Highlights
-
•
FIV-infected cats were treated with two protocols of rFeIFN-ω (sub-cutaneous vs oral).
-
•
The cytokine profile was evaluated in FIV-cats undergoing rFeIFN-ω therapy.
-
•
There was a decrease of IL-6 mRNA expression in cats treated with the oral protocol.
-
•
There was a reduction of IL-6 plasma levels in cats treated subcutaneously.
-
•
Independently of the protocol, rFeIFN seems to reduce pro-inflammatory stimuli.
Keywords: Interferon, Feline, FIV, Immunomodulator, Cytokine
Abstract
This study assesses viremia, provirus and blood cytokine profile in naturally FIV-infected cats treated with two distinct protocols of interferon omega (rFeIFN-ω).
Samples from FIV-cats previously submitted to two single-arm studies were used: 7/18 received the licensed/subcutaneous protocol (SC) while 11/18 were treated orally (PO). Viremia, provirus and blood mRNA expression of interleukin (IL)-1, IL-4, IL-6, IL-10, IL-12p40, Interferon-γ and Tumor Necrosis Factor-α were monitored by Real-Time qPCR. Concurrent plasma levels of IL-6, IL-12p40 and IL-4 were assessed by ELISA.
IL-6 plasma levels decreased in the SC group (p = 0.031). IL-6 mRNA expression (p = 0.037) decreased in the PO group, albeit not sufficiently to change concurrent plasma levels. Neither viremia nor other measured cytokines changed with therapy. Proviral load increased in the SC group (p = 0.031), which can be justified by a clinically irrelevant increase of lymphocyte count.
Independently of the protocol, rFeIFN-ω seems to act on innate immunity by reducing pro-inflammatory stimulus.
1. Introduction
Recombinant Feline Interferon Omega (rFeIFN-ω) is an immunomodulator commonly used in feline retroviral infections (de Mari et al, 2004, Doménech et al, 2011, Gil et al, 2013). It is produced as a recombinant protein by means of a baculovirus expression vector which contains the feline interferon omega (IFN-ω) sequence (Ueda et al., 1993). This baculovirus replicates in silkworms, permitting the production of the glycosylated molecule which, after purification, can be used therapeutically (Ueda et al., 1993).
The recommended protocol is based on 3 cycles of 5 daily subcutaneous administrations (1 MU/kg), beginning respectively on days 0, 14 and 60. Following initial in vitro studies (Truyen and Schultheiss, 2002), several authors have performed in vivo trials in order to assess its clinical and immune properties. The action of rFeIFN-ω in cats naturally infected with feline leukemia virus (FeLV) and co-infected with FeLV and feline immunodeficiency virus (FIV) has been described, showing that this compound induced an important clinical improvement and an increased survival time of treated cats (de Mari et al., 2004). Another group of authors reported that rFeIFN-ω improved the clinical condition of retroviral infected cats, although minor changes were observed on other parameters such as hypergammaglobulinemia, CD4/CD8 ratio, proviral load and viremia (Doménech et al., 2011). Thus, an overall improvement of innate immunity was suspected (Doménech et al., 2011). Recently, our group has reported that, in addition to improving clinical signs, rFeIFN-ω also induces a reduction of concurrent viral shedding (namely herpesvirus, coronavirus, parvovirus and calicivirus) which is particularly relevant in shelter medicine (Gil et al., 2013). In an attempt to understand the immune pathways underlying the therapeutic action of rFeIFN-ω, we also evaluated the effect of this compound on acute phase proteins (APPs) in naturally retrovirally infected cats, confirming that it potentiates the innate immune response (Leal et al., 2014).
Despite the clinical benefits of the licensed protocol in naturally retrovirus-infected cats, rFeIFN-ω can be cost-limitative in some cases and alternative protocols have been investigated. After some trials describing the use of lower oral doses of rFeIFN-ω in various conditions such as chronic gingivostomatitis (Hennet et al, 2011, Leal et al, 2013), an oral protocol was recently proposed in naturally FIV-infected cats (Gil et al., 2014). This was based on the daily oral administration of 0.1 MU/cat during 90 consecutive days and, in a similar way to the licensed protocol, revealed a significant clinical improvement of treated cats without relevant changes in hematology, serum biochemistry, serum protein electrophoresis or, in contrast to the licensed protocol, APPs (Gil et al., 2014). This apparent difference in the mechanism of action between the two protocols is in agreement with previous authors who suggested that oromucosal interferon (IFN) therapy seems to act by different mechanisms than parenteral protocols (Tovey, 2002). Therefore, while in the licensed protocol the increased APP seems to denote a potentiated innate immune response (Gil et al, 2014, Leal et al, 2014), in the oral protocol the immune mechanisms underlying the observed clinical improvement remain unclear.
Academically, the immune system can be divided into two general parts: the nonspecific (innate) response and the specific (acquired) immunity (Kennedy, 2010) that interact in order to maintain a competent immune system. This is achieved by the production and release of different cytokines which, being mediators of the immune response, have distinct functions including activation of pro-inflammatory and anti-inflammatory pathways (Day, 2012, Kennedy, 2010, Tizard, 2009a, Tizard, 2009b).
In spite of the fact that most cytokines are pleiotropic, each part of the immune system can be characterized by different cytokine patterns (Roitt and Delves, 2001). For instance, Interleukin-6 (IL-6), IL-1 and Tumor Necrosis Factor (TNF)-α are pro-inflammatory cytokines strongly involved in the innate immune response, potentiating nonspecific pathways such as acute phase response (APR) or fever (Ceron et al, 2005, Paltrinieri, 2008, Tizard, 2009a). Concerning the acquired immune pathways, IL-2, IL-12 and IFN-γ are strongly related to the cellular immune response (Th1 subset activation) (Locksley, Scott, 1991, Pedersen et al, 1998, Tizard, 2009b, VanCott et al, 1996) while the humoral antibody response (Th2 subset) is associated with IL-4, IL-5 and IL-10 production (Barnard et al, 1996, Osborne et al, 1996, Pedersen et al, 1998, Roitt, Delves, 2001, Romagnani et al, 1994).
In feline medicine, particularly in FIV, several studies have been performed not only in cell cultures but also in experimentally infected cats in order to characterize the cytokine profile after infection (Dean, Pedersen, 1998, Dean et al, 1998, Kipar et al, 2004, Lawrence et al, 1995, Lerner et al, 1998, Liang et al, 2000, Linenberger, Deng, 1999, Tompkins, Tompkins, 2008, Wood et al, 2012). Despite the fact that there is no clear Th1 to Th2 shift in response to FIV infection, this retrovirus induces a cytokine dysregulation with alterations in cytokine transcription, leading to an inadequate innate and cell-mediated immune response to other pathogens (Kipar et al, 2004, Levy et al, 1998, Tompkins, Tompkins, 2008).
To the authors' knowledge, no studies (in vivo) have been performed in order to assess the cytokine-based immunological pathways underlying the clinical improvement and restored control of other pathogens induced by rFeIFN-ω therapy (Gil et al, 2013, Gil et al, 2014). Therefore, this study aims to evaluate the anti-viral and immunomodulatory properties of rFeIFN-ω by monitoring changes in viremia, proviral load and blood cytokine profile in naturally FIV-infected cats receiving oral or subcutaneous rFeIFN-ω therapy.
2. Materials and methods
2.1. Animals and sample collection
The biological samples used in this study were collected from 18 naturally FIV-infected cats that had been previously enrolled in two past works from the group (Gil et al, 2013, Gil et al, 2014, Leal et al, 2014). In detail, 7/18 cats living in an animal shelter had received the licensed protocol (SC group) while 11/18 cats admitted/referred to the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine – University of Lisbon received the oral protocol (PO group), following the protocols previously described (Gil et al, 2013, Gil et al, 2014, Leal et al, 2014).
The animals had been monitored and submitted to blood collections before (D0) and after therapy (D65 and D90, respectively for SC and PO groups). All the procedures were approved by the Committee for Ethics and Animal Welfare of the Faculty of Veterinary Medicine – University of Lisbon (CEBEA – FMV-ULisboa).
Similar to studies previous published (Gil et al, 2013, Leal et al, 2014), a single-arm trial policy was applied in each group meaning that for each parameter, values on D0 were set as baseline and were taken as the individual control for each cat.
2.2. Relative quantification of cytokine expression by real-time qPCR
At each specified time point, whole blood was collected in RNA protected tubes (RNAprotect Animal Blood Tubes, Qiagen) and, according to the manufacturer's instruction, mRNA was extracted using specific kits (RNeasy protect animal blood kit, Qiagen). Thereafter, cDNA was synthesized using Transcriptor High Fidelity (Roche) following the manufacturer's instructions and used as a template for Real-Time quantitative Polymerase Chain Reaction (qPCR).
The primers used for each gene were published in the literature and the respective authors and sequences are presented in Table 1 . Despite the DNAse step performed during the RNA extraction, in order to preclude genomic DNA amplification, primers covered putative exon–exon junctions. Optimization experiments and efficiency assessments for each amplification system were previously performed (data not shown). Primers were obtained from a commercial manufacturer (STAB Vida, Portugal). Relative expression of each cytokine was quantified using Miner software (http://www.miner.ewindup.info), following the computed algorithm for Quantitative Real-time PCR system (Zhao et al., 1995). Beta-actin was set as the housekeeping/reference gene (Table 1).
Table 1.
Primers used to evaluate cytokine expression by Real-time qPCR in naturally FIV-infected cats treated with rFeIFN protocols.
| Gene | Oligo | Sequence (5′−3′) | Reference |
|---|---|---|---|
| Β-Actin | For | GACTACCTCATGAAGATCCTCACG | Scott et al., 2011 |
| Rev | CCTTGATGTCACGCACAATTTCC | ||
| IL-1β | For | ATTGTGGCTATGGAGAAACTGAAG | Scott et al., 2011 |
| Rev | TCTTCTTCAAAGATGCAGCAAAAG | ||
| IL-4 | For | CCCCTAAGAACACAAGTGACAAG | Taglinger, Van Nguyen, Helps, Day, & Foster, 2008 |
| Rev | CCTTTGAGGAATTTGGTGGAG | ||
| IL-6 | For | GTGTGACAACTATAACAAATGTGAGG | Scott et al., 2011 |
| Rev | GTCTCCTGATTGAACCCAGATTG | ||
| IL-10 | For | ACTTTCTTTCAAACCAAGGACGAG | Scott et al., 2011 |
| Rev | GGCATCACCTCCTCCAAATAAAAC | ||
| IL12p40 | For | TGGCCTTCTGAAGCGTGTTG | Scott et al., 2011 |
| Rev | GAAGTACACAGTGGAGTGTCAGG | ||
| IFN-γ | For | TGCAAGTAATCCAGATGTAGCAG | Taglinger et al., 2008 |
| Rev | GTTTTATCACTCTCCTCTTTCCAG | ||
| TNF-α | For | CACATGGCCTGCAACTAATC | Taglinger et al., 2008 |
Real-time qPCR was performed using the StepOne Plus real-time analyzer (Applied Biosystems). The PCR assays comprised, in each reaction, 2 µl of each primer (final concentration of 100 nM), 2 µl of cDNA, 4 µl of sterile water and 10 µl of SYBr (Applied Biosystems) in a total volume of 20 µl per reaction.
Thermocycling conditions consisted of an initial denaturation of 10 min at 95 °C, followed by 50 cycles of amplification (95 °C for 15 s and annealing at 60 °C for 1 min). A final melting curve stage consisted of 95 °C for 15 s, 60 °C for 1 min followed by a ramp rate and heating of samples until 95 °C with a 0.3 °C/s ramp rate. The melting curves obtained after each PCR were used to verify the specificity of each amplicon.
2.3. Measurement of plasma levels of IL-6, IL-12p40 and IL-4 cytokines
At each time point, whole blood was also collected in EDTA tubes which were centrifuged (5000 g for 10 minutes) to obtain plasma which was subsequently frozen at −20 °C until use. Plasma levels of IL-6, IL-12p40 and IL-4 were measured by specific ELISA kits and following manufacturer's instructions (SunRed Biotechnology Company).
2.4. Quantification of provirus
In order to assess proviral load, DNA was extracted from whole blood using a specific kit (DNeasy Blood & Tissue, Qiagen) by following the manufacturer's instructions. DNA was stored at −20 °C until use as a template for proviral load quantification by Real-time PCR.
Taking into account the major prevalence of FIV-subtypes A and B in southern Europe (Duarte, Tavares, 2006, Duarte et al, 2002), samples were screened for both subtypes. Primers used for FIV A subtype (gag gene) had been previously published and are presented in Table 2 (Leutenegger et al., 1999). For FIV B subtype, the gag gene nucleotide sequences available through their GenBank accession number, were aligned for identification of conserved regions using specific software (CLC Main Workbench). Primers were chosen using Primer Express software (Applied Biosystems) after visual inspection of the multiple alignment.
Table 2.
Real-time qPCR system to assess FIV provirus and viremia changes in naturally FIV-infected cats after rFEIFN therapy.
| Gene | Oligo | Sequence (5′–3′) | Reference |
|---|---|---|---|
| FIV-A subtype | For | GCC TTC TCT GCA AAT TTA ACA CCT | Leutenegger et al., 1999 |
| Rev | GAT CAT ATT CTG CTG TCA ATT GCT TT | ||
| Probe | FAM* CATGGCCACATTAATAATGGCCGCA* TAMRA | ||
| FIV-B subtype | For | AGACCGCTGCCCTATTTCACT | – |
| Rev | TTCTGGCTGGTGCAAATCTG | ||
| Probe | FAM*TGCCTGTTGTTCTTGAGTTAATCCTATTCCCA*TAMRA |
Real-time qPCR was performed using StepOne Plus real-time analyzer (Applied Biosystems). Fifty nanograms of DNA template was used in a total volume of 20 µl, comprising 10 µl of TaqMan PCR Master Mix (Applied Biosystems). Optimization of different primer and probe concentrations were performed. For the FIV-B system, a final concentration of 300 nM for each primer and 250 nM for the probe was used. For the FIV-A system, 900 nM for the primers and 250 nM for the probe was used.
Absolute quantification was assessed by real-time PCR using respective standard curves based on ten-fold dilutions of positive controls. For the FIV-B subtype, previously published plasmids were used (Duarte et al., 2002). For FIV-A, purified amplicons obtained from FIV-Petaluma CRFK infected cells were used.
For the FIV-A subtype, thermocycling conditions consisted of an initial denaturation (95 °C/3 min) followed by five cycles of 95 °C/30 s and 60 °C/30 s and 40 cycles of 85 °C/30 s and 60 °C/60 s. For the FIV-B subtype, thermocycling conditions began with an initial denaturation (95 °C/10 min) followed by 50 cycles of 95 °C/15 s, 58 °C/20 s and 72 °C/20 s.
2.5. Quantification of viremia
For viremia quantification, viral RNA was extracted from plasma samples (previously obtained by centrifugation of the blood collected for EDTA tubes at 5000 g for 10 minutes) using a specific kit (QIAmp Ultrasens Virus Kit). Plasma viral RNA was stored at −80 °C until use as a template on Real-time qPCR. Similarly to proviral load, the StepOne Plus Real-time analyzer (Applied Biosystems) was used.
A one-step Real-time qPCR was performed using 100 ng of RNA in a total volume of 20 µl of reaction using one-step PCR kit (MyTaq One-Step RT-PCR kit). Taking into account the provirus subtype results, the respective system was applied to assess concurrent viremia levels. The same concentrations of primers and probe were used.
Thermocycling conditions used for one-step Real-Time qPCR were similar to that previously described for provirus, with an additional initial step of reverse-transcription of 48 °C/15 min at the beginning.
2.6. Statistical analysis
For each measured parameter, the two groups were compared using the Mann–Whitney–Wilcoxon test for independent samples. The comparison between the end and the beginning of therapy in each group was carried out by the Mann–Whitney–Wilcoxon test for paired samples with appropriate small sample size correction. The significance level was set at 5%. A descriptive statistical analysis was also performed when appropriate. In order to assess potential correlations between measured parameters, a spearman correlation was also performed when suitable. All the statistical analyses were carried out using R-software.
3. Results
3.1. Cytokine expression
Relative quantification revealed very low levels in all the measured cytokines. In terms of mRNA expression, the groups were indistinguishable on D0 for all the evaluated cytokines (p = 0.55, 0.71, 0.24, 0.26, 0.70, 0.51 and 1 for IL-1, IL-4, IL-6, IL-10, IL-12p40, IFN-γ and TNF-α, respectively).
When comparing cytokine mRNA expression before and after therapy in both groups, in spite of an overall decreasing tendency only IL-6 expression significantly decreased and only in the PO group (p = 0.037). With the exception of this cytokine, no significant changes were observed in the cytokine profile of either group (D0 versus D65 for SC group: p = 0.58, 0.10, 0.18, 1, 0.37, 0.18, 1 for IL-1, IL-4, IL-6, IL-10, IL-12p40, IFN-γ and TNF-α, respectively; and D0 versus D90 for PO group: p = 0.28, 0.058, 1, 0.14, 0.55, 0.67 for IL-1, IL-4, IL-10, IL-12p40, IFN-γ and TNF-α, respectively).
On D0, cats from both groups showed a minimal expression of IL-1, IL-4, IL-6, IL-12p40 and IFN-γ. TNF-α expression was only quantified in one cat from the SC group and in two from the PO group. At the end of therapy, no cytokine expression other than IL-1 (which was measured in one cat) was observed in the SC group. Therefore, cytokine expression was set as zero for all the quantified cytokines in this group. Conversely, in the PO group, minimal mRNA expression of IL-1, IL-4, IL-6, IL-12p40, IFN-γ and TNF-α could still be measured at the end of therapy.
IL-10 expression was negligible in both groups and therefore these results were not charted. In detail, only two cats from the SC group and one cat from the PO group showed detectable mRNA expression on D0. On D90, only two other cats from the PO group, which tested negative before, expressed IL-10. The detailed results for the other cytokines are shown in Fig. 1 .
Fig. 1.
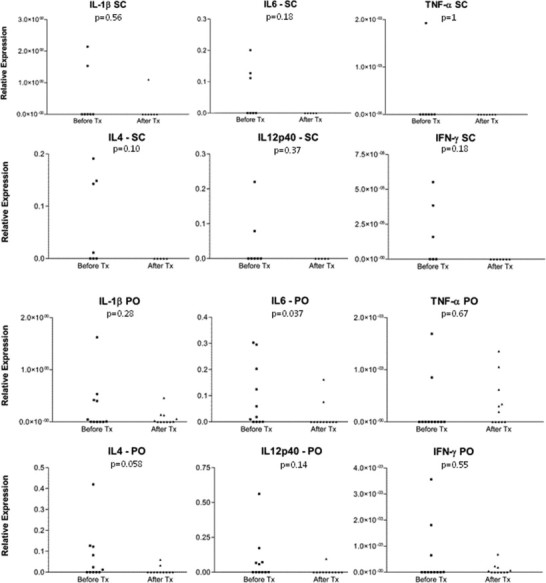
Detailed cytokine mRNA variation in naturally FIV-infected cats submitted to two different rFeIFN-ω protocols. SC refers to cats treated with the subcutaneous rFeIFN-ω licensed protocol and PO to cats receiving the oral protocol. The values represent the expression of each cytokine using a housekeeping gene (beta-actin) for normalization and relative quantification. p values refer to statistical comparison between the end and the beginning of therapy for each cytokine.
3.2. Plasma levels of IL-6, IL-12p40 and IL-4 cytokines
Concerning plasma levels of measured cytokines, the groups were similar on D0 for IL-12p40 and IL-6 (p = 0.82 and p = 0.22 respectively). For IL-4, plasma levels on D0 were significantly higher in the SC group than in the PO one (p = 0.013).
Comparing the beginning and the end of therapy in the SC group, there was a significant decrease of IL-6 plasma levels (p = 0.037). No statistical differences were observed for IL-12p40 and IL-4 plasma levels in this group (p = 0.87 and p = 0.24, respectively). In the PO group, no changes were observed in any of the measured plasmatic ILs (p = 0.062, 0.248, 0.074 respectively for IL-4, IL-12p40 and IL-6). The detailed results are shown in Fig. 2 .
Fig. 2.

Mean ± SE of plasma IL-12p40, IL-4 and IL-6 concentrations in naturally FIV-infected cats submitted to two different protocols of rFeIFN-ω. SC refers to cats treated with the subcutaneous rFeIFN-ω licensed protocol and PO to cats receiving the oral protocol. The groups were statistically similar at baseline values except for IL-4 concentration which was higher in the SC group than in the PO group (◊p = 0.013 – comparison between groups). The SC group showed a statistically significant decrease of IL-6 concentration (*p = 0.037 – comparison between the end and beginning of therapy).
3.3. Quantification of provirus
Regarding the primers and probes used, proviral load was quantified by the FIV-B system in 8/18 cats (3 from the SC group and 5 from the PO group), while the FIV-A system worked successfully in the other 10 cats (4 from the SC group and 6 from the PO group). To assess overall changes in the proviral load, the results of both subsystems were taken together and are shown in Fig. 3 . There was no statistical difference between the groups at D0 before therapy (p = 0.07). Also, at this time-point, a significant positive correlation could be observed in both groups between proviral loads and clinical condition (detailed data referring to clinical scores previously published on Gil et al, 2013, Gil et al, 2014) (CC = 88% and 61% for the SC and PO groups respectively).
Fig. 3.
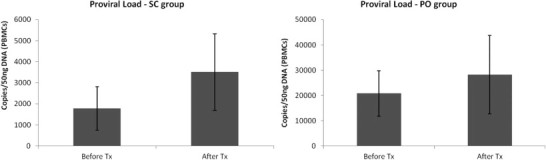
Mean ± SE of the proviral load of FIV in cats receiving two different rFeIFN-ω protocols. SC refers to cats treated with the subcutaneous rFeIFN-ω licensed protocol and PO to cats receiving the oral protocol.
After therapy there was a statistically significant increase in the SC group (p = 0.031). In contrast, in the PO group, although the proviral load tended to increase it was not statistically significant (p = 0.46).
3.4. Quantification of viremia
There was a low level of viremia at both time points for both groups. On D0 the groups were similar (p = 1).
In detail, only 7/18 cats (3/7 from the SC group and 4/11 from the PO group) showed detectable viremia on D0. Detailed individual values are presented in Fig. 4 .
Fig. 4.

Individual viremia changes of 18 FIV-infected cats submitted to two rFeIFN-ω protocols. 7/18 cats (SC group) received the subcutaneous licensed protocol while 11/18 cats were treated with oral rFeIFN-ω (PO group). *Refers to time-points in which viremia was undetectable.
In the SC group, the four cats with undetectable viremia levels on D0 remained negative after therapy (D65). Of the three cats which had detectable viremia on D0, two became negative while one reduced it.
In the PO group, of the seven cats which had undetectable viremia on D0, three remained negative while four became positive after therapy (D90). Considering the four cats which tested positive on D0, three of them reduced their viremia levels while one slightly increased it.
Despite this, when comparing the levels at the beginning and the end of therapy in each group, no significant differences were obtained for viremia measurements (p = 0.52 and 0.18 for the PO and SC groups respectively).
In contrast to what was seen with the provirus levels, no correlation was noted between the viremia levels and clinical health status (CC = 0.57/p = 0.18 for the SC group and CC = 0.351/p = 0.29 for the PO group on D0). Also no correlation was established between viremia and provirus at both time points (D0: CC = 0.39/p = 0.382 for the SC group and CC = 0.50/p = 0.11 for the PO group; after therapy: CC = 0.41/p = 0.36 for the SC group and CC = 0.46/p = 0.15 for the PO group).
4. Discussion
This study evaluated the effect of two distinct rFeIFN-ω protocols on blood cytokine profile, viremia and proviral load. The cytokines were chosen taking into account their main functions on the immune system and were considered biomarkers of the innate or the acquired immune response. Therefore, IL-1, IL-6 and TNF-α were measured in order to evaluate the innate pro-inflammatory pathways, IL-12p40 and IFN-γ were chosen as indicators of Th1 pathway activation while IL-4 and IL-10 were used as indicators of a Th2 response. IL-12 is a heterodimeric cytokine composed of two chains (p40 and P35) (Trinchieri et al., 2003). The measurement of the IL-12p40 subunit was chosen by the authors due to the fact that it was the only subunit available for complementary ELISA measurement.
Even if quantification was possible, mRNA expression was very low in all the animals. The groups were indistinguishable on D0 for mRNA expression of all the measured cytokines, which made the SC group, submitted to the licensed protocol, a reliable positive control. Comparing the beginning and the end of therapy in both groups, there were no marked differences in mRNA expression for the majority of cytokines, although a decreasing tendency was observed. This decrease was only significant for IL-6 expression in FIV-infected cats after oral rFeIFN-ω therapy. Although not statistically significant, IL-1 and TNF-α also tended to decrease in these animals. These findings are consistent with an overall reduction of pro-inflammatory pathways in cats treated with the oral protocol. The same is observed in the SC group in which IL-1, TNF-α and IL-6 expression showed a decreasing tendency. Therefore, also in the licensed protocol, the pro-inflammatory pathways of the innate immune response tend to be reduced. Correlating these findings with other biomarkers of the innate immunity, our group had shown that the licensed subcutaneous rFeIFN-ω therapy is associated with a concurrent increase of APPs (Leal et al., 2014). In opposition, the oral protocol did not change APP profile in treated cats (Gil et al., 2014). Due to the fact that IL-6 is strongly involved in the stimulation of APP production, it is surprising that pro-inflammatory pathways tended to decrease in these animals. A possible explanation relies on the fact that although IL-6, IL-1 and TNF-ω are thought to be the main inducers of this acute phase reaction (Martínez-Subiela et al, 2001, Paltrinieri, 2008), they are not the only cytokines involved in this phenomenon. Recognizing that APPs have several protective functions (Ceron et al, 2005, Hochepied et al, 2003, Paltrinieri, 2008, Petersen et al, 2004, Steel, Whitehead, 1994), various pathways other than pro-inflammatory stimuli can lead to their production. Therefore, it seems reasonable to state that this paradoxical increase of APPs in cats with evidence of a reduced pro-inflammatory stimuli may be induced by other mediators than IL-6, IL-1 or TNF-α, such as IL-18 (Duan et al., 2004). Further studies are needed to fully understand the mechanisms behind the observed APP increase in these cats.
Concerning the mRNA levels of the Th1 measured cytokines (IL-12p40 and IFN-γ), no changes were observed in either group meaning that the activation of the cellular immunity does not seem to be the way by which rFeIFN-ω therapy provides a benefit for FIV-infected cats. The same was observed for the Th2 quantified cytokines (IL-4 and IL-10). In particular, IL-10 was undetectable in the majority of animals, making the results for this cytokine negligible by this method. Nonetheless, IL4 mRNA expression allowed a reasonable quantification of Th2 activation. Although non-significant, a decreasing trend was noted in both protocols for IL-4 mRNA expression. This was closest to significance for the oral protocol (p = 0.058), which, incidentally, may partially explain the benefit seen with oral rFeIFN-ω therapy in a recently published study on canine atopic dermatitis where a high IL-4/IFN-γ ratio is believed to contribute to the disorder (Litzlbauer et al., 2014). Therefore, other than this potential trend for reduced IL-4 mRNA expression, our results did not demonstrate any significant impact of rFeIFN-ω therapy on the Th1 and Th2 responses in FIV-infected cats, independently of the chosen protocol.
Concurrently to mRNA expression monitoring, the plasma levels of IL-6, IL-12p40 and IL-4 were also measured. Similarly to the basal values of cytokine expression, groups were similar on D0 for IL-6 and IL-12p40. The exception was IL-4 which was significantly higher in animals from the SC group than in the PO group. This can be explained by different factors such as their environment once cats from the SC group were living in a cattery whereas cats from the PO group were mainly indoor animals with less exposure to other cats. Another relevant factor is the presence of opportunistic infections, which were more evident in the SC group than in the PO one (Gil et al, 2013, Gil et al, 2014). In cats from animal shelters it is expected that the Th2 response will be increased due to the constant stimuli from concurrent infections and environmental challenges. However, as the effect of therapy was assessed by monitoring plasma levels of each cytokine before and after therapy and the cats did not alter their living conditions, it is not anticipated that this difference at baseline will have any relevant impact on the results. Nevertheless, the changes seen in IL-4 and IL-12p40 plasma levels were not statistically significant and the overall tendencies were considered negligible. They can probably be explained by a high individual variability that induced slight fluctuations on the overall results of these cytokines. Interestingly, IL-6 plasma levels significantly reduced in the SC group while it remained stable in cats treated with the oral protocol. Correlating these results with mRNA expression profile, it is observed that in the SC group, although the decrease of IL-6 mRNA expression was not significant, rFeIFN-ω induced an important reduction of its concurrent plasma levels. In contrast, the significant decrease noted in IL-6 mRNA expression in cats submitted to oral therapy was not reflected in their plasma levels of this cytokine. This can be explained by eventual post-transcriptional events (Vogel and Marcotte, 2012) that delay the translation and impair a direct correlation between IL-6 mRNA expression and its concurrent plasma level in cats treated orally even though, albeit with some differences, these results suggest that IL-6 cytokine production is affected in FIV-infected cats during rFeIFN-ω therapy, independently of the protocol applied. It seems reasonable to state that higher pulsate subcutaneous doses seem to be more effective than lower continuous oral therapy for reducing pro-inflammatory stimuli in FIV-infected cats. However continuous oral therapy also altered IL-6 expression meaning that this immune modulation protocol retains some anti-inflammatory properties. These results reinforce the beneficial aspects of rFeIFN-ω as an immunomodulatory therapy, in light of the facts that basal levels of pro-inflammatory cytokines tend to be increased in FIV-infected cats (Lawrence et al., 1995).
Proviral load on D0 was similar in both groups and an expectable correlation was established between this parameter on D0 and clinical presentation, meaning that cats in worse condition showed higher levels of provirus. It has been previously reported that the licensed protocol does not induce significant changes in proviral load (Doménech et al., 2011). However in this study we found a significant increase of proviral load in the SC group. An increasing, but non-significant, tendency was also noted with the oral protocol. A possible explanation for this finding lies in the fact that, in both groups, lymphocyte numbers tended to increase with therapy. Although this increase was within the reference range, it may explain the subsequent increase of proviral load, especially in light of the improved clinical condition in both groups. (Gil et al, 2013, Gil et al, 2014).
Viremia was only detected in a small proportion of animals of both groups on D0. This is not surprising in a study using naturally infected cats with an unknown duration of infection. Chronically FIV-infected cats eventually cease to have detectable viral RNA due to viral latency (Murphy et al, 2012, Tomonaga et al, 1995). No correlation was established between viremia and clinical scores. The main clinical signs in the symptomatic animals were therefore most likely due to opportunistic infections rather than directly induced by FIV replication. This suggests that the beneficial effects of rFeIFN-ω in FIV-infected cats is unlikely to be related to a direct anti-viral action on the FIV virus in either protocol, but rather to improved control of secondary infections in addition to reduction of pro-inflammatory pathways.
As for all clinical trials, there were a few limitations that must be stated. Even if the groups did not differ in the majority of measured variables, cats that received the SC group were living in a cattery/animal shelter while cats treated with oral rFeIFN-ω were mainly indoor/owned cats. Therefore, the groups were exposed to different environmental and human stimuli which cannot be precisely determined. However, recognizing that D0 results were set as the baseline value and the individual control for each cat, the overall tendency was analyzed for each group which minimized this limitation. Another point to consider is that cytokine expression results depended on blood mRNA collection and extraction. It is important to note that a reliable measurement of circulating mRNA is difficult as RNAases are present ubiquitously which degrade it (Etheridge et al., 2013). Furthermore, mRNA extraction efficiency varies according to the method applied and can be affected by multiple external variables such as blood clots and sampling conditions which determine its quality (Wong et al., 2004). To the authors' knowledge, this is the first study reporting cytokine profiles based on blood mRNA measurements in naturally FIV-infected cats. Considering the low values obtained, it is reasonable to say that blood mRNA does not seem to be as effective as plasma levels or even in vitro studies for assessing this aspect of the immune response (Robert-Tissot et al., 2011). A concurrent evaluation and stimulation of PBMCs from naturally-FIV infected cats would have been helpful in clarifying these data. Interestingly, a recent study validated a microsphere immunoassay for the detection of plasma IL12/23 (Wood et al., 2012). Perhaps in the near future the evaluation of the cytokine profile of FIV cats will be easier and more helpful in the monitoring of infected cats and therapies.
5. Conclusion
Although the antiviral effect of rFeIFN-ω on FIV seems to be minor in FIV-infected cats, this study helped to enlarge our understanding of the role of this immunomodulator on the cytokine profile of these animals. Among the measured cytokines, this work revealed that IL-6 production was significantly affected in FIV-infected cats treated with subcutaneous or oral rFeIFN-ω protocols. While the high pulse scheme of the SC protocol leads to an important reduction on IL-6 plasma levels, the continuous lower doses of the oral protocol induce a decrease on IL-6 expression, although not sufficiently to be reflected in significant reductions of its plasma levels. In summary, the acquired immune-response, namely Th1/Th2 pathways, was not found to be the major means by which rFeIFN-ω acted in this study. Its main action seems to be on the innate immune response where it reduces the pro-inflammatory stimuli. This anti-inflammatory action can in part justify the observed clinical improvement induced by this immunomodulator.
Acknowledgements
Authors would like to thank Centro de Investigação Interdisciplinar em Sanidade Animal, União Zoófila de Lisboa and the owners of cats involved in this study.
References
- Barnard A., Mahon B.P., Watkins J., Redhead K., Mills K.H. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceron J.J., Eckersall P.D., Martýnez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Veterinary Clinical Pathology. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- de Mari K., Maynard L., Sanquer A., Lebreux B., Eun H.-M. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. Journal of Veterinary Internal Medicine. 2004;18:477–482. doi: 10.1892/0891-6640(2004)18<477:teorfi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Day M. Basic immunology. In: Day M., editor. Clinical Immunology of the Dog and Cat. Manson Publishing; London, UK: 2012. pp. 11–61. [Google Scholar]
- Dean G.A., Pedersen N.C. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. Journal of Virology. 1998;72:9436–9440. doi: 10.1128/jvi.72.12.9436-9440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean G.A., Bernales J.A., Pedersen N.C. Effect of feline immunodeficiency virus on cytokine response to Listeria monocytogenes in vivo. Veterinary Immunology and Immunopathology. 1998;65:125–138. doi: 10.1016/s0165-2427(98)00148-2. [DOI] [PubMed] [Google Scholar]
- Doménech A., Miró G., Collado V.M., Ballesteros N., Sanjosé L., Escolar E. Use of recombinant interferon omega in feline retrovirosis: from theory to practice. Veterinary Immunology and Immunopathology. 2011;143:301–306. doi: 10.1016/j.vetimm.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Yarmush D.M., Jayaraman A., Yarmush M.L. Dispensable role for interferon-gamma in the burn-induced acute phase response: a proteomic analysis. Proteomics. 2004;4:1830–1839. doi: 10.1002/pmic.200300696. [DOI] [PubMed] [Google Scholar]
- Duarte A., Tavares L. Phylogenetic analysis of Portuguese Feline Immunodeficiency Virus sequences reveals high genetic diversity. Veterinary Microbiology. 2006;114:25–33. doi: 10.1016/j.vetmic.2005.11.056. [DOI] [PubMed] [Google Scholar]
- Duarte A., Marques M.I., Tavares L., Fevereiro M. Phylogenetic analysis of five Portuguese strains of FIV. Archives of Virology. 2002;147:1061–1070. doi: 10.1007/s00705-002-0785-7. [DOI] [PubMed] [Google Scholar]
- Etheridge A., Gomes C.P.C., Pereira R.W., Galas D., Wang K. The complexity, function and applications of RNA in circulation. Frontiers in Genetics. 2013;4:115. doi: 10.3389/fgene.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil S., Leal R.O., Duarte A., McGahie D., Sepúlveda N., Siborro I. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Research in Veterinary Science. 2013;94:753–763. doi: 10.1016/j.rvsc.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil S., Leal R.O., McGahie D., Sepúlveda N., Duarte A., Niza M.M.R.E. Oral Recombinant Feline Interferon-Omega as an alternative immune modulation therapy in FIV positive cats: clinical and laboratory evaluation. Research in Veterinary Science. 2014;96:79–85. doi: 10.1016/j.rvsc.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet P.R., Camy G.A.L., McGahie D.M., Albouy M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: a randomised, multi-centre, controlled, double-blind study in 39 cats. Journal of Feline Medicine and Surgery. 2011;13:577–587. doi: 10.1016/j.jfms.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochepied T., Berger F.G., Baumann H., Libert C. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine and Growth Factor Reviews. 2003;14:25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Kennedy M.A. A brief review of the basics of immunology: the innate and adaptive response. The Veterinary Clinics of North America. Small Animal Practice. 2010;40:369–379. doi: 10.1016/j.cvsm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Kipar A., Boretti F.S., Meli M.M., Failing K., Reinacher M., Lutz H. Reduced constitutive cytokine transcription in isolated monocytes of clinically healthy cats, infected with an FIV strain of low pathogenicity. Veterinary Immunology and Immunopathology. 2004;98:215–221. doi: 10.1016/j.vetimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lawrence C.E., Callanan J.J., Willett B.J., Jarrett O. Cytokine production by cats infected with feline immunodeficiency virus: a longitudinal study. Immunology. 1995;85:568–574. [PMC free article] [PubMed] [Google Scholar]
- Leal R.O., Gil S., Brito M.T., McGahie D., Niza M.M., Tavares L. The use of oral recombinant feline interferon omega in two cats with type II diabetes mellitus and concurrent feline chronic gingivostomatitis syndrome. Irish Veterinary Journal. 2013;66:19. doi: 10.1186/2046-0481-66-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal R.O., Gil S., Sepúlveda N., McGahie D., Duarte A., Niza M.M.R.E. Monitoring acute phase proteins in retrovirus infected cats undergoing feline interferon-ω therapy. The Journal of Small Animal Practice. 2014;55:39–45. doi: 10.1111/jsap.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D.L., Grant C.K., de Parseval A., Elder J.H. FIV infection of IL-2-dependent and -independent feline lymphocyte lines: host cells range distinctions and specific cytokine upregulation. Veterinary Immunology and Immunopathology. 1998;65:277–297. doi: 10.1016/S0165-2427(98)00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger C.M., Klein D., Hofmann-Lehmann R., Mislin C., Hummel U., Böni J. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system. Journal of Virological Methods. 1999;78:105–116. doi: 10.1016/s0166-0934(98)00166-9. [DOI] [PubMed] [Google Scholar]
- Levy J.K., Ritchey J.W., Rottman J.B., Davidson M.G., Liang Y.H., Jordan H.L. Elevated interleukin-10-to-interleukin-12 ratio in feline immunodeficiency virus-infected cats predicts loss of type 1 immunity to Toxoplasma gondii. The Journal of Infectious Diseases. 1998;178:503–511. doi: 10.1086/515632. [DOI] [PubMed] [Google Scholar]
- Liang Y., Hudson L.C., Levy J.K., Ritchey J.W., Tompkins W.A., Tompkins M.B. T cells overexpressing interferon-gamma and interleukin-10 are found in both the thymus and secondary lymphoid tissues of feline immunodeficiency virus-infected cats. The Journal of Infectious Diseases. 2000;181:564–575. doi: 10.1086/315226. [DOI] [PubMed] [Google Scholar]
- Linenberger M.L., Deng T. The effects of feline retroviruses on cytokine expression. Veterinary Immunology and Immunopathology. 1999;72:343–368. doi: 10.1016/s0165-2427(99)00147-6. [DOI] [PubMed] [Google Scholar]
- Litzlbauer P., Weber K., Mueller R.S. Oral and subcutaneous therapy of canine atopic dermatitis with recombinant feline interferon omega. Cytokine. 2014;66:54–59. doi: 10.1016/j.cyto.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunology Today. 1991;12:A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Martínez-Subiela S.T.V.F., Parra Muñoz M.D., Cerón J.J.M. Proteínas de fase aguda: conceptos básicos y principales aplicaciones clínicas en medicina veterinaria [Acute phase proteins: general concepts and main clinical applications in veterinary medicine] Anales de Veterinaria de Murcia. 2001;17:97–113. [Google Scholar]
- Murphy B., Vapniarsky N., Hillman C., Castillo D., McDonnel S., Moore P. FIV establishes a latent infection in feline peripheral blood CD4+ T lymphocytes in vivo during the asymptomatic phase of infection. Retrovirology. 2012;9:12. doi: 10.1186/1742-4690-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne J., Hunter S.J., Devaney E. Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi. Infection and Immunity. 1996;64:3461–3466. doi: 10.1128/iai.64.9.3461-3466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S. The feline acute phase reaction. Veterinary Journal (London, England: 1997) 2008;177:26–35. doi: 10.1016/j.tvjl.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Dean G.A., Bernales J., Sukura A., Higgins J. Listeria monocytogenes and Serratia marcescens infections as models for Th1/Th2 immunity in laboratory cats. Veterinary Immunology and Immunopathology. 1998;63:83–103. doi: 10.1016/s0165-2427(98)00085-3. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Veterinary Research. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Robert-Tissot C., Rüegger V.L., Cattori V., Meli M.L., Riond B., Gomes-Keller M.A. The innate antiviral immune system of the cat: molecular tools for the measurement of its state of activation. Veterinary Immunology and Immunopathology. 2011;143:269–281. doi: 10.1016/j.vetimm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I.M., Delves P.J. The production of effectors. In: Roitt I.M., Delves P.J., editors. Essential Immunology. Blackwell Publishing; Massachusetts: 2001. pp. 147–164. [Google Scholar]
- Romagnani S., Del Prete G., Manetti R., Ravina A., Annunziato F., De Carli M. Role of TH1/TH2 cytokines in HIV infection. Immunological Reviews. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Scott V.L., Shack L.A., Eells J.B., Ryan P.L., Donaldson J.R., Coats K.S. Immunomodulator expression in trophoblasts from the feline immunodeficiency virus (FIV)-infected cat. Virology Journal. 2011;8:336. doi: 10.1186/1743-422X-8-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel D.M., Whitehead A.S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology Today. 1994;15:81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Taglinger K., Van Nguyen N., Helps C.R., Day M.J., Foster A.P. Quantitative real-time RT-PCR measurement of cytokine mRNA expression in the skin of normal cats and cats with allergic skin disease. Veterinary Immunology and Immunopathology. 2008;122:216–230. doi: 10.1016/j.vetimm.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Tizard I.R. Veterinary Immunology: An Introduction. Saunders; Missouri: 2009. Cell signaling: cytokines and their receptors. [Google Scholar]
- Tizard I.R. Veterinary Immunology: An Introduction. Saunders; Missouri: 2009. Helper T-cells and their response to Antigen. [Google Scholar]
- Tomonaga K., Inoshima Y., Ikeda Y., Mikami T. Temporal patterns of feline immunodeficiency virus transcripts in peripheral blood cells during the latent stage of infection. The Journal of General Virology. 1995;76(Pt 9):2193–2204. doi: 10.1099/0022-1317-76-9-2193. [DOI] [PubMed] [Google Scholar]
- Tompkins M.B., Tompkins W.A. Lentivirus-induced immune dysregulation. Veterinary Immunology and Immunopathology. 2008;123:45–55. doi: 10.1016/j.vetimm.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M.G. Oromucosal cytokine therapy: mechanism(s) of action. Taehan Kan Hakhoe chi. 2002;8:125–131. [PubMed] [Google Scholar]
- Trinchieri G., Pflanz S., Kastelein R.A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Truyen U., Schultheiss U. A study of the anti-viral activity of interferon omega against selected canine and feline viruses. Der Praktische Tierarzt. 2002;10:862–864. [Google Scholar]
- Ueda Y., Sakurai T., Kasama K., Satoh Y., Atsumi K., Hanawa S. Pharmacokinetic properties of recombinant feline interferon and its stimulatory effect on 2',5'-oligoadenylate synthetase activity in the cat. The Journal of Veterinary Medical Science. 1993;55:1–6. doi: 10.1292/jvms.55.1. [DOI] [PubMed] [Google Scholar]
- VanCott J.L., Staats H.F., Pascual D.W., Roberts M., Chatfield S.N., Yamamoto M. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. The Journal of Immunology. 1996;156:1504–1514. [PubMed] [Google Scholar]
- Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Reviews. Genetics. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.C.C., Lo E.S.F., Cheung M.T. An optimised protocol for the extraction of non-viral mRNA from human plasma frozen for three years. Journal of Clinical Pathology. 2004;57:766–768. doi: 10.1136/jcp.2003.007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B.A., Troyer R.M., Terwee J.A., Vandewoude S. Microsphere immunoassay for the detection of cytokines in domestic cat (Felis catus) plasma: elevated IL-12/23 in acute feline immunodeficiency virus infections. Veterinary Immunology and Immunopathology. 2012;145:604–610. doi: 10.1016/j.vetimm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Gebhard D., English R., Sellon R., Tompkins M., Tompkins W. Enhanced expression of novel CD57+CD8+ LAK cells from cats infected with feline immunodeficiency virus. Journal of Leukocyte Biology. 1995;58:423–431. doi: 10.1002/jlb.58.4.423. [DOI] [PubMed] [Google Scholar]


