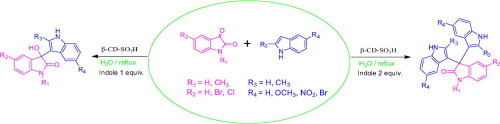
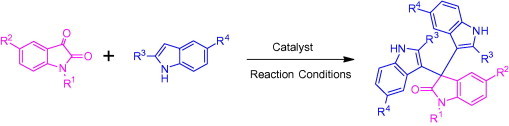
Graphical abstract
Keywords: Sulfonated-β-cyclodextrin; 3,3-Di(indolyl)indolin-2-ones; Recyclable catalyst
Abstract
Sulfonated-β-cyclodextrin (β-CD-SO3H) promoted efficient and fast electrophilic substitution reaction of indoles with various isatins reflux in water is reported affording various 3-indolyl-3-hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones in good to excellent yields in short reaction time.
Indole derivatives are important compounds that are widespread in nature as well as exhibit significant biological activities.1 3-Substituted 3-hydroxyoxindoles are encountered in a large variety of natural products with a wide spectrum of biological activities.2 3,3-di(indolyl)indolin-2-one derivatives were assessed as anticancer,3 anti-HIV,4 antiviral,5 anti-tumor,6 antifungal,7, 8 anti-angiogenic,9 anticonvulsants,10 anti-Parkinson’s disease therapeutic,11 and effective SARS corona virus 3CL protease inhibitor.12 Furthermore, a large number of bis (indolyl) methanes have been isolated from natural sources,13 and some of these natural products, for example, vibrindole have shown promising biological activity.14
Recently a number of methods for the synthesis of 3-substituted-3-hydroxyoxindoles and 3,3-di(indolyl)indolin-2-ones have been reported in the literature involving the use of K2CO3,15a β-cyclodextrin,15b triton-B,15c ZnO nano-rods,15d LiClO4,15e Sc/In (OTF),15f cupreine,15g ionic liquids,16a bismuth(III) triflate,16b indium(III) acetylacetonate,16c silica sulfuric acid,16d Bronsted acidic ionic liquid,16e ruthenium,16f ceric ammonium nitrate (CAN) under ultrasound irradiation,16g iodine,16h KSF,16i water–ethanol,16j and water16k at reflux temperature. These reported methodologies produce good results in many instances. However, some of the synthetic strategies suffer from metal catalyst, expensive reagents, long reaction time, environmentally hazardous, harsh reaction condition, tedious work-up procedure, unsatisfactory yield and use of homogeneous catalyst which are difficult to separate from the reaction mixture and reuse.
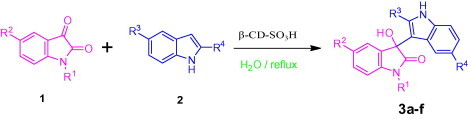
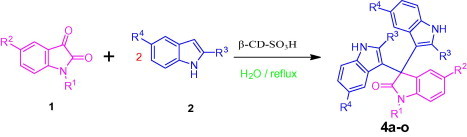
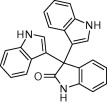
Aqueous phase organic synthesis has attracted the attention of chemists as it overcomes the harmful effects associated with the organic solvents and is environmentally benign. These reactions become more sophisticated if they can be performed under supramolecular catalysis. In view of the above, the development of a generally applicable and environmentally benign methodology for the synthesis of 3-indolyl-3-hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones derivatives is highly desirable. We report, herein, an aqueous phase synthesis of 3-indolyl-3-hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones from isatins and indoles in the presence of sulfonated-β-cyclodextrin (β-CD-SO3H) (Fig. 1 and Scheme 1 ).
Figure 1.
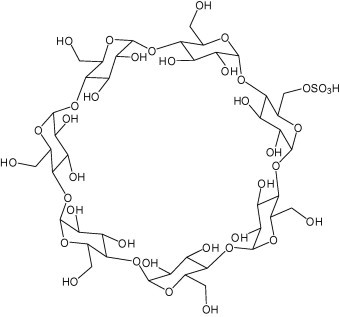
Chemical structure of β-CD-SO3H.
Scheme 1.
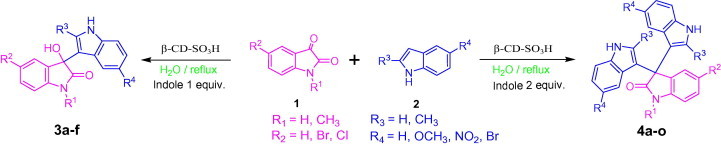
General scheme for the synthesis of 3-indolyl-3-hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones.
Supramolecular catalysis is a discipline in chemistry which involves intermolecular interactions where covalent bonds are not established between the interacting species which can be molecules, ions or radicals.17 The most accessible β-cyclodextrin (β-CD) is a cyclic oligosaccharide consisting of seven glucose units. The cavity size and the inner hydrophobicity are suitable for encapsulating a variety of guests such as aromatic compounds.18 The improvement of the reaction rate and selectivity with β-CD inclusion complexes has been reported in a number of organic reactions.19 β-cyclodextrin mediated reactions in water are very useful tool for economic as well as environmental points of view.20, 21 Sulfonated-β-cyclodextrin shows good results over β-cyclodextrin in the synthesis of 2,3-dihydroquinazolin-4(1H)-one22 and 3,4-dihydropyrimidine-2(1H)-one.23
In continuation of our work on β-CD,24 we envisioned β-CD-SO3H as a supramolecular catalyst and study its application on the synthesis of 3-substituted 3-hydroxyoxindoles and 3,3-di(indolyl)indolin-2-ones to develop a simple and efficient method in aqueous media.
Initially, the β-CD-SO3H was synthesized according to the method reported recently.22, 23 The –SO3H content obtained was in agreement with the proposed method, the value was 0.52 mequiv g−1, and it matches with the literature report22, 23 confirmed the sulfonation of β-CD. The catalytic role β-CD-SO3H for the synthesis of 3-substituted 3-hydroxyoxindoles and 3,3-di(indolyl)indolin-2-ones has been compared with various reported catalysts and found fast conversion within 5 min with yield up to 96% (Table 1, Table 2 ).
Table 1.
| Entry | Catalyst | Reaction conditions | Time (h/min) | Yield (%) | Refs. |
|---|---|---|---|---|---|
| 1 | K2CO3 | 20 mol %, rt, in water | 1 h | 91 | 15a |
| 2 | Triton-B | 7 mol %, rt, in water | 15 min | 94 | 15c |
| 3 | ZnO nano-rods | 10 mol %, 80 °C, in water | 1.5 h | 95 | 15d |
| 4 | LiClO4 | 10 mol %, 60 °C, in ethanol | 4 h | 93 | 15e |
| 5 | β-Cyclodextrin | 100 mol %, 40 °C, in water | 1 h | 93 | 15b |
| 6 | β-CD-SO3H | 10 mol %, reflux in water | 5 min | 96 | This work |
Table 2.
| Entry | Catalyst | Reaction conditions | Time (h/min) | Yield (%) | Refs. |
|---|---|---|---|---|---|
| 1 | Ionic liquid | 60 mol % of ([BMIM][BF4]LiCl), rt | 1 h | 93 | 16a |
| 2 | Bismuth(III) Triflate | 2 mol %, CH3CN, rt | 3 h | 92 | 16b |
| 3 | Indium(III) acetylacetonate | 10 mol %,(H2O:CH3CN 4:1), rt | 2.5 h | 92 | 16c |
| 4 | Silica sulfuric acid | 0.2 g, CH2Cl2, rt | 2 h | 92 | 16d |
| 5 | Ruthenium trichloride | 5 mol %, MeOH, 50 °C | 2 h | 75 | 16f |
| 6 | Ceric ammonium nitrate (CAN) | 10 mol %, US, EtOH, rt | 3 h | 95 | 16g |
| 7 | I2 (Iodine) | 10 mol %, CH2Cl2, rt | 14 h | 82 | 16h |
| 8 | KSF | 0.1 g, reflux in EtOH | 0.5 h | 90 | 16i |
| 9 | β-CD-SO3H | 10 mol %, H2O, reflux | 5 min | 96 | This work |
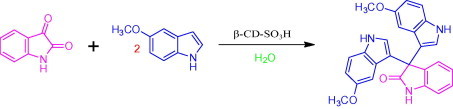
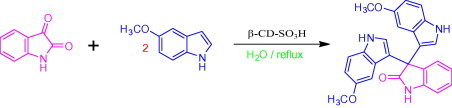
In order to optimize the reaction conditions and the performance of β-CD-SO3H as a catalyst for the synthesis of 3-substituted 3-hydroxyoxindoles, we studied 5-methoxy indole with isatin as a model reaction. The reaction proceeds in the absence of β-CD-SO3H to give lower yield (60%) with longer reaction time and in presence of 100 mol % β-CD and required 60 min to get 93% of yield.15b The best result was obtained for 10 mol % of β-CD-SO3H affording 96% of within 5 min (Table 3, Table 4 ). Encouraged by the initial success, we applied the optimal protocol to a variety of isatins and indoles (Table 5 ). Generally, the reactions were performed using 10 mol % of β-cyclodextrin-SO3H in H2O at reflux temperature for 5–15 min to give the desired products in good to excellent yields;25 the results are summarized in Table 5.
Table 3.
| Entry | Temp (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|
| 1 | Rt | 300 | 40 |
| 2 | 40 | 240 | 60 |
| 3 | 60 | 120 | 80 |
| 4 | 80 | 30 | 84 |
| 5 | 100 | 5 | 96 |
Reaction condition: isatin (1.0 mmol), 5-methoxy indole (2.0 mmol), β-CD-SO3H (0.1 mmol) and water (2 mL).
Isolated yield.
Table 4.
| Entry | Catalyst (mmol) | Time (min) | Yieldb (%) |
|---|---|---|---|
| 1 | β-CD-SO3H (0.00) | 120 | 60 |
| 2 | β-CD-SO3H (0.05) | 20 | 90 |
| 3 | β-CD-SO3H (0.10) | 5 | 96 |
| 4 | β-CD-SO3H (0.20) | 5 | 94 |
| 5 | β-CD-SO3H (0.30) | 5 | 94 |
Reaction condition: isatin (1.0 mmol), 5-methoxy indole (2.0 mmol), water (2 mL), refluxed.
Isolated yield.
Table 5.
| Sr. No. | Isatin | Indole | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 3a | 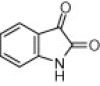 |
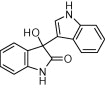 |
5 | 86 | |
| 3b | 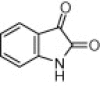 |
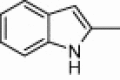 |
 |
5 | 92 |
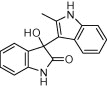
| 3c |  |
 |
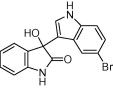 |
15 | 88 |
| 3d | 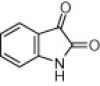 |
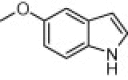 |
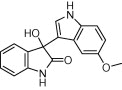 |
5 | 96 |
| 3e |  |
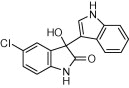 |
10 | 86 | |
| 3f | 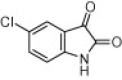 |
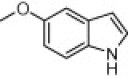 |
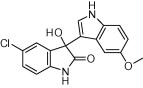 |
5 | 88 |
Reaction condition: isatin (1.0 mmol), indole (1.0 mmol), β-CD-SO3H (0.1 mmol), water (2 mL).
Isolated yield.
In order to optimize the reaction condition and the performance of β-CD-SO3H as a catalyst for this 3,3-di(1H-indol-3-yl)indolin-2-one the reaction between simple isatin and 5-methoxy indole was selected as a model reaction by using different reaction parameters and various amounts of catalyst (Table 3, Table 4). The reaction proceeds in the absence of β-CD-SO3H to give less yield (60%). The best result was obtained for 10 mmol % of β-CD-SO3H (Table 6 , entry 4c), affording 96% of 3,3-bis(5-methoxy-1H-indol-3-yl)indolin-2-one within 5 min. A further increase in the amount of catalyst has no significant effect on the yield and reaction time (Table 4, entries 4 and 5). The role of β-CD-SO3H as the catalyst has been confirmed when a similar reaction was carried out in the absence of catalyst (Table 4, entry 1), giving only 60% yield with a longer reaction time 2 h. It indicates that β-CD-SO3H not only improves the yield of the product but also accelerates the rate of reaction. The significant presence of β-CD-SO3H has a great influence on the reaction time as well as the yield (Table 4, entry 3). Temperature plays an important role, as at low temperature there is only a trace amount of product formed and required longer reaction time and as the temperature increases from 40 °C to reflux the yields also increase with decrease in reaction time (Table 3).
Table 6.
| Sr. No. | Isatin | Indole | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 4a | 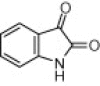 |
 |
10 | 95 | |
| 4b |  |
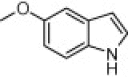
 |
 |
15 | 95 |
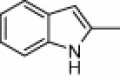
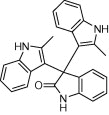
| 4c | 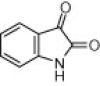 |
 |
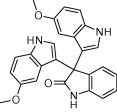 |
5 | 96c, 94d, 92e |
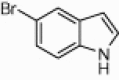
| 4d |  |
 |
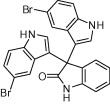 |
45 | 94 |
| 4e | 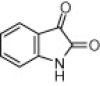 |
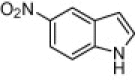 |
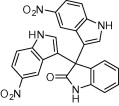 |
130 | 80 |
| 4f | 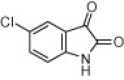 |
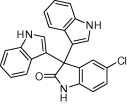 |
30 | 91 | |
| 4g | 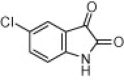 |
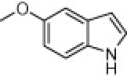 |
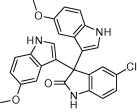 |
15 | 93 |
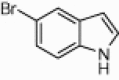
| 4h | 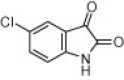 |
 |
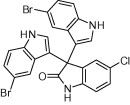 |
30 | 93 |
| 4i | 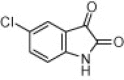 |
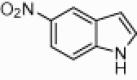 |
 |
130 | 91 |
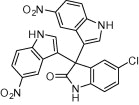
| 4j |  |
 |
30 | 85 | |
| 4k | 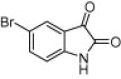 |
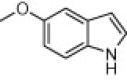 |
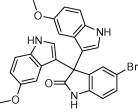 |
30 | 88 |
| 4l | 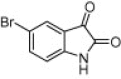 |
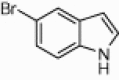 |
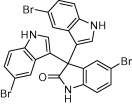 |
45 | 87 |
| 4m | 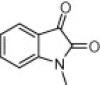 |
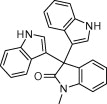 |
15 | 94 | |
| 4n | 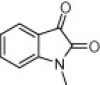 |
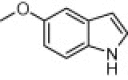 |
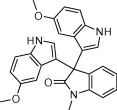 |
10 | 95 |
| 4o | 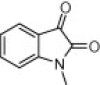 |
 |
 |
45 | 90 |
Reaction condition: isatin (1.0 mmol), indole (1.0 mmol), β-CD-SO3H (0.1 mmol), water (2 mL).
Isolated yield.
Yield after I, II, and III recycle of catalyst.
A variety of structurally divergent isatins possessing different substituents were selected to understand the scope and generality of the β-CD-SO3H promoted reaction to form 3,3-di(indolyl)indolin-2-ones.26 The results obtained are summarized in Table 6. For all the entries water was used as the solvent and the reaction was conducted under reflux condition. In all cases, the conversion was completed within 5–45 min with good to excellent yields except for 5-nitroindole (Table 6 entry 4e, 80% and entry 4i, 91%). A further increase in reaction time had no significant effect on the yields. In addition, the substituent on the aromatic indoles showed slightly different effects on the yields, reactions of aromatic indoles with electron-donating groups afforded little better yields of products than those with the electron-withdrawing groups (Table 6 entry 4e, 80% and entry 4i, 91%).
β-CD-SO3H was chosen as the catalyst since it is recyclable, environmentally benign, easily accessible and due to presence of the –SO3H group it possesses greater solubility than β-CD in water which enhances the rate of reaction greater than β-CD and shows good results over β-CD. The supramolecular β-CD have tendency to form inclusion complex with isatin which is reported by Rama Rao15b similarly due to presence of the –SO3H group in β-CD-SO3H it possesses greater solubility than β-CD and also forms inclusion complex more effectively with isatin to enhance the rate of reaction.
The catalyst recovery and reusability27 were studied by three cycles including the use of fresh catalyst for the synthesis of 3,3-bis(5-methoxy-1H-indol-3-yl)indolin-2-one (Table 6, entry 4c). In every cycle, the catalyst was almost quantitatively recovered and after second and third use of catalyst decrease in yield is not much more significant which is shown in Figure 2 . The FTIR spectra (Fig. 3 ) of fresh and recovered β-CD-SO3H were also measured and no change was found in the functional group as well as in fingerprint region, indicating that no reaction occurs with β-CD-SO3H.
Figure 2.
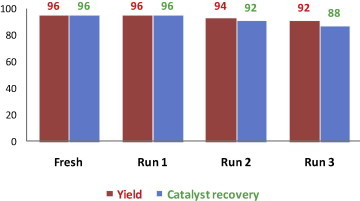
Catalyst β-CD-SO3H recyclability data (for Table 6, entry 4c).
Figure 3.
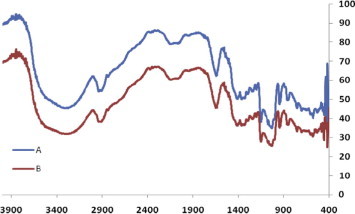
FTIR of (a) fresh β-CD-SO3H and (b) recovered β-CD-SO3H (for Table 6, entry 4c).
In conclusion, we report β-CD-SO3H as a highly efficient, reusable, environmentally benign catalyst for the synthesis of 3-substituted 3-hydroxyoxindoles and 3,3-di(indolyl)indolin-2-ones. The advantages of this catalyst are good to excellent yields of product, short reaction times, simple and clean work-up of the desired product without column chromatography, easy recovery and reuse of the catalyst.
Acknowledgments
One of the authors (Y.A.T.) is thankful to the ‘Rajiv Gandhi National Fellowship’, University Grants Commission, New Delhi, India for JRF fellowship award, and also Sophisticated Analytical Instrumentation Facility, Chandigarh University, Punjab and University of Pune, Pune for providing characterization facility for this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2014.12.012.
Supplementary data
Experimental procedures and spectral data.
References and notes
- 1.(a) Sundberg R.J. Academic Press; San Diego: 1996. Indoles. [Google Scholar]; (b) Cacchi S., Fabrizi G. Chem. Rev. 2005;105:2873. doi: 10.1021/cr040639b. [DOI] [PubMed] [Google Scholar]; (c) Joucla L., Djakovitch L. Adv. Synth. Catal. 2009;351:673. [Google Scholar]; (d) Bandini M., Eichholzer A. Angew. Chem., Int. Ed. 2009;48:9608. doi: 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]; (e) Bartoli G., Bencivenni G., Dalpozzo R. Chem. Soc. Rev. 2010;39:4449. doi: 10.1039/b923063g. [DOI] [PubMed] [Google Scholar]; (f) Kamano Y., Zhang H.P., Ichihara Y., Kizu H., Komiyama K., Pettit G.R. Tetrahedron Lett. 1995;36:2783. [Google Scholar]
- 2.(a) Rasmussen H.B., MacLeod J.K. J. Nat. Prod. 1997;60:1152. doi: 10.1021/np970006a. [DOI] [PubMed] [Google Scholar]; (b) Balk-Bindseil W., Helmke E., Weyland H., Laatsch H. Liebigs Ann. 1995:1291. [Google Scholar]; (c) Monde K., Sasaki K., Shirata A., Tagusuki M. Phytochemistry. 1991;30:2915. [Google Scholar]; (d) Suzuki H., Morita H., Shiro M., Kobayashi M. Tetrahedron. 2004;60:2489. [Google Scholar]; (e) Frechard A., Fabre N., Pean C., Montaut S., Fauvel M.T., Rollin P., Fouraste I. Tetrahedron Lett. 2001;42:9015. [Google Scholar]; (f) Kohno J., Koguchi Y., Nishio M., Nakao K., Kuroda M., Shimizu R., Ohnuki T., Komatsubara S. J. Org. Chem. 2000;65:990. doi: 10.1021/jo991375+. [DOI] [PubMed] [Google Scholar]
- 3.Kamal A., Srikanth Y.V.V., Khan M.N.A., Shaik T.B., Ashraf Md. Bioorg. Med. Chem. Lett. 2010;20:5229. doi: 10.1016/j.bmcl.2010.06.152. [DOI] [PubMed] [Google Scholar]
- 4.Bal R.T., Anand B., Yogeeswari P., Sriram Dh. Bioorg. Med. Chem. Lett. 2005;15:4451. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Jiang T., Kuhen K.L., Wolff K., Yin H., Bieza K., Caldwell J., Bursulaya B., Tuntland T., Zhang K., Karanewsky D., He Y. Bioorg. Med. Chem. Lett. 2006;16:2109. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Tripathy R., Reiboldt A., Messina P.A., Iqbal M., Singh J., Bacon E.R., Angeles Th.S., Yang Sh.X., Albom M.S., Robinson C., Chang H., Ruggeri B.A., Mallamo J.P. Bioorg. Med. Chem. Lett. 2006;16:2158. doi: 10.1016/j.bmcl.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 7.Amal R.A., Raghunathan R., Sridevikumaria M.R., Raman N. Bioorg. Med. Chem. 2003;11:407. doi: 10.1016/s0968-0896(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Arguelles M.C., Mosquera-Vazaquez S., Touron-Touceda P., Sanmartin-Matalobos J., Garcia-Deibe A.M., Belicchi-Ferraris M., Pelosi G., Pelizzi C., Zani F. J. Inorg. Biochem. 2007;101:138. doi: 10.1016/j.jinorgbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Maskell L., Blanche E.A., Colucci M.A., Whatmore J.L., Moody Ch. J. Bioorg. Med. Chem. Lett. 2007;17:1575. doi: 10.1016/j.bmcl.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 10.Verma M., Nath Pandeya S., Nand Singh K., Stables J.P. Acta Pharm. 2004;54:49. [PubMed] [Google Scholar]
- 11.Igosheva N., Lorz C., O’Conner E., Glover V., Mehmet H. Neurochem. Int. 2005;47:216. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Chen L.R., Wang Y.Ch., Lin Y.W., Chou Sh.Y., Chen Sh.F., Liu L.T., Wu Y.T., Kuo Ch.J., Chen T.Sh., Juang Sh.H. Bioorg. Med. Chem. Lett. 2005;15:3058. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Bell R., Carmeli S., Sar N. J. Nat. Prod. 1994;57:1587. doi: 10.1021/np50113a022. [DOI] [PubMed] [Google Scholar]; (b) Osawa T., Namiki M. Tetrahedron Lett. 1983;2:4719. [Google Scholar]
- 14.Hong C., Firestone G.L., Bjeldanes L.F. Biochem. Pharmacol. 2002;63:1085. doi: 10.1016/s0006-2952(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 15.(a) Shanthi G., Vidhya L.N., Paramasivan T.P. ARKIVOC. 2009:121. [Google Scholar]; (b) Pavan Kumar V., Prakash Reddy V., Sridhar R., Srinivas B., Narender M., RamaRao K. J. Org. Chem. 2008;73:1646. doi: 10.1021/jo702496s. [DOI] [PubMed] [Google Scholar]; (c) Meshram H.M., Aravind Kumar D., Ramesh Goud P., Chennakesava Reddy B. Synth. Commun. 2010;40:39. [Google Scholar]; (d) Hosseini-Sarvari M., Tavakolian M. Appl. Catal. A: General. 2012;441–442:65. [Google Scholar]; (e) Hanhan N.V., Sahin A.H., Toby W., Chang T.W., Fettinger J.C., Franz A.K. Angew. Chem., Int. Ed. 2010;49:744. doi: 10.1002/anie.200904393. [DOI] [PubMed] [Google Scholar]; (f) Rad-Moghadama K., Gholizadeh S. Iran. J. Catal. 2014;4:41. [Google Scholar]; (g) Deng J., Zhang S., Ding P., Jiang H., Wang W., Jian Li. Adv. Synth. Catal. 2010;352:833. [Google Scholar]
- 16.(a) Rad-Moghadam K., Sharifi-Kiasaraie M., Taheri-Amlashi H. Tetrahedron. 2010;66:2316. doi: 10.1016/j.tet.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yadav J.S., Subba Reddy B.V., Gayathri K.U., Syeda Meraj, Prasad A.R. Synthesis. 2006;24:4121. [Google Scholar]; (c) Sharma R.K., Sharma C. J. Mol. Catal. A: Chem. 2010;332:53. [Google Scholar]; (d) Azizian J., Mohammadi Ali A., Karimi N., Mohammadizadeh M.R., Karimi Ali R. Catal. Commun. 2006;7:752. [Google Scholar]; (e) Karimi N., Oskooi N., Heravi H., Saeedi M., Zakeri M., Niloofar M.T. Chin. J. Chem. 2011;29:321. [Google Scholar]; (f) Tabatabeinan K., Mamaghani M., Khorshidi A. Can. J. Chem. 2009;87:1213. [Google Scholar]; (g) Shun-Yi W., Shun-Jun Ji. Tetrahedron. 2006;62:1527. [Google Scholar]; (h) Subba Reddy B.V., Rajeswari N., Sarangpani M., Ganji R.J., Addlagatta A. Bioorg. Med. Chem. Lett. 2012;22:2460. doi: 10.1016/j.bmcl.2012.02.011. [DOI] [PubMed] [Google Scholar]; (i) Nikpassand M., Mamaghani M., Tabatabaeian K., Samimi H.A. Synth. Commun. 2010;40:3552. [Google Scholar]; (j) Deb M.L., Bhuyan P.J. Synth. Commun. 2009;39:2240. [Google Scholar]; (k) Srihari G., Murthy M.M. Chem. Lett. 2008;37:268. [Google Scholar]
- 17.Szejtli J. Chem. Rev. 1998;98:1743. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 18.(a) Bender M.L., Komiyama M. Springer; New York: 1978. Cyclodextrin Chemistry. [Google Scholar]; (b) Takahashi K. Chem. Rev. 1998;98:2013. doi: 10.1021/cr9700235. [DOI] [PubMed] [Google Scholar]
- 19.(a) Hedges A.R. Chem. Rev. 1998;98:2035. doi: 10.1021/cr970014w. [DOI] [PubMed] [Google Scholar]; (b) Sakuraba H., Maekawa H. J. Incl. Phenom. Macrocyclic Chem. 2006;54:41. [Google Scholar]; (c) Bhosale S.V., Bhosale S.V. Mini-Rev. Org. Chem. 2007;4:231. [Google Scholar]; (d) Hapiot F., Tilloy S., Monflier E. Chem. Rev. 2005;106:767. doi: 10.1021/cr050576c. [DOI] [PubMed] [Google Scholar]
- 20.(a) Reddy M.A., Bhanumathi N., Rao K.R. Chem. Commun. 2001:1974. doi: 10.1039/b106736m. [DOI] [PubMed] [Google Scholar]; (b) Narender M., Reddy M.S., Kumar V.P., Srinivas B., Shridhar R., Nageswar Y.V.D., Rao K.R. Synthesis. 2007;22:3469. [Google Scholar]; (c) Murthy S.N., Madhav B., Kumar A.V., Rao K.R., Nageswar Y.V.D. Tetrahedron. 2009;65:5251. [Google Scholar]
- 21.Shapiro N., Vigalok A. Angew. Chem., Int. Ed. 2008;120:2891. [Google Scholar]
- 22.Jian Wu, Xianli Du, Juan Ma, Yuping Zhang, Qingcai Shi, Lijun Luo, Baoan Song, Song Yanga, Deyu Hu. Green Chem. 2014;16:3210. [Google Scholar]; General procedure for preparation of sulfonated β-cyclodextrin: To a magnetically stirred mixture of β-cyclodextrin (5.107 g, 4.5 mmol) in CH2Cl2 (20 mL), chlorosulfonic acid (1.048 g, 9 mmol) was added slowly drop by drop at 0 °C during 3 h. After completion of addition, the mixture was stirred for 2 h to remove HCl from reaction vessel. Then, the mixture was filtered and washed with methanol (30 mL) and dried at room temperature to obtain sulfonated β-cyclodextrin as white powder (5.28 g). The –SO3H content was measured by titration method and showed 0.52 mequiv g−1.
- 23.Asghar S., Tajbakhsh M., Kenari B.J., Khaksar S. Chin. Chem. Lett. 2011;22:127. [Google Scholar]
- 24.(a) Patil D.R., Wagh Y.B., Ingole P.G., Singh K., Dalal D.S. New J. Chem. 2013;37:3261. [Google Scholar]; (b) Patil D.R., Dalal D.S. Chin. Chem. Lett. 2012;23:1125. [Google Scholar]; (c) Patil D.R., Ingole P.G., Singh K., Dalal D.S. J. Incl. Phenom. Macrocyclic Chem. 2013;76:327. [Google Scholar]; (d) Patil D.R., Dalal D.S. Synth. Commun. 2013;43:118. [Google Scholar]
- 25.General procedure for the preparation of 3-indolyl-3-hydroxy oxindoles (3a–f): To the reaction mixture containing isatin (1 mmol) and indole (1 mmol) in water (2 mL), catalytic amount of β-CD-SO3H (10 mol %) was added and stirred at reflux temperature for appropriate time and monitored through the TLC. After completion of reaction, it was cool at room temperature and filter to get the precipitate. The crude product was recrystallized from aqueous ethanol (60:40) giving pure 3-indolyl-3-hydroxy oxindoles.
- 26.General procedure for the preparation of 3,3-di(indolyl)indolin-2-ones (4a–o): Sulfonated-β-cyclodextrin (β-CD-SO3H) (0.1 mmol) was dissolved in water (2 mL) at room temperature by stirring to get the clear solution. Then the reaction was shifted to reflux with addition of isatin (1 mmol) and indole (2 mmol) with constant stirring. The progress of the reaction was monitored by TLC. After completion of reaction, it was cooled to room temperature and filtered, to get the solid. The crude product was recrystallized from aqueous ethanol (60:40) giving pure 3,3-di(indolyl)indolin-2-ones.
- 27.Catalyst recovery and reuse: The catalyst recovery is convenient and easy to perform. The filtered aqueous layer was evaporated under vacuum and the crude catalyst was collected. The recovered crude catalyst was washed with diethyl ether to obtained pure catalyst, dried it and reused for next reaction.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and spectral data.


