Abstract
The development of intestinal lesions after inoculation with Brachyspira hyodysenteriae was followed by repeated endoscopy and biopsy sampling through a caecal cannula. Seven eight-week-old pigs were cannulated and inoculated, two were cannulated but not inoculated, and two pigs were inoculated but not cannulated. Endoscopy, biopsy, and blood sampling to determine SAA (serum amyloid A), haptoglobin, cortisol, and WBC counts were performed at scheduled time-points. At the third day of disease, endoscopy showed a hyperaemic, perturbed mucosa and excessive amount of mucus. Histologically, crypt hyperplasia, depletion of goblet cell mucus, and erosions were noted. Simultaneously, elevated acute phase proteins and circulating monocytes, and decreased number of intraepithelial CD3+ cells were observed. After five days the pigs recovered. Intestinal lesions were demarcated and interspersed among apparently normal mucosa and blood parameters returned to initial values. Endoscopy through an intestinal cannula made it possible to follow the development of intestinal alterations in vivo and describe the sequential events during the course of swine dysentery. The number of animals used in a study could thus be minimised and the precision of the experiment increased.
Keywords: Caecal cannulation, SAA, Haptoglobin, Cortisol, Monocytes, Intraepithelial lymphocytes, Immunohistochemistry
1. Introduction
Swine dysentery is a widespread enteric disease caused by the spirochaete Brachyspira hyodysenteriae. Clinical signs range from mild, mucous diarrhoea and unaltered general condition to severe haemorrhagic diarrhoea with a mortality rate of 50–90% (Moreng et al., 1980, Raynaud et al., 1980, Whipp et al., 1979). The lesions are confined to the large intestine and vary from mild catarrh to marked inflammation with excessive mucus production, necrosis, haemorrhages and pseudomembranes. Microscopically, hyperplastic mucus-filled crypts, goblet cell hyperplasia, and mucosal oedema with congested blood vessels are frequently seen (Albassam et al., 1985, Glock and Harris, 1972, Kinyon et al., 1977). In progressive cases, superficial erosion and mucofibrinous exudate with neutrophils and debris develop (Albassam et al., 1985, Glock and Harris, 1972). Initially, the local inflammatory cell response is minimal, but within a few days a mixed population of inflammatory cells appears in the superficial lamina propria. Spirochaetes are sometimes seen lying free in the cytoplasm of goblet cells and enterocytes (Albassam et al., 1985).
Despite the well-characterised clinical and morphological features, the pathogenesis is poorly understood (ter Huurne and Gaastra, 1995). Concomitant synergistic infection with certain gut bacteria seems to be important but their role is not clear (Harris et al., 1978, Meyer et al., 1975). Opportunists might also exacerbate the lesions (Albassam et al., 1985, Lysons et al., 1984). Further, the importance and precise role of virulence factors such as chemotaxis, attachment, invasion, endotoxins or toxin production remains to be elucidated (ter Huurne and Gaastra, 1995). The bacterium is suggested to primarily invade the goblet cells, thereby causing excessive mucus secretion (Pohlenz et al., 1983). Alternatively, invasion is preceded by initial damage to tight junctions (Kang and Olander, 1990, ter Huurne and Gaastra, 1995). In addition, the host’s immune response might exacerbate the lesions (Hontecillas et al., 2005, Nuessen et al., 1983).
Results regarding the local immune response differ. In experimental infections, Pohlenz et al. (1983) reported a weak cellular response consisting of a few eosinophils and neutrophils together with oedema, and an increase in lymphocytes, macrophages and mast cells in response to necrosis. Meyer et al., 1975, Pohlenz et al., 1984 suggested that neutrophil infiltration occurred in response to secondary infections. However, the specific T cell immune response appears to be important (Galvin et al., 1997, Jonasson et al., 2003, Waters et al., 1999, Waters et al., 2000b). In the systemic immune response, a switch between different T cell subpopulations in response to experimental inoculation has been reported (Jonasson et al., 2003, Waters et al., 1999, Waters et al., 2000a). Further, a depletion of γδ T cells from the epithelial layer and an aggregation of CD4+ T cells in lamina propria have been described (Hontecillas et al., 2005).
The pathogenesis of infectious enteric diseases consists of series of interactions between the microorganism and the host, and repeated samplings of, e.g. blood, faeces and target tissues are required to understand the mechanisms. Therefore, serial necropsies of a large number of experimentally inoculated animals, sacrificed at various time points of infection, are generally used (Albassam et al., 1985, Glock and Harris, 1972, Kinyon et al., 1980, Pohlenz et al., 1983). In a previous study (Jacobson et al., 2001), the possibility of following events in the porcine gut by repeated endoscopy through a caecal cannula was evaluated. This technique enabled repeated biopsy sampling from the same individual, thus reducing the number of experimental animals required and increasing the precision of the experiment.
The aim of the present study was to follow the development of intestinal lesions in pigs after an experimental inoculation with B. hyodysenteriae. Thus, repeated endoscopy and biopsy samplings through an intestinal cannula were performed. The number of mucosal B- and T- cells was examined during the course of disease and the systemic response to infection was studied by monitoring the number of peripheral white blood cells and concentrations of the acute phase proteins haptoglobin and serum amyloid A (SAA).
2. Materials and methods
2.1. Experimental design
The experimental design was approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden. The study included 11 pigs that were allowed a 2-week acclimatisation period after arrival. Seven pigs were cannulated in the caecum and inoculated. Two pigs were not cannulated but inoculated, and two animals were cannulated but not inoculated. The nine pigs that underwent surgery had a 2-week post-operative recovery period prior to oral inoculation. Endoscopy and biopsy sampling were performed as described below. The pigs were usually sacrificed one to five days after cessation of clinical signs or, due to animal welfare considerations, during disease. Non-infected controls were sacrificed four to five days after the last biopsy occasion. The pigs were stunned with a captive bolt and bled, and necropsy was performed.
2.1.1. Animals and accommodations
Crossbred pigs (Swedish Landrace × Yorkshire) of both sexes, ∼8 weeks old at arrival, were used. They originated from a conventional herd of well-known health status, free from B. hyodysenteriae and Salmonella spp. The microbes Brachyspira pilosicoli, B. innocens and Lawsonia intracellularis had previously been isolated in the herd. The animals were kept individually within sight and sound of each other. Pens were provided with infrared lamps and supplied with straw or fiber-fur blankets. The non-infected pigs were kept in a separate unit. Except for a short period before and during inoculation (see below), the animals were fed a commercial finisher diet without growth promoters (Singel Flex®, Granngården, Sweden) ad lib and given free access to water. A clinical health examination including recording of rectal temperature was performed daily and the pigs became adjusted to petting and handling. The animals were weighed once a week and the daily weight gain (d.w.g.) was calculated.
2.2. Surgery
Surgery was performed under strict aseptic conditions and using a method previously described (Jacobson et al., 2001). However, in the present study general anaesthesia was maintained by inhalation of isofluran (Isoflo™ vet, Schering-Plough, Kent, UK). An analgesic protocol was also evaluated and reported by Malavasi et al. (2006).
2.3. Inoculation
Five days prior to inoculation, the finisher diet was replaced by pure soybean meal (Fori HB, Lidköping, Sweden) every second feeding for 7 days since this dietary regimen has been shown to increase susceptibility to swine dysentery (Jacobson et al., 2004). The pigs were orally inoculated on three consecutive days. The inocula consisted of 30 ml trypticase soy broth containing 107–109 organisms/ml of B. hyodysenteriae B204 (GenBank Accession No. U14932). The two cannulated, non-infected pigs were given sterile broth. From the day of inoculation onwards, the infected animals were moved in between the pens four times daily to increase susceptibility.
2.4. Endoscopy and biopsy sampling
Endoscopy was performed once before beginning the soybean-meal feeding, at the first sign of diarrhoea, and every second or third day thereafter until sacrifice. A large variation in incubation time was expected, and therefore endoscopy started at the first sign of diarrhoea. Anaesthesia was provided as described above. Initially, the pigs were starved for 20 h, and 12 and 2 h prior to endoscopy, 45 ml of an osmotically active laxative (Phosphoral™, Ferring, Limhamn, Sweden) were given per os. During disease, the gut was usually empty but if indicated, a water enema was given prior to endoscopy. The two non-inoculated pigs were submitted to endoscopy before beginning the soybean-meal feeding, 12 days after inoculation with sterile broth, and then every second or third day during 2 weeks. A video colonoscope with a diameter of 10 mm and a length of one meter (Fujinon video colonoscope EC-200 MP, Fujinon, Saitama, Japan) and a biopsy forceps 2.2 mm thick and 240 cm long and with a spike (RadialJaw®3, Boston Scientific Corporation, Watertown, MA, USA), were used. Biopsies were taken from the proximal spiral colon; 20, 30, and 40 cm from the caeco-colic orifice. At each location, five to seven biopsies were taken.
2.5. Necropsy
A complete necropsy was performed in all pigs. Specimens for light microscopy were taken from several sites of all major intestinal segments, as well as other relevant organs.
2.5.1. Histopathology
The biopsy and necropsy specimens were fixed in 10% buffered formalin, embedded in paraffin, cut 4 μm thick, and stained with hematoxylin and eosin (HE) for light microscopy. Intestinal specimens from each pig were also stained with Alcian blue-periodic acid Schiff for mucosubstances, and, from a few pigs, with Warthin–Starry for microorganisms. All slides were examined for pathological changes by a pathologist that was blinded for the group affiliation and identity of the animals. On all colonic sections with transversely cut mucosa and full mucosal thickness, measurements of crypt length were performed utilising a calibrated eyepiece micrometer.
2.6. Immunohistochemistry
From four experimental pigs with typical lesions of swine dysentery and from two control pigs, paraffined biopsies taken 30 cm from the caeco-colic orifice were used for immunohistochemistry for T and B lymphocytes. Sections for T cells were pre-treated with 0.05% pronase (DAKO A/S, Glostrup, Denmark) for antigen retrieval. Sections in duplicate were consecutively treated with 1.5% hydrogen peroxide to quench endogenous peroxidase and with normal horse serum to minimise non-specific protein binding (Vector Laboratories inc., California, USA). The sections were then incubated with rabbit polyclonal anti-human CD3ε diluted 1:100 (T-cell marker) or with mouse monoclonal anti-human CD79a diluted 1:100 (activated B-cell marker, Novocastra Laboratories Ltd, Newcastle upon Tyne, United Kingdom), respectively. The sections were further incubated with biotinylated panspecific horse immunoglobulins diluted 1:50 as secondary antibody (Vectastain®, Vector Laboratories inc., California, USA), followed by streptavidin-biotin-complex (DAKO A/S, Glostrup, Denmark). Sections incubated with non-immune rabbit immunoglobulins (DAKO A/S, Glostrup, Denmark) and mouse IgG2 was used as specificity control. Diaminobenzidine was used as chromogen and hematoxylin as counterstain. From each section, counts were performed in five well-oriented crypts. The CD3+ intraepithelial cells were counted and related to the crypt height. CD3+ and CD79a+ cells were counted in two areas (2500 μm2) in the lamina propria immediately above the lamina muscularis mucosae, and in two areas
(2500 μm2) immediately below the luminal epithelium. The sums of the counts in the areas (10,000 μm2) located between two transversely cut crypts were calculated.
2.7. Faecal analyses
For analyses of Brachyspira spp, faecal swabs (Transsystem Amies W CH, Copan Italia, Brescia, Italy) were taken before the study started, the first day of inoculation and once daily thereafter. The samples were analysed in accordance with Fellström and Gunnarsson (1995). Faecal samples taken before the start of the study and at slaughter were also analysed for Campylobacter spp., L. intracellularis, Yersinia enterocolitica, Escherichia coli, Salmonella spp., Clostridium spp., parasites and rotavirus by the respective routine procedures (including culture, PCR or serology) validated and used at the National Veterinary Institute (the rotavirus/coronavirus EIA kit, SVANOVA Biotech, Uppsala, Sweden; Anon, 1986, Jacobson et al., 2000, NCFA, 1987, NCFA, 1990, NCFA, 1999, Quinn et al., 1994, Söderlind et al., 1988, Yoo et al., 1997).
2.8. Blood analyses
Blood samples were collected from the jugular vein before the start of the study, at onset of diarrhoea, 12 h after onset, on occurrence of haemorrhagic diarrhoea and then once daily until slaughter. Vacuum tubes with EDTA were used for analyses of total WBC and differential counts (Cell-Dyn 3500, Abbott, Wiesbaden, Germany). Samples from the two inoculated, non-cannulated pigs were also analysed by flow cytometry for sub-populations of lymphocytes and these results have been presented elsewhere (Jonasson et al., 2003). Sera were extracted from tubes without additives and stored at −80 °C until analysed for cortisol and the acute phase proteins SAA and haptoglobin by ELISA (Tridelta Phase™ range Haptoglobin Assay, Tridelta Phase™ range SAA kit, Tridelta Development Limited, Greystones, Wicklow, Ireland), or RIA kits (Coat-A-Count, Diagnostic Products Co, Los Angeles, USA; Jacobson et al., 2001, Makimura and Suzuki, 1982).
2.9. Statistics
Comparisons between the experimental and control pigs were performed by the Mann–Whitney rank sum test, and comparisons before and after inoculation were performed by the Wilcoxon signed rank test (SigmaStat statistical software, SPSS Science, Chicago, USA). Statistical significance was considered at p < 0.05. The values are presented as mean ± standard deviation, if not otherwise indicated.
3. Results
3.1. Clinical examination and daily weight gain
The inoculated animals were clinically healthy until the development of dysentery and the non-inoculated controls were healthy throughout the study. The mean incubation period was 12 days (range 5–19 days). The first sign of disease was a greyish-brown loose stool, followed, usually within 1 day, by mucohaemorrhagic diarrhoea. Eight out of nine pigs developed haemorrhagic diarrhoea that lasted for 2–5 days, before the pigs started to recover. In seven pigs, the general condition was slightly to moderately depressed during the disease, as indicated by a decreased appetite, gnashing of teeth and a tendency to rest more than usual. One pig (no. 7) was more severely depressed and showed anorexia for 3 days and was unwilling to rise. One pig developed non-haemorrhagic diarrhoea only and recovered after 1 week. In addition, one animal (no. 5) developed a prolapse of the intestine in the cannulated area on the fourth day of disease and was promptly sacrificed. With one exception, the body temperature among all pigs ranged from 37 °C to 40.1 °C throughout the study. Disease did not alter the body temperatures (range 37.5–39.8 °C), except for one pig with a body temperature of 40.6 °C on the last day of haemorrhagic diarrhoea.
On arrival, the average weight was 14.7 kg (range 10.5–18.0 kg) and the average d.w.g. during the acclimatisation period was 385 ± 191 g. The d.w.g. from surgery to inoculation was 551 ± 175 g in the cannulated pigs, and 676 ± 188 g in the two non-cannulated pigs. The d.w.g. from inoculation until the development of dysentery was 666 ± 262 g in the seven cannulated and inoculated pigs, 750 ± 253 g in the two non-cannulated, inoculated pigs, and 804 ± 25 g in the two cannulated, non-inoculated control pigs. During the endoscopy period the cannulated, inoculated pigs lost 611 ± 392 g/day, and the non-cannulated, inoculated pigs lost 263 ± 194 g/day. The cannulated, non-inoculated control pigs gained 412 ± 77 g/day.
3.2. Haematologic analyses
The changes in haptoglobin, SAA and cortisol are illustrated in Fig. 1 . The SAA values of the cannulated, non-inoculated pigs were below the detection limit of the method (<9.8 mg/L) on all sampling occasions. The haptoglobin values in these pigs ranged from 1.0 to 2.3 g/L throughout the study. In all inoculated pigs (n = 9), SAA was below the detection limit before the start of the study, and the mean haptoglobin concentration was 2.5 ± 0.7 g/L. An increase (p < 0.01) to 185 ± 284 mg/L SAA and to 3.9 ± 1.9 g/L haptoglobin was detected at onset of diarrhoea. When haemorrhagic diarrhoea occurred, a further increase to 710 ± 1012 mg/L SAA (p < 0.01) and 6.0 ± 1.7 g/L haptoglobin (p < 0.001) was seen. Twelve hours after the onset of diarrhoea, the mean values for the 2 pigs that had not yet developed haemorrhagic diarrhoea were 183.6 mg/L for SAA and 4.85 g/L for haptoglobin. Thereafter, the values in all pigs ranged from 22 to >5000 mg/L SAA and from 4.9 to 12.3 g/L haptoglobin during the course of haemorrhagic dysentery. At the start of recovery, a decrease to 64 mg/L SAA (p < 0.05) and 5.4 ± 0.8 g/L haptoglobin (not significant) was seen. At sacrifice, the mean haptoglobin was 5.2 ± 1.5 g/L and the mean SAA was 40 mg/L.
Fig. 1.
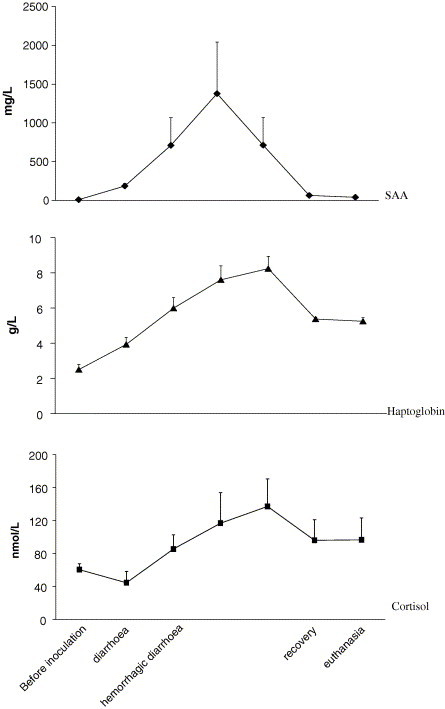
The serum concentrations of the acute phase proteins serum amyloid A (SAA), haptoglobin and cortisol in nine pigs that developed swine dysentery after an experimental challenge with Brachyspira hyodysenteriae. Samples were collected before inoculation, at onset of diarrhoea, when hemorrhagic diarrhoea occurred and once daily thereafter until the day of euthanasia.
Before inoculation, the mean cortisol value was 60 ± 66 nmol/L (range 10–215 nmol/L) in all pigs. In non-inoculated cannulated pigs, the values ranged from 56 to 200 nmol/L during the study. At onset of diarrhoea, the values ranged from 19 to 81 nmol/L. The mean cortisol values peaked on the third day of haemorrhagic diarrhoea with a mean value of 137 ± 88 nmol/L. The differences were not significant.
The total and differential white blood cell counts are shown in Table 1 . The total white blood cell count in the non-inoculated, cannulated pigs varied between 10.1 and 28.5 × 109 cells/L during the study, the highest value being recorded prior to the start of the study. In the experimentally inoculated pigs, monocytes increased (p < 0.05) when diarrhoea occurred. A further increase was seen during haemorrhagic diarrhoea (p < 0.001), also reflected in an increase in the total white blood cell counts (p < 0.05). When the pigs started to recover, as judged by their general condition and the consistency and colour of the faeces, the number of monocytes was still increased (p < 0.01). In addition, during the recovery phase, eosinophils increased (p < 0.05). No other differences were observed.
Table 1.
The total and differential leucocyte counts in nine pigs experimentally inoculated with Brachyspira hyodysenteriae, and in two non-inoculated pigs
| Before inoculation | Diarrhoea | Haemorrhagic diarrhoea (n = 8) | Recovery | ||
|---|---|---|---|---|---|
| Dysentery (n = 9) | WBC (109 cells/L) | 14.5 ± 4.9 | 17.7 ± 3.0 | 21.4 ± 4.5⁎ | 22.6 ± 7.2 |
| Neutrophils (109 cells/L) | 6.5 ± 2.5 | 8.0 ± 2.7 | 10.1 ± 4.4 | 12.6 ± 7.5 | |
| Lymphocytes (109 cells/L) | 6.5 ± 2.1 | 6.7 ± 2.2 | 6.5 ± 2.4 | 6.8 ± 1.3 | |
| Monocytes (109 cells/L) | 1.4 ± 0.6 | 2.8 ± 1.3⁎ | 4.0 ± 0.8⁎⁎⁎ | 3.1 ± 1.1⁎⁎ | |
| Eosinophils (109 cells/L) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.5 ± 0.3⁎ | |
| Healthy (n = 2) | WBC (109 cells/L) | 24.2 ± 6.1 | 11.8 ± 2.4 | 15.1 ± 2.5 | 15.8 ± 2.1 |
| Neutrophils (109 cells/L) | 10.4 ± 5.1 | 4.8 ± 2.2 | 6.6 ± 2.0 | 6.8 ± 2.0 | |
| Lymphocytes (109 cells/L) | 11.0 ± 0.6 | 5.4 | 6.6 ± 0.1 | 6.9 ± 0.1 | |
| Monocytes (109 cells/L) | 2.6 ± 0.3 | 1.4 ± 0.2 | 1.6 ± 0.4 | 1.8 ± 0.1 | |
| Eosinophils (109 cells/L) | 0.2 ± 0.1 | 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 |
The samples compared were collected before inoculation, when diarrhoea occurred, at the first sign of haemorrhagic diarrhoea and when the pigs started to recover. The healthy, non-inoculated pigs were sampled at corresponding time points.
WBC, white blood cell count.
vs. day 0: p < 0.05
vs. day 0: p < 0.01.
vs. day 0: p < 0.001.
3.3. Faecal analyses
Before the start of the study, all pigs cultured negative to B. hyodysenteriae. In three cannulated and one non-cannulated pig, B. innocens and/or B. murdochii were isolated. Haemolytic E. coli O45 K88− 99− was detected in one non-inoculated pig. In one cannulated, inoculated pig, small amounts of Strongyloides sp. were found. Further, haemolytic E. coli O8 K88− 99− was confirmed in two cannulated, inoculated pigs at sacrifice. L. intracellularis was not demonstrated. During the inoculation period, B. hyodysenteriae was isolated in faeces from one pig. In other animals, the first isolation was 5–15 days after challenge. In six animals, the shedding ceased when the animals were recovering clinically and the consistency of the faeces started to increase. At sacrifice, three animals were positive for B. hyodysenteriae, and two of them were still clinically affected.
3.4. Endoscopy
Complete emptying of the colon was sometimes difficult to achieve in healthy pigs, however, an adequate inspection of the mucosa and the collection of biopsy specimens were still possible. Before inoculation, the mucosa was smooth with a pale pink colour and distinct vessels (Fig. 2 a). A similar picture was seen on all occasions in the cannulated non-inoculated animals. The macroscopic lesions seen by endoscopy generally normalised within a week. The following alterations were observed: when diarrhoea was first observed, the mucosa was reddish and with a glistening, rough and perturbed appearance, the vessels were indistinct or invisible, and increased mucus with flecks of a yellowish fibrin-like material dispersed over the mucosa were seen (Fig. 2b). Two days later, at the second day of haemorrhagic diarrhoea, a similar but aggravated picture was present with more prominent yellowish flecks and scattered haemorrhagic areas (Fig. 2c). Increased haemorrhage was noted at the biopsy sites. On the fifth day of clinical disease, the mucosa was still red and glistening, but areas with a smooth appearance and distinct vessels were seen alternating with flecks of yellowish material and dark, brownish-red demarcated spots (Fig. 2d). In two pigs examined on the seventh day, the mucosa appeared normal. In one pig, an excessive amount of mucus was present at the first endoscopy and before onset of haemorrhagic diarrhoea (Fig. 2e). This was also the case in the inoculated pig not developing haemorrhagic diarrhoea. Two days later, the intestine was covered with confluent areas of mucus and yellowish material (Fig. 2f). On the fifth day, mucus and yellowish material were less abundant and the underlying mucosa had a pale pink colour.
Fig. 2.
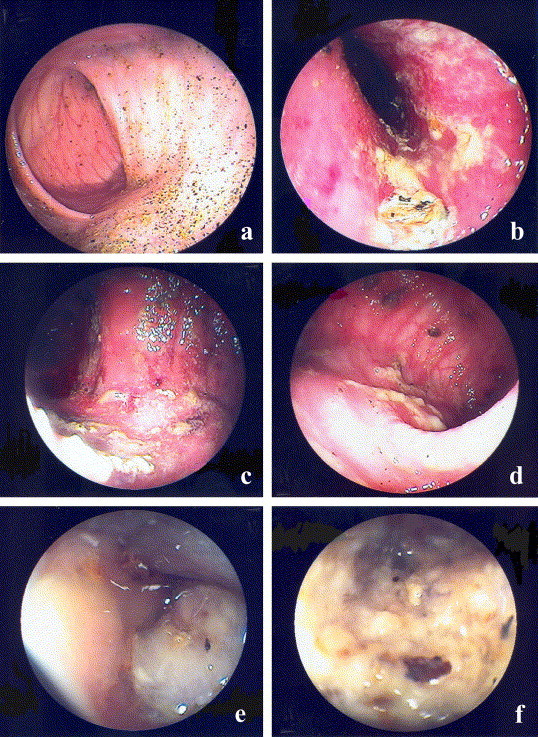
The general endoscopic appearance of the spiral colon of a healthy pig (a) before inoculation and in pigs with overt swine dysentery (b, c, d) after an experimental challenge with Brachyspira hyodysenteriae. (a) Shows the mucosa with a smooth surface, pale colour and distinct vessels before inoculation. (b) Shows the mucosa during the first day of diarrhoea with a perturbed appearance, indistinct vessels and increased amounts of mucus and yellowish material. (c) Shows the mucosa on the third day when haemorrhagic diarrhoea was present. Note the more prominent lesions and areas of haemorrhages. (d) Visualises the gut on the fifth day of disease. The mucosa is still hyperaemic but areas with a smooth appearance and distinct vessels are seen alternating with flecks of yellowish material and demarcated dark spots. (e) Shows the excessive amount of mucus occasionally occurring in the lumen. (f) Shows the intestine of a pig that developed diarrhoea without haemorrhages. On the third day of disease, the mucosa was covered with confluent areas of mucus and yellowish material.
3.5. Qualities of biopsy specimens
The sizes of the biopsies was in general 3–4 mm2 (range 1–4 mm2). Most sections had areas of well-oriented mucosa, showing full mucosal thickness, but tangentially cut areas were common. Submucosal tissue was often included, though with marked variations in depth, and was not evaluated. On three occasions, the ileum was accidentally sampled instead of the colon and these specimens were not included in the study.
3.6. Histology in biopsies
-
I.
Control pigs and pre-inoculation biopsies from infected pigs
In all biopsies, crypts abutted to the lamina muscularis mucosae. The maximum crypt length in the different biopsy specimens was 330–440 μm (mean 369 μm).
A marked predomination of goblet cells containing acid, neutral or mixed mucins were noted in the epithelium throughout the crypts. Crypt lumina were generally not, or only slightly, dilated by mucus (Fig. 3 a–b). The cellularity of lamina propria mainly included lymphocytes and plasma cells, admixed with macrophages. In about 30% of the biopsies from all but one pig, minimal to mild multifocal infiltrates of neutrophils were recorded in the crypt epithelium or the lamina propria. No consistent morphological variation related to biopsy sites or sampling occasion was noted.
-
II.
Pigs inoculated with B. hyodysenteriae
Histological findings are summarised in Table 2 . Generally, the overall cellularity of the lamina propria did not increase, as compared to the non-inoculated pigs. Except in one pig where the maximum crypt length had not increased at the first endoscopy p.i. (Fig. 3c, d), crypt hyperplasia was evident on all occasions after onset of diarrhoea, and from the maximal crypt length recorded in individual pigs (Fig. 3e–g) it seemed that crypt hyperplasia were more marked after a few days of diarrhoea. A marked depletion of goblet cell mucins at the crypt base was noted especially during the first 3 days and did sometimes involve the major length of the crypts (Fig. 3f, g). This was often associated with prominent accumulates of mucins in dilated crypt lumina (Fig. 3f) and on the mucosal surface. Goblet cell hyperplasia, i.e. hyperplastic crypts with almost complete predominance of crowded and intact goblet cells, was seen only in biopsies from the fifth day. The surface epithelium was often pleomorphic and markedly attenuated (Fig. 3e). Shallow mucosal erosions with sero- and fibrinocellular to haemorrhagic exudates were frequently observed on the first and second biopsy occasions and were abundant in some biopsies (Fig. 3h). Mild infiltrates of neutrophils were seen at least once in all pigs. In three pigs at the first and in one pig at the third sampling, marked neutrophil infiltration occurred in the superficial lamina propria, sometimes adjacent to erosions. Large pools of mucopurulent and fibrinocellular to fibrinonecrotic exudate often occurred on the mucosal surface, especially at 1–3 days after onset of diarrhoea. Warthin Starry staining revealed foci of numerous coiled spirochetes on the mucosal surface.
Fig. 3.
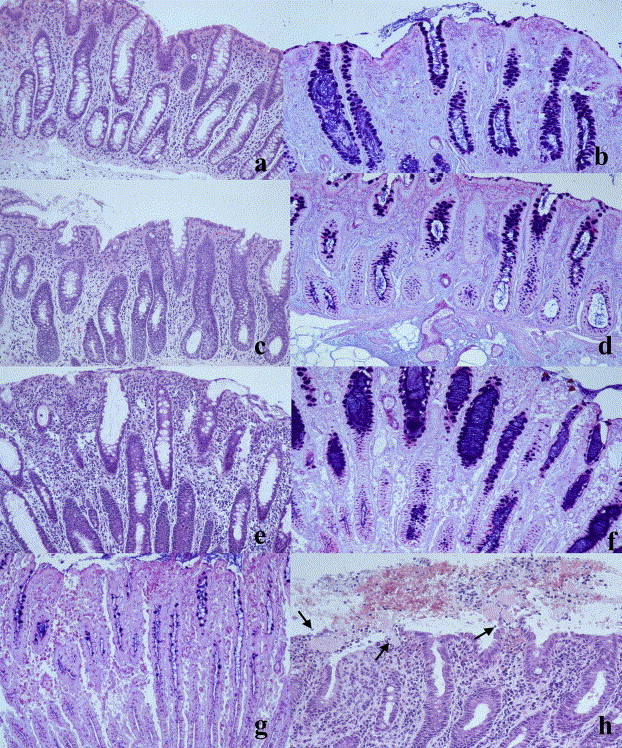
Histology of biopsies of colonic mucosa. (a) and (b): non-inoculated control pig. Marked predominance of goblet cells throughout the length of the crypts. Some mucin in crypt lumina and on mucosal surface (b). (c) and (d): Brachyspira hyodysenteriae-infected pig, first day of diarrhoea. Goblet cells of lower part of crypts depleted of mucins (d) but crypt length within the variation observed in non-inoculated pigs. (e) and (f): B. hyodysenteriae-infected pig with hemorrhagic diarrhoea, fifth day of disease. (for endoscopical picture, see Fig. 2d). Hyperplastic dilated crypts and markedly attenuated surface epithelium (e). Depletion of goblet cell mucins, mainly in basal crypts areas, and mucin-filled crypt lumina (f). (g): B. hyodysenteriae-infected pig, first day of diarrhoea. Hyperplastic crypts markedly depleted of goblet cell mucins in their major part. (h): B. hyodysenteriae-infected pig, first day of diarrhoea. Superficial erosions with fibrinocellular and haemorrhagic exudation (arrows). a, c, e: H&E × 220; h:H&E, × 275; b, d, f, g: Alcian blue-PAS × 220.
Table 2.
Light microscopical features of biopsy specimens before (biopsy 0) and after inoculation with Brachyspira hyodysenteriae
| Pig no. | 0 |
1 |
2 |
3 |
4 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum crypt length (μm) | Maximum crypt length (μm) | Depletion of goblet cell mucusa | Excess luminal mucus | Goblet cell hyperplasia | Mucosal erosions | Exudate on mucosal surface | Maximum crypt length (μm) | Depletion of goblet cell mucusa | Excess luminal mucus | Goblet cell hyperplasia | Mucosal erosions | Exudate on mucosal surface | Maximum crypt length (μm) | Depletion of goblet cell mucusa | Excess luminal mucus | Goblet cell hyperplasia | Mucosal erosions | Exudate on mucosal surface | Maximum crypt length (μm) | Depletion of goblet cell mucusa | Excess luminal mucus | Goblet cell hyperplasia | Mucosal erosions | Exudate on mucosal surface | |
| 1 | 352 | 682 | +A | + | − | − | − | 715 | +A | + | − | + | + | 605 | +A | + | + | − | + | ||||||
| 2 | 385 | 550 | +A | − | − | + | + | 770 | +A | + | − | − | + | 660 | +A | − | − | − | − | 660 | +A | + | + | − | − |
| 3 | 440 | 660 | +B | + | − | + | + | ||||||||||||||||||
| 4 | 385 | 660 | +A | + | − | + | + | 550 | +A | + | − | + | + | ||||||||||||
| 5 | 440 | 660 | +A | − | − | + | − | ||||||||||||||||||
| 6 | 440 | 715 | +B | + | − | − | + | 880 | +A | + | − | − | + | 770 | +A | + | + | − | − | ||||||
| 7 | 440 | 440 | +A | + | − | − | − | 825 | +B | + | − | + | + | 660 | +A | − | + | + | − | ||||||
Biopsies 1–4 taken with 2–3 days interval starting 1 day after onset of diarrhoea (biopsy 1).
A, in lower part of crypts; B, in major part of crypts.
3.7. Immunohistochemistry
The mean numbers of intraepithelial CD3+ cells per unit length (50 μm) in all pigs before inoculation and in the control pigs throughout the study was 65 ± 25 cells. In the experimentally inoculated pigs, a decrease was seen at the first (37 ± 27, p < 0.05), second (27 ± 19, p < 0.001) and third (10 ± 5, p < 0.001) sampling occasion after the onset of diarrhoea. No distinct pattern was noted for lamina propria CD3+cells or CD79a+ cells.
3.7.1. Necropsy
In the two non-infected, cannulated pigs, no other gross or microscopic lesions were found and the maximum crypt length in the spiral colon was 462 and 495 μm, respectively. Major macroscopic colonic lesions and light microscopic features of the proximal spiral colon of inoculated pigs are shown in Table 3 . Individual pigs also had similar or less pronounced microscopic lesions, consistent with B. hyodysenteriae infection, in other parts of the spiral colon and sometimes in the caecum. Two pigs showed intramural microabscesses in the caecum, and one of these also had microabscesses in regional lymph nodes. The other pig had focal caecal ulceration and an acute intussusception of small bowel into the caecum. Five cannulated pigs showed small, focal gastroesophageic ulcers. Occasional findings included moderate stasis of neutrophils in pulmonary small blood vessels and mild acute glomerulitis. Local, generally grossly indolent, fibrous peritonitis in the area of surgery was the rule in cannulated pigs. A few, about 0.5 cm long, sharply delineated, rodshaped active mucosal ulcers corresponding to biopsy sites were observed in the spiral colon in both inoculated and non-inoculated pigs.
Table 3.
Necropsy findings in the spiral colon of pigs inoculated with Brachyspira hyodysenteriae
| Major gross findings in spiral colon | Light microscopy of proximal spiral colon | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pig no. | Group | Day(s) post-end of diarrhoea | Maximum crypt length (μm) | Depletion of goblet cell mucusa | Excessive luminal mucus | Goblet cell hyperplasia | Exudate on mucosal surface | Mucosal ulceration | |
| 1 | 5 | None | 770 | − | + | + | − | − | |
| 2 | 1 | Excess mucus, hyperaemia, edema, thick granular luminal folds | 1210 | ++ | + | − | − | +b | |
| 3 | 0 | Semifluid ingesta | 880 | − | + | + | − | − | |
| 4 | Infected, cannulated | 3 | None | 682 | − | − | + | − | − |
| 5 | 0 | Excess mucus, hyperaemia | 990 | − | + | + | − | − | |
| 6 | 3 | Semifluid ingesta, focal confluent mucosal ulcers | 1012 | + | + | − | + | + | |
| 7 | 1 | Granular luminal folds, melaena | 759 | + | + | + | − | +b | |
| 10 | Infected, not cannulated | 1 | Excess mucus, hyperemia, edema, dull mucosa | 968 | + | + | − | + | − |
| 11 | 0 | Excess mucus, hyperaemia, edema, granular luminal folds | 990 | + | + | − | + | − | |
Seven pigs were cannulated in caecum and subjected to endoscopy at scheduled time points during the course of disease. Six pigs were sacrificed after cessation of clinical signs, and two pigs (no. 5 and 11) during disease.
+, in lower part of crypts only; ++, in major part of crypts.
Few rodshaped biopsy-related ulcers.
4. Discussion
Endoscopy provided a detailed three-dimensional, vivid macroscopic picture of the gut. The procedure did not seem to influence the development of intestinal lesions as judged by comparisons with the inoculated non-cannulated pigs, as well as with previous findings (Jacobson et al., 2001, Jacobson et al., 2004). It was also possible to relate clinical signs, acute phase proteins and white blood cell counts to the development of lesions. Observations made during endoscopy was largely consistent with previous observations made in studies on serial necropsies (Glock and Harris, 1972, Meyer, 1978, Wilcock and Olander, 1979), although the time-points for single observations differed somewhat. All but one pig started to recover within a week and therefore usually two to three endoscopies were performed during disease.
Altogether, the development of lesions in the pigs followed a similar pattern: Initially, the animals’ general condition was unaltered and greyish-brown diarrhoea was accompanied by a hyperaemic, oedematous and perturbed mucosa with crypt hyperplasia and excessive shedding of mucus. On histology, the overall cellularity of the lamina propria appeared unchanged compared to controls but the number of intraepithelial lymphocytes was decreased. Superficial erosions were common. Further, the systemic immune response was newly activated. After 1 day, the disease usually aggravated to haemorrhagic diarrhoea with slightly depressed general condition. On the third day, the mucosa was covered by granular yellow debris and haemorrhagic areas with erosions were frequently seen. The crypt length had increased and the number of intraepithelial lymphocytes still decreased. Systemically, an increase in numbers of monocytes and acute phase proteins was noted. Five days after the development of clinical signs, the pigs started to recover; the lesions in the intestine were demarcated and interspersed among areas of apparently normal mucosa. On histology, crypt hyperplasia was still evident and goblet cells in the lower part of the crypts were depleted of mucus. The cellular infiltration in the mucosa was not prominent, and the number of monocytes and levels of SAA and haptoglobin in plasma decreased. After one week, the pigs that had a longer course of disease was clinically recovered with a normal mucosa but crypt hyperplasia and an increased amount of mucus were still evident.
Lesions in the pig not developing haemorrhagic diarrhoea were consistent with previous observations by Wilcock and Olander (1979), described as mucosal oedema with a thick layer of tenacious mucin and few erosions or haemorrhages. The lesions in pigs with a moderately to severely depressed condition did not differ macroscopically from lesions in pigs with slightly depressed general behaviour. When viewed by light microscopy, the lesions have been reported to progress from catarrhal to fibrinonecrotic enteritis as the disease progresses (Glock et al., 1974). Others report that the severity of the lesions does not increase with duration of the disease (Wilcock and Olander, 1979). Crypt hyperplasia is a consistent feature of the disease (Glock et al., 1974, Wilcock and Olander, 1979). In the present study, it seemed that crypt hyperplasia was more marked after a few days of diarrhoea and goblet cell hyperplasia became prominent on the fifth and seventh day after the development of diarrhoea. Crypt elongation has been reported to be caused by increased regeneration of cells in areas with surface necrosis (Wilcock and Olander, 1979). However, in the present study crypt hyperplasia was found independently of erosions, as also reported by Jensen et al. (1998). Among infected pigs, the crypt length found at necropsy was generally increased, as compared to the length measured in the biopsies. The significance of this is unclear but may be caused by mucosal contraction when the biopsy was gripped (van der Gaag and Happé, 1990). Some authors have suggested that the disease begins with acute necrosis of the surface epithelium induced by toxin production (Albassam et al., 1985, Hughes et al., 1975). However, mucosal erosions were not a consistent feature in the biopsies of the early diarrhoeic phase in the present study. The depletion of mucus from the goblet cells in the basal part of the crypts is commonly reported (Lysons et al., 1984, Pohlenz et al., 1983, Wilcock and Olander, 1979), however, it is unclear whether this results from an increased expulsion of mucus or by diminished mucigen formation in immature proliferating goblet cells (Wilcock and Olander, 1979). Goblet cell hyperplasia and mucus hypersecretion was prominent in later stages, as also reported by Lysons et al. (1984) but in contrast to Glock et al. (1974), who regarded this as pronounced feature of the early course of the disease. In the present study, the overall cellularity of the lamina propria did not appear to be altered. This is consistent with Hughes et al., 1975, Albassam et al., 1985, who reported that the initial local inflammatory cell response was minor but that numerous macrophages and neutrophils were present in response to necrosis in later stages.
A prominent elevation of the acute phase proteins in response to haemorrhagic diarrhoea confirmed previous observations (Jacobson et al., 2004). In the present study, SAA peaked on the second to fourth day after the beginning of diarrhoea, whereas haptoglobin peaked on the third to fifth day (Fig. 1). In previous studies, SAA peaked the first day and haptoglobin the second day after surgery (Jacobson et al., 2001). Taken together, this suggests that the acute phase response to swine dysentery was induced in response to intestinal lesions. The pig that never developed haemorrhagic diarrhoea had histological mucosal erosions in the first and second endoscopy during overt disease and concomitantly increased levels of SAA and haptoglobin (data not shown).
There was no strict relationship between the magnitude of the acute phase protein response and the severity of disease although the four pigs with the most severe clinical signs also had the highest levels. In addition, the increase of the two proteins was not always correlated. However, ceasing to eat was associated with very high levels of one or both of the acute phase proteins studied (SAA > 5000 mg/L and haptoglobin 12.1 g/L, respectively). Thus, there seems to be an individual variation in the magnitude of the response, probably related to various expressions of the cytokines IL-1 and IL-6 that are main inducers of haptoglobin and SAA (Jacobson et al., 2004, Mackiewicz et al., 1991, Uhlar and Whitehead, 1999). These cytokines are referred to as endogenous pyrogens and act on the temperature-regulating centre in hypothalamus (Mims et al., 2001). Thus, an elevated body temperature might be expected. Hughes et al. (1975) reported an increase of body temperature of 1.1–1.7 °C during experimentally induced dysentery. However, except for one pig with a peak haptoglobin of 9.1 g/L correlated to a body temperature of 40.6 °C, no increase in body temperature was recorded in the present study. Thus, fever does not seem to be a prominent feature of swine dysentery.
Glucocorticoids enhance the induction of the APP synthesis and exert a negative feed back on cytokine production (Higuchi et al., 1994, Uhlar and Whitehead, 1999). In the present study, no significant increase in cortisol in response to disease was detected. However, compared with samples collected before inoculation, the mean cortisol level was 113 nmol/L higher during the course of haemorrhagic diarrhoea (Fig. 1). This elevation was rather small and a large variation was seen between both individuals and the day of the peak value in relation to disease, which might explain why no significance was apparent.
The decrease in the numbers of epithelial CD3+ cells in the inoculated pigs following the development of diarrhoea is in agreement with Hontecillas et al. (2005). In that study, a depletion of intraepithelial γδ lymphocytes was seen during disease. This is also a feature of other enteric diseases (MacIntyre et al., 2003) and warrants further investigation.
The development of dysentery was associated with severe weight losses probably caused partly by decreased appetite and partly because of altered metabolism due to the intestinal disorder (Somchit et al., 2003). The average incubation period and the bacterial shedding preceding the development of diarrhoea is in agreement with previous studies (Hughes et al., 1975, Fisher and Olander, 1981, Jensen et al., 1998) and with observations that some animals carry the bacteria in the intestine for several weeks without shedding (Hampson et al., 1992). Possibly, alterations in the intestinal environment caused by dietary changes such as inclusion of large quantities of soybean meal might produce favourable conditions for the bacteria, allowing it to multiply and induce clinical signs of disease. Alternatively, an imbalance in the intestinal eco-system causes the expulsion of the bacteria allowing it to be detected in the faeces.
In conclusion, this method enables repeated sampling of quality biopsies that can be used in detailed study of the kinetics of swine dysentery using small number of experimental animals allowing a high precision of the experiment. The method will be a useful model in future studies on the pathogenesis and control of swine dysentery.
Acknowledgements
This study was financed by grants from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning. We express our gratitude to Ulla Hammarström and Briitta Ojava for laboratory assistance, to Folke Lindberg, who supported us with animals, and to Jan Erik Lindberg and Bengt Pettersson for providing the cannulas.
References
- Albassam M.A., Olander H.J., Thacker H.L., Turek J.J. Ultrastructural characterization of colonic lesions in pigs inoculated with Treponema hyodysenteriae. Canadian Journal of Comparative Medicine. 1985;49:384–390. [PMC free article] [PubMed] [Google Scholar]
- Anon . Manual of veterinary parasitological laboratory techniques. third ed. Her Majesty’s Stationary Office; London: 1986. pp. 11–12. [Google Scholar]
- Fellström C., Gunnarsson A. Phenotypic characterisation of intestinal spirochaetes isolated from pigs. Research in Veterinary Science. 1995;59:1–4. doi: 10.1016/0034-5288(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Fisher L.F., Olander H.J. Shedding of Treponema hyodysenteriae, transmission of disease, and agglutinin response of pigs convalescent from swine dysentery. American Journal of Veterinary Research. 1981;42:450–455. [PubMed] [Google Scholar]
- Galvin J.E., Harris D.L., Wannemuehler M.J. In: Intestinal Spirochaetes in Domestic Animals and Humans. Prevention and Control of Intestinal Spirochaetal Disease: Immunological and Pharmacological Mechanisms. Hampson D.J., Stanton T.B., editors. CAB International; Wallingford, UK: 1997. pp. 343–374. [Google Scholar]
- Glock R.D., Harris D.L. Swine dysentery II. Characterization of lesions in pigs inoculated with Treponema hyodysenteriae in pure and mixed culture. Veterinary Medicine/Small Animal Clinician. 1972:65–68. [PubMed] [Google Scholar]
- Glock R.D., Harris D.L., Kluge J.P. Localization of spirochetes with the structural characteristics of Treponema hyodysenteriae in the lesions of swine dysentery. Infection and Immunity. 1974;9:167–178. doi: 10.1128/iai.9.1.167-178.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D.J., Cutler R., Lee B.J. Virulent Serpulina hyodysenteriae from a pig in a herd free of clinical swine dysentery. Veterinary Record. 1992;131:318–319. doi: 10.1136/vr.131.14.318. [DOI] [PubMed] [Google Scholar]
- Harris D.L., Alexander T.J.L., Whipp S.C., Robinson I.M., Glock R.D., Matthews P.J. Swine Dysentery: Studies of gnotobiotic pigs inoculated with Treponema hyodysenteriae, Bacteroides vulgatus, and Fusobacterium necrophorum. Journal of American Veterinary Medical Association. 1978;172:468–471. [PubMed] [Google Scholar]
- Higuchi H., Katoh N., Miyamoto T., Uchida E., Yuasa A., Takahashi K. Dexamethasone-induced haptoglobin release by calf liver parenchymal cells. American Journal of Veterinary Research. 1994;55:1080–1085. [PubMed] [Google Scholar]
- Hontecillas R., Bassaganya-Riera J., Wilson J., Hutto D.L., Wannemuehler M.J. CD4+ T-cell responses and distribution at the colonic mucosa during Brachyspira hyodysenteriae – induced colitis in pigs. Immunology. 2005;115:127–135. doi: 10.1111/j.1365-2567.2005.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R., Olander H.J., Williams C.B. Swine dysentery: pathogenicity of Treponema hyodysenteriae. American Journal of Veterinary Research. 1975;36:971–977. [PubMed] [Google Scholar]
- Jacobson, M., Fellström, C., Heldtander, M., Gunnarsson, A., 2000. The prevalence of Lawsonia intracellularis in Swedish pigs submitted for autopsy. In: The Sixteenth International Pig Veterinary Society Congress, Melbourne, Australia, p. 74.
- Jacobson M., Fellström C., Lindberg R., Wallgren P., Jensen-Waern M. Experimental swine dysentery – comparison between infection models and studies of the acute phase protein response to infection. Journal of Medical Microbiology. 2004;53:273–280. doi: 10.1099/jmm.0.05323-0. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Lindberg J.E., Lindberg R., Hård af Segerstad C., Wallgren P., Fellström C., Hultén C., Jensen-Waern M. Intestinal cannulation: model for study of the midgut of the pig. Comparative Medicine. 2001;51:163–170. [PubMed] [Google Scholar]
- Jensen T.K., Boye M., Möller K., Leser T.D., Jorsal S.E. Association of Serpulina hyodysenteriae with the colonic mucosa in experimental swine dysentery studied by fluorescent in situ hybridization. Acta Pathologica, Microbiologica et Immunologica Scandinavica (APMIS) 1998;106:1061–1068. [PubMed] [Google Scholar]
- Jonasson R., Johannisson A., Jacobson M., Fellström C., Jensen-Waern M. Differences in lymphocyte subpopulations and cell counts before and after experimentally induced swine dysentery. Journal of Medical Microbiology. 2003;53:267–272. doi: 10.1099/jmm.0.05359-0. [DOI] [PubMed] [Google Scholar]
- Kang, B., Olander, H.J., 1990. Scanning electron microscopy of the colon inoculated with Treponema hyodysenteriae in colonic loops of swine. In: Eleventh International Pig Veterinary Society Congress, Lausanne, Switzerland, p. 117.
- Kinyon J.M., Harris D.L., Glock R.D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infection and Immunity. 1977;15:638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon, J.M., Harris, D.L., Glock, R.D., 1980. Isolation of Treponema hyodysenteriae from experimentally infected pigs at various intervals post-inoculation. In: The Sixth International Pig Veterinary Society Congress, Copenhagen, Denmark, p. 232.
- Lysons, R.J., Pohlenz, J.F.L., Harris, D.L., Whipp, S.C., Burrows, M.R., Lemcke, R.M., 1984. Treponema hyodysenteriae in large intestinal disease. In: The Eighth International Pig Veterinary Society Congress, Ghent, Belgium, p. 179.
- MacIntyre N., Smith D.G.E., Shaw D.J., Thomson J.R., Rhind S. Immunopathogenesis of experimentally induced proliferative enteropathy in pigs. Veterinary Pathology. 2003;40:421–432. doi: 10.1354/vp.40-4-421. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Speroff T., Ganapathi M.K., Kuschner I. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. The Journal of Immunology. 1991;146:3032–3037. [PubMed] [Google Scholar]
- Makimura S., Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Japanese Journal of Veterinary Science. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- Malavasi L.M., Nyman G., Augustsson H., Jacobson M., Jensen-Waern M. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Laboratory Animal. 2006;40:16–27. doi: 10.1258/002367706775404453. [DOI] [PubMed] [Google Scholar]
- Meyer R.C. Swine dysentery: a perspective. Advances in Veterinary Science and Comparative Medicine. 1978;22:133–158. [PubMed] [Google Scholar]
- Meyer R.C., Simon J., Byerly C.S. The etiology of swine dysentery. III. The role of selected gram-negative obligate anaerobes. Veterinary Pathology. 1975;12:46–54. doi: 10.1177/030098587501200107. [DOI] [PubMed] [Google Scholar]
- Mims C.A., Nash A., Stephen J. Mims’ pathogenesis of infectious disease. 5th ed. Academic Press; London, UK: 2001. p. 474. [Google Scholar]
- Moreng N.T., Quarles C.L., Fagerberg D.J., Moeller D.J. Pathogenesis and lesions of swine dysentery induced by artificial methods in early weaned pigs. Veterinary Medicine/Small Animal Clinician. 1980;75:1841–1844. [PubMed] [Google Scholar]
- NCFA, Nordic Committee on Food Analysis., 1987. Yersinia enterocolitica. Detection in food. Report No 117, second ed.
- NCFA, Nordic Committee on Food Analysis., 1990. Campylobacter jejuni/coli detection in foods. Report No 119, second ed.
- NCFA, Nordic Committee on Food Analysis. 1999. Salmonella detection in foods. Report No 71, fifth ed.
- Nuessen M.E., Joens L.A., Glock R.D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. Journal of Immunology. 1983;131:997–999. [PubMed] [Google Scholar]
- Pohlenz J.F.L., Whipp S.C., Robinson I.M. Zur Pathogenese der durch Treponema hyodysenteriae verursachten Dysenterie des Schweines. Deutsche Tierarztliche Wochenschrift. 1983;90:363–367. [PubMed] [Google Scholar]
- Pohlenz, J.F.L., Whipp, S.C., Robinson, I.M., Fagerland, J.A., 1984. A hypothesis on the pathogenesis of Treponema hyodysenteriae induced diarrhea in pigs. In: the Eighth International Pig Veterinary Society Congress, Ghent, Belgium, p. 178.
- Quinn P.J., Carter M.E., Markey B.K., Carter G.R. Mosby International; London, England: 1994. Clinical Veterinary Microbiology: Clostridium species. pp. 191–208. [Google Scholar]
- Raynaud J.P., Brunault G., Philippe J. Swine dysentery. Comparison of experimental diseases produced by infection with colonic mucosa or with Treponema hyodysenteriae, french strains, and of “natural” disease. Annales de Recherches Vétérinaires. 1980;11:69–87. [PubMed] [Google Scholar]
- Söderlind O., Thafvelin B., Möllby R. Virulence factors in Escherichia coli strains isolated from Swedish piglets with diarrhea. Journal of Clinical Microbiology. 1988;26:879–884. doi: 10.1128/jcm.26.5.879-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somchit A., Jensen-Waern M., Jacobson M., Essén-Gustavsson B. On the carbohydrate metabolic response to an experimental infection with Brachyspira hyodysenteriae (swine dysentery) in pigs. Scandinavian Journal of Laboratory Animal Science. 2003;30:57–64. [Google Scholar]
- ter Huurne A.A.H.M., Gaastra W. Swine dysentery: more unknown than known. Veterinary Microbiology. 1995;46:347–360. doi: 10.1016/0378-1135(95)00049-g. [DOI] [PubMed] [Google Scholar]
- Uhlar C.M., Whitehead A.S. Serum amyloid A, the major vertebrate acute-phase reactant. European Journal of Biochemistry. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- van der Gaag I., Happé R. The histological appearance of peroral small intestinal biopsies in clinically healthy dogs and in dogs with chronic diarrhoea. Journal of Veterinary Medicine A. 1990;37:401–416. [PubMed] [Google Scholar]
- Waters W.R., Sacco R.E., Dorn A.D., Hontecillas R., Zuckermann F.A., Wannemuehler M.J. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Veterinary Immunology and Immunopathology. 1999;69:75–87. doi: 10.1016/s0165-2427(99)00043-4. [DOI] [PubMed] [Google Scholar]
- Waters W.R., Hontecillas R., Sacco R.E., Zuckermann F.A., Harkins K.R., Bassaganya-Riera J., Wannemuehler M.J. Antigen-specific proliferation of porcine CD8aa cells to an extracellular bacterial pathogen. Immunology. 2000;101:333–341. doi: 10.1046/j.1365-2567.2000.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters W.R., Pesch B.A., Hontecillas R., Sacco R.E., Zuckermann F.A., Wannemuehler M.J. Cellular immune responses of pigs induced by vaccination with either a whole cell sonicate or pepsin-digested Brachyspira (Serpulina) hyodysenteriae bacterin. Vaccine. 2000;18:711–719. doi: 10.1016/s0264-410x(99)00266-2. [DOI] [PubMed] [Google Scholar]
- Whipp S.C., Robinson I.M., Harris D.L., Glock R.D., Matthews P.J., Alexander T.J.L. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infection and Immunity. 1979;26:1042–1047. doi: 10.1128/iai.26.3.1042-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock B.P., Olander H.J. Studies on the pathogenesis of swine dysentery. I. Characterization of the lesions in colons and colonic segments inoculated with pure cultures or colonic content containing Treponema hyodysenteriae. Veterinary Pathology. 1979;16:450–465. doi: 10.1177/030098587901600409. [DOI] [PubMed] [Google Scholar]
- Yoo H.S., Lee S.U., Park K.Y., Park Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology. 1997;35:228–232. doi: 10.1128/jcm.35.1.228-232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


