Abstract
Canine inflammatory bowel disease (IBD) is an intractable autoimmune disorder that results in various gastrointestinal and systemic symptoms. Mesenchymal stem cells (MSCs), which release immunomodulatory factors such as tumor necrosis factor-α (TNF-α)-induced gene/protein 6 (TSG-6) and prostaglandin E2 (PGE2), have been suggested as an alternative therapeutic option for IBD treatment in veterinary medicine. Furthermore, although it is known that MSCs pre-treated with pro-inflammatory cytokines show enhanced anti-inflammatory properties via the secretion of soluble factors, the underlying mechanisms of IBD remain unclear. The aim of this study was to demonstrate the therapeutic effects and corresponding mechanisms of canine adipose tissue-derived (cAT)-MSCs stimulated with TNF-α in mouse models of IBD. Mice with dextran sulfate sodium (DSS)- or dinitrobenzene sulfonic acid (DNBS)-induced colitis were injected intraperitoneally with cAT-MSCs pre-treated with TNF-α. Colitis severity was assessed and colon tissues were collected for histopathological, enzyme-linked immunosorbent assay, and flow cytometry analysis. cAT-MSCs stimulated with TNF-α secreted higher concentrations of immunomodulatory factors such as TSG-6 and PGE2, which play a key role in inducing phenotypic alterations in macrophages. Consequently, TNF-α-pre-treated cAT-MSCs further regulated colonic inflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-10, and ameliorated DSS- or DNBS-induced colitis in mice. Additionally, we demonstrated that M1 macrophages (F4/80+/iNOS+ cells) were decreased in colon tissues from mice treated with TNF-α-pre-treated cAT-MSCs, whereas M2 macrophages (F4/80+/CD206+ cells) were increased. These results may suggest a new cell-based therapeutic option for treating IBD.
Keywords: Mesenchymal stem cells, Dog, Inflammatory bowel disease, Macrophage, Immunomodulation, Cell therapy
Highlights
-
•
Canine AT-MSCs stimulated with TNF-α enhanced immunomodulatory factor secretion.
-
•
TNF-α-stimulated cAT-MSCs showed enhanced anti-inflammatory effects during experimental colitis.
-
•
TNF-α-stimulated cAT-MSCs induced M2 macrophage phenotypic alterations in the colon.
-
•
Preconditioning canine AT-MSCs with TNF-α could be applicable to dogs with IBD.
1. Introduction
Canine inflammatory bowel disease (IBD), which leads to gastrointestinal or systemic clinical signs, is diagnosed by ruling out the possibility of other diseases such as infection or tumor and performing histopathological assessment (Cerquetella et al., 2010; Craven et al., 2004). IBD is an intractable autoimmune disease and immunosuppressive drugs are used to reduce inflammation (Allenspach et al., 2006; Dossin and Lavoue, 2011). However, no alternative treatments exist for dogs with IBD that do not respond to the conventional therapies. Therefore, mesenchymal stem cells (MSCs) that can effectively modulate inflammation might be an alternative therapeutic option (Iyer and Rojas, 2008).
Recent studies have revealed that soluble factors released by MSCs such as prostaglandin E2 (PGE2), hepatocyte growth factor, indoleamine 2,3-dioxygenase, and TNF-stimulated gene/protein 6 (TSG-6) contribute to immunomodulation (Bassi et al., 2012; Montemurro et al., 2016; Teng et al., 2015). Therefore, MSCs exert strong anti-inflammatory effects, although injected MSCs did not migrate into inflamed tissue in a previous study (Sala et al., 2015). Kang et al. and Chae et al. also demonstrated that canine and feline MSCs secrete soluble immunomodulatory factors (Chae et al., 2017; Kang et al., 2008). In addition, our previous studies have shown that TSG-6 released from human and canine MSCs ameliorates colitis in mice (Song et al., 2018; Song et al., 2017b).
Our previous study demonstrated that canine MSCs pre-treated with tumor necrosis factor (TNF)-α and interferon (IFN)-γ exerted enhanced anti-inflammatory effects in vitro by releasing higher concentrations of PGE2, an immunomodulatory factor (Yang et al., 2018). Fan et al. also revealed that human MSCs stimulated with interleukin (IL)-1β showed enhanced efficacy in mice with colitis (Fan et al., 2012). In addition, recent studies have demonstrated that MSCs pre-treated with pro-inflammatory cytokines showed enhanced secretory abilities (Broekman et al., 2016; Heo et al., 2011). However, few studies have assessed the therapeutic effects of pro-inflammatory cytokine-stimulated canine MSCs.
Therefore, in this study, we used canine adipose tissue (cAT)-MSCs stimulated with TNF-α, and revealed the therapeutic effects and their mechanisms in two mouse models of IBD.
2. Materials and methods
2.1. Isolation, culture, and characterization of cAT-MSCs
Canine adipose tissues were obtained from healthy 4-year-old dogs using protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University performed in accordance with approved guidelines. The dogs were negative for canine parvovirus, canine coronavirus, and canine distemper virus infections. MSCs were isolated and cultured as previously described (Kim et al., 2016). Briefly, adipose tissue samples were washed five times in Dulbecco's phosphate buffered saline (DPBS; PAN-Biotech, Aidenbach, Germany) containing 1% penicillin-streptomycin (PS; PAN-Biotech), and cut into small pieces in a petri dish. The samples were digested with collagenase type IA (0.1%, Gibco/Life Technologies, Carlsbad, CA, USA) for 60 min at 37 °C. The samples were neutralized with Dulbecco's modified Eagle's medium (DMEM; PAN-Biotech) containing 10% fetal bovine serum (FBS; PAN-Biotech). After centrifuging the adipose tissue mixture at 1200 ×g for 5 min, the pellet containing MSCs was passed through a 70-μm cell strainer (Thermo Fisher Scientific, Rockford, IL, USA) to remove undigested debris. Cells were resuspended in DMEM containing 10% FBS and 1% PS, seeded onto a cell culture dish at a density of 3000 cells/cm2, and incubated at 37 °C and 5% CO2. After 5 days, cultures were washed with DPBS to remove non-adherent cells and incubated with fresh medium. The culture medium was changed every 2–3 days until cells reached 70–80% confluence. The cells were then subcultured and seeded at a density of 10,000 cells/cm2 in culture dishes.
Before experimentation, the cells were characterized using flow cytometry to evaluate the expression of several stem cell markers. Cells were suspended in DPBS and monoclonal antibodies against the following proteins: cluster of differentiation (CD)29- fluorescein isothiocyanate (FITC), CD31-FITC, CD34-phycoerythrin (PE), CD73-PE (BD Biosciences, San Diego, CA, USA), CD45-FITC, and CD90-allophycocyanin (eBiosciences, San Diego, CA, USA). Cell fluorescence was analyzed with a FACS Aria II system (BD Biosciences). Additionally, the cells' differentiation abilities were evaluated using Stempro adipogenesis, osteogenesis, and chondrogenesis differentiation kits (Gibco, Grand Island, NY, USA) according to the manufacturer's instructions. The differentiated cells were stained with Oil Red O, Alizarin Red, and Alcian Blue.
2.2. TNF-α stimulation of cAT-MSCs
cAT-MSCs at approximately 60–70% confluence were stimulated with canine recombinant TNF-α (10 ng/mL; PROSPEC Protein Specialists, NJ, USA) for 24 h. The cells were used for TNF-α-stimulated cAT-MSC groups.
2.3. Animal experiments, colitis induction
C57BL/6 J mice (male, 5-week-old) were purchased from Nara Biotech (Seoul, Korea) and housed under standard conditions (controlled temperature, humidity, and light cycle). All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Seoul National University (protocol no. SNU-171123-2), and the protocols were performed in accordance with approved guidelines. Two different mouse models for IBD were used for this study (dextran sulfate sodium (DSS)-, and dinitrobenzene sulfonic acid (DNBS)-induced colitis models), and each colitis model was made as previously described (Kim et al., 2013; Martín et al., 2014; Morampudi et al., 2014; Solomon et al., 2010). For the first experiment, colitis was induced by 3% DSS (36–50 kDa; MP Biomedical, Solon, OH, USA) in the drinking water from day 0 to day 7, whereas mice offered normal water were used as the naive group. The following experiments were performed on day 1: cAT-MSCs (2 × 106) stimulated with TNF-α in 200 μL PBS; cAT-MSCs (2 × 106) in 200 μL PBS; or an identical volume of PBS was injected intraperitoneally into the DSS-induced colitis mice. For this experiment, mice were randomly divided to the following four groups: Naïve (n = 4), DSS + PBS (n = 6), DSS + cAT-MSC (n = 6), DSS+ TNF-α-cAT-MSC (n = 6). For the second experiment, DNBS (Sigma-Aldrich, St. Louis, MO, USA) colitis was induced by rectal administration of DNBS (5 mg/mouse in 50% ethanol) into mice. Six hours after DNBS infusion, cAT-MSCs were administered intraperitoneally as described above. For this experiment, mice were divided into five groups: Naïve (n = 4), Ethanol (sham; n = 4), DNBS+PBS (n = 6), DNBS+cAT-MSC (n = 6), DNBS+TNF-α-cAT-MSC (n = 6). The mice were sacrificed on day 10 (for DSS-induced colitis experiments) or day 3 (for DNBS-induced colitis experiments), and colon tissues were collected for further processing.
2.4. Evaluation of colitis severity
The disease activity index (DAI) was determined by a scoring system described previously (Song et al., 2018). Briefly, the body weight loss (grades 0–4), stool consistency (grades 0–2), rectal bleeding (grades 0–2), and general activity (grades 0–2) were monitored every 24 h. For histological analysis, colon tissue samples were fixed in 4% phosphate-buffered formaldehyde for 48–72 h, followed by embedding in paraffin, cutting into 4-μm sections, and staining with hematoxylin and eosin. Ten fields per group were randomly selected and histological examinations were performed. Colitis severity was calculated using the previously described scoring system. Briefly, the extent of bowel wall thickening (grades 0–3), crypt damage (grades 0–3), and inflammatory cell infiltration (grades 0–2) were examined in a blind manner.
2.5. Enzyme-linked immunosorbent assay (ELISA)
TSG-6 and PGE2 in the supernatants from TNF-α-pre-treated or non-treated cAT-MSCs were measured using a TSG-6 ELISA kit (MyBiosource, San Diego, CA, USA) and PGE2 ELISA Kit (Cusabio Biotech, MD, USA), respectively. Additionally, for in vivo experiments, total proteins were extracted from colon tissue samples using PRO-PREP Protein Extraction Solution (Intron Biotechnology, Seongnam, Korea) and the concentrations of IL-1β, IL-6, and IL-10 were measured using commercial ELISA kits (all from eBiosciences) according to the manufacturer's instructions.
2.6. Flow cytometry analysis
To evaluate the mouse macrophage population, the following monoclonal antibody mixtures were used for the experiments: anti-F4/80-FITC and anti-inducible nitric oxide synthase (iNOS)-PE, or anti-F4/80-FITC and anti-CD206-PE (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were incubated with cells isolated from digested colon tissues. Flow cytometry was performed using a FACS Aria II system (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
2.7. Statistical analysis
Data are shown as the mean ± standard deviation. Mean values among different groups were compared by one-way analysis of variance using GraphPad Prism software (v.6.01; GraphPad, Inc., La Jolla, CA, USA). P value <.05 was considered statistically significant.
3. Results
3.1. Phenotypic characterization of cAT-MSCs
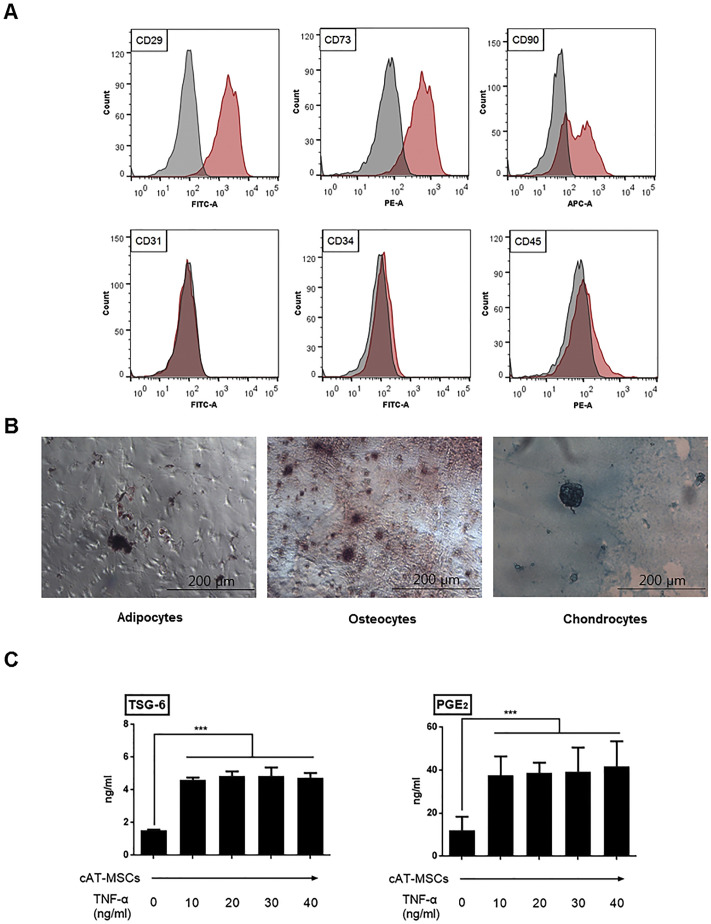
Cells isolated from canine adipose tissue were assessed for MSC characteristics. Flow cytometry analysis showed that stem cell markers such as CD29, CD73, and CD90 were highly expressed in these cells. In contrast, there was no detectable expression of hematopoietic markers, including CD31, CD34, and CD45 (Fig. 1A). Additionally, the cells could be differentiated into adipocytes, osteocytes, and chondrocytes (Fig. 1B). According to criteria established by International Society for Cellular Therapy, the cells used in this study represent MSCs.
Fig. 1.
(A, B) Cells isolated from canine adipose tissues were characterized before their use in this study by flow cytometry analysis (A), as well as adipogenic, osteogenic, and chondrogenic differentiation analysis (B). (C) Canine adipose tissue-derived mesenchymal stem cells (cAT-MSCs) stimulated with TNF-α released higher concentrations of immunomodulatory factors such as TSG-6 and PGE2 compared to levels released by naive cAT-MSCs. Results are shown as the mean ± standard deviation of three independent experiments. ***P < .001.
3.2. Enhanced secretory abilities for immunomodulatory factors of TNF-α-stimulated cAT-MSCs
Our previous studies demonstrated that secretory factors from canine MSCs, such as TSG-6 and PGE2, play a key role in modulating inflammation. Therefore, cAT-MSCs were stimulated with TNF-α, a pro-inflammatory cytokine, to produce more immunomodulatory factors. TSG-6 and PGE2 concentrations determined from the cAT-MSCs pre-treated with TNF-α were significantly higher than those measured from the naïve cAT-MSCs (Fig. 1C).
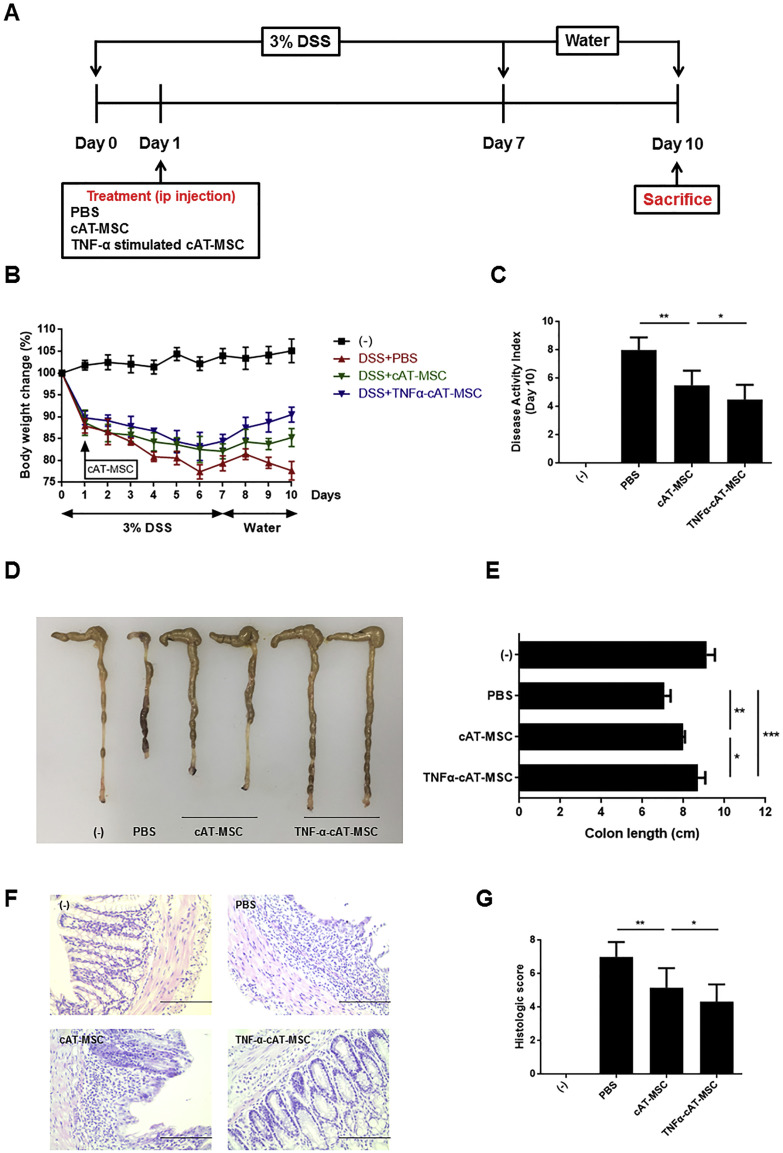
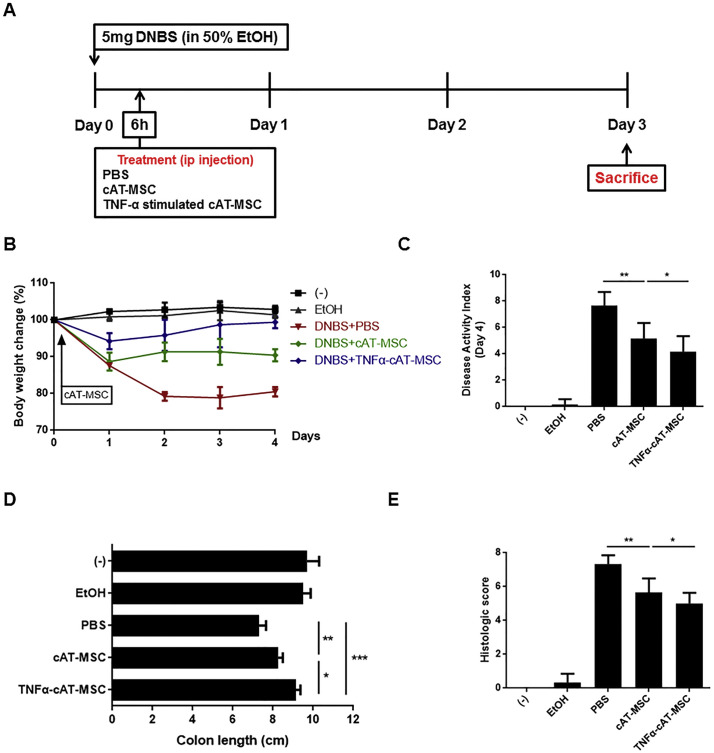
3.3. Improved therapeutic effects of TNF-α-stimulated cAT-MSCs in mice with DSS- or DNBS-induced colitis
Next, we evaluated whether administering cAT-MSCs or TNF-α-stimulated cAT-MSCs could reduce colitis severity in mice. Consistent with previous studies, administering cAT-MSCs resulted in a general reduction in body weight loss, DAI, and colon length shortening in mice with DSS- or DNBS-induced colitis (Fig. 2A–C, 3A–C ). In addition, further improvement was confirmed in mice with colitis injected with TNF-α-stimulated cAT-MSCs (Fig. 2A–C, 3A–C).
Fig. 2.
Canine adipose tissue-derived mesenchymal stem cells (cAT-MSCs) stimulated with TNF-α showed enhanced therapeutic effects on mice with dextran sodium sulfate (DSS)-induced colitis. Therapeutic abilities of cAT-MSCs were assessed by measuring body weight changes (A), disease activity index (B), colon length (C), and histopathologic analysis (D). Four to six mice per group were used. Results are shown as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
Fig. 3.
Canine adipose tissue-derived mesenchymal stem cells (cAT-MSCs) stimulated with TNF-α showed enhanced therapeutic effects on mice with dinitrobenzene sulfonic acid (DNBS)-induced colitis. Therapeutic abilities of cAT-MSCs were assessed by body weight changes (A), disease activity index (B), colon length (C), and histopathologic analysis (D). Four to six mice per group were used. Results are shown as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
Additionally, histopathology in the inflamed colon tissue was evaluated. Colons of mice treated with DSS or DNBS showed severe submucosal thickening, crypt damage, and infiltration of inflammatory cells. In contrast, administering cAT-MSCs to mice with colitis resulted in a slight improvement, which was additionally enhanced by administering TNF-α-stimulated cAT-MSCs (Fig. 2D, 3D).
3.4. Enhanced anti-inflammatory effects of TNF-α-primed cAT-MSCs on mice with DSS-induced colitis
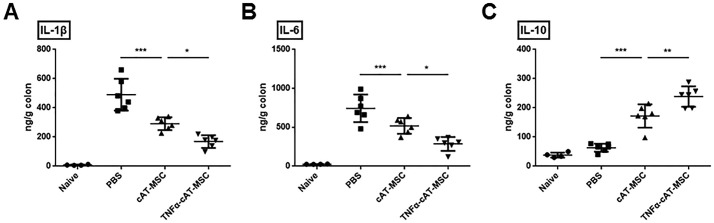
We next explored whether cAT-MSCs or TNF-α-stimulated cAT-MSCs could improve the anti-inflammatory effects in DSS-induced colitis. In the colons of mice treated with naïve cAT-MSCs, the production of IL-1β and IL-6 was significantly decreased, and production of IL-10 was increased considerably compared to that in the colons of mice treated with PBS, as expected from previous studies. Similar to the above results, intestinal inflammation was further improved in colons from mice treated with TNF-α-stimulated cAT-MSCs (Fig. 4A-C).
Fig. 4.
Canine adipose tissue-derived mesenchymal stem cells (cAT-MSCs) stimulated with TNF-α decreased expression of pro-inflammatory cytokines (A; IL-1β, B; IL-6) and increased expression of one anti-inflammatory cytokine (C; IL-10) in colon samples from mice with dextran sodium sulfate (DSS)-induced colitis. Results are shown as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
3.5. Increased alteration of macrophage phenotype in the inflamed colons of mice treated with TNF-α-primed cAT-MSCs
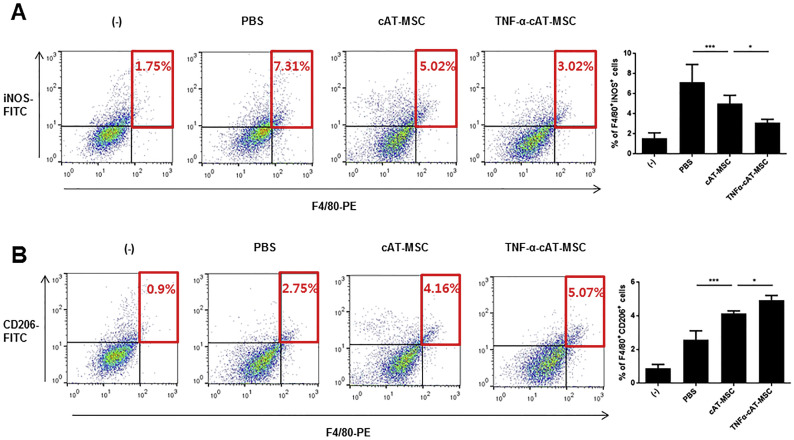
Given that the cytokines (IL-1β, IL-6, and IL-10) modulated in the above results are mainly secreted from macrophages, we further investigated whether cAT-MSCs or TNF-α-stimulated cAT-MSCs could alter macrophage phenotypes in inflamed colon tissue. Our previous study demonstrated that TSG-6 secreted from cAT-MSCs could increase the number of M2 macrophages in the colons of mice treated with DSS. Consistent with these results, our study showed that the M2 macrophage population (F4/80+/CD206+) in colons of DSS-induced colitis mice treated with cAT-MSCs was significantly increased compared with that in mice treated with PBS. In addition, the M1 macrophage population (F4/80+/iNOS+) was decreased in the cAT-MSCs-treated group. The phenotypic population of macrophages was further altered in the TNF-α-stimulated cAT-MSCs-treated group (Fig. 5 ).
Fig. 5.
Canine adipose tissue-derived mesenchymal stem cells (cAT-MSCs) stimulated with TNF-α further decreased the number of M1 macrophages (A; F4/80+/iNOS+ cells) and increased M2 macrophages (B; F4/80+/CD206+ cells) more so than naïve cAT-MSCs in colons from mice with dextran sodium sulfate (DSS)-induced colitis. Results are shown as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001.
4. Discussion
Recently, a number of studies have shown that MSCs can effectively ameliorate IBD in rodent models (Gonzalez-Rey et al., 2009; Tanaka et al., 2008; Wang et al., 2014). Furthermore, administering MSCs as a therapeutic option for IBD in small clinical trials (both in humans and dogs) has also shown considerable promise (Kim et al., 2017; Perez-Merino et al., 2015). Our previous studies have demonstrated that TSG-6 released from human and canine MSCs plays a crucial role in immunomodulation by inducing an M2 macrophage switch (Song et al., 2018; Song et al., 2017b). In addition, we have shown that canine MSCs stimulated with TNF-α and IFN-γ released higher concentration of PGE2 which exert anti-inflammatory effects (Yang et al., 2018). Therefore, by up-regulating the secretion of theses soluble factors, MSCs can enhance the immunomodulatory effects. Overall, these findings highlight the efficacy of TNF-α-stimulated cAT-MSCs against DSS- or DNBS-induced colitis in mice.
In our study, we demonstrated that intraperitoneal injection of TNF-α stimulated cAT-MSCs resulted in higher therapeutic efficacy than injecting naïve cAT-MSCs in two mouse models of IBD. For example, body weight loss and DAI were further improved in the TNF-α-stimulated cAT-MSCs-treated mice by 6% and 20%, respectively, compared to the improvements in the naive cAT-MSCs-treated group. In addition, evaluation of colon length and histopathologic analysis highlighted the increased therapeutic effects of TNF-α-stimulated cAT-MSCs. In addition, concentrations of inflammatory cytokines in the inflamed colons were significantly altered in the TNF-α-cAT-MSC group compared with those in the cAT-MSC group.
Previous studies have revealed that human MSCs stimulated with pro-inflammatory cytokines (such as TNF-α and IL-1β) can improve the secretory effects of immunomodulatory soluble factors (Broekman et al., 2016; Heo et al., 2011). Here, we also showed that TNF-α-stimulated cAT-MSCs released significantly higher concentrations of TSG-6 (2.5-fold) and PGE2 (3-fold), relative to naive cAT-MSCs. TSG-6 and PGE2 are well-known immunomodulatory factors secreted from human and canine MSCs, and recent studies have demonstrated that these factors play important roles in ameliorating atopic dermatitis, rheumatoid arthritis, acute pancreatitis, and IBD (Kim et al., 2016; Kim et al., 2015; Mao et al., 2017; Shin et al., 2016; Song et al., 2017a). Consistent with these studies, our results indicated that cAT-MSCs stimulated with TNF-α further reduced DSS- or DNBS-induced colitis by releasing higher concentrations of TSG-6 and PGE2.
Macrophages are important innate immune cells that play a key role in releasing inflammatory cytokines and transferring information to acquired immune cells such as T cells. It is well-established that two types of macrophages (M1 and M2) are observed in inflamed tissues (Mosser and Edwards, 2008; Stout and Suttles, 2004) and these cells play an important role in regulating inflammatory responses. Melief et al. demonstrated that human MSCs promote the transition of monocytes into CD206+ M2 macrophages, and consequently increase Foxp3+ regulatory T cells (Melief et al., 2013). In addition, recent studies have revealed that soluble factors (such as TSG-6 and PGE2) released from human MSCs could promote the M2 macrophage phenotype and reduce inflammation in mouse models of rheumatoid arthritis, wound healing, and IBD (Shin et al., 2016; Song et al., 2017b; Zhang et al., 2010). Additionally, we previously demonstrated that TSG-6 released from canine MSCs can induce M2 macrophage phenotypic changes in vitro and in vivo (Song et al., 2018). In this study, TNF-α stimulated cAT-MSCs increased macrophage alteration to the M2 phenotype (F4/80+/CD206+) in the colons of mice with IBD, whereas numbers of M1 macrophages (F4/80+/iNOS+) decreased in the inflamed colons. Consistent with previous studies and our results, TNF-α-stimulated cAT-MSCs reduced inflammation through altering macrophage phenotypic changes by secreting higher concentrations of TSG-6 and PGE2.
Recent studies have suggested other mechanisms by which MSCs might help reduce colitis severity. For example, MSCs may stimulate epithelial regeneration (Sémont et al., 2013; Sémont et al., 2006; Valcz et al., 2011). It is well-established that MSCs upregulate Ki67+ cells in inflamed tissues (Nakagawa et al., 2015; Wu et al., 2007). In addition, Chen et al. demonstrated that MSCs increase Lgr5+ intestinal stem cells in colonic tissues of IBD model mice (Chen et al., 2013). Another potential mechanism of MSC-dependent improvement in IBD involves microbiome changes. However, in this study, TNF-α-stimulated MSCs, which release higher concentrations of soluble immunomodulatory factors, showed further improved colitis in mice (Soontararak et al., 2018). Therefore, we demonstrated here that the anti-inflammatory effects of MSCs play an important role in their overall therapeutic effects on colitis, although MSCs might reduce IBD through various mechanisms.
Previous studies have revealed that a high frequency of intraperitoneally infused MSCs aggregated with immune cells in the peritoneal cavity (Sala et al., 2015; Bazhanov et al., 2016). In addition, our previous studies have shown that <0.5% of intraperitoneally injected MSCs were detected in the heart, lung, liver, spleen, kidney, brain, and colon tissues (Song et al., 2017b; Song et al., 2018). Based on these previous studies, it is tempting to speculate that most of intraperitoneally infused TNF-α-stimulated cAT-MSCs formed aggregates and ameliorated IBD at sites distant from the inflamed colon by releasing soluble factors such as TSG-6 and PGE2.
It should be acknowledged that we were not able to perform microarray screening of TNF-α-stimulated cAT-MSCs, although we evaluated increased TSG-6 and PGE2 from TNF-α-stimulated cAT-MSCs. However, it is well demonstrated that TSG-6 and PGE2 secreted from MSCs could induce macrophage phenotypic alterations. Therefore, our findings suggest that increased TSG-6 and PGE2 released from TNF-α stimulated cAT-MSCs play a key role in reducing inflammation in a mouse model of IBD.
5. Conclusion
In summary, we demonstrated that TNF-α-stimulated cAT-MSCs further ameliorated IBD via their enhanced anti-inflammatory effects over naïve cAT-MSCs. Additionally, we showed that cAT-MSCs pre-treated with TNF-α could release higher levels of immunomodulatory factors such as TSG-6 and PGE2, which contributed to induce macrophage phenotypic alterations. These results may represent a novel cell-based therapeutic option for treating autoimmune diseases such as IBD.
Declaration of Competing Interests
Declarations of interest: none.
Funding sources
This study was supported by the Research Institute for Veterinary Science and BK21 PLUS Program for Creative Veterinary Science Research.
Acknowledgements
We are very grateful to the Research Institute for Veterinary Science of Seoul National University, and the BK21 PLUS Program for Creative Veterinary Science Research.
References
- Allenspach K., Rüfenacht S., Sauter S., Gröne A., Steffan J., Strehlau G., Gaschen F. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J. Vet. Med. 2006;20:239–244. doi: 10.1892/0891-6640(2006)20[239:paceoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bassi Ê.J., de Almeida D.C., Moraes-Vieira P.M.M., Câmara N.O.S. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev. Rep. 2012;8:329–342. doi: 10.1007/s12015-011-9311-1. [DOI] [PubMed] [Google Scholar]
- Bazhanov N., Ylostalo J., Bartosh T., Tiblow A., Mohammadipoor A., Foskett A., Prockop D. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res Ther. 2016;7(1):27–40. doi: 10.1186/s13287-016-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman W., Amatngalim G.D., de Mooij-Eijk Y., Oostendorp J., Roelofs H., Taube C., Stolk J., Hiemstra P.S. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir. Res. 2016;17(3):1–12. doi: 10.1186/s12931-015-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquetella M., Spaterna A.S., Laus F., Tesei B., Rossi G., Antonelli E., Villanacci V., Bassotti G. Inflammatory bowel disease in the dog: differences and similarities with humans. World J. Gastroenterol. 2010;16(9):1050–1056. doi: 10.3748/wjg.v16.i9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H.-K., Song W.-J., Ahn J.-O., Li Q., Lee B.-Y., Kweon K., Park S.-C., Youn H.-Y. Immunomodulatory effects of soluble factors secreted by feline adipose tissue-derived mesenchymal stem cells. Vet. Immunol. Immunopathol. 2017;191:22–29. doi: 10.1016/j.vetimm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Chen Q.-Q., Yan L., Wang C.-Z., Wang W.-H., Shi H., Su B.-B., Zeng Q.-H., Du H.-T., Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013;19:4702–4717. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven M., Simpson J., Ridyard A., Chandler M.L. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995–2002) J. Small Anim. Pract. 2004;45:336–342. doi: 10.1111/j.1748-5827.2004.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Dossin O., Lavoue R. Protein-losing enteropathies in dogs. Vet Clin. Small Anim. 2011;41(2):399–418. doi: 10.1016/j.cvsm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Fan H., Zhao G., Liu L., Liu F., Gong W., Liu X., Yang L., Wang J., Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol. Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E., Gonzalez M.A., Rico L., Buscher D., Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Heo S.C., Jeon E.S., Lee I.H., Kim H.S., Kim M.B., Kim J.H. Tumor necrosis factor-α-activated human adipose tissue–derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Invest. Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- Iyer S.S., Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert. Opin. Biol. Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- Kang J.W., Kang K.-S., Koo H.C., Park J.R., Choi E.W., Park Y.H. Soluble factors–mediated immunomodulatory effects of canine adipose tissue–derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–694. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Shin T.H., Lee B.C., Yu K.R., Seo Y.J., Lee S., Seo M.S., Hong I.S., Choi S.W., Seo K.W., Nunez G., Park J.H., Kang K.S. Human Umbilical Cord Blood Mesenchymal Stem Cells Reduce Colitis in Mice by Activating NOD2 Signaling to COX2. Gastroenterology. 2013;145:1392–1403. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Yun J.W., Shin T.H., Lee S.H., Lee B.C., Yu K.R., Seo Y., Lee S., Kang T.W., Choi S., Seo K.W., Kang K.S. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33:1254–1266. doi: 10.1002/stem.1913. [DOI] [PubMed] [Google Scholar]
- Kim H.-W., Song W.-J., Li Q., Han S.-M., Jeon K.-O., Park S.-C., Ryu M.-O., Chae H.-K., Kyeong K., Youn H.-Y. Canine adipose tissue-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating T cells in rats. J. Vet. Sci. 2016;17:539–548. doi: 10.4142/jvs.2016.17.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Lee J.H., Roh K.H., Jun H.J., Kang K.S., Kim T.Y. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35:248–255. doi: 10.1002/stem.2401. [DOI] [PubMed] [Google Scholar]
- Mao F., Tu Q., Wang L., Chu F., Li X., Li H.S., Xu W. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8:38008–38021. doi: 10.18632/oncotarget.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R., Chain F., Miquel S., Lu J., Gratadoux J.J., Sokol H., Verdu E.F., Bercik P., Bermúdez-Humarán L.G., Langella P. The Commensal Bacterium Faecalibacterium prausnitzii Is Protective in DNBS-induced Chronic Moderate and Severe Colitis Models. Inflammatory Bowel Disease. 2014;20:417–430. doi: 10.1097/01.MIB.0000440815.76627.64. [DOI] [PubMed] [Google Scholar]
- Melief S.M., Schrama E., Brugman M.H., Tiemessen M.M., Hoogduijn M.J., Fibbe W.E., Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- Montemurro T., Viganò M., Ragni E., Barilani M., Parazzi V., Boldrin V., Lavazza C., Montelatici E., Banfi F., Lauri E., Giovanelli S., Baccarin M., Guerneri S., Giordiano R., Lazzari L. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur. J. Cell Biol. 2016;95:228–238. doi: 10.1016/j.ejcb.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Morampudi V., Bhinder G., Dai C., Sham H.P., Vallance B.A. Jacobson K., DNBS/TNBS Colitis Models: Providing Insights Into Inflammatory Bowel Disease and Effects of Dietary Fat. Journal of Visualized Experiments. 2014;84:e51297. doi: 10.3791/51297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Muneta T., Kondo S., Mizuno M., Takakuda K., Ichinose S., Tabuchi T., Koga H., Tsuji K., Sekiya I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr. Cartil. 2015;23:1007–1017. doi: 10.1016/j.joca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Perez-Merino E.M., Uson-Casaus J.M., Zaragoza-Bayle C., Duque-Carrasco J., Marinas-Pardo L., Hermida-Prieto M., Barrera-Chacon R., Gualtieri M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: clinical and laboratory outcomes. Vet. J. 2015;206:385–390. doi: 10.1016/j.tvjl.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Sala E., Genua M., Petti L., Anselmo A., Arena V., Cibella J., Zanotti L., D'Alessio S., Scaldaferri F., Luca G., Arato I., Calafiore R., Sgambato A., Rutella S., Locati M., Danese S., Vetrano S. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterol. 2015;149:163–176. doi: 10.1053/j.gastro.2015.03.013. (e120) [DOI] [PubMed] [Google Scholar]
- Sémont A., François S., Mouiseddine M., François A., Saché A., Frick J., Thierry D., Chapel A. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. In: Fisher J.P., editor. Tissue Engineering. Springer Publishing; USA: 2006. pp. 19–30. [DOI] [PubMed] [Google Scholar]
- Sémont A., Demarquay C., Bessout R., Durand C., Benderitter M., Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T.-H., Kim H.-S., Kang T.-W., Lee B.-C., Lee H.-Y., Kim Y.-J., Shin J.-H., Seo Y., Choi S.W., Lee S., Shin K., Seo K.W., Kang K.S. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. doi: 10.1038/cddis.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L., Mansor S., Donnelly E., Hoper M., Loughrey M., Kirk S., Gardiner K. The Dextran Sulphate Sodium (DSS) Model of Colitis: an overview. Comparative Clinical Pathology. 2010;19:235–239. [Google Scholar]
- Song J.Y., Kang H.J., Hong J.S., Kim C.J., Shim J.Y., Lee C.W., Choi J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci. Rep. 2017;7:9412. doi: 10.1038/s41598-017-09827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.-J., Li Q., Ryu M.-O., Ahn J.-O., Bhang D.H., Jung Y.C., Youn H.-Y. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci. Rep. 2017;7:5187. doi: 10.1038/s41598-017-04766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W.-J., Li Q., Ryu M.-O., Ahn J.-O., Bhang D.H., Jung Y.C., Youn H.-Y. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res Ther. 2018;9(91):1–12. doi: 10.1186/s13287-018-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontararak S., Chow L., Johnson V., Coy J., Wheat W., Regan D., Dow S.J. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl. Med. 2018;7:456–467. doi: 10.1002/sctm.17-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R.D., Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka F., Tominaga K., Ochi M., Tanigawa T., Watanabe T., Fujiwara Y., Ohta K., Oshitani N., Higuchi K., Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771–779. doi: 10.1016/j.lfs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Teng X., Chen L., Chen W., Yang J., Yang Z., Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- Valcz G., Krenács T., Sipos F., Leiszter K., Tóth K., Balogh Z., Csizmadia A., Műzes G., Molnár B., Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Pathol. Oncol. Res. 2011;17:11–16. doi: 10.1007/s12253-010-9262-x. [DOI] [PubMed] [Google Scholar]
- Wang C., Chen J., Sun L., Liu Y. TGF-beta signaling-dependent alleviation of dextran sulfate sodium-induced colitis by mesenchymal stem cell transplantation. Mol. Biol. Rep. 2014;41:4977–4983. doi: 10.1007/s11033-014-3364-6. [DOI] [PubMed] [Google Scholar]
- Wu Y., Chen L., Scott P.G., Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Yang H.-M., Song W.-J., Li Q., Kim S.-Y., Kim H.-J., Ryu M.-O., Ahn J.-O., Youn H.-Y. Canine mesenchymal stem cells treated with TNF-α and IFN-γ enhance anti-inflammatory effects through the COX-2/PGE2 pathway. Res. Vet. Sci. 2018;119:19–26. doi: 10.1016/j.rvsc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Z., Su W.R., Shi S.H., Wilder-Smith P., Xiang A.P., Wong A., Nguyen A.L., Kwon C.W., Le A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]