Graphical abstract
Keywords: Arenes, Cyclizations, Sulfones, Regioselectivity, Silyl enol ethers
Abstract
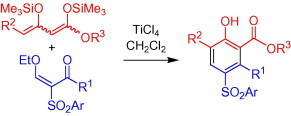
The formal [3+3] cyclization of 1,3-bis(silyloxy)-1,3-butadienes with readily available 2-arylsulfonyl-3-ethoxy-2-en-1-ones resulted in regioselective formation of 4-(arylsulfonyl)phenols.
4-(Arylsulfonyl)phenols are of considerable pharmacological relevance. This includes antibacterial activity,1 inhibition of phospholipidase A2,2 inhibition of catechol O-methyltransferase,3 inhibition of dihydropteroate synthase of Escherichia coli,4 hypolipidemic activity,5 cytotoxicity against HeLa cells and the antipicornavirus,6 neuropeptide Y1 receptor binding activity,7 anti-HIV activity,8 anticholesteremic activity,9 binding to human muscarinic M1 and M2 receptors,10 histamine H3-receptor antagonistic activity,11 antiprotozoal activity,12 binding to neuroblastoma cells,13 binding to the human cannabinoid CB1 receptor,14 and inhibition of the main protease of the recombinant SARS coronavirus.15
Diaryl sulfones are available by oxidation of diaryl sulfides. For example, 4-(phenylsulfonyl)anisole has been prepared by oxidation of 4-(phenylthio)anisole with hydrogen peroxide.16 An alternative approach to this compound relies on the aluminum(III)chloride-mediated reaction of anisole with phenylsulfonic acid chloride.17 However, this approach suffers from the formation of a regioisomeric mixture, which is difficult to be separated. The reaction of phenol with benzenesulfonic acid has been reported to give 4-(phenylsulfonyl)phenol.18 However, no yield was given, and the reaction required harsh conditions (240 °C). Recently, an efficient approach to 4-(phenylsulfonyl)phenol, based on the CuI/proline-mediated reaction of aryl iodides with sodium benzenesulfinate, has been reported.19 4-(Phenylsulfonyl)anisole has been prepared by Suzuki reaction of 4-methoxybenzeneboronic acid with phenylsulfonic acid chloride.20 Recently, its synthesis by Cu(OAc)2-catalyzed reaction of 4-methoxybenzeneboronic acid with sodium benzenesulfinate in the presence of 1,10-phenanthroline and oxygen has been reported.21 All of these reactions rely on the coupling of two arene moieties. The application of these reactions to the synthesis of sterically encumbered or functionalized products can be difficult in some cases. In addition, the synthesis of the required starting materials, functionalized arenes, can be a tedious task.
Chan and coworkers were the first to report22 the TiCl4-mediated [3+3] cyclization23 of 1,3-bis(trimethylsilyloxy)-1,3-butadienes24 with 3-silyloxy-2-en-1-ones, which allows a convenient synthesis of salicylates. In recent years, the application of this methodology to the synthesis of various functionalized arenes has been reported.23 Herein, we report, for the first time, the synthesis of 4-(arylsulfonyl)phenols by [3+3] cyclocondensations of 1,3-bis(silyloxy)-1,3-butadienes with 2-arylsulfonyl-3-ethoxy-2-en-1-ones. These reactions allow a convenient and regioselective access to functionalized 4-(arylsulfonyl)phenols, which are not readily available by other methods. In contrast to the C–S coupling reactions that are outlined above, the method reported herein involves the formation of one of the two arene moieties by formation of two C–C bonds.
1,3-Bis(silyloxy)-1,3-butadienes 3a–g were prepared from the corresponding β-ketoesters in two steps.22 2-Arylsulfonyl-3-ethoxy-2-en-1-ones 2a–d were prepared, following a known procedure,25 by reaction of β-ketosulfones 1a–d with triethyl orthoformate and acetic anhydride (Scheme 1 ).
Scheme 1.
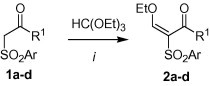
Synthesis of 2a–d. Reagents and conditions: (i) 1a–d (1.0 equiv), HC(OEt)3 (1.2 equiv), Ac2O, reflux, 2 h.
The TiCl4-mediated cyclization of 2a with 3a afforded the novel 4-(arylsulfonyl)phenol 4a in up to 80% yield (Scheme 2 ). The best yield was obtained when the reaction was carried out, in a highly concentrated solution.26 It is worth to be noted that the cyclization proceeded with excellent regioselectivity. The formation of product 4a might be explained by TiCl4-mediated attack of the terminal carbon atom of 3a onto 2a to give intermediate A, cyclization via the central carbon (intermediate B) and subsequent aromatization.
Scheme 2.
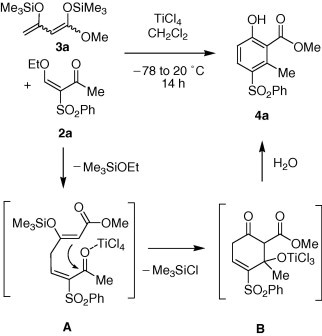
Possible mechanism of the formation of 4a.
The formal [3+3] cyclization of 2-arylsulfonyl-3-ethoxy-2-en-1-ones 2a–d with 1,3-bis(silyloxy)-1,3-butadienes 3a-g afforded the 4-(arylsulfonyl)phenols 4a–n in 45–80% yield (Scheme 3 , Table 1 ). The aryl groups located at the sulfonyl group of enones 2 have some influence on the yields. The best yields were obtained for products 4a–c derived from phenyl-substituted enone 2a. In contrast, the presence of a substituent located at carbon atom C-4 of the 1,3-bis(silyloxy)-1,3-butadiene has no significant effect on the yield. Products 4a–m, derived from enones 2a–c, contain a methyl group located at carbon C-3 of the phenol moiety. Product 4n, containing an aryl group located at C-3, was prepared from enone 2d. The yield was slightly lower than the yield of 4d (which also contains, like 4n, a tosyl group located at C-4). All products were formed with excellent regioselectivity.
Scheme 3.
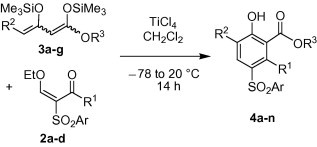
Synthesis of 4a–n.
Table 1.
Synthesis of 4a–n
| 2 | 3 | 4 | Ar | R1 | R2 | R3 | %a |
|---|---|---|---|---|---|---|---|
| a | a | a | Ph | Me | H | Me | 80 |
| a | b | b | Ph | Me | nBu | Me | 76 |
| a | c | c | Ph | Me | nHept | Me | 75 |
| b | a | d | 4-MeC6H4 | Me | H | Me | 57 |
| b | d | e | 4-MeC6H4 | Me | Me | Me | 65 |
| b | b | g | 4-MeC6H4 | Me | nBu | Me | 65 |
| b | c | h | 4-MeC6H4 | Me | nHept | Me | 60 |
| b | e | f | 4-MeC6H4 | Me | nOct | Me | 59 |
| c | a | i | 4-ClC6H4 | Me | H | Me | 47 |
| c | d | j | 4-ClC6H4 | Me | Me | Me | 48 |
| c | f | k | 4-ClC6H4 | Me | Et | Et | 47 |
| c | g | l | 4-ClC6H4 | Me | nHex | Me | 50 |
| c | e | m | 4-ClC6H4 | Me | nOct | Me | 52 |
| d | a | n | 4-MeC6H4 | 4-(O2N)C6H4 | H | Me | 45 |
Yields of isolated products.
The structures of all products were confirmed by spectroscopic methods. The structure of 4d was independently confirmed by X-ray crystal structure analysis (Fig. 1 ).27
Figure 1.
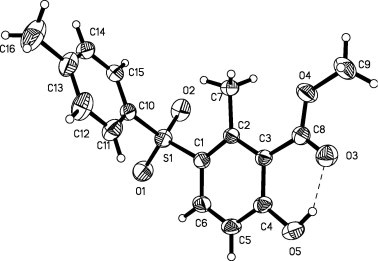
Ortep plot of 4d (30% probability level).
In conclusion, we have reported a convenient and regioselective synthesis of 4-(arylsulfonyl)phenols by what are, to the best of our knowledge, the first formal [3+3] cyclizations of 1,3-bis(silyloxy)-1,3-butadienes with 2-arylsulfonyl-3-ethoxy-2-en-1-ones. The reactions are easy to be carried out and the starting materials are readily available. We currently study the preparative scope of the methodology and applications to the synthesis of pharmacologically active products.
Acknowledgment
Financial support by the State of Mecklenburg-Vorpommern (Landesgraduierten-scholarship for M.S.) is gratefully acknowledged.
References and notes
- 1.(a) Shrimali S.S., Joshi B.C., Kishore D. J. Indian Chem. Soc. 1988;65:438. [Google Scholar]; (b) Upadhyay P.S., Vansdadia R.N., Baxi A.J. Indian J. Chem., Sect. B. 1990;29:793. [Google Scholar]
- 2.Teshirogi I., Matsutani S., Shirahase K., Fujii Y., Yoshida T. J. Med. Chem. 1996;39:5183. doi: 10.1021/jm960437a. [DOI] [PubMed] [Google Scholar]
- 3.Paulini R., Lerner C., Diederich F., Jakob-Roetne R., Zuercher G., Borroni E. Helv. Chim. Acta. 2006;89:1856. [Google Scholar]
- 4.(a) de Benedetti P.G., Iarossi D., Menziani C., Caiolfa V., Frassineti C., Cennamo C. J. Med. Chem. 1987;30:459. doi: 10.1021/jm00386a004. [DOI] [PubMed] [Google Scholar]; (b) de Benedetti P.G., Iarossi D., Folli U., Frassineti C., Menziani M.C., Cennamo C. J. Med. Chem. 1989;32:2396. doi: 10.1021/jm00130a028. [DOI] [PubMed] [Google Scholar]
- 5.Sircar I., Hoefle M., Maxwell R.E. J. Med. Chem. 1983;26:1020. doi: 10.1021/jm00361a015. [DOI] [PubMed] [Google Scholar]
- 6.Markley L.D., Tong Y.C., Dulworth J.K., Steward D.L., Goralski C.T. J. Med. Chem. 1986;29:427. doi: 10.1021/jm00153a020. [DOI] [PubMed] [Google Scholar]
- 7.Wright J., Bolton G., Creswell M., Downing D., Georgic L. Bioorg. Med. Chem. Lett. 1996;6:1809. [Google Scholar]
- 8.(a) Neamati N., Mazumder A., Zhao H., Sunder S., Burke T.R., Jr., Schultz R.J., Pommier Y. Antimicrob. Agents Chemother. 1997;41:385. doi: 10.1128/aac.41.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chan J.H., Hong J.S., Hunter R.N., Orr G.F., Cowan J.R., Sherman D.B., Sparks S.M., Reitter B.E., Andrews C.W., Hazen A., Richard J., Clair M.S. J. Med. Chem. 2001;44:1866. doi: 10.1021/jm0004906. [DOI] [PubMed] [Google Scholar]; (c) Tagat J.R., McCombie S.W., Steensma R.W., Lin S.-I., Nazareno D.V., Baroudy B., Vantuno N., Xu S., Liu J. Bioorg. Med. Chem. Lett. 2001;11:2143. doi: 10.1016/s0960-894x(01)00381-x. [DOI] [PubMed] [Google Scholar]
- 9.Stanton J.L., Cahill E., Dotson R., Tan J., Tomaselli H.C., Wasvary J.M., Stephan Z.F., Steele R.E. Bioorg. Med. Chem. Lett. 2000;10:1661. doi: 10.1016/s0960-894x(00)00309-7. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kozlowski J.A., Zhou G., Tagat J.R., Lin S.-I., McCombie S.W., Ruperto V.B., Duffy R.A., McQuade R.A., Crosby G., Taylor L.A., Billard W. Bioorg. Med. Chem. Lett. 2002;12:791. doi: 10.1016/s0960-894x(02)00023-9. [DOI] [PubMed] [Google Scholar]; (b) Wang Y., Chackalamannil S., Hu Z., Clader J.W., Greenlee W., Billard W., Binch H., Crosby G., Ruperto V., Duffy R.A., McQuade R., Lachowicz J.E. Bioorg. Med. Chem. Lett. 2000;10:2247. doi: 10.1016/s0960-894x(00)00457-1. [DOI] [PubMed] [Google Scholar]; (c) Boyle C.D., Chackalamannil S., Chen L.-Y., Dugar S., Pushpavanam P., Billard W., Binch H., Crosby G., Cohen-Williams M., Coffin V.L., Duffy R.A. Bioorg. Med. Chem. Lett. 2000;10:2727. doi: 10.1016/s0960-894x(00)00553-9. [DOI] [PubMed] [Google Scholar]; (d) Wang Y., Chackalamannil S., Chang W., Greenlee W., Ruperto V., Duffy R.A., McQuade R., Lachowicz J.E. Bioorg. Med. Chem. Lett. 2001;11:891. doi: 10.1016/s0960-894x(01)00100-7. [DOI] [PubMed] [Google Scholar]; (e) Tagat J.R., McCombie S.W., Steensma R.W., Lin S.-I., Nazareno D.V., Baroudy B., Vantuno N., Xu S., Liu J. Bioorg. Med. Chem. Lett. 2001;11:2143. doi: 10.1016/s0960-894x(01)00381-x. [DOI] [PubMed] [Google Scholar]; (f) Boyle C.D., Chackalamannil S., Clader J.W., Greenlee W.J., Josien H.B., Kaminski J.J., Kozlowski J.A., McCombie S.W., Nazareno D.V., Tagat J.R., Wang Y. Bioorg. Med. Chem. Lett. 2001;11:2311. doi: 10.1016/s0960-894x(01)00435-8. [DOI] [PubMed] [Google Scholar]; (g) Boyle C.D., Vice S.F., Campion J., Chackalamannil S., Lankin C.M., McCombie S.W., Billard W., Binch H., Crosby G., Williams M.-C., Coffin V.L. Bioorg. Med. Chem. Lett. 2002;12:3479. doi: 10.1016/s0960-894x(02)00742-4. [DOI] [PubMed] [Google Scholar]
- 11.Sasse A., Ligneau X., Sadek B., Elz S., Pertz H.H., Ganellin C.R., Arrang J.-M., Schwartz J.-C., Schunack W., Stark H. Arch. Pharm. (Weinheim Ger.) 2001;334:45. doi: 10.1002/1521-4184(200102)334:2<45::aid-ardp45>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Langler R.F., Paddock R.L., Thompson D.B., Crandall I., Ciach M., Kain K.C. Aust. J. Chem. 2003;56:1127. [Google Scholar]
- 13.Clark R.D., Jahangir A., Severance D., Salazar R., Chang T., Chang D., Jett M.F., Smith S., Bley K. Bioorg. Med. Chem. Lett. 2004;14:1053. doi: 10.1016/j.bmcl.2003.10.070. [DOI] [PubMed] [Google Scholar]
- 14.Lavey B.J., Kozlowski J.A., Hipkin R.W., Gonsiorek W., Lundell D.J., Piwinski J.J., Narula S., Lunn C.A. Bioorg. Med. Chem. Lett. 2005;15:783. doi: 10.1016/j.bmcl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Lu I.-L., Mahindroo N., Liang P.-H., Peng Y.-H., Kuo C.-J., Tsai K.-C., Hsieh H.-P., Chao Y.-S., Wu S.-Y. J. Med. Chem. 2006;9:5154. doi: 10.1021/jm060207o. [DOI] [PubMed] [Google Scholar]
- 16.Joseph J.K., Jain S.L., Sain B. Synth. Commun. 2006;36:2743. [Google Scholar]
- 17.(a) Chen D.-W., Kubiak R.J., Ashley J.A., Janda K.D. J. Chem. Soc., Perkin Trans. 1. 2001;21:2796. [Google Scholar]; (b) Marquie J., Laporterie A., Dubac J., Roques N., Desmurs J.-R. J. Org. Chem. 2001;66:421. doi: 10.1021/jo0010173. [DOI] [PubMed] [Google Scholar]; (c) Repichet S., Le Roux C., Dubac J. Tetrahedron Lett. 1999;40:9233. [Google Scholar]; (d) Hajipour A.R., Zarei A., Khazdooz L., Pourmousavi S.A., Mirjalili B.B.F., Ruoho A.E. Phosphorus, Sulfur Silicon Relat. Elem. 2005;180:2029. [Google Scholar]
- 18.Woroshzow V., Kutschkarow V. Zh. Obshch. Khim. 1949;19:1943. [Google Scholar]; Woroshzow V., Kutschkarow V. Chem. Abstr. 1950:1922. [Google Scholar]
- 19.Zhu W., Ma D. J. Org. Chem. 2005;70:2696. doi: 10.1021/jo047758b. [DOI] [PubMed] [Google Scholar]
- 20.Bandgar B.P., Bettigeri S.V., Phopase J. Org. Lett. 2004;6:2105. doi: 10.1021/ol049692c. [DOI] [PubMed] [Google Scholar]
- 21.Huang F., Batey R.A. Tetrahedron. 2007;63:7667. [Google Scholar]
- 22.(a) Chan T.-H., Brownbridge P. J. Am. Chem. Soc. 1980;102:3534. [Google Scholar]; (b) Brownbridge P., Chan T.-H., Brook M.A., Kang G.J. Can. J. Chem. 1983;61:688. [Google Scholar]
- 23.Feist H., Langer P. Synthesis. 2007:327. For a review of [3+3] cyclizations, see: [Google Scholar]
- 24.Langer P. Synthesis. 2002:441. For a review of 1,3-bis(silyl enol ethers), see: [Google Scholar]
- 25.(a) Reiter L.A. J. Org. Chem. 1984;49:3494. [Google Scholar]; (b) Lemcke T., Messinger P. Arch. Pharm. (Weinheim) 1995;328:269. [Google Scholar]
- 26.General procedure for the synthesis of 4-(arylsulfonyl)phenols4a–n: To a CH2Cl2 solution (2 mL/1 mmol of 2a–d) of 2a–d were added 3a–g (1.1 mmol) and, subsequently, TiCl4 (1.1 mmol) at 78 °C. The temperature of the solution was allowed to warm to 20 °C during 14 h with stirring. To the solution was added hydrochloric acid (10%, 20 mL) and the organic and the aqueous layer were separated. The latter was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were dried (Na2SO4), filtered and the filtrate was concentrated in vacuo. The residue was purifed by chromatography (silica gel, heptanes/EtOAc) to give 4a–n.Methyl 6-hydroxy-2-methyl-3-tosylbenzoate (4d): Starting with 2a (0.402 g, 1.5 mmol) and 3a (0.429 g, 1.65 mmol), 4d was isolated (0.274 g, 57%) as a yellowish solid (0.274 g, 57%), mp 109–110 °C. 1H NMR (250 MHz, CDCl3): δ 2.28 (s, 3H, CH3Ar), 2.47 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.87 (d, 3J = 8.4 Hz, 1H, Ar), 7.14–7.17 (m, 2H, Ar), 7.35–7.58 (m, 2H, Ar), 8.22 (d, 3J = 8.7 Hz, 1H, Ar), 11.03 (s, 1H, OH). 13C NMR (CDCl3, 75 MHz): δ 19.0, 21.5 (2 × CH3), 52.7 (OCH3), 115.1 (CCO2CH3), 115.6 (CHAr), 127.3 (2 × CHTol), 129.6 (2 × CHTol), 131.8 (CArSO2), 135.2 (CHAr), 138.9 (CArCH3), 142.6 (CTolSO2), 143.8 (CTolCH3), 165.2 (COH), 171.0 (CO). IR (KBr, cm−1): ν = 3072 (w), 3029 (w), 2953 (w), 2922 (w), 2852 (w), 1715 (w), 1673 (m), 1592 (m), 1574 (m), 1495 (w), 1435 (m), 1348 (m), 1300 (m), 1286 (m), 1218 (m), 1188 (m), 1155 (m), 1142 (s), 1109 (m), 1081 (m), 1040 (w), 1018 (w), 997 (m), 939 (m), 848 (w), 815 (m), 759 (w), 709 (m), 692 (m), 649 (m), 597 (w), 587 (m), 565 (m), 549 (m), 533 (s). GC–MS (EI, 70 eV): m/z (%) = 320 ([M]+, 34), 289 (27), 288 (100), 271 (23), 269 (18), 256 (9), 255 (48), 224 (16), 223 (20), 222 (17), 181 (10), 152 (11), 149 (12), 121 (12), 105 (10), 91 (19), 77 (22), 65 (20), 51 (14). HRMS (EI): Calcd for C16H16O5S ([M]+): 320.07130; found: 320.071076. All products gave correct elemental analyses and/or HRMS data.
- 27.CCDC-699679 contains all crystallographic details of this publication and is available free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html or can be ordered from the following address: Cambridge Crystallographic Data Centre, 12 Union Road, GB-Cambridge CB21EZ; Fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk.


