Abstract
Akabane virus (AKAV) belongs to the Simbu serogroup of the genus Orthobunyavirus in the family Bunyaviridae. It has been shown that AKAV induces apoptosis in mammalian cells. It is necessary to understand the signaling pathways involved in AKAV-induced apoptosis to further elucidate the molecular virology of AKAV. c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) are mediators of apoptosis; therefore, we investigated the roles of JNK and p38 MAPK cascades in AKAV-infected cells. We found that JNK and p38 MAPK as well as their downstream substrates, c-Jun and heat shock protein 27 (HSP27), were phosphorylated in response to AKAV infection. A JNK inhibitor (SP600125) inhibited AKAV-mediated apoptosis whereas a p38 MAPK inhibitor (SB203580) did not. We conclude that AKAV infection activates the JNK and p38 MAPK signaling pathways, and the JNK cascade plays a crucial role in AKAV-induced apoptosis in vitro.
Keywords: Akabane virus, Apoptosis, Mitogen-activated protein kinase (MAPK)
Highlights
-
•
JNK and p38 MAPK were phosphorylated in response to Akabane virus infection.
-
•
Downstream substrates, c-Jun and heat shock protein 27, were also phosphorylated by viral infection.
-
•
JNK inhibitor (SP600125) inhibited AKAV-mediated apoptosis whereas a p38 MAPK inhibitor (SB203580) did not.
1. Introduction
Akabane virus (AKAV) is transmitted by arthropod vectors such as Culicoides oxystoma (Kurogi et al., 1987) and it is the etiological agent that causes Akabane disease. AKAV causes abortions, stillbirths, and congenital disorders in cows, goats, and sheep (Kurogi et al., 1987). AKAV has been detected in Japan, Korea, China, Israel, Australia, Turkey, Sudan, and Kenya (Kurogi et al., 1975, Lee et al., 2002, Jun et al., 2012, Stram et al., 2004, Della-Porta et al., 1976, Taylor and Mellor, 1994, Elhassan et al., 2014, Davies and Jessett, 1985). AKAV belongs to the Simbu serogroup of the genus Orthobunyavirus in the family Bunyaviridae. In addition to AKAV, the Simbu serogroup includes Aino virus, Peaton virus, and Shamonda virus. Recently, a novel Orthobunyavirus called Schmallenberg virus emerged in Europe, which is associated with reduced milk production and diarrhea in adult cattle, as well as congenital malformations in cows and sheep (Garigliany et al., 2012, van den Brom et al., 2012). The AKAV genome comprises three segments of negative sense single-stranded RNA: large (L), medium (M), and small (S). The L segment encodes an RNA-dependent RNA polymerase, the M segment encodes the precursor of glycoproteins (Gn and Gc) and a nonstructural protein (NSm), and the S segment encodes the nucleocapsid protein (N) and a nonstructural protein (NSs). The nonstructural proteins are assumed to interact with the vector/host immune system, thereby contributing to viral pathogenesis (Eifan et al., 2013, Bridgen et al., 2001).
Mitogen-activated protein kinase (MAPK) cascades are intracellular signal transduction pathways that respond to various extracellular stimuli, which are involved in the regulation of a wide variety of cellular process, including growth, proliferation, survival, and apoptosis (Strnisková et al., 2002). The three major MAPK cascades are the extracellular signal-regulated protein kinase cascade, the c-Jun N-terminal kinase (JNK) cascade, and the p38 MAPK cascade. JNK is a regulator of the transcription factor c-Jun and a mediator of intra or extracellular stresses such as heat shock, osmotic shock, cytokines, and UV, which is also known as the stress-activated protein kinase (SAPK) cascade (Robinson and Cobb, 1997). The p38 MAPK cascade is another SAPK signaling pathway; however, there is considerable cross-talk as well as shared components with the JNK cascade (Plotnikov et al., 2011). The activated JNK translocates to the nucleus where it phosphorylates and transactivates c-Jun, and the phosphorylated c-Jun then leads to the formation of activator protein 1 (AP-1). The formation of AP-1 is related to the transcription of a wide variety of proteins, including proapoptotic factors (Dhanasekaran and Reddy, 2008). p38 MAPK is responsible for the phosphorylation and activation of MAP kinase-activated protein kinase 2, heat shock protein 27 (HSP27), activating transcription factor 1, and cAMP response element-binding protein (Dorion and Landry, 2002, Cowan, 2003).
In general, activation of the JNK cascade and p38 MAPK cascade induces apoptosis. These two activated cascades mediate intracellular signaling and lead to caspase-3 activation, which is indispensable for apoptotic chromatin condensation and DNA fragmentation. Caspase-3 is synthesized as an inactive proenzyme, which is activated via cleavage at specific Asp residues to yield the active enzyme that contains large (p20) and small (p10) subunits (Cohen, 1997, Cho and Choi, 2002). JNK activation is the mediator of apoptosis induction by some viruses such as infectious bursal disease virus, porcine reproductive and respiratory syndrome virus, and equine influenza virus (Wei et al., 2011, Yin et al., 2012, Lin et al., 2001). Furthermore, some viruses such as herpes simplex virus type 1 and rotavirus can manipulate the JNK signaling pathway to regulate viral replication (McLean and Bachenheimer, 1999, Holloway and Coulson, 2006). The p38 MAPK signaling pathway is activated to induce apoptosis by bluetongue virus, soft-shelled turtle iridovirus, and other viruses as well as to control viral replication by viruses such as coxsackievirus B3 and varicella-zoster virus (Mortola and Larsen, 2010, Huang et al., 2011, Si et al., 2005, Rahaus et al., 2004). In case of severe acute respiratory syndrome-coronavirus, p38 MAPK induces cell death in Vero E6 cells and JNK is related to persistent infection (Mizutani et al., 2004, Mizutani et al., 2005). Viruses in the Simbu serogroup replicate and induce apoptosis in cultured cells (Lim et al., 2005, Barry et al., 2014, Varela et al., 2013).
AKAV infects cultured neuronal cells and astroglia cells and causes degenerative death (Kitani et al., 2000). AKAV induces cytopathic effects (CPE) and apoptosis via the activation of caspase-3 in Vero E6 cells (Lim et al., 2005). However, there have been no detailed studies of the signaling pathways involved in AKAV-induced apoptosis. Thus, the aim of the present study was to determine whether the JNK and p38 MAPK signaling pathways play crucial roles in AKAV-induced apoptosis.
2. Materials and methods
2.1. Cell line and virus
Vero E6 cells, a subclone of the African green monkey kidney epithelial cell line, were maintained in minimum essential medium (MEM, Gibco) containing 5% fetal calf serum (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated at 37 °C with 5% CO2. For the infection experiments, the cells were prepared in 6-well or 24-well tissue culture plates.
AKAV strain JaGAR39, a prototype of AKAV, was used in this study. Cell monolayers were washed once with MEM and then infected with AKAV at a multiplicity of infection (MOI) of 0.5 for 1 h at 37 °C. Incubation with MEM served as the mock-infected control. After adsorption, the cells were incubated in serum-free medium.
2.2. Antibodies and reagents
Antibodies specific for phospho-JNK1/2 (Thr183/Tyr185) (1:2000, #9251), JNK1/2 (1:2000, #9252), phospho-p38 MAPK (Thr180/Tyr182) (1:2000, #9211), p38 MAPK (1:2000, #9212), phospho-c-Jun (Ser63) (1:1000, #9261), phospho-HSP27 (Ser82) (1:1000, #2401), cleaved caspase-3 (Asp175) (1:1000, #9661), GAPDH (1:4000, #2118), and anti-rabbit IgG HRP-linked secondary antibody (1:10,000 for detection of phospho-c-Jun, phospho-HSP27, and cleaved caspase-3 and 1:20,000 for detection of the others, #7074) were purchased from Cell Signaling Technology (Danvers, USA).
The JNK inhibitor SP600125 and the p38 MAPK inhibitor SB203580 were purchased from Wako (Osaka, Japan). We prepared 10 mM stocks of each inhibitor in dimethyl sulfoxide (DMSO). In the inhibition experiments, Vero E6 cells were infected with AKAV at an MOI of 0.5 for 1 h and then incubated in serum-free medium containing 20 μM of each inhibitor. Medium containing DMSO was used as the mock treatment control.
2.3. Western blotting
After viral infection at an MOI of 0.5 or mock infection, the cells were lysed by adding 1 × sodium dodecyl sulfate sample buffer and boiled at 100 °C for 5 min. Whole-cell extracts were electrophoresed on 12.5% polyacrylamide gels and transferred onto PVDF membranes (Bio-Rad). The membranes were blocked at room temperature with Starting Block T20 (TBS) Blocking Buffer (Thermo) for 1 h and then incubated overnight with specific primary antibodies at 4 °C. After three washes with PBST buffer, the membranes were incubated with secondary antibodies at room temperature for 1 h. Immunoreactive bands were detected using an enhanced chemiluminescence procedure with LAS-3000 (Fujifilm).
3. Results
3.1. Induction of apoptosis in Vero E6 cells by AKAV infection
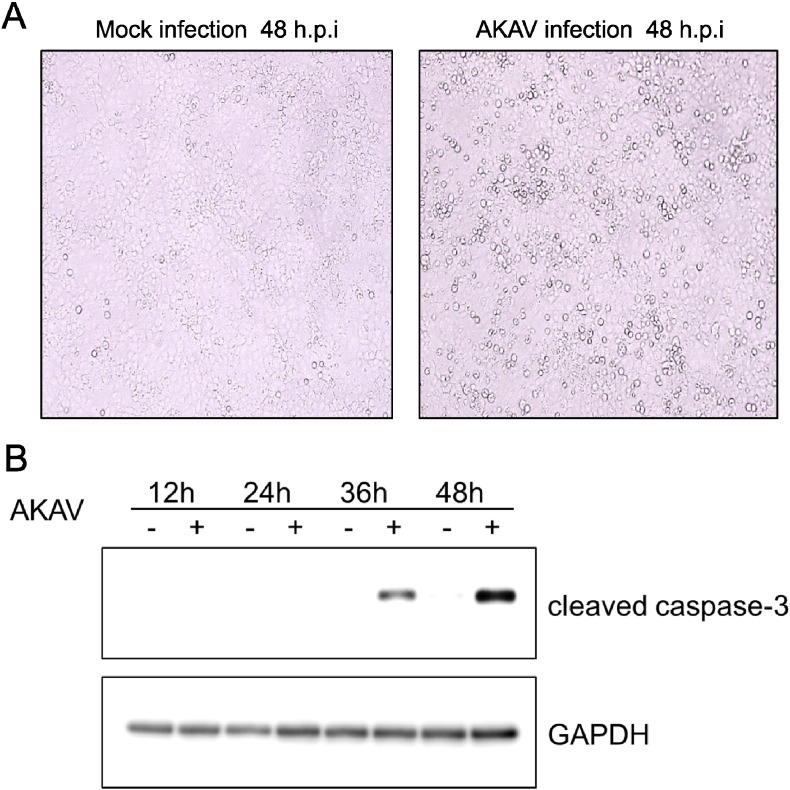
The cell death was observed at 48 h postinfection (hpi) in Vero E6 cells (Fig. 1A). To determine whether AKAV infection activated caspase-3 in Vero E6 cells, Western blot analysis of the cleaved caspase-3 was performed. As shown in Fig. 1B, cleaved caspase-3 was detected in AKAV-infected Vero E6 cells at 36 and 48 hpi, thereby indicating that AKAV infection induced apoptosis in Vero E6 cells.
Fig. 1.
Apoptosis induced by AKAV infection. (A) Vero E6 cells were mock-infected or infected with AKAV at an MOI of 0.5 and incubated for 48 h at 37 °C. (B) Western blot analysis of cleaved caspase-3 was performed using whole-cell extracts from mock-infected (−) or AKAV-infected (+) Vero E6 cells at 12, 24, 36, and 48 hpi. GAPDH was used as the control.
3.2. Phosphorylation of JNK and p38 MAPK in AKAV-infected cells
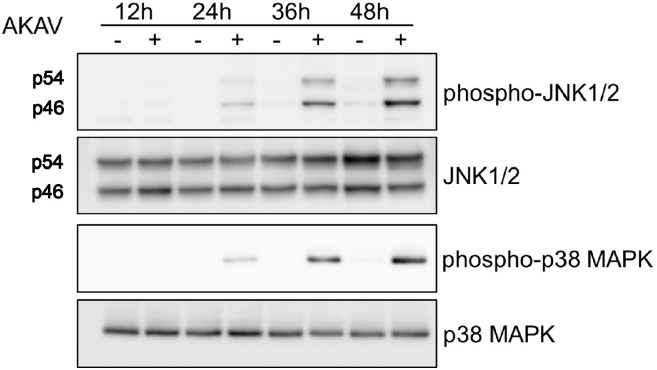
JNK and p38 MAPK are activated via phosphorylation at Thr183/Tyr185 and Thr180/Tyr182, respectively (Plotnikov et al., 2011). As shown in Fig. 2 , phosphorylated JNK and p38 MAPK were detected at 24 hpi and their levels increased gradually. In contrast, the protein levels of total JNK and p38 MAPK did not differ at each time point.
Fig. 2.
Phosphorylation of JNK and p38 MAPK in AKAV-infected Vero E6 cells. Whole-cell extracts from Vero E6 cells were mock-infected (−) or infected (+) with AKAV and prepared for Western blot analysis of the phosphorylation of JNK and p38 MAPK at 12, 24, 36, and 48 hpi.
3.3. Phosphorylation of c-Jun and HSP27 by AKAV infection
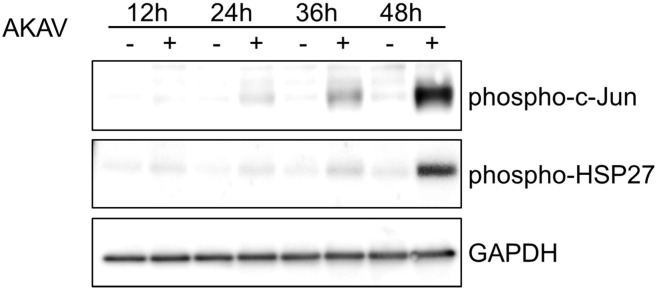
Activated JNK phosphorylates c-Jun, which regulates a range of cellular processes, including apoptosis and cell proliferation (Dunn et al., 2002). HSP27 interacts with key components of the apoptotic signaling pathway, especially during caspase activation, and it is phosphorylated as a result of the activation of p38 MAPK (Concannon et al., 2003). Western blot analyses were performed to confirm that JNK and p38 MAPK, which were phosphorylated as a result of AKAV infection, actually phosphorylated c-Jun and HSP27, respectively. As shown in Fig. 3 , c-Jun and HSP27 were phosphorylated in AKAV-infected cells where the kinetics agreed with those observed in JNK and p38 MAPK, respectively. These results suggest that the JNK and p38 MAPK signaling pathways were activated in response to AKAV infection.
Fig. 3.
Phosphorylation of c-Jun and HSP27 in AKAV-infected Vero E6 cells. Phosphorylated c-Jun and HSP27 were determined by Western blot analysis using whole-cell extracts from mock-infected (−) or AKAV-infected (+) Vero E6 cells at 12, 24, 36, and 48 hpi.
3.4. Inhibition of AKAV-induced apoptosis by a JNK inhibitor
To confirm that the JNK and p38 MAPK signaling pathways need to be activated for AKAV-induced apoptosis, we determined the effects of JNK and p38 MAPK inhibition on apoptosis induction by AKAV infection using a JNK inhibitor (SP600125) and a p38 MAPK inhibitor (SB203580). Vero E6 cells were treated with 20 μM of the inhibitors in serum-free medium for 48 h, and the cell viability was then assayed using Cell Counting Kit-8 (Doujin Chemical). The viabilities of the cells treated with SP600125 or SB203580 were > 90% and approximately 100%, respectively, compared with those of untreated cells. There was very little effect on cell viability in Vero E6 cells treated with both SP600125 and SB203580 at 20 μM.
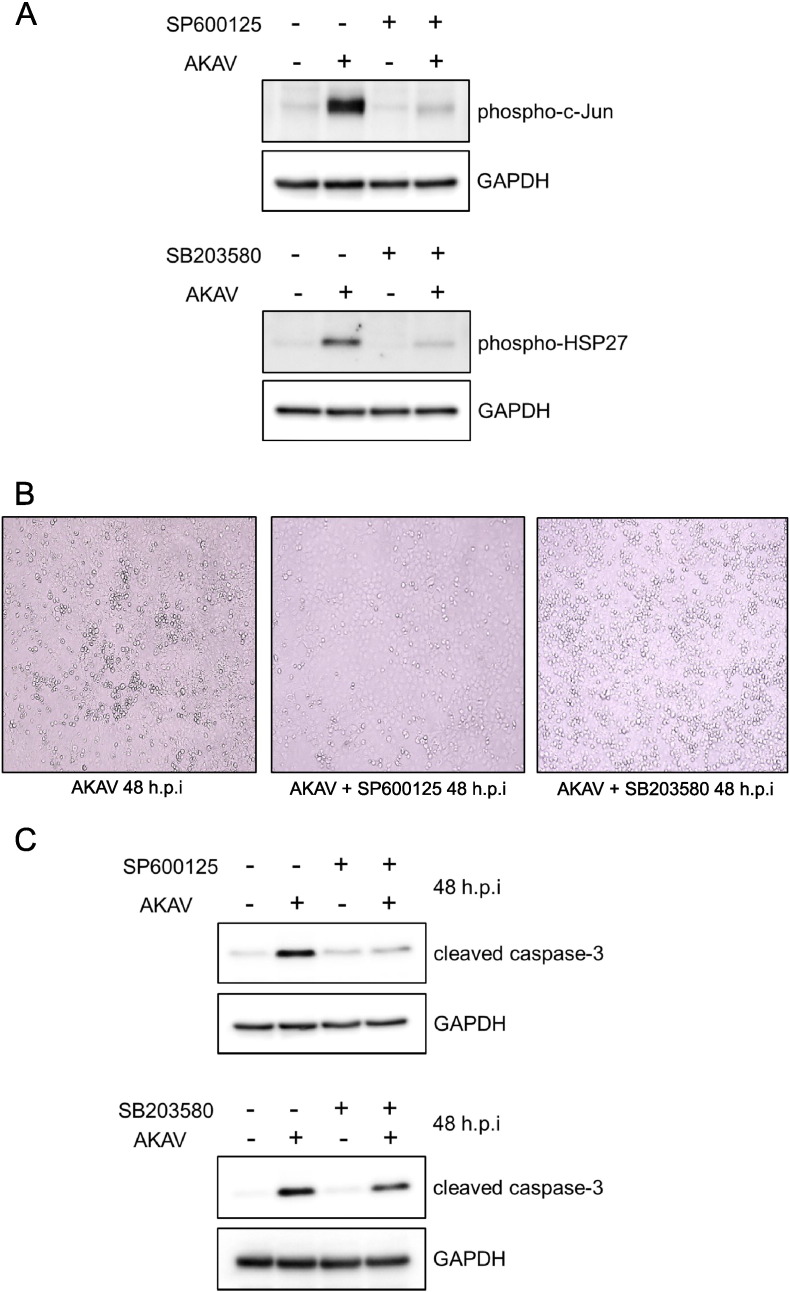
As shown in Fig. 4A, the Western blot analysis with anti-phospho-c-Jun antibody did not detect phosphorylated c-Jun in AKAV-infected Vero E6 cells treated with the JNK inhibitor, i.e., SP600125, at 36 hpi compared with the untreated cells. The p38 MAPK inhibitor, i.e., SB203580, was also sufficient to block the phosphorylation of HSP27 (Fig. 4A). These results indicate that the JNK and p38 MAPK signaling pathways were inhibited by each inhibitor at 20 μM.
Fig. 4.
Involvement of JNK and p38 MAPK activation in AKAV-induced apoptosis. (A) Mock-infected (−) or AKAV-infected (+) Vero E6 cells were not treated (−) or treated (+) with SP600125 (JNK inhibitor) and SB203580 (p38 MAPK inhibitor) for 36 h. Phosphorylation of c-Jun and HSP27 were determined by Western blotting. (B) Vero E6 cells infected with AKAV were not treated or treated with SP600125 and SB203580 for 48 h. (C) Western blot analysis with anti-cleaved caspase-3 antibody was performed using whole-cell extracts from the cells mock-infected (−) or infected (+) with AKAV in the absence (−) or presence (+) of SP600125 and SB203580 for 48 h.
Next, we investigated whether the JNK and p38 MAPK inhibitors reduced the production of CPE induced by AKAV infection. CPE inhibition was clearly observed at 48 hpi in AKAV-infected cells treated with SP600125 compared with that of untreated cells. However, SB203580 treatment did not inhibit CPE production at 48 hpi compared with that of untreated cells (Fig. 4B). We then examined whether the inhibition of JNK and p38 MAPK suppressed AKAV-mediated apoptosis based on Western blot analysis of the cleavage of caspase-3. As shown in Fig. 4C, the inhibition of the JNK signaling pathway by the addition of SP600125 led to marked inhibition of caspase-3 compared with that of untreated cells. In contrast, the amount of cleaved caspase-3 did not differ between control cells and those treated with SB203580 (Fig. 4C). The copy numbers of AKAV RNA in the AKAV-infected cells treated with the two inhibitors were almost the same as those in untreated AKAV-infected cells according to real-time PCR at 48 hpi (data not shown). These results suggest the involvement of the JNK signaling pathway in AKAV-induced apoptosis but not the p38 MAPK signaling pathway.
4. Discussion
Signaling pathways involved in apoptosis induction by AKAV are unclear. A better understanding of these signaling pathways is needed to further elucidate the molecular virology of AKAV. In the present study, we demonstrated that AKAV, a member of the Simbu serogroup, activated the JNK and p38 MAPK signaling pathways, where the activation of the JNK signaling pathway was shown to be a crucial mediator of AKAV-induced apoptosis.
MAPK cascades are activated by various viral infections (Mizutani et al., 2004, Yamane et al., 2009, Bendfeldt et al., 2007). In this study, we investigated whether AKAV infection induced JNK and p38 MAPK activation and found that both signaling pathways were activated persistently by AKAV infection. In MAPK cascades, activation of the JNK cascade and the p38 MAPK cascade tend to promote apoptosis, and several studies have demonstrated that virus-mediated activation of these two cascades leads to apoptosis (Franklin and McCubrey, 2000, Wang et al., 2014, Wei et al., 2009). Our data clearly showed that JNK inhibition by treatment with each specific inhibitor resulted in marked suppression of AKAV-induced apoptosis, whereas inhibition of p38 MAPK did not. Thus, the JNK cascade plays a crucial role in apoptosis caused by AKAV. Oropouche virus is a member of the Simbu serogroup and a cause of acute febrile illness in humans. Mitochondrial release of cytochrome C and activation of caspase-9 and -3 are detected during viral infection, and Oropouche virus infection causes apoptosis via an intracellular pathway that involves mitochondria (Acrani et al., 2010). JNK promotes the translocation of the Bcl-2 family pro-apoptotic protein, Bax, into mitochondria via the phosphorylation of 14-3-3 proteins that cause dissociation of Bax, where this translocation is essential for mediating the apoptotic release of cytochrome C (Tsuruta et al., 2004, Papadakis et al., 2006). Thus, we suggest that AKAV-induced JNK activation exerts a pro-apoptotic effect via the mitochondrial pathway, thereby regulating Bax translocation into the mitochondria, cytochrome C release, and caspase activation.
In conclusion, our results indicate that AKAV infection induces the phosphorylation of JNK and p38 MAPK as well as their downstream substrates, c-Jun and HSP27 in cultured cells. In particular, JNK activation plays a crucial role in AKAV-induced apoptosis. AKAV causes abortions, stillbirths, and congenital disorders in animals. This study is useful to understand the pathogenicity of AKAV.
Acknowledgements
This work was supported by management expenses grant of Tokyo University of Agriculture and Technology.
References
- Acrani G.O., Gomes R., Proença-Módena J.L., da Silva A.F., Carminati P.O., Silva M.L., Santos R.I., Arruda E. Apoptosis induced by Oropouche virus infection in HeLa cells is dependent on virus protein expression. Virus Res. 2010;149:56–63. doi: 10.1016/j.virusres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Barry G., Varela M., Ratinier M., Blomström A.L., Caporale M., Seehusen F., Hahn K., Schnettler E., Baumgärtner W., Kohl A., Palmarini M. NSs protein of Schmallenberg virus counteracts the antiviral response of the cell by inhibiting its transcriptional machinery. J. Gen. Virol. 2014;95:1640–1646. doi: 10.1099/vir.0.065425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendfeldt S., Ridpath J.F., Neill J.D. Activation of cell signaling pathways is dependant on the biotype of bovine viral diarrhea viruses type 2. Virus Res. 2007;126:96–105. doi: 10.1016/j.virusres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bridgen A., Weber F., Fazakerley J.K., Elliott R.M. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:664–669. doi: 10.1073/pnas.98.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.G., Choi E.J. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J. Biochem. Mol. Biol. 2002;35:24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- Cohen G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon C.G., Gorman A.M., Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- Cowan K.J. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Davies F.G., Jessett D.M. A study of the host range and distribution of antibody to Akabane virus (genus Bunyavirus, family Bunyaviridae) in Kenya. J. Hyg. 1985;95:191–196. doi: 10.1017/s0022172400062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Porta A.J., Murray M.D., Cybinski D.H. Congenital bovine epizootic arthrogryposis and hydranencephaly in Australia. Distribution of antibodies to Akabane virus in Australian Cattle after the 1974 epizootic. Aust. Vet. J. 1976;52:496–501. doi: 10.1111/j.1751-0813.1976.tb06983.x. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion S., Landry J. Activation of the mitogen-activated protein kinase pathways by heat shock. Cell Stress Chaperones. 2002;7:200–206. doi: 10.1379/1466-1268(2002)007<0200:aotmap>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C., Wiltshire C., MacLaren A., Gillespie D.A. Molecular mechanism and biological functions of c-Jun N-terminal kinase signalling via the c-Jun transcription factor. Cell. Signal. 2002;14:585–593. doi: 10.1016/s0898-6568(01)00275-3. [DOI] [PubMed] [Google Scholar]
- Eifan S., Schnettler E., Dietrich I., Kohl A., Blomström A.L. Non-structural proteins of arthropod-borne bunyaviruses: roles and functions. Viruses. 2013;5:2447–2468. doi: 10.3390/v5102447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhassan A.M., Mansour M.E., Shamon A.A., El Hussein A.M. A serological survey of Akabane virus infection in cattle in Sudan. ISRN Vet. Sci. 2014;2014 doi: 10.1155/2014/123904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R.A., McCubrey J.A. Kinases: positive and negative regulators of apoptosis. Leukemia. 2000;14:2019–2034. doi: 10.1038/sj.leu.2401967. [DOI] [PubMed] [Google Scholar]
- Garigliany M.M., Bayrou C., Kleijnen D., Cassart D., Jolly S., Linden A., Desmecht D. Schmallenberg virus: a new Shamonda/Sathuperi-like virus on the rise in Europe. Antivir. Res. 2012;95:82–87. doi: 10.1016/j.antiviral.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Holloway G., Coulson B.S. Rotavirus activates JNK and p38 signaling pathways in intestinal cells, leading to AP-1-driven transcriptional responses and enhanced virus replication. J. Virol. 2006;80:10624–10633. doi: 10.1128/JVI.00390-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Huang X., Cai J., Ye F., Qin Q. Involvement of the mitogen-activated protein kinase pathway in soft-shelled turtle iridovirus-induced apoptosis. Apoptosis. 2011;16:581–593. doi: 10.1007/s10495-011-0595-z. [DOI] [PubMed] [Google Scholar]
- Jun Q., Qingling M., Zaichao Z., Kuojun C., Jingsheng Z., Minxing M., Chuangfu C. A serological survey of Akabane virus infection in cattle and sheep in northwest China. Trop. Anim. Health Prod. 2012;44:1817–1820. doi: 10.1007/s11250-012-0168-3. [DOI] [PubMed] [Google Scholar]
- Kitani H., Yamakawa M., Ikeda H. Preferential infection of neuronal and astroglia cells by Akabane virus in primary cultures of fetal bovine brain. Vet. Microbiol. 2000;73:269–279. doi: 10.1016/s0378-1135(00)00158-9. [DOI] [PubMed] [Google Scholar]
- Kurogi H., Inaba Y., Goto Y., Miura Y., Takahashi H. Serologic evidence for etiologic role of Akabane virus in epizootic abortion-arthrogryposis-hydranencephaly in cattle in Japan, 1972–1974. Arch. Virol. 1975;47:71–83. doi: 10.1007/BF01315594. [DOI] [PubMed] [Google Scholar]
- Kurogi H., Akiba K., Inaba Y., Matumoto M. Isolation of Akabane virus from the biting midge Culicoides oxystoma in Japan. Vet. Microbiol. 1987;15:243–248. doi: 10.1016/0378-1135(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Park J.S., Choi J.H., Park B.K., Lee B.C., Hwang W.S., Kim J.H., Jean Y.H., Haritani M., Yoo H.S., Kim D.Y. Encephalomyelitis associated with akabane virus infection in adult cows. Vet. Pathol. 2002;39:269–273. doi: 10.1354/vp.39-2-269. [DOI] [PubMed] [Google Scholar]
- Lim S.I., Kweon C.H., Yang D.K., Tark D.S., Kweon J.H. Apoptosis in Vero cells infected with Akabane, Aino and Chuzan virus. J. Vet. Sci. 2005;6:251–254. [PubMed] [Google Scholar]
- Lin C., Zimmer S.G., Lu Z., Holland R.E., Dong Q., Chambers T.M. The involvement of a stress-activated pathway in equine influenza virus-mediated apoptosis. Virology. 2001;287:202–213. doi: 10.1006/viro.2001.1010. [DOI] [PubMed] [Google Scholar]
- McLean T.I., Bachenheimer S.L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun. 2004;319:1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. JNK and PI3K/Akt signaling pathways are required for establishing persistent SARS-CoV-infection in Vero E6 cells. Biochim. Biophys. Acta. 2005;1741:4–10. doi: 10.1016/j.bbadis.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola E., Larsen A. Bluetongue virus infection: activation of the MAP kinase-dependent pathway is required for apoptosis. Res. Vet. Sci. 2010;89:460–464. doi: 10.1016/j.rvsc.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Papadakis E.S., Finegan K.G., Wang X., Robinson A.C., Guo C., Kayahara M., Tournier C. The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 2006;580:1320–1326. doi: 10.1016/j.febslet.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Plotnikov A., Zehorai E., Procaccia S., Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Rahaus M., Desloges N., Wolff M.H. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 2004;85:3529–3540. doi: 10.1099/vir.0.80347-0. [DOI] [PubMed] [Google Scholar]
- Robinson M.J., Cobb M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;92:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Si X., Luo H., Morgan A., Zhang J., Wong J., Yuan J., Esfandiarei M., Gao G., Cheung C., McManus B.M. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. J. Virol. 2005;79:13875–13881. doi: 10.1128/JVI.79.22.13875-13881.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stram Y., Brenner J., Braverman Y., Banet-Noach C., Kuznetzova L., Ginni M. Akabane virus in Israel: a new virus lineage. Virus Res. 2004;104:93–97. doi: 10.1016/j.virusres.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Strnisková M., Barancík M., Ravingerová T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen. Physiol. Biophys. 2002;21:231–255. [PubMed] [Google Scholar]
- Taylor W.P., Mellor P.S. The distribution of Akabane virus in the Middle East. Epidemiol. Infect. 1994;113:175–185. doi: 10.1017/s0950268800051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F., Sunayama J., Mori Y., Hattori S., Shimizu S., Tsujimoto Y., Yoshioka K., Masuyama N., Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brom R., Luttikholt S.J., Lievaart-Peterson K., Peperkamp N.H., Mars M.H., van der Poel W.H., Vellema P. Epizootic of ovine congenital malformations associated with Schmallenberg virus infection. Tijdschr. Diergeneeskd. 2012;137:106–111. [PubMed] [Google Scholar]
- Varela M., Schnettler E., Caporale M., Murgia C., Barry G., McFarlane M., McGregor E., Piras I.M., Shaw A., Lamm C., Janowicz A., Beer M., Glass M., Herder V., Hahn K., Baumgärtner W., Kohl A., Palmarini M. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tan J., Zoueva O., Zhao J., Ye Z., Hewlett I. Novel pandemic influenza A (H1N1) virus infection modulates apoptotic pathways that impact its replication in A549 cells. Microbes Infect. 2014;16:178–186. doi: 10.1016/j.micinf.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhu Z., Wang J., Liu J. JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J. Virol. 2009;83:6039–6047. doi: 10.1128/JVI.00135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Zhu S., Ruan G., Hou L., Wang J., Wang B., Liu J. Infectious bursal disease virus-induced activation of JNK signaling pathway is required for virus replication and correlates with virus-induced apoptosis. Virology. 2011;420:156–163. doi: 10.1016/j.virol.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Yamane D., Zahoor M.A., Mohamed Y.M., Azab W., Kato K., Tohya Y., Akashi H. Activation of extracellular signal-regulated kinase in MDBK cells infected with bovine viral diarrhea virus. Arch. Virol. 2009;154:1499–1503. doi: 10.1007/s00705-009-0453-2. [DOI] [PubMed] [Google Scholar]
- Yin S., Huo Y., Dong Y., Fan L., Yang H., Wang L., Ning Y., Hu H. Activation of c-Jun NH(2)-terminal kinase is required for porcine reproductive and respiratory syndrome virus-induced apoptosis but not for virus replication. Virus Res. 2012;166:103–108. doi: 10.1016/j.virusres.2012.03.010. [DOI] [PubMed] [Google Scholar]