Abstract
Cryptosporidium spp. infections in neonatal dairy calves can cause diarrhoea and, in rare cases, death. The infection is usually self-limiting, but halofuginone lactate (HL) can be used prophylactically. Calves (n = 144) in the study were born during a 2-month period on one farm. A total of 901 serum and 767 faecal samples were collected. Based on HL treatment, the calves were divided into 3 groups: I) not treated, II) treated incorrectly (treatment started > 48 h after birth, or lasted < 7 days), and III) treated correctly (started < 48 h after birth, and lasted ≥ 7 days). Over the 3-month observation period, 14.6% (n = 21) of the calves died, of which most (67%) had not been treated with HL. Correctly performed treatment of cryptosporidiosis significantly delayed the onset of oocysts shedding (P < 0.001) and reduced haptoglobin (HP) and serum amyloid A (SAA) concentrations in the second week of life. HP concentration and HL treatment were negatively associated with weight gain at 3 months of age. Cryptosporidium positive faecal samples were significantly (P < 0.001) more likely to be diarrhoeic but Giardia or Eimeria positive samples were not. Correct prophylactic treatment with HL delayed the shedding of Cryptosporidium oocysts and improved survival, but was negatively associated with weight gain. Incorrect treatment had a low impact on mortality and resembled no treatment regarding the proportion of calves shedding oocysts. Acute phase response (APR) in the second week of life seemed to be positively associated with shedding high amounts of Cryptosporidium oocysts.
Keywords: Calf morbidity, Antiparasitic treatment, Neonatal, Gastrointestinal disorder, Innate immunity response
Highlights
-
•
Acute phase response was affected by halofuginone lactate treatment.
-
•
Haptoglobin concentration was more affected than serum amyloid A by Cryptosporidium.
-
•
Gamma-glutamyltransferase activity after birth had a positive association with survival.
-
•
Halofuginone lactate treatment had a negative effect on weight gain.
1. Introduction
Cryptosporidium can be found in cattle herds worldwide (O'Handley and Olson, 2006) and has also been found in Estonian dairy farms (Lassen et al., 2009). Cryptosporidium infection in dairy calves can lead to villous atrophy in the small intestine mucosa and increase intestinal permeability (Wyatt et al., 2010). Consequently, these pathologies can lead to diarrhoea and increased risk of mortality (Delafosse et al., 2015). Neonatal calves have a higher risk of being negatively affected and shed Cryptosporidium oocysts more frequently than adult livestock (Maddox-Hyttel et al., 2006, Featherstone et al., 2010). The incubation period of cryptosporidiosis varies on average from 5 to 7 days, but symptoms can start as early as 2 days post-infection (Abeywardena et al., 2015). Infected calves typically excrete oocysts with faeces for about 2 weeks (Fayer et al., 1998, O'Handley et al., 1999). Under experimental conditions, the Cryptosporidium oocysts count in faeces rises a day before the onset of diarrhoea, peaks, and drops 2 days before the diarrhoea becomes less severe (Operario et al., 2015). In previous longitudinal studies, the highest number of Cryptosporidium oocysts were found during the second or third weeks of the calves' lives (Santín et al., 2008, Coklin et al., 2010).
Giardia's role as a pathogen in production animals is debated (Geurden et al., 2010). Giardia infections can be chronic and last for months (Grit et al., 2014). Giardia and Eimeria have multifactorial pathogenesis that leads to microvilli alteration, diarrhoea, and weight loss in production animals (Olson et al., 1995, Geurden et al., 2010, Lassen et al., 2015). In a recent study, Giardia infection was also associated with haemorrhagic diarrhoea in calves (Lee et al., 2016). In cases of experimental inoculation with the parasite, dairy calves usually survive the infection with only minor repercussions (Grit et al., 2014). Calves who are infected after birth start shedding oocysts around the third week of life (O'Handley et al., 1999). The Giardia infection rate is related to the age and has been found to peak 6 weeks after birth (Winkworth et al., 2008, Coklin et al., 2010).
If Cryptosporidium and Giardia infections are concurrent, it could cause morphological damage to the jejunum to a lesser extent; this could be because of the antagonistic nature of the coinfection (Ruest et al., 1997). Very low doses of both parasites (around 10 oocysts/cysts) are required to mount an successful infection (Rendtorff, 1954, Okhuysen et al., 1999).
For the prophylactic treatment of Cryptosporidium, halofuginone lactate (HL) can be used; it has cryptosporidiostatic effect that in most cases has been proven to be effective in reducing the excretion of oocysts (Joachim et al., 2003, Silverlås et al., 2009).
The acute phase response (APR) is a series of complex physiological events occurring after tissue injuries or infections (Cray et al., 2009). In response to APR, the concentrations of specific acute phase proteins (APP), serum amyloid A (SAA) and haptoglobin (HP), can increase in ruminant serum > 1000-fold (Ceciliani et al., 2012, Eklund et al., 2012). In cases of viral (foot-and-mouth disease and bovine respiratory syncytial virus) or parasitic (Eimeria and Cryptosporidium) infections, APP concentrations can increase in domestic ruminants' blood serum (Orro et al., 2011, Pourjafar et al., 2011, Stenfeldt et al., 2011, Lassen et al., 2015). In neonatal calves, APPs go through significant changes during first 2 to 3 weeks of life (Orro et al., 2008, Tóthová et al., 2015), suggesting that APPs have a role in the adaptation of neonate calves to the new environment. In reindeer calves, lambs, and beef calves, high concentrations of SAA measured in the second week of life have been associated with lower weight gain recorded many months later (Orro et al., 2006, Peetsalu et al., 2013, Seppä-Lassila et al., 2015, Seppä-Lassila et al., 2017).
Cryptosporidium infection has been shown to increase the APP concentration in dairy calves (Pourjafar et al., 2011). However, the effect of Cryptosporidium infection combined with prophylactic treatment on the immune system and growth remains unknown. In this study, we examined the effects of untreated, incorrect treatment, and correct treatment with HL in an outbreak of cryptosporidiosis in neonatal calves.
2. Materials and methods
2.1. Ethics statement
This study was conducted based on ethical permission issued by the Ethical Committee of Animal Experiments in the Estonian Ministry of Agriculture (no. 7.2-11/2).
2.2. The farm
This study took place on a large dairy farm in Järvamaa County, Central-Estonia. The average milk production per cow in 2015 was 10,000 kg (Estonian Livestock Performance Recording Ltd., 2015). During the study, there were about 1800 dairy cows in the farm.
2.3. Animals
Inclusion criteria: all of the female calves born from January 21 to March 16, 2015, were included in the study (n = 145). Exclusion criteria: twins (1 pair of twins born) and male calves. One animal was dropped from the study because she died before any samples were collected.
The calves were separated from their mothers immediately after birth. In the first 4 weeks, the calves were kept in individual pens with wooden floors and straw bedding. After that, they were moved to group pens with concrete flooring and straw and sawdust bedding. Group pens were composed of 8–10 calves. Both individual and group pens were housed in the same building until the animals were 2 months old. Immediately after birth, the calves were weighed with a digital scale (MS4 PW, Excell Precision Co., Ltd, Vilnius, Lithuania). Additional weight measurements were taken around 1 and 3 months of age with a digital scale (KERN EOS 150K100NXL, Kern & Sohn GmbH, Balingen, Germany) and measuring tape (ANImeter, Albert Kerbl GmbH, Buchbach, Germany), respectively.
2.4. Feeding
The calves were fed 3 l of unpasteurised colostrum in the first 2 h of life. The colostrum given to the calves was collected from the dam and the quality examined visually and with a hydrometer (Kruuse colostrum densimeter, Jørgen Kruuse A/S, Langeskov, Denmark). If the colostrum was of unsatisfactory quality (n = 2), deep frozen colostrum from another dam was provided. The calves were fed 2–3 kg of warmed unpasteurised raw milk twice per day with free access to hay and starter feed (Prestarter, Agrovarustus OÜ, Tartu, Estonia) up to 15–17 days of age. Then their feed was switched to milk powder (Josera GoldenSpezial, Josera GmbH & Co. KG, Kleinheubach, Germany) solution (1 l of warm water + 140 g of milk powder) of 2 × 3 l/day for 1 week with free access to starter feed (Prestarter, Agrovarustus OÜ) and hay. At 1 month of age, the milk powder product was changed (Josera IgluStart, Josera GmbH & Co. KG) and decreased each week with 0.5 l per feeding. Around weaning time (70–80 days of age), the calves received 2 × 2 l/day. After weaning, the calves had free access to starter feed (Starter, Agrovarustus OÜ), hay, and silage. No significant changes were made to the feeding regiments or feed itself during the study period.
2.5. Treatments
All of the calves were vaccinated on the second day after birth against parainfluenza virus type 3 (PI3V) and bovine respiratory syncytial virus (BRSV) (Rispoval, Zoetis Belgium SA, Louvain-la-Neuve, Belgium). At 3 months of age, all of the calves were vaccinated against bovine herpesvirus-1 (BoHV-1) (Hiprabovis, Laboratorios HIPRA, S.A., Girona, Spain). Prophylactic treatment against Eimeria infection was done once by administrating toltrazuril (Cevazuril, Ceva Santé Animale, Libourne, France) to every calf between 29 and 65 days of age. Prophylactic treatment of the Cryptosporidium infection was done using HL (Halocur, Intervet International B.V., Boxmeer, Netherlands).
The study was designed as observational cohort study. Based on the HL treatment regime, the calves were divided retrospectively into 3 groups: I) not treated (n = 34), II) treated incorrectly (treatment started > 48 h after birth, or lasted < 7 days) (n = 45), and III) treated according to manufacturer's instructions (started < 48 h after birth, and lasted ≥ 7 days) (n = 65).
All animals in the study requiring medical treatment received it from the farm's veterinarian. Diarrhoea was treated by administering electrolyte solutions, and if needed, antibiotics were also given. Antibiotics were also used to treat respiratory infections.
No necropsies were performed on the study animals that died. The farm's veterinarian noted the most likely cause of death based on symptoms, such as diarrhoea, respiratory distress, or lameness.
2.6. Sample collection
Once per week, up to 6 weeks of age, serum and faecal samples were collected from each calf. Follow-up sample collection was done at around 3 months of age.
Faecal samples were collected with a clean disposable latex glove directly from the rectum and placed into clean sealable plastic cups and marked with the last 5 numbers of the animal's ear tag. If the rectum was found empty and the calf could not be stimulated to defecate using finger, the sample collection was abandoned (n = 158). In addition, 83 faecal samples were not collected due to the unexpected death of 21 calves. Faecal samples were stored in an insulated container with cooling elements for 2 h and then kept at 4 °C for a maximum of 48 h until analysis. In total, 767 faecal samples were collected from 144 calves.
Serum samples (n = 901) were collected from the jugular vein in sterile evacuated test tubes using an 18-G sterile needle. Blood samples were transported to the laboratory and centrifuged (3000 RCF for 10 min). All of the serum samples were then stored at − 20 °C until further analysis.
In order to avoid dehorning affecting the APP serum concentrations, all of the blood samples were collected immediately before the procedure. For technical reasons, 8 calves were not sampled before dehorning and were marked as compromised.
2.7. Parasites
Faecal samples were prepared for Cryptosporidium and Giardia detection in a similar method described for Eimeria detection by Lassen et al., 2009, but with slight modifications. In detail, sample preparation followed the same steps: weighing, mixing, diluting, and centrifuging, but after the supernatant was removed and before saturated sugar and solution (ρ = 1.26 g/cm3) was added, a 20 μl subsample of the 1 ml suspended pellet was fixed on glass slides well (14 mm diameter latex wells). Staining was done using fluorescein isothiocyanate (FITC) conjugated anti-Cryptosporidium and anti-Giardia monoclonal antibodies (Crypto/Giardia Cel, Cellabs Pty Ltd., Sydney, Australia). The slides were examined using an epifluorescence Nikon Eclipse 80i microscope using 200–400 × magnification. Cryptosporidium and Giardia oocysts were differentiated visually based on morphology, and considered positive if at least 1 oocyst or cyst was found. All of the oocysts and cysts on the slide were counted and the approximate number of oocysts per gram of faeces (OPG) was calculated, corrected to the total area of the well and to the dilution of the sample (De Waele et al., 2010). In case there were too many oocysts to count, 3 random visual fields on the slide were picked and all of the oocysts were counted in the field of view (Lassen and Lepik, 2014). The counts of each visual field were averaged and multiplied with the fraction of the visual field surface area divided by the total slide surface area to calculate the total number of oocysts on a slide.
DNA was extracted from 12 FITC Cryptosporidium-positive faecal samples collected at 3th March from calves borned between 21st January-20th February 2015 (mean age 21 days) using the PSP® Spin Stool DNA Kit (STRATEC Biomedical AG, Birkenfeld, Germany). The DNA was submitted to PCR amplification targeting the 18S rRNA gene of Cryptosporidum spp. as described by Zintl et al. (2007), and the 60 kDa glycoprotein (gp60) gene as described by Peng et al. (2001). The PCR products were run on a 2% ethidium bromide stained agarose gel and visualized under an UV transilluminator. Products of approximately 825 bp from the 18S rRNA and approximately 490 bp of the gp60 amplifications were cleaned and submitted to sequencing in two directions using Applied Biosystems® 3130xl Genetic Analyzer. Forward and reverse sequences were aligned using the BioEdit v7.2.5 software (Hall, 1999) to generate consensus sequences and correct potential mismatches. The GenBank BLASTn (Altschul et al., 1990) tool was used to find similarities between the sequences of our PCR products and with deposited nucleotide sequences in the library. Sequences of the 18S rRNA products were used to determine the species of Cryptosporidium, and gp60 was used to determine the subtype.
The faecal samples were classified as diarrhoeic or non-diarrhoeic based on visual examination. The remaining 1 ml of concentrated faecal sample from above was examined with a light microscope using the flotation method (Roepstorff and Nansen, 1998) for possible parasites (Eimeria spp. and intestinal nematodes). Eimeria spp. were differentiated visually based on morphology (Levine, 1985).
2.8. Acute phase proteins and gamma-glutamyltransferase
The concentration of SAA was measured by commercial ELISA kit (Phase BE kit, Tridelta Development Ltd., Dublin, Ireland). The HP concentration was assessed via the method defined by Makimura and Suzuki, 1982), with an alteration using tetramethylbenzidine (0.06 mg/ml) as a substrate and using microtitration plates (Alsemgeest et al., 1994). Bovine acute phase serum (pooled and lyophilised) were used to generate standard curves. Standard provided by the European Commission Concerted Action Project (number QLK5-CT-1999-0153) was used to standardise the assay of bovine plasma sample with a known HP concentration. The range of the standard curve was 75–1160 mg/l.
The intra-assay and inter-assay coefficients of variations for SAA were ˂11% and ˂13% and for HP were ˂13% and ˂10%, respectively.
Analysis of GGT activity was measured using the kinetic method with l-γ-glutamyl-3-carboxy-4-nitroanilide (Persijn and van der Slik, 1976) in a clinical chemistry analyzer (Accent-200 GGT, PZ Cormay S.A., Łomianki, Poland).
2.9. Statistical analysis
Linear regression models were used to check if HL treatments were associated with changes in the HP or SAA concentrations in the first 6 weeks of life. HP or SAA were the dependent variables and both were logarithmically transformed in order to meet the presumption of normal distribution. The explanatory variables were the age (days) at sample collection and HL treatment as a categorical variable.
A random-effects logistic regression model was constructed to investigate if Cryptosporidium- or Giardia-positive faecal samples in the first 6 weeks of life were more likely to be diarrhoeic. Eimeria was excluded from these models, as all the faecal samples from the first 6 weeks of life were negative. The sample being diarrhoeic was added as a binary dependent variable. Explanatory variables were Cryptosporidium-positive faecal samples, Giardia-positive samples, and age (days). Parasite-positive samples were categorised as follows: 0 = no oocysts or cysts found; 1 = the oocyst or cyst count in the sample below the median count; and 2 = the oocyst or cyst count in the sample above the median count. The calves were added to the model as random intercepts.
A logistic regression model was used to examine if Eimeria-positive calves were diarrhoeic at 3 months of age. The dependent variable was diarrhoea (binary) and the explanatory variables were the total number of Eimeria oocysts in 1 g of sample (OPG) and the age (days) at sample collection.
For assessing the odds of death within the first 6 weeks of age, a retrospective case-control logistic regression model was constructed. The case group (n = 14) consisted of animals who died before 43 days of age. The control group (n = 49) consisted of animals born ± 3 days to a matching case group of animals that did not die before 43 days of age and whose dams were also either primiparous or multiparous. The dependent variable was death; the explanatory continuous variables were Cryptosporidium oocyst count in faecal samples, birth weight, GGT, and APPs (SAA and HP) at the first week of life; and the independent variable was the dam being primiparous or multiparous. Backward step-wise elimination procedure was used for final model.
Linear regression models were used to evaluate if HP and SAA concentrations differed on weekly bases over the first 6 weeks of life based on the Cryptosporidium oocyst count found in the faecal samples. The dependent variables were HP or SAA, and both were logarithmically transformed in order to meet the presumption of normal distribution. The explanatory variables were age at sample collection (days) and Cryptosporidium infection intensity as a categorical variable (0 = no oocysts found in faecal sample; 1 = low; sample containing less than the median number of oocysts (OPG) when compared to other same weeks' positive results; and 2 = high; sample containing more than the median number of oocysts when compared to the other same weeks' positive results). Bonferroni's multiple comparison correction procedure was used to control Type I errors.
The average area under the curve (AUC) was calculated using the trapezoidal method for different APPs and the parasite oocyst count over 6-week periods as:
where ti = the time of observation, ti − 1 = the previous time of observation, fi = APP concentration at the time, and fi − 1 = APP concentration at the previous time. AUCs were used as summary measures for concentrations of APPs and the oocyst counts over time. The AUC value was divided with the calves' age in order to be comparable between different animals. AUCaverage = AUC/age at sample collection. The AUC calculation was performed if the calf had 4 observations or more and was not compromised (had serum sample collected prior to dehorning).
Multiple linear regression models were used to determine the association between APPs-AUC results and Cryptosporidium and Giardia infection. The SAA- and HP-AUC results were used as dependent variables. The independent variables of AUCs for both parasites were: the oocyst or cyst count in the faecal samples, the age and GGT concentration at the first sample collection, and HL treatment as categorical variable. The dependent variables SAA- and HP-AUC results were logarithmically transformed to meet the presumption of normal distribution.
Multiple linear regression models were used to describe the APPs and the Cryptosporidium and Giardia infections possible association with average daily weight gain (ADWG). The dependent variable was ADWG at the age of 1 month or at the age of 3 months. The independent variables were SAA, HP average-AUCs, Cryptosporidium and Giardia oocysts-AUCs, age (days) at the first collection of the first sample, age (days) at weight measurement, proportion of diarrhoeic faecal samples, HL treatment categories, and primiparous or multiparous dam's offspring as a categorical variable.
In the linear and logistic regression models, independent variables were selected according to their P values using backward stepwise elimination. Independent variables were eliminated from a model if P > 0.05. Variables that changed the coefficient of the remaining variables with > 10% were kept as confounders.
Statistical data analysis was done using STATA 14.1 (StataCorp LP, College Station, TX, USA). Basic data management was done using Excel 2013 (Microsoft, Redmond, WA, USA) and Python 3.5.1 (Anaconda 4.0.0 by Continuum Analytics, Austin, TX, USA). The level of a significant result was P ≤ 0.05.
3. Results
3.1. Parasite infection, diarrhoea, and halofuginone lactate treatment
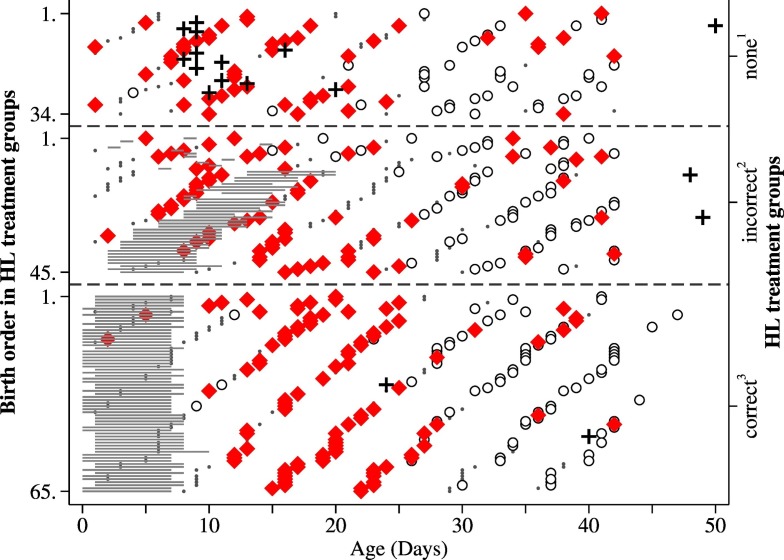
The treatment initiated to control the outbreak of diarrhoea in the calves with HL was started on February 17 and ended on March 22, 2015. In total, 110 calves were treated an average of 6 times (range 1 to 9). On average, the earliest treatment started on day 3 of life (range 0 to 14) (Fig. 1 ).
Fig. 1.
Cryptosporidium and Giardia infection patterns differentiated by halofuginone lactate (HL) treatments. Calves were assigned into groups retrospectively based on HL treatment regiments (separated by dashed lines on figure): 1) not treated (n = 34), 2) treated incorrectly (treatment started > 48 h after birth, or lasted < 7 days) (n = 45), and 3) treated according to manufacturer's instructions (started < 48 h after birth, and lasted ≥ 7 days) (n = 65). (◆) Cryptosporidium positive; (○) Giardia positive; (·) Giardia and Cryptosporidium negative; (+) death (n = 17); horizontal lines represent HL treatment and the length represents the treatment in days. The y-axis represents the birth order of calves in different HL treatment groups, starting with the oldest and ending with the youngest; x-axis represent the age of the calf.
In first six weeks of life, a total of 655 faecal samples (by HL treatment groups: I) no treatment: n = 131, II) incorrect treatment: n = 228, III) correct treatment: n = 296) and 774 serum samples (by HL treatment groups: I) no treatment: n = 156, II) incorrect treatment: n = 272, III) correct treatment: n = 346) were collected. Additionally, 112 faecal and 130 serum samples were collected at 3 months of age.
Cryptosporidium oocysts were found in 33.3% (218/655) of faecal samples and 84.7% (122/144) of calves. Giardia cysts were found in 30.8% (202/655) of faecal samples and 76.4% (110/144) calves. Protozoan infections detection and average age of first detection according to different HL treatment groups are presented in Table 2. Mixed protozoan infections were found in 5.8% (38/655) of faecal samples in 22.2% (32/144) of calves.
Table 2.
Calves tested for presence of Cryptosporidium oocysts and Giardia cysts in faeces and average age of first positive sample grouped by halofuginone lactate (HL) treatment regimentsa in the first six weeks of life.
| (n = no. of calves) |
Cryptosporidium |
Giardia |
||
|---|---|---|---|---|
| No. of animals tested positive | Average (± SD) age of first positive sample (days) | No. of animals tested positive | Average (± SD) age of first positive sample (days) | |
| Not treated (n = 34) | 26 (77%) | 11 ± 7 | 23 (68%) | 28 ± 10 |
| Incorrectly treated (n = 45) | 41 (91%) | 12 ± 7 | 41 (91%) | 31 ± 7 |
| Correctly treated (n = 65) | 55 (84%) | 16 ± 5 | 46 (71%) | 31 ± 8 |
| Total (n = 144) | 122 (85%) | 14 ± 6 | 110 (76%) | 30 ± 8 |
I) not treated; II) treatment start was delayed or duration was < 7 days; III) treatment was done correctly (started in the first 48 h of life and lasted ≥ 7 days of treatment).
The median OPG in a positive Cryptosporidium and Giardia faecal sample in the first six weeks of life, by HL treatment groups was: I) no treatment: 242,844 and 14,035 with range of 70–2,755,554 and 69–469,367, II) incorrect treatment: 333,027 and 79,112 with range of 70–3,646,621 and 70–1,401,208, III) correct treatment: 363,436 and 29,386 with range of 69–10,145,426 and 71–2,652,344.
Out of the 12 FITC Cryptosporidium-positive faecal samples the 18S rRNA gene was successfully amplified in six samples and sequence analysis identified these as C. parvum. Seven (7/12) samples successfully amplified the gp60 gene; five of which were positive and two that were negative in the amplification of the 18S rRNA gene. Sequence analysis of the gp60-positive samples identified them as C. parvum subtype IIaA18G1R1.
Diarrhoea was diagnosed in 53% (344/655) of samples and in 92% (132/144) of calves during the first 6 weeks of age and in 29% (32/112) of samples and in 29% (32/112) of calves at 3 months of age. For the full observation period, 92.4% (133/144) of calves had at least 1 sample that was considered diarrhoeic. The model indicated that in the first 6 weeks of the calves' lives, Cryptosporidium-positive samples were associated with diarrhoea but not Giardia (Table 1 ). The age of the calf was negatively associated with diarrhoea (OR = 0.98; P = 0.007). Calves shedding Eimeria oocysts did not have increased odds of being diarrhoeic (P = 0.2).
Table 1.
Logistic regression model examining the association between diarrhoea in 144 calves during the first 6 weeks of life and the concentration of Cryptosporidium, the concentration of Giardia oocysts in faecal samples, and age at sample collection. Calves were added as random intercepts. Final model is presented.
| Variable (n = no. of samples) | OR | Confidence interval 95% | P-value |
|---|---|---|---|
| Cryptosporidium negative (n = 437) | 1.0 | – | – |
| Cryptosporidium low (n = 109) | 1.93 | 1.23; 3.03 | 0.004 |
| Cryptosporidium high (n = 109) | 2.22 | 1.39; 3.56 | 0.001 |
| Giardia negative (n = 453) | 1.0 | – | – |
| Giardia low (n = 101) | 1.45 | 0.83; 2.55 | 0.192 |
| Giardia high (n = 101) | 1.58 | 0.91; 2.74 | 0.102 |
| Age at sample collection (days) | 0.98 | 0.96; 0.99 | 0.007 |
n (observations) = 655, average n (observations) per calf = 4.5.
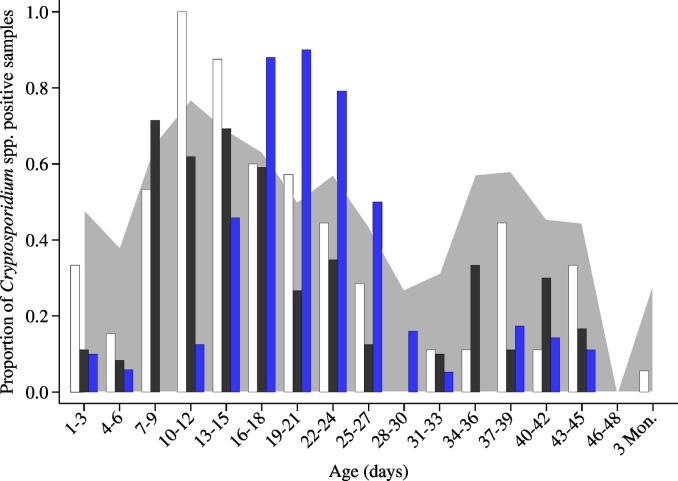
In general, the highest proportion of Cryptosporidium-positive samples was found in calves that were 16–18 days old. When looking at the proportion of animals shedding oocysts by treatment category, calves that got no treatment or were treated incorrectly peaked around 10–12 days of age, while correctly treated animals peaked around 19–21 days of age (Fig. 2 ).
Fig. 2.
Proportion of positive Cryptosporidium spp. faecal samples with different halofuginone lactate (HL) treatment category: (white) not treated (n = 34); (black) treatment start was delayed or duration was < 7 days (n = 45); (blue) treatment was done correctly (started in the first 48 h of life and lasted ≥ 7 days of treatment) (n = 65). Total proportion of diarrhoeic faecal samples presented as grey area in the background.
Eimeria oocysts were detected in 9.5% (73/767) of all the faecal samples collected. Eimeria was only detected in faecal samples collected at approximately three months of age (median age: 99 days). Four species of Eimeria were detected in a total of 73 faecal samples: E. bovis (71.2%, 52/73), E. zuernii (45.2%, 33/73), E. ellipsoidalis (37.0%, 27/73) and E. auburnensis (16.4%, 12/73). Additional results of Eimeria infection, grouped by different HL treatments can be found in Table 1S. No helminth eggs or nematode larvae were found.
3.2. Survival
In first 3 months of life, 21 calves (14.6%) died or were euthanised (Fig. 1 and Fig. 1S). The average age of death was 29 ± 24 days (median 16, range 8 to 83). The reasons listed by the farm veterinarian for mortalities were diarrhoea (n = 12), respiratory infection (n = 6), euthanised because of massive inflammation of the carpal joint or septic umbiliculitis (n = 2), and unknown cause (n = 1).
In total, 66.7% (14/21) of the calves in the group that got no HL treatment died. In the groups of calves that were treated incorrectly or correctly, 2 and 5 died, respectively (Fig. 1S). Based on the veterinarian's diagnosis, 71% of the deaths in the no treatment group were caused by diarrhoea, and in the other groups, 1 animal succumbed to diarrhoea.
In the retrospective case-control logistic regression model, the odds of a calf dying within the first 6 weeks of life increased with higher SAA concentrations (OR = 1.01; P = 0.041). Factors that decreased the odds of a calf dying were: higher GGT activity (OR = 0.99; P = 0.004), larger birth weight (OR = 0.76; P = 0.019), and having a primiparous mother (OR = 0.11; P = 0.022) (Table 3).
Table 3.
Retrospective case control logistic regression modelling of factors associated with mortality of calves up to 43 days of age. Final model is presented.
| Variable (n = no. of calves) | OR | Confidence interval 95% | P-value |
|---|---|---|---|
| SAA (mg/l)a | 1.013 | 1.001; 1.026 | 0.041 |
| GGT (IU/l)a | 0.993 | 0.988; 0.998 | 0.004 |
| Birth weight (kg) | 0.762 | 0.607; 0.957 | 0.019 |
| Multiparous (n = 23) | 1.0 | – | – |
| Primiparous (n = 40) | 0.111 | 0.017; 0.731 | 0.022 |
n (observations) = 63 (the case group (n = 14) and the control group (n = 49)), SAA = Serum amyloid A, GGT = gamma glutamyltransferase.
Sample collected first week of life.
3.3. Acute phase proteins
In the linear regression models, no associations were found between HP, SAA, or HL treatment in the parasitic infection category (average AUC) (P > 0.05) during the first 6 weeks (average AUC).
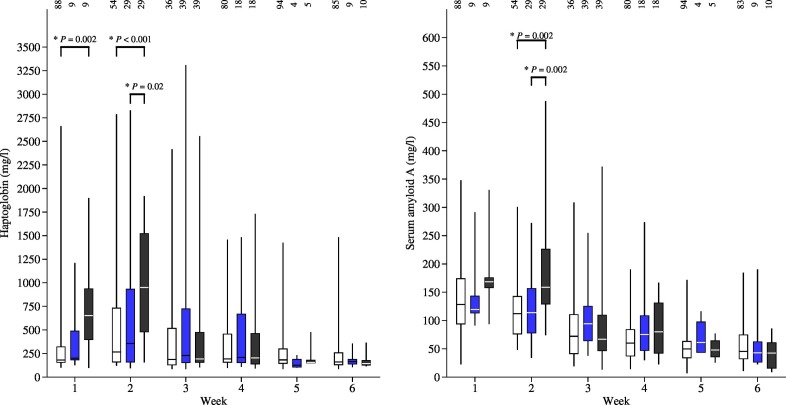
The average SAA concentration increased during the first 2 weeks of life and then decreased. The average HP concentration peaked in the second week of life. In the first 2 weeks of life, calves with a high number of Cryptosporidium oocysts in their faecal samples also had elevated serum concentrations of HP compared to calves in the groups with fewer oocysts in their faeces (Fig. 3 ). Similarly, in the second week of life, calves with a high number of Cryptosporidium oocysts in their faeces also had higher SAA serum concentrations in their serum compared to the other groups (Fig. 3).
Fig. 3.
Haptoglobin (HP) and serum amyloid A (SAA) concentrations in serum and different categories of Cryptosporidium oocyst counts in faecal samples. White = negative (no oocysts found), blue = low (below median oocysts per gram (OPG)), black = high (more than median OPG found in a faecal sample). The number of calves in each group is marked above each bar. Results from the 3 months of age were not presented because only one calf had a Cryptosporidium positive faecal sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
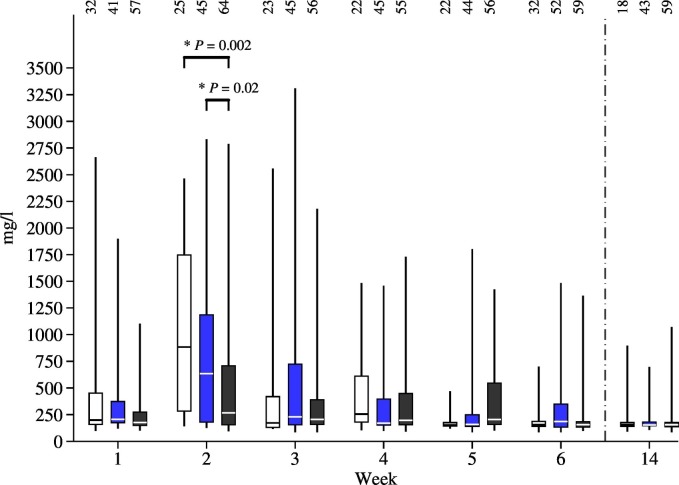
The HP concentration during the second week of life was higher in the HL untreated group compared to the incorrectly (P = 0.001) or correctly treated groups (P = 0.001) (Fig. 4 ). HL treatment in the second week of life was not associated with SAA (P > 0.05).
Fig. 4.
Haptoglobin (HP) concentrations in serum and different halofuginone lactate (HL) treatment groups. Statistically significant differences demonstrated with a horizontal bar on top of second week results. The number of animals in a group shown at the top of a bar. HL treatment groups: white = no treatment; blue = incorrect treatment (treatment start was delayed or was < 7 days long); black = correct treatment (started in the first 48 h of life and had ≥ 7 days of treatment). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Weight gain
The average birth weight was 41.19 ± 5.1 kg (range 27 to 52 kg). Linear regression predicted a negative association (P = 0.004) between HP-AUC and ADWG at 3 months of age. Correct treatment had a negative effect (P = 0.003) on ADWG after a 3-month period when compared to the group that did not receive treatment (Table 4). Information on weight and ADWG by different HL treatment groups is presented in Table 5.
Table 4.
Association of average daily weight gain (g/days) of 109 calves at 3 months of age, haptoglobin (HP) average area under the curve (AUC), halofuginone lactate (HL) treatmenta and age at weight measurement. Final model is presented.
| Variable (n = no. of calves) | Estimate | Confidence interval 95% | P-value |
|---|---|---|---|
| HP average AUC (mg/g/day) | − 0.16 | − 0.27; − 0.05 | 0.004 |
| Not HL treated (n = 18) | 0 | – | – |
| Incorrect HL treatment (n = 42) | − 55.53 | − 126.63; 15.58 | 0.125 |
| Correct HL treatment (n = 49) | − 107.22 | − 176.37; − 38.06 | 0.003 |
| Age at weight measurement (days) | 2.15 | − 0.75; 5.05 | 0.145 |
| Intercept | 708.69 | 390.65; 1026.72 | 0.000 |
| n (observations) = 109 |
I) not treated; II) treatment start was delayed or duration was < 7 days; III) treatment was done correctly (started in the first 48 h of life and lasted ≥ 7 days of treatment).
Table 5.
Results of weight measurement and average daily weight gain (ADWG) at 1 and 3 months (± SD) by different halofuginone lactate (HL) treatment groups.a
| HL treatment group | n (observations) | Age (days) | Weight (kg) | ADWG (g/day) |
|---|---|---|---|---|
| 1 month of age | ||||
| Not treated | 22 | 29.2 ± 4.4 | 54.1 ± 5.7 | 433.6 ± 160.2 |
| Incorrectly treated | 44 | 27.8 ± 3.8 | 53.4 ± 6.3 | 449.5 ± 168.2 |
| Correctly treated | 56 | 31.2 ± 4.6 | 53.9 ± 5.7 | 388.4 ± 122.8 |
| Total | 122 | 29.6 ± 4.5 | 53.8 ± 5.9 | 418.6 ± 148.9 |
| 3 months of age | ||||
| Not treated | 19 | 108.6 ± 12.2 | 134.5 ± 17.3 | 861.6 ± 121.0 |
| Incorrectly treated | 43 | 99.8 ± 12.9 | 120.4 ± 19.3 | 794.4 ± 129.4 |
| Correctly treated | 58 | 100.4 ± 6.6 | 117.7 ± 14.9 | 751.3 ± 123.7 |
| Total | 120 | 101.5 ± 10.6 | 121.3 ± 17.9 | 784.2 ± 130.3 |
I) not treated; II) treatment start was delayed or duration was < 7 days; III) treatment was done correctly (started in the first 48 h of life and lasted ≥ 7 days of treatment).
4. Discussion
Several pathogens potentially fit the differential diagnosis of diarrhoea in calves, including coronavirus, rotavirus, E. coli, and Salmonella. Before the start of the study, veterinarians on the farm had performed rapid pen-side tests with positive results for coronavirus, rotavirus, and Cryptosporidium. In addition, the herd had tested positive for bovine viral diarrhoea virus at the time of the study (personal communication from farm veterinarian). The significant increase in SAA and HP concentrations and presence of diarrhoea in calves has been observed in calves during rotavirus, coronavirus and E. coli infections (Balikci and Al, 2014). In case of naturally occurring rotavirus or coronavirus co-infection with Cryptosporidium versus mono-infection, significantly higher SAA and HP concentration increase has been previously reported (Pourjafar et al., 2011). Nevertheless, best to our knowledge there are no experimental studies where potential co-pathogenic synergism between rotavirus and Cryptosporidium infection has been demonstrated in calves. Our results indicated that Cryptosporidium infections effect to the production of APP was dose-dependent and associated clinical signs could be attributed to the Cryptosporidium infection (Fig. 4). This study reflects the conditions of a farm, and though the outbreak fits the picture of cryptosporidiosis, it is not possible to exclude the co-existence of other pathogens.
4.1. Parasite infection, diarrhoea, and halofuginone lactate treatment
This study investigated the dynamics and treatment of what the farm veterinarians considered an outbreak of cryptosporidiosis. Only C. parvum isotype IIaA18G1R1 was found and which has been previously detected in calves faeces (Misic and Abe, 2007, Plutzer and Karanis, 2007, Brook et al., 2009). We suspect that this subtype was the main cause of cryptosporidiosis in current study, but due to relatively small PCR sample size, which was collected in single time point during the study, it was difficult to say whether there were other subtypes present. Almost all of the calves (84%) were shedding Cryptosporidium spp. oocysts or Giardia spp. cysts in their faeces, but both parasites were found in only 6% of the faecal samples, and thus did not indicate an antagonistic effect. This may be explained by the differences in the parasites infection patterns (Xiao and Herd, 1994, Santín et al., 2008, Santín et al., 2009). However, studies of morphological changes of the jejunum have suggested the possibility of an antagonistic effect of the two parasites (Ruest et al., 1997). Most of Cryptosporidium infections happened before 1 month of age, similar to what has been reported previously in longitudinal studies (Harp and Goff, 1998, O'Handley et al., 1999, Geurden et al., 2007). Calves that were shedding high amounts of Cryptosporidium oocysts had higher odds of being diarrhoeic than calves shedding Giardia or Eimeria, supporting Cryptosporidium as the causative agent of the symptoms. Some authors have suggested that Giardia infection itself does not cause diarrhoea (Maddox-Hyttel et al., 2006, O'Handley and Olson, 2006). A previous study in dairy cattle on several Estonian farms found a negative correlation between diarrhoea and the presence of Eimeria spp. in faeces, but a positive correlation between diarrhoea and higher amounts of Cryptosporidium spp. oocysts (Lassen et al., 2009). In Finnish calves, the opposite was observed; Eimeria spp. was associated with diarrhoea, while Cryptosporidium and Giardia were not (Seppä-Lassila et al., 2015). This illustrates that intestinal parasites, including Cryptosporidium, are important agents of disease in calves in the area, but the general clinical picture varies.
The initiation of the Cryptosporidium infection's prophylactic treatment with HL exemplified the connection between APR and the parasitic infection under natural conditions. The HL treatment did not seem to decrease the number of Cryptosporidium oocysts shed in faeces, similar to what has been reported in one study (Weber et al., 2016), but contrary to another (Keidel and Daugschies, 2013). Nevertheless, the correctly performed prophylactic treatment had a delaying effect on the onset of shedding (Fig. 2 and Table 2), seemed to improve survival (Fig. 1S), but resulted in a poorer ADWG (Table 5). Previous investigations have also reported that HL can cause a delay in oocyst shedding (Jarvie et al., 2005, Trotz-Williams et al., 2011, Keidel and Daugschies, 2013), but not an impact on the survival of calves. Calves have higher risk of succumbing to dehydration and acidosis due to diarrhoea in their first week of life (Foster and Smith, 2009). Prophylactic HL treatment may delay the development of cryptosporidiosis and help calves cope with very strong infection pressure (Abeywardena et al., 2015). It has been suggested that HL may have a positive therapeutic effect in calves aged 8–14 days (Klein, 2008). Other authors (Silverlås et al., 2009, Almawly et al., 2013) have reported that the therapeutic treatment effect of HL on calves' health seems to be limited, similar to the findings in this study.
4.2. Survival
Most of the calves' deaths in the current study were concentrated in a relatively short period, and were found to be related to infections of the digestive system in the group that did not receive HL treatment. Shortly after the mass treatment with HL started, the death rate dropped (Fig. 1). This suggests that the mortalities were related. Cryptosporidium infections and the treatment may have reduced the severity of the illness and raised the chance of survival (Fig. 1S). Higher GGT activity had a positive effect on survival, which suggests colostrum quality and adsorption of antibodies had an important role in the animal's ability to survive the infection. It has been shown that high levels of immunoglobulin G and long fatty acids in colostrum have some protective effect against diarrhoea caused by Cryptosporidium, but not against the infection itself (Lopez et al., 1988, Schmidt and Kuhlenschmidt, 2008, Weber et al., 2016). This could mean that the animals who were at a weaker starting position due to poorer quality colostrum and lower birth weights more easily succumbed to Cryptosporidium infection. Only after starting the mass treatment with HL did the survival chances of these calves improve. Even incorrect treatment with HL seemed to have a positive effect on survival; thus, we could conclude that no treatment would be the worst option during a massive increase in cryptosporidiosis cases, especially when most of the deaths are diarrhoea-related.
The incubation period of Cryptosporidium infection is 5–7 days, which is so short that the adaptive immune response is unlikely to stop the development of clinical disease (Petry et al., 2010, Abeywardena et al., 2015). APR as an innate immune response is faster and more likely to play a role in controlling the infection and the development of disease in the early stages. Interestingly, higher SAA concentrations had a negative impact on the survival of calves. This suggests that APR was triggered more profoundly in severely affected animals (Fig. 3). Although we cannot rule out other common digestive system pathogens, the evidence suggests that Cryptosporidium played a major role in diarrhoeic calves, and that the correct HL treatment was able to delay the APR induction and decrease its magnitude (Fig. 4).
4.3. APPs
The HL prophylactic treatment delayed Cryptosporidium infection and seemed to affect the APR in the second week of life. HP, but not SAA, serum concentrations were significantly lower in the animal groups that were correctly treated compared to the untreated and incorrectly treated calves (Fig. 4). This increase in concentrations of APP coincided with an increased proportion of the calves shedding Cryptosporidium spp. oocysts (Fig. 2) and mortality (Fig. 1S) in the second week of life. The HP median concentration in heavily shedding animals was 4.8 times higher (950 mg/l) than the reference value (< 196 mg/l) of calves that age while the SAA median value (158 mg/l) did not exceed the reference value (< 178 mg/l) (Seppä-Lassila et al., 2013). We speculate that this drastic difference was caused by the nature of the Cryptosporidium infection and likely because localised damage to the small intestine was more prone to trigger an immunological response that increased HP rather than SAA concentrations. Previously, relatively small studies (1 to 6 animals) reported an increase in HP and SAA in dairy calves as a response to Cryptosporidium infections, especially before the onset of diarrhoea (Enemark et al., 2003a, Enemark et al., 2003b, Pourjafar et al., 2011). Although this study shed more light on the subject, there is a lack of research on the role of APR in Cryptosporidium and Giardia infections in cattle.
4.4. Weight gain
Although about 67% of the calves died in the group that did not receive HL treatment, it was surprising to find that the treatment had significant negative effects on the daily weight gain when the calves reached 3 months of age. In general, the calves' weight gain met the expectations of Holstein breed calves at 3 months of age (Retamal and Risco, 2011), averaging around 121.3 kg. We expected that Cryptosporidium infection would have a lasting negative effect on the growth of the surviving calves. The effect of the infection should have been most obvious in calves that were infected but not treated. However, the largest effect was observed in animals that had the correct treatment. Other authors have not found a significant positive or negative effect of HL treatment on the growth rate (Jarvie et al., 2005, Trotz-Williams et al., 2011). It is important to remember that HL treatment does not stop calves from being infected and shedding large numbers of Cryptosporidium oocysts, damaging the host's cells and consuming resources for replicating (Silverlås et al., 2009). There were no significant changes in the feeding regimens in first 3 months of the calves' lives, ruling out differences in nutrition as an explanation. A possible explanation for the observed effect is the delay and possible expansion of the parasites' life cycle in the host due to the effect of HL. The categorisation of different HL treatment groups was very strongly influenced by birth order. As a result, we were not able to exclude time as a confounding factor in average daily weight regression models.
Elevated concentrations of SAA in the second week of life have been negatively associated with the growth rate in reindeer calves (Orro et al., 2006), lambs (Peetsalu et al., 2013) and beef calves (Seppä-Lassila et al., 2017). In the current study, Cryptosporidium shedding was associated with higher serum concentrations of SAA and HP in the second week of the calves' life, indicating that this period of adaptation was critical. However, only the HP overall response had a negative association with short-term weight gain at 3 months of age. The elevated HP concentrations may have triggered a stronger APR as a response to the infection and consequently affected the growth of the calf.
5. Conclusions
In the outbreak, there was a strong association between Cryptosporidium infection and diarrhoea, but not with Giardia or Eimeria infections. Correctly performed prophylactic HL treatment against cryptosporidiosis delayed the onset of oocyst shedding and improved the chances of survival. However, the growth rate was negatively affected by correct treatment and a strong APR. Correct treatment was associated with lower HP concentrations in the second week of life. The study demonstrates a possible connection between Cryptosporidium infection and APR in dairy calves.
The following are the supplementary data related to this article.
Survival curves of the calves based on different halofuginone lactate (HL) treatment categories (no treatment (n = 34, thick grey line), incorrect treatment (n = 45, dashed line), correct treatment (n = 65, solid black line)). Total percentage of deaths (n = 21) in all the groups is shown at the top of figure by quartiles.
Different Eimeria species detected at three months of age in faecal samples (n = 112) and average number of oocysts per gram (OPG) by treatment group using halofuginone lactate (HL)1. Median age at diagnosing of Eimeria infection was 99 days of age.
Conflict of interest
None.
Acknowledgements
We would like to thank veterinary students for helping with sample collection, farm veterinarians Kärt Kalvet and Kadri Kaugerand, and laboratory help by Pille Paats, Külli Must, Azzurra Santoro and Niina Sidarova. The project was funded by the Estonian Research Council project IUT8-1.
References
- Abeywardena H., Jex A.R., Gasser R.B. Advances in Parasitology. Elsevier Ltd; 2015. A perspective on Cryptosporidium and Giardia, with an emphasis on bovines and recent epidemiological findings; pp. 243–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almawly J., Prattley D., French N.P., Lopez-Villalobos N., Hedgespeth B., Grinberg A. Utility of halofuginone lactate for the prevention of natural cryptosporidiosis of calves, in the presence of co-infection with rotavirus and Salmonella Typhimurium. Vet. Parasitol. 2013;197:59–67. doi: 10.1016/j.vetpar.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsemgeest S.P., Kalsbeek H.C., Wensing T., Koeman J.P., van Ederen A.M., Gruys E. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 1994;16:21–23. doi: 10.1080/01652176.1994.9694410. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Balikci E., Al M. Some serum acute phase proteins and immunoglobulins concentrations in calves with rotavirus, coronavirus, E. coli F5 and Eimeria species. Iran. J. Vet. Res. 2014;15:397–401. [PMC free article] [PubMed] [Google Scholar]
- Brook E.J., Anthony Hart C., French N.P., Christley R.M. Molecular epidemiology of Cryptosporidium subtypes in cattle in England. Vet. J. 2009;179:378–382. doi: 10.1016/j.tvjl.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Ceciliani F., Ceron J.J., Eckersall P.D., Sauerwein H. Acute phase proteins in ruminants. J. Proteome. 2012;75:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Coklin T., Farber J.M., Parrington L.J., Coklin Z., Ross W.H., Dixon B.R. Temporal changes in the prevalence and shedding patterns of Giardia duodenalis cysts and Cryptosporidium spp. oocysts in a herd of dairy calves in Ontario. Can. Vet. J. 2010;51:841–846. [PMC free article] [PubMed] [Google Scholar]
- Cray C., Zaias J., Altman N.H. Acute phase response in animals: a review. Comp. Med. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- De Waele V., Speybroeck N., Berkvens D., Mulcahy G., Murphy T.M. Control of cryptosporidiosis in neonatal calves: use of halofuginone lactate in two different calf rearing systems. Prev. Vet. Med. 2010;96:143–151. doi: 10.1016/j.prevetmed.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafosse A., Chartier C., Dupuy M.C., Dumoulin M., Pors I., Paraud C. Cryptosporidium parvum infection and associated risk factors in dairy calves in western France. Prev. Vet. Med. 2015;118:406–412. doi: 10.1016/j.prevetmed.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eesti Põllumajandusloomade Jõudluskontrolli AS (in Estonian with English summary) Estonian Livestock Performance Recording Ltd; Tartu: 2015. Results of Animal Recording in Estonia 2015. [Google Scholar]
- Eklund K.K., Niemi K., Kovanen P.T. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012;32:335–348. doi: 10.1615/critrevimmunol.v32.i4.40. [DOI] [PubMed] [Google Scholar]
- Enemark H.L., Bille-Hansen V., Lind P., Heegaard P.M., Vigre H., Ahrens P., Thamsborg S. Pathogenicity of Cryptosporidium parvum—evaluation of an animal infection model. Vet. Parasitol. 2003;113:35–57. doi: 10.1016/S0304-4017(03)00034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enemark H.L., Ahrens P., Bille-Hansen V., Heegaard P.M.H., Vigre H., Thamsborg S.M., Lind P. Cryptosporidium parvum: infectivity and pathogenicity of the “porcine” genotype. Parasitology. 2003;126:407–416. doi: 10.1017/s0031182003003032. [DOI] [PubMed] [Google Scholar]
- Fayer R., Gasbarre L., Pasquali P., Canals A., Almeria S., Zarlenga D. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 1998;28:49–56. doi: 10.1016/s0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Featherstone C.A., Giles M., Marshall J.A., Mawhinney I.C., Holliman A., Pritchard G.C. Cryptosporidium species in calves submitted for postmortem examination in England and Wales. Vet. Rec. 2010;167:979–980. doi: 10.1136/vr.c3948. [DOI] [PubMed] [Google Scholar]
- Foster D.M., Smith G.W. Pathophysiology of diarrhea in calves. Vet. Clin. North Am. Food Anim. Pract. 2009;25:13–36. doi: 10.1016/j.cvfa.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurden T., Berkvens D., Martens C., Casaert S., Vercruysse J., Claerebout E. Molecular epidemiology with subtype analysis of Cryptosporidium in calves in Belgium. Parasitology. 2007;134:1981–1987. doi: 10.1017/S0031182007003460. [DOI] [PubMed] [Google Scholar]
- Geurden T., Vercruysse J., Claerebout E. Is Giardia a significant pathogen in production animals? Exp. Parasitol. 2010;124:98–106. doi: 10.1016/j.exppara.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Grit G.H., Van Coppernolle S., Devriendt B., Geurden T., Dreesen L., Hope J., Vercruysse J., Cox E., Geldhof P., Claerebout E. Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet. Parasitol. 2014;202:145–155. doi: 10.1016/j.vetpar.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Harp J.A., Goff J.P. Strategies for the control of Cryptosporidium parvum infection in calves. J. Dairy Sci. 1998;81:289–294. doi: 10.3168/jds.S0022-0302(98)75578-X. [DOI] [PubMed] [Google Scholar]
- Jarvie B.D., Trotz-Williams L.A., McKnight D.R., Leslie K.E., Wallace M.M., Todd C.G., Sharpe P.H., Peregrine A.S. Effect of halofuginone lactate on the occurrence of Cryptosporidium parvum and growth of neonatal dairy calves. J. Dairy Sci. 2005;88:1801–1806. doi: 10.3168/jds.S0022-0302(05)72854-X. [DOI] [PubMed] [Google Scholar]
- Joachim A., Krull T., Schwarzkopf J., Daugschies A. Prevalence and control of bovine cryptosporidiosis in German dairy herds. Vet. Parasitol. 2003;112:277–288. doi: 10.1016/S0304-4017(03)00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel J., Daugschies A. Integration of halofuginone lactate treatment and disinfection with p-chloro-m-cresol to control natural cryptosporidiosis in calves. Vet. Parasitol. 2013;196:321–326. doi: 10.1016/j.vetpar.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. Preventive and therapeutic efficacy of halofuginone-lactate against Cryptosporidium parvum in spontaneously infected calves: a centralised, randomised, double-blind, placebo-controlled study. Vet. J. 2008;177:429–431. doi: 10.1016/j.tvjl.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen B., Lepik T. Isolation of Eimeria oocysts from soil samples: a simple method described in detail. Agraart. J. Agric. Sci. 2014;XXV:77–81. [Google Scholar]
- Lassen B., Viltrop A., Raaperi K., Järvis T. Eimeria and Cryptosporidium in Estonian dairy farms in regard to age, species, and diarrhoea. Vet. Parasitol. 2009;166:212–219. doi: 10.1016/j.vetpar.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Lassen B., Bangoura B., Lepik T., Orro T. Systemic acute phase proteins response in calves experimentally infected with Eimeria zuernii. Vet. Parasitol. 2015;212:140–146. doi: 10.1016/j.vetpar.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., VanBik D., Kim H.Y., Cho A., Kim J.W., Byun J.W., Oem J.K., Oh S.I., Kwak D. Prevalence and molecular characterisation of Giardia duodenalis in calves with diarrhoea. Vet. Rec. 2016;178:633–669. doi: 10.1136/vr.103534. [DOI] [PubMed] [Google Scholar]
- Levine N. Iowa State University Press; Iowa: 1985. Veterinary Protozoology; pp. 148–149. [Google Scholar]
- Lopez J.W., Allen S.D., Mitchell J., Quinn M. Rotavirus and Cryptosporidium shedding in dairy calf feces and its relationship to colostrum immune transfer. J. Dairy Sci. 1988;71:1288–1294. doi: 10.3168/jds.S0022-0302(88)79685-X. [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel C., Langkjær R.B., Enemark H.L., Vigre H. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs—occurrence and management associated risk factors. Vet. Parasitol. 2006;141:48–59. doi: 10.1016/j.vetpar.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Makimura S., Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nihon Juigaku Zasshi. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- Misic Z., Abe N. Subtype analysis of Cryptosporidium parvum isolates from calves on farms around Belgrade, Serbia and Montenegro, using the 60 kDa glycoprotein gene sequences. Parasitology. 2007;134:351–358. doi: 10.1017/S0031182006001508. [DOI] [PubMed] [Google Scholar]
- O'Handley R.M., Olson M.E. Giardiasis and cryptosporidiosis in ruminants. Vet. Clin. North Am. Food Anim. Pract. 2006;22:623–643. doi: 10.1016/j.cvfa.2006.07.002. [DOI] [PubMed] [Google Scholar]
- O'Handley R.M., Cockwill C., McAllister T.A., Jelinski M., Morck D.W., Olson M.E. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J. Am. Vet. Med. Assoc. 1999;214:391–396. [PubMed] [Google Scholar]
- Okhuysen P.C., Chappell C.L., Crabb J.H., Sterling C.R., DuPont H.L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- Olson M.E., McAllister T.A., Deselliers L., Morck D.W., Cheng K.J., Buret A.G., Ceri H. Effects of giardiasis on production in a domestic ruminant (lamb) model. Am. J. Vet. Res. 1995;56:1470–1474. [PubMed] [Google Scholar]
- Operario D.J., Bristol L.S., Liotta J., Nydam D.V., Houpt E.R. Correlation between diarrhea severity and oocyst count via quantitative PCR or fluorescence microscopy in experimental cryptosporidiosis in calves. Am. J. Trop. Med. Hyg. 2015;92:45–49. doi: 10.4269/ajtmh.14-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orro T., Nieminen M., Tamminen T., Sukura A., Sankari S., Soveri T. Temporal changes in concentrations of serum amyloid-A and haptoglobin and their associations with weight gain in neonatal reindeer calves. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:79–88. doi: 10.1016/j.cimid.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Orro T., Jacobsen S., LePage J.-P., Niewold T., Alasuutari S., Soveri T. Temporal changes in serum concentrations of acute phase proteins in newborn dairy calves. Vet. J. 2008;176:182–187. doi: 10.1016/j.tvjl.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Orro T., Pohjanvirta T., Rikula U., Huovilainen A., Alasuutari S., Sihvonen L., Pelkonen S., Soveri T. Acute phase protein changes in calves during an outbreak of respiratory disease caused by bovine respiratory syncytial virus. Comp. Immunol. Microbiol. Infect. Dis. 2011;34:23–29. doi: 10.1016/j.cimid.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peetsalu K., Kuks A., Orro T. 10th International Veterinary Immunology Symposium, Milan, Italy. 2013. Temporal changes of serum amyloid A (SAA) concentrations in newborn lambs: effect of colostrum SAA concentrations and associations to the weight gain; pp. 36–37. [Google Scholar]
- Peng M.M., Matos O., Gatei W., Das P., Stantic-Pavlinic M., Bern C., Sulaiman I.M., Glaberman S., Lal A.A., Xiao L. A comparison of Cryptosporidium subgenotypes from several geographic regions. J. Eukaryot. Microbiol. Suppl. 2001:28S–31S. doi: 10.1111/j.1550-7408.2001.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Persijn J.P., van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J. Clin. Chem. Clin. Biochem. 1976;14:421–427. doi: 10.1515/cclm.1976.14.1-12.421. [DOI] [PubMed] [Google Scholar]
- Petry F., Jakobi V., Tessema T.S. Host immune response to Cryptosporidium parvum infection. Exp. Parasitol. 2010;126:304–309. doi: 10.1016/j.exppara.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Plutzer J., Karanis P. Genotype and subtype analyses of Cryptosporidium isolates from cattle in Hungary. Vet. Parasitol. 2007;146:357–362. doi: 10.1016/j.vetpar.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Pourjafar M., Badiei K., Nazifi S., Naghib S.M. Acute phase response in Holstein dairy calves affected with diarrhoea. Bulg. J. Vet. Med. 2011;14:142–149. [Google Scholar]
- Rendtorff R.C. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am. J. Hyg. 1954;59:209–220. doi: 10.1093/oxfordjournals.aje.a119634. [DOI] [PubMed] [Google Scholar]
- Retamal P.M., Risco C.A. Dairy Production Medicine. Blackwell Publishing Ltd; Oxford: 2011. Nutritional management of dairy heifers; pp. 195–206. [Google Scholar]
- Roepstorff A., Nansen P. FAO Animal Health Manual. 1998. Epidemiology, diagnosis and control of helminth parasites of swine; pp. 51–56. Rome. [Google Scholar]
- Ruest N., Couture Y., Faubert G.M., Girard C. Morphological changes in the jejunum of calves naturally infected with Giardia spp. and Cryptosporidium spp. Vet. Parasitol. 1997;69:177–186. doi: 10.1016/S0304-4017(96)01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santín M., Trout J.M., Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet. Parasitol. 2008;155:15–23. doi: 10.1016/j.vetpar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Santín M., Trout J.M., Fayer R. A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet. Parasitol. 2009;162:40–45. doi: 10.1016/j.vetpar.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Kuhlenschmidt M.S. Microbial adhesion of Cryptosporidium parvum: identification of a colostrum-derived inhibitory lipid. Mol. Biochem. Parasitol. 2008;162:32–39. doi: 10.1016/j.molbiopara.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä-Lassila L., Orro T., LePage J.-P., Soveri T. Reference values for acute phase proteins in calves and its clinical application. Vet. Rec. 2013;173:319. doi: 10.1136/vr.101233. [DOI] [PubMed] [Google Scholar]
- Seppä-Lassila L., Orro T., Lassen B., Lasonen R., Autio T., Pelkonen S., Soveri T. Intestinal pathogens, diarrhoea and acute phase proteins in naturally infected dairy calves. Comp. Immunol. Microbiol. Infect. Dis. 2015;41:10–16. doi: 10.1016/j.cimid.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä-Lassila L., Eerola U., Orro T., Härtel H., Simojoki H., Autio T., Pelkonen S., Soveri T. Health and growth of Finnish beef calves and the relation to acute phase response. Livest. Sci. 2017;196:7–13. doi: 10.1016/j.livsci.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverlås C., Björkman C., Egenvall A. Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Prev. Vet. Med. 2009;91:73–84. doi: 10.1016/j.prevetmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Stenfeldt C., Heegaard P.M., Stockmarr A., Tjørnehøj K., Belsham G.J. Analysis of the acute phase responses of Serum Amyloid A, Haptoglobin and Type 1 Interferon in cattle experimentally infected with foot-and-mouth disease virus serotype O. Vet. Res. 2011;42:66. doi: 10.1186/1297-9716-42-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóthová C., Nagy O., Nagyová V., Kováč G. Changes in the concentrations of acute phase proteins in calves during the first month of life. Acta Vet. Brno. 2015;65:260–270. [Google Scholar]
- Trotz-Williams L.A., Jarvie B.D., Peregrine A.S., Duffield T.F., Leslie K.E. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. Vet. Rec. 2011;168:509. doi: 10.1136/vr.d1492. [DOI] [PubMed] [Google Scholar]
- Weber S.E., Lippuner C., Corti S., Deplazes P., Hässig M. Klinische epidemiologie der kälber- cryptosporidiose. Schweiz. Arch. Tierheilkd. 2016;158:341–350. doi: 10.17236/sat00062. (in German, with English abstract) [DOI] [PubMed] [Google Scholar]
- Winkworth C.L., Matthaei C.D., Townsend C.R. Prevalence of Giardia and Cryptosporidium spp in calves from a region in New Zealand experiencing intensification of dairying. N. Z. Vet. J. 2008;56:15–20. doi: 10.1080/00480169.2008.36799. [DOI] [PubMed] [Google Scholar]
- Wyatt C.R., Riggs M.W., Fayer R. Cryptosporidiosis in neonatal calves. Vet. Clin. North Am. Food Anim. Pract. 2010;26:89–103. doi: 10.1016/j.cvfa.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Xiao L., Herd R.P. Infection patterns of Cryptosporidium and Giardia in calves. Vet. Parasitol. 1994;55:257–262. doi: 10.1016/0304-4017(93)00645-f. [DOI] [PubMed] [Google Scholar]
- Zintl A., Neville D., Maguire D., Fanning S., Mulcahy G., Smith H.V., De Waal T. Prevalence of Cryptosporidium species in intensively farmed pigs in Ireland. Parasitology. 2007;134:1575–1582. doi: 10.1017/S0031182007002983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves of the calves based on different halofuginone lactate (HL) treatment categories (no treatment (n = 34, thick grey line), incorrect treatment (n = 45, dashed line), correct treatment (n = 65, solid black line)). Total percentage of deaths (n = 21) in all the groups is shown at the top of figure by quartiles.
Different Eimeria species detected at three months of age in faecal samples (n = 112) and average number of oocysts per gram (OPG) by treatment group using halofuginone lactate (HL)1. Median age at diagnosing of Eimeria infection was 99 days of age.