Abstract
Feline Immnunodeficiency (FIV) and Feline Leukemia (FeLV) viruses are common infectious agents in stray cats and shelter environments. Recombinant feline interferon-ω (rFeIFNω) has shown an antiviral action not only against FIV and FeLV but also against herpesvirus (FHV-1) and calicivirus (FCV).
Sixteen naturally infected FIV/FeLV cats were followed during rFeIFNω therapy in order to monitor clinical signs and to correlate with excretion of concomitant viruses (FCV, FHV-1, feline coronavirus (FCoV) and parvovirus (FPV)). Cats were submitted to clinical evaluations and concomitant virus excretion assessement.
Comparing D0–D65, 10/16 cats improved clinical scores. Of the 10 cats positive for FHV-1 on D0, 4 were negative and 6 reduced viral loads. Of the 11 FCoV positive cats, 9 reduced viral loads. The 13 FCV positive cats and the FPV positive cat were negative on D65.
In conclusion, rFeIFNω improves clinical signs and reduces concurrent viral excretion in naturally infected retroviral cats.
Keywords: Feline immunodeficiency virus, Feline Leukemia Virus, Interferon, Therapy, Shelter, Feline herpesvirus, Feline coronavirus, Feline calicivirus
1. Introduction
Feline Immunodeficiency Virus (FIV) and Feline Leukemia Virus (FeLV) are two important retroviruses that infect domestic cats (Hosie et al., 2009, Lutz et al., 2009). Their prevalence differs according to geographic regions and indoor/outdoor status (Gleich et al., 2009, Norris et al., 2007). Common risk factors are geriatric cats, male gender, mixed breeding, aggressive behavior, co-infection with other retroviruses and outdoor contact with non-hierarchical cat communities (Murray et al., 2009). Even with more sensitive diagnostic tests and a good therapeutic approach, retroviruses remain a problem among animal rescue shelters (Hosie et al., 2009, Lutz et al., 2009).
The clinical signs observed in cats infected with these retroviruses are nonspecific and mainly due to immune suppression (Hartmann, 2011). In FeLV infected cats, clinical signs usually develop in viraemic animals some months to years after the infection (Lutz et al., 2009). In FIV infected cats, most of the clinical signs are not directly caused by the viraemia, but they result from secondary infections (Gleich and Hartmann, 2009). Furthermore, some FIV-infected cats may even show clinical signs that result from imbalanced stimulation of parts of the immune response, such as immune-mediated glomerulonephritis (Hosie et al., 2009). Although many retroviral infected cats go on to develop clinical signs, others may remain in good health for several years (Hosie et al., 2009).
The immune suppression induced by retroviruses may predispose cats to clinical infection with multiple opportunistic agents to which they would normally be resistant. Moreover, it is also possible to trigger an exacerbated response to some common bacterial, fungal and protozoal pathogens, occasionally (Dunham and Graham, 2008, Reche et al., 2010). In rescue cat shelters, viruses such as Feline herpesvirus type I (FHV-1), Feline calicivirus (FCV), Feline coronavirus (FCoV) and Feline parvovirus (FPV) are also important infectious agents to consider. They are particularly exacerbated when incoming animals are introduced to the shelters. However, even in stable resident shelters, intermittent excretion of these viruses may contribute to continuous spreading to the environment. These concurrent viral infections are potentiated in retroviral infected shelters and easily contribute to a general worsening of the clinical condition of infected cats (Addie et al., 2009, Radford et al., 2009, Thiry et al., 2009, Truyen et al., 2009). General management of retroviral infected cats should include an isolation policy, neutering and regular health check-ups, particularly in rescue shelters. For symptomatic cats, supportive general treatment should always be considered (Hosie et al., 2009, Lutz et al., 2009). Antiviral and immune modulation therapies are important options that should also be considered (Collado et al., 2006).
Recognizing the similarity between FIV and Human Immunodeficiency Virus, there are multiple drugs such as zidovudine, fozivudine or human Interferon-α, commonly used in humans, that can be applied in retroviral infected cats. However some of these drugs can have significant side effects (Domenech et al., 2011, Fogle et al., 2011). Interferons are a family of species-specific compounds that act not only as anti-viral drugs but also as immune modulators and anti-tumor agents (Gerlach et al., 2009, Tompkins, 1999). They can be classified in type I or type II interferons (IFNs) according to their biological properties (Collado et al., 2006, Pestka et al., 2004).
Type I IFNs are produced by virally infected cells and have immunomodulating effects (Domenech et al., 2011). This is due to interaction with specific cell-receptors and concurrent induction of the expression of specific genes that encode cytokines involved in innate immunity. Moreover, type I IFNs also have anti-viral effects, anti-proliferative and anti-inflammatory actions (Domenech et al., 2011, Gerlach et al., 2009, Gerlach et al., 2006). Type II IFNs are mainly immunomodulatory with only a low level of anti-viral effects, meaning that they are less useful in clinical practice. Currently there are two important IFNs used in veterinary medicine: Human Interferon-α and Recombinant Feline Interferon-ω (rFeIFNω), both of them type I IFNs (Collado et al., 2006).
Although it was proven that Human Interferon-α increases survival time in FIV and FeLV- cats (Pedretti et al., 2006, Weiss et al., 1991), the development of specific neutralizing antibodies may decrease its efficiency (Muller, 2002, Zeidner et al., 1990). More recently, rFeIFNω was licensed for use in veterinary medicine, namely for treatment of canine parvovirus and feline retroviral (FIV and FeLV) infections. As an homologous feline molecule, it has a good safety index and does not induce production of neutralizing antibodies (de Mari et al., 2004). However, despite its license, there is limited published information about the use of rFeIFNω in retroviral infections. Initially, its use in asymptomatic FIV cats was described (Caney et al., 2003) but the first conclusive results were provided by a study that revealed a clinical improvement and an increased survival time in FeLV and co-infected symptomatic cats (de Mari et al., 2004). A more recent study demonstrated that rFeIFNω improves the clinical condition and haematologic parameters not only in FeLV but also in FIV infected cats (Domenech et al., 2011). While there are few in-vivo studies to support its expected benefits, the use of rFeIFNω has been extended to other viral infections namely FHV-1, FCoV, FCV and FPV (Hennet et al., 2011, Ishida et al., 2004, Paltrinieri et al., 2007, Ritz et al., 2007, Thiry et al., 2009).
This study aims to evaluate the role of rFeIFNω on clinical improvement of naturally infected FIV and FeLV cats living in a rescue shelter and to clarify whether this therapy also reduces concurrent viral excretion of FCV, FHV-1, FCoV and FPV.
2. Materials and methods employed
2.1. Animals
Sixteen neutered domestic short-hair cats (11 males and 5 females), living in a Lisbon animal shelter and previously determined as FIV and/or FeLV positive status were selected for the study. Nevertheless, at inclusion, all the cats were retested to confirm their FIV/FeLV infections by Enzyme Linked Immunoabsorbent Assay (ViraCHEK/FIV and ViraCHEK/FeLV, Synbiotics).
The cats were living in good conditions, in agreement with current ethical and welfare standards. All the procedures involving the manipulation of these animals were consented and approved not only by the Committee for Ethics and Animal Welfare of the Faculty of Veterinary Medicine – Technical University of Lisbon (CEBEA) but also by the clinical direction of the referred animal shelter (União Zoófila de Lisboa).
Taking into account that animals’ origins were unknown, age and past information were considered irrelevant for this study. Nevertheless, all the animals were adults with ages estimated in a range of 3–8 year old. Animals were housed in two different catteries, correlated with their FIV or FeLV status. Due to previous shelter facilities, Co-infected animals were housed in the FeLV cattery. Subsequently, cats were divided in three different groups according to their retroviral status: FIV positive cats (n = 7; 5 males and 2 females), FeLV positive cats (n = 6; 4 males and 2 females) and Co-infected animals (n = 3; 2 males and 1 female). Based on previous studies (de Mari et al., 2004), the inclusion criteria were the following: (1) cats of any age, breed or sex (heterogeneous population), (2) cats that showed at least one clinical sign potentially related to retroviral infections, (3) cats that had previously had a positive rapid immune-migration FIV/FeLV test result. Exclusion criteria were: (1) cats that showed clinical signs of malignancy (such as Lymphoma or Lymphoid leukemia), (2) cats having received immunomodulating drugs (such corticotherapy) during the 4 weeks before the study (3) cats having received antibiotics or non-steroidal anti-inflammatory drugs during the 2 weeks before the study and (4) cats that did not complete the therapeutic protocol.
2.2. Products
Vials of rFeIFNω (Virbagen Omega; Virbac) were reconstituted with the accompanying saline diluent according to the manufacturer’s recommendations immediately before each treatment.
2.3. Treatment protocol
Based on assumptions derived from two previously published double arm trials with rFeIFNω (de Mari et al., 2004, Domenech et al., 2011), a single arm study was performed.
All the animals were treated with rFeIFNω, according to the licensed protocol (3 cycles of injections at Day (D) 0, D14 and D60. Each treatment cycle consists of 5 subcutaneous injections: 1 MU/kg once per day for 5 days. Treatment was administered by two veterinary clinicians from the research team.
Assessments before therapy were designated in our report as D0 and considered representative of the stage of each animal before treatment.
2.4. Supportive treatment
Despite the exclusion criteria applied, some animals needed supportive treatment during therapy. Consequently, potentiated amoxicillin, hepatic protectants (ursodeoxycholic acid, Sylimarin or S-Adenilmethionine) and/or fluid therapy were allowed. Antibiotics (other than potentiated amoxicillin), corticosteroids and non-steroidal anti-inflammatory drugs were not permitted to avoid any possible immunomodulation effects.
2.5. Clinical evaluation and scoring
At D0 (before therapy), D10, D30 and D65 after the beginning of the protocol, all the cats were submitted to regular clinical evaluations.
In order to reduce subjectivity, the findings of the clinical evaluations were scored according to a clinical-score scale (Table 1 ). This scale included the most important clinical parameters typically presented in retroviral infections (Fig. 1 ) such as oral ulcers/gingivitis (score 0-2), caudal stomatitis/palatitis (score 0-2), ophthalmic abnormalities (score 0-2), lymphadenopathy (score 0-2), ocular and nasal discharge (score 0-2), mucous membrane color (score 0-2), coat appearance (score 0-1), body score (score 0-2), faecal appearance (score 0-1) and concurrent diseases/co-morbidities (score 0-2). After each parameter was assessed, a sum score of 11 criteria was obtained for each animal to reflect the overall clinical condition of the animal. These scores were then compared at each time point.
Table 1.
Clinical score – scale used for cats’ clinical evaluation.
| Clinical parameter | Classification |
|---|---|
| Oral ulcers/Gingivitis | 0 – No evidence of oral lesions |
| +1 – Mild to moderate oral lesions | |
| +2 – Severe oral gingivitis | |
| Caudal stomatitis/Palatitis | 0 – No evidence of caudal stomatitis |
| +1 – Mild to moderate hyperemia and caudal stomatitis | |
| +2 – Severe hyperemia and caudal stomatitis | |
| Ophthalmology abnormalities | 0 – No evidence of ophthalmology changes |
| +1 – Mild conjunctival hyperemia (mainly unilateral), mild keratitis | |
| +2 – Severe conjunctival hyperemia (mainly bilateral), active keratitis | |
| Lymphadenopathy | 0 – No evidence of lymphadenopathy |
| +1 – Mild localized lymphadenopathy | |
| +2 – Generalized lymphadenopathy | |
| Ocular discharge | 0 – No evidence of ocular discharge |
| +1 – Serous ocular discharge | |
| +2 – Muco-purulent ocular discharge | |
| Nasal discharge | 0 – No evidence of nasal discharge |
| +1 – Serous nasal discharge | |
| +2 – Muco-purulent nasal discharge | |
| Pale Mucous membranes | 0 – No evidence of pale mucous membranes |
| +1 – Mild pale mucous membranes | |
| +2 – Severe pale mucous membranes | |
| Dry coat/Seborrhea | 0 – Normal coat condition |
| +1 – Dry coat and/or seborrhea | |
| Body condition score | 0 – Normal or fat: body condition score 4/6 to 6/6 |
| +1 – Mildly reduced body condition score 3/6 | |
| +2 – Underweight animal with a body score of 1/6-2/6 | |
| Faecal appearence | 0 – no evidence of diarrhea |
| +1 – clinical evidence of diarrhea | |
| Concurrent diseases or Co-morbidities | 0 – No evidence of concurrent diseases |
| +1 – Clinical evidence of concurrent disease | |
| +2 – Severe prostration/global weakness | |
Fig. 1.
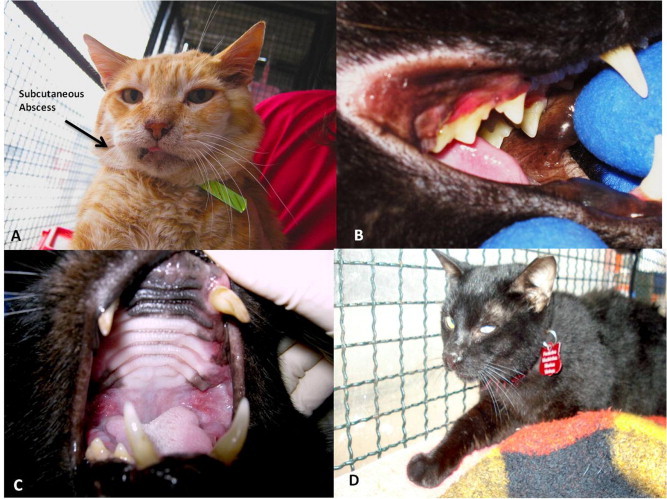
Clinical parameters observed in some cats evaluated in this study: namely subcutaneous abscess (A – Cat 1), oral ulcers/gingivitis (B – Cat 3), caudal stomatitis (C – Cat 10) and ocular and nasal discharge (D – Cat 13).
2.6. Blood sample collection and treatment
Blood samples were collected by venipuncture of the jugular vein at the same time as the clinical evaluations (D0, 10, 30, 65). Samples were analysed for complete blood-cell count (CBC), hepatic enzymes (alanine-transaminase, aspartate-transaminase) and renal function (creatinine, urea). CBC and biochemistry was performed on Cell-Dyn 3700 (Abbott diagnostics division) and Kone Optima 4.2 (Kemia Cientifica) respectively.
To allow a better evaluation and simpler blood sample collections, cats were submitted to mild tranquilization with 0.2–0.5 mg/kg of butorphanol solution (Dolorex, Intervet Portugal), sub-cutaneously.
2.7. Survey of concomitant pathogens
Cats from all groups were checked for FIV antibodies and FeLV antigen by ELISA using serum or plasma at D0 (ViraCHEK/FIV and ViraCHEK/FeLV, Synbiotics).
At each time point (D0, 10, 30, 65) oral swabs for the feline respiratory tract viruses (FHV-1; FCV) and rectal swabs for the digestive tract viruses (FPV, FCoV), were also obtained. Oral and rectal swabs were processed for viral DNA/RNA extraction (QIAamp MinElute Virus Spin Kit, Qiagen, Portugal).
The determination and quantification of concurrent viral excretion were performed using the diagnostic procedures available in the Virology Laboratory of the Faculty of Veterinary Medicine – Technical University of Lisbon.
Screening and quantification of FHV-1 was assessed by Real Time Polymerase Chain Reaction (PCR) amplification (Applied 7300 instrument, Applied Biosystems), using a specific gene expression kit (TaqMan gene expression Kit (Applied Biosystems). Primers and Cycling conditions are described in Table 2, Table 3 . Serial tenfold dilutions of the recombinant plasmid DNA were used to estimate the FHV-1 target copy number, using a specific software (7300 System SDS software) and generating a standard curve obtained with 101–106 DNA dilution of recombinant plasmids, with a correlation efficiency of r 2 = 0.997.
Table 2.
Primer nucleotide sequences used for the amplification of FCV, FHV-1, FPV and FCoV.
| Virus | Sequence |
|---|---|
| FCVa | Primer forward: GNA AAG CWC AAC AAA TTG AATT Primer reverse: CHTGTACCCTYTGCTCAAG |
| FHV-1b | Primer forward: ACGTGGTGAATTATCAGCTGAAG Primer reverse: AAGGTATGGTGCGGCAAATC Probe: TGCTGCCTATATCACCGCCCACTATCAA |
| FPVb | Primer forward: CAGGAAGATATCCAGAAGGA Primer reverse: GGTGCTAGTTGATATGTAATAAACA |
| FCoVb | Primer forward: TGGTCATCGCGCTGTCTACT Primer reverse: AGGGTTGCTTGTACCTCCTATTACA Probe:TTGTACAGAATGGTAAGCAC |
In house designed.
Table 3.
PCR and real time PCR amplification conditions.
| Virus | Reverse transcription | Initial denaturation | Denaturation | Annealing | Extension | Last Extension |
|---|---|---|---|---|---|---|
| FCV | 42 °C/30 mn | 95 °C/5 mn | 95 °C/30 s 35 Cycles |
52 °C/30 s 35 Cycles |
68 °C/30 s 35 Cycles |
68 °C/10 mn |
| FHV-1 | 95 °C/10 mn | 95 °C/15 s | 60 °C/1 mn 45 Cycles |
65 °C–95 °C (0.1 °C/s ramp rate) |
||
| FPV | 95 °C/10 mn | 94 °C/30 s 40 Cycles |
50 °C/1 mn 40 Cycles |
68 °C/1 mn 40 Cycles |
68 °C/10 mn | |
| FCoV | 48 °C/15 mn | 95 °C/10 mn | 95 °C/15 s | 60 °C/1 mn 45 Cycles |
65 °C–95 °C (0.1 °C/s ramp rate) |
|
FCV presence was assessed using conventional reverse transcriptase PCR (Desario et al., 2005, Wilhelm and Truyen, 2006) using 20 ng of viral RNA. Primers and Cycling conditions are described in Table 2, Table 3. A live vaccine (Fevaxyn Pentofel, Pfizer) was used as a positive control.
FPV presence was assessed using conventional PCR (Desario et al., 2005, Wilhelm and Truyen, 2006). Ten ng of viral DNA was used to amplify sequences within the VP2 gene. Primers and cycling conditions are described in Table 2, Table 3.
For screening and quantification of FCoV, primers and probe (TaqMan, Applied Biosystems) were chosen using a specific software (Primer Express, Applied Biosystems), within the 177 bp fragment included in the 3′ UTR region of FCoV (Herrewegh et al., 1995), previously cloned in the pGEM plasmid (Duarte et al., 2009). FCoV quantification was assessed by one step real-time PCR (TaqMan RNA-to-CT™ 1-Step Kit, Applied Biosystems), using cycling conditions described in Table 3. Serial tenfold dilutions of the recombinant plasmid DNA were used to estimate the FCoV target copy number, using the referred specific softwareh and generating a standard curve with a correlation efficiency of r 2 = 0.997.
2.8. Statistical analysis
To compare clinical scores and viral loads between different treatment days, the non parametric Friedman Test was applied. The significance level was set at 5%. For the remaining analyses, the mean values and the respective standard errors were reported.
3. Results
3.1. Clinical evaluation and scoring
Cats were submitted to clinical evaluation at D0, 10, 30 and 65 after starting therapy with rFeIFNω using a score scale (table 1).
Oral ulcers and gingivitis were the most frequent clinical sign at D0 in all the groups, Caudal stomatitis was also a prevalent finding at the beginning of the study, particularly with FIV positive cats. These were also the clinical signs that improved most consistently with therapy. Concerning the other parameters, they were variable during therapy and nonspecific fluctuations were observed. The detailed individual clinical scores are recorded in Table 4, Table 5, Table 6 .
Table 4.
Total group and detailed individual clinical score values for each parameter evaluated in FIV cats during rFeIFNω therapy.
| Detailed individual clinical scores | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat 1 | Cat 2 | Cat 3 | Cat 4 | Cat 5 | Cat 6 | Cat 7 | ||||||||||||||||||||||
| Clinical parameter/Day | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 |
| Oral ulcers | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 |
| Caudal stomatitis | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ophthalmological abnormalities | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular discharge | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nasal discharge | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucous membranes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry coat/seborrhea | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Body condition | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Faecal Appearance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Concurrent Diseases | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total (individual) | 13 | 10 | 8 | 6 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 7 | 7 | 5 | 2 | 5 | 5 | 4 | 2 | 1 | 1 | 1 | 1 |
| Total group clinical scores | ||||||||||||||||||||||||||||
| Day | 0 | 10 | 30 | 65 | ||||||||||||||||||||||||
| Total (sum) | 33 | 30 | 23 | 15 | ||||||||||||||||||||||||
Table 5.
Total group and detailed individual clinical score values for each parameter evaluated in FeLV cats during rFeIFNω therapy.
| Detailed individual clinical scores | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat 8 | Cat 9 | Cat 10 | Cat 11 | Cat 12 | Cat 13 | |||||||||||||||||||
| Clinical parameter/Day | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 |
| Oral ulcers | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Caudal Stomatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ophthalmological abnormalities | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 1 |
| Lymphadenopathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular discharge | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| Nasal discharge | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 2 | 0 |
| Mucous membranes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Dry coat/seborrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Body condition | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 |
| Faecal Appearance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Concurrent Diseases | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 |
| Total (individual) | 2 | 2 | 5 | 2 | 3 | 2 | 3 | 3 | 6 | 5 | 11 | 4 | 2 | 2 | 2 | 1 | 3 | 14 | 9 | 3 | 11 | 8 | 6 | 6 |
| Total group clinical scores | ||||||||||||||||||||||||
| Day | 0 | 10 | 30 | 65 | ||||||||||||||||||||
| Total (sum) | 27 | 33 | 36 | 19 | ||||||||||||||||||||
Table 6.
Total group and detailed individual clinical score values for each parameter evaluated in Co-Infected cats during rFeIFNω therapy.
| Detailed individual clinical scores | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat 14 | Cat 15 | Cat 16 | ||||||||||
| Clinical parameter/Day | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 | 0 | 10 | 30 | 65 |
| Oral ulcers | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 |
| Caudal stomatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 |
| Ophthalmological abnormalities | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphadenopathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ocular discharge | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nasal discharge | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucous membranes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry coat/seborrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Body condition | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Faecal Appearance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Concurrent Diseases | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Total (individual) | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 7 | 7 | 5 | 3 |
| Total group clinical scores | ||||||||||||
| Day | 0 | 10 | 30 | 65 | ||||||||
| Total (sum) | 10 | 10 | 8 | 4 | ||||||||
Considering the total values of clinical scores over the course of the study, FIV and Co-infected cats improved homogeneously during therapy. Conversely, FeLV cats showed important fluctuations during therapy meaning that, at D10 and 30, the cats’ mean scores worsened. Despite these results, comparing total scores of D0 and D65, a global improvement was observed.
Comparing D0 and D65, the beginning and end of treatment respectively, the overall improvement in clinical scores (indicating a better clinical condition) for the 16 naturally retroviral infected cats is statistically significant (p = 0.00066, Friedman Test). In particular, 10 cats improved their clinical conditions while 6 cats maintained the same clinical status. No cats experienced worsening of their scores.
Regarding the FIV group, the clinical improvement was also statistically significant (p = 0.025, Friedman Test). In particular, 4 out of 7 cats showed a marked improvement (final score >50% better than initial), 1/7 revealed a mild to moderate improvement (final score up to 50% better than initial) and 2/7 remained stable. Concerning FeLV infected cats, the clinical improvement was not statistically relevant (p = 0.32, Friedman Test). Nevertheless, it is observed that 3/6 showed a mild to moderate improvement and 3/6 remained with the same initial score. Two out of three co-infected cats showed a marked improvement and one out of three remained stable. However, due to the low number of animals in this group, these results were not statistically significant (p = 0.16, Friedman Test).
3.2. Blood sample collection and treatment
3.2.1. Haematology
Although some mild fluctuations were observed, hematologic parameters remained within reference ranges during therapy.
One FIV positive cat developed a very mild anemia at D65, which was clinically irrelevant. One FeLV positive cat revealed a moderate anemia also at D65. Mean values for red-blood cells concentration (with standard error (±SE)) are shown in Fig. 2 .
Fig. 2.
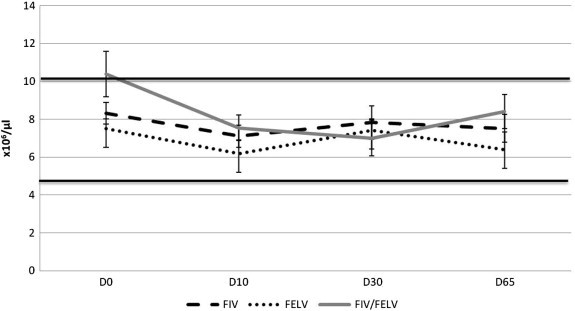
Average ± standard error of red blood cell count variation in FIV, FeLV and Co-infected cats under treatment with rFeIFNω (reference range is in between 5, 0–10, 0 × 106/μl, which is represented by continuous black lines). p = 0.32, Friedman Test for comparison of D0 and D65.
Despite some irrelevant occasional variations, all the animals had normal leucocyte levels during therapy. Mean values (±SE) are displayed in Fig. 3 .
Fig. 3.
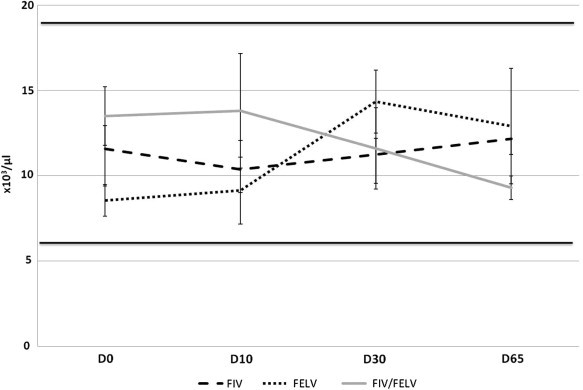
Average ± standard error of white blood cell count variation in FIV, FeLV and Co-infected cats under treatment with rFeIFNω (reference range is in between 5, 5–19, 5 × 103/μl, which is represented by continuous black lines). p = 0.62, Friedman Test for comparison of D0 and D65.
3.2.2. Biochemistry analysis
In all cats, renal parameters and liver enzymes remained stable and within reference range during therapy.
3.3. Survey of concomitant pathogens
Oral swabs for FCV detection were processed for viral RNA as described above. Amplification showed that prevalence of FCV shedding was 13/16 (4/7 FIV, 6/6 FeLV (Fig. 4 ) and 3/3 Co-infected) at D0 and 5/16 (1/7 FIV, 3/6 FeLV and 1/3 Co-infected) at D10. At D30 and D65 all the cats tested negative. All the FIV cats that tested positive for FCV (4/7) had gingivostomatitis in contrast with FeLV and Co-infected groups in which not all the FCV positive animals showed this clinical sign.
Fig. 4.
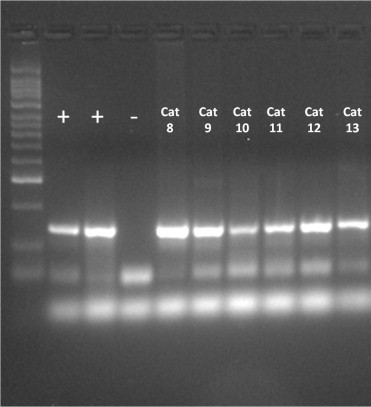
FCV PCR amplification for FeLV group on D0. (+) Vaccine positive control. (−) Negative control. Cats are listed as presented in Table 5.
As previously described, oral swabs for FHV-1 were processed for viral DNA extraction. Quantification of FHV-1 revealed 10/16 positive cats (4/7 FIV, 4/6 FeLV and 2/3 Co-infected) at D0. Detailed results are summarized in Fig. 5 . Comparing D0 with D65, 3 of the 4 FIV infected cats reduced their FHV-1 excretion and 1 cat tested negative (Fig. 5). Also in this group, one cat showed a punctual excretion on D10 but tested negative on D65. In FeLV group, on D65 2 of the 4 FHV-1 positive cats tested negative. The other two were still excreting, although at a lower level than D0 (Fig. 5). Also in this group, 2 cats revealed a punctual FHV-1 excretion on D30 but on D65 one reduced and the other tested negative. In the Co-infected cats, 2/3 cats were FHV-1 positive. On D65, 1 cat was still excreting FHV-1, at lower levels than D0, and the other cat tested negative (Fig. 5).
Fig. 5.
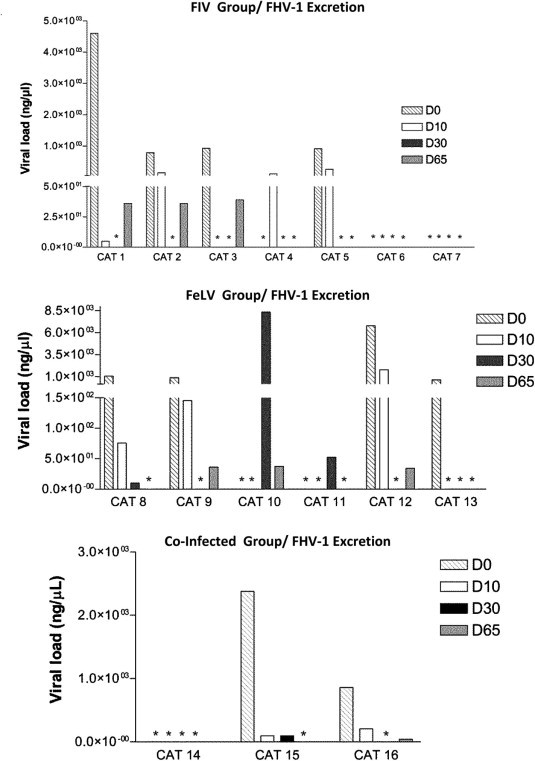
Real-time PCR viral load quantification (ng/μl) of FHV-1 excretion in FIV, FeLV and co-infected cats under rFeIFNω therapy (D0, D10, D30, D65). p-Values are respectively: p = 0.046; p = 0.18; p = 0.16 (Friedman Test). Overall p = 0.0066 (Friedman Test). (∗) refers to zero values.
Comparing D0 with D65, the overall reduction of FHV-1 excretion is statistically significant for the 16 naturally retroviral infected cats (p = 0.0066, Friedman Test). When correlating FHV-1 viral status with individual clinical scores, it is observed that 5/16 cats (31%; Confidence Interval 95% = 11%; 59%) showed a concurrent clinical improvement and a reduction in FHV-1 excretion.
Rectal swabs used for screening and quantification of FCoV showed 11/16 positive (5/7 FIV, 4/6 FeLV and 2/3 Co-infected) on D0. On D65, nine of them decreased their viral excretion. In detail, 4 FIV infected cats reduced their FCoV viral excretion while 1 cat increased it (Fig. 6 ). In the FeLV group, comparing D0 with D65, 3 of the 4 FCoV infected cats reduced their viral load, while 1 enhanced it. Two cats which were negative on D0, revealed punctual excretions on D10 and D30 but decreased to zero or values near zero at D65. Both Co-infected cats which were FCoV positive on D0 reduced viral excretion at D65. Interestingly the one Co-Infected cat which was negative for FCoV excretion at D0 revealed an increased excretion at D30, which reduced at D65 (Fig. 6). Comparing D0 with D65, overall group results are not statistically significant (p = 0.17, Friedman Test).
Fig. 6.
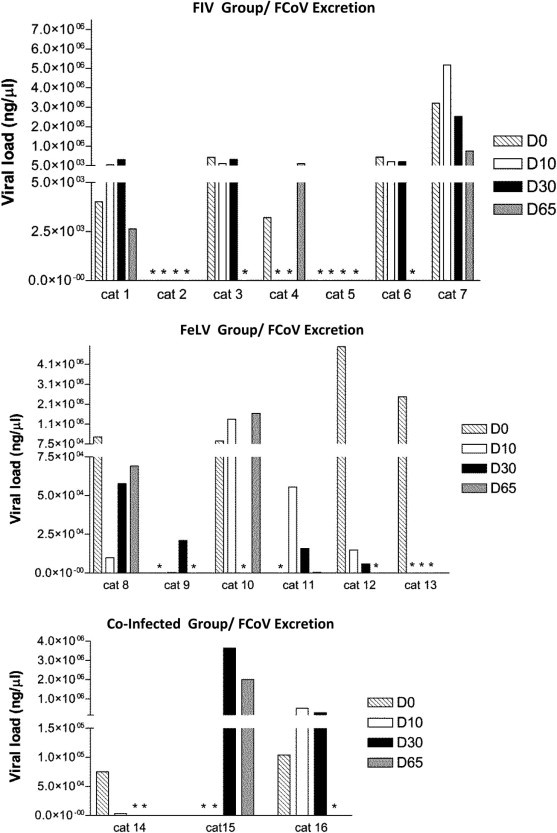
Real-time PCR viral load quantification (ng/μl) of FCoV excretion in FIV, FeLV and co-infected cats under rFeIFNω therapy (D0, D10, D30, D65). p-Values are respectively: p = 0.18; p = 0.65; p = 0.56 (Friedman Test). Overall p = 0.17 (Friedman Test). (∗) refers to zero values.
Correlating FCoV excretion with individual clinical scores, it is observed that 5/16 cats (31%; Confidence Interval 95% = 11%; 59%) showed a concurrent clinical improvement and a reduction in FCoV excretion.
Rectal swabs were also used for FPV screening. At D0 only one FeLV infected cat tested positive by conventional PCR for parvovirus excretion. From D10 till the end of therapy all cats remained negative.
4. Discussion and conclusions
FIV/FeLV naturally infected cats were followed before, during and after rFeIFNω treatment. Parameters such as clinical evaluation, haematological analysis, and viral loads were assessed in each individual cat before therapy (at D0). These assessments were designated as D0 and were representative of the stage of each animal before therapy.
As rFeIFNω is a licensed product and its efficacy had been described in multiple double arm studies (de Mari et al., 2004, Domenech et al., 2011), a single arm trial was performed in order to extend our understanding of the improvement of retroviral infected cats under this therapy. A control other than the D0 results, such as a group without treatment or placebo was considered of limited interest and ethically controversial in the context. The control group would be eventually important if we were studying experimental infected animals, where all the cats should be at the same stage of infection. In this case, we have a heterogeneous group of cats and there was difficult to know the point and stage of infection. Even more, these cats were located in a shelter environment where the inclusion of a placebo group was even more controversial ethically. Indeed, the whole point is the therapy in the shelter environment. It is believed that this study reflects the crude reality of cat shelters. In this sense, the decision of establishing a control group before therapy was deliberated and assumed. In our viewpoint, measurements on D0 in the shelter environment are potentially more representative than a placebo with different stages of infection group. Therefore, each cat at day 0 was considered its best untreated control.
Regarding the length of the study, it would be also interesting to evaluate viral excretion a few weeks after the end of the therapy (D65). However, the inclusion of new animals in the catteries could not be excluded after this time-point, being impossible to consider a reliable follow-up of the group viral excretion, when re-infections could occur. Therefore, although the animals were followed after this time-point, sample collection for viral excretion were concluded at this time point.
In accordance with previous studies (Domenech et al., 2011), the clinical condition of the majority of the cats improved with rFeIFNω treatment. This improvement was more pronounced in cats with higher initial clinical scores. 6/16 cats were mildly symptomatic at the start of the study and remained stable. No cat got worse at the end of the study than at the start. When compared with FeLV cats, FIV cats presented a more evident general improvement. The fluctuations observed with the FeLV cats during the course of the study contributed to the worsening of clinical scores at interim time points for certain cats. Recognizing that rFeIFNω is an immune-modulator, a possible explanation refers to the fact that FeLV cats have a more compromised immune response in comparison to FIV cats (Gleich and Hartmann, 2009, Pardi et al., 1991). This is mainly due to the fact that FeLV infected cats seem to have a more pronounced defect of helper T-cells. Consequently, these animals have a reduced humoral immune response (Gleich and Hartmann, 2009).
Despite the low number of animals, the co-infected group also showed a good improvement when compared with FeLV cats. In those animals, rFeIFNω also seems to have an important imunomodulatory effect, resulting in significant improvement.
The most evident clinical sign at inclusion in all groups was the oral ulcers and gingivitis/gingivostomatitis, both of which improved during rFeIFNω therapy. Although the condition has a multifactorial etiology, rFeIFNω is well known as a prescribed therapy for Feline Chronic Gingivostomatits Syndrome (FCGS) (Dowers et al., 2010) and these results corroborate the relevance of its use. However it is interesting to note that all previously published studies on the use of rFeIFNω in the management of FCGS have focused on retrovirus negative cats (Hennet et al., 2011), and this is the first time, to our knowledge, that evidence has been presented for efficacy in FeLV or FIV positive cats.
Previous studies suggested that rFeIFNω is effective on retrovirus-induced anemia in cats (de Mari et al., 2004) and improves haematological profiles (Domenech et al., 2011). In this study, all the cats had normal haematologic values on D0 and consequently no significant changes were observed during therapy. Even the FeLV cats, which are often lymphopenic (Gleich and Hartmann, 2009), were normal at baseline and showed no relevant changes during treatment. Two cats (1/7 FIV and 1/6 FeLV) developed anaemia at D65. Nevertheless, both cats were closely monitored after the end of the study and two weeks later CBC were repeated and showed normal values suggesting a temporary irrelevant fluctuation of the values.
Renal function and transaminases were also assessed. All the cats had a normal biochemistry profile and this did not significantly change during therapy. According to the European Medicines Agency’s published scientific discussion, rFeIFNω may lead to a temporary leucopenia, thrombocytopenia, anaemia and an increase in alanine aminotransferase. Other than the mild short-term anaemia and sporadic clinical irrelevant trombocytopenias, none of these other side-effects were observed.
One of the main goals of this study was the evaluation of concurrent viral excretion in cats under rFeIFNω therapy. Some authors have suggested that FIV-positive cats that are co-infected with other viruses such as FCV and/or FeLV seem to have a higher prevalence of oral infections and severe oral lesions (Tenorio et al., 1991). This agrees with the presented results of this study. In fact, all the FIV cats that tested positive for FCV (4/7) had gingivostomatitis. In the FeLV group, 4 of the 6 FCV positive cats had severe gingivostomatitis, 1/6 had mild gingivitis while 1/6 had no observed oral lesions. For Co-infected cats, all 3 animals tested FCV positive and 2 of them had oral signs.
FCV status can also be associated with the extension of gingivitis to the palate and the mucosa lateral to the palatoglossal arches (caudal stomatitis/palatitis). Three out of four FIV/FCV positive cats presented this sign. Even with a low sample size, these findings agree with a previous study which reported that a greater proportion of cats with caudal stomatitis are FCV positive (Hennet and Boucraut-Baralon, 2005). In contrast, in FeLV and Co-infected animals, only one cat in each group presented with caudal stomatitis.
Recently, a study described the use of rFeIFNω by oral administration in refractory cases of caudal stomatitis (Hennet et al., 2011). To the authors’ knowledge, there are no studies that describe the use of rFeIFNω, in its licensed protocol, in concurrent retroviral and FCV-infected cats. 13/16 animals were positive for FCV at D0 (4/7 FIV, 6/6 FeLV and 3/3 Co-infected). At D30 and D65, all cats tested negative for FCV, meaning that rFeIFNω, administered according to this protocol, was associated with a remission of FCV excretion in these cats. Furthermore, only 3 of those cats revealed persistent oral lesions at the end of therapy. The remaining FCV positive animals improved their gingivostomatitis and palatitis. Although FCGS may have a multifactorial etiology, the improvement of oral clinical signs was evident and agreed with the observed reduction in FCV excretion. It is likely that the observed improvement of both the clinical signs and the shedding of FCV was due to a combination of the immunomodulating and the antiviral effects of the treatment. This is especially important in animal shelters where gingivostomatitis is frequently associated with active FCV infection, and animals may also be more likely to experience some stress-induced reduction in immune competence.
Recognizing that FHV-1 is ubiquitous in catteries (Thiry et al., 2009), its prevalence among retroviral infected cats was expected to be elevated. At D0, 10/16 animals tested positive. During rFeIFNω therapy, a reduction of FHV-1 excretion was observed (p = 0.0066, Friedman Test). Curiously, shedding of FHV-1 was even completely suppressed in 4 animals during therapy. Typical respiratory and ocular signs were not very remarkable in these shelter cats. However, the concurrent clinical improvement and reduction in FHV-1 excretion observed in some cats (31%) lead to conclude that, in particular cases, the reduction of FHV-1 shedding may contribute for a clinical improvement. Despite these good clinical findings for animal shelters, FHV-1 may remain latent and the suppression of viral excretion may not lead to complete cure (Dowers et al., 2010). Also in these animals, this effect may be temporary. Therefore, not only a clinical follow-up but also a reduction in input/output of animals within catteries should be considered.
It has been reported that 70% of the feline population in catteries are FCoV positive (Addie et al., 2009). As expected, in our study 11/16 cats tested positive at D0. Infected cats may shed high viral loads of FCoV without showing relevant symptoms (Foley et al., 1997, Meli et al., 2004). Despite promising results in an initial study using rFeIFNω therapy in cats with clinical feline infectious peritonitis (Ishida et al., 2004) a further one was not able to reveal a significant clinical improvement (Ritz et al., 2007). However, viral excretion was not assessed. To our knowledge, this is the first study that describes the effect of rFeIFNω on FCoV excretion levels in naturally FIV and FeLV infected cats. Despite some fluctuations which can explain non statistical significant results (p > 0.05, Friedman test), viral excretion decreased in most animals (9/11 positive FCoV cats) showing that rFeIFNω therapy seems to be helpful in this situation.
As remarked for FHV-1, several cats showed a concurrent clinical improvement and a reduction in FCoV excretion (31%). Therefore, FCoV status may also contribute for the better clinical condition observed in these cases.
Not only in FCoV but also in FHV-1, viral excretion is not a continuous process and some fluctuations were observed. In fact, some cats showed punctual excretions of both viruses namely on D30. A possible explanation may reside in the licensed rFeIFNω protocol. It is worth noting that D30 is the only follow-up time point during this study at which the animal had not experienced rFeIFNω administration on the previous days.
In the FPV screening, only one cat tested positive on conventional PCR and it was negative 10 days after treatment. In fact, Parvovirus PCR detection is not species-specific. As recently reported (Clegg et al., 2011), healthy cats may shed canine parvovirus, being an important reservoirs of this virus namely in animal shelters. Due to lack of clinical signs, in agreement with this study, this cat was assumed to be a subclinical carrier.
This study corresponds to the common reality of animal shelters where the overflow of cats and continuous resident rotation (stray animals that are introduced and others that are adopted) may contribute to potentiate different ubiquitous viral infections. Concerning these results, rFeIFNω seems to be able to contribute to the management of this reality by improving clinical signs and decreasing concurrent viral excretion. In summary, rFeIFNω therapy may be beneficial in naturally retroviral infected cats, particularly in the shelter/rescue context, where prevalence of concomitant infections is higher.
Conflict of interest statement
Mc Gahie, D: employee Virbac, Carros, France. His contribution for this work was mainly as a Consultant.
Acknowledgments
This work was supported by the Project CIISA 50; Rodolfo Leal is a PhD fellow (FCT SFRH/BD/62917/2009 Portugal); Solange Gil is a research assistant under Programa Ciência 2007 (FCT-Portugal).
The authors would like to thank União Zoófila (Lisboa) and Laboratório de Análises Clínicas Prof. Dr. Braço Forte.
Footnotes
Parts of this work were presented in abstract form at 21st European College of Veterinary Internal Medicine (ECVIM) Congress, September 2011 and Southern European Veterinary Conference/46th AVEPA NATIONAL CONGRESS, October 2011.
References
- Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Radford A.D., Thiry E., Truyen U., Horzinek M.C. Feline infectious peritonitis. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:594–604. doi: 10.1016/j.jfms.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caney, S., Helps,C., Finerty, S., Tasker, S., Gruffydd-Jones, TJ. 2003. Treatment of asymptomatic chronically FIV-infected cats with recombinant feline interferon omega. In: Proceedings of the Annual Conference of the American College of Veterinary Internal Medicine, Charlotte, USA.
- Clegg S.R., Coyne K.P., Dawson S., Spibey N., Gaskell R.M., Radford A.D. Canine parvovirus in asymptomatic feline carriers. Vet Microbiology. 2011;157:78–85. doi: 10.1016/j.vetmic.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Collado V.M., Doménech A., Gómez-Lucía E., Tejerizo G., Miró G. Usos de interferón en la clínica de pequeños animales. Pequeños animales. 2006;63:68–75. [Google Scholar]
- de Mari K., Maynard L., Sanquer A., Lebreux B., Eun H.M. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. Journal of Veterinary Internal Medicine. 2004;18:477–482. doi: 10.1892/0891-6640(2004)18<477:teorfi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? Journal of Virological Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Domenech A., Miro G., Collado V.M., Ballesteros N., Sanjose L., Escolar E., Martin S., Gomez-Lucia E. Use of recombinant interferon omega in feline retrovirosis: from theory to practice. Veterinary Immunology and Immunopathology. 2011;143:301–306. doi: 10.1016/j.vetimm.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowers K.L., Hawley J.R., Brewer M.M., Morris A.K., Radecki S.V., Lappin M.R. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. Journal of Feline Medicine and Surgery. 2010;12:314–321. doi: 10.1016/j.jfms.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A., Veiga I., Tavares L. Genetic diversity and phylogenetic analysis of Feline Coronavirus sequences from Portugal. Veterinary Microbiology. 2009;138:163–168. doi: 10.1016/j.vetmic.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham S.P., Graham E. Retroviral infections of small animals. Veterinary Clinics of North America Small Animal Practice. 2008;38(ix):879–901. doi: 10.1016/j.cvsm.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Fogle J.E., Tompkins W.A., Campbell B., Sumner D., Tompkins M.B. Fozivudine tidoxil as single-agent therapy decreases plasma and cell-associated viremia during acute feline immunodeficiency virus infection. Journal of Veterinary Internal Medicine. 2011;25:413–418. doi: 10.1111/j.1939-1676.2011.0699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. Journal of American Veterinary Medical Association. 1997;210:1307–1312. [PubMed] [Google Scholar]
- Gerlach N., Gibbert K., Alter C., Nair S., Zelinskyy G., James C.M., Dittmer U. Anti-retroviral effects of type I IFN subtypes in vivo. European Journal of Immunology. 2009;39:136–146. doi: 10.1002/eji.200838311. [DOI] [PubMed] [Google Scholar]
- Gerlach N., Schimmer S., Weiss S., Kalinke U., Dittmer U. Effects of type I interferons on Friend retrovirus infection. Journal of Virology. 2006;80:3438–3444. doi: 10.1128/JVI.80.7.3438-3444.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich S., Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats. Journal of Veterinary Internal Medicine. 2009;23:552–558. doi: 10.1111/j.1939-1676.2009.0303.x. [DOI] [PubMed] [Google Scholar]
- Gleich S.E., Krieger S., Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. Journal of Feline Medicine and Surgery. 2009;11:985–992. doi: 10.1016/j.jfms.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Veterinary Immunology and Immunopathology. 2011;143:190–201. doi: 10.1016/j.vetimm.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet, P., Boucraut-Baralon C. 2005. Relationship between oral calicivirus and herpesvirus carriage and palatoglossitis. In: Proceedings of the Nineteenth Annual Veterinary Dental Forum, Orlando, USA.
- Hennet P.R., Camy G.A., McGahie D.M., Albouy M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: a randomised, multi-centre, controlled, double-blind study in 39 cats. Journal of Feline Medicine and Surgery. 2011;13:577–587. doi: 10.1016/j.jfms.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., de Groot R.J., Cepica A., Egberink H.F., Horzinek M.C., Rottier P.J. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. Journal of Clinical Microbiology. 1995;33:684–689. doi: 10.1128/jcm.33.3.684-689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Radford A.D., Thiry E., Truyen U., Horzinek M.C. Feline immunodeficiency. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:575–584. doi: 10.1016/j.jfms.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Shibanai A., Tanaka S., Uchida K., Mochizuki M. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. Journal of Feline Medicine and Surgery. 2004;6:107–109. doi: 10.1016/j.jfms.2003.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz H., Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Marsilio F., Pennisi M.G., Radford A.D., Thiry E., Truyen U., Horzinek M.C. Feline leukaemia. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:565–574. doi: 10.1016/j.jfms.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli M., Kipar A., Muller C., Jenal K., Gonczi E., Borel N., Gunn-Moore D., Chalmers S., Lin F., Reinacher M., Lutz H. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (FCoV) type I and in naturally FCoV-infected cats. Journal of Feline Medicine and Surgery. 2004;6:69–81. doi: 10.1016/j.jfms.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. Interferon therapy in dogs and cats. Kleintiermedizin. 2002;8:334–337. [Google Scholar]
- Murray J.K., Roberts M.A., Skillings E., Morrow L.D., Gruffydd-Jones T.J. Risk factors for feline immunodeficiency virus antibody test status in Cats Protection adoption centres (2004) Journal of Feline Medicine and Surgery. 2009;11:467–473. doi: 10.1016/j.jfms.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J.M., Bell E.T., Hales L., Toribio J.A., White J.D., Wigney D.I., Baral R.M., Malik R. Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. Journal of Feline Medicine and Surgery. 2007;9:300–308. doi: 10.1016/j.jfms.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S., Crippa A., Comerio T., Angioletti A., Roccabianca P. Evaluation of inflammation and immunity in cats with spontaneous parvovirus infection: consequences of recombinant feline interferon-omega administration. Veterinary Immunology and Immunopathology. 2007;118:68–74. doi: 10.1016/j.vetimm.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi D., Hoover E.A., Quackenbush S.L., Mullins J.I., Callahan G.N. Selective impairment of humoral immunity in feline leukemia virus-induced immunodeficiency. Veterinary Immunology and Immunopathology. 1991;28:183–200. doi: 10.1016/0165-2427(91)90114-r. [DOI] [PubMed] [Google Scholar]
- Pedretti E., Passeri B., Amadori M., Isola P., Di Pede P., Telera A., Vescovini R., Quintavalla F., Pistello M. Low-dose interferon-alpha treatment for feline immunodeficiency virus infection. Veterinary Immunology and Immunopathology. 2006;109:245–254. doi: 10.1016/j.vetimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunological Reviews. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Thiry E., Truyen U., Horzinek M.C. Feline calicivirus infection. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:556–564. doi: 10.1016/j.jfms.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reche A., Jr., Daniel A.G., Lazaro Strauss T.C., Taborda C.P., Vieira Marques S.A., Haipek K., Oliveira L.J., Monteiro J.M., Kfoury J.R., Jr. Cutaneous mycoflora and CD4:CD8 ratio of cats infected with feline immunodeficiency virus. Journal of Feline Medicine and Surgery. 2010;12:355–358. doi: 10.1016/j.jfms.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz S., Egberink H., Hartmann K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. Journal of Veterinary Internal Medicine. 2007;21:1193–1197. doi: 10.1111/j.1939-1676.2007.tb01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio A.P., Franti C.E., Madewell B.R., Pedersen N.C. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Veterinary Immunology and Immunopathology. 1991;29:1–14. doi: 10.1016/0165-2427(91)90048-h. [DOI] [PubMed] [Google Scholar]
- Thiry E., Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Radford A.D., Truyen U., Horzinek M.C. Feline herpesvirus infection. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:547–555. doi: 10.1016/j.jfms.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins W.A. Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. Journal of Interferon & Cytokine Research. 1999;19:817–828. doi: 10.1089/107999099313325. [DOI] [PubMed] [Google Scholar]
- Truyen U., Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Radford A.D., Thiry E., Horzinek M.C. Feline panleukopenia. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2009;11:538–546. doi: 10.1016/j.jfms.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.C., Cummins J.M., Richards A.B. Low-dose orally administered alpha interferon treatment for feline leukemia virus infection. Journal of American Veterinary Medical Association. 1991;199:1477–1481. [PubMed] [Google Scholar]
- Wilhelm S., Truyen U. Real-time reverse transcription polymerase chain reaction assay to detect a broad range of feline calicivirus isolates. Journal of Virological Methods. 2006;133:105–108. doi: 10.1016/j.jviromet.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zeidner N.S., Myles M.H., Mathiason-DuBard C.K., Dreitz M.J., Mullins J.I., Hoover E.A. Alpha interferon (2b) in combination with zidovudine for the treatment of presymptomatic feline leukemia virus-induced immunodeficiency syndrome. Antimicrobial Agents and Chemotherapy. 1990;34:1749–1756. doi: 10.1128/aac.34.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]


