Abstract
Serotonin is a critical monoamine neurotransmitter molecule stored and released from enterochromaffin (EC) cells into the gut submucosa, transmitting the vomiting signal to the brain. We studied one mechanism by which vomiting is induced in pigs infected with porcine epidemic diarrhea virus (PEDV) by characterization of swine EC cells by immunohistochemistry. Conventional or gnotobiotic (Gn) 9-day-old pigs [PEDV-inoculated (n = 12); Mock (n = 14)] were inoculated orally (8.9–9.2 log10 genomic equivalents/pig) with PEDV PC21A strain or mock. This is the first identification of serotonin-positive EC cells in swine by immunohistochemistry and mainly in intestinal crypts, regardless of infection status. They were morphologically triangular-shaped or round cells with or without apical cytoplasmic extensions, respectively. At post-inoculation hour (PIH) 16 or 24, when vomiting was first or frequently observed, respectively, PEDV infection resulted in significantly reduced numbers of serotonin-positive EC cells in duodenum, mid-jejunum, ileum, or colon. However, two of three PEDV-inoculated Gn pigs that did not yet show vomiting at PIH 16 had numbers of serotonin-positive EC cells in duodenum, ileum and colon similar to those in the negative controls. These findings suggest that serotonin release from EC cells (increased serotonin levels) into the gut submucosa might occur early PEDV post-infection to stimulate the vagal afferent neurons, followed by vomiting. Serotonin might be involved in the mechanisms related to vomiting in PEDV-infected piglets. We also found that mid-jejunum was the primary site of acute PEDV infection, and that systemic innate and pro-inflammatory cytokine responses were induced during the acute stage of PEDV infection.
Keywords: PEDV, Pathogenesis, Vomiting, Serotonin, Enterochromaffin cell, Immune response
Highlights
-
•
Serotonin is the vomiting-related neurotransmitter secreted from enterochromaffin (EC) cells.
-
•
Acute PEDV infection significantly resulted in reduced numbers of serotonin-positive EC cells in the small and large intestines.
-
•
PEDV infection induced systemic innate and pro-inflammatory cytokine responses in gnotobiotic pigs.
-
•
Serotonin might be involved in the mechanisms related to vomiting in PEDV-infected nursing pigs.
1. Introduction
Vomiting is a defensive reaction of the body to rapidly remove ingested toxins from the gastrointestinal tract. Vomiting is generally induced when either of the two medullary centers in the brain, integrative vomiting center and chemoreceptor trigger zone (CRTZ), is activated via a variety of their surface chemoreceptors, such as 5-HT3 (Endo et al., 2000; Sikander et al., 2009; Spiller, 2008). The integrative vomiting center and CRTZ are triggered by activation of vagal afferent neurons in the gastrointestinal tract and circulating toxins in blood, respectively. There are vagal or enteric afferent neurons in the gut submucosa. Vagal afferent neurons are stimulated by a monoamine neurotransmitter molecule, serotonin (5-hydroxytryptamine, 5-HT), released from enterochromaffin (EC) cells in the gastrointestinal tract. Subsequently, vomiting signals are transmitted to the integrative vomiting center and then to the central nervous system (CNS). Emetic efferent signals from the CNS reach the gastrointestinal and abdominal muscles, leading to their expulsive actions by which vomiting is induced (Endo et al., 2000; Sikander et al., 2009; Spiller, 2008).
In humans, approximately 95% and 5% of serotonin is stored in the gut and brain, respectively. In the gut, 90% of serotonin is deposited in the secretory granules of EC cells mainly located in intestinal crypts, and 10% is in neurons in the submucosa (Endo et al., 2000; Spiller, 2008). In humans or rats, by immunohistochemistry (IHC) using antibodies against serotonin, EC cells were serotonin-positive and triangular-shaped with apical cytoplasmic extensions (Gustafsson et al., 2006; Spiller, 2008). The EC cells likely originate from intestinal crypt stem cells. A mechanical, biological, or chemical stimulus causes EC cells to secret serotonin into the lamina propria or lumen that acts on serotonin receptors (5-HT3) at the terminals of the vagal afferent neurons (Endo et al., 2000; Gustafsson et al., 2006; Spiller, 2008). Serotonin also functions to promote immune activation through the receptors expressed on macrophages, dendritic cells and T and B cells (Li et al., 2011; Shajib and Khan, 2015).
Porcine epidemic diarrhea virus (PEDV) (family Coronaviridae, genus Alphacoronavirus) causes acute diarrhea, vomiting, decreased or loss of appetite, dehydration and high mortality in neonatal piglets (Jung and Saif, 2015). Diarrhea is frequently accompanied by vomiting in PEDV-infected nursing piglets during the acute stage of infection, exacerbating dehydration (Jung and Saif, 2015). However, the mechanisms by which vomiting is induced in PEDV infection are poorly understood. We hypothesized that: i) serotonin is involved in the mechanisms related to vomiting; ii) serotonin release from EC cells into the intestinal lamina propria or lumen occurs early PEDV post-infection to stimulate the vagal afferent neurons, followed by vomiting; and iii) expression levels of serotonin in intestinal EC cells or numbers of serotonin-positive EC cells in the small or large intestine differ between PEDV-infected and uninfected pigs. The in situ distribution and characterization of EC cells in swine intestine are also unknown. In our study, therefore, we aimed to develop an IHC to detect and characterize EC cells in swine intestine and to determine whether PEDV infection alters the number of serotonin-positive EC cells in the small and large intestines (primary sites of PEDV infection) of infected gnotobiotic (Gn) or conventional, 9-day-old piglets during disease progression. We also aimed to detail the pathogenesis of acute PEDV infection, including intestinal distribution of PEDV antigen and serum innate and pro-inflammatory cytokine profiles in infected Gn pigs to understand the relationship of acute PEDV infection with the frequency of serotonin-positive EC cells in the small and large intestines.
2. Materials and methods
2.1. Virus
The US virulent (wild-type) PEDV strain PC21A was obtained from intestinal contents of a diarrheic 1-day-old piglet in an Ohio farm in June 2013 (Jung et al., 2014). The original sample was serially passaged 2 times in Gn pigs, and the intestinal contents were negative for other enteric viruses [transmissible gastroenteritis virus, porcine deltacoronavirus (PDCoV), porcine rotavirus groups A-C, etc.] by PCR/RT-PCR and electron microscopic examination. The titer of Gn pig 2nd-passaged PC21A was 11.8 log10 GE/ml and was used as virus inoculum after dilution in minimal essential medium (MEM) (Invitrogen, Carlsbad, CA, USA), as described previously (Jung et al., 2015).
2.2. Gnotobiotic pigs and experimental pig infection
Six Large White × Duroc crossbred Gn pigs were acquired by hysterectomy from a PEDV-seronegative pregnant sow obtained from a PEDV-free, specific-pathogen-free (SPF) (confirmed by history and seronegative sows; lack of qRT-PCR-positive fecal samples) swine herd of The Ohio State University. The SPF herd was seronegative for antibodies to porcine reproductive and respiratory syndrome virus, porcine respiratory coronavirus, transmissible gastroenteritis virus and porcine circovirus type 2. Six 9-day-old Gn piglets were randomly assigned to one of two groups: PEDV-infected (n = 3; pigs 1–3) and Mock (n = 3; pigs 4–6). Pigs were inoculated orally with 2 ml of PEDV strain PC21A [8.9 log10 genomic equivalents (GE)/ml] [9.2 log10 GE (≈3.2 log10 PFU) per pig], a dose similar to that (8.9 log10 GE/pig) used in the previous study (Jung et al., 2015), or mock inoculated with MEM. After PEDV inoculation, the pigs were monitored frequently for clinical signs, such as diarrhea, appetite, activity, etc., especially vomiting. Inoculated and negative control pigs (n = 3/group at each time-point) were euthanized for virological and pathological examination at an acute-stage of infection when or shortly after vomiting was first detected, i.e. approximately post-inoculation hour (PIH) 16 in this study. Diarrhea was assessed by scoring fecal consistency. Fecal consistency was scored as follows: 0 = solid; 1 = pasty; 2 = semi-liquid; 3 = liquid, with scores of 2 or more considered diarrheic. The Institutional Animal Care and Use Committee (IACUC) of The Ohio State University approved all protocols related to the animal experiments in this study.
2.3. Archival intestinal tissues
The other tissue samples tested were archival formalin-fixed, paraffin-embedded tissues acquired from twenty 9-day-old [PEDV-infected (n = 9) and Mock (n = 11)] conventional pigs inoculated orally with 8.9 log10 GE of PEDV strain PC21A or mock (MEM) (Jung et al., 2015). The clinical disease, fecal virus shedding, and gross and histopathology were described in a previous paper (Jung et al., 2015). However, the previous report included only limited information to understand intestinal distribution of serotonin-positive EC cells at the different locations of the intestine and the relationship of the frequency of serotonin-positive cells with vomiting. Therefore, more detailed clinical and histopathological observations relevant to the aims of the current study were described in the Results section. Pigs (n = 3–4/time-point) were euthanized for pathologic examination at post-inoculation days (PIDs) 1, 3, and 5.
2.4. Analysis of PEDV RNA titers in fecal and serum samples
Rectal swabs and serum samples were collected from Gn pigs 1–6 at PIH 16. Two rectal swabs were suspended in 4 ml MEM. The RNA was extracted from 200 μl of centrifuged (2000 ×g for 30 min at 4 °C) fecal suspensions using the Mag-MAX Viral RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. PEDV RNA titers in rectal swabs and serum samples were determined as described previously (Jung et al., 2014).
2.5. Analysis of innate and pro-inflammatory cytokine levels in serum samples
There were increases in serum innate (IFNα) and pro-inflammatory (TNFα and IL-12) cytokine levels in PEDV-infected conventional pigs (10-day-old) at PID 1 (Annamalai et al., 2015) and increased mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and TNFα) in IPEC-J2 cells infected with PEDV (Lin et al., 2017). Therefore, serum innate (IFNα and IL-22) cytokines, which are known to play a role in antiviral immune responses (Gimeno Brias et al., 2016; Zhang and Yoo, 2016), and pro-inflammatory (TNFα, IL-6, and IL-12) cytokines were evaluated in Gn pigs 1–6 at PIH 16 to confirm the previous findings as well as to investigate whether acute PEDV infection induces systemic innate and pro-inflammatory immune responses.
Serum IFNα, IL-6, IL-12, IL-22, and TNFα cytokine levels were quantitated by ELISA in the serum samples collected from Gn pigs 1–6 at PIH 16, as described previously (Annamalai et al., 2015; Azevedo et al., 2006; Chattha et al., 2013). Briefly, Nunc Maxisorp 96-well plates were coated with anti-porcine IL-6 (0.75 μg/ml, goat polyclonal antibody), anti-porcine IL-12 (0.75 μg/ml, goat polyclonal antibody), anti-porcine IFNα (2.5 μg/ml, clone K9) (R&D systems, Minneapolis, MN), anti-porcine IL-22 (2.0 μg/ml, rabbit polyclonal antibody), and anti-porcine TNFα (1.5 μg/ml, goat polyclonal antibody) (Kingfisher biotech, Saint Paul, MN) overnight at 4 °C or 37 °C (for IFN-α only). Biotinylated anti-porcine IL-6 (0.1 μg/ml, goat polyclonal antibody), anti-porcine IL-12 (0.2 μg/ml, goat polyclonal antibody), anti-porcine IFNα (3.75 μg/ml, clone F17) (R&D systems, Minneapolis, MN), anti-porcine IL-22 (0.5 μg/ml, rabbit polyclonal antibody), and anti-porcine TNFα (0.4 μg/ml, goat polyclonal antibody) (Kingfisher biotech, Saint Paul, MN) were used as detection antibodies. Porcine IFN-α detection antibody was biotinylated using a commercial kit, as described previously (Chattha et al., 2013). Plates were developed and cytokine concentrations were calculated, as previously described (Annamalai et al., 2015; Azevedo et al., 2006). The samples were tested in duplicate, and cytokine levels were expressed as the mean values. Detection limits of our ELISA were 1 pg/ml for IFNα and IL-12, 4 pg/ml for TNFα and IL-22, and 16 pg/ml for IL-6, respectively.
2.6. Gross and histological analysis and immunohistochemistry for the detection of PEDV antigen
Small intestinal tissues [duodenum, proximal (distant by 15–20 cm from the pylorus), middle (mid-location of the small intestine (duodenum to ileum) dissociated from the mesentery) and distal jejunum (distant by 15–20 cm from the ileocecal junction), and ileum] and large (cecum/colon) intestinal tissues were examined grossly and histologically. IHC results from only the mid-jejunum and cecum/colon of the conventional pigs were reported in the previous paper (Jung et al., 2015). Therefore, duodenal, mid-jejunal, ileal, and colonic tissues of conventional or Gn pigs were also conducted or re-tested by IHC, as described previously (Jung et al., 2007; Jung et al., 2014), for the detection of PEDV antigen (using monoclonal antibody 6C8-1 specific to PEDV provided by Dr. Daesub Song, Korean Research Institute of Bioscience and Biotechnology, Korea) (Jung et al., 2014). For Gn pigs 1-6 euthanized at PIH 16, proximal and distal jejunum were additionally tested by IHC for the detection of PEDV antigen to clarify the tissue tropism of the virus during the early stage of infection. Duodenal, mid-jejunal, ileal, and colonic tissues of conventional or Gn pigs were conducted by IHC, as described previously (Jung et al., 2007; Jung et al., 2014), for the detection of serotonin [using monoclonal antibody 5HT-H209 against human serotonin (Novus Biologicals, Littleton, CO, USA)]. Monoclonal antibodies 6C8-1 and 5HT-H209 were diluted 1 in 200 and 1 in 50, respectively, in phosphate-buffered saline. Mean ratios of jejunal or ileal villous height to crypt depth (VH:CD) were calculated in Gn pigs, as described previously (Jung et al., 2015). Only well-orientated, hematoxylin and eosin-stained jejunal or ileal sections were measured and care was taken to ensure that only transverse sections cut perpendicularly from the villus tip to the muscularis mucosa were included. Villous height and crypt depth were estimated by measuring 4–5 villi and crypts throughout the section from each Gn pig.
2.7. Morphometric analysis
PEDV antigen-positive scores were computed by estimating the number of IHC-positive cells in the intestinal section, as described previously (Jung et al., 2014), as follows: +/− (few but clearly positive), <1% of villous epithelial cells showed staining; + (low), 1%–29% of villous epithelial cells showed staining; ++ (moderate), 30%–59% of villous epithelial cells showed staining; +++ (high), 60%–100% villous epithelial cells showed staining; and – (negative), no cells showed staining. Mean numbers of serotonin-positive EC cells were evaluated per microscopic area, at ×250 magnification by a Leitz light microscope, where approximately 10–18 intestinal crypts and 4–7 entire or lower half of villi were included. The variable numbers were dependent on the location of intestinal tissues. In PEDV-inoculated pigs at PIDs 1–5, unlike mock-inoculated pigs, entire jejunal and ileal villi were infrequently observed even at ×250 magnification, because the villi were severely atrophied. Only single crypt layer-included areas in well-orientated intestinal tissue sections were evaluated to count the number of serotonin-positive EC cells. Mean numbers of serotonin-positive EC cells were estimated by measuring at least 4 different tissue areas for each PID and from PEDV-inoculated or mock-inoculated pigs.
2.8. Data analysis
All values were expressed as the means ± standard deviation of the means (SDM). Mean numbers of serotonin-positive EC cells per microscopic area in duodenum, mid-jejunum, ileum, and colon between PEDV- and mock-inoculated pigs at the same time-points were analyzed and compared by a Student's t-test using GraphPad Prism software (GraphPad Prism Inc.). A value of P < .05 was considered statistically significant.
3. Results
3.1. Clinical observations and histologic lesions in conventional 9-day-old nursing pigs inoculated orally with PEDV
Watery diarrhea (9/9 pigs) and vomiting (7/9 pigs) were first detected at PID 1 in PEDV-inoculated piglets. At PIDs 3–5, however, all the inoculated piglets exhibited watery diarrhea, lethargy, and dehydration, but not all showed vomiting, whereas none of the mock-inoculated pigs showed clinical signs. In PEDV-inoculated piglets, histologic lesions were limited to the jejunum and ileum, and included diffuse, severe atrophic enteritis at PIDs 1–5. The duodenum showed only mild villous atrophy at PIDs 1–5. No histologic lesions were evident in the large intestine and other organs of the inoculated nursing pigs and negative controls.
3.2. Intestinal distribution of PEDV antigen in conventional 9-day-old nursing pigs inoculated orally with PEDV
PEDV antigens were mostly found in the villous epithelial cells. Of 3 PEDV-inoculated pigs at PID 1, 2 pigs showed moderate numbers of PEDV antigen-positive cells in the mid-jejunum and ileum only, and 1 pig showed low numbers of PEDV antigen-positive cells in all intestinal segments (duodenum to colon). At PID 3, all 3 PEDV-inoculated pigs showed high numbers of PEDV antigen-positive cells in the mid-jejunum and ileum and few to low numbers of positive cells in the colon. However, there were no PEDV antigen-positive cells in the duodenum. At PID 5, all 3 PEDV-inoculated pigs tested showed high numbers of PEDV antigen-positive cells in the mid-jejunum and ileum only. No PEDV antigen-positive cells were detected in the intestines of the negative control pigs.
3.3. Identification, characterization, and distribution of serotonin-positive enterochromaffin cells in duodenum, mid-jejunum, ileum, and colon of conventional 9-day-old nursing pigs inoculated orally with PEDV or mock
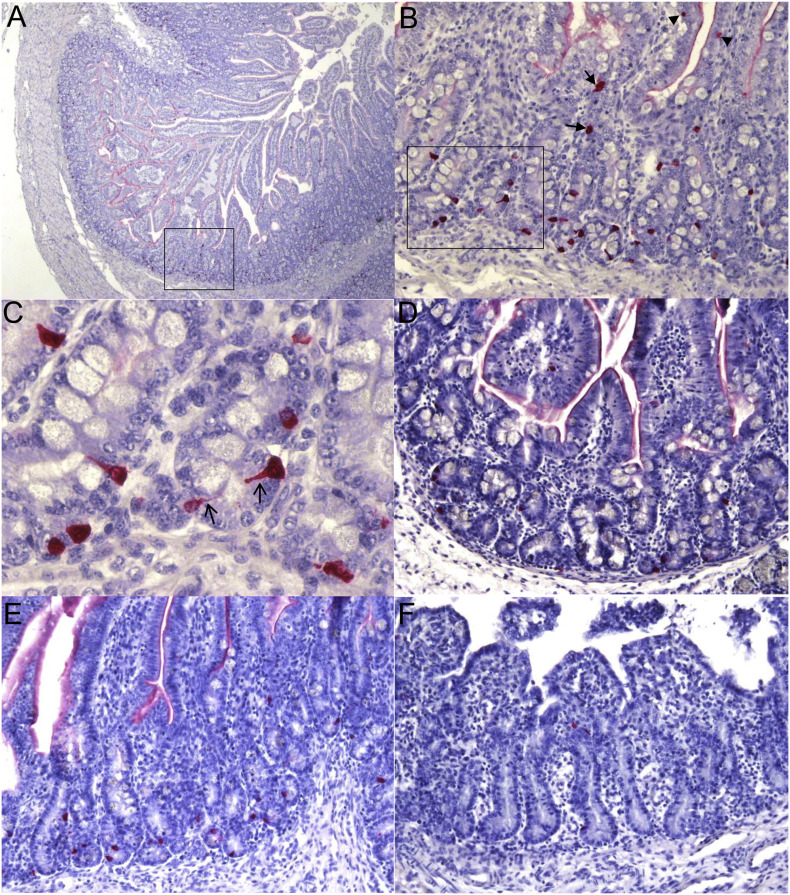
In duodenum, mid-jejunum, ileum, and colon of mock-inoculated nursing pigs at PIDs 1–5 (10–14 days of age), serotonin-positive EC cells were found mainly in the crypts (Fig. 1A–E) and occasionally, in the lamina propria or epithelium of the lower half of the villi (Fig. 1B) (except for colon, where EC cells were detected in the entire colonic epithelium). In intestinal crypts, the majority of serotonin-positive EC cells were triangular-shaped or round cells with or without apical cytoplasmic extensions, respectively, that project into the lumen (Fig. 1B and C). Occasionally, serotonin-positive EC cells were closely associated with the basement membrane of crypt cells. They were spindle cells with extensions surrounding mucus in crypt cells. In the lamina propria or epithelium of villi, serotonin-positive EC cells mostly appeared to be round cells, whereas no serotonin-positive cells were detected in the submucosa. On the other hand, in the duodenum, mid-jejunum, ileum, and colon of PEDV-inoculated nursing pigs at PIDs 1–5 (10–14 days of age), the morphology and intestinal distribution of serotonin-positive EC cells were similar to those found in the mock-inoculated control pigs. However, a few serotonin-positive EC cells were also detected in the entire epithelium of the atrophied jejunal or ileal villi (Fig. 1F).
Fig. 1.
Serotonin-positive enterochromaffin (EC) cells in the duodenum and mid-jejunum of conventional 9-day-old nursing pigs inoculated with mock (A, B, C and E) or virulent US porcine epidemic diarrhea virus (PEDV) strain PC21A (D and F) detected by immunohistochemistry. (A) Duodenum of a mock-inoculated nursing pig at post-inoculation day (PID) 1, showing high numbers of serotonin-positive EC cells (red color) mainly distributed in the intestinal crypt layer and occasionally, in the lower half of villi. Original magnification, ×40 (B) Higher magnification of highlighted area (black box) in panel A, highlighting serotonin-positive EC cells (red color) mainly localized in the intestinal crypts and occasionally, in the lamina propria (arrows) and villous epithelium (arrowheads). Original magnification, ×200 (C) Higher magnification of highlighted area (black box) in panel B, highlighting triangular-shaped or round EC cells positive for serotonin (red color), with or without apical cytoplasmic extensions (open arrows) that project into the lumen. Original magnification, ×600 (D) Duodenum of a PEDV-inoculated nursing pig at PID 1, showing lower numbers of serotonin-positive EC cells (red color) in the intestinal crypts, compared with the corresponding negative control pig (panel B). Original magnification, ×200 (E) Mid-jejunum of a mock-inoculated nursing pig at PID 1, showing moderate numbers of serotonin-positive EC cells (red color) in the intestinal crypts. Original magnification, ×200 (F) Mid-jejunum of a PEDV-inoculated nursing pig at PID 1, showing lower numbers of serotonin-positive EC cells (red color) in the intestinal villi and crypts, compared with the corresponding negative control pig (panel E). Original magnification, ×200. Fast Red, Gill's hematoxylin counterstaining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Serotonin-positive EC cells in duodenum, mid-jejunum, ileum, and colon of conventional 9-day-old nursing pigs inoculated orally with PEDV or mock during disease progression
At PID 1 when vomiting was observed in PEDV-inoculated pigs, mean numbers of serotonin-positive EC cells per microscopic area (×250) were significantly (P < .05) reduced in the duodenum, mid-jejunum, ileum, and colon, compared with the corresponding negative controls (Table 1 ; Fig. 1B, D, E, and F). At PID 3 when vomiting had ceased, mean numbers of serotonin-positive EC cells per microscopic area (×250) were significantly (P < .05) increased in duodenum but reduced in ileum of the PEDV-inoculated pigs, compared with the corresponding negative controls, but they did not differ in mid-jejunum and colon (Table 1). At PID 5, mean numbers of serotonin-positive EC cells per microscopic area (×250) in duodenum, mid-jejunum, ileum, and colon did not differ between the PEDV- and mock-inoculated nursing pigs (Table 1).
Table 1.
Mean numbers (±SDM) of serotonin-positive enterochromaffin cells by immunohistochemistry in the crypt layers and entire or lower half of villi of duodenum, mid-jejunum, ileum, and colon per microscopic area, at ×250 magnification, of conventional 9-day-old nursing pigs inoculated with virulent US PEDV strain PC21A or mock at post-inoculation days (PIDs) 1, 3, and 5.
| PID 1 (10 days of age) | PID 3 (12 days of age) | PID 5 (14 days of age) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEDV-inoculateda, b |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
|
15.0 (3.0)c |
8.4 (4.1) |
8.9 (3.2) |
8.8 (2.6) |
23.7 (4.0) |
11.2 (3.6) |
9.6 (3.0) |
9.4 (2.6) |
18.1 (4.0) |
13.7 (3.0) |
9.6 (4.4) |
5.5 (1.2) |
|
| Mock-inoculateda |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
Duodenum |
Mid-jejunum |
Ileum |
Colon |
| 23.1 (6.6) |
15.9 (3.6) |
16.3 (4.1) |
13.7 (5.1) |
18.4 (5.5) |
11.8 (4.5) |
13.1 (4.3) |
10.2 (4.5) |
20.2 (3.5) |
12.5 (3.5) |
11.1 (3.2) |
4.9 (1.4) |
|
Mock-inoculated, n = 3 at PID 1 and n = 4 at each PID 3 and 5; PEDV-inoculated, n = 3 at each PID.
In PEDV-inoculated pigs at PIDs 1–5, unlike mock-inoculated pigs, entire jejunal and ileal villi were infrequently observed even at ×250 magnification, because their villi were severely atrophied during the period.
Bold numbers, P < .05 (statistically significant differences between the PEDV-inoculated and mock-inoculated pigs by Student's t-test).
3.5. Clinical observations and PEDV RNA titers in fecal and serum samples in gnotobiotic 9-day-old pigs inoculated orally with PEDV during the subclinical incubation stage of infection (prior to PID 1–5 when conventional 9-day-old pigs showed clinical signs)
The significantly reduced numbers of serotonin-positive EC cells in the small and large intestines of PEDV-inoculated conventional piglets at PID 1, compared with the corresponding negative controls, might be a result of the early release of serotonin from EC cells into the intestinal lamina propria or lumen to stimulate the vagal afferent neurons, followed by vomiting. In an attempt to explore this effect, a further study using six 9-day-old Gn pigs inoculated orally with 9.2 log10 GE/pig (pigs 1–3) or mock (pigs 4–6) was conducted to investigate alterations in the number of serotonin-positive EC cells in the small and large intestines at an earlier time-point concurrent with or shortly after vomiting was first detected, as compared with PID 1 when most of the conventional 9-day-old pigs tested already exhibited vomiting. After virus inoculation, vomiting and other clinical signs such as diarrhea were monitored frequently to determine an earlier time-point appropriate for euthanasia. In our experiments, at PIH 16, pig 3 began to exhibit vomiting (but no diarrhea), whereas pigs 1 and 2 did not show any clinical signs. Thus, inoculated pigs 1–3 and negative control pigs 4–6 were euthanized at PIH 16 for virological and pathological examination to determine their infection status and to understand the relationship of acute PEDV infection and frequency of serotonin-positive EC cells in the small and large intestines. The results are summarized in Table 2 . At PIH 16, by qRT-PCR, no PEDV-inoculated Gn pigs 1–3 or negative control pigs 4–6 had detectable viral RNA (<4.8 log10 GE/ml) in the feces. However, pigs 1–3 had moderate viral RNA titers in serum at PIH 16, ranging from 8.1 to 8.5 log10 GE/ml, whereas no negative control pigs 4–6 had detectable viral RNA (<3.8 log10 GE/ml) in the serum.
Table 2.
Design and results of a pathology study of the original US PEDV strain PC21A in gnotobiotic 9-day-old pigs at post-inoculation hour (PIH) 16 when or shortly after vomiting was first detected.
| Pig no. | 1 | 2 | 3 | 4, 5 and 6 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral inoculum, log10 GE/ml |
2 ml, 8.9 (9.2 log10 GE/pig) |
2 ml, 8.9 (9.2 log10 GE/pig) |
2 ml, 8.9 (9.2 log10 GE/pig) |
2 ml, mock |
||||||||||||||||||||
| Onset of vomitinga |
None (>PIH 16) |
None (>PIH 16) |
Around PIH 16 |
None |
||||||||||||||||||||
| Fecal consistency score at PIH 16b |
0 |
0 |
1 |
0 |
||||||||||||||||||||
| PEDV RNA in serum, log10 GE/ml, at PIH 16c |
8.4 |
8.1 |
8.5 |
<3.8 |
||||||||||||||||||||
| Distribution of PEDV-induced histologic lesions based on intestinal locations (as also determined by IHC data)d |
D |
PJ |
MJ |
DJ |
I |
C |
D |
PJ |
MJ |
DJ |
I |
C |
D |
PJ |
MJ |
DJ |
I |
C |
D |
PJ |
MJ |
DJ |
I |
C |
| − |
− |
+ |
− |
+ |
− |
− |
− |
+ |
− |
+ |
− |
− |
+ |
+ |
− |
+ |
− |
− |
− |
− |
− |
− |
− |
|
| Antigen detection by IHC in FFPE intestinal tissue sectionse |
+/− |
+ or ++ |
+++ |
+ or ++ |
++ or +++ |
− |
− |
+ |
+++ |
+ |
+ |
− |
+ |
+ or ++ |
+++ |
+ or ++ |
+ or ++ |
− |
− |
− |
− |
− |
− |
− |
D, duodenum; PJ, proximal jejunum; MJ, mid-jejunum; DJ, distal jejunum; I, ileum; C, colon; IHC, immunohistochemistry; FFPE, formalin-fixed, paraffin-embedded.
Pig 3 had only one frequency of vomiting until euthanasia. None of the pigs 1–6 had other clinical signs until euthanasia.
Fecal consistency was scored as follows: 0 = solid; 1 = pasty; 2 = semi-liquid; 3 = liquid, with scores of 2 or more considered diarrheic.
Detected by real-time reverse transcription PCR with a detection limit of 4.8 log10 GE/ml for fecal samples and 3.8 log10 GE/ml for serum samples.
Detected by histopathology as evaluated along with IHC data. +, swelling and coagulative necrosis of villous epithelial cells, subepithelial edema, exfoliation of enterocytes, and/or villous atrophy; and -, no such lesions or lipid deposit in enterocytes.
Detected by IHC in multiple tissue sections, resulting in a range of IHC score. +/− (few), <1% of villous epithelial cells showed staining; + (low), 1%–29% of villous epithelial cells showed staining; ++ (moderate), 30%–59% of villous epithelial cells showed staining; +++ (high), 60%–100% villous epithelial cells showed staining; and -, no cells showed staining.
3.6. Trend toward increased innate (IFNα and IL-22) and pro-inflammatory (TNFα, IL-6, and IL-12) cytokine levels in the sera of gnotobiotic 9-day-old pigs inoculated orally with PEDV during the subclinical incubation stage of infection
As compared with uninfected Gn pigs 4–6 at PIH 16, mean serum IFNα, IL-22, TNFα, IL-6, and IL-12 levels in the infected Gn pigs 1–3 at PIH 16 were increased by 45-fold, 56-fold, 6-fold, 3-fold, and 3-fold, respectively (Table 3 ). Since there were large standard deviations of mean values of most of the cytokines in infected pigs as well as too few pigs (n = 3) in each group, statistical analysis was not done. Interestingly, the infected Gn pig 3 with vomiting at PIH 16 showed increased or higher serum IFNα, IL-22, TNFα, IL-6, and IL-12 cytokine levels compared with the other infected Gn pigs 1 and 2 with no vomiting, or uninfected Gn pigs 4–6. As compared with uninfected Gn pigs 4–6 at PIH 16, serum IFNα, IL-22, TNFα, IL-6, and IL-12 cytokine levels in the infected Gn pig 3 at PIH 16 were increased by 119-fold, 123-fold, 12-fold, 6-fold, and 6-fold, respectively (Table 3). The data clearly indicate that infected Gn pigs exhibited systemic innate and pro-inflammatory cytokine responses to acute PEDV infection concurrent with the detection of PEDV RNA in serum.
Table 3.
Innate and pro-inflammatory cytokine profiles by ELISA in sera of gnotobiotic 9-day-old pigs inoculated with virulent US PEDV strain PC21A or mock at post-inoculation hour (PIH) 16 when or shortly after vomiting was first detected.
| Group | Pig no. | Clinical signs | Histological characteristics | Innatea |
Pro-inflammatorya |
|||
|---|---|---|---|---|---|---|---|---|
| IFNα (pg/ml) | IL-22 (pg/ml) | TNFα (pg/ml) | IL-6 (pg/ml) | IL-12 (pg/ml) | ||||
| PEDV-infected |
1 |
None |
Enterocyte necrosis is in progress, with incomplete villous atrophy |
1 |
186 |
10 |
213 |
27 |
| 2 |
None |
14 |
155 |
14 |
331 |
41 |
||
|
3 |
Vomitingb |
Complete villous atrophy |
119b |
943 |
46 |
817 |
102 |
|
| Mean (SD)c |
45 (65) |
428 (446) |
23 (20) |
454 (320) |
57 (40) |
|||
| Mock-infected |
4 |
None |
None |
1 |
4 |
4 |
151 |
19 |
| 5 |
None |
None |
1 |
4 |
4 |
66 |
8 |
|
| 6 |
None |
None |
1 |
15 |
4 |
209 |
26 |
|
| Mean (SD)c |
1 (0) |
8 (6) |
4 (0) |
142 (72) |
18 (9) |
|||
The samples were tested in duplicate, and cytokine levels were expressed as the mean values. Detection limits of our ELISA were 1 pg/ml for IFNα and IL-12, 4 pg/ml for TNFα and IL-22, and 16 pg/ml for IL-6, respectively.
Bold fonts and numbers, pig 3 with vomiting at PIH 16 showed higher serum IFNα, TNFα, IL-6, IL-12, and IL-22 cytokine levels compared with the other infected Gn pigs 1 and 2 with no vomiting, or uninfected Gn pigs 4–6.
Since there were no or little standard deviations of mean values of some cytokines in mock-inoculated pigs and large standard deviations of mean values of most of the cytokines in infected pigs as well as too few animals in each group, statistical analysis was not conducted.
3.7. Gross and histologic lesions and intestinal distribution of PEDV antigen in gnotobiotic 9-day-old pigs inoculated orally with PEDV during the subclinical incubation stage of infection (prior to PID 1–5 when conventional 9-day-old pigs showed clinical signs)
At PIH 16, neither PEDV-inoculated Gn pigs 1 or 2 exhibited gross lesions in the small and large intestines, whereas PEDV-inoculated Gn pig 3 had accumulation of moderate amounts of watery liquid in the small intestinal lumen, but not large intestine. No gross lesions were evident in the other organs of the PEDV-inoculated Gn pigs 1–3 and negative controls 4–6. Histologic lesions and the distribution based on intestinal locations are summarized in Table 2. Duodenum and proximal jejunum of PEDV-inoculated Gn pigs 1 and 2 showed normal mucosa, submucosa, and serosa. However, their mid-jejunum and ileum showed acute, diffuse, mild (pig 2) to moderate (pig 1) swelling and coagulative necrosis of villous epithelial cells lining up to 100% of the villous epithelium, accompanied by diffuse, mild subepithelial edema and multifocal, mild exfoliation of enterocytes located on the villous tip. Also, for pig 1, multifocal, moderate suppurative inflammation was evident among the affected villi in the lumen. The length of the villi appeared normal. Distal jejunum showed diffuse, lipid accumulation-related, cytoplasmic vacuolation of enterocytes lining up to 100% of villous epithelium, with normal length of the villi. On the other hand, PEDV-inoculated Gn pig 3 had more progressed lesions compared with pigs 1 and 2. Duodenum and proximal jejunum mostly had normal mucosa, submucosa, and serosa; however, mild, multifocal subepithelial edema was observed in villi of the proximal jejunum. Mid-jejunum showed acute, diffuse, severe coagulative necrosis of villous epithelial cells, and the affected villi was shortened, with villous height: crypt depth (VH:CD) ratios ranging from 3 to 4 (vs. 6.8 to 7.9 in negative controls). Distal jejunum showed diffuse, lipid accumulation-related, cytoplasmic vacuolation of enterocytes lining up to 100% of the villous epithelium, with normal length of the villi. Ileum showed acute, mild, diffuse swelling and coagulative necrosis of villous epithelial cells, accompanied by diffuse, moderate subepithelial edema and diffuse, mild exfoliation of enterocytes located on villous tip. However, the length of the villi appeared to be within the normal range, as the VH:CD ratios ranged from 5 to 6 (vs. 5.0 to 5.6 in negative controls). No histologic lesions were evident in the large intestine and other organs of the PEDV-inoculated Gn pigs 1–3 and negative controls 4–6.
3.8. Intestinal distribution of PEDV antigen in gnotobiotic 9-day-old pigs inoculated orally with PEDV during the subclinical incubation stage of infection (prior to PID 1–5 when conventional 9-day-old pigs showed clinical signs)
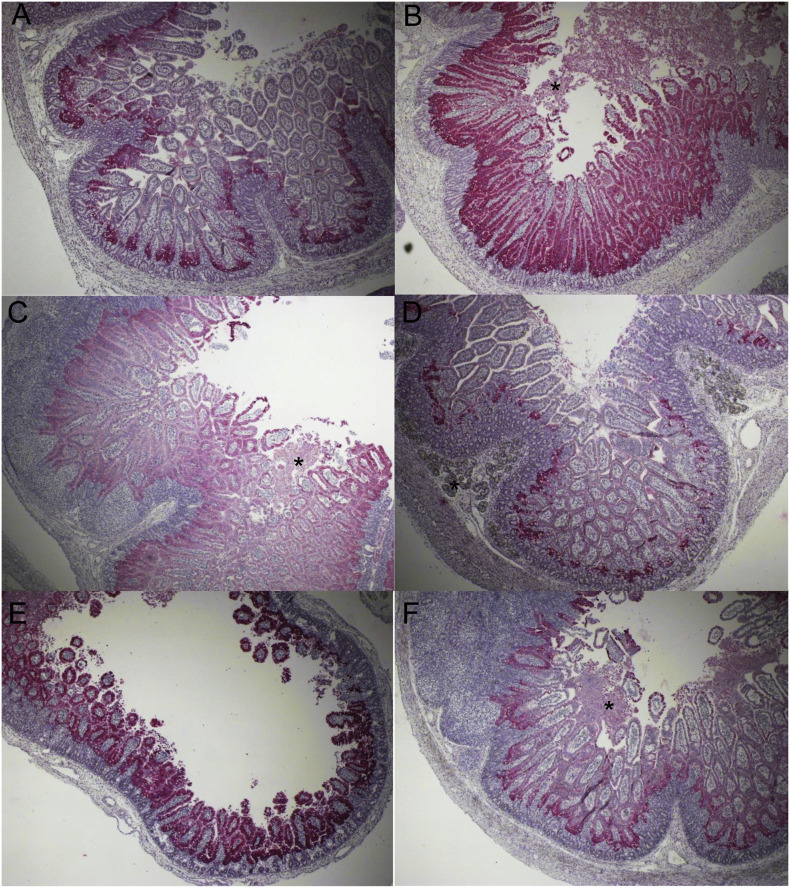
Like PEDV-inoculated conventional pigs tested earlier, PEDV antigens were mostly found in the villous epithelial cells, not the crypt epithelial cells (Fig. 2A–F). At PIH 16, PEDV-inoculated Gn pig 1 showed moderate to high numbers of PEDV antigen-positive cells in up to 100% of the mid-jejunal and ileal epithelium (Fig. 2B and C), low to moderate numbers of PEDV antigen-positive cells in the villus-crypt interface of the proximal and distal jejunum (Fig. 2A), few positive cells in the villus-crypt interface of the duodenum, and no positive cells in the colon. PEDV-inoculated Gn pig 2 showed high numbers of PEDV antigen-positive cells in up to 100% of the mid-jejunal epithelium, low numbers of PEDV antigen-positive cells in the villus-crypt interface of the proximal and distal jejunum and ileum, and no positive cells in the duodenum and colon. PEDV-inoculated Gn pig 3 showed high numbers of PEDV antigen-positive cells in up to 100% of the epithelium of atrophied mid-jejunal villi (Fig. 2E), low to moderate numbers of PEDV antigen-positive cells in the villus-crypt interface of the proximal and distal jejunum and ileum (Fig. 2F), low numbers of positive cells in the villus-crypt interface of the duodenum (Fig. 2D), and no positive cells in the colon. No PEDV antigen-positive cells were detected in the intestines of the negative control pigs.
Fig. 2.
Immunohistochemical detection and distribution of PEDV antigen-positive cells in the duodenum, proximal jejunum, mid-jejunum, or ileum of gnotobiotic 9-day-old pigs inoculated with the original US PEDV strain PC21A. (A) Proximal jejunum of PEDV-inoculated pig 1 at post-inoculation hour (PIH) 16, showing low numbers of PEDV antigen-positive cells (red color) in the villus-crypt interface (B) Mid-jejunum of PEDV-inoculated pig 1 with no clinical signs (vomiting and diarrhea) at PIH 16, showing high numbers of PEDV antigen-positive cells (red color) in most of the villous epithelium. Note normal villous length and exfoliation of enterocytes on the villous tips (asterisk) (C) Ileum of PEDV-inoculated pig 1 at PIH 16, showing high numbers of PEDV antigen-positive cells (red color) in most of the villous epithelium. Note normal villous length and exfoliation of enterocytes on the villous tips (asterisk) (D) Duodenum of PEDV-inoculated pig 3 with vomiting (but no diarrhea) at PIH 16, showing low numbers of PEDV antigen-positive cells (red color) in the villus-crypt interface. Duodenal gland (asterisk) (E) Mid-jejunum of PEDV-inoculated pig 3 at PIH 16, showing high numbers of PEDV antigen-positive cells (red color) in most of the epithelium of atrophied villi (F) Ileum of PEDV-inoculated pig 3 at PIH 16, showing moderate numbers of PEDV antigen-positive cells (red color) in the villous epithelium. Note exfoliation of enterocytes on the villous tips (asterisk). Original magnification, all ×40. Fast Red, Gill's hematoxylin counterstaining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.9. Serotonin-positive EC cells in duodenum, mid-jejunum, ileum, and colon of gnotobiotic 9-day-old pigs inoculated orally with PEDV or mock during the subclinical incubation stage of infection (prior to PID 1–5 when conventional 9-day-old pigs showed clinical signs)
The morphology and intestinal distribution of serotonin-positive EC cells identified in PEDV or mock-inoculated Gn pigs 1–6 were similar to those observed in the PEDV- or mock-inoculated conventional pigs. At PIH 16, mean numbers of serotonin-positive EC cells per microscopic area (×250) were significantly (P < .05) reduced in duodenum, mid-jejunum, and colon, but not ileum, of PEDV-inoculated Gn pigs 1–3, compared with the corresponding negative controls (Table 4 ). However, further analyses were done based on presence or absence of onset of vomiting in the PEDV-inoculated Gn pigs tested. In PEDV-inoculated Gn pigs 1 and 2 with no vomiting, mean numbers of serotonin-positive EC cells per microscopic area (×250) in duodenum, ileum, and colon, but not mid-jejunum, were comparable to those in the corresponding negative controls (Table 4). Like PEDV-inoculated conventional pigs tested at PID 1, however, PEDV-inoculated Gn pig 3 with vomiting exhibited a trend toward reduced numbers of serotonin-positive EC cells per microscopic area (×250) in the duodenum, mid-jejunum, ileum, and colon, compared with the corresponding negative controls (Table 4).
Table 4.
Mean numbers (±SDM) of serotonin-positive enterochromaffin cells by immunohistochemistry in the crypt layers and lower half of villi of duodenum, mid-jejunum, ileum, and colon per microscopic area, at ×250 magnification, of gnotobiotic 9-day-old pigs inoculated with virulent US PEDV strain PC21A or mock at post-inoculation hour (PIH) 16 when or shortly after vomiting was first detected.
| PEDV-inoculated Gn pigs at PIH 16 (10 days of age) |
Mock-inoculated Gn pigs 4–6 (10 days of age) (n = 3) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Based on the time-point, PIH 16 |
Based on presence or absence of vomiting at PIH 16 |
||||||||||||||
| Pigs 1–3 (n = 3) |
Pigs 1 and 2 with no vomiting (n = 2) |
Pig 3 with onset of vomiting (n = 1) |
|||||||||||||
| D | MJ | I | C | D | MJ | I | C | D | MJ | I | C | D | MJ | I | C |
|
19.8 (6.6)a |
11.4 (3.1) |
16.6 (4.5) |
8.7 (2.8) |
24.6 (3.6) |
10.9 (2.4)b |
20.0 (2.7) |
9.7 (3.0) |
12.8 (1.9) |
12.4 (4.5) |
13.1 (3.1) |
6.8 (1.1) |
27.2 (4.8) |
16.9 (3.7) |
20.2 (3.6) |
12.1 (3.1) |
D, duodenum; MJ, mid-jejunum; I, ileum; C, colon.
Bold numbers, P < .05 [statistically significant differences between the PEDV-inoculated (n = 3) and Mock-inoculated Gn pigs (n = 3) at PIH 16 by Student's t-test].
Underlined numbers, trends (based on mean values and their standard deviations) toward reduced numbers of serotonin-positive EC cells in the PEDV-inoculated Gn pigs compared with Mock-inoculated Gn pigs.
4. Discussion
Our study demonstrates that the monoclonal antibody 5HT-H209 is useful for the detection of serotonin in EC cells in swine intestinal tissues. Similar to serotonin-positive intestinal EC cells identified by IHC in humans or rats (Gustafsson et al., 2006; Spiller, 2008), swine EC cells were also mainly localized in intestinal crypts, and they were morphologically triangular-shaped or round cells with or without apical cytoplasmic extensions, respectively. Like EC cells in humans, swine intestinal EC cells were the most frequent in duodenum compared with the other intestinal segments (Table 1, Table 4) (Endo et al., 2000; Spiller, 2008). At PIH 16 or PID 1, when vomiting was first or frequently observed in PEDV-inoculated Gn or conventional pigs, respectively, the number of serotonin-positive EC cells was significantly reduced in the small and large intestines, compared with the negative controls. However, two of three PEDV-inoculated Gn pigs (pigs 1 and 2) that did not exhibit vomiting at PIH 16 concomitantly showed numbers of serotonin-positive EC cells in the small (but not mid-jejunum) and large intestines similar to those in the negative controls. These findings suggest that serotonin release from EC cells into the intestinal lamina propria or lumen (increased serotonin levels in the gut submucosa) occurred early post-infection to stimulate the vagal afferent neurons, followed by vomiting. The procedure may be essential for inducing vomiting in PEDV-infected piglets. At PID 1, reduced numbers of serotonin-positive EC cells were observed from the duodenum to the colon of conventional pigs, although extensive villous atrophy with a large amount of PEDV antigen was confined to the jejunum and ileum. In contrast, the duodenum and colon showed no or mild villous atrophy with little PEDV antigen at PID 1. We speculate that regardless of the tissue tropism of the virus, EC cells in all intestinal segments (duodenum to colon) might be involved in the induction of vomiting in PEDV-infected piglets.
In our study, the detailed pathogenesis of PEDV in infected Gn pigs was examined to understand the relationship of acute PEDV infection and frequency of serotonin-positive EC cells in the small and large intestines. Also, there was little information on the pathogenesis of acute PEDV infection in young piglets prior to onset of clinical signs. At PIH 16, PEDV RNA was not detected in feces but was detected in serum (8.1–8.5 log10 GE/ml) of PEDV-inoculated Gn pigs. Our observations are similar to a previous study, showing that only 1 of 20 (5%) 1-day-old, caesarean-derived, colostrum-deprived (CDCD) pigs inoculated orally with 10 3 PEDV plaque-forming units/pig were positive for PEDV RNA in the feces at PIH 12 (Madson et al., 2016). In the same study, however, 4 of 5 pigs (80%) were positive for PEDV RNA in serum at the same time-point. Based on these and our observations, viremia (viral RNA) may be detected earlier than fecal virus RNA shedding in PEDV-infected piglets. As our IHC study also revealed large amounts of PEDV antigens in the small intestine of PEDV-inoculated Gn pigs 1–3 at PIH 16, viremia might be a result of diffusion or spread of replicated PEDV from the infected intestine to the blood, although the detailed mechanisms need to be studied further. Based on our histopathologic and IHC observations in PEDV-inoculated Gn pigs 1–3 at PIH 16, PEDV initially and mainly infected the mild-jejunum and to a lesser extent, ileum; however, PEDV infection of the duodenum, proximal jejunum, and distal jejunum was less compared with the mid-jejunum and ileum. In addition, our IHC study did not find any PEDV antigen in the pylorus of the PEDV-inoculated pigs 1–3. Our pilot study also included another location of proximal jejunum distant by approximately 30 cm from the pylorus. We found that infectivity of PEDV in this intestinal segment was more similar to that found in the mid-jejunum compared with the other proximal jejunum segment (distant by 15–20 cm from the pylorus) closer to the pylorus. These observations underscore that the mid-jejunum and ileum may be the primary sites of acute PEDV infection (Jung and Saif, 2015), although the exact cause of the distinct tissue tropism remains obscure in terms of virus-host cell interactions at the molecular level.
It is debatable whether PEDV induces innate immune or pro-inflammatory responses in infected pigs, because most of the research has been conducted in cell cultures in vitro (Zhang et al., 2016; Zhang and Yoo, 2016). However, our previous study showed increased serum IFNα and IL-12 at PID 1 in conventional 9-day-old nursing pigs infected with PEDV (Annamalai et al., 2015). Our current study also clearly showed that infected Gn pigs exhibited systemic innate (IFNα and IL-22) and pro-inflammatory (IL-6, TNFα, and IL-12) cytokine responses after acute PEDV infection, similar to the reported increased mRNA levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-8 and TNFα) in IPEC-J2 cells infected with PEDV (Lin et al., 2017). In particular, 3- to 6-fold increases in the pro-inflammatory cytokines observed in infected Gn pigs 1–3 coincided with the decreased or loss of appetite following acute PEDV infection in young suckling piglets (Langhans, 2000). EC cells secrete serotonin in response to mechanical stimulation as well as recognition of pathogens via toll-like receptors (Worthington, 2015). Several studies showed that serotonin functions to promote immune activation through the receptors expressed on macrophages, dendritic cells, and T and B cells (Li et al., 2011; Shajib and Khan, 2015). In our pilot study, increased serum levels of pro-inflammatory and innate cytokines coincided with reduced numbers in serotonin-positive intestinal EC cells, i.e. serotonin release from EC cells in the gut submucosa, during the early stage of infection (PIH 16 to PID 1) (Table 3, Table 4). This suggests a possible involvement of EC cells and serotonin, not only in triggering an emetic response but also in development of innate or pro-inflammatory immune responses to acute PEDV infection. However, the potentially beneficial or detrimental effects or roles of those innate and pro-inflammatory cytokines and serotonin in infected piglets need to be studied further.
As tested in PEDV-inoculated Gn pigs 1–3 at PIH 16, our IHC observations revealed that early localization of PEDV antigen is evident in the villous-crypt interface of the duodenum, jejunum, and ileum during the acute or incubation stage of infection (Fig. 2A–F), similar to previous observations (Madson et al., 2016). A similar observation was also detected by IHC in 1-day-old, CDCD pigs inoculated with a porcine deltacoronaivrus at PIH 12–24 (Jung and Saif, unpublished), implying a similar tissue tropism between these two viruses during the early stages of infection. The PEDV antigen-positive regions appeared first in the villus-crypt interface and subsequently expanded to the upper and then entire villous epithelium of the jejunum to ileum. Our data indicate that enzymatically immature (young) enterocytes in the villous-crypt interface of the small intestine, relative to the enzymatically mature (older) enterocytes located at the villous tips, might be the major infection site of PEDV. During the incubation period, similar to vibrio cholera (cholera toxin) or rotavirus (non-structural viral protein 4) (Spiller, 2008), PEDV itself or undefined secondary mediators from the infected host might act to trigger serotonin release from EC cells. Because the infection site of PEDV is close to the crypt layer where the majority of serotonin-positive EC cells are located, it might be beneficial for PEDV itself or secondary mediators to access EC cells in the crypt layers. In our study, although PEDV antigen-positive cells were confined to villous epithelium (not crypt epithelium where the majority of serotonin-positive EC cells were localized), whether PEDV can also infect EC cells needs to be studied further. At PID 5 when vomiting disappeared completely, the numbers of serotonin-positive EC cells in the small and large intestines of the conventional pigs recovered and were comparable to those in the negative controls. The rapid recovery could imply a rapid turnover of EC cells to compensate for the reduced numbers. However, the turnover time of EC cells (15–150 days) is much slower than for enterocytes (2–4 days) in rats (de Bruine et al., 1992). Thus, the rapid recovery of the number of serotonin-positive EC cells might be a result of increased synthesis and deposition of serotonin in EC cells.
5. Conclusion
Collectively, serotonin might be involved in the mechanisms related to vomiting in PEDV-infected nursing pigs. Serotonin receptor (5-HT3) antagonists may be useful targets as a therapeutic intervention to suppress acute vomiting in PEDV-infected pigs, especially in cases when the clinical signs are severe. Therefore, further studies are needed to test whether 5-HT3 antagonists are effective to inhibit or suppress acute vomiting in PEDV-infected pigs. To evaluate the disease mechanisms and comparative pathogenesis of PEDV strains in young piglets during the acute or incubation stage of PEDV infection, the mid-jejunum and ileum are the most critical and useful intestinal locations for histological analysis. Our study also revealed that acute PEDV infection with evidence of PEDV RNA in serum, induces systemic innate (IFNα and IL-22) and pro-inflammatory (IL-6, TNFα, and IL-12) cytokine responses in young Gn pigs.
Conflict of interest statement
None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
We thank Dr. Juliette Hanson, Andrew Wright, Megan Strother, and Ronna Wood for assistance with animal care; and Xiaohong Wang, Zhongyan Lu, John Blakenship, and Thavamathi Annamalai for technical assistance. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
Contributor Information
Kwonil Jung, Email: jung.221@osu.edu.
Linda J. Saif, Email: saif.2@osu.edu.
References
- Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015;168:193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo M.S., Yuan L., Pouly S., Gonzales A.M., Jeong K.I., Nguyen T.V., Saif L.J. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J. Virol. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattha K.S., Vlasova A.N., Kandasamy S., Rajashekara G., Saif L.J. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J. Immunol. 2013;191:2446–2456. doi: 10.4049/jimmunol.1300678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruine A.P., Dinjens W.N., Zijlema J.H., Lenders M.H., Bosman F.T. Renewal of enterochromaffin cells in the rat caecum. Anat. Rec. 1992;233:75–82. doi: 10.1002/ar.1092330110. [DOI] [PubMed] [Google Scholar]
- Endo T., Minami M., Hirafuji M., Ogawa T., Akita K., Nemoto M., Saito H., Yoshioka M., Parvez S.H. Neurochemistry and neuropharmacology of emesis - the role of serotonin. Toxicology. 2000;153:189–201. doi: 10.1016/s0300-483x(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Gimeno Brias S., Stack G., Stacey M.A., Redwood A.J., Humphreys I.R. The role of IL-22 in viral infections: paradigms and paradoxes. Front. Immunol. 2016;7:211. doi: 10.3389/fimmu.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B.I., Bakke I., Tommeras K., Waldum H.L. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand. J. Gastroenterol. 2006;41:390–395. doi: 10.1080/00365520500331281. [DOI] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Alekseev K.P., Zhang X., Cheon D.S., Vlasova A.N., Saif L.J. Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J. Virol. 2007;81:13681–13693. doi: 10.1128/JVI.01702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Annamalai T., Lu Z., Saif L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet. Microbiol. 2015;178:31–40. doi: 10.1016/j.vetmic.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W. Anorexia of infection: current prospects. Nutrition. 2000;16:996–1005. doi: 10.1016/s0899-9007(00)00421-4. [DOI] [PubMed] [Google Scholar]
- Li N., Ghia J.E., Wang H., Mcclemens J., Cote F., Suehiro Y., Mallet J., Khan W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Li B., Chen L., Ma Z., He K., Fan H. Differential protein analysis of IPEC-J2 cells infected with porcine epidemic diarrhea virus pandemic and classical strains elucidates the pathogenesis of infection. J. Proteome Res. 2017;16:2113–2120. doi: 10.1021/acs.jproteome.6b00957. [DOI] [PubMed] [Google Scholar]
- Madson D.M., Arruda P.H., Magstadt D.R., Burrough E.R., Hoang H., Sun D., Bower L.P., Bhandari M., Gauger P.C., Stevenson G.W., Wilberts B.L., Wang C., Zhang J., Yoon K.J. Characterization of porcine epidemic diarrhea virus isolate US/Iowa/18984/2013 infection in 1-day-old cesarean-derived colostrum-deprived piglets. Vet. Pathol. 2016;53:44–52. doi: 10.1177/0300985815591080. [DOI] [PubMed] [Google Scholar]
- Shajib M.S., Khan W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015;213:561–574. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- Sikander A., Rana S.V., Prasad K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Worthington J.J. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 2015;43:727–733. doi: 10.1042/BST20150090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yoo D. Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus Res. 2016;226:128–141. doi: 10.1016/j.virusres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Shi K., Yoo D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology. 2016;489:252–268. doi: 10.1016/j.virol.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]