Graphical abstract
A facile and high-yielding protocol for diverse benzotriazoles through intramolecular N-arylation of different o-chloro-1,2,3-triazenes using CuI/Cs2CO3 has been developed under mild reaction condition.

Keywords: Diazonium salt, Benzotriazole, Amines, CuI, N-Arylation
Abstract
A facile and high-yielding protocol for diverse benzotriazoles through intramolecular N-arylation of different o-chloro-1,2,3-benzotriazenes using CuI/Cs2CO3 has been developed.
There is an increased demand for significant amount of benzotraizole-containing molecules because of their interesting chemistry and tremendous chemotherapeutic values.1, 2, 3, 4 Potential drugs like Vorozole1a and Alizapride1b contain a benzotriazole skeleton. Several simple benzotriazole derivatives have been identified as agonists of the human orphan G-protein-coupled receptor GPR109b (HM74),2a inhibitors against different kinases,2b inactivators of severe acute respiratory syndrome 3CL protease,2c light-activable DNA cleaving agents2d and are also known to display an interesting selectivity profile in radio ligand-binding experiments.2e Furthermore, benzotriazole has proved to be a versatile synthetic auxiliary due to several advantages over other methodologies including inexpensive, non-toxic, highly stable, and operational simplicity.3 Thus the methodology nowadays recognized as the most successful synthetic protocol has grown from an obscure level to the level of very high popularity, since it can easily be introduced into the molecules by a variety of reactions, activates molecules toward numerous transformations, recognized as sufficiently stable during the course of the reaction, and finally can easily be removed too at the end of the reaction sequence.3, 4 In light of this importance, a facile and efficient method for the synthesis of benzotriazoles is an attractive objective.
The common synthetic methods for benzotriazole and their derivatives (Scheme 1 )5, 6, 7, 8, 9, 10, 11, 12 generally involve the reaction of azides with diazotized anthranilic acid as the benzyne precursor which suffers the harsh reaction condition and thus limiting the exploration of these reactions.5 Recently 1,2,3-triazoles6 were obtained by the reaction of aromatic amines with t-butyl nitrite and azidotrimethylsilane followed by CuI-catalyzed azide-alkyne 1,3-dipolar cycloaddition (click chemistry), and later on, similar chemistry has successfully been extended for an easy access to functionalized benzotriazoles.7 Nowadays, fluoride-triggered azide-benzyne cycloaddition strategies are being used for the synthesis of various benzotriazoles by treating substituted azides with 2-(trimethylsilyl) phenyl triflate in the presence of CsF.8, 9, 10
Scheme 1.

Synthesis of N1-benzotriazole under Click reaction condition.
A similar strategy was employed to obtain the triazoles with fused aromatic skeleton via 2,3-didehydronaphthalene intermediate.11 Solid-phase synthesis of 1H-benzotriazoles using Hartwig–Buchwald amination is another recent development in this promising area.12 Recently, Pereira et al. has described the ultrasound-assisted synthesis of 1-acylbenzotriazoles from diazotization of o-phenylenediamine.13 Carta constructed the triazole ring of 4-aminotriazolo[4,5-f]quinolines from 8-acetylamino-6-chloro-5-nitroquinoline through the reaction with ammonia or hydrazines at 150 °C14a and later on the method was extended for the synthesis of related heterocyclic skeletons.(b), (c)
Despite the advantages of the above described protocols for the synthesis of functionalized benzotriazoles, there are still some shortcomings like the use of hazardous chemicals, long reaction times, low reaction yields, requirement of absolute anhydrous conditions, limited availability of starting materials, harsh reaction conditions, and limited stability of the benzyne intermediate. Moreover, the hazardous nature of low molecular weight azides, warrant the search for a simple, short, and high-yielding alternate protocol to synthesize substituted benzotriazoles. Herein, a facile and high-yielding protocol for diverse benzotriazoles through intramolecular N-arylation of different o-chloro-1,2,3-benzotriazenes using CuI/base has been described.
Further to our ongoing research on benzotriazole-mediated novel synthetic methodologies,15 we commenced our synthetic strategy with environmentally benign o-chloroaniline, which on diazotization and in situ treatment with different aromatic amines including p-anisidine, 2-napthylamine, p-toluedine, and aniline afforded the intermediates o-chloro-1,2,3-benzotriazenes in good yields. The benzotriazenes (3a–f) on further treatment with CuI in the presence of Cs2CO3 led to the formation of various N1-subsituted benzotriazoles (4a–d, f) in good yields (Scheme 2 ). Thus, the synthesis of N1-subsutituted benzotriazoles was achieved in two steps. Step 1 includes the formation of intermediate 3a through diazotization and in situ coupling of amines whereas, step 2 involves the formation of desired N1-substituted benzotriazoles through N-arylation of the intermediates. In our early attempts to synthesize N1-substituted benzotriazole, we did not succeed in converting the benzotriazene intermediates into the desired products. The reaction was investigated carefully and we noticed that the intermediate formed after the coupling of diazonium salt of o-chloroaniline with amine 1 was not sufficiently stable. It was getting converted into p-aminoazoaryne, a thermodynamically favoured rearranged product after keeping at 45 °C for a short period of time. Hence, the reaction was carried out at 20 °C initially for 1 h to avoid decomposition and then allowed further to continue at 55 °C.
Scheme 2.

Synthesis of N1-substituted benzotriazoles via intramolecular N-arylation.
Having established a protocol for the synthesis of benzotriazole derivatives, we shifted our focus toward the role of solvents like CH2Cl2, CHCl3, DMF, and toluene upon yield and the reaction time. The results illustrated that the reaction in toluene did not give the desired benzotriazole, whereas, the reaction in CHCl3 was slow and did not proceed smoothly. However, for this cyclization CH2Cl2 was found to be good in terms of yield and handling but took a slightly longer time to afford the products. Eventually, DMF emerged as a solvent of choice for N-arylation of o-chloro-1,2,3-benzotriazenes by affording the desired product in very good yield. N-Arylation reactions are greatly influenced by the base used, therefore, to find out the appropriate base, we examined K2CO3 and Cs2CO3 in the cyclization reaction of 3a and found that the reaction in the presence of K2CO3 afforded the N1-substituted benzotriazole in 72% yield after a prolonged reaction (10 h) whereas Cs2CO3 gave this product in 80% yield. We believe that carbonate with a bigger counter cation may be more dissociated in aprotic solvents and consequently is more reactive. In order to enhance the yield further, we reviewed some more bases and noticed that for a similar type of cyclization, DABCO with ligand 1,10-phenanthroline has been successfully used.16 When the cyclization reaction 3a was carried out with DABCO in presence of 1,10-phenanthroline ligand, the yield of N1-substituted benzotriazole dropped drastically from 80% to 30% (Table 1 ). Therefore we explored another ligand, 1,8-naphthyridine which provides a 1,3 chelating site but this also gave a poor yield of the product.
Table 1.
Benzotriazole derivatives (4a–f) via intramolecular N-arylation
| Entry | Amine (2a, 1, 2c–f) | Condition | Product (4a–f) | Yielda (%) |
|---|---|---|---|---|
| 1 | 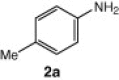 |
CuI, Cs2CO3, DMF, 8 h | 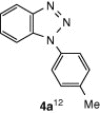 |
80 |
| 2 | 2a | CuI, Cs2CO3, DCM, 10 h | 4a | 70 |
| 3 | 2a | CuI, Cs2CO3, toluene, 8 h | NPb | — |
| 4 | 2a | CuI, K2CO3, DMF, 10 h | 4a | 72 |
| 5 | 2a | CuI, DABCO, 1, 10-phenanthroline, DMF, 8 h | 4a | 30 |
| 6 | 2a | CuI, DABCO , 1,8-naphthyridine, DMF, 8 h | 4a | 28 |
| 7 | 2a | CuI, DBU, DMF, 8 h | 4a | Trace amount |
| 8 | 2a | DIB, DMF, 24 h | 4a | Trace amount |
| 9 | 2a | Cs2CO3, DMF, 10 h | NPb | — |
| 10 | 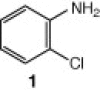 |
CuI, Cs2CO3, DMF, 8 h | 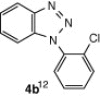 |
78 |
| 11 | 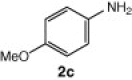 |
CuI, Cs2CO3, DMF, 8 h | 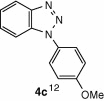 |
76 |
| 12 | 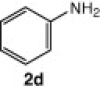 |
CuI, Cs2CO3, DMF, 8 h | 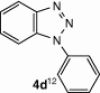 |
78 |
| 13 | 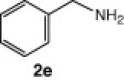 |
CuI, Cs2CO3, DMF, 8 h | NRb | — |
| 14 | 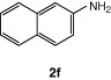 |
CuI, Cs2CO3, DMF, 8 h | 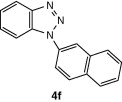 |
75 |
The reaction yield refers to product isolated through column chromatography (SiO2).
No desired product formation, confirmed by comparison with known compound.
The cyclization reaction of 3a in anhydrous DMF in the presence of Cs2CO3 without CuI was investigated and it resulted in the formation of undesired rearranged product. The failure of the reaction to afford the desired product confirmed the feasibility of this cyclization only under the catalysis of CuI. Then the cyclization of 3a through diacetoxyiodobenzene17 was tried and the reaction again did not occur smoothly and yielded the compound 4a in trace amounts. All the above studies suggest that CuI in combination with Cs2CO3 in anhydrous DMF is well suited for intramolecular N-arylation reaction. In another reaction, o-chloroaniline 1 was coupled in situ with its own diazonium salt to afford intermediate o-chloro-1,2,3-benzotriazene 3b in good yield which on treatment with CuI/Cs2CO3 in anhydrous DMF afforded 2-cholorophenyl benzotriazole 4b in good yield (78%). Unfortunately similar CuI-catalyzed cyclization reaction with benzyl-substituted compound did not provide the desired benzotriazole. Under similar reaction conditions a series of diverse N1-substituted benzotriazoles (4a–d, and f) were obtained in good to high yields using different amines such as p-toluedine, o-chloroaniline, p-anisidine, aniline, and 2-napthylamine (Scheme 2).18
In conclusion, a simple, efficient and novel method has been developed for an easy access to diverse N1-aryl benzotriazoles through intramolecular N-arylation of different o-chloro-1,2,3-benzotriazenes using CuI/Cs2CO3. The protocol offers several advantages including (a) mild reaction conditions; (b) simple work-up procedure; (c) moderately high yields of the desired products; and finally (d) the use of explosive azides is avoided. To the best of our knowledge this is the first report of this kind of intramolecular N-arylation reaction for an easy access to functionalized benzotriazoles. Efforts to widen the scope of the process on fused heterocycles as well as carbohydrate-based molecules of great chemotherapeutic value are under progress in our laboratory.
Acknowledgments
We thank CISC, BHU, and RSIC, CDRI for providing spectroscopic and analytical data of synthesized compounds; Dr. R. P. Tripathi, CDRI, Lucknow for useful suggestions and Professor K. N. Singh, BHU for providing MW facility. Grant-in-Aid from University Grant Commission, New Delhi, India is gratefully acknowledged.
References and notes
- 1.(a) Carlini R., Bria E., Giannarelli D., Ferretti G., Felici A., Papaldo P., Fabi A., Nistico C., Cosimo S. Di.; Ruggeri, E. M.; Milella, M.; Mottolese, M.; Terzoli, E.; Cognetti, F. Cancer. 2005;104:1335–1557. doi: 10.1002/cncr.21339. [DOI] [PubMed] [Google Scholar]; (b) Semple G., Skinner P.J., Cherrier M.C., Webb P.J., Sage C.R., Tamura S.Y., Chen R., Richman J.G., Connolly D.T. J. Med. Chem. 2006;49:1227–1230. doi: 10.1021/jm051099t. [DOI] [PubMed] [Google Scholar]
- 2.(a) Dipesa A.J., Donahue K.M., Dombroski M.A., Elliott N.C., Gabel C.A., Han S., Hynes T.R., LeMotte P.K., Mansour M.N., Marr E.S., Letavic M.A., Pandit J., Ripin D.B., Sweeney F.J., Tan D., Tao Y. J. Med. Chem. 2005;48:5728–5737. doi: 10.1021/jm050346q. [DOI] [PubMed] [Google Scholar]; (b) Wu C.Y., King K.Y., Fang J.M., Wu Y.T., Ho M.Y., Liao C.L., Shie J., Liang P.H., Wong C.H. Chem. Biol. 2006;13:261–268. doi: 10.1016/j.chembiol.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Katritzky A.R., Jiang J., Urogdi L. Tetrahedron Lett. 1989;25:3303–3306. [Google Scholar]; (d) Wender P.A., Touami S.M., Alayrac C., Philipp U.C. J. Am. Chem. Soc. 1996;118:6522–6523. [Google Scholar]; (e) Caliendo G., Greco G., Grieco P., Novellino E., Perissuttil E., Santagada V., Barbarul D., Esposit E., De Blas A. Eur. J. Med. Chem. 1996;31:207–213. [Google Scholar]
- 3.(a) Katritzky A.R., Lan X., Jason Z., Yang Z., Denisko O.V. Chem. Rev. 1998;98:409–548. doi: 10.1021/cr941170v. [DOI] [PubMed] [Google Scholar]; (b) Katritzky A.R., Manju K., Singh S.K., Meher N.K. Tetrahedron. 2005;61:2555–2581. [Google Scholar]; (c) Katritzky A.R., Rogovoy B.V. Chem. Eur. J. 2003;19:4586–4593. doi: 10.1002/chem.200304990. [DOI] [PubMed] [Google Scholar]; (d) Katritzky A.R., Lan X. Chem. Soc. Rev. 1994;23:363–373. [Google Scholar]; (e) Katritzky A.R., Henderson S.A., Yang B. J. Heterocycl. Chem. 1998;35:1123–1159. [Google Scholar]
- 4.(a) Verma A.K., Kesharwani T., Singh J., Tandon V., Larock R.C. Angew. Chem., Int. Ed. 2009;48:1138–1143. doi: 10.1002/anie.200804427. [DOI] [PubMed] [Google Scholar]; (b) Kale R.R., Prasad V., Mohapatra P.P., Tiwari V.K. Monatsh. Chem. 2010 [Google Scholar]
- 5.Reynolds G.A. J. Org. Chem. 1964;29:3733–3734. [Google Scholar]
- 6.Karine B., Adam D.M., John E.M. Org. Lett. 2007;9:1809–1811. [Google Scholar]
- 7.Zhang F., Moses J.E. Org. Lett. 2009;11:1587–1590. doi: 10.1021/ol9002338. [DOI] [PubMed] [Google Scholar]
- 8.Shi F., Waldo J.P., Chen Y., Larock R.C. Org. Lett. 2008;10:2409–2412. doi: 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandrasekhar S., Seenaiah M., Rao C.L., Reddy C.R. Tetrahedron. 2008;64:11325–11327. [Google Scholar]
- 10.Campbell-Verduyn L., Elsinga P.H., Mirfeizi L., Dierckx R.A., Feringa B.L. Org. Biomol. Chem. 2008;6:3461–3463. doi: 10.1039/b812403e. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T., Fukatsu N., Fujiwara Y. J. Org. Chem. 1998;63:8579–8581. [Google Scholar]
- 12.Zimmermann V., Brase S. J. Comb. Chem. 2007;9:1114–1137. doi: 10.1021/cc700105p. [DOI] [PubMed] [Google Scholar]
- 13.Pereira C.M.P., Stefani H.A., Guzenc K.P., Orfaoa A.T.G. Lett. Org. Chem. 2007;4:43–46. [Google Scholar]
- 14.(a) Sanna P., Carta A., Paglietti G. Heterocycles. 1999;50:693–702. [Google Scholar]; (b) Carta A., Boatto G., Paglietti G., Poni G., Setzu M.G., Caredda P. Heterocycles. 2003;60:833–842. [Google Scholar]; (c) Corona P., Piras S., Palomba M., Carta A. Mini-Rev. Org. Chem. 2008;5:295–302. [Google Scholar]
- 15.(a) Kale R.R., Prasad V., Tiwari V.K. Lett. Org. Chem. 2010;7:136–143. [Google Scholar]; (b) Tiwari V.K., Singh A., Hussain H.A., Mishra B.B., Tripathi V. Monatsh. Chem. 2007;138:1297–1302. [Google Scholar]; (c) Tiwari V.K., Hussain H.A., Mishra B.B., Singh D.D., Tripathi V. Med. Chem. Res. 2007;15:325–327. [Google Scholar]; (d) Singh A., Kale R.R., Tiwari V.K. Trends Carbo. Res. 2009;1:80–85. [Google Scholar]; (e) Tiwari V.K., Singh D.D., Hussain H.A., Mishra B.B., Singh A. Monatsh. Chem. 2008;139:43–48. [Google Scholar]
- 16.Ding Q., He X., Wu J. J. Comb. Chem. 2009;11:587–591. doi: 10.1021/cc900027c. [DOI] [PubMed] [Google Scholar]
- 17.Prasad V., Kale R.R., Mishra B.B., Tiwari V.K. Trends Carbohydrate Res. 2009;1:43–49. [Google Scholar]
- 18.Typical experimental procedure for the synthesis of functionalized benzotriazoles (4): 2-Chloroaniline 1 (1 mmol), concd HCl (0.1 ml) and 0.5 ml of water were mixed in a R.B. flask and stirred for 10 min followed by the addition of 0.4 g of crushed ice. Then a precooled solution of NaNO2 (1 mmol) in 1 ml of ice cold water was added dropwise for 5 min with constant stirring at 0 °C. After complete addition of precooled NaNO2 solution, the reaction was further stirred for 15 min. The diazonium salt thus precipitated was added slowly into the solution of p-toludine 2a (1 mmol) and CH3COONa (2 mmol) over a period of 5–10 min with constant stirring at 0–5 °C. The solution was stirred for another 1 h maintaining the same reaction temperature. The solid product o-chloro-1,2,3-benzotriazenes (3a) obtained in quantitative yield was filtered immediately at below 10 °C and kept for drying in desiccator.CuI (0.2 mmol) was added to a solution of freshly prepared o-chloro-1,2,3-benzotriazines (3a, 1 mmol) in anhydrous DMF (2 ml) and the solution was stirred maintaining the temperature at 20 °C for 15 min. Cs2CO3 (2 mmol) was added and the reaction was stirred magnetically initially for 1 h at 20 °C and then allowed to warm up to 55 °C for another 7 h. The progress of the reaction was monitored by TLC using 12% EtOAc in n-hexane. The reaction mixture was filtered through Celite, the solvent was evaporated under reduced pressure. The residue was washed with water (and then extracted with EtOAc (2 × 100 ml). The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude mass thus obtained was subjected to flash column chromatography that furnished the pure product, 1-(4-methylphenyl-1H-benzo[d][1,2,3] triazole (4a): Yield 80%; IR (KBr): νmax 2928, 2847, 1452, 1101 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 8.01 (d, J = 6 Hz, 2 H), 7.70 (d, J = 9.9 Hz, 1 H), 7.49 (d, J = 7.8 Hz, 1 H), 7.38 (d, J = 8.4 Hz, 2 H), 7.15 (two d merged and appeared as m, J = 8.1 Hz, 2 H), 2.32 (s, 3 H, CH3) ppm; 13C NMR (CDCl3, 75 MHz): δ = 146.6, 138.8, 134.7, 132.5, 130.5, 128.2, 124.3, 123.0, 120.4, 110.4, and 21.3 ppm. 1-(2-Chlorolphenyl-1H-benzo[d][1,2,3]triazole (4b): Yield 78%; IR (KBr): ν 2945, 2843, 1542 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.76 (d, J = 8.7 Hz, 1 H), 7.58 (m, 1 H), 7.41–7.35 (m, 4 H), 7.14 (d, J = 8.6 Hz, 1 H), 7.07 (m, J = 7.0 Hz, 1 H) ppm. 1-(4-Methoxyphenyl-1H-benzo[d] [1,2,3]triazole (4c): Yield 76%; IR (KBr): ν 2931, 2853, 1559 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.67 (ddd, J = 8.2 Hz, 2 H), 7.53 (d, J = 8.0 Hz, 1 H), 7.14 (d, J = 8.6 Hz, 1 H), 7.07 (m, J = 7.5 Hz, 4 H), 3.84 (s, 3 H, OCH3) ppm; 13C NMR (CDCl3, 75 MHz): δ = 142.3, 133.5, 129.6, 128.1, 123.8, 123.57, 123.53, 119.2, 114.1, 113.9, 112.4, 111.9, and 55.3 ppm. 1-Phenyl-1H-benzo[d][1,2,3]triazole (4d): Yield 78%, brown solid: mp 84 °C; IR (KBr): ν 2933, 2841, 1546 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 8.13 (d, J = 8.2 Hz, 1 H), 7.77 (d, J = 8.0 Hz, 2 H), 7.73 (d, J = 8.6 Hz, 1 H), 7.59 (t, J = 7.5 Hz, 2 H), 7.46–7.54 (m, 2 H), 7.41 (t, J = 8.2 Hz, 1 H); 13C NMR (CDCl3, 75 MHz): δ = 146.5, 136.9, 132.3, 129.9, 128.7, 128.3, 124.4, 122.8, 122.4, and 110.4 ppm. 1-(2-Naphthyl-1H-benzo[d][1,2,3]triazole (4f): Yield 75%; IR (KBr): ν 2929, 2845, 1537 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 8.67 (d, J = 8.4 Hz, 1 H), 8.09–7.85 (m, 3 H), 7.76 (d, J = 9.3 Hz, 1 H), 7.64–7.39 (m, 5 H), 6.94 (d, J = 9.6 Hz, 1 H); 13C NMR (CDCl3, 75 MHz): δ 142.8, 139.6, 133.7, 133.5, 132.8, 130.2, 129.8, 128.8, 128.2, 127.0, 126.4, 125.6, 124.4, 121.7, 118.4, 116.5 ppm.


