radix isatidis polysaccharide (RIPS), anthranilic acids and total amino acids are highly responsible for anti-influenza virus effect of I.indigotica, and these compounds should be considered as indicators of quality assessment. Measuring the total bioactive and chemical composition of I.indigotica is an effective way to assess quality.
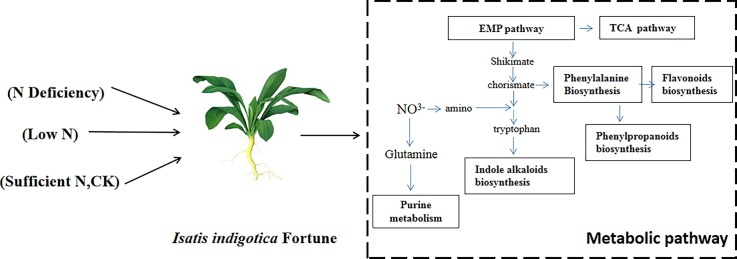
The development of metabolomics approaches and multivariate statistics analysis provides useful methods to obtain a complete picture of a plant's metabolites. We used metabolomics methods to assess the effects of nitrogen deficiency on I.indigotica. We compared metabolomics profile of leaves with roots in three field nitrogen application levels: severe nitrogen deficiency (0 kg/hm2), medium-nitrogen deficiency (337.5 kg/hm2), and normal-nitrogen (675 kg/hm2). Then, we characterized the most relevant metabolic pathways and metabolites to low nitrogen tolerance. We also focused on some quality-related compounds of I.indigotica, which were though not being discriminated among the treatments: indigo-indirubin, epigoitrin and anthranilic acids. Thus we could fully characterize the effect of nitrogen deficiency on I.indigotica.
2. Results and discussion
2.1. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA)
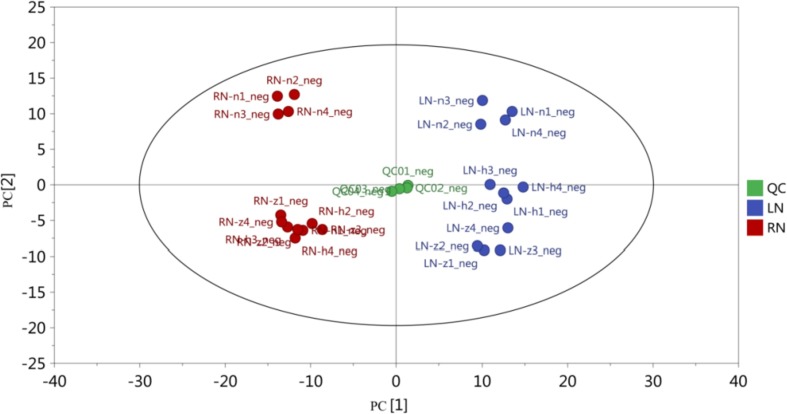
The original data contained four quality control (QC) samples and 24 experimental samples, and 1914 peaks were extracted. The original data were then adjusted by missing value recoding and normalization analysis. In total, 447 peaks were reserved and annotated. To provide an overview of the similarities and differences among the samples, we used PCA to annotate the compounds of I.indigotica (Fig. 1 ).The total variance rate explained by PC1 and PC2 were 51.8% and 14.6%, respectively. In this model, the quality control (QC) samples gathered in the center of the score plot and the test samples of three nitrogen groups were separated obviously. The samples were all in the 95% confidence interval (Hotelling's T-squared ellipse), reflecting the stable detection conditions and good repeatability of the analysis process. The PCA plot of untargeted liquid chromatography coupled with mass spectrometry (UHPLC-QTOF-MS) data showed that the metabolic profiles of I.indigotica changed significantly in N-deficiency stress.
Fig. 1.
Score scatter plot for PCA model TOTAL with QC of all samples under different nitrogen levels. The abscissa PC [1] and the ordinate PC [2] represent the scores of the first and second ranked principal components, respectively, and the scatter color and shape represent experimental groupings of the samples. The differences between the visible groups are significantly different on the top principal components. The samples are all within the 95% confidence interval (Hotelling's T-squared ellipse).
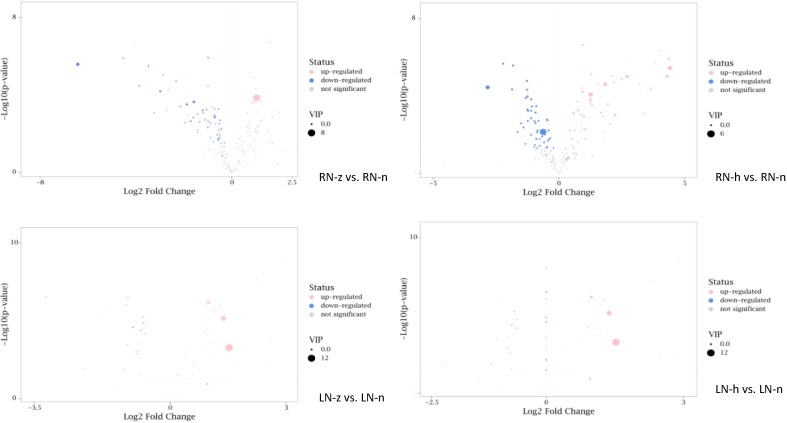
To further investigate the effect of N-deficiency on metabolites of I.indigotica and to obtain group discrimination, we subjected the sample data to orthogonal partial least square discriminant analysis (OPLS-DA). By OPLS-DA, we could filter out orthogonal variables in metabolites that were not related to categorical variables, and analyzed non-orthogonal variables and orthogonal variables separately to obtain more reliable meta-group differences and experiments (Trygg and Wold, 2002). All models passed the displacement test. To identify differential metabolites of the N deficient treatments in root and leaf samples, we performed multivariate data analysis with Student's t-test and fold change methods. As the screening criteria, we use the p<0.05 (t-test) and VIP>1 at the same time to obtain the differential metabolites between the low nitrogen treatments and CK. Then we visualized the results of screening differential metabolites in the form of volcano plots (Fig. 2 ).
Fig. 2.
Volcano plots of differential metabolites in nitrogen deficiency treatments. Each point in the volcano plot represents a metabolite, the abscissa represents the fold change of the group compared to the substance (take the base 2 logarithm), and the ordinate represents the p-value of the Student's t-test (take the base 10 Logarithm), the size of scatter represents the VIP value of the OPLS-DA model. The larger the scatter is, the larger the VIP value. The scatter color represents the final screening result, the significantly up-regulated metabolites are shown in red, the significantly down-regulated metabolites are shown in blue, and the non-significantly different metabolites are gray.
The differential metabolites of roots in nitrogen deficient treatments were greater than those in leaves, and the up-regulated metabolites were greater than the down-regulated metabolites in N-deficient treatments. These results indicated that the roots had a more positive response to low nitrogen stress.
2.2. Differential metabolic pathway screening
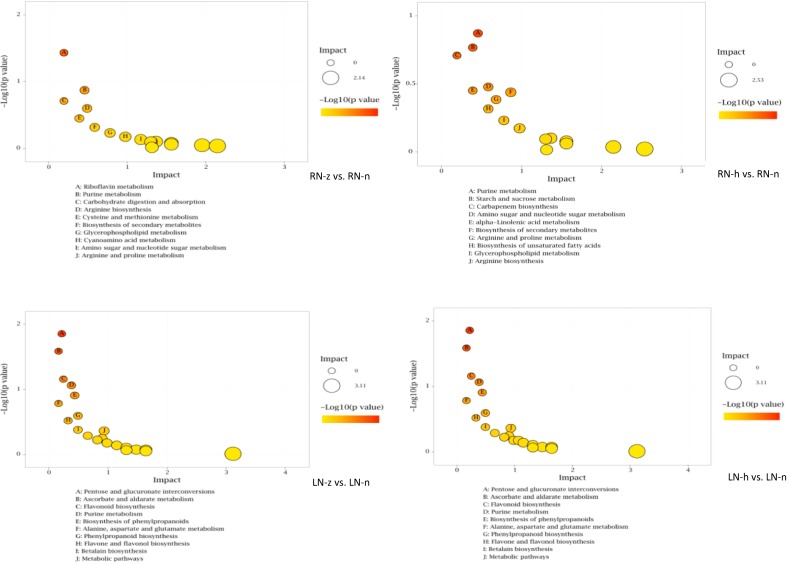
We compared the differential metabolites that were screened by the OPLS-DA model with the KEGG database (www.kegg.jp/kegg/pathway.html), and we organized all the metabolic pathways in which the differential metabolites mapped. To determine the most relevant metabolic pathways in the different nitrogen conditions, enrichment analysis and topological analysis of differential metabolites were required to find the key pathways most relevant to the differential metabolites. The results were shown by bubble plots (Fig. 3 .).In roots, purine metabolism changed notably in RN-z and RN-h. Amino sugar and nucleotide metabolism, arginine metabolism and secondary metabolism also differentiated the nitrogen deficient treatments and normal nitrogen treatment. Analogously, in leaves, carbohydrate metabolism, purine metabolism, arginine metabolism and secondary metabolism significantly changed in LN-z and LN-h. Flavonoid biosynthesis, flavone and flavonol biosynthesis were notably induced only in leaves. To unravel the changes of these pathways and decipher the effect on quality formation of I.indigotica, we concluded the relative differential metabolites in roots and leaves, respectively (Table 2 and Table 3).
Fig. 3.
The bubble plots of differential metabolic pathway in nitrogen deficiency. Each bubble represents a metabolic pathway respectively, the abscissa and the size of the bubble indicates the size of the influence factor of the pathway in the topological analysis; the larger the size, the larger the influence facto. The ordinate of the bubble and the bubble color indicated the p value (take negative natural logarithm, lnp-value) in the enrichment analysis, the deeper the color, the smaller the p value, and the more significant the enrichment.
Table 2.
Related differential metabolites and the relative content identified in leaves between the nitrogen deficiency treatments and CK.
| Differential metabolites | metabolic pathway | LN-z | LN-h | LN-n |
|---|---|---|---|---|
| L-Arabinose | Pentose and glucuronate interconversions | 1.1709 | 1.2335 | 0.6721 |
| Ascorbate and aldarate metabolism | ||||
| Glycerol | Pentose and glucuronate interconversions | 2.2697 | 2.7722 | 1.6469 |
| Ribitol | Pentose and glucuronate interconversions | 0.9405 | 1.6283 | 0.1765 |
| L-Threonine | Ascorbate and aldarate metabolism | 3.9229 | 2.7810 | 4.2008 |
| Adenosine | Purine metabolism | 3.5233 | 3.4536 | 1.5008 |
| Deoxyadenosine | Purine metabolism | 18.0737 | 12.3745 | 24.5579 |
| Hypoxanthine | Purine metabolism | 0.9451 | 0.7762 | 1.4389 |
| L-Glutamate | Alanine, aspartate and glutamate metabolism | 0.7398 | 0.8032 | 1.4367 |
| Biosynthesis of plant secondary metabolites | ||||
| Vitexin | Flavone and flavonol biosynthesis | 34.1606 | 25.6962 | 22.9451 |
| Flavonoid biosynthesis | ||||
| Xanthohumol | Flavone and flavonol biosynthesis | 0.5990 | 1.0521 | 0.3456 |
| 3,5-Dimethoxy-4-hydroxy-cinnamic acid | Phenylpropanoid biosynthesis | 5.6138 | 2.6434 | 1.5430 |
| Sinapyl alcohol | Phenylpropanoid biosynthesis | 5.4348 | 5.1813 | 3.0867 |
| Dopamine | Betalain biosynthesis | 6.3209 | 20.5738 | 53.5003 |
| Biosynthesis of alkaloids derived from shikimate pathway | ||||
| L-Tryptophan | Biosynthesis of alkaloids derived from shikimate pathway | 2.2762 | 2.9887 | 1.9359 |
| Phenylpropanoid biosynthesis |
Table 3.
List of sampling information of root and leaf of I.indigotica.
| index | Nitrogen level | Nitrogen amount kg/hm2 | Organ |
|---|---|---|---|
| LN-z | severe nitrogen deficiency | 0 | Leaf |
| LN-h | medium-nitrogen deficiency | 337.5 | |
| LN-n | Normal-nitrogen (CK) | 675 | |
| RN-z | severe nitrogen deficiency | 0 | Root |
| RN-h | medium-nitrogen deficiency | 337.5 | |
| RN-n | Normal-nitrogen (CK) | 675 |
2.3. Primary metabolic pathway and metabolites analysis
One strategy of plants to tolerate low nitrogen stress may relate to carbohydrate transport and accumulation. Chen et al. (2015) reported that plants accumulate a large amount of sugar in the early stages of aging in low nitrogen stress, a condition which is obviously manifested as carbon-nitrogen imbalance. In addition, more sucrose is transported to the roots and it is involved in the root growth regulation, whereby the roots provide carbon sources and energy to plants in stressful conditions (Krapp et al., 2011). In this research, we found statistically differences in carbohydrate levels in Isatis roots. ADP-glucose, maltotriose and glycogen were found in relatively high concentrations in RN-h. L-arabinose abundance was significantly altered in roots and leaves. L-arabinose is an important constituent of Radix Isatidis polysaccharide (RIPS) (Jia et al., 2016). Interestingly, in this study, levels of L-arabinose were high in N-deficient treatments in Isatis leaf, which the trend was opposite to that in roots. L-arabinose is a good carbon source in plants, and it has a key function in nitrogen-fixation (Novick and Tyler, 1982).Thus, we speculate that the changes in L-arabinose abundance were related to up-regulation in carbohydrate synthesis of Isatis leaf under low N conditions.
The high flexibility of the amino acid library is the embodiment of a plant's ability to adapt to the external environment. We found that the abundances of L-citrulline, L-proline and S-adenosyl-L-homocysteine were significantly changed in roots; the contents showed a trend of first decreasing and then increasing with a decrease in nitrogen availability. Amino acid content is closed related to nitrogen, yet some amino acids also act as regulators to alleviate abiotic stress (Dietz and Sharma, 2006).According to a study on Arabidopsis, L-citrulline produces N compounds in specific tissues to serve as endogenous N sources for growth (Ludwig, 1993). L-proline is related to plant cell osmotic regulation in stress conditions (Singh et al., 2002). L-glutamate and L-tryptophan in leaves showed an opposite trend. An increase in L-tryptophan content in N-deficiency conditions is probably associated with amino acids acting synergically to mitigate N-deficiency damage of plants. Purine metabolism is part of nitrogen metabolism, and the relative metabolites synthesis is closely linked to nitrogen availability (Ma et al., 2016). We found that the abundances of adenosine, deoxyadenosine and hypoxanthine were significantly different both in roots and leaves. Only adenosine accumulated in N deficiency. Adenosine is a principal nucleoside in I.indigotica, which has the effect of enhancing immunity (Zuo et al., 2007).The accumulation of adenosine may be related to purine metabolism acting to regulate nitrogen and reactive oxygen metabolism to alleviate damage (Brychkova et al., 2008, Koprivova et al., 2008). However, further studies are needed to investigate the regulatory mechanism of purine metabolism in nitrogen deficiency conditions.
2.4. Specialized metabolic pathway and metabolites analysis
With respect to higher plants, the shikimate acid pathway could provide precursor substances for some special amino acids and various secondary metabolites (Herrmann, 1995).The downstream pathways include tryptophan metabolism, phenylpropanoids biosynthesis, and flavonoid biosynthesis, etc. (Herrmann and Weaver, 1999). We found the differential metabolite related to the shikimate acid pathway was dopamine, and dopamine content in the LN-n group was significantly higher than that in the low-nitrogen group in leaves. Dopamine is an intermediate for the synthesis of alkaloids and its synthesis is closely linked to tyrosine. Dopamine has stress resistance functions (Świędrych et al., 2004), and it regulates plant growth by affecting the oxidation process of IAA (Elstner et al., 1976). Associated with the high content of L-tryptophan in N-deficient conditions, we speculate that the up-regulation of tryptophan metabolism and insufficiency of its precursor tyrosine which was due to low nitrogen application may explain the steep change in dopamine abundance.
In addition to physiological functions, some specialized compounds in herbs may also be potential medicinal ingredients. Matairesinol and dictamnine are validated to have antitumor and antiviral effects, matairesinol is also a potent inhibitor of HIV Type-1 Integrase (Fischer et al., 2004). The abundances of matairesinol and dictamnine were significantly in roots in nitrogen deficiency. As a down-stream production of phenylpropanoid biosynthesis, sinapyl alcohol is also an important secondary metabolite in I.indigotica, which was proved to have anti-inflammatory and ant nociceptive effects (Choi et al., 2004). Its content is positively correlated with the content of cinnamic acid (Crosby, 2005). We found that 3, 5-dimethoxy-4-hydroxycinnamic acid and sinapyl alcohol both accumulated in low-N stress. This accumulation was possibly related to a reduction in demand for organic acids in low-N conditions and an accumulation of organic acids (Paul and Driscoll, 2010).
Flavonoid biosynthesis is a major part of plant secondary metabolism. Derived from the phenylpropanoid pathway, the flavonoid pathway is hypothesized to have evolved for protection against abiotic stresses by regulating the content of flavonoid (Albert et al., 2018). Flavonoids are also important bio-active compounds in medicinal plants, and they have variety of physiological effects, such as anti-oxidation, anti-cancer and anti-virus (Albert et al., 2018, Zhang et al., 2015).We found that the metabolic pathway of flavonoids was induced only in leaves by N-deficiency and low nitrogen. The accumulations of vitexin and xanthohumol were enhanced, by about 1.5 to 3-foldbetween low-N treatments and CK. The isomer of vitexin, isovitexin is the main flavonoids of I.indigotica and is responsible for the antitumor effect (Zou et al., 2005). Similarly, xanthohumol is also validated to have anti-cancer and anti-tumor effects (Wang et al., 2004).
Most plant research of tryptophan metabolism concerns its defensive function, which produced toxic metabolites in common with phenylpropanoids (Ishihara et al., 2008, Nomura et al., 2002, Zhao and Last, 1996). In addition, tryptophan metabolism is closely related with the biosynthesis of indole alkaloids in several plants, such as Arabidopsis and I.indigotica (Maugard et al., 2001). Here we found that the concentration of 5-hydroxytryptamine (serotonin) in roots was higher in low nitrogen treatments (Table 1 ). Being synthesized from tryptophan, 5-hydroxytryptamine has a vital function in tryptophan metabolism to alleviate biotic and abiotic stress (Ishihara et al., 2008). Pharmacological studies show that 5-hydroxytryptamine also has an important neuromodulatory function in physiological responses and mental processes in human (Hensler, 2012).
Table 1.
Related differential metabolites and the relative content identified in roots between the nitrogen deficiency treatments and CK.
| Differential metabolites | metabolic pathway | RN-z | RN-h | RN-n |
|---|---|---|---|---|
| Adenosine | Purine metabolism | 3.1275 | 6.8881 | 0.9724 |
| Deoxyadenosine | Purine metabolism | 7.2720 | 4.9004 | 11.9217 |
| Hypoxanthine | Purine metabolism | 0.5072 | 0.4723 | 1.9469 |
| L-Citrulline | Agrine metabolism | 1.4217 | 0.7875 | 5.2285 |
| Ribitol | Riboflavin metabolism | 0.3824 | 0.3191 | 0.2893 |
| Riboflavin | Riboflavin metabolism | 0.0712 | 0.0854 | 0.1066 |
| Maltotriose | Carbohydrate digestion and absorption | 2.6778 | 4.0549 | 4.4395 |
| 5-Hydroxyindoleacetate | Tryptophan metabolism | 1.0192 | 1.1472 | 0.4855 |
| Matairesinol | Biosynthesis of phenylpropanoids | 0.3302 | 0.2307 | 0.1659 |
| Dictamnine | Biosynthesis of alkaloids derived from shikimate pathway | 0.0733 | 0.0641 | 0.0354 |
| Glycerophosphocholine | Glycerophosphlipid metabolism | 0.0332 | 0.0333 | 0.0658 |
| Prunasin | Cyanoamino acid metabolism | 0.0800 | 0.0853 | 0.0345 |
| ADP-glucose | Amino sugar and nucleotide sugar metabolism | 0.0486 | 0.0753 | 0.0208 |
| Starch and sucrose metabolism | ||||
| L-Arabinose | Amino sugar and nucleotide sugar metabolism | 0.0960 | 0.0710 | 0.1489 |
| Glycogen | Starch and sucrose metabolism | 0.0357 | 0.0532 | 0.0420 |
| Carbohydrate digestion and absorption | ||||
| 4-Guanidinobutyric acid; | Agrine and proline metabolism | 3.4244 | 2.8916 | 4.8994 |
| L-Proline | Agrine and proline metabolism | 0.8593 | 0.5766 | 1.4069 |
| Carbapenem biosynthesis | ||||
| Traumatic acid | alpha-linolenic acid metabolism | 0.0392 | 0.0405 | 0.6521 |
| Eicosapentaenoic acid | Unsaturated fatty acids metabolism | 0.0686 | 0.0763 | 0.1419 |
| S-Adenosyl-L-homocysteine | Cysteine and methionine metabolism | 0.3255 | 0.2422 | 0.4603 |
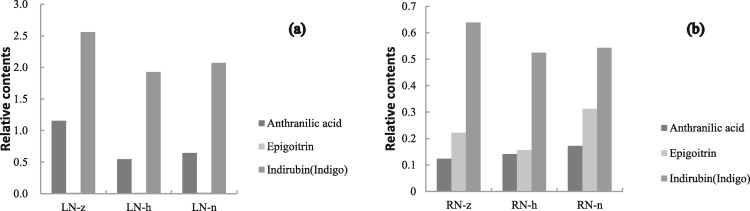
2.5. Contents of indictor components based on metabolomics data
As is shown in Fig. 4 , the contents of indirubin-indigo and anthranilic acid was maintained at high levels under LN-z, and there was a similar trend with an increase in nitrogen levels: a steep decrease then slight increase. A similar trend for indirubin-indigo abundance was also observed in roots. Although epigoitrin content was not high and did not vary significantly in leaves, a distinct trend was observed in roots—first a decrease then an increase with the decrease of N levels. The content of anthranilic acid showed positive correlations with nitrogen levels in roots and the content was the highest in RN-n. Anthranilic acid and indirubin-indigo are recognized as main bioactive compounds in Isatis leaf, while in Isatis root was epigoitrin. Our results showed that low nitrogen application enhanced accumulation of these quality components.
Fig. 4.
Relative contents of anthranilic acid, epigoitrin and indirubin (indigo) in Isatis leaf and Isatis root under different nitrogen levels. (a) Isatis leaf; (b) Isatis root.
3. Conclusion
In this investigation, I.indigotica was cultivated in nature conditions in the field, with the changes in metabolites thereof being more complex and variable. Thus our investigation has a more practical meaning for the popularization and application of low-nitrogen conditions.
By employing LC-MS/MS metabolomics profiling with multivariate analysis methods, this research has clearly shown the biochemical response of I.indigotica in nitrogen deficient conditions. The roots had a more positive role in low-N tolerance. Purine metabolism was tightly correlated with nitrogen levels. Analogously, specialized metabolic pathways such as phenylpropanoids biosynthesis, the shikimate pathway, tryptophan metabolism and flavonoid biosynthesis were induced to adapt to the low-N conditions. Carbohydrate content was maintained at high levels in medium-nitrogen deficiency. The different distribution of L-arabinose between roots and leaves may be related to low-N tolerance. Severe and medium nitrogen treatments were negatively correlated with the accumulations of most amino acids, whereas L-tryptophan accumulated under low-N stress. The contents of L-proline and L-citrulline showed steep increases in severe-N treatment compared with medium-N treatment, a response which may be related to their special functions in alleviating cell damage. The amino acids were speculated to act synergistically to regulate low-N stress.
The up-regulation of specialized metabolic pathway is thought to be a plant adaptive mechanism to stress, such as salt stress, drought stress and nutrient stress. We found that some specialized metabolites, such as matairesinol and dictamnine 5-hydroxytryptamine (serotonin), vitexin, xanthohumol, sinapyl alcohol were maintained at high concentrations in N-deficiency treatments. Most of these metabolites have been validated to have pharmacological activities, and presumably be the potential medicinal constituents of I.indigotica. Quality indicators: indirubin-indigo, epigoitrin and anthranilic acid were negatively correlated with nitrogen level, and their concentrations were maintained relatively high in low nitrogen treatments. Similarly, vitexin notably accumulated in LN-z and LN-h. Vitexin and isovitexin are the main active-flavonoids in I.indigotica. Thus, we speculate that the moderate nitrogen deficiency induced the accumulation of pharmacologically related metabolites and had a positive effect on the medicinal value of I.indigotica.
4. Experimental
4.1. Chemicals
Chemicals: indigo, indirubin and (R,S)- epigoitrin (National Institute for Food and Drug Control, China).
Reagents: Methanol, acetonitrile, ammonium acetate, ammonium hydroxide (CNW Technologies, LC-MS grade), 2-Chloro-L-phenylalanine (Shanghai Hengbai Biotech Co., Ltd).
4.2. Plant material culture
Field experiments were performed at Jurong Country, Jiangsu Province, China (120°E, 32°N). The soil had an organic matter content of 10.03 g kg−1, a total nitrogen content of 0.80 g/Kg and pH of 6.3. The experimental material was Isatis indigotica Fortune from Shanxi province, China. Seeds of I.indigotica were sown on April 16, 2017 (warm season). I.indigotica plants were subjected to the following three treatments: severe nitrogen deficiency (0 kg/hm2), medium nitrogen deficiency (337.5 kg/hm2) and normal nitrogen application (665 kg/hm2) (Table 1). Phosphorus and potassium fertilizers were treated in the same amount, which were all KH2PO4 180 kg/hm2. Quadruplicate experiments were carried out for each treatment. There were 12 plots with a size of 1.5 m × 4 m. Random block design was used for routine field management. Both nitrogen fertilizer and phosphorus potassium fertilizer were applied twice with the same amounts each time. The first topdressing was on June 16, 2017 and the second topdressing was on Sept.16, 2017. Nitrogen fertilizer, phosphorus and potassium fertilizer were urea (nitrogen 46%) and KH2PO4 (including P 26.7%, including K 16.4%), respectively.
Sample were collected on Dec.16, 2017 (ten individual plants were randomly selected from each plot), the samples were washed and dried to measure the leaf and root fresh weight, and placed in an oven at 105 °C for 15 min, then dried to constant weight at 60 °C. The dry leaves and roots were pulverized with a pulverizer and the powders were sieved through 60 mesh (250 μm) and 100 mesh (180 μm), respectively for metabolomics analysis.
4.3. Sample extraction
Approximately 30 mg of sample was transferred to a EP tube, then 1 mL extraction liquid (V methanol: V acetonitrile: V water = 2:2:1, which was kept at −20 °C before extraction) was added and 5 μL internal standard (IS, 2-Chloro-L-phenylalanine). The extraction was repeated three times for each treatment. The mixtures were homogenized in a ball mill for 4 min at 45Hz, and then subjected to ultrasound for 5min (incubated in ice water). After homogenization for three times, the solutions were incubated for 1 h at −20 °C to precipitate proteins. Then solutions were centrifuged at 12000 rpm for 15 min at 4 °C and the supernatants (500 μL) were transferred into fresh EP tubes. The extracts were dried in a vacuum concentrator without heating, then dissolved by adding 500 μL extraction liquid (V acetonitrile: V water = 1:1) and vortexing for 30s followed by ultra-sonication 10min (4 °C water bath). The solutions were centrifuged for 15 min at 12000 rpm (4 °C), and then the supernatants (60 μL) were transferred into fresh 2 mL LC/MS glass vials. Aliquots of 10 μL were taken from each sample and pooled as QC samples. Aliquots supernatant (60 μL) and the QC samples were used for UHPLC-QTOF-MS analysis.
4.4. LC-MS/MS analysis
LC-MS/MS analysis was performed with an UHPLC system (1290, Agilent Technologies) and a UPLC BEH Amide column (1.7 μm 2.1*100 mm, Waters) coupled to TripleTOF 5600 (Q-TOF, AB Sciex). The mobile phase consisted of 25 mM NH4OAc and 25 mM NH4OH in water (A) and acetonitrile (B) was carried with elution gradient as follows: 0 min, 95% B; 7min, 65% B; 9 min, 40% B; 9.1 min, 95% B; 12 min, 95% B, which was delivered at 0.5 mL min−1. The injection volume was 2 μL. The Triple TOF mass spectrometer was used because of its ability to acquire MS/MS spectra on an information-dependent basis (IDA) during an LC/MS experiment. In this mode, the acquisition software (Analyst TF 1.7, AB Sciex) continuously evaluates the full scan survey of MS data as it collects and triggers the acquisition of MS/MS spectra depending on preselected criteria. In each cycle, 12 precursor ions with intensity greater than 100 were chosen for fragmentation at collision energy (CE) of 30 V (15 MS/MS events with product ion accumulation time of 50 msec. each). ESI source conditions were set as following: ion source gas 1 as 60 Psi, ion source gas 2 as 60 Psi, curtain gas as 35 Psi, source temperature 650 °C, Ion Spray Voltage Floating (ISVF) 5000 V or −4000 V in positive or negative modes, respectively.
4.5. Data preprocessing and annotation
MS raw data files were converted to the mz XML format using Proteo Wizard, and processed by R package XCMS (version 3.2). The preprocessing results generated a data matrix that consisted of the retention time (RT), mass to-charge ratio (m/z) values, and peak intensity. R package CAMERA was used for peak annotation after XCMS data processing. An in-house MS2 database was applied in metabolites identification. Metabolites putatively identified in leaves and roots of I.indigotica in different nitrogen treatments were subject to principle component analysis (PCA) and orthogonal partial least square discriminant analysis (OPLS-DA) using SIMCA14.1 chemometric software package. The differential metabolites screened by the OPLS-DA were compared with the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Database. The relative content of each metabolites was calculated based on the peak area of the internal standard.
Author contributions
Tang X.-Q. conceived, designed and revised the experiment; Cao Y.-W. analyzed the data and drafted the manuscript; Miao Y.-J. and Qu R.-J. helped perform the experiment; Zhou Y. assisted with drafting the manuscript; Wang L. and Geng L. helped the experiment material culture. All authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Research involving human and animal participants
This article does not contain any studies with human and/or animal participants performed by any of the authors.
Acknowledgement
This work was supported by National Natural Science Foundation of China (31171486) and Zhenjiang Jinshan Talents in Jiangsu Province of China- Modern Agricultural Leaders (Innovation) Project (2018).
References
- Albert N.W., Thrimawithana A.H., McGhie T.K., Clayton W.A., Deroles S.C., Schwinn K.E., Bowman J.L., Jordan B.R., Davies K.M. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytol. 2018;218:554–566. doi: 10.1111/nph.15002. [DOI] [PubMed] [Google Scholar]
- Brychkova G., Alikulov Z., Fluhr R., Sagi M. A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J. 2008;54:496–509. doi: 10.1111/j.1365-313X.2008.03440.x. [DOI] [PubMed] [Google Scholar]
- Cao X., Zhu C., Zhong C., Hussain S., Zhu L., Wu L., Jin Q. Mixed-nitrogen nutrition-mediated enhancement of drought tolerance of rice seedlings associated with photosynthesis, hormone balance and carbohydrate partitioning. Plant Growth Regul. 2018;84:451–465. [Google Scholar]
- Chen D., Wang S., Xiong B., Cao B., Deng X. Carbon/nitrogen imbalance associated with drought-induced leaf senescence in sorghum bicolor. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhou J., Tang X., Wang K. [Effect of water stress on content of four organic acids in different cultivated populations of Isatis indigotica] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chinese Materia Medica. 2009;34:3195–3198. [PubMed] [Google Scholar]
- Choi J., Shin K.-M., Park H.-J., Jung H.-J., Kim H.J., Lee Y.S., Rew J.-H., Lee K.-T. Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med. 2004;70:1027–1032. doi: 10.1055/s-2004-832642. [DOI] [PubMed] [Google Scholar]
- Crosby G.A. Lignans in food and nutrition. Food Technol. 2005;59:32–36. [Google Scholar]
- Dai H., Xiao C., Liu H., Hao F., Tang H. Combined NMR and LC−DAD-MS analysis reveals comprehensive metabonomic variations for three phenotypic cultivars of salvia miltiorrhiza bunge. J. Proteome Res. 2010;9:1565–1578. doi: 10.1021/pr901045c. [DOI] [PubMed] [Google Scholar]
- Dietz K.-J., Sharma S.S. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006;57:711–726. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- Elstner E.F., Konze J.R., Selman B.R., Stoffer C. Ethylene formation in sugar beet leaves. Plant Physiol. 1976;58:163. doi: 10.1104/pp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J., Reynolds A.J., Sharp L.A., Sherburn M.S. Radical carboxyarylation approach to lignans. Total synthesis of (−)-Arctigenin, (−)-Matairesinol, and related natural products. Org. Lett. 2004;6:1345–1348. doi: 10.1021/ol049878b. [DOI] [PubMed] [Google Scholar]
- Frink C.R., Waggoner P.E., Ausubel J.H. Nitrogen fertilizer: retrospect and prospect. Proc. Natl. Acad. Sci. Unit. States Am. 1999;96:1175. doi: 10.1073/pnas.96.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good A.G., Beatty P.H. Fertilizing nature: a tragedy of excess in the commons. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford M., Horst W., Kichey T., Lambers H., Schjoerring J., Møller I.S., White P. Chapter 6 - functions of macronutrients. In: Marschner P., editor. Marschner's Mineral Nutrition of Higher Plants. third ed. Academic Press; San Diego: 2012. pp. 135–189. [Google Scholar]
- He D., Wang M., Zhao S., Shu Y., Zeng H., Xiao C., Lu C., Liu Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia. 2017;119:136–149. doi: 10.1016/j.fitote.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Hensler J.G. In: Chapter 15 - Serotonin in Brady. eighth ed. S.T., Siegel G.J., Albers R.W., Price D.L., editors. Academic Press; New York: 2012. pp. 300–322. (Basic Neurochemistry). [Google Scholar]
- Herrmann K.M. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995;7:907–919. doi: 10.1105/tpc.7.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K.M., Weaver L.M. THE SHIKIMATE PATHWAY. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Hirel B., Le Gouis J., Ney B., Gallais A. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007;58:2369–2387. doi: 10.1093/jxb/erm097. [DOI] [PubMed] [Google Scholar]
- Hu Z.,L., Meng H., Li Y., Wang X., L., Liu J.,H. Study on anti human cytomegalovirus effect of isatis root by MTT colorimetry. J. Shandong Univ. Tradit. Chin. Med. 1999;02:137–138. [Google Scholar]
- Ishihara A., Hashimoto Y., Tanaka C., Dubouzet J.G., Nakao T., Matsuda F., Nishioka T., Miyagawa H., Wakasa K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008;54:481–495. doi: 10.1111/j.1365-313X.2008.03441.x. [DOI] [PubMed] [Google Scholar]
- Jie C., Luo Z., Chen H., Wang M., Yan C., Mao Z.-F., Xiao G.-K., Kurihara H., Li Y.-F., He R.-R. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget. 2017;8:105615–105629. doi: 10.18632/oncotarget.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Cao R.-A., Du Y.-H., Zhao Z.-L., Li L.-Y. Review of the researches on polysaccharides from Isatis indigatica Fort. Sci. Technol. Food Ind. 2016;37:378–383. [Google Scholar]
- Kant S., Bi Y.-M., Rothstein S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011;62:1499–1509. doi: 10.1093/jxb/erq297. [DOI] [PubMed] [Google Scholar]
- Kaur R., Manjal S.K., Rawal R.K., Kumar K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017;25:4533–4552. doi: 10.1016/j.bmc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Koprivova A., North K.A., Kopriva S. Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008;146:1408. doi: 10.1104/pp.107.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Berthomé R., Orsel M., Mercey-Boutet S., Yu A., Castaings L., Elftieh S., Major H., Renou J.-P., Daniel-Vedele F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011;157:1255. doi: 10.1104/pp.111.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea P.J., Azevedo R.A. Nitrogen use efficiency. 1. Uptake of nitrogen from the soil. Ann. Appl. Biol. 2006;149:243–247. [Google Scholar]
- Li J., Zhou B., Li C., Chen Q., Wang Y., Li Z., Chen T., Yang C., Jiang Z., Zhong N., Yang Z., Chen R. Lariciresinol-4-O-β-D-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J. Ethnopharmacol. 2015;174:379–386. doi: 10.1016/j.jep.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Li L., Dong T.,Y., Li X.,L., Qiao C.,Z. Study on the quality control of Daqingye(Folium strobilanthes),Banlangen(Radix strobilanthes) and their preparations. Acta Pharm. Sin. 1994;29:128–131. [Google Scholar]
- Lin C.-W., Tsai F.-J., Tsai C.-H., Lai C.-C., Wan L., Ho T.-Y., Hsieh C.-C., Chao P.-D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R.A. Arabidopsis chloroplasts dissimilate L-arginine and L-citrulline for use as N source. Plant Physiol. 1993;101:429. doi: 10.1104/pp.101.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang W., Bittner F., Schmidt N., Berkey R., Zhang L., King H., Zhang Y., Feng J., Wen Y., Tan L., Li Y., Zhang Q., Deng Z., Xiong X., Xiao S. Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell. 2016;28:1108. doi: 10.1105/tpc.15.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae T. Physiological nitrogen efficiency in rice: nitrogen utilization, photosynthesis, and yield potential. Plant Soil. 1997;196:201–210. [Google Scholar]
- Maugard T., Enaud E., Choisy P., Legoy M.D. Identification of an indigo precursor from leaves of Isatis tinctoria (Woad) Phytochemistry. 2001;58:897–904. doi: 10.1016/s0031-9422(01)00335-1. [DOI] [PubMed] [Google Scholar]
- Meng L., Guo Q., Liu Y., Chen M., Li Y., Jiang J., Shi J. Indole alkaloid sulfonic acids from an aqueous extract of Isatis indigotica roots and their antiviral activity. Acta Pharm. Sin. B. 2017;7:334–341. doi: 10.1016/j.apsb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan L., Raghav D., Ashraf S.M., Sebastian J., Rathinasamy K. Indirubin, a bis-indole alkaloid binds to tubulin and exhibits antimitotic activity against HeLa cells in synergism with vinblastine. Biomed. Pharmacother. 2018;105:506–517. doi: 10.1016/j.biopha.2018.05.127. [DOI] [PubMed] [Google Scholar]
- Muhammad S., Sanden B.L., Saa S., Lampinen B.D., Smart D.R., Shackel K.A., DeJong T.M., Brown P.H. Optimization of nitrogen and potassium nutrition to improve yield and yield parameters of irrigated almond (Prunus dulcis (Mill.) D. A. webb) Sci. Hortic. 2018;228:204–212. [Google Scholar]
- Nomura T., Ishihara A., Imaishi H., Endo T., Ohkawa H., Iwamura H. Molecular characterization and chromosomal localization of cytochrome P450 genes involved in the biosynthesis of cyclic hydroxamic acids in hexaploid wheat. Mol. Genet. Genom. 2002;267:210–217. doi: 10.1007/s00438-002-0653-x. [DOI] [PubMed] [Google Scholar]
- Novick N.J., Tyler M.E. L-arabinose metabolism in Azospirillum brasiliense. J. Bacteriol. 1982;149:364. doi: 10.1128/jb.149.1.364-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M.J., Driscoll S.P. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 2010;20:110–116. [Google Scholar]
- Ruan J., Gerendás J., Härdter R., Sattelmacher B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea ( camellia sinensis ) plants. Ann. Bot. 2007;99:301–310. doi: 10.1093/aob/mcl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.-H., Xie Z.-Y., Wang R., Huang S.-J., Li Y.-M., Wang Z.-T. Quantitative and chemical fingerprint analysis for the quality evaluation of isatis indigotica based on ultra-performance liquid chromatography with photodiode array detector combined with chemometric methods. Int. J. Mol. Sci. 2012;13 doi: 10.3390/ijms13079035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.B., Foley R.C., Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Stewart A.J., Chapman W., Jenkins G.I., Graham I., Martin T., Crozier A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001;24:1189–1197. [Google Scholar]
- Świędrych A., Lorenc-Kukuła K., Skirycz A., Szopa J. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol. Biochem. 2004;42:593–600. doi: 10.1016/j.plaphy.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Tilman D., Cassman K.G., Matson P.A., Naylor R., Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- Wang Q., Ding Z.-H., Liu J.-K., Zheng Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004;64:189–194. doi: 10.1016/j.antiviral.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yang L., Wang G., Wang M., Jiang H., Chen L., Zhao F., Qiu F. Indole alkaloids from the roots of Isatis indigotica and their inhibitory effects on nitric oxide production. Fitoterapia. 2014;95:175–181. doi: 10.1016/j.fitote.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zheng X., Cheng N., Gai W., Xue X., Wang Y., Gao Y., Shan J., Yang S., Xia X. Isatis indigotica root polysaccharides as adjuvants for an inactivated rabies virus vaccine. Int. J. Biol. Macromol. 2016;87:7–15. doi: 10.1016/j.ijbiomac.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang H., Zhao X., Lv Q., Sun C., Li X., Chen K. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods. 2015;14:144–153. [Google Scholar]
- Zhao D., Reddy K.R., Kakani V.G., Reddy V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005;22:391–403. [Google Scholar]
- Zhao J., Last R.L. Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell. 1996;8:2235. doi: 10.1105/tpc.8.12.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Zhang X.-Y. Research progress of Chinese herbal medicine radix isatidis (banlangen) Am. J. Chin. Med. 2013;41:743–764. doi: 10.1142/S0192415X1350050X. [DOI] [PubMed] [Google Scholar]
- Zou P., Hong Y., Koh H. Chemical fingerprinting of Isatis indigotica root by RP-HPLC and hierarchical clustering analysis. J. Pharm. Biomed. Anal. 2005;38:514–520. doi: 10.1016/j.jpba.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Zuo L., Li J.-b., Xu J., Yang J.-z., Zhang D.-m., Tong Y.-l. [Studies on chemical constituents in root of Isatis indigotica] Zhongguo Zhong yao za zhi = China J. Chinese Materia Medica. 2007;32:688–691. [PubMed] [Google Scholar]