Abstract
Certain viruses, bacteria, fungi and parasites target dendritic cells through the interaction with the cellular attachment factor DC-SIGN, making this C-type lectin an attractive target for therapeutic intervention. Studies on DC-SIGN function would be greatly aided by the establishment of a mouse model, however, it is unclear if the murine (m) homologue of human (h) DC-SIGN also binds to pathogens. Here, we investigated the interaction of mDC-SIGN, also termed CIRE, with the Ebolavirus glycoprotein (EBOV-GP), a ligand of hDC-SIGN. We found that mDC-SIGN neither binds EBOV-GP nor enhances infection by reporterviruses pseudotyped with EBOV-GP. Analysis of chimeras between mDC-SIGN and hDC-SIGN provided evidence that determinants in the carbohydrate recognition domain and in the neck domain of mDC-SIGN inhibit a functional interaction with EBOV-GP. Moreover, mDC-SIGN was found be monomeric, suggesting that lack of multimerization, which is believed to be required for efficient pathogen recognition by hDC-SIGN, might be one factor that prevents binding of mDC-SIGN to EBOV-GP. Our results suggest that mDC-SIGN on murine dendritic cells is not an adequate model for pathogen interactions with hDC-SIGN.
Keywords: DC-SIGN, CIRE, Ebolavirus, Human immunodeficiency virus, Attachment factor, Dendritic cell
Introduction
Dendritic cells (DCs) are key to the establishment of effective innate and adaptive immune responses against invading pathogens (Banchereau and Steinman, 1998). The C-type (i.e., calcium-dependent) lectin DC-SIGN, which is expressed at high levels on DCs, might play an important role in these processes (Geijtenbeek et al., 2000b, Geijtenbeek et al., 2000c). DC-SIGN recognizes high-mannose glycans and fucose containing structures present on the surface of pathogens and self antigens (Feinberg et al., 2001, Guo et al., 2004). Binding of DC-SIGN to pathogens can lead to antigen uptake and processing for MHC presentation (Engering et al., 2002). In contrast, DC-SIGN binding to the natural ligands ICAM-2 on endothelial cells (Geijtenbeek et al., 2000a), and ICAM-3 on T-cells (Geijtenbeek et al., 2000c), might promote establishment of close cell contact, required for extravasation and antigen presentation, respectively. Despite its role as an antigen uptake receptor, a variety of pathogens, among them human immunodeficiency virus (HIV), Ebolavirus (EBOV) and dengue virus, misuse DCs via DC-SIGN to promote their dissemination in the host (van Kooyk and Geijtenbeek, 2003). The importance of DC-SIGN for pathogen spread is particularly underlined by recent reports demonstrating an association between polymorphisms in the DC-SIGN gene and susceptibility to dengue virus and HIV infection (Liu et al., 2004, Martin et al., 2004, Sakuntabhai et al., 2005).
For the analysis of DC-SIGN function and the evaluation of potential inhibitors, a mouse model for human (h) DC-SIGN on DCs is highly desirable. Therefore, the characterization of murine (m) homologues of hDC-SIGN is an important task. Baribaud and colleagues first reported the identification of a DC-SIGN variant in mice, which they termed mDC-SIGN (Baribaud et al., 2001). This molecule was shown to bind to HIV but failed to enhance infectivity for adjacent T-cells (Baribaud et al., 2001). Subsequent studies demonstrated, however, that this lectin, then termed mSIGNR1, is only one out of five murine homologues of hDC-SIGN (Parent et al., 2002, Park et al., 2001) and is expressed on macrophages but not on mDCs (Geijtenbeek et al., 2002a, Kang et al., 2003). One of the murine isoforms of hDC-SIGN was indeed shown to be expressed on mDCs and is believed to constitute the murine homologue of hDC-SIGN (Caminschi et al., 2001, Park et al., 2001). This variant is now termed mDC-SIGN or CIRE. While it has been demonstrated that mSIGNR1 binds to pathogens and to murine ICAM2 (Geijtenbeek et al., 2002a, Kang et al., 2003, Takahara et al., 2004, Taylor et al., 2004) and plays a role in the defense against pathogens (Koppel et al., 2005c, Lanoue et al., 2004), the ligands and thus the function of mDC-SIGN are unclear (Koppel et al., 2005b).
mDC-SIGN and hDC-SIGN exhibit a similar domain organization (Caminschi et al., 2001, Park et al., 2001). The N-terminus of both lectins is located in the cytoplasm and is followed by a transmembrane domain, which inserts the proteins into the cytoplasmic membrane. The extracellular domain consists of a neck region followed by a carbohydrate recognition domain (CRD) containing an EPN motif required for binding to mannose containing carbohydrates (Koppel et al., 2005b). However, also differences between mDC-SIGN and hDC-SIGN sequences are apparent. Maybe most strikingly, the 191 amino acids encompassing hDC-SIGN neck domain consists of 7.5 blocks of a repeating sequence and mediates tetramerization, which is likely required for high-avidity binding to pathogens (Feinberg et al., 2001, Mitchell et al., 2001). In contrast, the neck domain of mDC-SIGN only comprises a 29 amino acid sequence (Caminschi et al., 2001, Park et al., 2001) sharing considerable homology with that of a single repeat unit in the neck domain of hDC-SIGN, and its function is unknown. Despite similarities in expression and domain organization, mDC-SIGN and hDC-SIGN might therefore exhibit differences in the interaction with pathogens.
Here, we analyzed mDC-SIGN interactions with the glycoprotein (GP) of EBOV, a ligand of hDC-SIGN. We show that mDC-SIGN does not bind to EBOV-GP and does not enhance EBOV-GP-dependent infection. Analysis of chimeric proteins between human and mDC-SIGN revealed that determinants in the neck and CRD of mDC-SIGN prevent a functional interaction with EBOV-GP. Finally, evidence was obtained that cellular mDC-SIGN is monomeric, which might generally impede efficient binding to multivalent ligands like viral GPs.
Results
mDC-SIGN does not interact with the GPs of EBOV, severe acute respiratory syndrome coronavirus (SARS-CoV) and HIV-1
In order to analyze mDC-SIGN interactions with EBOV-GP, we thought to transiently express the lectin on 293T cells. However, in contrast to published data (Takahara et al., 2004), we found that mDC-SIGN is not expressed on 293T cells to appreciable levels (Fig. 3), while robust expression was observed on Chinese hamster ovary (CHO) cells (Fig. 3). CHO cells stably expressing mDC-SIGN or hDC-SIGN were therefore used for further analysis. In order to investigate lectin mediated enhancement of EBOV-GP driven infection, these cells were inoculated with lentiviral reporterviruses pseudotyped with the EBOV-GP of the Zaire strain (so called pseudotypes). These viruses encode the luciferase gene in place of nef and luciferase is only expressed upon successful integration of the proviral genome into the host cell chromosome. In agreement with previous results (Alvarez et al., 2002, Baribaud et al., 2002b, Simmons et al., 2003), expression of hDC-SIGN strongly enhanced EBOV-GP driven infection compared to control cells (Fig. 1A). Augmentation of infection was specific, since pretreatment with mannan, a mannose polymer produced in yeast, diminished infection to levels observed with control cells. In contrast, expression of mDC-SIGN did not enhance EBOV-GP-dependent infection (Fig. 1A). Comparable results were obtained when binding of soluble EBOV-GP to mDC-SIGN and hDC-SIGN was analyzed (Fig. 1B, upper panel), indicating that mDC-SIGN does not interact with EBOV-GP. Similarly, mDC-SIGN failed to bind to soluble SARS-CoV spike (S) protein and HIV-1 gp120 (Fig. 1C), further underlining that mDC-SIGN exhibits defects in the capture of pathogens known to bind to hDC-SIGN. Finally, induced expression of mSIGNR1 on 293 T-REx cells augmented binding of EBOV-GP (Fig. 1B, lower panel) and, in agreement with our previous results (Marzi et al., 2004), enhanced EBOV-GP-dependent infection (data not shown), indicating that hDC-SIGN and mSIGNR1, but not mDC-SIGN, function as attachment factors for pathogens.
Fig. 3.
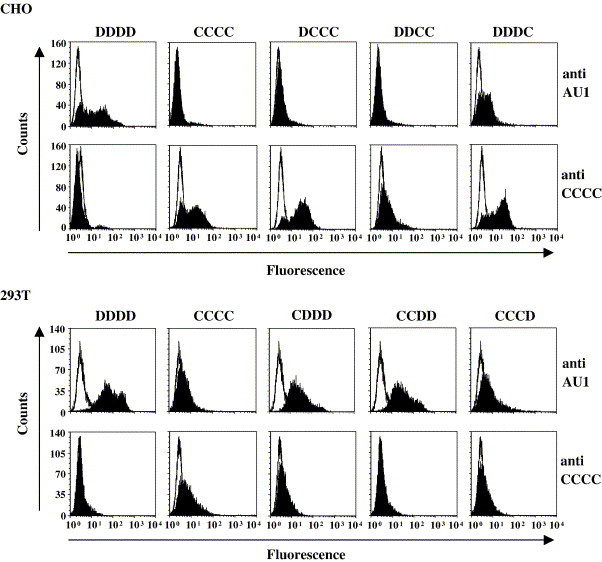
Expression of chimeric lectins. Expression of the indicated lectins on stably transfected CHO cells (upper panel) and transiently transfected 293T cells (lower panel) was analyzed by FACS. Monoclonal antibodies directed against the C-terminal AU1 antigenic tag or against the mDC-SIGN protein were used for staining as indicated. The results of a single experiment is shown, the results were confirmed in at least two independent experiments.
Fig. 1.

mDC-SIGN does not interact with EBOV-GP. (A) mDC-SIGN does not augment EBOV-GP-dependent infection. CHO cell lines stably expressing the indicated lectins or CHO control cells were preincubated with PBS or mannan and inoculated with pseudotypes bearing EBOV-GP. Luciferase activity in cell lysates was determined 3 days after infection. The results of a representative experiment are shown, similar results were obtained in two independent experiments. Error bars indicate standard deviation (SD). (B and C) mDC-SIGN does not bind to soluble viral GPs. CHO cells or 293 T-REx cells expressing the indicated lectins were incubated with culture supernatants containing chimeric proteins in which the EBOV-GP1 subunit, the SARS-CoV-S1 subunit or HIV-1 gp120 were fused to the Fc portion of human immunoglobulin, and bound protein was detected by FACS analysis. Binding to lectin-expressing cells is shown in white, while binding to control cells is shown in black. Representative experiments are presented, comparable results were obtained in two independent experiments.
Expression of mDC-SIGN-hDC-SIGN chimeras
In order to investigate why mDC-SIGN (CCCC), despite its considerable sequence homology with hDC-SIGN (DDDD), does not interact with EBOV-GP, we analyzed chimeras between both proteins. Fragments containing the cytoplasmic domain (variant CDDD), the cytoplasmic and transmembrane domain (variant CCDD) and the cytoplasmic, transmembrane and neck domain of mDC-SIGN (variant CCCD) were introduced into hDC-SIGN and vice versa (variants DCCC, DDCC, DDDC) (Fig. 2 ). A C-terminal AU1 antigenic tag was added to all chimeras in order to allow detection of expression. Expression studies demonstrated that constructs harboring at least the mDC-SIGN CRD were not appreciably expressed on the surface of 293T cells (data not shown). Therefore, CHO cells were generated that stably expressed chimeras containing the mDC-SIGN CRD. Staining with a monoclonal antibody specific for the mDC-SIGN CRD indicated that these constructs were expressed robustly and to comparable degrees, with the exception of chimera DDCC, which exhibited reduced expression (Fig. 3 , upper panel). In contrast, when an antibody reactive against the AU1 antigenic tag was used for staining, only expression of DDDC could be detected (Fig. 3, upper panel). These observations suggest that the C-terminus of mDC-SIGN (in chimeras CCCC, DCCC and DDCC) is not accessible to the anti AU1 antibody in its wild type conformation, while the introduction of the hDC-SIGN neck domain (chimera DDDC) seems to alter the spatial orientation of the mDC-SIGN CRD, thereby making its C-terminus accessible to the antibody. In turn, expression of all constructs harboring the hDC-SIGN CRD (CDDD, CCDD and CCCD) was readily detected on transfected 293T cells by staining with the anti AU1 antibody, albeit expression of CCCD was reduced (Fig. 3, lower panel). In contrast, no staining was observed with the anti mDC-SIGN antibody (Fig. 3, lower panel), which is in agreement with the specificity of this antibody for the mDC-SIGN CRD (Caminschi et al., submitted for publication).
Fig. 2.
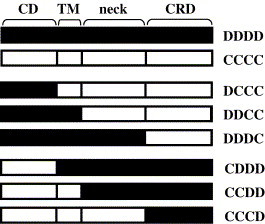
Schematic representation of the chimeras between mDC-SIGN/CIRE (“C”) and hDC-SIGN (“D”). CD, cytoplasmic domain; TM, transmembrane domain; neck, neck domain; CRD, carbohydrate recognition domain.
The neck domain and the CRD of mDC-SIGN impede the interaction with EBOV-GP
We next investigated the ability of the chimeric proteins to enhance infection by EBOV-GP bearing pseudotypes. All lectin-expressing cells analyzed were readily permissive to infection driven by the GP of vesicular stomatitis virus (VSV-G) and, in agreement with previous results (Simmons et al., 2003), lectin expression did not modulate VSV-G-dependent infection (Fig. 4 ). In contrast, hDC-SIGN but not mDC-SIGN expression efficiently and specifically augmented EBOV-GP mediated infectious entry, while all chimeras harboring the mDC-SIGN CRD did not enhance EBOV-GP-dependent infection (Fig. 4, left panel). These results suggest that the mDC-SIGN CRD does not recognize EBOV-GP and that this defect cannot be rescued by linking the CRD to the hDC-SIGN neck. Insertion of the mDC-SIGN cytoplasmic domain and transmembrane domain into hDC-SIGN was compatible with enhancement of infection, while the additional insertion of the mDC-SIGN neck abrogated augmentation of infection (Fig. 4, right panel). However, the respective variant, CCCD, was not efficiently expressed on 293T cells (Fig. 3, lower panel), and we therefore repeated the infection with a CHO cell line stably expressing this lectin to appreciable levels (Fig. 5A). Again, CCCD was defective in the interaction with EBOV-GP bearing pseudotypes (data not shown), suggesting that the mDC-SIGN neck prevents the interaction with virion-associated EBOV-GP.
Fig. 4.
The neck domain and CRD of mDC-SIGN are not compatible with enhancement of EBOV-GP-dependent infection. CHO cells stably expressing the indicated lectins (left panel) or 293T cells transiently expressing the indicated lectins (right panel) were inoculated with EBOV-GP or VSV-G harboring pseudotypes and the luciferase activity in cellular lysates was determined 3 days after infection. A representative experiment is presented, similar results were obtained in two independent experiments. Error bars indicate SD.
Fig. 5.
Replacement of the mDC-SIGN CRD with the CRD of hDC-SIGN confers binding to soluble EBOV-GP. (A) Expression of chimeric lectins. The expression of the indicated lectins on stably transfected CHO cells (white) was analyzed by FACS upon staining with hDC-SIGN (for detection of DDDD and CCCD) or mDC-SIGN (for detection of DDDC) specific antibodies. CHO cells stably transfected with empty vector served as negative controls (black). A single experiment is shown, the results are representative for at least two independent experiments. (B) Binding of soluble EBOV-GP to chimeric lectins. Binding of a control Ig protein and of soluble EBOV-GP to CHO lines stably expressing the indicated lectins (white) or control CHO cells (black) was analyzed. A representative experiment is shown, similar results were obtained in an independent experiment.
To further characterize the impact of the mDC-SIGN neck domain on the interaction with EBOV-GP, we assessed binding of soluble EBOV-GP to chimeras, in which the CRDs were exchanged between hDC-SIGN and mDC-SIGN (chimeras CCCD, DDDC). Expression of all chimeras was readily detectable on stably transfected CHO cells (Fig. 5A). However, only hDC-SIGN and, unexpectedly, chimera CCCD-expressing cells bound to EBOV-GP, while no binding to cells stably transfected with chimera DDDC was detected (Fig. 5B). These results indicate that the mDC-SIGN neck domain interferes with the interaction with virion-associated EBOV-GP trimers, but is compatible with binding to soluble EBOV-GP. In contrast, the neck domain of hDC-SIGN did not rescue EBOV-GP recognition by the mDC-SIGN CRD (Fig. 5B), indicating that the CRD is not capable of binding virion-associated or soluble EBOV-GP.
Cellular mDC-SIGN is monomeric
The neck domain of hDC-SIGN drives tetramerization of the protein, which is required for high-avidity binding to ligands displaying a high number of appropriate glycans on their surface (Feinberg et al., 2005, Mitchell et al., 2001). We therefore investigated if mDC-SIGN also forms multimers. SDS gelelectrophoresis under reducing and non-reducing conditions revealed that hDC-SIGN forms multimers (Figs. 6A, B), as expected from previous reports (Feinberg et al., 2005, Mitchell et al., 2001), while no evidence for multimerization was obtained for mDC-SIGN (Fig. 6A). Absence of multimerization was linked to the presence of the mDC-SIGN neck domain (variants DDDC, CCCD; Figs. 6A, B), suggesting that, apart of determinants in the CRD, the absence of neck domain induced mDC-SIGN clustering on the cell surface might prevent this protein from interacting with EBOV-GP.
Fig. 6.
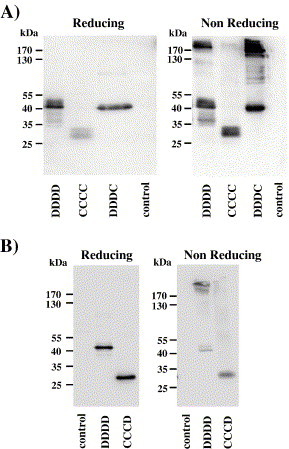
Cellular mDC-SIGN is monomeric. Lysates from CHO cells (A) or 293T cells (B) expressing the indicated lectins were separated by SDS gel-electrophoresis under reducing and non-reducing conditions, and lectin expression was analyzed by Western blot. Comparable results were obtained in four independent experiments.
Discussion
Here, we show that in contrast to hDC-SIGN, mDC-SIGN does not interact with EBOV-GP and other viral GPs. Determinants which inhibit the interaction were mapped to the neck domain and the CRD. Moreover, evidence was obtained that cellular mDC-SIGN is monomeric, which might generally hamper efficient recognition of multivalent ligands. Our results suggest, that mDC-SIGN expressed on murine DCs is not suitable as a model for hDC-SIGN function.
hDC-SIGN and the related lectin DC-SIGNR (also termed L-SIGN) (Bashirova et al., 2001, Pöhlmann et al., 2001c) are encoded by adjacent genes located on chromosome 19p13 and share 77% amino acid sequence identity. Although hDC-SIGN exhibits an extended carbohydrate specificity compared to DC-SIGNR (Guo et al., 2004), both lectins recognize high-mannose glycans and interact with much the same ligands (Baribaud et al., 2002a, van Kooyk and Geijtenbeek, 2003). However, DC-SIGNR is not expressed on DCs, but has been detected in the endothelium of liver and lymph node sinusoids as well as in placental villi (Bashirova et al., 2001, Pöhlmann et al., 2001c). DC-SIGNR might capture pathogens present in blood and lymph fluid and might promote infection of adjacent cells or of the lectin-expressing cells. Thus, hDC-SIGN and the related receptor DC-SIGNR facilitate attachment of pathogens and might promote dissemination of pathogens in infected individuals (Pöhlmann et al., 2001a).
Albeit controversial findings regarding the importance of hDC-SIGN for pathogen interactions with DCs have been reported (Geijtenbeek et al., 2000b, Gummuluru et al., 2003, Wu et al., 2002), several recent studies strongly support an important function of hDC-SIGN in pathogen spread (Arrighi et al., 2004a, Arrighi et al., 2004b, Hu et al., 2004). Maybe most strikingly, a polymorphism in the hDC-SIGN promoter has been shown to modulate the susceptibility to primary dengue virus infection and the risk of HIV infection upon parenteral transmission (Martin et al., 2004, Sakuntabhai et al., 2005). Similarly, a polymorphism in the hDC-SIGN neck domain was found to be associated with reduced risk of HIV infection (Liu et al., 2004). These observations underline that hDC-SIGN is an attractive target for therapeutic intervention and call for the development of small animal models to analyze DC-SIGN function and to test potential inhibitors.
Establishment of a mouse model for hDC-SIGN interactions with pathogens is particularly desirable. However, such efforts are complicated by the expression of five DC-SIGN homologues in mice, which are termed mDC-SIGN and mSIGNR1 to mSIGNR4 (Park et al., 2001). The genes for the murine isoforms of hDC-SIGN are located in the same chromosomal locus and encode proteins with a comparable domain organization (Koppel et al., 2005b). However, the neck region of these proteins is of variable length and could not be detected in mSIGNR2, which also does not contain a transmembrane domain and might be secreted (Park et al., 2001). All lectins except mSIGNR4 contain a EPN motif in the CRD, suggesting that they might bind to mannosylated ligands (Koppel et al., 2005b). Indeed, both mSIGNR1 and mSIGNR3 were found to interact with yeast-derived zymosan particles and binding was inhibited by mannan (Takahara et al., 2004), and it has been demonstrated that mSIGNR1 exhibits specificity for high-mannose carbohydrates (Galustian et al., 2004, Koppel et al., 2005a). mSIGNR1 was also shown to capture blood-borne pathogens like HIV and to interact with ICAM-2 (Baribaud et al., 2001, Geijtenbeek et al., 2002a, Takahara et al., 2004). These findings are complemented by our observation that mSIGNR1 binds to EBOV-GP (Fig. 1A) and augments EBOV-GP-dependent entry (Marzi et al., 2004), suggesting that this lectin might be a functional equivalent of hDC-SIGN. However, the expression pattern of mSIGNR1, which is found on macrophages in lymph nodes and spleen as well as on liver sinusoidal endothelial cells, resembles that of DC-SIGNR (Koppel et al., 2005b). In turn, mDC-SIGN was detected on plasmacytoid preDCs and is the only known murine DC-SIGN isoform expressed on DCs (Caminschi et al., 2001, O'Keeffe et al., 2002), indicating that mDC-SIGN is the murine homologue of hDC-SIGN. However, previous studies did not detect binding of mDC-SIGN to mannosylated ligands (Takahara et al., 2004), suggesting that the carbohydrate specificity of mDC-SIGN differs from that of hDC-SIGN. Our observation that mDC-SIGN fails to complex EBOV-GP, SARS-CoV-S and HIV-1 gp120 (Figs. 1B, C) corroborates these findings and suggests that mDC-SIGN might not function as an adhesion receptor for pathogens and is thus not suitable as a model for DC-SIGN engagement by pathogens.
Which determinants impede pathogen binding to mDC-SIGN? The finding that the introduction of the mDC-SIGN CRD into hDC-SIGN abrogates binding to EBOV-GP, while the converse exchange allows the interaction with soluble EBOV-GP (Fig. 4, Fig. 5), indicates that the mDC-SIGN CRD is not compatible with the recognition of pathogens. At present, it is unclear, however, if lack of binding of mDC-SIGN to EBOV-GP is due to the absence of the appropriate type of glycans on EBOV-GP, or if the spatial orientation of the glycans is not compatible with recognition by mDC-SIGN. In this regard it is of interest, that despite the considerable sequence identity between the CRDs of hDC-SIGN and mDC-SIGN, several amino acid exchanges are found in the mDC-SIGN sequences corresponding to the primary (Feinberg et al., 2001, Geijtenbeek et al., 2002b) and secondary (Guo et al., 2004) carbohydrate binding sites in hDC-SIGN (Fig. 7 ). Particularly, the V351L exchange in the primary binding site of mDC-SIGN might narrow the carbohydrate specificity of this lectin, since V351 in hDC-SIGN is important for the extended carbohydrate specificity of hDC-SIGN compared to DC-SIGNR (Guo et al., 2004).
Fig. 7.

The CRD of mDC-SIGN harbors amino acids exchanges in the primary and secondary ligand binding site as compared to hDC-SIGN. An alignment of the CRDs of hDC-SIGN and mDC-SIGN is shown. Triangles mark key residues in the primary and circles indicate important residues in the secondary ligand binding site of hDC-SIGN. Amino acid exchanges in the ligand binding sites of mDC-SIGN are boxed. A conserved EPN motif important for binding to mannose containing carbohydrates is marked in bold. The numbering is shown relative to that of the full length proteins.
Another determinant which might prevent mDC-SIGN from binding to EBOV-GP is the neck domain. Thus, introduction of the cytoplasmic and transmembrane domains of mDC-SIGN into hDC-SIGN was compatible with augmentation of EBOV-GP-dependent infection, while the additional introduction of the neck domain abrogated augmentation of infection (Fig. 4), suggesting that the neck domain inhibits a functional interaction with EBOV-GP bearing pseudovirions. However, the mDC-SIGN variant harboring the CRD of hDC-SIGN was still capable of binding to soluble EBOV-GP (Fig. 5). This discrepancy might have several reasons. For one, virion-associated and soluble EBOV-GP might exhibit slight differences in glycosylation or exposure of carbohydrate moieties. Second, it can formally not be excluded that the expression of the respective variant was insufficient for augmentation of infection, but allowed binding to soluble GP. Third, the defect in multimerization exhibited by this variant (Fig. 6B), which is most likely caused by the neck domain, might prevent functional interactions with virion associated GPs resulting in augmentation of infection, but might be compatible with capture of soluble GP. Thus, the 191 amino acids comprising neck domain of hDC-SIGN consists of repeating sequences that drive tetramerization, which is critical for the efficient interaction with ligands modified with a high number of appropriate glycans (Feinberg et al., 2005, Mitchell et al., 2001). In contrast, the neck domain of mDC-SIGN consists only of 29 amino acids, does not contain repetitive elements and does not drive tetramerization (Fig. 6A), indicating that the neck domain of mDC-SIGN might impede efficient binding to multivalent ligands by failing to mediate lectin multimerization. In this regard it is of interest that mSIGNR1 was shown to form oligomers (Kang et al., 2003), further substantiating the importance of lectin multimerization for pathogen capture. Finally, a negative role of the mDC-SIGN neck domain in pathogen binding might be explained by inadequate spacing of the CRD. Thus, it has been suggested that the neck domain of hDC-SIGN projects the lectin domain about 200Å over the cellular membrane and thereby prevents binding to ligands present in the same membrane (Feinberg et al., 2005). The small neck domain of mDC-SIGN, however, might orient the CRD in a way that allows binding to ligands present in the same cellular membranes, which might explain the absence of binding to pathogens.
In summary, we provided evidence that mDC-SIGN is not an attachment factor for pathogens and that features of the neck domain and CRD might impede binding to multivalent ligands. Further studies are required to define the natural ligands and function of mDC-SIGN and to attain these goals the generation and characterization of mDC-SIGN knock-out mice would be particularly helpful.
Materials and methods
Cell culture
293T cells were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin. 293 T-REx cells expressing DC-SIGN and mSIGNR1 were described previously (Marzi et al., 2004, Pöhlmann et al., 2001b) and maintained in DMEM medium containing 10% FBS, 50 μg/ml zeocin (Invitrogen, CA, USA), 2.5 μg/ml blasticidin (Invitrogen, CA, USA), penicillin and streptomycin. Expression was induced by culturing the cells in medium containing 0.1 μg/ml doxycycline (SIGMA-Aldrich, Germany). 293 T-REx parental cells were maintained in the same medium as lectin-expressing cell lines, however, no zeocin was added. Chinese Ovary Hamster-KI cells (CHO) were cultivated in RPMI 1640 medium containing 10% FBS, penicillin and streptomycin. Lectin-expressing CHO cells were generated by transfection of CHO cells with pcDNA3.1 Zeo (Invitrogen, CA, USA) plasmids harboring the lectin ORFs employing FuGENE6 transfection reagent (Roche, IN, USA) according to the manufacturer's instructions. Transfectants were allowed to recover for 24 h before selection with 250 mg/ml zeocin (Invitrogen, CA, USA) commenced. Three days later, the concentration of zeocin was increased to 500 mg/ml and the cells were maintained until outgrowth of resistant cells was observed. The selected cells were analyzed for lectin expression by fluorescence activated cell sorting (FACS) as described below and cell populations expressing high levels of lectin were isolated and expanded. All cells were grown at 37 °C and 5% CO2.
Plasmid construction and in vitro mutagenesis
Expression plasmids encoding hDC-SIGN were described previously (Pöhlmann et al., 2001b). To construct mDC-SIGN expression vectors, the ORF of mDC-SIGN (GeneBank file AY049062) was amplified by PCR and cloned into pcDNA3.1Zeo (Invitrogen, CA, USA). Chimeras between hDC-SIGN and mDC-SIGN were generated by overlap extension PCR mutagenesis. For convenient detection of lectin expression, carboxy-terminal AU1 antigenic tags were added to the ORFs by PCR mutagenesis. The GP expression plasmids employed for the generation of pseudotyped viruses have been described previously (Simmons et al., 2003). For expression of soluble EBOV-GP, the extracellular part of the GP ORF was fused to the Fc part of human IgG1 via PCR and cloned in frame with the amino-terminal murine IgG kappa signal peptide in the eukaryotic expression vector pAB61 (Birkmann et al., 2001). All PCR amplified sequences were confirmed by automated sequence analysis.
Analysis of lectin mediated enhancement of EBOV-GP-dependent infection
Lentiviral pseudotypes bearing the GP of the EBOV subspecies Zaire or VSV-G were generated as described elsewhere (Simmons et al., 2003). In brief, 293T cells were transiently cotransfected with pNL4-3 E−R− Luc (Connor et al., 1995) and an expression plasmid for EBOV-GP or VSV-G using the calcium phosphate method. The culture medium was changed after 16 h and then harvested 48 h post transfection. The supernatants were passed through 0.4 μm filters, aliquotted and stored at −80 °C. Lectin mediated enhancement of viral infection was assessed employing CHO transfectants or transiently transfected 293T cells. To analyze the impact of lectin expression on EBOV-GP or VSV-G driven infection, the indicated cell lines were seeded in 96-well plates and infected with the indicated pseudotypes normalized for comparable infectivity. After overnight incubation the infection medium was changed and the cells cultivated for 3 days. Subsequently, the cells were lysed and luciferase-activities determined using a commercially available kit (Promega, MA, USA).
Analysis of lectin expression by flow cytometry
To assess cell surface expression of lectins, fluorescence activated cell sorting analysis (FACS) was performed. Transfected CHO cells, 293T cells or doxycycline-induced T-REx cell lines were harvested, washed and resuspended in ice-cold FACS buffer (PBS with 3% FBS; 0.01% NaN3). Approximately 2 × 105 cells were incubated with either a monoclonal antibody (MAb) specific for the AU1 tag (Covance, CA, USA), or MAb 526 specific for hDC-SIGN (Baribaud et al., 2002b) at a final concentration of 10 μg/ml or rat MAb 5H10 (Caminschi et al., submitted for publication) raised against a peptide derived from the mDC-SIGN lectin domain in a total volume of 100 μl FACS buffer for 45 min on ice. Cells were washed and incubated with phycoerythrin-conjugated anti-mouse IgG or fluorescein-conjugated anti-rat IgG (both from Vector Laboratories, CA, USA) at a final concentration of 5 μg/ml for 45 min on ice. Thereafter, the cells were washed, reconstituted in FACS buffer and analyzed by FACS employing a FACScalibur flow cytometer (Becton Dickinson).
Binding of soluble viral glycoproteins to lectin-expressing cells
Soluble EBOV-GP-Ig or control-Ig were obtained from supernatants of 293T cells transiently expressing these proteins. The fusion proteins were concentrated by employing Centricon Plus-20 centrifugal filters (Millipore, USA). To measure binding to lectin expressing or parental CHO cells, comparable amounts of soluble protein, as judged by Western blot analysis, were incubated with the different cell lines for 45 min on ice. After washing with FACS buffer, cells were stained with Cyan5-conjugated anti-human IgG (Jackson ImmunoResearch, USA) at a final concentration of 150 μg/ml for 45 min on ice. Cells were then washed, reconstituted in FACS buffer and analyzed by flow-cytometry using a FACScalibur flow cytometer (Becton Dickinson).
Western blot analysis of lectin expression
Lysates from CHO cells stably expressing the indicated lectins or control CHO cells were generated under reducing and non-reducing conditions. For reducing conditions samples were dissolved in sodium dodecyl sulphate (SDS)-Laemmli buffer containing β-mercaptoethanol and boiled for 15 min at 95 °C, whereas under non-reducing conditions, cells were lysed in 10 mM Tris–HCl, 1 mM EDTA, 1 mM PMSF and 0.5% TritonX100 for 60 min at 4 °C and diluted in SDS-Laemmli buffer without β-mercaptoethanol. Samples were separated via SDS gel-electrophoresis and transferred onto nitrocellulose membranes (Schleicher and Schüll, Germany). The indicated lectins were detected by staining with AU1 specific MAb (1 μg/ml final concentration) and a peroxidase-conjugated anti-mouse IgG (Jackson ImmunoResearch, USA), concentrated 0.3 μg/ml. Chemiluminescence detection was performed using a commercially available kit according to the manufacturer's protocol (ECL Western detection kit; Amersham Pharmacia, Germany).
Acknowledgments
We thank B. Fleckenstein and K. von der Mark for constant support. T.G., A.W., H.H. and S.P. were supported by SFB 466.
References
- Alvarez C.P., Lasala F., Carrillo J., Muniz O., Corbi A.L., Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi J.F., Pion M., Garcia E., Escola J.M., van Kooyk Y., Geijtenbeek T.B., Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi J.F., Pion M., Wiznerowicz M., Geijtenbeek T.B., Garcia E., Abraham S., Leuba F., Dutoit V., Ducrey-Rundquist O., van Kooyk Y., Trono D., Piguet V. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 2004;78:10848–10855. doi: 10.1128/JVI.78.20.10848-10855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Baribaud F., Doms R.W., Pöhlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets. 2002;6:423–431. doi: 10.1517/14728222.6.4.423. [DOI] [PubMed] [Google Scholar]
- Baribaud F., Pöhlmann S., Leslie G., Mortari F., Doms R.W. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 2002;76:9135–9142. doi: 10.1128/JVI.76.18.9135-9142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribaud F., Pöhlmann S., Sparwasser T., Kimata M.T., Choi Y.K., Haggarty B.S., Ahmad N., Macfarlan T., Edwards T.G., Leslie G.J., Arnason J., Reinhart T.A., Kimata J.T., Littman D.R., Hoxie J.A., Doms R.W. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 2001;75:10281–10289. doi: 10.1128/JVI.75.21.10281-10289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Eilering J.B., Martin M.P., Wu L., Martin T.D., Viebig N., Knolle P.A., KewalRamani V.N., van Kooyk Y., Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmann A., Mahr K., Ensser A., Yaguboglu S., Titgemeyer F., Fleckenstein B., Neipel F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 2001;75:11583–11593. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I., Lucas K.M., O'Keeffe M.A., Hochrein H., Laabi Y., Brodnicki T.C., Lew A.M., Shortman K., Wright M.D. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8alpha(−) splenic dendritic cells. Mol. Immunol. 2001;38:365–373. doi: 10.1016/s0161-5890(01)00067-0. [DOI] [PubMed] [Google Scholar]
- Caminschi, I., Corbett, A.J., Lucas, K.M., Sofi, M., Vremec, D., Gramberg, T., Pöhlmann, S., Curtis, J., Handman, E., van Dommelen, S.L.H., Fleming, P., Degli-Esposti, M.a., Shortman, K., Wright, M.D., submitted for publication. Functional comparison of mouse CIRE/mouseDC-SIGN and human DC-SIGN.
- Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Engering A., Geijtenbeek T.B., van Vliet S.J., Wijers M., Van Liempt E., Demaurex N., Lanzavecchia A., Fransen J., Figdor C.G., Piguet V., van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Mitchell D.A., Drickamer K., Weis W.I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Guo Y., Mitchell D.A., Drickamer K., Weis W.I. Extended neck regions stabilize tetramers of the receptors DC-SIGN and DC-SIGNR. J. Biol. Chem. 2005;280:1327–1335. doi: 10.1074/jbc.M409925200. [DOI] [PubMed] [Google Scholar]
- Galustian C., Park C.G., Chai W., Kiso M., Bruening S.A., Kang Y.S., Steinman R.M., Feizi T. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int. Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A., van Vliet S.J., van Duijnhoven G.C., Grabovsky V., Alon R., Figdor C.G., van Kooyk Y. DC-SIGN–ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R., Figdor C.G., van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Torensma R., van Vliet S.J., van Duijnhoven G.C., Adema G.J., van Kooyk Y., Figdor C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Groot P.C., Nolte M.A., van Vliet S.J., Gangaram-Panday S.T., van Duijnhoven G.C., Kraal G., van Oosterhout A.J., van Kooyk Y. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Krieger E., Vriend G., Figdor C.G., van Kooyk Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 2002;277:11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- Gummuluru S., Rogel M., Stamatatos L., Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 2003;77:12865–12874. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Feinberg H., Conroy E., Mitchell D.A., Alvarez R., Blixt O., Taylor M.E., Weis W.I., Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- Hu Q., Frank I., Williams V., Santos J.J., Watts P., Griffin G.E., Moore J.P., Pope M., Shattock R.J. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 2004;199:1065–1075. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.S., Yamazaki S., Iyoda T., Pack M., Bruening S.A., Kim J.Y., Takahara K., Inaba K., Steinman R.M., Park C.G. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- Koppel E.A., Ludwig I.S., Appelmelk B.J., van Kooyk Y., Geijtenbeek T.B. Carbohydrate specificities of the murine DC-SIGN homologue mSIGNR1. Immunobiology. 2005;210:195–201. doi: 10.1016/j.imbio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Koppel E.A., van Gisbergen K.P., Geijtenbeek T.B., van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell. Microbiol. 2005;7:157–165. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- Koppel E.A., Wieland C.W., Berg V.C., Litjens M., Florquin S., Kooyk Y.V., Poll T.V., Geijtenbeek T.B. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur. J. Immunol. 2005;35:2962–2969. doi: 10.1002/eji.200526216. [DOI] [PubMed] [Google Scholar]
- Lanoue A., Clatworthy M.R., Smith P., Green S., Townsend M.J., Jolin H.E., Smith K.G., Fallon P.G., McKenzie A.N. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J. Exp. Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Hwangbo Y., Holte S., Lee J., Wang C., Kaupp N., Zhu H., Celum C., Corey L., McElrath M.J., Zhu T. Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J. Infect. Dis. 2004;190:1055–1058. doi: 10.1086/423209. [DOI] [PubMed] [Google Scholar]
- Martin M.P., Lederman M.M., Hutcheson H.B., Goedert J.J., Nelson G.W., van Kooyk Y., Detels R., Buchbinder S., Hoots K., Vlahov D., O'Brien S.J., Carrington M. Association of DC-SIGN promoter polymorphism with increased risk for parenteral, but not mucosal, acquisition of human immunodeficiency virus type 1 infection. J. Virol. 2004;78:14053–14056. doi: 10.1128/JVI.78.24.14053-14056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A., Gramberg T., Simmons G., Moller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pöhlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A., Fadden A.J., Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- O'Keeffe M., Hochrein H., Vremec D., Caminschi I., Miller J.L., Anders E.M., Wu L., Lahoud M.H., Henri S., Scott B., Hertzog P., Tatarczuch L., Shortman K. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent S.A., Zhang T., Chrebet G., Clemas J.A., Figueroa D.J., Ky B., Blevins R.A., Austin C.P., Rosen H. Molecular characterization of the murine SIGNR1 gene encoding a C-type lectin homologous to human DC-SIGN and DC-SIGNR. Gene. 2002;293:33–46. doi: 10.1016/s0378-1119(02)00722-9. [DOI] [PubMed] [Google Scholar]
- Park C.G., Takahara K., Umemoto E., Yashima Y., Matsubara K., Matsuda Y., Clausen B.E., Inaba K., Steinman R.M. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Pöhlmann S., Baribaud F., Doms R.W. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 2001;22:643–646. doi: 10.1016/s1471-4906(01)02081-6. [DOI] [PubMed] [Google Scholar]
- Pöhlmann S., Baribaud F., Lee B., Leslie G.J., Sanchez M.D., Hiebenthal-Millow K., Munch J., Kirchhoff F., Doms R.W. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 2001;75:4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhlmann S., Soilleux E.J., Baribaud F., Leslie G.J., Morris L.S., Trowsdale J., Lee B., Coleman N., Doms R.W. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuntabhai A., Turbpaiboon C., Casadémont I., Chuansumrit A., Lowhnoo T., Kajaste-Rudnitski A., Kalayanarooj S.M., Tangnararatchakit K., Vasanawathana S., Chaiyaratana W., Yenchitsomanus P., Suriyaphol P., Avirutnan P., Chokephaibulkit K., Matsuda F., Yoksan S., Jacob Y., Lathrop G.M., Malasit P., Desprès P., Julier C. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Grogan C.C., Vandenberghe L.H., Baribaud F., Whitbeck J.C., Burke E., Buchmeier M.J., Soilleux E.J., Riley J.L., Doms R.W., Bates P., Pöhlmann S. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Takahara K., Yashima Y., Omatsu Y., Yoshida H., Kimura Y., Kang Y.S., Steinman R.M., Park C.G., Inaba K. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int. Immunol. 2004;16:819–829. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Brown G.D., Herre J., Williams D.L., Willment J.A., Gordon S. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J. Immunol. 2004;172:1157–1162. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., Geijtenbeek T.B. DC-SIGN: escape mechanism for pathogens. Nat. Rev., Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Wu L., Bashirova A.A., Martin T.D., Villamide L., Mehlhop E., Chertov A.O., Unutmaz D., Pope M., Carrington M., KewalRamani V.N. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1568–1573. doi: 10.1073/pnas.032654399. [DOI] [PMC free article] [PubMed] [Google Scholar]


