Abstract
Memory CD8+ T cells are comprised of CD122hi IL-15-dependent and CD122lo IL-15-independent subsets. Induction and retention of IL-15-independent memory CD8+ T cells was assessed in IL-15−/− and wild-type (wt) mice immunized with recombinant vaccinia virus (rVV) or Sindbis virus (rSIN) vectors expressing the identical foreign epitope. Both vectors induced epitope-specific CD8+ T cell expansion and function, independent of IL-15. Similar kinetics of rVV clearance confirmed effective CD8+ T cell function in IL-15−/− mice. CD44hi CD122hi CD8+ T cells, mainly of the CD62L−/lo phenotype, increased more dramatically and declined more rapidly in IL-15−/− mice, independent of the vector. Rapid IL-15-independent memory CD8+ T cell expansion following challenge of immune mice compensated for the limited memory CD8+ populations in IL-15−/− mice. However, despite expansion and expression of potent effector function, viral clearance was delayed in the absence of IL-15, coinciding with a rapid loss in cytolytic function.
Keywords: IL-15, CD8 T cells, Memory, Sindbis virus, Vaccinia virus
Introduction
Induction of memory CD8+ T cells constitutes an important parameter of vaccine-induced protective immunity against microbial infection. CD8+ T cell memory is influenced by the magnitude of the acute response (Ahmed and Gray, 1996, Flynn et al., 1998, Hou et al., 1994, Murali-Krishna et al., 1998), the presence of survival factors during the contraction and memory phases (Grayson et al., 2000, Jameson, 2002, Lanzavecchia and Sallusto, 2002), and by subsequent homologous or heterologous infections (Welsh and Selin, 2002). The majority of antigen primed memory cells appear to be replenished by proliferation, independent of class I interactions (Jameson, 2002, Murali-Krishna et al., 1999, Prlic et al., 2002). Prominent factors regulating CD8+ memory T cell homeostasis are IL-15 and IL-7, but their roles are not necessarily overlapping (Tan et al., 2002). A major role for IL-15 in regulating distinct lymphocyte subsets was revealed by the absence of NK cells and diminished memory CD8+ T cells in IL-15−/− mice (Kennedy et al., 2000). The inability of IL-15−/− mice to control infection with the virulent WR strain of vaccinia virus, a lytic infection controlled by CD8+ T cells (Kennedy et al., 2000), implied additional roles for IL-15 during the CD8+ T cell expansion, effector, or contraction phases. However, initial expansion of virus-specific responses is only marginally affected during vesicular stomatitis virus (VSV) infection (Schluns et al., 2002), and no defects were noted following lymphocytic choriomeningitis virus (LCMV) infection (Becker et al., 2002). Both VSV and LCMV infections were controlled and the initial virus-specific memory pool was not severely compromised. More recently, a redundant role for IL-15 during CD8 T cell expansion and virus clearance was confirmed following gammaherpes virus MHV-68 infection (Obar et al., 2004). However, longitudinal analysis in models where virus is cleared revealed a progressive loss of virus-specific memory CD8+ T cells due to defective homeostatic renewal (Becker et al., 2002, Schluns et al., 2002). The slowly dividing residual memory CD8+ T cells were nevertheless capable of recall cytokine responses and control of secondary viral challenge (Becker et al., 2002). By contrast, IL-15 was not required for homeostatic maintenance of virus-specific memory CD8 T cells following latent MHV-68 infection (Obar et al., 2004). IL-15 overexpression confirmed a positive regulatory role, specifically in memory CD8+ T cell homeostasis (Yajima et al., 2002). However, if given during a secondary response, IL-15 may have detrimental effects on preexisting, nonboosted memory T cells (Chapdelaine et al., 2003), questioning the adjuvant effect of IL-15 for vaccination purposes.
IL-15-dependent and -independent CD44hi CD8+ memory populations are not only evident following viral infection, but also in naive wt mice (Judge et al., 2002). These populations vary prominently in expression of the IL-15Rβ chain, CD122, which together with the common IL-2/IL-15R γc chain is shared by the IL-2R (Nelson and Willerford, 1998). In wt mice, IL-15-dependent CD122hi memory CD8+ T cells constitute the most prominent subset, whereas the minor IL-15-independent subset is CD122lo (Judge et al., 2002). VSV- and LCMV-specific memory CD8+ T cells in IL-15−/− mice are also severely impaired in the CD122hi subset (Becker et al., 2002, Schluns et al., 2002). Importantly, IL-15 is not required to convert naïve antigen-specific CD122lo CD8+ T cells into CD122hi cells during antigen-driven expansion/activation (Judge et al., 2002). However, a rapid decline of the CD122hi population in the absence of IL-15 is consistent with a role of IL-15 in promoting survival by up-regulating the anti apoptotic protein, Bcl-2 (Wu et al., 2002). CD122 inhibition only decreased turnover by ∼50%, suggesting an IL-15- and IL-2-independent pathway for sustaining CD8+ T cell memory (Ku et al., 2000). Indeed IL-2Rβ-deficient mice, incapable of either IL-2- or IL-15-mediated signaling, mount primary and secondary CD8+ T cell responses, albeit with limited effector function (Yu et al., 2003). IL-15-independent survival of memory CD8+ T cells may be partially attributed to IL-7 (Goldrath et al., 2002, Jameson, 2002, Prlic et al., 2002, Tan et al., 2002). However, while a clear role for IL-7 in homeostasis was not confirmed in IL-7-deficient mice, deprivation of both IL-15 and IL-7 completely inhibited homeostatic proliferation (Goldrath et al., 2002, Ku et al., 2000, Tan et al., 2002).
Distinct infections evoke unique cytokine profiles; therefore, IL-15-independent generation and survival of memory CD8+ T cell subsets was analyzed using recombinant vaccinia (rVV) and Sindbis (rSIN) viruses. These prototype DNA and RNA vectors were chosen based on their differential susceptibilities to IFNα/β and CD8+ T cell immunity. IFNα/β, a strong inducer of IL-15 severely, limits SIN replication and spread (Ryman et al., 2000), whereas CD8+ T cells control rVV infection (Binder and Kündig, 1991). Although primed by alphaviruses, CD8+ T cells do not appear to play a prominent role in providing in vivo protection (Linn et al., 1998). Nevertheless, despite the paucity of anti Sindbis virus CD8+ T cell responses (Müllbacher and Blanden, 1978), rSIN vectors efficiently prime protective CD8+ T cells to foreign microbial epitopes (Tsuji et al., 1998, Villacres et al., 2000). IL-15−/− and wt mice were infected with the respective recombinant viruses expressing the Db restricted S510 epitope derived from the murine coronavirus spike protein as an index epitope (Bergmann et al., 1996). Primary epitope-specific CD8+ T cell responses in IL-15−/− mice were similar to wt mice independent of the vector, confirming that IL-15 does not affect primary CD8+ T cell effector function (Becker et al., 2002). A dramatic increase in CD122hi CD44hi CD8+ T cells further supported the redundancy for IL-15 in expanding this phenotype. Although the rapid emergence of the CD122hi CD44hi CD8+ T cell subset was transient in the absence of IL-15, the frequency of antigen-specific memory CD8+ T cells remained similar to wt mice, independent of the type of infection. Maintenance of virus-specific memory CD8+ T cells thus appears to be independent of virus-induced factors and governed by constraints similar to those that regulate endogenous memory CD8+ T cells. The data demonstrate that rapid expansion of IL-15-independent CD8+ T cells into potent effector CD8+ T cells upon primary or secondary antigen challenge compensates for impaired IL-15-driven homeostatic maintenance of antigen-specific memory CD8+ T cells.
Results
IL-15 deficiency does not compromise antigen-specific CD8+ T cell expansion
Distinct viral infections of IL-15−/− mice revealed different outcomes in expansion of virus-specific CD8+ T cells as well as control of virus replication. Unlike vaccinia virus (Kennedy et al., 2000), acute VSV, LCMV, and MHV-68 infections were controlled, despite reduced expansion of VSV-specific CD8+ T cells in the absence of IL-15 (Becker et al., 2002, Obar et al., 2004, Schluns et al., 2002). To assess whether IL-15-independent CD8+ T cell responses are influenced by the type of viral infection, rVV or rSIN expressing the heterologous S510 indicator epitope were tested as immunogens in IL-15−/− and wt mice. No significant differences in the relative percentages of S510-specific cells within splenic CD8+ T cells from IL-15−/− and wt mice were evident during the acute response to either vJS510 or SINJS510 (Figs. 1A and B). Frequencies of tetramer+ cells within the CD8+ T cell population remained similar throughout days 6–8 during the response to vJS510. While the absolute numbers of S510-specific CD8+ T cells were also similar in IL-15−/− compared to wt mice following vJS510 infection, they were consistently reduced following SINJS510 infection (Fig. 1C). These differential effects, and the overall higher numbers of S510-specific T cells following rVV infection, likely reside in the more robust host CD8+ T cell response to rVV compared to rSIN (Villacres et al., 2000).
Fig. 1.

Primary antigen-specific CD8+ T cells in the absence of IL-15. C57BL/6 wt and IL-15−/− mice were infected intraperitoneally with either 5 × 107 PFU vJS510 or SINJS510. Splenocytes obtained at the peak of the acute responses, day 8 pi for vJS510 and day 6 for SINJS510, were stained with FITC-conjugated anti-CD8 mAb and PE-conjugated Db-S510 tetramer and compared to splenocytes from naïve controls. (A) Representative dot plots gated on CD8 T cells for one of three to four mice per infected or naïve group. Tetramer+ CD8+ T cells are circled and numbers represent their percentages within CD8+ T cells. (B) Average percentages of tetramer+ cells within the CD8+ T cell population ± SD and (C) average absolute numbers of tetramer+ CD8+ T cells per spleen ± SD (n = 3–4 mice per group). Black bars represent wt and white bars IL-15−/− mice. The data are representative of three to four independent experiments.
To confirm efficient induction of functional CD8+ T cells, the frequency of S510-specific IFN-γ producing CD8+ T cells was determined (Fig. 2A). Similar to tetramer analysis, no significant differences in percentages of CD8+ T cells secreting IFN-γ in IL-15−/− and wt mice were detected following either vJS510 or SINJS510 priming, respectively. Neither vector induced detectable ex vivo S510-specific cytolytic activity during acute infection (data not shown). However, the cytolytic potency of splenocytes from rVV-infected mice was identical in IL-15−/− and wt mice following 4 days in vitro activation (Fig. 2B) and was only slightly reduced in cultures derived from IL-15−/− mice at day 6. Thus, there was no evidence for impaired epitope-specific CD8+ T cell expansion or function following infection of IL-15−/− mice with these two distinct recombinant vectors. However, IL-15 is pivotal in regulating NK cell and NK T cell activities (Kennedy et al., 2000). In addition, IL-15 secretion by activated macrophages, dendritic cells, and stromal cells may affect other early events during viral infection (Fawaz et al., 1999, Fehniger and Caligiuri, 2000, Mattei et al., 2001, Nguyen et al., 2002). Nevertheless, rVV was cleared from ovaries of infected IL-15−/− mice with the same kinetics as in wt mice (Fig. 3 ). Therefore, the absence of IL-15 did not impair the primary antiviral response governed by CD8+ T cell immunity, as exemplified by rVV infection.
Fig. 2.

Effector functions of antigen-specific CD8+ T cells in IL-15−/− mice. Splenocytes were obtained from vJS510 or SINJS510 immunized mice as described in Fig. 1. (A) Cells were stimulated for 5–6 h in the presence of 1 μM S510 peptide and stained for surface CD8 and production of intracellular IFN-γ. Dot plots, gated on CD8 T cells, are representative of three to four individual mice. IFN-γ+ CD8+ T cells are confined by a rectangle and their percentages within CD8+ T cells indicated. IFN-γ staining of unstimulated CD8+ T cells was below 0.5%. (B) Splenocytes from vJS510 immunized mice harvested at day 5 pi were cultured in the presence of 1μM peptide S510 and tested for S510-specific cytolytic activity at day 4 (left panel) and day 6 (right panel) using S510 peptide-coated or untreated EL-4 cells as targets. Lysis of untreated targets was below 5%. Data are representative of two independent experiments.
Fig. 3.

Clearance of rVV is not impaired in IL-15−/− mice. Wt (solid squares) and IL-15−/− mice (open circles) were infected intraperitoneally with 5 × 107 PFU vJS510. Ovaries were obtained at the indicated time points and virus titers measured by plaque assays on BSC-1 cells. Each symbol represents the log-transformed virus titer of an individual mouse. The dashed line represents limit of detection. Data are representative of two experiments.
Enhanced, but transient, expansion of activated CD122hi CD44hi CD62Llo/− CD8+ T cells in the absence of IL-15
The relative percentages of total CD8+ T cells are reduced twofold in lymphoid organs of IL-15−/− compared to wt mice, despite similar cellularity (Kennedy et al., 2000). This difference cannot be solely attributed to a defect in memory CD8+ T cells, which only comprise ∼6% in IL-15−/− compared to ∼20% in wt mice (Kennedy et al., 2000). Efficient CD8+ T cell expansion and induction of S510-specific CD8+ T cells in response to vJS510 or SINJS510 in IL-15−/− mice, despite initially compromised naïve T cell numbers, thus suggested a compensatory effect during expansion. Both IL-2 and IL-15 promote T cell expansion, although these two cytokines differentially regulate CD8+ T cell differentiation (Manjunath et al., 2001). IL-2 is secreted during both viral infections (Villacres et al., 2000) and may thus exert more activity in the absence of IL-15. CD8+ T cells were analyzed for expression of the IL-2R/IL-15R β chain, CD122, in conjunction with the activation markers CD44 and CD62L. In naïve wt mice, CD122 is predominantly expressed on CD44hi and CD62Lhi CD8+ T cells (Fig. 4A). By contrast, the severe reduction of CD44hi cells coincident with reduced CD122hi CD8+ T cells in naïve IL-15−/− mice (Fig. 4A) is consistent with constitutive IL-15-driven CD122 expression in the memory CD8+ T cell subset. By contrast, low CD122 levels on naïve T cells in both groups suggests that CD122 up-regulation during infection may reflect IL-2-driven expansion in IL-15−/− mice. Indeed, CD122 was strongly up-regulated on splenic CD8+ T cells following both infections, resulting in nearly equivalent CD122hi CD8+ T cell populations in IL-15−/− and wt mice (Fig. 4B). Taking the already existing CD122hi endogenous memory population in wt mice into account, these data suggest that naïve CD8+ T cells respond more vigorously in the absence of IL-15. Enhanced CD8+ T cell activation and expansion was confirmed by both similar levels of CD44hi CD8+ T cell subsets in infected IL-15−/− compared to wt mice, irrespective of the preexisting memory pool in naïve wt mice, and more prominent CD62L down-regulation in CD8+ T cells from infected IL-15−/− mice (Fig. 4B). Finally, infected IL-15−/− mice exhibited a significantly higher proportion of CD43+ CD8+ T cells, associated with expression of CD8+ T cell effector function (Harrington et al., 2000). Analysis of the expression of five markers associated with T cell activation thus revealed enhanced CD8+ T cell responsiveness following infection of IL-15−/− mice. A substantial loss of CD62L expression and increased CD43 expression by CD8+ T cells in IL-15−/− mice was especially pronounced following rSIN infection. Overall, increased frequencies of activated CD8+ T cells following rVV compared to rSIN infection likely reflect the more virulent nature of the infection (Villacres et al., 2000).
Fig. 4.
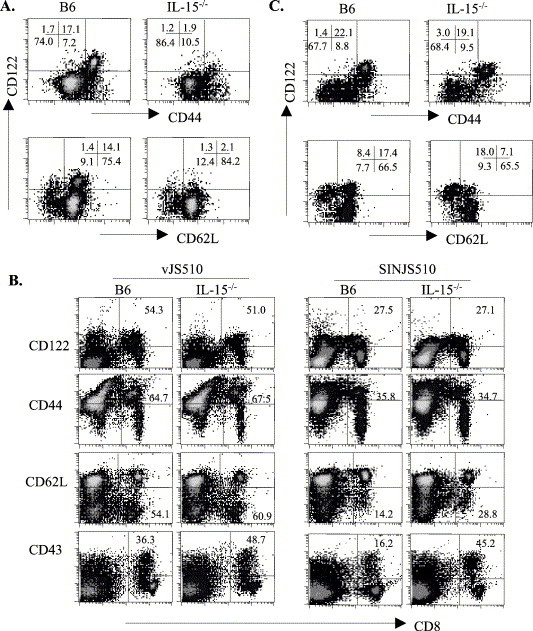
Phenotype of activated CD8+ T cells in IL-15−/− mice following primary infection. Splenocytes were obtained from either naïve (A) or infected (B and C) IL-15−/− and wt mice and stained with various combinations of anti-CD8, CD122, CD44, and CD62L mAb. Representative plots of three to four individual mice of each group are shown. (A) Three-color stains of naïve splenocytes using CyChromeC-conjugated anti-CD8, PE-conjugated anti-CD122, and FITC-conjugated CD44 (top panel) or CDL2L (bottom panel). Plots are gated on CD8+ T cells; numbers depict percentages within in each quadrant. (B) Splenocytes obtained from vJS510-infected mice at day 8 pi and SINJS510-infected mice at day 6 pi were surface stained with FITC-conjugated anti-CD8 and PE-conjugated anti-CD122, CD44, CD62L, or CD43. Plots are gated on live total lymphocytes; numbers represent the relative percentages of activated cells within the CD8+ T cell population. Similar results were obtained in three separate experiments. (C) Splenocytes obtained from SINJS510-infected mice at day 6 pi were analyzed as described above in panel A.
The CD122hi populations induced by rSIN in IL-15 mice expressed the classical CD44hi CD62Llo/− phenotype (Fig. 4C). CD44hi CD122hi CD8+ T cells comprised ∼20% of CD8+ T cells, constituting a 10-fold increase compared to naïve mice. By contrast, this population only increased slightly (from 17% to 22%) in infected wt mice. Furthermore, while the majority (72%) of CD122hi CD8+ T cells in IL-15−/− mice lacked CD62L expression, only 33% of CD122hi CD8+ T cells in wt mice expressed a CD62Llo/− phenotype (Fig. 4C). This population presumably reflects recently activated cells, as CD62L is down-regulated during activation and remains low on a substantial population of memory CD8+ T cells (Tripp et al., 1995, Wherry et al., 2003). Reversion of a subset of virus-specific memory cells to the CD62Lhi phenotype (Wherry et al., 2003) suggests that the CD122hi CD62Lhi subset in wt naïve mice (Fig. 4A) represents preestablished memory cells. The more prominent expansion/accumulation of CD8+ T cells with a classic CD44hi CD122hi CD62Llo/− activation phenotype in IL-15−/− compared to wt mice was also observed following rVV infection (data not shown).
Increased decline of CD8+ T cell responses in the absence of IL-15
IL-15 has been suggested to provide CD8+ T cells with resistance to activation-induced cell death or apoptosis (Marks-Konczalik et al., 2000, Wu et al., 2002). To determine how the absence of IL-15 influenced the CD8+ T cell contraction phase following viral infection, the relative proportion of CD44hi CD8+ T cells was measured throughout the acute response to rVV infection. Total CD8+ T cell percentages in IL-15−/− mice increased threefold by day 6 postinfection (pi), reaching levels similar to those found in wt mice (Fig. 5 ). CD8+ T cell decline was already clearly evident by day 8 pi and by day 10 pi had decreased to ∼50% of those in wt mice. The increase in total CD8+ T cells in IL-15−/− mice at day 6 pi was largely attributed to activated CD8+ T cells. Relative to naïve mice, the overall increase in activation marker expression was more prominent on CD8+ T cells derived from IL-15−/− mice. Whereas the CD44hi subset increased 20-fold in IL-15−/− mice by day 6 pi, it increased only eightfold in wt mice (Fig. 5A). The progressive decline in CD8+ T cells in IL-15−/− mice by day 12 pi correlated with an increased loss of CD44hi CD8+ T cells in IL-15−/− versus wt mice. Similar to increased CD44 expression, CD122 expression was maximal at ∼70% of CD8+ T cells by day 6 pi, independent of IL-15 (Fig. 5B). However, the CD122hi phenotype was lost more rapidly than the CD44hi phenotype in both groups. The rapid increase and subsequent decrease of CD122+ CD8+ T cells in infected IL-15−/− mice are emphasized by presenting total numbers per spleen (Fig. 5C).
Fig. 5.

Kinetics of primary CD8+ T cell responses to vJS510 infection in IL-15−/− mouse. Splenocytes from naïve and vJS510-infected wt (B6, gray bars) and IL-15−/− mice (white bars) were analyzed for CD44 (A) and CD122 (B) expression on CD8+ T cells at the indicated time points pi. Each full-length bar represents the average percentage of CD8+ T cells within total splenocytes. The portion of CD44hi CD8+ T cells or CD122hi CD8+ T cells, respectively, is indicated by striped portions in each bar. SD were below 10% of the average and are not shown. (C) Bars indicate total numbers of CD122hi CD8+ T cells per spleen ± SD (n = 3–4 mice).
Rapid reactivation of memory epitope-specific CD8+ T cells
Despite the importance of IL-15 as a survival factor for CD8+ T cell homeostatic memory (Becker et al., 2002), recent studies established a subset of IL-15-independent memory CD8+ T cells (Judge et al., 2002). The magnitude of S510-specific memory CD8+ T cell responses, and their recall responses, was thus examined in a secondary challenge model. Following either rVV or rSIN infection, the relative percentages within total CD8+ T cells were low, but not significantly different between mouse groups (Fig. 6 ). However, total S510-specific CD8+ T cells per spleen were ∼50% decreased in IL-15−/− mice compared to wt mice at 6 weeks pi, consistent with the lower frequencies of CD8+ T cells in naïve IL-15−/− mice. Furthermore, there were no significant differences in the ability of S510-specific CD8+ T cells from IL-15−/− mice to produce IFN-γ as assessed by IC staining (data not shown). Analysis of activation markers revealed a similar discrepancy in CD44hi CD122hi populations as in naïve mice. The relative populations of CD62L-/lo CD8+ T cells were slightly higher in IL-15−/− mice (data not shown).
Fig. 6.

Retention of antigen-specific memory CD8+ T cells in IL-15−/− mice. Splenocytes were obtained from wt (black bars) and IL-15−/− mice (open bars) infected with 5 × 107 PFU vJS510 or SINJS510 at least 5 weeks pi. Cells were stained with anti-CD8 mAb and tetramer as in Fig. 1. (A) Representative dot plots gated on CD8 T cells for one of three to four mice. Numbers represent the percentages of tetramer+ CD8+ T cells within CD8+ T cells as shown by the circled population. (B) Bars represent the average ± SD absolute numbers of tetramer+ CD8+ T cells per spleen (n = 3).
To assess defects in anti-viral function of memory CD8+ T cells in the absence of IL-15, rSIN primed mice were challenged with vJS510. IL-15−/− mice revealed no impairment in memory CD8+ T cell expansion compared to wt mice (Fig. 7A). Total numbers of tetramer+ CD8+ T cells were prominent by day 4 pi, comprising ∼20% and ∼35% of CD8+ T cells in wt and IL-15−/− mice, respectively. Numbers further increased slightly to day 6 pi, when tetramer percentages were maximal within the CD8 population. Total numbers remained elevated through day 10 postchallenge. Similar to the primary response, CD8+ T cell activation coincided with a more prominent increase of CD122hi cells in IL-15−/− mice. Furthermore, frequencies of antigen-specific IFN-γ secreting CD8+ T cells were similar in challenged IL-15−/− and wt mice, suggesting no defects in functional responsiveness (Fig. 7B). In contrast to the primary rVV response, secondary CD8+ T cell responses in rSIN primed mice were associated with ex vivo S510-specific cytolytic activity in both wt and IL-15−/− mice (Fig. 7C). Consistent with a higher percentage of tetramer+ CD8+ T cells in IL-15−/− mice, cytolysis was also elevated compared to wt mice at day 6 pi. Adjustment of E:T ratios to tetramer+ CD8+ T cells further indicated that enhanced cytolytic activity was not solely explicable by higher frequencies, suggesting increased cytolytic capacity at the single cell level. Nevertheless, increased cytolytic activity was transient in the IL-15−/− mice and decreased to levels below those exhibited by cells from wt mice by day 8 pi. By day 10 pi, ex vivo cytolytic activity had decreased to barely detectable levels in both groups (Fig. 7C). Despite efficient expansion and expression of effector function by memory cells in IL-15−/− mice, viral clearance was delayed compared to wt mice (Fig. 8 ). Although replication at all time points was reduced in both groups compared to naive mice (see Fig. 3), residual virus was still detectable in both ovaries of all challenged IL-15−/− mice by day 14 pi. In contrast, the majority of wt mice had cleared virus to levels below detection by day 12 pi. Similar results were obtained when mice were primed with a 10-fold higher dose of SINJS510. Thus, rapid, but transient accumulation of S510-specific CD8+ T cells upon challenge is insufficient to provide optimal viral protection in the absence of IL-15. The reduced frequency of epitope-specific memory CD8+ T cells, in addition to their apparent premature loss of cytolytic function, may contribute to the delayed viral clearance. IL-15-independent memory CD8+ T cells are thus functional but appear to have less sustained responsiveness upon secondary antigen exposure.
Fig. 7.

Functional recall responses of memory CD8+ T cells in the absence of IL-15. Wt and IL-15−/− mice primed with 5 × 107 PFU SINJS510 were challenged with 1 × 107 PFU vJS510 6 weeks post-SINJS510 immunization. (A) Reactivation of S510-specific CD8+ T cells was determined in splenocytes by tetramer staining at the indicated time points. Bars represent the average ± SD numbers of tetramer+ CD8+ T cells per spleen of individual mice (n = 3–4) and numbers above each bar indicate average percentages of tetramer+ CD8+ T cells within CD8+ T cells. (B) Representative plots of three mice per group comparing tetramer+ and IFN-γ+ CD8+ populations at the peak of the response at day 6. IC IFN-γ staining was performed as in Fig. 2. Similar results were observed in two other separate experiments. (C) S510-specific ex vivo cytolytic activity of reactivated CD8+ T cells post-vJS510 challenge. Splenocytes were directly tested for cytolysis of peptide-coated EL-4 target cells without in vitro stimulation at the indicated time points postchallenge. Solid squares represent wt and open circles IL-15−/− splenocytes. E:T ratios are presented on the x-axis based on the entire splenocyte population or calculated per tetramer+ cell as indicated by ratios above the symbols.
Fig. 8.

Delayed clearance of vJS510 in SINJS510-primed IL-15−/− mice. Mice primed with 5 × 107 PFU SINJS510 were challenged with 5 × 107 PFU vJS510 6 weeks later. Virus titers from individual ovaries were analyzed separately for each of three mice per group per time point. Average titers ± SD are shown in black bars for wt and white bars for IL-15−/− mice.
Discussion
IL-15 and IL-2 share many biological activities but play disparate roles in homeostasis of both activated and memory CD8+ T cells (Ku et al., 2000, Waldman et al., 2001). Both IL-2 and IL-15 support T cell proliferation after in vitro activation. However, IL-2 signaling induces high levels of T cell activation and expression of effector markers, whereas T cells stimulated by IL-15 resemble memory cells (Manjunath et al., 2001). While IL-2 is a growth factor, it also down-regulates highly activated CD8+ T cells by enhancing activation-induced cell death (Cousens et al., 1995, Kundig et al., 1993, Marks-Konczalik et al., 2000, Waldman et al., 2001). By contrast, IL-15 plays a minor role in CD8+ T cell expansion but contributes significantly to proliferative renewal and survival of memory CD8+ T cells (Becker et al., 2002, Schluns et al., 2002). The reduction of primary CD8+ T cell responses to VSV infection (Schluns et al., 2002), but not LCMV or MHV-68 infection (Becker et al., 2002, Obar et al., 2004) in IL-15−/− mice, suggested that the type of viral infection may influence IL-15-dependent CD8+ T cell activation. Indeed, distinct viruses induce different levels of IL-15 in human PMBC (Fawaz et al., 1999), suggesting that the immune system may have adapted to different types of viral infections by distinct IL-15 and NK cell-dependent responses.
To uncover potential IL-15-dependent differences in primary as well as secondary CD8+ T cell responses to distinct viruses, rVV and rSIN expressing the heterologous S510 CD8+ T cell epitope were tested as vaccine vectors. Similar frequencies of S510-specific cells in CD8+ splenocytes from rVV-infected IL-15−/− and wt mice support the notion that the absence of IL-15 does not impede CD8+ T cell responses to a viral infection associated with potent inflammatory responses. Although alphaviruses are strong inducers of type I interferons (Ryman et al., 2000) and thus IL-15 expression (Mattei et al., 2001, Nguyen et al., 2002), the absence of IL-15 also did not impair S510-specific CD8+ T cell induction following rSIN immunization. Nevertheless, reduced absolute numbers of antigen-specific CD8+ T cells per spleen following VSV (Schluns et al., 2002) and rSIN infection of IL-15−/− mice suggest that IL-15 may play a more prominent role in these viral infections compared to rVV and LCMV (Becker et al., 2002).
Efficient primary CD8+ T cell responses to viral infection in the absence of IL-15 coincided with a high percentage of activated CD122hi CD44hi CD62Llo/− CD8+ cells compared to wt mice. Naïve CD44lo CD8+ T cells express little CD122 in either uninfected IL-15−/− or wt mice. Therefore, substantially increased numbers of CD122hi CD8+ T cells during both infections relative to naïve mice likely reflects a compensatory mechanism. The CD122 IL-2R/IL-15R β chain is uniquely shared by IL-2 and IL-15 (Fehniger and Caligiuri, 2000, Nelson and Willerford, 1998), suggesting the possibility of enhanced IL-2 responsiveness in the absence of competing IL-15. Both rVV and rSIN induce substantial IL-2 secretion by CD4+ T cells (Villacres et al., 2000), likely promoting CD8+ T cell expansion. This notion is supported by increased IL-2R α chain (CD25) expression on CD8+ T cells derived from IL-15−/− mice (data not shown). A subset of the CD122hi population may constitute CD8+ T cells specific for the vector virus in the case of rVV; however, SIN-induced responses have not been identified in H-2b mice (Müllbacher and Blanden, 1978). The observation that the CD122hi population substantially exceeded the S510-specific population thus implies that CD122 up-regulation not only reflects virus-specific CD8+ T cells, but indicates a bystander effect, potentially enhancing proliferation of heterologous memory CD8+ T cells. Despite an apparent role for CD122 during CD8+ T cell activation, IL-2- and IL-15-independent proliferation and differentiation of CD8+ T cells were recently demonstrated using OT-1 TCR-transgenic T cells (Teague et al., 2004). Furthermore, rVV infection of mice deficient in both IL-2 and IL-15 signaling induced surprisingly effective antigen-specific cell expansion (Yu et al., 2003). Although these data substantiate cytokine redundancy in promoting CD8+ T cell activation, the absence of IL-2Rβ signaling severely impaired CD8+ T cell IFN-γ secretion (Yu et al., 2003). The redundancy of IL-15 in antigen-induced IFN-γ secretion in our results is consistent with a prominent role of IL-2, but not IL-15, in enhancing IFN-γ production during anti-viral responses (Seder et al., 1994, Su et al., 1998).
IL-2 and IL-15 have contrasting effects on antiviral CD8+ T cells during the decline phase. IL-2 induces apoptosis of activated CD8+ T cells, while IL-15 enhances resistance to apoptosis, potentially through Bcl-2 up-regulation (Marks-Konczalik et al., 2000, Wu et al., 2002). The notion that IL-15 augments differentiation into memory CD8+ T cells (Waldman et al., 2001, Yajima et al., 2002) is supported by the accelerated decline of activated CD8+ T cells in rVV-infected IL-15−/− compared to wt mice. Whether this is due to increased IL-2-driven apoptosis or the inability of IL-15 to counteract apoptosis is unclear (Wu et al., 2002). However, the disproportionately faster decline of CD122hi compared to CD44hi CD8+ T cells in IL-15−/− mice favors the latter. The presence of S510-specific memory CD8+ T cells established following either rVV or rSIN infection at 6 weeks pi in the absence of IL-15 confirmed IL-15-independent survival of a memory CD8+ T cell subset. The possibility that superior CD4+ T cell helper responses contribute to effective maintenance of antigen-specific CD8+ T cells in the absence of IL-15 was excluded based on identical activation of CD4+ T cells in both types of mice throughout infection (data not shown). Thus, it remains unclear if survival of IL-15-independent antigen-specific CD8+ T cells is mediated by IL-7, which has been implicated in maintaining both naïve and memory CD8+ T cells (Goldrath et al., 2002, Kieper et al., 2002, Prlic et al., 2002).
In summary, these results demonstrate that expansion and function of activated antigen-specific CD8+ T cells are IL-15-independent following challenge with two distinct recombinant viral vectors, rVV and rSIN. Furthermore, consistent with VSV and LCMV infections (Becker et al., 2002, Schluns et al., 2002), IL-15 was not absolutely required to establish a functional antigen-specific memory CD8+ T cell population. Efficient induction and expansion of both primary and recall CD8+ T cell responses was tightly linked with CD122 expression and down-regulation of CD62L, suggesting a transient role for IL-2 in CD8+ T cell activation in the absence of IL-15. Nevertheless, reduced protective immunity to challenge in IL-15−/− mice compared to wt mice was associated with both an accelerated loss of antigen-specific CD8+ T cells and cytolytic potency on a per cell basis. These data suggested a critical role of IL-15 in maintaining effector function in highly activated antigen-specific CD8+ T cells.
Materials and methods
Mice and infections
Homozygote C57BL/6-IL-15−/−(IL-15−/−) mice were provided by Immunex Corporation (Seattle, WA) (Kennedy et al., 2000). C57Bl/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD) at 5–6 weeks of age. Mice were housed in an accredited animal facility at the University of Southern California and were used between 6 and 8 weeks of age. For each experiment, mice were both sex- and age-matched and groups of three to four individual mice analyzed per time point. For measurement of primary responses, mice were injected intraperitoneally with either 5 × 107 PFU of rVV or rSIN expressing the S510 epitope derived from the murine coronavirus spike protein, designated vJS510 or SINJS510, respectively. For secondary responses, mice primed with 5 × 107 PFU SINJS510 were challenged with 1 × 107 or 5 × 107 PFU vJS510 6 weeks post immunization as indicated in figure legends.
Viruses and cell lines
VJS510, a rVV expressing the Db restricted S510 CD8+ T cell epitope derived from murine coronavirus, was generated and titered by plaque assay on BSC-1 cells as described previously (Bergmann et al., 1999). SINJS510 was generated by inserting hybridized oligonucleotides encoding the S510 epitope (5′ CTAGATGTGTTCTCTTTGGAATGGGC CCCATTTGTGA 3′ and 5′ CTAGTCACAAATGGGG CCCATTCCAAAGAGAACACAT 3′) into the XbaI site of the parent SIN vector pTE3′2J (Hahn et al., 1992). SINJS510 virus was generated via transfection of BHK-21 cells with in vitro transcribed mRNA from XhoI-linearized plasmid pSINJS510 and plaque assayed on BHK-21 cells (Hahn et al., 1992). Titers of rVV in ovaries were measured by plaque assay on BSC-1 monolayers as described (Binder and Kündig, 1991).
Flow cytometry
Expression of cell surface markers on splenocytes was determined as described previously (Bergmann et al., 1999, Villacres et al., 2000). Briefly, cells were blocked with purified anti-mouse CD16/CD32 (2.4G2, Pharmingen) for 15 min at 4 °C prior to staining. For three color flow cytometric analysis, cells were stained with FITC-, PE-, or CyC-conjugated mAb at 4 °C for 30 min in PBS containing 0.1% BSA using mAb specific for CD8 (53-6.7), CD4 (GK1.5), CD19 (1D3), NK cells (clone DX5), NK1.1 (PK136), CD44 (IM7), CD62L (MEL-14), and CD122 (TM-β1) (all from BD PharMingen, San Diego, CA). S510-specific CD8+ T cells were detected with PE-labeled Db-S510 class I tetramer as previously described (Bergmann et al., 1999). Stained cells were fixed with 2% paraformaldehyde and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). Forward and side scatter signals obtained in linear mode were used to establish a gate containing intact lymphocytes. A minimum of 2.5 × 105 viable cells were stained and 0.5–1 × 105 events per sample analyzed using CellQuestPro software (Becton Dickinson). Data represent the average of three to four individual mice per time point. Error bars indicate SD.
Intracellular (IC) IFN-γ staining and CTL assays
IC IFN-γ staining was performed as described (Murali-Krishna et al., 1998). Briefly, 1 × 106 spleen cells were stimulated in 200 μl of RPMI 1640 with 10% FCS, 1 μM S510 peptide, and 1 μl/ml Golgistop (BD PharMingen) for 5 h. Cells were surface-stained with anti-CD8 mAb, fixed and permeabilized with cytofix/cytoperm reagents (BD PharMingen), and finally stained with anti-IFN-γ mAb (XMG1.2) according to instructions provided by the supplier (BD PharMingen). Splenocytes were evaluated for ex vivo cytolytic activity at the indicated time points postchallenge. Briefly, EL-4 (H-2b) target cells were labeled with Na51CrO4, washed, and 1 μM S510 peptide added prior to addition of effector splenocytes at various E:T ratios. Cr51 release was determined in 100 μl of supernatant following 4 h incubation at 37 °C. Specific lysis is defined as 100 × (experimental release − spontaneous release)/(detergent release − spontaneous release). Spontaneous release values were <20% of total release in all experiments.
Acknowledgments
This work was supported by grants NS 18146 and AI 33314 from the National Institutes of Health.
We sincerely thank Dr. Mary Kennedy and Immunex (Amgen) Corporation for providing IL-15−/− mice.
References
- Ahmed R., Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Becker T.C., Wherry E.J., Boone D., Murali-Krishna K., Antia R., Ma A., Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C.C., Yao Q., Lin M., Stohlman S.A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted cytotoxic T cell response. J. Gen. Virol. 1996;77:315–325. doi: 10.1099/0022-1317-77-2-315. [DOI] [PubMed] [Google Scholar]
- Bergmann C.C., Altman J.D., Hinton D., Stohlman S.A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- Binder D., Kündig T.M. Antiviral protection by CD8+ versus CD4+ T cells: CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in anti vaccinia virus protection than CD4-dependent IL. J. Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- Chapdelaine Y., Smith D.k., Pedras-Vasconcelos J.A., Krishnan L., Sad S. Increased CD8+ T cell memory to concurrent infection at the expense of increased erosion of pre-existing memory: the paradoxical role of IL-15. J. Immunol. 2003;171:5454–5460. doi: 10.4049/jimmunol.171.10.5454. [DOI] [PubMed] [Google Scholar]
- Cousens L.P., Orange J.S., Biron C.A. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J. Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- Fawaz L.M., Sharif-Askari E., Menezes J. Up-regulation of NK-cytotoxic activity via IL-15 induction by different viruses: a comparative study. J. Immunol. 1999;163:4473–4480. [PubMed] [Google Scholar]
- Fehniger T.A., Caligiuri M.A. Interleukin 15: biology and relevance to human disease. Blood. 2000;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Goldrath A.W., Sivakumar P.V., Glaccum M., Kennedy M.K., Bevan M.J., Benoist C., Mathis D., Butz E.A. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson J.M., Zajac A.J., Altman J.D., Ahmed R. Cutting edge: increased expression of Bcl-2 in Ag-specific memory CD8+ T cells. J. Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- Hahn C.S., Hahn Y.S., Braciale T.J., Rice C.M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L.E., Galvan M., Baum L.G., Altman J.D., Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Hyland L., Ryan K.W., Portner A., Doherty P.C. Clonal burst size determines virus specific CD8+ T cell memory. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Jameson S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- Judge A.D., Zhang X., Fujii H., Surh C.D., Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R., Brasel K., Morrissey P.J., Stocking K., Schuh J.C., Joyce S., Peschon J.J. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper W.C., Tan J.T., Bondi-Boyd B., Gapin L., Sprent J., Ceredig R., Surh C.D. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.C., Murakami M., Sakamoto A., Kappler J., Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Kundig T.M., Schorle H., Bachmann M.F., Hengartner H., Zinkernagel R.M., Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Linn M.L., Mateo L., Gardner J., Suhrbier A. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J. Virol. 1998;72:5146–5153. doi: 10.1128/jvi.72.6.5146-5153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N., Shankar P., Wan J., Weninger W., Crowley M.A., Hieshima K., Springer T.A., Fan X., Shen H., Lieberman J., von Andrian U.H. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Konczalik J., Dubois S., Losi J.M., Sabzevari H., Yamada N., Feigenbaum L., Waldmann T.A., Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei F., Schiavoni G., Belardelli F., Tough D.F. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- Müllbacher A., Blanden R.V. Murine cytotoxic T-cell response to alphavirus is associated mainly with H-2Dk. Immunogenetics. 1978;7:551–561. doi: 10.1007/BF01844044. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D., Zajac A., Miller J., Slansky J., Ahmed R. Counting Ag-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Lau L.L., Sambhara S., Lemonnier F., Altman J., Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;28:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Nelson B.H., Willerford D.M. Biology of the interleukin-2 receptor. Adv. Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- Nguyen K.B., Salazar-Mather T.P., Dalod M.Y., Van Deusen J.B., Wei X.Q., Liew F.Y., Caligiuri M.A., Durbin J.E., Biron C.A. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;16:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Obar J.J., Crist S.G., Leung E.K., Usherwood E.J. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- Prlic M., Lefrancois L., Jameson S.C. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin IL-7 and IL-15. J. Exp. Med. 2002;12:F45–F52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman K.D., Klimstra W.B., Nguyen K.B., Biron C.A., Johnston R.E. Alpha/Beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S., Williams K., Ma A., Zheng X.X., Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory Ag specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Seder R.A., Germain R.N., Linsley P.S., Paul W.E. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. J. Exp. Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.C., Cousens L.P., Fast L.D., Slifka M.K., Bungiro R.D., Ahmed R., Biron C.A. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J. Immunol. 1998;160:5007–5017. [PubMed] [Google Scholar]
- Tan J.T., Ernst B., Kieper W.C., LeRoy E., Sprent J., Surh C.D. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague R.M., Tempero R.M., Thomas S., Murali-Krishna K. Proliferation and differentiation of CD8+ T cells in the absence of IL-2/15 receptor beta-chain expression or STAT5 activation. J. Immunol. 2004;173:3131–3139. doi: 10.4049/jimmunol.173.5.3131. [DOI] [PubMed] [Google Scholar]
- Tripp R.A., Hou S., Doherty P. Temporal loss of activated L-selectin-low phenotype for virus-specific CD8 memory T cells. J. Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- Tsuji M., Bergmann C.C., Takita-Sonoda Y., Murata K., Rodrigues E.G., Nussenzweig R.S., Zavala F. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J. Virol. 1998;72:6907–6910. doi: 10.1128/jvi.72.8.6907-6910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacres M.C., Zuo J., Bergmann C.C. Maintenance of CD8 T cell memory following infection with recombinant Sindbis and vaccinia viruses. Virology. 2000;270:54–64. doi: 10.1006/viro.2000.0255. [DOI] [PubMed] [Google Scholar]
- Waldman T.A., Dubois S., Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- Welsh R.M., Selin L.K. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Teichgraber V., Becker T.C., Masopust D., Kaech S.M., Antia R., von Andrian U.H., Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;43:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Wu T.S., Lee J.M., Lai Y.G., Hsu J.C., Tsai C.Y., Lee Y.H., Liao N.S. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. J. Immunol. 2002;168:705–712. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- Yajima T., Nishimura H., Ishimitsu R., Watase T., Busch D.H., Pamer E.G., Kuwano H., Yoshikai Y. Overexpression of IL-15 in vivo increases Ag-driven memory CD8+ T cells following microbe exposure. J. Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]
- Yu A., Zhou J., Marten N., Bergmann C.C., Mammolenti M., Levy R.B., Malek T.R. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J. Immunol. 2003;170:236–242. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]


