Abstract
SARS coronavirus (SARS-CoV) administered intranasally and intratracheally to rhesus, cynomolgus and African Green monkeys (AGM) replicated in the respiratory tract but did not induce illness. The titer of serum neutralizing antibodies correlated with the level of virus replication in the respiratory tract (AGM>cynomolgus>rhesus). Moderate to high titers of SARS-CoV with associated interstitial pneumonitis were detected in the lungs of AGMs on day 2 and were resolving by day 4 post-infection. Following challenge of AGMs 2 months later, virus replication was highly restricted and there was no evidence of enhanced disease. These species will be useful for the evaluation of the immunogenicity of candidate vaccines, but the lack of apparent clinical illness in all three species, variability from animal to animal in level of viral replication, and rapid clearance of virus and pneumonitis in AGMs must be taken into account by investigators considering the use of these species in efficacy and challenge studies.
Keywords: Replication, SARS, Respiratory tract
Introduction
Severe acute respiratory syndrome (SARS) was recognized in late 2002, and by the end of the outbreak in July 2003 more than 8000 cases and 774 deaths were attributed to SARS worldwide (Kuiken et al., 2003). A new coronavirus, termed the SARS coronavirus (SARS-CoV), was isolated from patients with SARS (Drosten et al., 2003, Ksiazek et al., 2003, Poutanen et al., 2003). Soon after the disease was recognized, the ability to experimentally infect and induce interstitial pneumonitis in cynomolgus macaques with SARS-CoV was demonstrated, thus fulfilling Koch's postulates and confirming that SARS-CoV was the causative agent of SARS (Fouchier et al., 2003, Kuiken et al., 2003). Efforts to develop an animal disease model for SARS have continued. BALB/c mice have been established as a good rodent model for the replication of SARS-CoV (Subbarao et al., 2004) and viral replication has also been demonstrated in ferrets and cats (Martina et al., 2003). Although pigs and chickens could be experimentally infected with SARS-CoV, these species did not support efficient virus replication (Weingartl et al., 2004). In addition to the importance of establishing the microbial etiology of a disease, animal models are critical to the study of disease pathogenesis and the evaluation of treatment and control strategies. Non-human primates have been useful in evaluating vaccines and studying disease pathogenesis for several respiratory viruses including influenza, respiratory syncytial virus, and human parainfluenza viruses (Crowe et al., 1993, Durbin et al., 2000, Murphy et al., 1982, Rimmelzwaan et al., 2001, Skiadopoulos et al., 2002). These studies demonstrated that the susceptibility of different monkey species to specific respiratory viruses is variable. However, assessment of the level of virus replication in the respiratory tract of these models has been very informative.
The primary purpose of this study was to identify the most permissive non-human primate species that could serve as a reproducible and informative model for the evaluation of SARS vaccines and immunotherapy. We evaluated the level of virus replication and serologic response to infection with SARS-CoV in three species of Old World monkeys.
Results
Comparison of level of replication of SARS-CoV in three species of nonhuman primates
We first undertook a comparison of the level of replication of SARS-CoV administered via the respiratory route to three species of Old World monkeys (4 African Green, 4 rhesus and 4 cynomolgus monkeys). Clinical signs of the febrile respiratory illness that defined SARS in humans or afebrile respiratory tract illness were not observed in any of the monkeys following administration of 106.3 tissue culture infectious doses (TCID50) of SARS CoV. Rectal temperatures of all monkeys remained in the normal range (98–102 °F) for the duration of the study with the exception of one African Green monkey (AGM) that had a fever of 105 °F on day 3.
The level of replication of SARS-CoV in the upper and lower respiratory tract of monkeys of all the three species detected from combined nose and throat (NT) swabs and tracheal lavage (TL) samples is presented in Fig. 1 . The level of viral replication in AGMs was greater than that in cynomolgus monkeys, which was greater than that in rhesus monkeys. Mean peak virus titers in AGMs were 103.1 and 103 TCID50/ml in the upper and lower respiratory tract, respectively (panels E and F).
Fig. 1.
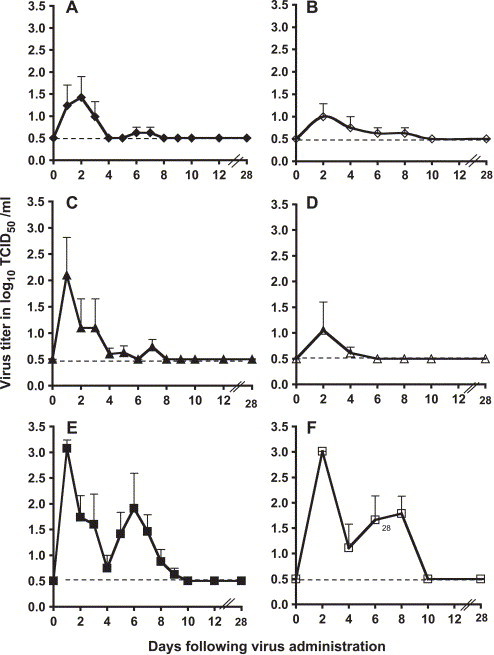
SARS-CoV replication in the respiratory tract of monkeys. Mean titers of virus (expressed as log10 TCID50/ml of sample; y axis) detected on indicated days (x axis) in the upper respiratory tract (left panels, A, C, and E, closed symbols) and lower respiratory tract (right panels, B, D, and F, open symbols) of rhesus (panels A and B, ◆, ◇), cynomolgus (panels C and D, ▴, ▵), and African Green (panels E and F, ■, □) monkeys following intranasal and intratracheal administration of 106 TCID50 of SARS-CoV. Error bars associated with each data point indicate standard error, and the dotted line indicates the lower limit of detection of virus (100.5 TCID50/ml).
Plasma, urine, and fecal specimens from rhesus monkeys and AGMs did not yield infectious virus but viral genome was detected by reverse-transcriptase polymerase chain reaction (RT-PCR) from fecal samples of the four SARS-infected AGMs between days 8 and 20 (data not shown). Laboratory evaluations in AGMs revealed two findings that were not observed in rhesus or cynomolgus monkeys and were suggestive of systemic infection and may warrant further investigation. First, the hemoglobin concentrations and hematocrit of the four AGMs decreased by a mean of 25% by days 8–10 following infection and returned to baseline by day 27. The total white blood cell and platelet counts did not demonstrate a similar pattern. Second, liver enzyme elevations (alanine amino transferase and isocitrate dehydrogenase) were noted in the serum of two AGMs with peak levels at day 4 post-infection; these values returned to baseline by day 27.
Serologic response to SARS-CoV infection
Serum IgG antibody titers against recombinant baculovirus-expressed spike (S) protein of SARS-CoV were measured in an ELISA assay (Table 1 ). Four-fold rises in titer were detected in all monkeys following primary infection but titers in pre-infection (day 0) sera ranged from 1:640 to 1:2560, suggesting that although the ELISA assay detected four-fold rises following infection, it also detected some pre-existing cross-reactive antibodies.
Table 1.
Serum neutralizing and SARS-S specific ELISA IgG antibody responses following intranasal and intratracheal administration of SARS-CoVa
| Monkey |
Serum antibody titer on indicated day |
||||
|---|---|---|---|---|---|
| Neutralizing Ab |
ELISA Ab |
||||
| Species | ID # | Day 0 | Day 28 | Day 0 | Day 28 |
| Rhesus | 97 | <1:8b | 1:37c | 1:640 | 1:10240 |
| 9R | <1:8 | 1:18 | 1:640 | 1:2560 | |
| 8V | <1:8 | 1:17 | 1:640 | 1:2560 | |
| 6K | <1:8 | 1:36 | 1:2560 | 1:10240 | |
| Cynomolgus | 18 | <1:8 | 1:32 | 1:640 | 1:10240 |
| 1806 | <1:8 | <1:8 | 1:640 | 1:2560 | |
| 396 | <1:8 | 1:16 | 1:640 | 1:10240 | |
| 972 | <1:8 | 1:71 | 1:160 | 1:40960 | |
| African Green | V130 | <1:8 | 1:35 | 1:2560 | 1:40960 |
| V131 | <1:8 | 1:64 | 1:2560 | 1:10240 | |
| V240 | <1:8 | 1:64 | 1:640 | 1:10240 | |
| V304 | <1:8 | 1:64 | 1:640 | 1:10240 | |
Monkeys received 1 ml each of 106 TCID50 of SARS-CoV by intranasal and intratracheal administration on day 0 and sera were collected before virus administration and 28 days later.
Sera were tested starting with a dilution of 1:8. Serum samples that did not neutralize virus infectivity at the starting dilution were assigned a titer of 1:4 in determining four-fold rises.
Four-fold rises in titer are indicated in bold type.
All monkeys were seronegative (titer < 1:8) for SARS-specific neutralizing antibodies (Nt Ab) prior to virus administration (Table 1). The mean titers of Nt Ab achieved on day 28 in the three species of monkeys correlated with the levels of virus replication in the respiratory tract; the mean titer was 1:27 in rhesus monkeys, 1:31 in cynomolgus monkeys, and 1:57 in African Green monkeys (Table 1). In our assay, the mean neutralizing antibody titer in post-infection sera from mice was 1:25 to 1:49 (Subbarao et al., 2004) and the titer in a convalescent human serum sample was 1:71 (unpublished data). Serologic evidence of infection, defined as a four-fold rise in Nt Ab titer, was observed in 4 of 4 rhesus, 3 of 4 cynomolgus, and 4 of 4 AGMs.
Virologic and histopathologic findings at necropsy in SARS-CoV infected African Green monkeys
Although the study described above indicated that all three species of monkeys were infected with SARS-CoV, there were significant discrepancies between our findings and published reports of cynomolgus macaques infected with SARS-CoV; Kuiken et al. (2003) reported clinical illness and pathologic evidence of disease in cynomolgus macaques. We did not euthanize the monkeys in our first study, so we were unable to determine whether there were any histologic changes associated with virus replication. In order to resolve these differences, we performed an additional study in six AGMs, where four AGMs received the same dose (106.3 TCID50) of SARS-CoV by intranasal and intratracheal administration and two were mock-infected. Two infected and 1 mock-infected animal were euthanized and necropsied on day 2 and the remaining two infected and one mock-infected animals were euthanized and necropsied on day 4. Combined NT swabs were collected daily and TL fluids were collected every other day till the animals were euthanized, so that we could determine how accurately NT swabs and TL samples reflected the level of virus replication in the lungs. At necropsy, trachea, nasal turbinates, and 12 to 14 samples from each animal's lungs were assayed for virus titration and histopathology and samples from the liver and spleen were examined for histopathologic changes.
In three of four SARS-CoV-infected AGMs, virus titers in TL samples did not accurately reflect the titer of virus present in tracheal or lung tissue (Table 2 ) and consistently higher titers of virus were seen in lung, tracheal, or nasal turbinate tissue homogenates than in NT swabs or TL samples (Table 2). Moderate (AGM ID #443) to high (AGM ID #400) titers of virus were present in the tissue samples from the right lung on day 2, but the detection of virus was more patchy and titers were significantly lower on day 4 (Table 2). Virus titers in samples from the right lung were higher than in samples from the left lung, presumably because the right main stem bronchus is straighter and more of the intratracheally administered virus inoculum reached the right lung. There was no difference between the titer of virus in samples collected from the hilar region compared to those from the periphery of the lungs (data not shown).
Table 2.
Comparison of virus titers in NT swabs, TL fluids, and tissue samples collected at necropsy from the respiratory tract of AGMsa
| Monkey ID # | Sacrificed on indicated day post-infection | Virus titer in upper respiratory tractb |
Virus titer in lower respiratory tractb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT swab on days |
Nasal turbinate tissue at necropsy | TL on days |
Tracheal tissue at necropsy | Right lungc |
Left lungc |
||||||||
| 1 | 2 | 3 | 4 | 2 | 4 | Titer | # samples positive/total | Titer | # samples positive/total | ||||
| 400 | 2 | 1.5 | <0.5d | 5.5 | <0.5d | 3.5 | 7.2 ± 0.29 | 8/8 | 2.8 ± 0.78 | 3/4 | |||
| 443 | 2 | <0.5d | <0.5d | 3.7 | 3.2 | 4.2 | 4.0 ± 0.36 | 8/8 | 2.9 ± 0.41 | 5/6 | |||
| 420 | 4 | 1 | 3.0 | 2.2 | <0.5d | 4.0 | <0.5d | <0.5d | 2.5 | 2.3 ± 0.36 | 5/8 | 2.2 ± 0.36 | 3/6 |
| 568 | 4 | 1.5 | 1 | 2.0 | <0.5d | 3.7 | <0.5d | <0.5d | 4.0 | 3.3 ± 0.47 | 7/8 | ≤1.5 ± 0d | 0/6 |
Four AGMs received 106.3 TCID50 of SARS-CoV by intranasal and intratracheal administration on day 0.
Virus titers are expressed as log10 TCID50/ml from secretions and as log10 TCID50/g from 10% w/v tissue homogenates.
Titers in lung are expressed as mean ± standard error for 4–8 samples obtained from each lobe of the lung.
Virus was not detected; the lower limit of detection in tissue homogenates was 101.5 TCID50/g and in NT swabs and TL was 100.5 TCID50/ml.
Microscopic examination of lungs of AGMs sacrificed on day 2 post-infection revealed focal interstitial mononuclear inflammatory infiltrates (Fig. 2 ) and edema in the lung. Immunohistochemical assays (IHC) showed focal distribution of viral antigens in tracheal and bronchiolar epithelial cells, pneumocytes (Figs. 3A–C), and macrophages on day 2 post-infection. IHC with double staining confirmed that the majority of SARS-CoV-antigen-positive cells were type I pneumocytes (co-labeled with cytokeratin and not co-labeled with surfactant, Fig. 4A) and some scattered macrophages (co-labeled with antibodies to CD68, Fig. 4B). The degree of inflammation and the amount of viral antigen were significantly reduced in the lungs of animals sacrificed 4 days post-infection compared to findings on day 2 post-infection (data not shown). Prominent histopathologic changes and immunostaining were not observed in liver and spleen on day 2 or day 4 post-infection.
Fig. 2.
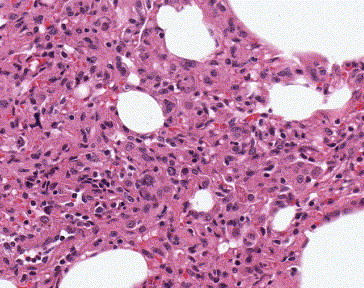
Histopathological findings in AGM lung 2 days post-infection. Histopathologic examination of lungs shows edema and interstitial mononuclear inflammatory infiltrates. Hematoxylin and eosin stain; original magnification: ×100.
Fig. 3.

Immunohistochemical staining for SARS-CoV antigens in AGM tissues obtained 2 days post-infection. SARS-CoV antigens stained red were detected in: (A) tracheal epithelial cells; (B) bronchiolar epithelial cells; (C) pneumocytes. Immunoalkaline phosphatase staining, naphthol fast red substrate with light hematoxylin counterstain; original magnification: A, B: ×158; C: ×100.
Fig. 4.

Immunohistochemical double staining to localize SARS-CoV antigens in AGM tissues obtained 2 days post-infection. (A) SARS-CoV antigen and cytokeratin in pneumocytes in AGM lungs 2 days post-infection. Red stain: SARS-CoV; brown stain: cytokeratin; original magnification: ×158. (B) SARS-CoV and CD68 antigens in alveolar macrophage. Red stain: SARS-CoV; brown stain: CD68; original magnification: ×158. Double-stain IHC with immunoalkaline phosphatase and peroxidase polymer.
Response of AGMs previously infected with SARS-CoV to re-challenge
The ability of primary infection to prevent re-infection was evaluated in the AGMs. The four AGMs from the first study were challenged with the same dose (106.3 TCID50) of SARS-CoV administered via the intratracheal and intranasal route 2 months following primary infection. This study was performed before we established that virus titer in tissue homogenates exceeded titers seen in respiratory secretions, so only NT swabs and TL were collected from these animals following challenge. Virus replication was highly restricted in the respiratory tract upon challenge; virus was not isolated from the upper respiratory tract of any monkey and was isolated at a single time point (day 2) at the lowest detectable titer in the lower respiratory tract of 2 AGMs (ID #240 and #304). The possibility that this low titer represents residual virus inoculum cannot be ruled out. The AGMs had measurable Nt Ab present prior to challenge, but two of the AGMs (ID #131 and #240) developed a four-fold rise in titer (data not shown) suggesting that, although virus replication was restricted in the respiratory tract upon challenge, at least two monkeys were re-infected.
Discussion
We compared the responses of three species of Old World monkeys to experimental infection with SARS-CoV and established that although clinical illness was not present in any of the three species, the rhesus, AGMs and all but one cynomolgus monkey could be infected. SARS-CoV replicated to a higher titer and for a longer time in the respiratory tract of AGMs than in rhesus or cynomolgus monkeys, and the Nt Ab response correlated with the level of virus replication detected in the respiratory tract. Consistently higher titers of virus were seen in tissue homogenates at necropsy than in NT swabs or TL fluid collected before the animals were sacrificed, indicating that more virus was present in the tissues than in secretions. This observation is consistent with the clinical finding that severe lung disease can occur early in SARS cases, at a time when virus is not easily detected in respiratory secretions (Peiris et al., 2003). Histopathologic examination from AGM lungs revealed interstitial pneumonitis in association with SARS-CoV on day 2 that was resolving by day 4. IHC staining for SARS-CoV antigens and double staining showed that tracheal and bronchial epithelial cells and type 1 pneumocytes were involved early in infection. These findings are consistent with our findings in mice and hamsters infected with SARS-CoV, where antigen is detected in epithelial cells early in infection (Roberts et al., 2004, Subbarao et al., 2004). Primary infection protected AGMs from re-infection with SARS-CoV.
These findings are consistent with those reported by Kuiken et al. and Haagmans et al., in appearance and patchiness, though we see more evidence of pneumonitis at an earlier time point post-infection than reported by Kuiken et al. (2003) or Haagmans et al. (2004) (day 2 instead of day 6 or 4, respectively). Kuiken et al. (2003) also found that one of four macaques did not have detectable virus in the lungs at day 6. The key difference that remains unresolved between our findings and those of Kuiken et al. is that we did not observe lethargy, skin rash, or respiratory distress in cynomolgus macaques (or rhesus or AGMs) infected with the Urbani strain of SARS-CoV. Kuiken et al. (2003) infected cynomolgus monkeys by administration of a Hong Kong SARS-CoV strain into the nose, trachea, and conjunctiva, while our studies were done with the Urbani strain administered into the nose and trachea. Possible reasons that could account for the differences in our findings and those reported by Kuiken et al. (2003) in cynomolgus monkeys are differences in the strains of SARS-CoV used and differences in the subspecies of monkeys in the two studies. Both strains of SARS-CoV were isolated from fatal human cases and subsequently underwent a similar number of passages in Vero cells. However, it is possible that the strain used in the studies of Kuiken et al. is more virulent for cynomolgus monkeys; this question can be resolved by evaluating both strains in parallel.
There are concerns about the risk of enhanced disease on re-exposure to SARS, as has been described with feline infectious peritonitis virus infection (FIPV) in cats, where accelerated and enhanced disease can occur on re-exposure to FIPV in seropositive cats (Pederson and Boyle, 1980, Weiss and Scott, 1981a, Weiss and Scott, 1981b). Infection of macrophages by FIPV is believed to be important in the pathogenesis of accelerated disease. SARS-CoV primarily infects epithelial cells in the lungs of AGMs but there is evidence of infection in some macrophages as well that warrants further study. However, the lack of clinical illness and active viral replication on challenge of AGMs is reassuring. We followed the AGMs for 2 months after challenge and saw no evidence of enhanced disease and no evidence of virus replication in the lungs, liver, kidneys, spleen, or intestines at necropsy 2 months following challenge. These observations are consistent with our findings in mice and hamsters, where primary infection with SARS-CoV confers resistance to reinfection and we have not detected evidence of enhanced disease upon re-exposure to SARS-CoV (Roberts et al., 2004, Subbarao et al., 2004).
We have carefully considered the optimal use of these three non-human primate species in the context of other available animal models for SARS including mice, hamsters, and ferrets. Serologic data from our study confirm that non-human primates will be useful for vaccine immunogenicity studies (Gao et al., 2003) and our virologic data and histopathologic findings indicate that AGMs can be used for vaccine efficacy studies (Bukreyev et al., 2004). SARS-CoV titers in lung tissue homogenates were high in one AGM and moderately high in the other at day 2 but were significantly lower in both animals sampled on day 4. An early peak of viral replication, seen in the respiratory tract of AGMs, has also been reported in mice (Subbarao et al., 2004), ferrets, and cats (Martina et al., 2003). It is not entirely clear how these kinetics compare with those in humans because the only report of viral load in respiratory secretions of SARS patients was based on samples from subjects who were treated with steroids and ribavirin (Peiris et al., 2003). AGMs support replication of SARS-CoV, with associated evidence of pneumonitis for at least 2 days but both viral infection and pneumonitis are patchy and not as consistent as the corresponding findings in experimentally infected mice and hamsters (Roberts et al., 2004, Subbarao et al., 2004). The animal-to-animal variability, rapid clearance of virus from the lungs, and rapid resolution of pneumonitis in AGMs are considerations that preclude this species from being the ideal model for the evaluation of the efficacy of vaccines against viral challenge.
Materials and methods
Virus and cells
Drs. L. J. Anderson and T. G. Ksiazek from the Centers for Disease Control and Prevention (CDC), Atlanta, GA kindly provided the SARS-CoV (Urbani strain) used in this study (Ksiazek et al., 2003). The virus was isolated and passaged twice in Vero E6 cells at the CDC and was passaged twice in Vero cells in our laboratory. The Vero cells were maintained in OptiPro SFM (Invitrogen, Carlsbad, CA). All in vitro manipulations with infectious virus were performed inside a biosafety cabinet, in a biosafety containment level 3 facility, and personnel wore powered air purifying respirators (3M HEPA AirMate, Saint Paul, MN) as previously described (Subbarao et al., 2004).
Virus titers in secretions and tissues were determined in Vero cell monolayers as previously described (Subbarao et al., 2004) and are expressed as TCID50/ml or TCID50/g and the lower limits of detection were 100.5 TCID50/ml and 101.5 TCID50/g, respectively.
Animal studies
The NIH Animal Care and Use Committee approved the monkey studies that were carried out in an animal biosafety level 3 facility. All personnel entering the facility wore powered air-purifying respirators (3M HEPA AirMate, Saint Paul, MN). Four juvenile African Green monkeys (Cercopithecus atheiops or Chlorocebus sabeus), four rhesus monkeys (Macaca mulata) and four cynomolgus monkeys (Macaca fasicularis) were inoculated intranasally and intratracheally with 1 ml of 106 TCID50 of SARS-CoV in each site.
NT samples from the upper respiratory tract were collected daily from day 0 (prior to inoculation) to day 10 and on days 12, 14, 20, 23, and 27 post-inoculation. TL samples from the lower respiratory tract were collected on days 2, 4, 6, 8, 10, 14, 20, and 27 post-inoculation. Feces, plasma, and urine samples were collected on days 2, 4, 6, 8, 10, 14, 20, and 27 post-inoculation. Duplicate samples were flash frozen and stored at −70 °C until all samples were available for virus titration. NT, TL, plasma, urine and fecal samples were assayed for presence of infectious SARS-CoV. Piperacillin (Sigma Aldrich Co., St. Louis, MO), gentamicin (Invitrogen, Grand Island, NY) and amphotericin B (Quality Biological, Gaithersburg, MD) were added to the tissue culture medium at final concentrations of 0.4, 0.1, 5 mg/L, respectively, when determining virus titers from monkey samples.
Monkeys were observed daily for signs of clinical illness from day 0 to day 10, and on days 12, 14, 20, 23, and 27 post-inoculation. Rectal temperatures of monkeys were recorded, and blood was collected on days 0, 2, 4, 6, 8, 10, 14, 20, and 28 for a complete blood count and alanine amino transferase (ALT), isocitrate dehydrogenase (ICD), and gamma glutamyl transferase (GGT) levels.
To determine whether primary infection protected monkeys from subsequent challenge, AGMs were inoculated intranasally and intratracheally with 106 TCID50 of SARS-CoV 2 months after the end of the primary infection study. NT and TL were collected and titered as described above for primary infection.
The rhesus and cynomolgus monkeys were euthanized 2 months after primary infection and the AGMs were euthanized 2 months following challenge infection and tissue samples were obtained from liver, spleen, kidneys, and lungs for virus isolation.
An additional study was undertaken in AGMs in order to determine whether the replication of SARS-CoV was associated with histopathologic changes in the lungs and to compare the level of virus replication in lung tissue with that seen in NT swabs and TL fluid. Four AGMs were inoculated intranasally and intratracheally with 106 TCID50 of SARS-CoV, and two were mock infected with Leibovitz 15 medium (Invitrogen) on day 0. Two days later, two SARS-CoV infected and one mock-infected AGM were euthanized and the remaining two SARS-CoV infected and one mock-infected AGM were euthanized on day four. Monkeys were observed daily for signs of clinical illness, rectal temperatures were recorded, and blood was collected on days 0, 2, and 4 for a complete blood count and a determination of the level of liver enzymes. NT swabs were collected daily and TL fluids were collected every other day till the animals were euthanized. At necropsy, trachea, nasal turbinates, and tissue blocks of 1 cm3 from the hilar and peripheral regions of each lobe of the lungs were frozen and assayed for virus titration. Similar lung tissue blocks were fixed in 2.5% glutaraldehyde for electron microscopy and the remaining tissues were placed in formalin for histopathologic examination. Additional tissue samples that were collected for histopathologic examination include liver, spleen, kidney, heart, bone marrow, and intestines.
RNA extraction from fecal samples
Fecal samples from AGM and cynomolgus monkeys were thawed and 0.1 to 0.2 g of feces was suspended in sterile phosphate buffered saline for a final 10% w/v solution. RNA was extracted as per manufacturer's protocol (Qiagen Viral RNA Kit, Valencia, CA) from clarified supernatants and stored at −80 °C. Fecal samples from rhesus monkeys were not tested.
Reverse transcriptase PCR for SARS-CoV
Reverse transcription and real-time PCR analysis was performed using the Stratagene Brilliant Plus Two-Step Quantitative RT-PCR Core Reagent Kit as per manufacturer's protocol on a MX4000 thermocycler (Stratagene, LaJolla, CA). Primers and TaqMan probe (synthesized by Qiagen) were used to amplify and detect a small 70-bp fragment located in the SARS CoV N-ORF (Accession number AY278741). The sequences of the primers used were as follows: forward: 5′-CCG AAG AGC TAC CCG ACG-3′; reverse: 5′-GAA GTA CCA TCT GGG GCT GAG-3′ and probe 5′-Hex-CTC TTT CAT TTT GCC GTC ACC ACC AC-BHQ1-3′. Samples from which the 70-bp fragment was consistently amplified in two or more independent assays (each performed in duplicate or triplicate) were reported as positive for detection of SARS-CoV viral RNA.
Neutralizing antibody assay
Serum samples were assayed for the presence of neutralizing antibody in a microneutralization assay starting with a dilution of 1:8. The serum dilution that completely neutralized infectivity of 100 TCID50 of virus was calculated by the method of Reed and Muench as described previously (Subbarao et al., 2004). Serum samples that did not neutralize virus infectivity at the starting dilution were assigned a titer of 1:4 to determine four-fold rises in titer.
ELISA antibody assay
Serial four-fold dilutions of heat-treated sera were assayed for the presence of IgG antibodies against the spike (S) protein of SARS-CoV in an ELISA assay. Serum dilutions beginning at 1:40 were applied to wells of Immulon 1B plates (Dynex Technologies, Inc., Chantilly, VA) that were coated with 40 ng per well of purified recombinant baculovirus-expressed SARS S protein (Protein Sciences, Inc. Meridian, CT). The second antibody used was rabbit anti-monkey IgG at a dilution of 1:30,000 (ICN, Aurora, OH) and the ELISA was developed using alkaline phosphatase-labeled goat anti-rabbit antibody at 1:8000 (ICN) followed by substrate. The ELISA antibody titer recorded was the serum dilution at which the optical density reading at 405 nm exceeded 0.2 and exceeded the reading from wells that were not coated with antigen by two-fold.
Immunohistochemistry
A colorimetric immunoalkaline phosphatase IHC method was used as previously described (Subbarao et al., 2004). Double-stain IHC was performed by using peroxidase polymer (DakoCytomation Inc., Carpinteria, CA) labeled antibodies against cytokeratin, surfactant, or CD68, followed by the mouse anti-SARS-CoV antibody labeled with immunoalkaline phosphatase polymer.
Statistics
Log transformed virus titers were compared in a two-tailed t test and statistical significance was assigned to differences with P values <0.05.
Acknowledgments
We thank Dr. Randy Elkins for advice and assistance in procuring animals and thank Tammy Tobery, Steve Harbaugh, Kenny Stockman and Josh Moore from Bioqual Inc. for expert technical assistance in the primate studies.
References
- Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L., Elkins W.R., St. Claire M., Murphy B.R., Subbarao K., Collins P.L. Mucosal immunization of nonhuman primates with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J.E., Jr., Collins P.L., London W.T., Chanock R.M., Murphy B.R. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine. 1993;11(14):1395–1404. doi: 10.1016/0264-410x(93)90168-w. [DOI] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Durbin A.P., Elkins W.R., Murphy B.R. African green monkeys provide a useful nonhuman primate model for the study of human parainfluenza virus types-1,-2, and-3 infection. Vaccine. 2000;18(22):2462–2469. doi: 10.1016/s0264-410x(99)00575-7. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stohr K., Osterhaus A.D. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423(6937):240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Tamin A., Soloff A., D'Aiuto L., Nwanegbo E., Robbins P.D., Bellini W.J., Barratt-Boyes S., Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A.M., Rimmelzwaan G.F., Amerongen G.V., Riel D.V., Jong T.D., Itamura S., Chan K.-H., Tashiro M., Osterhaus A.D.M.E. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., Group S.W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A.M., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K.S., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S.M., Osterhaus A.D.M.E. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina B.E.E., Haagmans B.L., Kuiken T., Fouchier R.A.M., Rimmelzwaan G.F., Amerongen G.V., Peiris J.S.M., Lim W., Osterhaus A.D.M.E. SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B.R., Hinshaw V.S., Sly D.L., London W.T., Hosier N.T., Wood F.T., Webster R.G., Chanock R.M. Virulence of avian influenza A viruses for squirrel monkeys. Infect. Immun. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson N.C., Boyle J.F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am. J. Vet. Res. 1980;41:868–876. [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., Group H.U.S.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J., National Microbiology Laboratory, C, Canada and Canadian Severe Acute Respiratory Syndrome Study Team Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Kuiken T., Amerongen G.V., Bestebroer T.M., Fouchier R.A.M., Osterhaus A.D.M.E. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 2001;75(14):6687–6691. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Vogel L., Guarner J., Hayes N., Murphy B., Zaki S., Subbarao K. SARS coronavirus infection of golden Syrian hamsters. J. Virol. 2004 doi: 10.1128/JVI.79.1.503-511.2005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiadopoulos M.H., Tatem J.M., Surman S.R., Mitcho Y., Wu S.L., Elkins W.R., Murphy B.R. The recombinant chimeric human parainfluenza virus type 1 vaccine candidate, rHPIV3-1cp45, is attenuated, immunogenic, and protective in African green monkeys. Vaccine. 2002;20(13):1846–1852. doi: 10.1016/s0264-410x(02)00038-5. [DOI] [PubMed] [Google Scholar]
- Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.-J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of SARS coronavirus in the respiratory tract of mice. J. Virol. 2004;78(7):3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H.M., Copps J., Derebot M.A., Marszal P., Smith G., Gren J., Andonova M., Pasick J., Kitching P., Czub M. Susceptibility of pigs and chickens to SARS coronavirus. Emerging Infect. Dis. 2004;10(2):179–184. doi: 10.3201/eid1002.030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Pathogenesis of feline infectious peritonitis: nature and development of viremia. Am. J. Vet. Res. 1981;42:382–390. [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Pathogenesis of feline infectious peritonitis: pathologic changes and immunofluorescence. Am. J. Vet. Res. 1981;42:2036–2048. [PubMed] [Google Scholar]


