Abstract
A cascade reaction that involves a unique C–C bond cleavage has been discovered. This protocol affords an unusual and facile method for the synthesis of 1,3-oxazin derivatives under mild conditions.
Keywords: Pyridine-mediated; Pyrane; 1,3-Oxazin; C–C bond cleavage
Graphical abstract

1. Introduction
Carbon–carbon bond cleavage is a challenging target in modern organic chemistry.1 Up to date, the carbon–carbon bond cleaving reactions have been achieved by the following catalytic methods: enzyme-catalyzed,2 photocatalytic,3 organocatalytic,4 and transition-metal catalyzed reactions.5 Most of these reactions reported are classified into ring strain, β-carbon elimination, chelation assistance, skeletal rearrangement, the stabilization of the aromatic system, and miscellaneous phenomena.6 However, those reports focused on the metal-catalyzed approaches, while the design of new C–C bond cleaving reactions without the use of metal catalysts, especially those can avoid the use of expensive catalyst and harsh reaction conditions, has not been placed a high value.
Various 1,3-oxazine derivatives have shown a wide variety of bioactivities, such as anti-human coronavirus activity,7 inhibition of cholesterol esterase and acetylcholinesterase,8 inhibition of human leukocyte elastase,9 and nonsteroidal progesterone receptor antagonists.10 A well-known oxazine is Efavirenz, a non-nucleoside reverse transcriptase inhibitor that was approved by the FDA in 1998 and is currently in clinical use for treatment of AIDS.11 These biological activities have prompted synthetic chemists to establish novel 1,3-oxazine ring formation methods to find promising bioactive oxazine compounds.12 There are several synthetic methods for preparation of 1,3-oxazine derivatives, such as the [4+2] cycloaddition of an alkene and an N-acylimine,13 intramolecular hydroamination of trichloroacetimidate,14 cycloaddition reactions of 2-azadienes with alkenes,15 Ritter reaction of a diol with a nitrile,16 intramolecular cyclization of N-thioacyl 1,3-amino alcohols with Bu4NF and EtI.17 However, most of these reactions require harsh reaction conditions, expensive starting materials or reagents and longer reaction times.
Since organocatalytic cascade reaction has demonstrated several advantages including operational simplicity, significant reduction in reaction time, less formation of by-products, and easier work-up compared to transition-metal catalyzed reactions, it has been recognized as an efficient, green chemical method for building up the diversity of the compound library.18 Recently, we have found an unusual cascade reaction. This reaction could convert pyran ring into 1,3-oxazin ring during the reaction of pyrane derivatives with acetic anhydride in the present of pyridine (Scheme 1 ). The attractive aspect of the cascade reaction is that the novel construction of 1,3-oxazine and the direct C–N bond formation from C–C bond can be easily achieved via pyridine-mediated acylation in a one-pot operation.
Scheme 1.

2. Results and discussion
The 2-amimo-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 1 is a versatile and readily available reagent, and its chemistry has received considerable attention in recent years.19 As we reported before, our strategies of synthesizing this compound were through the reaction of aromatic aldehydes, malononitrile with 5,5-dimethyl-1,3-cyclohexanedione.19e In order to further modify this compound, we recently investigated the reaction of the compound 1 with acetic anhydride 3 in the presence of a substoichiometric amount of pyridine. The product of this reaction was expected to be compound 6.20 However, to our surprise the sole product was 2-benzo[e][1,3]oxazine derivatives 4.
In the initial studies, the mixtures of compound 1 and acetic anhydride 3 were heated at the temperature in the range of 60–120 °C without a catalyst. Only trace amount of products were formed even extending the reaction time to 12 h. To improve the yield, the reaction conditions were optimized by varying the catalysts. The results showed that the pyridine-catalyzed reaction gave the highest yields, as illustrated in Table 1 .
Table 1.
Catalyst optimization for the synthesis of 4a
| Entry | Catalyst (mol %) | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | — | 120 | 12 | Trace |
| 2 | Sodium acetate (0.15) | 95 | 8 | 34 |
| 3 | Pyridine(0.1) | 85 | 6 | 68 |
| 4 | Diethylamine(0.1) | 85 | 7 | 51 |
| 5 | Triethylamine(0.1) | 80 | 7 | 58 |
The results of the reaction of 1 with 3 in the presence of pyridine are summarized in Table 2 . The structures of 4 have been confirmed by 1H NMR, 13C NMR, and elemental analysis. Furthermore, the structure of 4c was proven by single-crystal X-ray analysis (Fig. 1 ).
Table 2.

| Entry | 1 | R | Time (h) | Yield (%)b |
|---|---|---|---|---|
| 1 | 1a | Ph | 6 | 4a (68) |
| 2 | 1b | 3-NO2C6H4 | 7 | 4b (65) |
| 3 | 1c | 3,4-(MeO)2C6H3 | 6.5 | 4c(72) |
| 4 | 1d | 3-MeC6H4 | 7 | 4d (52) |
| 5 | 1e | 4-MeC6H4 | 6 | 4e (73) |
| 6 | 1f | 3,4-(Me)2C6H3 | 7.5 | 4f (55) |
| 7 | 1g | 4-ClC6H4 | 5 | 4g (74) |
| 8 | 1h | 4-BrC6H4 | 5.5 | 4h (71) |
| 9 | 1i | 3-MeOC6H4 | 6 | 4i (64) |
| 10 | 1j | 4-NO2C6H4 | 5.5 | 4j (71) |
The reactions were carried out with 1 (1 mmol), pyridine(0.1 mL), acetic anhydride (1 mL) under reflux at 85 °C.
All the yields were isolated yields.
Fig. 1.

ORTEP drawing of the X-ray crystal structure of 4c.
Inspired by the interesting results, we further explore the applicability of this method. The 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile 2 were used to react with acetic anhydride 3 under the same reaction conditions, 2-(4-aryl-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile 5 were produced in high yields with the results listed in Table 3 . The new products were characterized with 1H NMR, 13C NMR and elemental analysis. Furthermore, the structure of 5b was further confirmed by single-crystal X-ray analysis (Fig. 2 ).
Table 3.

| Entry | 2 | R | Time (h) | Yield (%)b |
|---|---|---|---|---|
| 1 | 2a | Ph | 5 | 5a (68) |
| 2 | 2b | 4-MeOC6H4 | 5 | 5b (77) |
| 3 | 2c | 4-ClC6H4 | 6 | 5c (71) |
| 4 | 2d | 3-NO2C6H4 | 5 | 5d (76) |
| 5 | 2e | 3-MeC6H4 | 6.5 | 5e (52) |
| 6 | 2f | 4-MeC6H4 | 5.5 | 5f (73) |
| 7 | 2g | 3,4-(Me)2C6H4 | 7 | 5g (55) |
| 8 | 2h | 3-MeOC6H4 | 5 | 5h (74) |
| 9 | 2i | 4-BrC6H4 | 5.5 | 5i (65) |
| 10 | 2j | 3,4-(MeO)2C6H4 | 7 | 5j (71) |
The reactions were carried out with 2(1 mmol), pyridine(0.1 mL), acetic anhydride(1 mL) under reflux at 85 °C.
All the yields were isolated yields.
Fig. 2.

ORTEP drawing of the X-ray crystal structure of 5b.
Single-crystal X-ray diffraction analysis reveal that classical hydrogen bonds, N(1)–H(1)…O(3) and N(1)–H(1)…O(5), exist in the molecular structure of 4c and 5b, respectively. The hydrogen bond length is 2.66 Å.
Although the detailed mechanism of the above reaction remains to be unclear, a possible mechanism was proposed in Scheme 2 . Initially, pyridine rapidly reacts with the anhydride to form N-acyl pyridine onium salt. Then nucleophilic attack of the active carbonyl of acyl pyridine cation by compound A gives intermediate B, eliminating 1 equiv of pyridine to form the unstable intermediate C. Due to the strongly electron-withdrawing group (C O) at the β-position of cyclic double bonds in the compounds C, an addition–elimination reaction occurs when pyridine attacks a vinylic carbon atom of C; this leads to open chain E-enamine isomer E because of intramolecular hydrogen bond. The intermediate E undergoes cyclization to afford the final products 4 or 5.
Scheme 2.
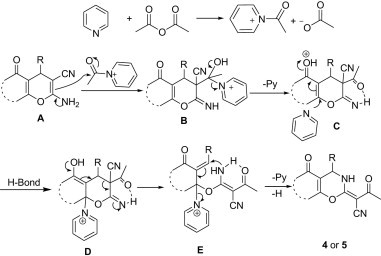
Possible reaction mechanism of the reaction.
3. Conclusion
In summary, a novel cascade reaction to convert pyrane derivatives into 1,3-oxazine derivatives bearing an exocyclic double bond has been discovered. The reaction involves an unusual carbon–carbon bond cleavage, and a facile C–N bond formation in one pot. We believe this method will broaden the scope of the synthesis of 1,3-oxazine moiety. Further studies for exploration of the detailed mechanism and changing the cyanide group to other electron-withdrawing group are underway in our laboratory.
4. Experimental section
4.1. General
All reagents purchased from commercial sources were used as received. 2-amimo-4-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 1 and 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile 2 were prepared according to literature procedures, respectively.(e), 21 TLC analysis was run on commercial TLC plates (Qingdao, China, 60 F254) using UV light to visualize the compounds. Melting points were measured on an X-4 microscopic melting point apparatus and are uncorrected. The 1H NMR and 13C NMR spectra were recorded on a Varian INOVA-400 MHz spectrometer and are referenced to the residual solvent signals. Elemental analysis were performed on an Elementar Vario EL cube instrument.
4.2. Synthesis
4.2.1. General procedure for the synthesis of 4
In an oven-dried 25 mL flask, 2-amimo-4-phenyl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyran-3-carbonitrile (1 mmol), acetic anhydride (1 mL), and pyridine (0.1 mL) were mixed and stirred at 85 °C until TLC indicated total consumption of the starting material. Upon completion, the reaction mixture was cooled to room temperature and then poured into 250 mL water. The solid product was removed by filtration and purified by recrystallization from 95% ethanol to afford the pure product 4a.
4.2.1.1. 2-(7,7-Dimethyl-5-oxo-4-phenyl-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4a)
Colorless crystals. Mp 242–243 °C. IR (KBr, ν, cm−1): 3412, 2911, 2209, 1690, 1666, 1577, 1450, 1373, 1208, 969, 832. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.80 (s, 1H, NH), 7.27–7.38 (m, 5H, ArH), 5.37 (s, 1H, CH), 2.61–2.63 (m, 2H, CH2), 2.34 (s, 3H, CH3), 2.28 (d, J=5.3 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.06 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 162.1, 161.8, 141.0, 129.3, 128.9, 127.6, 118.1, 113.1, 71.3, 50.2, 48.9, 32.6, 28.5, 28.2, 27.4. Anal. calcd for C20H20N2O3: C 71.41, H 5.99, N 8.33; found: C 71.22, H 5.68, N 8.27.
4.2.1.2. 2-(7,7-Dimethyl-4-(3-nitrophenyl)-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4b)
A colorless solid. Mp 253–255 °C. IR (KBr, ν, cm−1): 3402, 2957, 2203, 1665, 1577, 1374, 1208, 1057, 971, 823. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.97 (s, 1H, NH), 8.19–8.21 (m, 1H, ArH), 8.14 (s, 1H, ArH), 7.66–7.68 (m, 1H, ArH), 7.55–7.59 (m, 1H, ArH), 5.49 (s, 1H, CH), 2.65–2.68 (m, 2H, CH2), 2.37 (s, 3H, CH3), 2.29 (d, J=9.2 Hz, 2H, CH2), 1.17 (s, 3H, CH3), 1.08 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.1, 194.1, 162.4, 161.9, 148.2, 142.9, 134.3, 131.0, 123.8, 123.0, 118.0, 112.0, 71.6, 50.1, 48.5, 32.6, 28.5, 28.3, 27.5. Anal. calcd for C20H19N3O5: C 62.99, H 5.02, N 11.02; found: C 62.78, H 4.53, N 11.05.
4.2.1.3. 2-(4-(3,4-Dimethoxyphenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4c)
Colorless crystals. Mp 209–211 °C. IR (KBr, ν, cm−1): 3430, 2960, 2199, 1691, 1664, 1585, 1375, 1207, 1139, 974, 801. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.78 (s, 1H, NH), 6.76–6.83 (m, 3H, ArH), 5.31 (s, 1H, CH), 3.87 (s,3H, OCH3), 3.85 (s,3H, OCH3), 2.61–2.62 (m, 2H, CH2), 2.35 (s, 3H, CH3), 2.29 (d, J=3.5 Hz, 2H, CH2), 1.16 (s, 3H, CH3), 1.08 (s, 3H, CH3).
13C NMR (100 MHz, DMSO-d 6) δ: 195.1, 194.0, 161.9, 161.8, 149.3, 149.2, 133.2, 119.6, 118.2, 112.9, 112.4, 111.5, 71.2, 56.0, 56.0, 50.2, 48.6, 32.5, 28.6, 28.2, 27.4. Anal. calcd for C22H24N2O5: C 66.65, H 6.10, N 7.07; found: C 66.51, H 5.66, N 7.10.
4.2.1.4. 2-(7,7-Dimethyl-4-(3-methylphenyl)-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4d)
A colorless solid. Mp 251–253 °C. IR (KBr, ν, cm−1): 3446, 2956, 2207, 1666, 1575, 1370, 1204, 969, 814. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.76 (s, 1H, NH), 7.22 (m, 1H, ArH), 7.11 (m, 1H, ArH), 7.04 (m, 2H, ArH), 5.32 (s, 1H, CH), 2.61–2.64 (m, 2H, CH2), 2.34 (s, 6H, 2CH3), 2.28 (d, J=3.1 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.08 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 162.0, 161.8, 140.8, 138.5, 129.5, 129.2, 128.1, 124.6, 118.1, 112.9, 71.3, 50.2, 48.9, 32.6, 28.5, 28.2, 27.4, 21.4. Anal. calcd for C21H22N2O3: C 71.98, H 6.33, N 7.99; found: C 70.21, H 6.07, N 7.65.
4.2.1.5. 2-(7,7-Dimethyl-4-(4-methylphenyl)-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4e)
A colorless solid. Mp 226–228 °C. IR (KBr, ν, cm−1): 3448, 2952, 2209, 1664, 1575, 1371, 1209, 968, 820. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.76 (s, 1H, NH), 7.26 (s, 2H, ArH), 7.14 (s, 2H, ArH), 5.33 (s, 1H, CH), 2.60–2.62 (m, 2H, CH2), 2.33 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.27 (d, J=5.9 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.06 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 162.0, 161.7, 138.3, 138.1, 129.8, 127.5, 118.2, 113.1, 71.3, 50.2, 48.7, 32.6, 28.6, 28.2, 27.4, 21.1. Anal. calcd for C21H22N2O3: C 71.98, H 6.33, N 7.99; found: C 71.70, H 5.97, N 7.93.
4.2.1.6. 2-(4-(3,4-Dimethylphenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4f)
A colorless solid. Mp 210–212 °C. IR (KBr, ν, cm−1): 3491, 2956, 2210, 1662, 1571, 1451, 1374, 1205, 1152, 931, 892. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.73 (s, 1H, NH), 7.08–7.10 (m, 1H, ArH), 6.96–7.00 (m, 2H, ArH), 5.28 (s, 1H, CH), 2.61–2.64 (m, 2H, CH2), 2.33 (s, 3H, CH3), 2.28 (d, J=3.5 Hz, 2H, CH2), 2.24 (s, 3H, CH3), 2.22 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.08 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 161.9, 161.7, 138.3, 137.1, 137.1, 130.4, 128.6, 124.9, 118.2, 113.0, 71.2, 50.2, 48.7, 32.6, 28.6, 28.2, 27.5, 19.9, 19.5. Anal. calcd for C22H24N2O3: C 72.50, H 6.64, N 7.69; found: C 72.12, H 6.39, N 7.71.
4.2.1.7. 2-(4-(4-Chlorophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4g)
A colorless solid. Mp 252–253 °C. IR (KBr, ν, cm−1): 3735, 2949, 2209, 1664, 1574, 1374, 1208, 969, 826. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.82 (s, 1H, NH), 7.33 (d, J=8.4 Hz, 2H, ArH), 7.21 (d, J=8.4 Hz, 2H, ArH), 5.35 (s, 1H, CH), 2.61–2.62 (m, 2H, CH2), 2.35 (s, 3H, CH3), 2.28 (d, J=7.4 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.05 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.1, 194.0, 162.0, 162.0, 140.0, 133.5, 129.7, 129.3, 118.1, 112.7, 71.5, 50.2, 48.4, 32.6, 28.5, 28.3, 27.5. Anal. calcd for C20H19ClN2O3: C 64.78, H 5.16, N 7.55; found: C 64.59, H 5.23, N 7.64.
4.2.1.8. 2-(4-(4-Bromophenyl)-7,7-dimethyl-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4h)
A colorless solid. Mp 243–244 °C. IR (KBr, ν, cm−1): 3421, 2948, 2208, 1664, 1578, 1373, 1207, 1056, 823. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.83 (s, 1H, NH), 7.48 (d, J=8.4 Hz, 2H, ArH), 7.15 (d, J=8.4 Hz, 2H, ArH), 5.34 (s, 1H, CH), 2.61–2.62 (m, 2H, CH2), 2.35 (s, 3H, CH3), 2.28 (d, J=7.4 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.05 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 161.9, 140.3, 132.1, 129.9, 122.1, 118.1, 112.6, 72.9, 63.5, 50.1, 48.4, 32.6, 28.5, 28.2, 27.5. Anal. calcd for C20H19BrN2O3: C 57.84, H 4.61, N 6.75; found: C 54.079, H 4.74, N 6.51.
4.2.1.9. 2-(7,7-Dimethyl-4-(3-methoxyphenyl)-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4i)
A colorless solid. Mp 218–220 °C. IR (KBr, ν, cm−1): 3745, 2360, 2207, 1668, 1559, 939, 821. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.79 (s, 1H, NH), 7.24–7.28 (m, 1H, ArH), 6.80–6.84 (m, 3H, ArH), 5.33 (s, 1H, CH), 3.79 (s, 3H, OCH3), 2.60–2.63(m, 2H, CH2), 2.34 (s, 3H, CH3), 2.29 (d, J=2.3 Hz, 2H, CH2), 1.15 (s, 3H, CH3), 1.08 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.0, 162.0, 160.0, 142.3, 130.5, 119.3, 118.1, 113.8, 113.7, 112.8, 71.3, 55.6, 50.2, 48.7, 32.5, 28.5, 28.2, 27.4. Anal. calcd for C21H22N2O4: C 68.83, H 6.05, N 7.65; found: C 68.51, H 5.99, N 7.71.
4.2.1.10. 2-(7,7-Dimethyl-4-(4-nitrophenyl)-5-oxo-3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazin-2-ylidene)-3-oxobutanenitrile (4j)
A colorless solid. Mp 227–229 °C. IR (KBr, ν, cm−1): 3542, 2975, 2207, 1665, 1526, 1375, 1203, 1051, 875, 810, 734. 1H NMR (400 MHz, CDCl3) δ (ppm): 11.96 (s, 1H, NH), 8.23 (d, J=8.8 Hz, 2H, ArH), 7.49 (d, J=8.8 Hz, 2H, ArH), 5.49 (s, 1H, CH), 2.64 (s, 2H, CH2), 2.37 (s, 3H, CH3), 2.23 (d, J=12.1 Hz, 2H, CH2), 1.17 (s, 3H, CH3), 1.05 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 195.0, 194.1, 162.2, 162.0, 147.8, 147.7, 129.3, 124.4, 118.0, 112.2, 71.7, 50.1, 48.5, 32.6, 28.5, 28.3, 27.5. Anal. calcd for C20H19N3O5: C 62.99, H 5.02, N 11.02; found: C 63.03, H 4.75, N 10.96.
4.2.2. General procedure for the synthesis of 5
In an oven-dried 25 mL flask, 2-amino-4-aryl-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carbonitrile (1 mmol), acetic anhydride (1 mL), and pyridine (0.1 mL) were mixed and magnetically stirred at 85 °C until TLC indicated total consumption of the starting material. Upon completion, the reaction mixture was cooled to room temperature and then poured into 250 mL water. The solid product was removed by filtration and purified by recrystallization from 95% ethanol to afford the pure product 5a.
4.2.2.1. 2-(7-Methyl-5-oxo-4-phenyl-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5a)
A colorless solid. Mp 229–232 °C. IR (KBr, ν, cm−1): 3106, 2362, 2213, 1714, 1671, 1649, 1492, 1396, 1159, 982, 833, 758, 693. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.57(s, 1H, NH), 7.33–7.40 (m, 5H, ArH), 6.62 (s, 1H, CH), 5.55 (s, 1H, CH), 2.27 (s, 3H, CH3), 2.23 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.3, 161.7, 160.1, 157.0, 140.1, 129.3, 129.1, 127.8, 117.7, 100.4, 98.1, 71.7, 49.7, 28.2, 19.9. Anal. calcd for C18H14N2O4: C 67.07, H 4.38, N 8.69; found: C 67.32, H 4.36, N 8.29.
4.2.2.2. 2-(4-(4-Methoxyphenyl)-7-methyl-5-oxo-3,4-dihydropy-rano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5b)
A yellow solid. Mp 221–222 °C. IR (KBr, ν, cm−1): 3446, 3102, 2205, 1731, 1672, 1576, 1391, 1244, 1023, 984, 810. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.54(s, 1H, NH), 7.30 (d, J=8.8 Hz, 2H, ArH), 6.92 (d, J=8.8 Hz, 2H, ArH), 6.60 (s, 1H, CH), 5.49 (s, 1H, CH), 3.74(s, 3H, CH3), 2.27 (s, 3H, CH3), 2.22 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.1, 161.5, 160.1, 156.0, 156.8, 132.2, 129.2, 117.8, 114.7, 100.5, 98.1, 71.6, 55.7, 49.1, 28.2, 19.9. Anal. calcd for C19H16N2O5: C 64.86, H 4.56, N 7.94; found: C 64.52, H 4.42, N 8.18.
4.2.2.3. 2-(4-(4-Chlorophenyl)-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5c)
A yellow solid. Mp 219–220 °C. IR (KBr, ν, cm−1): 3436, 3101, 2213, 1716, 1648, 1574, 1394, 1160, 983, 837, 781. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.59(s, 1H, NH), 7.43 (d, J=8.4 Hz, 2H, ArH), 7.32 (d, J=8.4 Hz, 2H, ArH), 6.61 (s, 1H, CH), 5.52 (s, 1H, CH), 2.27 (s, 3H, CH3), 2.23 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.4, 161.5, 160.1, 157.1, 139.1, 133.8, 130.0, 129.2, 117.8, 99.9, 98.2, 71.8, 49.1, 28.3, 20.0. Anal. calcd for C18H13Cl N2O4: C 60.60, H 3.67, N 7.85; found: C 59.84, H 3.59, N 7.77.
4.2.2.4. 2-(7-Methyl-4-(3-nitrophenyl)-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5d)
A light yellow solid. Mp 242–245 °C. IR (KBr, ν, cm−1): 3088, 2362, 2208, 1724, 1673, 1643, 1536, 1352, 1184, 1162, 983, 823, 744. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.64(s, 1H, NH), 8.19–8.33 (m, 2H, ArH), 7.86 (d, J=7.8 Hz, 1H, ArH), 7.68 (t, J=7.8 Hz, 1H, ArH), 6.63 (s, 1H, CH), 5.71 (s, 1H, CH), 2.29 (s, 3H, CH3), 2.24 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.6, 161.5, 160.2, 157.3, 148.1, 142.0, 134.7, 130.9, 124.0, 123.4, 117.8, 99.2, 98.2, 71.9, 49.2, 28.3, 19.9. Anal. calcd for C18H13N3O6: C 58.86, H 3.57, N 11.44; found: C 58.81, H 3.56, N 7.88.
4.2.2.5. 2-(7-Methyl-4-(3-methylphenyl)-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5e)
A colorless solid. Mp 237–239 °C. IR (KBr, ν, cm−1): 3166, 2366, 2214, 1719, 1670, 1393, 1186, 982, 959, 823, 776. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.56(s, 1H, NH), 7.25–7.29 (m, 1H, ArH), 7.15–7.19 (m, 3H, ArH), 6.61 (s, 1H, CH), 5.50 (s, 1H, CH), 2.30 (s,3H, CH3), 2.28 (s,3H, CH3), 2.22 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.2, 165.2, 161.4, 160.1, 156.9, 140.0, 138.6, 129.8, 129.2, 128.2, 125.0, 117.8, 100.2, 98.1, 71.6, 49.6, 28.2, 21.4, 19.9. Anal. calcd for C19H16N2O4: C 67.58, H 4.79, N 8.33; found: C 67.91, H 4.68, N 8.27.
4.2.2.6. 2-(7-Methyl-4-(4-methylphenyl)-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5f)
A light yellow solid. Mp 217–219 °C. IR (KBr, ν, cm−1): 3442, 3105, 2361, 2215, 1715, 1646, 1607, 1397, 1185, 983, 842, 787. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.54(s, 1H, NH), 7.25 (d, J=7.8 Hz, 2H, ArH), 7.17 (d, J=8.0 Hz, 2H, ArH), 6.61 (s, 1H, CH), 5.55 (s, 1H, CH), 2.28 (s,3H, CH3), 2.27 (s,3H, CH3), 2.22 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.2, 161.5, 160.1, 156.9, 138.6, 137.2, 129.8, 127.7, 117.8, 100.4, 98.1, 71.6, 49.4, 28.3, 21.2, 19.9. Anal. calcd for C19H16N2O4: C 67.58, H 4.79, N 8.33; found: C 67.26, H 4.63, N 7.99.
4.2.2.7. 2-(4-(3,4-Dimethylphenyl)-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5g)
A colorless solid. Mp 227–229 °C. IR (KBr, ν, cm−1): 3101, 3002, 2361, 2213, 1724, 1673, 1606, 1395, 1185, 981, 813, 771. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.54(s, 1H, NH), 7.12–7.14 (m, 2H, ArH), 7.06–7.08 (m, 1H, ArH), 6.61 (s, 1H, CH), 5.47 (s, 1H, CH), 2.28 (s, 3H, CH3), 2.19–2.21 (m, 9H, 3CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.1, 161.4, 160.1, 156.9, 137.5, 137.4, 137.2, 130.3, 128.7, 125.3, 117.8, 100.3, 98.1, 71.5, 49.4, 28.2, 19.9, 19.9, 19.5. Anal. calcd for C20H18N2O4: C 68.56, H 5.18, N 8.00; found: C 68.25, H 4.88, N 7.91.
4.2.2.8. 2-(4-(3-Methoxyphenyl)-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5h)
A light yellow solid. Mp 219–221 °C. IR (KBr, ν, cm−1): 3429, 3094, 2966, 2210, 1723, 1672, 1645, 1393, 1183, 1043, 983, 872, 788. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.57(s, 1H, NH), 7.30 (t, J=8.2 Hz, 1H, ArH), 6.90–6.96 (m, 3H, ArH), 6.61 (s, 1H, CH), 5.52 (s, 1H, CH), 3.75 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.23 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.3, 161.6, 160.2, 159.9, 157.1, 141.6, 130.6, 119.6, 117.8, 114.2, 114.1, 100.1, 98.2, 71.7, 55.7, 49.5, 28.3, 20.0. Anal. calcd for C19H16N2O5: C 64.77, H 4.58, N 7.95; found: C 64.60, H 4.43, N 7.93.
4.2.2.9. 2-(4-(4-Bromophenyl)-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5i)
A yellow solid. Mp 224–226 °C. IR (KBr, ν, cm−1): 3445, 3108, 2217, 1726, 1652, 1574, 1395, 1162, 987, 832, 793. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.55(s, 1H, NH), 7.58 (d, J=8.6 Hz, 2H, ArH), 7.36 (d, J=8.6 Hz, 2H, ArH), 6.62 (s, 1H, CH), 5.53 (s, 1H, CH), 2.27 (s, 3H, CH3), 2.23 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.3, 165.3, 161.5, 160.1, 157.0, 139.0, 133.8, 130.0, 129.2, 117.8, 99.8, 98.2, 71.7, 49.1, 28.3, 20.0. Anal. calcd for C18H13Br N2O4: C 53.89, H 3.27, N 6.98; found: C 53.74, H 3.31, N 6.95.
4.2.2.10. 2-(4-(3,4-Dimethoxyphenyl)-7-methyl-5-oxo-3,4-dihydropyrano[3,4-e][1,3]oxazin-2(5H)-ylidene)-3-oxobutanenitrile (5j)
A colorless solid. Mp 217–220 °C. IR (KBr, ν, cm−1): 3735, 3567, 2369, 2212, 1711, 1668, 1578, 1391, 1288, 1016, 986, 838. 1H NMR (400 MHz, DMSO-d 6) δ (ppm): 11.55(s, 1H, NH), 7.02 (d, J=2.2 Hz, 1H, ArH), 6.92–6.94 (m, 1H, ArH), 6.82–6.85 (m, 1H, ArH), 6.61 (s, 1H, CH), 5.49 (s, 1H, CH), 3.74 (s,3H, OCH3), 3.73 (s,3H, OCH3), 2.23 (s, 3H, CH3), 2.22(s, 3H, CH3). 13C NMR (100 MHz, DMSO-d 6) δ: 194.2, 165.0, 161.4, 160.1, 156.9, 149.5, 149.1, 132.4, 119.9, 117.8, 112.4, 111.9, 100.2, 98.1, 71.5, 56.1, 56.0, 49.3, 28.2, 19.9. Anal. calcd for C20H18N2O6: C 62.82, H 4.74, N 7.33; found: C 62.78, H 4.78, N 7.45.
Acknowledgements
We are grateful to financial support from the National Natural Science Foundation of China (No. 81071144) and Natural Science Foundation of the Guangdong Province (No. 9451806001002961).
Footnotes
Copies of 1H NMR and 13C NMR spectra for all new compounds. Supplementary data related to this article can be found online at doi:10.1016/j.tet.2012.05.089.
Supplementary data
Copies of 1H NMR and 13C NMR spectra for all new compounds.
References and notes
- 1.For selected reports on carbon–carbon bond cleavage.; (a) Rybtchinski B., Milstein D. Angew. Chem., Int. Ed. 1999;38:870–883. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (b) Jun C.-H. Chem. Soc. Rev. 2004;33:610–618. doi: 10.1039/b308864m. [DOI] [PubMed] [Google Scholar]; (c) Matsuda T., Shigeno M., Makino M., Murakami M. Org. Lett. 2006;8:3379–3381. doi: 10.1021/ol061359g. [DOI] [PubMed] [Google Scholar]; (d) Wang J.-Y., Hu Y., Wang D.-X., Pan J., Huang Z.-T., Wang M.-X. Chem. Commun. 2009:422–424. doi: 10.1039/b816007d. [DOI] [PubMed] [Google Scholar]; (e) Sattler A., Parkin G. Nature. 2010;463:523–526. doi: 10.1038/nature08730. [DOI] [PubMed] [Google Scholar]; (f) Nakai K., Kurahashi T., Matsubara S. J. Am. Chem. Soc. 2011;133:11066–11068. doi: 10.1021/ja203829j. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kluger R. Chem. Rev. 1990;90:1151–1169. [Google Scholar]; (b) Johnson A.E., Tanner M.E. Biochemistry. 1998;37:5746–5754. doi: 10.1021/bi972984j. [DOI] [PubMed] [Google Scholar]; (c) Fleming S.M., Robertson T.A., Langley G.J., Bugg T.D.H. Biochemistry. 2000;39:1522–1531. doi: 10.1021/bi9923095. [DOI] [PubMed] [Google Scholar]; (d) Drahl C. Chem. Eng. News. 2009;87 8–8. [Google Scholar]; (e) Millic D., Demidkina T.V.N., Faleev G., Phillips R.S., Calogovict D.M., Antson A.A. J. Am. Chem. Soc. 2011;133:16468–16476. doi: 10.1021/ja203361g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Li X.-J., Cubbage J.W., Tetzlaff T.A., Jenks W.S. J. Org. Chem. 1999;64:8509–8524. [Google Scholar]; (b) Maldotti A., Molinari A., Amadelli R. Chem. Rev. 2002;102:3811–3836. doi: 10.1021/cr010364p. [DOI] [PubMed] [Google Scholar]; (c) Fagnoni M., Dondi D., Ravelli D., Albini A. Chem. Rev. 2007;107:2725–2756. doi: 10.1021/cr068352x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ghosh S., Banerjee S. Arkivoc. 2002;vii:8–20. [Google Scholar]; (b) Zhang N., Vozzolo J. J. Org. Chem. 2002;67:1703–1704. doi: 10.1021/jo016145l. [DOI] [PubMed] [Google Scholar]; (c) Han C., Kim E.H., Colby D.A. J. Am. Chem. Soc. 2011;133:5802–5805. doi: 10.1021/ja202213f. [DOI] [PubMed] [Google Scholar]
- 5.(a) Garcia J.J., Jones W.D. Organmetallics. 2000;19:5544–5545. [Google Scholar]; (b) Szajna E., Arif A.M., Berreau L.M. J. Am. Chem. Soc. 2005;127:17186–17187. doi: 10.1021/ja056346x. [DOI] [PubMed] [Google Scholar]; (c) Mecinovic J., Hamed R.B., Schofield C.J. Angew. Chem., Int. Ed. 2009;48:2796–2800. doi: 10.1002/anie.200806296. [DOI] [PubMed] [Google Scholar]; (d) Seiser T., Cramer N. J. Am. Chem. Soc. 2010;132:5340–5341. doi: 10.1021/ja101469t. [DOI] [PubMed] [Google Scholar]; (e) Schmidt A.-K.C., Stark C.B.W. Org. Lett. 2011;13:5788–5791. doi: 10.1021/ol202354g. [DOI] [PubMed] [Google Scholar]; (f) Murakami M., Matsuda T. Chem. Commun. 2011:1100–1105. doi: 10.1039/c0cc02566f. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T., Mitsudo T. Chem. Lett. 2005;34:1462–1467. [Google Scholar]
- 7.Hsieh P.W., Chang F.R., Chang C.H., Cheng P.W., Chiang L.C., Zeng F.L., Lin K.H., Wu Y.C. Bioorg. Med. Chem. Lett. 2004;14:4751–4754. doi: 10.1016/j.bmcl.2004.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietsch M., Gutschow M. J. Med. Chem. 2005;48:8270–8288. doi: 10.1021/jm0508639. [DOI] [PubMed] [Google Scholar]
- 9.Gutschow M., Kuerschner L., Neumann U., Pietsch M., Loeser R., Koglin N., Eger K. J. Med. Chem. 1999;42:5437–5447. doi: 10.1021/jm991108w. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fensome A., Bender R., Chopra R., Cohen J., Collins M.A., Hudak V., Malakian K., Lockhead S., Olland A., Svenson K., Terefenko E.A., Unwalla R.J., Wilhelm J.M., Wolfrom S., Zhu Y., Zhang Z., Zhang P., Winneker R.C., Wrobel J. J. Med. Chem. 2005;48:5092–5095. doi: 10.1021/jm050358b. [DOI] [PubMed] [Google Scholar]; (b) Kern J.C., Terefenko E.A., Fensome A., Unwalla R., Wrobel J., Zhu Y., Cohen J., Winneker R., Zhang Z., Zhang P. Bioorg. Med. Chem. Lett. 2007;17:189–192. doi: 10.1016/j.bmcl.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 11.Vrouenraets S.M., Wit W.F., Tongeren van J., Lange J.M. Expert Opin. Pharmacother. 2007;8:851–871. doi: 10.1517/14656566.8.6.851. [DOI] [PubMed] [Google Scholar]
- 12.For reviews, see:; (a) Feng E., Zhou Y., Zhang D., Zhang L., Sun H., Jiang H., Liu H. J. Org. Chem. 2010;75:3274–3282. doi: 10.1021/jo100228u. [DOI] [PubMed] [Google Scholar]; (b) Mulzer M., Coates G.W. Org. Lett. 2011;13:1426–1428. doi: 10.1021/ol200111a. [DOI] [PubMed] [Google Scholar]; (c) Baltork I.M., Moghadam M., Tangstaninejad S., Mirkhani V., Eskandari Z. Ultrason. Sonochem. 2010;17:857–862. doi: 10.1016/j.ultsonch.2010.02.005. [DOI] [PubMed] [Google Scholar]; (d) Benaamane N., Nedjar-Kolli B., Bentarzi Y., Hammal L., Geronikaki A., Eleftheriou P., Lagunin A. Bioorg. Med. Chem. 2008;16:3059–3066. doi: 10.1016/j.bmc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Toujas J.L., Jost E., Vaultier M. Bull. Soc. Chim. Fr. 1997;134:713–715. [Google Scholar]
- 14.Kang J.E., Kim H.B., Lee J.W., Shin S. Org. Lett. 2006;8:3537–3540. doi: 10.1021/ol061307r. [DOI] [PubMed] [Google Scholar]
- 15.Palacios F., Herran E., Rubiales G., Ezpeleta M.J. J. Org. Chem. 2002;67:2131–2135. doi: 10.1021/jo016273+. [DOI] [PubMed] [Google Scholar]
- 16.Tillmanns E.J., Ritter J.J. J. Org. Chem. 1957;22:839–840. [Google Scholar]
- 17.Murai T., Sano H., Kawai H., Aso H., Shibahara F. J. Org. Chem. 2005;70:8148–8153. doi: 10.1021/jo051378o. [DOI] [PubMed] [Google Scholar]
- 18.For recent reviews on cascade reactions, see:; (a) Tietze L.F. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; (b) Wasilke J.-C., Obrey S.J., Baker T., Bazan G.C. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou K.C., Edmonds D.J., Bulger P.G. Angew. Chem., Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; (d) Kim J.K., Kim Y.H., Nam H.T., Kim B.T., Heo J.-N. Org. Lett. 2008;10:3543–3546. doi: 10.1021/ol801291k. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou K.C., Chen J.S. Chem. Soc. Rev. 2009;38:2993–3009. doi: 10.1039/b903290h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Khodaei M.M., Bahrami K., Farrokhi A. Synth. Commun. 2010;40:1492–1499. [Google Scholar]; (b) Gong K., Wang H.L., Luo J., Liu Z.L. J. Heterocycl. Chem. 2009;46:1145–1150. [Google Scholar]; (c) Gao S., Tsai C.H., Tseng C., Yao C.F. Tetrahedron. 2008;64:9143–9149. [Google Scholar]; (d) Fotouhi L., Heravi M.M., Fatehi A., Bakhtiari K. Tetrahedron Lett. 2007;48:5379–5381. [Google Scholar]; (e) Tu S.-J., Jiang H., Zhuang Q.-Y., Miao C.-B., Shi D.-Q., Wang X.-S., Gao Y. Chin. J. Org. Chem. 2003;23:488–490. [Google Scholar]; (f) Jiang B., Li C., Tu S.-J., Shi F. J. Comb. Chem. 2010;12:482–487. doi: 10.1021/cc1000192. [DOI] [PubMed] [Google Scholar]
- 20.Abdelrazek F.M., Metz P., Kataeva O., Jager A., El-Mahrouky S.F. Arch. Pharm. Chem. Life Sci. 2007;340:543–548. doi: 10.1002/ardp.200700157. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X.L., Huang Y., Li Z.N., Lu Y.J., Gao Y. Chin. J. Org. Chem. 2006;26:1434–1436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


