Abstract
The acute phase of respiratory distress caused by porcine reproductive and respiratory syndrome virus (PRRSV) is likely a consequence of the release of inflammatory cytokines in the lung. IL-8, the main chemokine and activator of neutrophils, might be related to the lung injury upon PRRSV infection. In this study, we showed that PRRSV induced IL-8 expression in vivo and in vitro. Subsequently, we demonstrated that JNK and NF-κB pathways were activated upon PRRSV infection and required for the enhancement of IL-8 expression. We further verified that PRRSV-activated TAK-1 was essential for the activation of JNK and NF-κB pathways and IL-8 expression. Moreover, we revealed an AP-1 binding motif in the cloned porcine IL-8 (pIL-8) promoter, and deletion of this motif abolished the pIL-8 promoter activity. Finally, we found that the JNK-activated AP-1 subunit c-Jun was critical for the up-regulation of IL-8 expression by PRRSV. These data suggest that PRRSV-induced IL-8 production is likely through the TAK-1/JNK/AP-1 pathways.
Keywords: PRRSV, IL-8, TAK-1, JNK, AP-1
Highlights
-
•
PRRSV infection induces IL-8 expression in vitro and in vivo.
-
•
PRRSV up-regulates IL-8 expression through TAK-1/JNK/AP-1 pathways.
-
•
AP-1 element in porcine IL-8 promoter is essential for PRRSV induced IL-8 expression.
1. Introduction
Since described in USA in 1987, porcine reproductive and respiratory syndrome (PRRS) characterized with respiratory illness in piglets and severe reproductive problems in sows and gilts (Music and Gagnon, 2010) has now been one of the most important diseases in pigs, leading to significant economic losses in swine industry worldwide (Li et al., 2007). PRRS virus (PRRSV) is an enveloped positive single stranded RNA virus and belongs to the genus Arterivirus, family Arteriviridae, order Nidovirales (Cavanagh, 1997). PRRSV genome is approximately 15.4 kb in length and has 10 open reading frames (ORFs) (Yun and Lee, 2013). PRRSV exhibits a highly restricted host cell tropism for the cells of the monocyte/macrophage/dendritic lineages (Duan et al., 1997). Since 2006, there have been devastating outbreaks of atypical PRRS in China, which is characterized by high fever, high morbidity, and high mortality. A highly pathogenic PRRSV (HP-PRRSV) isolate with a 30-amino-acid (30 aa) deletion in non-structural protein (nsp2) was identified as the causative agent (Tian et al., 2007).
The acute phase of PRRSV infection primarily targets alveolar macrophages. The mechanistic basis for the acute phase of respiratory distress is likely a consequence of the release of inflammatory cytokines in the lung (Chand et al., 2012). The intensity of the disease appears to vary among isolates and variation in the pathogenicity of PRRSV has been observed in experimentally infected animals (Cho and Dee, 2006, Han et al., 2014). HP-PRRSV is reported to cause severe acute lung injury (Han et al., 2014). The lesions include destruction of lung structure with extensive hemorrhage and a large number of inflammatory cell infiltration into the alveolar spaces. Additionally, interstitial pneumonia also occurs, which is characterized by a marked thickening of the alveolar septa (Shang et al., 2013). Upon infection, neutrophils are always the first to be recruited to the focus and cause capillary leak and pulmonary edema (Segel et al., 2011). Compared with the pigs infected typical PRRSV strains, the number of neutrophils in the lungs of HP-PRRSV-infected pigs was significantly increased (Guo et al., 2013, Han et al., 2014). IL-8 is the main chemokine and activator of neutrophils (Pease and Sabroe, 2002). Upon receiving inflammatory stimuli, IL-8 can be up-regulated in many different cell types, including fibroblasts, monocytes, and hepatocytes (Mukaida, 2000). Thus, we assume that IL-8 production might be induced and be related in the lung lesion upon PRRSV infection (Bohnet et al., 1997, Fujimori et al., 2003, Harada et al., 1994, Hay and Sarau, 2001, Jundi and Greene, 2015, Mukaida et al., 1998).
Given the important significance of IL-8 in the pathology of virus infection,we dissected the underlying mechanism of IL-8 expression under PRRSV infection in the present study. We demonstrate that HP-PRRSV induces IL-8 expression through the TAK-1/JNK/AP-1 pathways.
2. Results
2.1. PRRSV induces IL-8 production both in vitro and in vivo
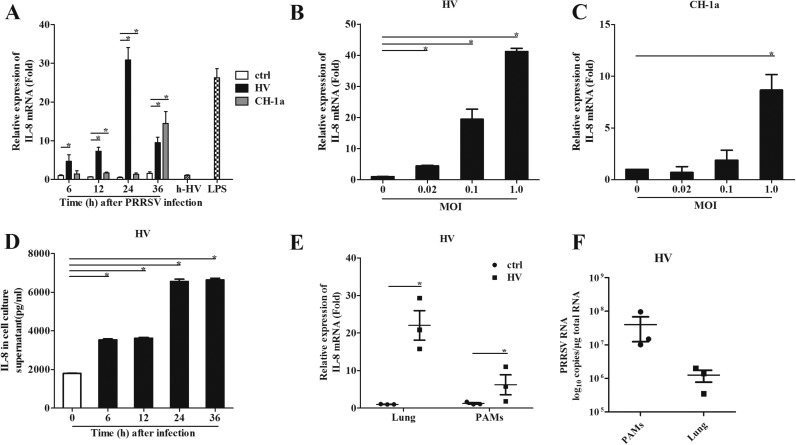
To investigate whether PRRSV can induce IL-8 production, we infected porcine alveolar macrophages (PAMs) with different strains of PRRSV, including typical PRRSV strain CH-1a and HP-PRRSV strain HV. Our results showed that HV up-regulated IL-8 mRNA expression about 5, 8, 30, and 10 folds compared to the uninfected controls at 6, 12, 24, and 36 h post infection (hpi), respectively ( Fig. 1A). CH-1a also induced IL-8 expression, but at a lower level. These data suggest that PRRSV-induced IL-8 production is not strain-dependent, but varies among stains. To confirm this result, we infected PAMs with HV and CH-1a at an MOI of 0.02, 0.1 or 1. Our results indicated that the up-regulation of IL-8 by both HV and CH-1a was in a dose dependent manner (Fig. 1B and C). We also detected the cell viability after PRRSV infection, and found that infection with an MOI of 1 for 24 h or an MOI of 0.1 for 36 h led to significant PAM death (data not shown). Thus, we used MOI of 0.1 to infect PAMs in the following experiment. Additionally, the IL-8 protein level induced by PRRSV was significantly increased (Fig. 1D).
Fig. 1.
PRRSV induces IL-8 expression in vitro and in vivo. (A) PAMs were inoculated with HP-PRRSV isolate HV, CH-1a strain, heat inactivated HV (h-HV) at a multiplicity of infection (MOI) of 1, LPS, or medium alone. Total RNA was extracted from cell lysates at 6, 12, 24, and 36 h post inoculation. (B, C) PAMs were infected with HV or CH-1a at an MOI of 0.02, 0.1 and 1, and then the Total RNA was extracted from cell lysates at 24 and 36 hpi. IL-8 mRNA was quantified by real-time PCR, and results were normalized to GAPDH and expressed as fold induction over medium alone. (D) Supernatants were harvested at 6, 12, 24, and 36 hpi post PRRSV infection (MOI=1), and levels of IL-8 (pg/ml) released were determined by ELISA. (E) Pigs were infected intranasally with 2 ml (105 TCID50 virus/ml) HV. Samples were collected at 5 days post infection. IL-8 mRNA was quantified by real-time PCR. And results were normalized to GAPDH and expressed as fold induction over samples from uninfected pigs. (F) PRRSV ORF-7 mRNA was quantified by real-time PCR. Differences were evaluated by Student's t-test (*, P<0.05).
To investigate whether HP-PRRSV has the ability to induce IL-8 production in vivo, four-week old pigs were intranasally infected with 2 ml of HV (105TCID50/ml) for each piglet, and samples were collected at 5 days post infection for IL-8 and PRRSV analysis. Our results showed that IL-8 mRNA expression was significantly induced in lungs and PAMs from PRRSV-infected piglets (Fig. 1E). PRRSV infection was also analyzed in lungs and PAMs from PRRSV-infected piglets (Fig. 1F). Collectively, these data indicate that PRRSV infection remarkably induces IL-8 production both in vivo and in vitro.
2.2. JNK and NF-κB signaling pathways are required for PRRSV induced IL-8 expression
To dissect the signaling pathways involved in PRRSV-induced IL-8 production, PAMs were pretreated for 1 h with the inhibitors of the key signaling pathways, including JNK, NF-κB, MEK, p38 MAPK, PI3K and PKC before PRRSV infection. Twenty-four hours later, the expression of IL-8 mRNA was analyzed. As shown in Fig. 2A, inhibitors of JNK (SP60125), p38 (SB203580), and NF-κB (BAY11-7082) significantly inhibited IL-8 production induced by HV by 70%, 50%, and 90%, respectively. While inhibition of MEK (PD98059), PKC (GF109203X) or PI3K (LY294002) only slightly decreased IL-8 mRNA expression. These findings suggest that the JNK and NF-κB pathways might be involved in PRRSV-induced IL-8 production. To confirm this, PAMs were treated with JNK inhibitor and NF-κB inhibitor at different concentrations before PRRSV infection. As shown in Fig. 2B, the inhibitory effects of both inhibitors were in a dose dependent manner. In addition, they also impaired IL-8 protein secretion (Fig. 2C). We also analyzed PRRSV replication in the presence of JNK and NF-κB inhibitors. Our results showed that JNK and NF-κB inhibitors at the used concentrations did not affect PRRSV replication (Fig. 2D). To further investigate whether JNK and NF-κB signaling pathways are activated in PRRSV-infected PAMs, JNK and IκB phosphorylations were analyzed by western blot. As shown in Fig. 2E and F, the phosphorylated JNK and IκB were significantly increased at 12 h and 24 h post infection. Meanwhile, IκB was gradually degraded in PRRSV-infected cells. Together, these results suggest that PRRSV infection induces IL-8 production by activating the JNK and NF-κB pathways.
Fig. 2.
PRRSV up-regulates IL-8 expression through JNK and NF-κB pathways. (A) PAMs were pretreated with inhibitors of JNK (SP600125 (SP), 10 μM), NF-κB (BAY11-7082 (BAY), 1 μM), P38 (SB202190 (SB), 5 μM), MEK (PD98059 (PD), 10 μM), PKC (GF-109203X (GF), 1 μM), PI3K (LY294002 (LY), 5 μM), or DMSO control for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, total RNAs were extracted for IL-8 mRNA analysis by real-time PCR. (B) PAMs were pretreated with JNK or NF-κB inhibitor at different doses (1, 5, 10 μM for SP and 0.1, 0.5, 1 μM for BAY) or DMSO control for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, total RNAs were extracted for IL-8 mRNA analysis by real-time PCR. (C) PAMs were pretreated with JNK (5 μM) or NF-κB (0.5 μM) inhibitor or DMSO control for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). IL-8 production in cell supernatants was detected at 24 hpi by ELISA. (D) PRRSV ORF-7 mRNA was analyzed by real-time PCR. (E, F) PAMs were inoculated with PRRSV (MOI=0.1), and cells were harvested at 6, 12, 24, and 36 hpi for p-JNK, JNK (D), p-IκB and IκB (E) analysis by Western blotting. Differences were evaluated by Student's t-test (*, P<0.05).
2.3. TAK-1 is essential for PRRSV-activated JNK and NF-κB signaling pathways
Upon inflammatory stimulation, TAK-1 is activated and subsequently activates MAPK and NF-κB signaling pathways. To investigate whether TAK-1 participates in PRRSV-induced IL-8 expression, PAMs were pretreated with TAK-1 inhibitor (5Z)-7-Oxozeaenol at different concentrations 1 h before PRRSV infection, and then IL-8 mRNA expression and protein production were analyzed. As shown in Fig. 3A and B, PRRSV-induced IL-8 expression was inhibited by (5Z)-7-Oxozeaenol in a dose dependent meaner. (5Z)-7-Oxozeaenol also down-regulated IL-8 protein production by about 47%. (5Z)-7-Oxozeaenol did not affect PRRSV replication (Fig. 3C). These results suggest that TAK-1 is essential for IL-8 production induced by PRRSV. To further verify this, we investigated whether TAK-1 signaling pathway was activated in PRRSV-infected PAMs. As shown in Fig. 3D, TAK-1 phosphorylation was significantly up-regulated by PRRSV infection. Next, to study whether JNK activation is through TAK-1, PAMs were pretreated with (5Z)-7-Oxozeaenol at different concentrations 1 h before PRRSV infection, and then JNK and IκB phosphorylations were analyzed. As shown in Fig. 3E and F, phosphorylations of JNK and IκB were inhibited by (5Z)-7-Oxozeaenol, suggesting that TAK-1 is required for PRRSV-induced JNK and NF-κB pathway activations.
Fig. 3.
TAK-1 is required in PRRSV- induced IL-8 expression. (A) PAMs were pretreated with the inhibitor of TAK-1 ((5Z)-7-Oxozeaenol) at different concentrations (0.5 and 1 μM), or DMSO control for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, total RNAs were extracted for IL-8 mRNA analysis by real-time PCR. (B) PAMs were pretreated with TAK-1 inhibitor at 1 μM. IL-8 production in cell supernatants was detected at 24 hpi using ELISA. (C) PAMs were pretreated with 1 μM (5Z)-7-Oxozeaenol or DMSO for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, PRRSV ORF-7 mRNA was analyzed by real-time PCR. (D) PAMs were inoculated with PRRSV (MOI=0.1). Cells were then harvested at 6, 12, 24, and 36 h for p-TAK-1 and TAK-1 analysis by Western blotting. (E) PAMs were pretreated with the TAK-1 inhibitor at doses of 1 or 2 μM for 1 h, and then infected with HP-PRRSV (MOI=0.1). At 24 hpi, cells were harvested for p-JNK and JNK analysis by Western blotting. (F) PAMs were pretreated with the TAK-1 inhibitor at doses of 0.1, 0.5, 1 or 2 μM for 1 h, and then infected with HV (MOI=0.1). At 24 hpi, cells were harvested for p-IκB and IκB analysis by Western blotting. Differences were evaluated by Student's t-test (*, P<0.05).
2.4. AP-1 is critical for IL-8 expression
To study the mechanisms underlying the transcriptional regulation of PRRSV-induced IL-8 production, a 2751-bp fragment flanking the 5′ region of porcine IL-8 gene was cloned. Using the bioinformatics approach (http://www.cbrc.jp/research/db/TFSEARCH.html), several putative transcriptional regulatory elements were found in the 5′-flanking region of the pIL-8 gene, including CREB, C/EBP β, AP-1, OCT-1, and NF-κB. To evaluate the porcine IL-8 promoter activity and determine the region responsive to PRRSV infection, serial deletions starting from the 5′-end of the promoter were generated and schematically shown in Fig. 4A. Luciferase reporter vectors were constructed, and then transfected into Marc-145 cells. The luciferase activities were assessed with or without PRRSV infection. Our results showed that deletion construct −187/123-luc exhibited a 66% up-regulation after PRRSV infection, while other constructs were not up-regulated by virus infection (Fig. 4B). When the promoter was truncated to −108/123 and −76/123, the basal-level luciferase activity was low and there were no significant changes for the luciferase activity after PRRSV infection. This observation suggests that the regulatory elements might exist in the region from −187 to −76 bp in porcine IL-8 promoter and the responsive elements could be the putative AP-1 (−138 to −130),C/EBP β (−107 to −90) and/or NF-κB (−92 to −82). We then deleted each of AP-1, NF-κB and C/EBP β, two or all three of the binding sites from −108/123-luc to generate new mutation vectors (Fig. 4C). Luciferase results showed that mutations with AP-1 binding site deletion significantly impaired IL-8 promoter activation (Fig. 4D), suggesting that AP-1 biding site in porcine IL-8 promoter is the main responsive element for PRRSV infection.
Fig. 4.
Cloning, sequence analysis, and characterization of porcine IL-8 promoter. (A) Cloning and sequence analysis of the 2751-bp porcine IL-8 promoter. The positions of the putative regulatory motifs are relative to the transcription initiation site. Schematic representation of the porcine IL-8 promoter and promoter deletion mutants inserted into pGL3 basic luciferase vectors: −2628/+123-luc, −1982/+123-luc, −1278/+123-luc, −939/+123-luc, −515/+123-luc, −187/+123-luc, −108/+123-luc, and −76/+123-luc porcine IL-8 promoter vectors. The relative lengths and positions of the 5′-ends of these fragments are indicated. (B) The porcine IL-8 promoter vectors or pGL3 empty vector were transfected into Marc-145 cells. Twenty-four hours later, cells were inoculated with PRRSV (MOI=1) or medium. Cells were harvested to determine luciferase activity at 36 hpi. (C) Schematic diagram represents porcine IL-8 deletion mutant constructs including −187/123(ΔAP-1)-luc, −187/123(ΔNF-κB)-luc, −187/123(ΔC/EBP β)-luc, −187/123(ΔAP-1, NF-κB)-luc, −187/123(ΔAP-1, C/EBP β)-luc, −187/123(ΔC/EBP β, NF-κB)-luc and −187/123(ΔAP-1, NF-κB, C/EBP β)-luc. (D) Marc-145 cells were transfected with IL-8 deletion mutant promoter or pGL3 empty vector. Twenty-four hours later, cells were inoculated with PRRSV (MOI=1) or medium control and then harvested for luciferase activity analysis at 24 hpi. Differences were evaluated by Student's t-test (*, P<0.05).
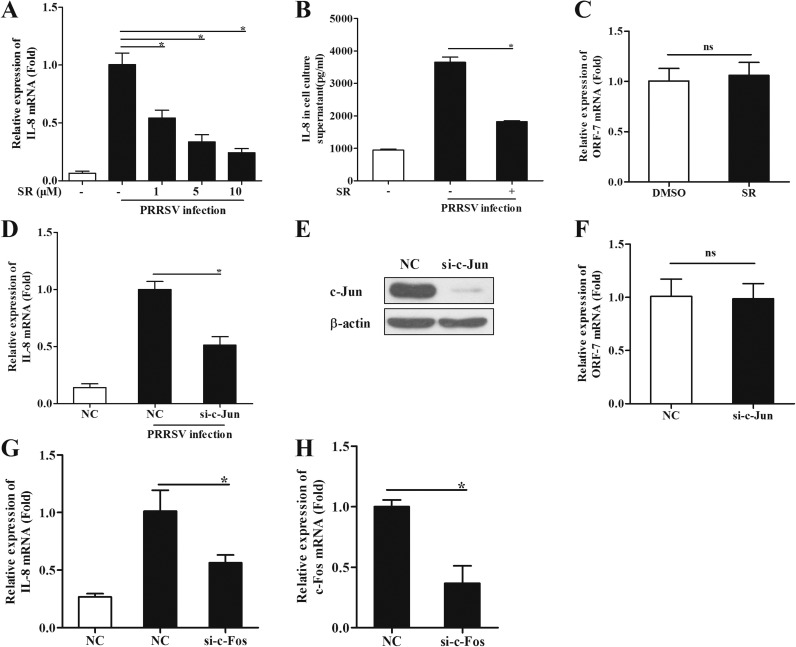
The transcription factor AP-1 has been extensively studied and believed to contribute to the production of pro-inflammatory cytokines and chemokines (Liu et al., 2007; Rahman et al., 2011). AP-1 is a dimeric complex of c-Jun and c-Fos. To further evaluate the role of AP-1 in IL-8 induction by PRRSV infection, PAMs were pretreated with the c-Jun inhibitor SR11302 1 h before PRRSV infection. Twenty-four hours later, IL-8 production was analyzed. Our results showed that c-Jun inhibitor remarkably suppressed IL-8 production both at mRNA and protein levels induced by PRRSV ( Fig. 5A and B). SR at the used concentrations did not affect PRRSV replication (Fig. 5C). The role of c-Jun was also confirmed with siRNA knockdown. PAMs were transfected with specific siRNA targeting c-Jun at 48 h before PRRSV infection, and IL-8 mRNA expression was then analyzed by real-time PCR at 24 hpi. As shown in Fig. 5D, knockdown of c-Jun significantly decreased the level of IL-8 expression by 49%. Meanwhile, the knockdown efficiency was also confirmed (Fig. 5E), and knockdown of c-Jun by siRNA did not affect PRRSV replication (Fig. 5F). Knockdown of c-Fos (Fig. 5H), the other subunit of AP-1, also suppressed IL-8 mRNA expression (Fig. 5G).
Fig. 5.
c-Jun is required for PRRSV- induced IL-8 expression. (A) PAMs were pretreated with the inhibitor of c-Jun (SR11302) at different doses (1, 5, and 10 μM), or DMSO for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, total RNAs were extracted for IL-8 mRNA analysis by real-time PCR. (B) PAMs were pretreated with the inhibitor SR11302 at 10 μM. IL-8 production in cell supernatants was analyzed at 24 hpi by ELISA. (C) PAMs were pretreated with the inhibitor SR11302 at 10 μM or DMSO for 1 h, and then cells were inoculated with or without PRRSV (MOI=0.1). Twenty-four hours later, PRRSV ORF-7 mRNA was analyzed by real-time PCR. (D) PAMs were transfected with siRNA targeting c-Jun for 48 h and then infected with HV (MOI=0.1). Total RNAs were extracted for IL-8 mRNA analysis by real-time PCR at 24 hpi. (E) C-Jun knockdown efficiency was determined by Western blotting. (F) ORF-7 mRNA analysis by real-time PCR. (G, H) PAMs were transfected with siRNA targeting c-Fos for 48 h and then infected with HV (MOI=0.1). Total RNAs were extracted for IL-8 and c-Fos mRNA analysis by real-time PCR at 24 hpi. Differences were evaluated by Student's t-test (*, P<0.05).
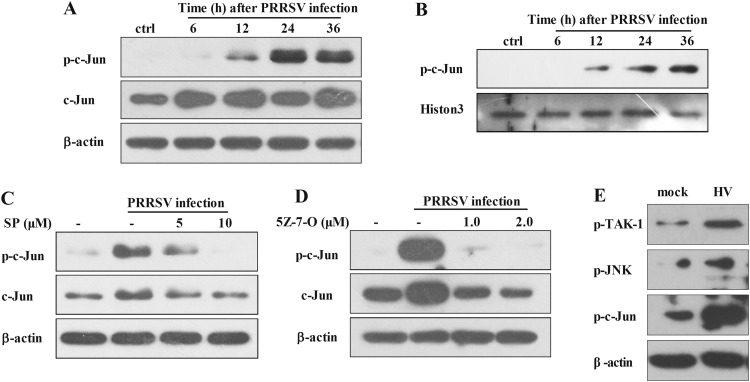
To investigate whether AP-1 pathway is activated in PAMs during the course of PRRSV infection, PAMs were infected with HV at an MOI of 0.5 and the phosphorylation of c-Jun was then evaluated using western blot. As shown in Fig. 6A, c-Jun phosphorylation was remarkably increased at 12, 24, and 36 hpi. Meanwhile, total c-Jun expression was also increased at 6 hpi and kept at high levels through the end of the experiment at 36 hpi. c-Jun activation was also confirmed by phosphorylated c-Jun accumulation in the nucleus (Fig. 6B).
Fig. 6.
PRRSV activates c-Jun through JNK. (A) PAMs were inoculated with PRRSV (MOI=0.1), and cells were harvested at 6, 12, 24, and 36 hpi for p-c-Jun and c-Jun analysis by Western blotting. (B) PAMs were inoculated with HV (MOI=0.1), and nuclear protein fractions were separated for p-c-Jun analysis at 6, 12, 24, and 36 hpi. (C) PAMs were pretreated with the JNK inhibitor at a dose of 5 or 10 μM for 1 h, and then infected with HV (MOI=0.1). At 24 hpi, cells were harvested for p-c-Jun and c-Jun analysis by Western blotting. (D) PAMs were pretreated with the TAK-1 inhibitor at a dose of 1 or 2 μM for 1 h, and then infected with HV (MOI=0.1). At 24 hpi, cells were harvested for p-c-Jun analysis by Western blotting. (E) Pigs were infected intranasally with 2 ml (105 TCID50 virus/ml) HV. PAMs were collected at 5 days post infection for p-TAK-1, P-JNK, and p-c-Jun analysis by Western blotting.
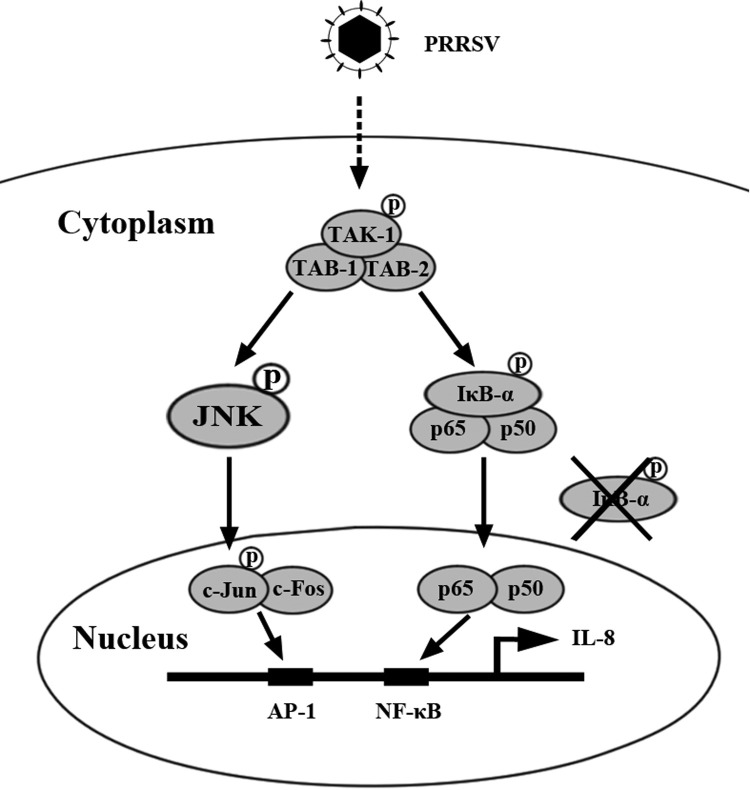
Previous studies have demonstrated that c-Jun is located downstream of JNK. To investigate whether the activation of JNK and TAK-1 signaling pathways is essential for c-Jun phosphorylation during PRRSV infection, PAMs were pretreated with different doses of SP or (5Z)-7-Oxozeaenol 1 h before PRRSV infection. At 24 h after PRRSV infection, c-Jun phosphorylation was analyzed by Western blot. As shown in Fig. 6C and D, c-Jun phosphorylation induced by PRRSV was significantly impaired by the JNK inhibitor SP and TAK-1 inhibitor (5Z)-7-Oxozeaenolin in a dose-dependent manner, suggesting that the activation of c-Jun by PRRSV is mediated by TAK-1/JNK pathways. To further confirm this observation in vivo, we collected the PAMs from HV-infected pigs, and western blot analysis showed that TAK-1, JNK, and c-Jun were also activated (Fig. 6E). Collectively, these data indicate that PRRSV induces IL-8 production through TAK-1/JNK/AP-1 signaling pathways ( Fig. 7).
Fig. 7.
A model showing that PRRSV induces IL-8 production mainly through TAK-1/JNK/AP-1 and NF-κB pathways.
3. Discussion
In this study, we investigated how PRRSV induced IL-8 production. We showed that PRRSV induced IL-8 production in PAMs both in vitro and in vivo. Subsequently, we demonstrated that PRRSV-induced IL-8 production was mainly dependent on the activation of TAK-1/JNK/AP-1 pathways. It was demonstrated that infection with a highly pathogenic strain of PRRSV elicited a significant elevation of cytokines including IL-8 in BALF, serum and TBLN homogenates of pigs (Guo et al., 2013). In our study, we found that PRRSV infection induced IL-8 expression both in vivo and in vitro. Besides, PAMs infected with HP-PRRSV isolate induced higher level of IL-8 expression in PAMs than CH-1a, which is in accordance with another in vivo report (Han et al., 2014). The extensive level of IL-8 and the neutrophils recruited to lungs should be of particular significance in the lung injury. After stimulation by pro-inflammatory cytokines, the activated neutrophils could release free radicals, inflammatory cytokines and proteases, which have contributions to lung lesions (Avasarala et al., 2013, Han et al., 2014, Ware and Matthay, 2000). Indeed, compared with traditional strain, HP-PRRSV isolate can cause much severer neutrophil infiltration and lung lesions (Han et al., 2014, Tian et al., 2007). Therefore, it is reasonable to speculate that upon HP-PRRSV infection macrophages secret higher levels of IL-8, which recruits more neutrophils and eventually leads to tissue damage and dysfunction of the lung.
Previous studies have indicated that maximal IL-8 protein expression requires NF-κB activation as well as the activation of the MAP kinases ERK, JNK, and p38 (Li et al., 2002). In our study, NF-κB inhibitor can reduce the up-regulated IL-8 production by PRRSV infection, indicating that PRRSV-induced IL-8 expression is partially dependent on NF-κB pathway. This is in agreement with the report regarding the significance of NF-κB pathway in IL-8 expression induced by other stimulators (Ohkuni et al., 2011). Moreover, our results suggest that JNK pathway is more essential for PRRSV induced IL-8 expression as JNK and c-Jun inhibitor and siRNA knockdown of c-Jun and c-Fos significantly reduced IL-8 expression. Similarly, under stimulation of HIV-1, HCV and Papillomavirus, the expression of IL-8 is also JNK dependent (Chen et al., 2015, Gangwani and Kumar, 2015, Saeed et al., 2015, Zhang et al., 2014). JNK isoforms are strongly activated in response to various cellular stresses (Cargnello and Roux, 2011). And indeed, we found that PRRSV infection activated JNK, which is in agreement with other reports (Huo et al., 2013, Jing et al., 2014, Lee and Lee, 2012, Yin et al., 2012). Here, we need to indicate that even though JNK inhibitor SP and NF-κB inhibitor BAY significantly inhibit IL-8 mRNA expression induced by PRRSV infection, they do not affect the protein level of IL-8 at the same level, suggesting that there may exist post transcription level regulations such as translation repress, which might be not affected by JNK and NF-kB inhibitors. Interestingly, it has been reported that ARE in IL-8 3′ UTR can recruit TTP, which can repress RNA translation (Anderson and Kedersha, 2009, Eulalio et al., 2007, Franks and Lykke-Andersen, 2008, Winzen et al., 2007). However, this needs to be investigated in the future. The transcription factor c-Jun is a well-described substrate for JNKs. The phosphorylation of c-Jun by JNK has been reported to potentiate the transcriptional capacity of c-Jun, thus enhancing its ability to engage in gene transcription as well as its own expression (Hazzalin and Mahadevan, 2002). Our results also show significant phosphorylation and expression of c-Jun upon PRRSV infection, which is dependent on JNK activation. It is reported that stabilization of IL-8 mRNA is modulated by the p38 mitogen-activated protein kinase pathway (Hoffmann et al., 2002, Winzen et al., 2007). Exposure to the inflammatory cytokine IL-1 has been shown to stabilize IL-8 mRNA through p38 mitogen-activated protein (MAP) kinase and MK2 (Winzen et al., 2007). Interestingly, we found that p38 inhibitor also reduced IL-8 mRNA expression.
In human and mouse IL-8 promoters, a sequence from nt +1 to −133 within the 5′flanking region of the IL-8 gene is essential and sufficient for the transcriptional regulation of the gene (Mukaida and Murayama, 1998). Analysis demonstrates that the promoter elements contain NF-κB, AP-1, and C/EBP β binding sites (Matsusaka et al., 1993, Mukaida and Murayama, 1998, Nourbakhsh et al., 2001). The promoter is regulated in a cell type-specific fashion, requiring a NF-κB element plus either an AP-1 or a C/EBP β element (Chang et al., 2004; Wu et al., 1997). We cloned the 2751 bp-sequence in the 5′ flanking region of the porcine IL-8 gene. Truncation mutations indicated that the region from −187 to +123 bp was essential for the porcine IL-8 promoter activity. However, the longer promoter was not stimulated by PRRSV. The reason could be that there might exist some negative regulation elements in the upstream of the −187 to +123 bp. Analysis showed that AP-1, C/EBP β, and NF-κB elements existed in the region from −187 to −76 bp. Deletion of these three elements suggested that AP-1 element was the most significant one in PRRSV induced IL-8 expression. Similar result was reported with SARS-CoV (Chang et al., 2004).
TAK1 is a key regulator of the innate immunity and the pro-inflammatory signaling pathway. Many viruses have been reported to modulate the activity of TAK1 and thereby the NF-κB and AP-1 pathways. For example, the X protein of hepatitis B virus, LMP1 of Epstein-Barr virus, vGPCR of KHSV, ICP0 of herpes simplex virus type 1, RSV, and Tax of human T cell leukemia virus type 1 can activate NF-κB or AP-1 signaling by targeting TAK1 (Bottero et al., 2011, Diao et al., 2005; Liu et al., 2014; Soni et al., 2007; Zhou et al., 2010). TAK1 regulates JNK, p38 MAPKs and IκB kinase (IKK) signaling pathways, leading to the activation of transcription factors AP-1 and NF-κB (Sakurai, 2002). Consistent with this, we found that PRRSV significantly induced TAK-1 activation and PRRSV-induced IL-8 expression was TAK-1 dependent. Similarly, TAK-1 participates in IL-8 expression under stimulation of other virus (Pera et al., 2012).
In summary, our study demonstrates that PRRSV inducesIL-8 expression is dependent on the activation of TAK-1/JNK/AP1 as well as NF-κB pathways, in which AP-1 element is shown to be critical for IL-8 expression. This work may provide some insights into the molecular mechanisms of IL-8 regulation in pigs during PRRSV infection.
4. Materials and methods
4.1. Cells and virus preparation
PAMs were obtained by postmortem lung lavage of 8-week-old specific-pathogen-free (SPF) pigs and maintained in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin. Marc-145 cells, which is a PRRSV-permissive cell line sub-cloned from MA-104 cells (African green monkey kidney cells), were maintained in Dulbecco's minimum essential medium (DMEΜ) supplemented with 10% heat-inactivated FBS and penicillin-streptomycin. All the cells were cultured and maintained at 37 °C with 5% CO2. PRRSV strain, CH-1a (the first type 2 PRRSV strain isolated in China), and HV (a highly pathogenic PRRSV isolate) were propagated in PAMs. Virus preparations were titrated, and then stored at −80 °C.
4.2. Real-time PCR
Total RNAs were extracted from cells or tissues with TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed using M-MLV reverse transcriptase (Promega) with oligo (dT) 18 primer. Real-time PCR was performed using SYBR Premix Ex Taq (TaKaRa) and a ViiA 7 real-time PCR system (Applied Biosystems). For quantitative analysis of PRRSV in cells and tissues, quantitative PCR (qPCR) was performed as previously described (Du et al., 2016). Relative analysis of gene expression was evaluated using the ΔΔCt method (Schmittgen and Livak, 2008). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set up as an endogenous control. The specific primers used were listed in Table 1.
Table 1.
Primers used in real-time PCR.
| Name | Sequence |
|---|---|
| ORF7 forward | CCCGGGTTGAAAAGCCTCGTGT |
| ORF7 reverse | GGCTTCTCCGGGTTTTTCTTCCTA |
| IL-8 forward | GGCAGTTTTCCTGCTTTCT |
| IL-8 reverse | CAGTGGGGTCCACTCTCAAT |
| C-Jun forward | GCATCGCTGCCTCCAAGT |
| C-Jun reverse | CCCAACAGTCTCGCCTCAAA |
| GAPDH forward | CCTTCCGTGTCCCTACTGCCAAC |
| GAPDH reverse | GACGCCTGCTTCACCACCTTCT |
4.3. Animal experiment
Four-week-old SPF piglets were obtained from the Beijing Center for SPF Swine Breeding and Management and randomly divided into two groups (three piglets per group). All animal studies were performed according to protocols approved by the Animal Welfare Committee of China Agricultural University. Piglets were intranasally inoculated with 2 ml of PRRSV strain HV (105TCID50 virus/ml). Samples were collected at 5 days post infection (dpi).
4.4. Reagents and antibodies
NF-κB inhibitor (BAY11-7082), protein kinase C (PKC) inhibitor (GF-109203X), MEK inhibitor (PD98059), p38 inhibitor (SB203580), JNK inhibitor (SP600125), and phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002) were purchased from Cell Signaling Technology, Inc. LPS was obtained from Sigma-Aldrich. Enzyme-linked immunosorbent assay (Serti et al., 2010) kit for porcine IL-8 was purchased from R&D Systems. All inhibitors were reconstituted in dimethyl sulfoxide (DMSO), and DMSO was used as the solvent control for all experiments involving treatment with inhibitors. A Dual-Glo luciferase assay system was purchased from Promega. Antibodies against c-Jun, p-c-Jun, JNK, p-JNK, TAK-1, p-TAK-1, IκBα and p-IκBα were from Cell Signaling Technology, Inc. Antibody against β-actin was purchased from Sigma.
4.5. Western blotting
Whole-cell extracts were prepared as follows: Cells were washed twice with ice-cold PBS, and then lysed in radioimmuneprecipitation assay (RIPA) lysis buffer (Beyotime) with 100 U proteinase inhibitor (Cwbiotech) and 20 M NaF for 15 min on ice. The lysates were centrifuged at 10,000g for 10 min and the supernatant was aliquoted and stored at −80 °C. Nuclei protein samples were extracted with a nuclear and cytosol fractionation kit (BioVision Incorporated). Protein levels in each sample were quantified with a bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Inc.). Similar amounts of protein from each extract were separated by 12% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyl difluoride (PVDF) membranes (Millipore).
4.6. siRNA knockdown
Cells were transfected with 200 pmol of small interfering RNA (siRNA) oligonucleotides specific for the c-Jun gene, c-Fos gene, or a nonspecific control (NC) (GenePharmaInc.) using HiPerFect Transfection Reagent (QIAGEN) according to the manufacturer's instructions. The efficiency of knockdown of protein expression was confirmed by Western blotting.
4.7. Construction of porcine IL-8 promoters
Genomic DNA was extracted from PAMs and purified by the use of a DNA extraction kit (TaKaRa). The fragment of the porcine IL-8 gene promoter flanking the 5′ IL-8 gene (NC_010450.3) was cloned with specific primers. The obtained 2751-bp Susscrofa IL-8 promoter sequence (nucleotides −2628 to +123) relative to the transcription initiation site (+1) was subcloned into the luciferase (luc) reporter vector pGL3-basic at the NheI site (pGL −2628/+123-luc), and the nucleotide sequence was determined by DNA sequencing. The truncated mutants of the IL-8 promoter were then constructed and inserted into the luciferase reporter vector pGL3-basic (named as pGL3-1892/+123-luc, pGL3-1278/+123-luc, pGL3-939/+123-luc, pGL3-515/+123-luc, pGL3-187/+123-luc, pGL3-108/+123-luc, and pGL3-76/+123-luc). The truncated mutants of the IL-8 promoter were constructed using the primers listed in Table 2. The AP-1, NF-κB, and C/EBP β element deletion IL-8 promoters including −187/123(ΔAP-1)-luc, −187/123(ΔNF-κB)-luc, −187/123(ΔC/EBP β)-luc, −187/123(ΔAP-1, NF-κB)-luc, −187/123(ΔAP-1, C/EBP β)-luc, −187/123(ΔC/EBP β, NF-κB)-luc and −187/123(ΔAP-1, NF-κB, C/EBP β)-luc were generated by overlapping PCR from the −187/123 promoter pGL3 vector. The mutated DNA was then subcloned into the pGL3-basic vector and verified by sequence analysis.
Table 2.
Primers for truncated mutants of IL-8 promoter.
| Name | Sequence |
|---|---|
| +123-HindⅢ | CCCAAGCTTGGAAAACTGCCAAGAAGG |
| −1892/+123-luc | CTAGCTAGCACAACAGTCCCTGACATCC |
| −1278/+123-luc | CTAGCTAGCCATTACCACTGAGCCACAA |
| −939/+123-luc | CTAGCTAGCACATCCTGGAGCATAAACAA |
| −515/+123-luc | CTAGCTAGCTCCCAAATGCTTAGAAATG |
| −187/+123-luc | CTAGCTAGCCTCAATGCTGCTGAAAACA |
| −108/+123-luc | CTAGCTAGCGGATGGTTGCGTAGTGTG |
| −76/+123-luc | CTAGCTAGCATGATGTAAGCATGAGGGC |
4.8. Luciferase reporter assays
Marc-145 cells were seeded in 24-well plates at a cell density of 4×104 cells per well. At 14–16 h after plating, cells were transfected with pGL3 and pRL-TK using Lipofectamine LTX and Plus reagents (Invitrogen). pRL-TK plasmid was used as a control for transfection efficiency. At 24 h after transfection, cells were infected with virus for 36 h or left uninfected. Cell extracts were prepared and analyzed for firefly and Renilla luciferase activities using the dual-luciferase reporter assay kit (Promega) according to the manufacturer's instructions.
4.9. Statistical analysis
All the experiments were performed with at least three independent replicates. Statistical analysis was performed using GraphPad Prism software, and differences in data were analyzed by Student's t-test. Statistically significant was allowed if the P value was less than 0.05. *, P<0.05.
Acknowledgement
This work was supported by the Beijing Natural Science Foundation (Grant no. 6151001), and the National Natural Science Foundation of China (Grant no. 31572516).
References
- Anderson P., Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009;10(6):430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Avasarala S., Zhang F., Liu G., Wang R., London S.D., London L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One. 2013;8(2):e57285. doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnet S., Kotschau U., Braun J., Dalhoff K. Role of interleukin-8 in community-acquired pneumonia: relation to microbial load and pulmonary function. Infection. 1997;25(2):95–100. doi: 10.1007/BF02113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero V., Kerur N., Sadagopan S., Patel K., Sharma-Walia N., Chandran B. Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J. Virol. 2011;85(5):1980–1993. doi: 10.1128/JVI.01911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142(3):629–633. [PubMed] [Google Scholar]
- Chand R.J., Trible B.R., Rowland R.R. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr. Opin. Virol. 2012;2(3):256–263. doi: 10.1016/j.coviro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Chang Y.J., Liu C.Y.Y., Chiang B.L., Chao Y.C., Chen C.C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant Baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 2004;173(12):7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Chen W.C., Tseng C.K., Chen Y.H., Lin C.K., Hsu S.H., Wang S.N., Lee J.C. HCV NS5A up-regulates COX-2 expression via IL-8-mediated activation of the ERK/JNK MAPK pathway. PLoS One. 2015;10(7):e0133264. doi: 10.1371/journal.pone.0133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.G., Dee S.A. Porcine reproductive and respiratory syndrome virus. Theriogenology. 2006;66(3):655–662. doi: 10.1016/j.theriogenology.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Diao L., Zhang B., Xuan C., Sun S., Yang K., Tang Y., Wang C. Activation of c-Jun N-terminal kinase (JNK) pathway by HSV-1 immediate early protein ICP0. Exp. Cell Res. 2005;308(1):196–210. doi: 10.1016/j.yexcr.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Liu Y., Du Y., Wang H., Zhang M., Du Y., Feng W.H. Porcine reproductive and respiratory syndrome virus (PRRSV) up-regulates IL-15 through PKCbeta1-TAK1-NF-kappaB signaling pathway. Virology. 2016;496:166–174. doi: 10.1016/j.virol.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Duan X., Nauwynck H.J., Pensaert M.B. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Microbiol. 1997;56(1–2):9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007;8(1):9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Franks T.M., Lykke-Andersen J. The control of mrna decapping and P-body formation. Mol. Cell. 2008;32(5):605–615. doi: 10.1016/j.molcel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori Y., Kataoka M., Tada S., Takehara H., Matsuo K., Miyake T., Tanimoto M. The role of interleukin-8 in interstitial pneumonia. Respirology. 2003;8(1):33–40. doi: 10.1046/j.1440-1843.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- Gangwani M.R., Kumar A. Multiple protein kinases via activation of transcription factors NF-kappaB, AP-1 and C/EBP-delta regulate the IL-6/IL-8 production by HIV-1 Vpr in astrocytes. PLoS One. 2015;10(8):e0135633. doi: 10.1371/journal.pone.0135633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Lager K.M., Henningson J.N., Miller L.C., Schlink S.N., Kappes M.A., Faaberg K.S. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology. 2013;435(2):372–384. doi: 10.1016/j.virol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Hu Y., Li L., Tian H., Chen Z., Wang L., Teng K. Highly pathogenic porcine reproductive and respiratory syndrome virus infection results in acute lung injury of the infected pigs. Vet. Microbiol. 2014;169(3–4):135–146. doi: 10.1016/j.vetmic.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Sekido N., Akahoshi T., Wada T., Mukaida N., Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994;56(5):559–564. [PubMed] [Google Scholar]
- Hay D.W., Sarau H.M. Interleukin-8 receptor antagonists in pulmonary diseases. Curr. Opin. Pharm. 2001;1(3):242–247. doi: 10.1016/s1471-4892(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Hazzalin C.A., Mahadevan L.C. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 2002;3(1):30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72(5):847–855. [PubMed] [Google Scholar]
- Huo Y., Fan L., Yin S., Dong Y., Guo X., Yang H., Hu H. Involvement of unfolded protein response, p53 and Akt in modulation of porcine reproductive and respiratory syndrome virus-mediated JNK activation. Virology. 2013;444(1–2):233–240. doi: 10.1016/j.virol.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Jing H., Fang L., Wang D., Ding Z., Luo R., Chen H., Xiao S. Porcine reproductive and respiratory syndrome virus infection activates NOD2–RIP2 signal pathway in MARC-145 cells. Virology. 2014;458–459:162–171. doi: 10.1016/j.virol.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Jundi K., Greene C.M. Transcription of Interleukin-8: how altered regulation can affect cystic fibrosis lung disease. Biomolecules. 2015;5(3):1386–1398. doi: 10.3390/biom5031386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee C. Stress-activated protein kinases are involved in porcine reproductive and respiratory syndrome virus infection and modulate virus-induced cytokine production. Virology. 2012;427(2):80–89. doi: 10.1016/j.virol.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Li J., Kartha S., Iasvovskaia S., Tan A., Bhat R.K., Manaligod J.M., Hershenson M.B. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283(4):L690–L699. doi: 10.1152/ajplung.00060.2002. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang X., Bo K., Wang X., Tang B., Yang B., Jiang P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet. J. 2007;174(3):577–584. doi: 10.1016/j.tvjl.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Liu R., Lin Y., Jia R., Geng Y., Liang C., Tan J., Qiao W. HIV-1 Vpr stimulates NF-kappaB and AP-1 signaling by activating TAK1. Retrovirology. 2014;11:45. doi: 10.1186/1742-4690-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Shepherd E.G., Nelin L.D. MAPK phosphatases--regulating the immune response. Nat. Rev. Immunol. 2007;7(3):202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA. 1993;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 2000;72(4):391–398. [PubMed] [Google Scholar]
- Mukaida N., Harada A., Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9(1):9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Murayama T. Molecular mechanism of interleukin-8 gene expression. Rinsho Byori. 1998;46(8):821–828. [PubMed] [Google Scholar]
- Music N., Gagnon C.A. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim. Health Res. Rev. 2010;11(2):135–163. doi: 10.1017/S1466252310000034. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh M., Kalble S., Dorrie A., Hauser H., Resch K., Kracht M. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)−1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. J. Biol. Chem. 2001;276(6):4501–4508. doi: 10.1074/jbc.M007532200. [DOI] [PubMed] [Google Scholar]
- Ohkuni T., Kojima T., Ogasawara N., Masaki T., Fuchimoto J., Kamekura R., Sawada N. Poly(I:C) reduces expression of JAM-A and induces secretion of IL-8 and TNF-alpha via distinct NF-kappaB pathways in human nasal epithelial cells. Toxicol. Appl. Pharm. 2011;250(1):29–38. doi: 10.1016/j.taap.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Pease J.E., Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am. J. Respir. Med. 2002;1(1):19–25. doi: 10.1007/BF03257159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera T., Atmaj C., van der Vegt M., Halayko A.J., Zaagsma J., Meurs H. Role for TAK1 in cigarette smoke-induced proinflammatory signaling and IL-8 release by human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012;303(3):L272–L278. doi: 10.1152/ajplung.00291.2011. [DOI] [PubMed] [Google Scholar]
- Rahman S., Connolly J.E., Manuel S.L., Chehimi J., Montaner L.J., Jain P. Unique cytokine/chemokine signatures for HIV-1 and HCV mono-infection versus Co-infection as determined by the Luminex(R) analyses. J. Clin. Cell Immunol. 2011;2(1) doi: 10.4172/2155-9899.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed U., Mazoor S., Jalal N., Zahid Piracha Z. Contemplating the importance of toll-like receptors I and II regarding human viral pathogenesis. Jundishapur J. Microbiol. 2015;8(1):e13348. doi: 10.5812/jjm.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H., Nishi A., Sato N., Mizukami J., Miyoshi H., Sugita T. TAK1–TAB1 fusion protein a novel constitutively active mitogen-activated protein kinase kinase kinase that stimulates AP-1 and NF-κB signaling pathways. Biochem. Biophys. Res. Commun. 2002;297(5):1277–1281. doi: 10.1016/s0006-291x(02)02379-3. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Segel G.B., Halterman M.W., Lichtman M.A. The paradox of the neutrophil's role in tissue injury. J. Leukoc. Biol. 2011;89(3):359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serti E., Doumba P.P., Thyphronitis G., Tsitoura P., Katsarou K., Foka P., Georgopoulou U. Modulation of IL-2 expression after uptake of hepatitis C virus non-enveloped capsid-like particles: the role of p38 kinase. Cell. Mol. Life Sci. 2010;68(3):505–522. doi: 10.1007/s00018-010-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Wang G., Yin S., Tian H., Du P., Wu J., Liu X. Pathogenic characteristics of three genotype II porcine reproductive and respiratory syndrome viruses isolated from China. Virol. J. 2013;10:7. doi: 10.1186/1743-422X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni V., Cahir-McFarland E., Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv. Exp. Med. Biol. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- Tian K., Yu X., Zhao T., Feng Y., Cao Z., Wang C., Gao G.F. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One. 2007;2(6):e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Winzen R., Thakur B.K., Dittrich-Breiholz O., Shah M., Redich N., Dhamija S., Holtmann H. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol. Cell Biol. 2007;27(23):8388–8400. doi: 10.1128/MCB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.L., Huang E.J., Wen N., XM Oct-1 and CCAAT enhancer-binding protein (CEBP) bind to overlapping elements within the interleukin-8 promoter. J. Biol. Chem. 1997;272(4):2396–2403. [PubMed] [Google Scholar]
- Yin S., Huo Y., Dong Y., Fan L., Yang H., Wang L., Hu H. Activation of c-Jun NH(2)-terminal kinase is required for porcine reproductive and respiratory syndrome virus-induced apoptosis but not for virus replication. Virus Res. 2012;166(1–2):103–108. doi: 10.1016/j.virusres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Yun S.I., Lee Y.M. Overview: replication of porcine reproductive and respiratory syndrome virus. J. Microbiol. 2013;51(6):711–723. doi: 10.1007/s12275-013-3431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E., Feng X., Liu F., Zhang P., Liang J., Tang X. Roles of PI3K/Akt and c-Jun signaling pathways in human papillomavirus type 16 oncoprotein-induced HIF-1alpha, VEGF, and IL-8 expression and in vitro angiogenesis in non-small cell lung cancer cells. PLoS One. 2014;9(7):e103440. doi: 10.1371/journal.pone.0103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang S., Ma J.W., Lei Z., Zhu H.F., Lei P., Huang B. Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes. J. Biol. Chem. 2010;285(16):12159–12168. doi: 10.1074/jbc.M109.067629. [DOI] [PMC free article] [PubMed] [Google Scholar]