Abstract
Infectious bronchitis (IB) generated by the infectious bronchitis virus (IBV) causes economic difficulties for livestock farmers. The 3D8 single chain variable fragment (scFv) protein is a recombinant antibody with nuclease activity that shows antiviral effects against various DNA and RNA viruses in mice and chickens. In this experiment, 3D8 scFv G2 transgenic chickens produced by crossing 3D8 scFv G1 transgenic rooster and wild type hens were screened by genomic PCR and immunohistochemistry analysis. 3D8 scFv transgenic chickens, wild type sibling chickens, and SPF chickens were directly infected with IBV (5 chickens per group) and indirectly infected by airborne propagation (15 chickens per group). The relative IBV shedding titers were measured by quantitative real-time PCR using oropharyngeal and cloacal swabs on days 3 and 5 after intraocular infection. The viral load was significantly decreased in the 3D8 scFv transgenic chickens from the contact transmission group. Additionally, blood was collected from each group on day 17 post-infection. The ELISA results showed a marked reduction of the antibody titer against IBV in the 3D8 scFv transgenic chickens from the contact transmission group. These results suggest that the 3D8 scFv protein potentially inhibits infectious bronchitis virus transmission in chickens.
Keywords: Infectious bronchitis virus, 3D8 scFv, Chicken, Transgenic, Shedding
Highlights
-
•
Produced G2 3D8 single chain variable fragment (scFv) transgenic chickens.
-
•
3D8 scFv transgenic chickens showed reduced infectious bronchitis viral shedding level in the contact transmission group.
-
•
3D8 scFv transgenic chickens were 40% lower than the response in the control groups in IBV serum antibody titer.
1. Introduction
Infectious bronchitis virus (IBV) is the causative agent of infectious bronchitis (IB), has a positive-sense, single-stranded RNA genome (approximately 27 kilobases), and belongs to the coronavirus family (Lai and Cavanagh, 1997). The genome of IBV is an enveloped virus encoding four major structural proteins: the spike protein (S), which forms the prominent coronavirus spikes, the membrane protein (M), which is the most abundant component of the coronavirus, the envelope protein (E), which is a minor but critical component in virion assembly (De Haan et al., 1998), and the nucleocapsid protein (N), which binds to the viral RNA with high affinity (Spencer and Hiscox, 2006; Liu et al., 2006).
IBV causes economically important upper respiratory and urogenital tract diseases in chickens, resulting in tracheal rales, sneezing, coughing, reduced weight gain, and reduction of egg production (Cavanagh and Naqi, 1997) IBV is classified as a member of the family Coronaviridae and is found worldwide (Lai and Cavanagh, 1997). In addition, although the use of vaccines has reduced large-scale IBV outbreaks, the vaccine alone is not sufficient to inhibit IBV (Jackwood et al., 2005). Therefore, antiviral drug studies of IBV have been conducted using various extracts and proteins. Huawei showed that forsythoside A is an antiviral agent that protects primary chicken embryo kidney cells against IBV (Li et al., 2011). Zhang et al. (2017) demonstrated that chicken mannose binding lectin (cMBL) plays a role in immunity, induces viral aggregation, and reduces IBV infection (Zhang et al., 2017). Another study reported using swine intestine antimicrobial peptides (SIAMP) in chick embryos; with inhibition of viral replication and decreases in the pathological changes induced by IBV (Sun et al., 2010).
Through collaboration with several research groups, we have developed a catalytic antibody fragment (3D8 scFv) that exhibits non-specific nuclease activity toward both DNA and RNA (Kim et al., 2006). 3D8 scFv, which is based on an anti-DNA monoclonal antibody originating from an auto-immune Murphy Roths Large (MRL) mouse, is a recombinant single chain antibody that links the variable region of the heavy chain (VH) and variable region of the light chain (VL) through linker peptides (Kwon et al., 2002).
The antiviral effects of 3D8 scFv were previously confirmed in a porcine kidney 15 cell line expressing the 3D8 scFv protein that was subsequently challenged with the classical swine fever virus (CSFV), which is an RNA virus (Jun et al., 2010). We also observed antiviral effects in a human cell line (HeLa cells) and in mice harboring 3D8 scFv based on the detection of DNase activity in the nucleus and RNase activity in the cytoplasm against DNA viruses (herpes simplex virus and pseudorabies virus) (Lee et al., 2014). Byun et al. (2017) reported that 3D8 scFv transgenic (tg) chickens in the contact transmission group exhibited inhibition of viral shedding in oropharyngeal and cloacal swab samples after challenge with the avian influenza virus (AIV).
The aim of this study was to investigate in chickens the antiviral effect against IBV of 3D8 scFv. We confirmed the antiviral effects by real-time reverse transcription polymerase chain reaction and ELISA analysis in direct infection and contact transmission groups. Based on the results of this study, the 3D8 scFv protein is considered important for the development for antiviral studies.
2. Materials and methods
2.1. Establishment of 3D8 scFv transgenic chickens
3D8 scFv G1 tg rooster generated with the pLenti-CBA (chicken β-actin promoter)-3D8 scFv-HA (human influenza hemagglutinin tag)-IRES-puro vector was mated with wild type hens to produce G2 tg chickens as previously described (Byun et al., 2017). Genomic DNA (gDNA) was isolated from whole blood (1 ml) of chickens. After the addition of 3 ml of cell lysis solution, the mixture was centrifuged to recover white blood cell (WBC). The supernatant was removed and gDNA was purified from the WBC pellet using Wizard Genomic Purification Kit (Promega, Madison, USA). The amount of gDNA used in PCR reactions was 50 ng. The presence of 3D8 scFv in the G2 chickens was confirmed by genomic PCR analysis using a forward primer targeting the CBA promoter region and a reverse primer targeting the 3D8 scFv region. The primer set (forward: 5′- CCTCTGCTAACCATGTTCATGCCTTC - 3′ and reverse: 5′- GCTAGTGAATGTGTATCCAGAAGCCTT- 3′) was designed for the amplification. The PCR conditions were as follows: 35 cycles at 95 °C for 20 s (denaturation), 60 °C for 40 s (annealing), and 72 °C for 30 s (extension).
2.2. Detection of 3D8 scFv protein expression in transgenic chickens by immunohistochemical staining
To verify 3D8 scFv protein expression in the G2 tg chickens, tracheal tissues from the chickens were sliced to a 3-μm thickness and mounted onto silane-coated slides. A three-week-old chicken not infected with IBV was used for staining. Blocking was performed in normal serum for 1 h at room temperature. Polyclonal rabbit anti-HA antibodies (Abcam, Cambridge, UK) were used as the primary antibody (1:1000) to detect the 3D8 scFv-fused HA tag. The prediluted primary antibodies were applied for 12 h at 4 °C. The biotinylated secondary antibodies were applied for 1 h at 4 °C. The ABC kit (Vector Laboratories, Burlingame, CA, USA) was used with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen to detect expression (10 min), and counterstaining was performed with hematoxylin (20 min). The sections were dehydrated in an alcohol series, cleared in xylene, and covered with a coverslip. Light microscopy was conducted on the Leica DE/DMI6000B equipped with a Leica Microsystems CMS GmbH D-35578 Wetzlar digital camera (Leica, Germany) to capture images (Devi and Ohno, 2014).
2.3. In vivo IBV infection and transmission studies
The infectious bronchitis virus K40/09 strain isolated from a broiler farm was provided by Konkuk University of Korea (Lim et al., 2011). Three-week-old G2 3D8 scFv tg chickens, non-transgenic chickens (non-TG), and SPF chickens (positive control) were housed in containment cages in a biosafety level 2 (BSL2) animal facility. All chickens were identified using individual tags, and feed and water were supplied ad libitum. Five chickens per group were challenged intraocularly with 30 μL containing 104.5 EID50 of IBV. One day later, fifteen chickens per group were co-housed in the same containment isolator. To assess viral shedding, oropharyngeal and cloacal swab samples were collected at 3 and 5 days post-infection (dpi) and suspended in 1 ml of PBS. To detect anti-IBV antibodies, serum samples were collected 17 days after challenge for an ELISA assay. All animal study procedures were reviewed and approved by the Korea National Institute of Animal Science Institutional Animal Care and Use Committee (IACUC) (2015–111).
2.4. Quantitation of IBV shedding by real-time reverse transcription polymerase chain reaction
Total RNA was isolated from the oropharyngeal and cloacal swabs at days 3 and 5 post-infection with IBV using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) (Spackman et al., 2003). cDNA was synthesized from 2 μg of total RNA using oligo dT and the Superscript III First-Strand Synthesis System (Invitrogen, MA, USA). Primers targeting the IBV gene were designed using the Primer3 program. A primer set (forward: 5′- TCATGGCAAGCGGTAAGG - 3′ and reverse: 5′- TTCAGGTTAGCGGCTGGTC - 3′) was designed to amplify the IBV nucleocapsid gene. Real-time RT-PCR amplification was analyzed with the SYBR Premix Ex Taq kit (Takara, Japan) in the Rotor gene 3000 (Corbett Research, Sydney, Australia). The PCR conditions were as follows: 30 cycles at 94 °C for 1 min (denaturation), 55 °C for 1 min (annealing), and 72 °C for 1 min (extension). The PCR data was analyzed according to (Yuk et al., 2016).
2.5. Enzyme-linked immunosorbent assay (ELISA) for IBV antibody detection
An anti-IBV antibody analysis was performed with serum samples from each group 17 days after the IBV challenge using the IBV Ab ELISA kit (Bionote, Hwaseong, Korea). Diluted serum samples and control serum in a volume of 100 μl were added to each well of an IBV antigen-coated test plate and incubated at room temperature for 30 min. After washing with 100 μl of phosphate-buffered saline (PBS), 100 μl of the enzyme conjugates was added and incubated at room temperature for 30 min. After washing with 100 μl of PBS, 100 μl of the substrate solution was added to each well and incubated at room temperature for 15 min. After adding 100 μl of stopping solution, IBV antibody levels were measured based on the absorbance at 405 nm. The status of a sample was evaluated by the sample-to-positive ratio (S/P ratio = [OD samples - OD of negative control]/[OD positive control – OD of negative control]).
2.6. Statistical analysis
All analyses were conducted using the GraphPad Prism statistical software (GraphPad Software). One-way ANOVA and Tukey's post hoc t-test were used for the statistical analyses. The data are presented as the mean ± SE or SD.
3. Results and discussion
3.1. Production of G2 3D8 scFv transgenic chickens
In a previous study, we constructed the CBA-3D8 scFv-HA-IRES-puro lentiviral vector and transfected chickens to establish G0 tg chickens (Byun et al., 2017). We produced a G1 tg rooster using established G0 tg chickens crossed with wild type hens. G2 tg chickens for antiviral experiments against IBV were produced by breeding a G1 tg rooster and wild type hens. The presence or absence of the 3D8 scFv gene was evaluated by genomic PCR, and 22 of the 40 G2 tg chickens showed the presence of the 3D8 scFv gene (Fig. 1A). In addition, immunohistochemistry staining was performed to confirm expression of the 3D8 scFv protein by the G2 tg chickens. The tracheal tissues of the 3D8 scFv tg chickens exhibited a dark brown color after DAB staining (Fig. 1B, C).
Fig. 1.
Verification of G2 3D8 scFv tg expression in chickens. (A) Genomic PCR analysis of the blood from the G2 3D8 scFv tg progeny chickens. The PCR product size is 227 bp. M: 100 bp ladder marker, NC: negative control, PC: positive control. (B) Immunohistochemistry of the tracheal tissues from wild type chickens. No staining was observed in the wild type chickens. (C) Immunohistochemistry of the tracheal tissues from the G2 3D8 scFv tg progeny chickens. The 3D8 scFv protein was visualized with DAB. All scale bars indicate 20 μm.
The chicken β-actin promoter used in this study is generally expressed uniformly in chicken tissues (Hitoshi et al., 1991). However, Fig. 1C showed that 3D8 scFv was expressed in the epithelium of the trachea. As mentioned in the previous report, this result suggests that overexpression of the 3D8 scFv protein may be life-threatening to the organism during the developmental stage (Byun et al., 2017). Therefore, only chicken embryos with low-level expression survived. When very small amounts were expressed in the tissues, different expression patterns were seen, as shown in (Fig. 1C).
3.2. Antiviral effects of the 3D8 scFv protein in the IBV transmission group
3D8 scFv transgenic, sibling wild type, and positive control (SPF) chickens were used to confirm the antiviral effect against IBV in 3D8 scFv tg chickens. To assess whether the IBV-infected chickens successfully transmitted IBV to the contact-exposed chickens in this study, SPF chickens were included as a positive control. In each group, 5 chickens were directly infected with IBV using an intraocular inoculation method, and 15 chickens were placed in the same space to measure the contact infection rate. Oropharyngeal and cloacal swabs were collected from both the direct infection and contact transmission groups on the 3rd and 5th days after IBV infection, and the viral shedding levels were assessed by quantitative real-time PCR. The directly infected group showed similar viral RNA transcription levels among the 3D8 scFv tg, sibling wild type, and positive control (SPF) chickens (data not shown). However, the 3D8 scFv tg chickens in the contact transmission group exhibited resistance against IBV (Fig. 2 ). The shedding levels in the oropharyngeal swabs of the 3D8 scFv tg chickens were significantly lower than those of the wild type and SPF chickens at 3 and 5 dpi. Additionally, the cloacal swab samples showed significant differences between the 3D8 scFv tg and wild type chickens at 3 dpi (Fig. 2).
Fig. 2.
Inhibition of the IBV shedding levels in the 3D8 scFv tg chicken oropharyngeal and cloacal swabs of the contact transmission group. Three-week-old G2 3D8 scFv tg chickens, non-transgenic chickens (non-TG), and SPF chickens (positive control) were housed in containment cages in a biosafety level 2 (BSL2) animal facility. Oropharyngeal and cloacal swab samples were harvested at 3 and 5 days post-inoculation (dpi) from the contact transmission group. The IBV RNA was investigated by real-time RT-PCR. Mean viral shedding titers were calculated. Data bars represent the mean ± standard error. *, *** indicate significant differences compared to the control chickens or positive control chickens at p < 0.05 and p < 0.001, respectively (one-way analysis of variance followed by Tukey's post hoc t-test).
In the present study, the 3D8 scFv transgenic chickens showed reduced viral shedding levels in the contact transmission group. However, confirming the effects was difficult in the direct infection group. In our previous study on the antiviral effect against AIV, we noted that “because the level of 3D8 scFv expression failed to induce apoptosis, the transgenic cells did not undergo apoptosis” (Byun et al., 2017). Moreover, cytosolically localized 3D8 scFv triggered apoptotic cell death via its nuclease activity (Jang et al., 2009). In other words, a high 3D8 scFv protein expression level cannot be expected in chickens. If 3D8 scFv tg chickens are challenged with a viral titer exceeding the critical point at which an antiviral effect occurs, antiviral effects against the virus will not be observed in the tg chickens. However, in the case of the contact transmission group, the amount of virus derived from the directly infected chickens will represent the exposure, and the exposure dose might be lower than that inoculated into directly-infected chickens. Therefore, antiviral effects can be expected to occur in chickens with low 3D8 scFv expression.
3.3. Reduced IBV antibody titers in 3D8 scFv tg chicken sera in the transmission group
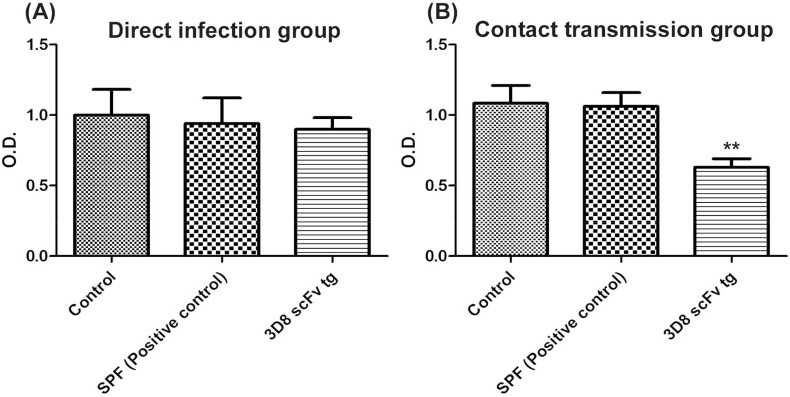
At 17 days post-IBV infection, the IBV serum Ab titer was estimated using an ELISA assay. In the direct infection group, IBV Ab accumulation in the 3D8 scFv tg chickens (OD mean: 0.9) decreased by 10% and 4.6% compared with that in the control chickens (OD mean: 1.0) and positive control chickens (OD mean: 0.94), respectively (Fig. 3A), but these differences did not reach significance. Conversely, in the contact transmission group, the IBV Ab responses of the 3D8 scFv tg chickens (OD mean: 0.63) were 41% and 40% lower than the responses in the control chickens (OD mean: 1.07) and positive control chickens (OD mean: 1.06), respectively (Fig. 3B).
Fig. 3.
Reduction of the anti-IBV antibody titers in the 3D8 scFv tg chickens of the contact transmission group. Serum samples were collected at 17 days post-inoculation (dpi) and measured with an ELISA assay. (A) In the direct infection group, no difference was identified. (B) In the contact transmission group, the titers in the 3D8 scFv tg chickens were significantly reduced compared to the titers in the control and positive control groups. Data bars represent the mean ± standard error. ** indicates significant differences from the control chickens at p < 0.01 (one-way analysis of variance followed by Tukey's post hoc t-test).
IBV is a coronavirus that causes respiratory signs related to infectious bronchitis (Raj and Jones, 1997; Matthijs et al., 2008). Also, it is infected in the respiratory tract, gut, kidney, and reproductive system of chicken which ultimately affects meat and egg production (Awad et al., 2014). A number of antiviral studies have been conducted against IBV in response to these problems (Harrison et al., 2007; Jackwood et al., 2010; Sun et al., 2010; Li et al., 2011; Chen et al., 2014; Shojai et al., 2016; Zhang et al., 2017). In the present study, we investigated resistance against IBV in chickens using 3D8 scFv. The 3D8 scFv tg chickens exhibited a reduction in shedding based on quantitation of viral RNA in the oropharyngeal and cloacal swabs and reduced antibody titers against IBV. Many studies have confirmed the antiviral effects based on the nuclease activity of 3D8 scFv in rats, humans, and chickens (Jun et al., 2010; Lee et al., 2014; Lee et al., 2015; Byun et al., 2017). The nuclease activity of 3D8 scFv may be used as an antiviral agent for most viruses that have DNA or RNA as their genetic material. The development of a broad range of therapeutic agents for viruses with various mutations is very urgent and necessary. Ensuing studies will examine whether 3D8 scFv is effective against a variety of chicken viruses.
Acknowledgements
This study was supported by the 2017-2019 RDA Fellowship Program of the National Institute of Animal Science, Rural Development Administration, Republic of Korea. This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01094401)” of the Rural Development Administration, Republic of Korea.
References
- Awad F., Chhabra R., Baylis M., Ganapathy K. An overview of infectious bronchitis virus in chickens. Worlds. Poult. Sci. J. 2014;70:375–384. [Google Scholar]
- Byun S.J., Yuk S.S., Jang Y.J., Choi H., Jeon M.H., Ochir T.E., Kwon J.H., Noh J.Y., Kim J.S., Yoo J.G., Song C.S. Transgenic chickens expressing the 3D8 single chain variable fragment protein suppress avian influenza transmission. Sci. Rep. 2017;7:5938. doi: 10.1038/s41598-017-05270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S.A. Infectious bursal disease. In: Calnek B.W., Barnes H.J., Beard C.W., McCougald L.R., Saif Y.M., editors. Diseases of Poultry. 10th ed. Iowa state University Press; Ames: 1997. pp. 511–526. [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC. Vet. Rec. 2014;10:24. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan C.A., Kuo L., Masters P.S., Vennema H., Rottier P.J. Coronavirus particle assembly: primary structure requirements of the membrane protein. J. Virol. 1998;72:6838–6850. doi: 10.1128/jvi.72.8.6838-6850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L., Ohno M. PERK mediates eIF2α phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2014;35:2272–2281. doi: 10.1016/j.neurobiolaging.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.M., Tarpey I., Rothwell L., Kaiser P., Hiscox J.A. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathol. 2007;36:109–114. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi N., Ken-Ichi Y., Jun-Ichi M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Lee C.W., Kwon H.M., Callison S.A., Moore K.M., Moscoso H., Sellers H., Thayer S. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- Jackwood M., Rosenbloom R., Petteruti M., Hilt D., Mccall A., Williams S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149:86–94. doi: 10.1016/j.virusres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.Y., Jeong J.G., Jun H.R., Lee S.C., Kim J.S., Kim Y.S., Kwon M.H. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell. Mol. Life Sci. 2009;66:1985–1997. doi: 10.1007/s00018-009-9179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H.R., Pham C.D., Lim S.I., Lee S.C., Kim Y.S., Park S., Kwon M.H. An RNA-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem. Biophys. Res. Commun. 2010;395:484–489. doi: 10.1016/j.bbrc.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Kim Y.R., Kim J.S., Lee S.H., Lee W.R., Sohn J.N., Lee S.C., Kwon M.H., Kim Y.S. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double-and single-stranded DNAs without sequence specificity. J. Biol. Chem. 2006;281:15287–15295. doi: 10.1074/jbc.M600937200. [DOI] [PubMed] [Google Scholar]
- Kwon M.H., Lee M.S., Kim K.H., Park S., Shin H.J., Jang Y.J., Kim H.I. Production and characterization of an anti-idiotypic single chain Fv that recognizes an anti-DNA antibody. Immunol. Investig. 2002;3:205–218. doi: 10.1081/imm-120016241. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Yu J., Cho S., Byun S.J., Kim D.H., Lee T.K., Kwon M.H., Lee S. A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and C57BL/6 mice. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Cho S., Hoang P.M., Kim D., Lee Y., Kil E.J., Byun Jang S.J., Lee T.K., Kim D.H., Kim S., Lee S. Therapeutic strategy for the prevention of pseudorabies virus infection in C57BL/6 mice by 3D8 scFv with intrinsic nuclease activity. Mol. Cell. 2015;38:773–780. doi: 10.14348/molcells.2015.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu J., Zhang Z., Ma Y., Liao F., Zhang Y., Wu G. Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phytother. Res. 2011;25:338–342. doi: 10.1002/ptr.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T.H., Lee H.J., Lee D.H., Lee Y.N., Park J.K., Youn H.N. An emerging recombinant cluster of nephropathogenic strains of avian infectious bronchitis virus in Korea. Infect. Genet. Evol. 2011;11:678–685. doi: 10.1016/j.meegid.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Chen J., Han Z., Zhang Q., Shao Y., Kong K., Tong G. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- Matthijs M., Bouma A., Velkers F., Van Eck J., Stegeman J. Transmissibility of infectious bronchitis virus H120 vaccine strain among broilers under experimental conditions. Avian Dis. 2008;52:461–466. doi: 10.1637/8204-010708-Reg.1. [DOI] [PubMed] [Google Scholar]
- Raj G.D., Jones R. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojai T.M., Langeroudi A.G., Karimi V., Barin A., Sadri N. The effect of Allium sativum (Garlic) extract on infectious bronchitis virus in specific pathogen free embryonic egg. Avicenna J. Phytomed. 2016;6:458. [PMC free article] [PubMed] [Google Scholar]
- Spackman E., Senne D., Bulaga L., Myers T., Perdue M., Garber P., Lohman K., Daum L.T., Suarez D.L. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003;47:1079–1082. doi: 10.1637/0005-2086-47.s3.1079. [DOI] [PubMed] [Google Scholar]
- Spencer K.A., Hiscox J.A. Characterisation of the RNA binding properties of the coronavirus infectious bronchitis virus nucleocapsid protein amino-terminal region. FEBS Lett. 2006;580:5993–5998. doi: 10.1016/j.febslet.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Wang K., She R., Ma W., Peng F., Jin H. Swine intestine antimicrobial peptides inhibit infectious bronchitis virus infectivity in chick embryos. Poult. Sci. 2010;89:464–469. doi: 10.3382/ps.2009-00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk S.S., Kwon J.H., Noh J.Y., Hong W.T., Gwon G.B., Jeong J.H., Jeong S., Youn H.N., Heo Y.H., Lee J.B., Park S.Y., Choi I.S., Song C.S. Comparison between dot-immunoblotting assay and clinical sign determination method for quantifying avian infectious bronchitis virus vaccine by titration in embryonated eggs. J. Virol. Methods. 2016;230:13–17. doi: 10.1016/j.jviromet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bouwman K.M., Van Beurden S.J., Ordonez S.R., Van Eijk M., Haagsman H.P., Verheije M.H., Veldhuizen E.J.A. Chicken mannose binding lectin has antiviral activity towards infectious bronchitis virus. Virology. 2017;509:252–259. doi: 10.1016/j.virol.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]