Abstract
Three ionic liquids, [BMIM][BF4] doped with 60 mol % of LiCl ([BMIM][BF4]–LiCl), N,N,N,N-tetramethylguanidinium trifluoroacetate (TMGT), and N,N,N,N-tetramethylguanidinium triflate (TMGTf) were found useful as catalyst solvents for controlled 3-indolylation of isatins. Our investigation revealed that the reaction between isatin and indoles in [BMIM][BF4]–LiCl or TMGTf media stops at the step of addition of the two components providing 3-indolyl-3-hydroxyindolin-2-ones while the ionic liquid TMGT runs the reaction further through accompanying Friedel–Crafts substitution to afford symmetrical 3,3-di(indol-3-yl)indolin-2-ones. To take advantage of the difference between the effects of these ionic liquids on the reaction progress, we planned a two-step protocol for the efficient synthesis of unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones.
Keywords: Indole, Isatin, Oxindole, Ionic liquid
Graphical abstract
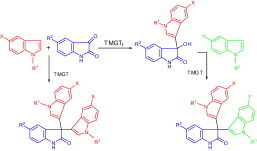
1. Introduction
The versatile properties and the variety of available ionic liquids have made them promising substances for application in most chemistry areas.1, 2, 3 Ionic liquids are new generation solvents composed of bulky organic cations having liquid range at/or close to room temperature, hence they are well positioned to fill the polarity gap between molecular liquids and the molten metal salts. The unique nature of ionic liquids allows virtually all possible interactions between the solutes and the ions thus have an ability to dissolve both organic and inorganic materials. On other hand, the high polarity of ionic liquids ensures immiscibility with many organic solvents, offering unique opportunities for phase separation and recycling. An important feature of ionic liquids is the possibility to tune their chemical properties and also to adjust their solubility to enable phase separation. Today, ionic liquids have been shown to be more than simple solvents, showing significant roles in controlling reactions as catalysts.4, 5 The early use of ionic liquids as catalysts was merely based on their role as polar media to facilitate organic reactions having polar transition states. The application now is extended by the so-called task-specific ionic liquids either through immobilization of the effective ionic catalysts in ionic liquids6, 7 or by incorporating a functional group responsible for catalysis in their ionic partners.8, 9, 10 Synthesis by the aid of immobilized metal catalysts in ionic liquids11, 12 recently has received considerable attention because such ‘liquid phase’ synthesis retains the advantages of homogenous catalysis, and still permits the fairly facile purification of the products and recycling of the catalysts.
In continuation of our studies directed toward the use of ionic liquids as catalysts in synthesis of organic compounds,13 herein we describe the efficiencies of three ionic liquids, [BMIM][BF4] doped with 60 mol % of LiCl ([BMIM][BF4]–LiCl), N,N,N,N-tetramethylguanidinium trifluoroacetate (TMGT), and N,N,N,N-tetramethylguanidinium triflate (TMGTf) for the promotion of the reaction between isatin and indoles providing the catalytic controlled syntheses of 3-(indol-3-yl)-3-hydroxyindolin-2-ones and symmetrical or unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones.
Isatin is a privileged lead molecule for designing potential bioactive agents, and its derivatives have been shown to possess a broad spectrum of bioactivity as many of which were assessed anti-HIV,14 antiviral,15 anti-tumor,16, 17, 18 antifungal,19, 20 anti-angiogenic,21 anticonvulsants,22 anti-Parkinson's disease therapeutic,23 and effective SARS coronavirus 3CL protease inhibitor.24 These interesting properties prompted many efforts toward the synthesis and pharmacological screening of isatin derivatives. During these investigations, the indolin-2-one (oxindole) moiety has been recognized as a biologically active framework.25, 26 Oxindole is an integral constituent of many natural products.27, 28, 29, 30, 31 Thus, it is not surprising that access to several members of this class may be the goal of many research laboratories. Oxindole derivatives have been prepared by the reaction of isatin or its derivatives with barbituric acid,32 aromatics in triflic acid,33 pyrazolones,34 and other routes.35, 36, 37, 38 The 3,3-di(indol-3-yl)indolin-2-ones can be formed by the reaction of isatin and indoles in acidic conditions and several methods have been developed for the synthesis of these compounds.39, 40, 41 On the other hand, a number of oxindole alkaloids having a hydroxyl substituent at the C-3 position possess various bioactivities,42, 43 and were synthesized by a variety of methods.38, 44, 45
2. Result and discussion
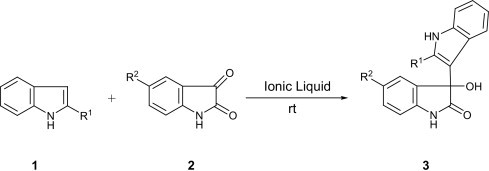
The 3-indolylation of isatin, which was found very sluggish in nature is essentially a Friedel–Crafts reaction. This recognition implies that the reaction could be assisted by enhancing the nucleophilic character of indoles in basic media, or by activating the carbonyl group of isatin with the aid of acidic species. In this view, we envisaged that the acid–base guanidinium ionic liquids could be suitable choices for performing the dual role. Our initial trials on the model reaction between indole and isatin in TMGTf was very encouraging as it proceeds smoothly at room temperature and stops at the stage of monoindolylation of isatin to afford 3-(indol-3-yl)-3-hydroxyindolin-2-one 3a in fairly high yield. We have also performed the model reaction in either of the neutral ionic liquids, [BMIM]Cl and [BMIM]BF4 or in the absence of ionic liquids to respond the issue if the high polarity of TMGTf is responsible for catalysis of the reaction. Of note, the model reaction has not proceeded in either of the above imidazolium ionic liquids or absence of them, confirming the functional role of TMGTf in catalysis of the reaction. To learn more about this function and on expectation that Li+ could play the same role as H+ in TMGTf we intended to test our model reaction in [BMIM]BF4 doped with LiCl. As expected, the LiCl contained in [BMIM]BF4 efficiently catalyzed the addition of indole onto isatin to provide 3-(indol-3-yl)-3-hydroxyindolin-2-one 3a in a few minutes (Table 1 ).
Table 1.
| Entry | R1 | R2 | Ionic liquid | Product | Reaction time | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | H | H | [BMIM]Cl | — | 12 h | — |
| 2 | H | H | [BMIM]BF4 | — | 12 h | — |
| 3 | H | H | [BMIM]BF4–LiCl | 3a | 20 min | 90 |
| 4 | H | H | TMGTf | 3a | 10 min | 92 |
| 5 | Me | H | [BMIM]Cl | — | 12 h | — |
| 6 | Me | H | [BMIM]BF4 | — | 12 h | — |
| 7 | Me | H | [BMIM]BF4–LiCl | 3b | 20 min | 92 |
| 8 | Me | H | TMGTf | 3b | 10 min | 93 |
Several indoles and isatin derivatives, having electron donor and electron withdrawing substituents, likewise underwent the same reaction efficiently in both ionic liquids, TMGTf and [BMIM]BF4–LiCl.
The results are summarized in Table 2 . All the compounds 3a–f are formed solely under these conditions and were not contaminated with the production of 3,3-di(indol-3-yl)indolin-2-ones 4 even when 3 equiv of indoles were employed in the reactions.
Table 2.
Synthesis of 3-(indol-3-yl)-3-hydroxyindolin-2-ones 3a–f in TMGTf or [BMIM][BF4]–LiCl ionic liquids
| Entry | R1 | R2 | Product | Yield (%) |
|
|---|---|---|---|---|---|
| [BMIM]BF4–LiCl | TMGTf | ||||
| 1 | H | H | 3a | 90 | 92 |
| 2 | Me | H | 3b | 92 | 93 |
| 3 | H | Br | 3c | 88 | 89 |
| 4 | Me | Br | 3d | 90 | 87 |
| 5 | H | F | 3e | 89 | 90 |
| 6 | Me | F | 3f | 91 | 89 |
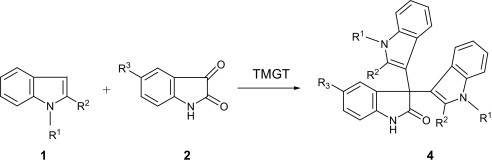
Delighted by this result, we intended to examine the effect of another guanidinium ionic liquid. For this purpose the reaction involving a 1:1 mmol ratio of isatin and indole was similarly carried out in N,N,N,N-tetramethylguanidinium trifluoroacetate (TMGT), in place of TMGTf, at room temperature. Monitoring of this reaction by TLC revealed a rather slow disappearance of the reactants, compared to the reaction in TMGTf or [BMIM]BF4–LiCl, and still signifying an efficient conversion giving both 3-(indol-3-yl)-3-hydroxyindolin-2-one 3a and 3,3-di(indol-3-yl)indolin-2-one 4a in about an hour. Formation of 3,3-di(indol-3-yl)indolin-2-one 4a as a by-product obviously accounts for the capability of TMGT to catalyze the Friedel–Crafts reaction between 3-(indol-3-yl)-3-hydroxyindolin-2-one 3a and another indole molecule, meanwhile showing a need for optimization of conditions to gain better yield of 4a. In order to optimize the reaction conditions, we performed the model reaction using different quantities of reactants and the ionic liquid. The best results were obtained with 1:2 mmol ratios of isatin and indoles in the presence of 1 mL of TMGT at room temperature. Therefore, we set out a method comprising the room temperature reaction of a 2:1 mixture of indole 1 and isatin 2 without using any solvent or additional catalyst in TMGT and thereby 3,3-di(indol-3-yl)indolin-2-ones 4a–f were obtained in fairly high yields (Table 3 ). Here again both electron donor as well as electron withdrawing substituents exert no considerable effects on reactivity of reactants under catalysis of TMGT.
Table 3.
| Entry | R1 | R2 | R3 | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 | H | H | H | 4a | 93 |
| 2 | Me | H | OMe | 4b | 91 |
| 3 | Me | H | Br | 4c | 86 |
| 4 | H | Me | H | 4d | 90 |
| 5 | Me | H | NO2 | 4e | 88 |
The condensation of indoles with isatin derivatives is better rationalized by an initial nucleophilic addition of the indole onto the ketonic carbonyl group of isatin to yield the corresponding 3-(indol-3-yl)-3-hydroxyindolin-2-one 3. Dehydration of this adduct gives the intermediate 6, which subsequently undergoes nucleophilic addition of the second indole molecule to provide the final products 3,3-di(indol-3-yl)indolin-2-ones 4 (Scheme 1 ).
Scheme 1.
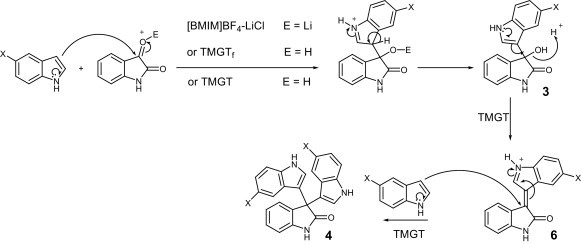
Proposed mechanism for the reaction between isatin and indole derivatives in the ionic liquids.
In the next phase of this investigation we planned to take advantage of the difference between catalytic activities of TMGT and TMGTf for the synthesis of unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones in a more controlled manner. In this protocol the initially synthesized 3-(indol-3-yl)-3-hydroxyindolin-2-ones 3, on reaction of an isatin derivatve with an indole in TMGTf or [BMIM]BF4–LiCl ionic liquids, was treated with 1 equiv of another indole derivate in TMGT to give the desired unsymmetrical 3,3-di(indol-3-yl)indolin-2-one 5. The results of the application of this procedure are summarized in Table 4 .
Table 4.
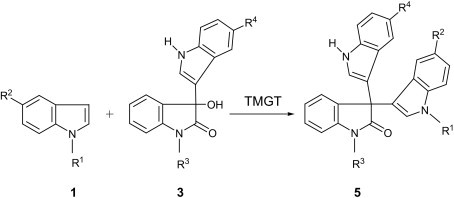
| Entry | R1 | R2 | R3 | R4 | Yield (%) | Product |
|---|---|---|---|---|---|---|
| 1 | H | H | H | H | 5a | 90 |
| 2 | H | H | H | CN | 5b | 88 |
| 3 | H | H | H | Br | 5c | 88 |
| 4 | H | Br | Me | H | 5d | 87 |
| 5 | Me | H | H | H | 5e | 91 |
3. Conclusion
We have demonstrated the application of three ionic liquids in the synthesis of 3-(indol-3-yl)-3-hydroxyindolin-2-ones, symmetrical and unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones of biological interests at room temperature. The reaction of an indole and an isatin derivative even in 3:1 mole ratio under catalysis of either TMGTf or [BMIM]BF4–LiCl ionic liquids gave solely the 1:1 adduct, 3-(indol-3-yl)-3-hydroxyindolin-2-ones 3a–f, in fairly high yields at room temperature, while similar reaction in TMGT favored to form solely symmetrical 3,3-di(indol-3-yl)indolin-2-ones 4a–e. We have also devised the reaction between the adducts 3 and indoles in TMGT to synthesize some unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones 5a–e at room temperature. Experimental simplicity associated with the high yield of products, recyclability of the ionic liquids, and short reaction times render the methods presented here highly competitive compared to existing procedures. We believe that the simple and novel methods presented here could be of broad interest for synthetic and medicinal chemists.
4. Experimental section
4.1. General
All of the solvents and reagents were purchased from Fluka or Merck chemical companies. Melting points were measured on an Electrothermal apparatus and are uncorrected. IR spectra were obtained in KBr discs on a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were measured with Brucker DRX-500, DRX-400 or DRX-300 AVANCE spectrometers. Chemical shifts of 1H and 13C NMR spectra were expressed in parts per million downfield from tetramethylsilane. Mass spectra were recorded on a Shimadzu QP1100EX mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses for C, H, and N were performed using a Foss Heraus CHN-O-rapid analyzer.
4.2. Procedure for preparation of [BMIM]BF4–LiCl
1-Methylimidazole (5.1 g, 62.1 mmol) was added to 32 mL of 1-chlorobutane. The mixture was heated to reflux for 24 h and then cooled to room temperature, the obtained oily product was separated from reaction mixture by decanting, washed with EtOAc (2× 20 mL), and the solvent of the collected organic phase was removed under reduced pressure to give 1-butyl-3-methylimidazolium chloride ([BMIM]Cl). In second step, to [BMIM]Cl (9.3 g, 53 mmol) dissolved in anhydrous acetone (40 mL) was added LiBF4 (5 g, 53 mmol). The mixture was then stirred for 48 h at room temperature to get a solution and then stored at 4 °C over 2 days in refrigerator giving the excess of the dissolved LiCl crystals to precipitate. After separation of the precipitated LiCl crystals (0.87 g, 20.5 mmol) the solvent of the filtered solution was removed under reduced pressure thereby the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate doped with 60 mol % of LiCl ([BMIM]BF4–LiCl) was obtained as a yellow oil.
4.3. General procedure for preparation of 3-(indol-3-yl)-3-hydroxyindolin-2-ones (3a–f)
A mixture of the indole (1 mmol) and an isatin derivative (1 mmol) was added to a vial containing a magnetic stir bar and the ionic liquid (TMGTf or [BMIM][BF4]–LiCl, 1 mL). The reaction mixture was sealed and stirred at room temperature until disappearance of the starting materials (20 min for [BMIM][BF4]–LiCl, 10 min for TMGTf), as monitored by TLC on silica gel using 1:2 mixture of ethyl acetate/n-hexane. After completion of the reaction, the residue was washed with 2×15 mL of cold water to extract the ionic liquids. The solid residues were recrystallized from ethanol (95.5%) to afford pure products 3a–f. The ionic liquids were recovered from the aqueous extracts by evaporation of water in reduced pressure and reused in the next cycles. All the known products have spectral and physical data consistent with those reported in literatures as well as the samples prepared from previously reported methods.
4.3.1. 3-Hydroxy-3-(1H-indol-3-yl)indolin-2-one (3a)
White solid (0.243 g, 92%), mp 293–295 °C, lit.44 mp 294–296 °C; ν max (KBr) 3457, 3261, 1702, 1618, 1476, 1182 cm−1; δ H (300 MHz, DMSO) 6.32 (1H, s, OH), 6.85 (1H, t, J 8.0 Hz), 6.90 (1H, d, J 7.6 Hz), 6.96 (1H, t, J 6.7 Hz), 7.02 (1H, t, J 7.0 Hz), 7.06 (1H, d, J 2.6 Hz), 7.23 (1H, d, J 6.0 Hz), 7.25 (1H, t, J 6.5 Hz), 7.32 (1H, d, J 8.2 Hz), 7.34 (1H, d, J 9.4 Hz), 10.32 (1H, s, amidic N–H), 10.97 (1H, br s, N–H).
4.3.2. 3-Hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one (3b)
Dark red solid (0.259 g, 93%), mp 181–183 °C, lit.44 mp 178–180 °C; ν max (KBr) 3398, 3352, 3297, 1705, 1617 cm−1; δ H (300 MHz, DMSO) 2.41 (3H, s, CH 3), 6.27 (1H, s, OH), 6.73 (1H, t, J 7.5 Hz), 6.89–6.97 (4H, m), 7.18–7.26 (3H, m), 10.34 (1H, s, amidic N–H), 10.87 (1H, br s, N–H).
4.3.3. 5-Bromo-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3c)
White solid (0.306 g, 89%), mp 200 °C (dec). Found: C, 56.16; H, 3.31; N, 8.08. C16H11BrN2O2 requires C, 56.00; H, 3.23; N, 8.16%; ν max (KBr) 3457, 3261, 1703, 1615, 1472, 1168 cm−1; δ H (300 MHz, DMSO) 6.55 (1H, br s, OH), 6.88–6.96 (2H, m), 7.05 (1H, t, J 7.7 Hz, 5′-H), 7.12 (1H, d, J 2.3 Hz, 2′-H of indole), 7.33 (1H, d, J 2.0 Hz, 4-H), 7.36 (2H, d, J 8.3 Hz), 7.44 (1H, dd, J 8.2 and 2.0 Hz, 6-H), 10.30 (1H, br, N–H), 11.07 (1H, s, N–H); δ C (100.62 MHz, DMSO) 75.4 (C-3), 112.1, 112.3, 113.8, 115.1, 119.2, 120.5, 121.7, 124.1, 125.2, 127.8, 132.2, 136.3, 137.3, 141.4, 178.4 (C O).
4.3.4. 5-Bromo-3-hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one (3d)
Brown solid (0.321 g, 90%), mp 136–138 °C. Found: C, 57.22; H, 3.72; N, 7.68. C17H13BrN2O2 requires C, 57.17; H, 3.67; N, 7.84%; ν max (KBr) 3387 (br), 1721, 1615, 1465, 1178 cm−1; δ H (300 MHz, DMSO) 2.41 (3H, s, CH 3), 6.47 (1H, s, OH), 6.77 (1H, t, J 7.5 Hz, 5′-H), 6.86–6.98 (3H, m,), 7.21 (1H, d, J 8.1 Hz, 7-H), 7.24 (1H, d, J 2.0 Hz, 4-H), 7.41 (1H, dd, J 8.1 and 2.0 Hz, 6-H), 10.54 (1H, s, amidic N–H), 10.95 (1H, s, N–H); δ C (100.62 MHz, DMSO) 13.7 (CH3), 76.4 (C-3), 109.1, 110.9, 112.3, 113.8, 118.9, 119.3, 120.4, 126.8, 127.9, 132.1, 134.0, 135.3, 137.0, 141.3, 178.6 (C O).
4.3.5. 5-Fluoro-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one (3e)
White solid (0.254 g, 90%), mp 194–196 °C, lit.44 mp 196–198 °C; ν max (KBr) 3425, 3355, 1720, 1623, 1484, 1189 cm−1; δ H (300 MHz, DMSO) 6.50 (1H, br s, OH), 6.80–6.91 (2H, m), 7.01–7.12 (3H, m), 7.11 (1H, br s, 2′-H of indole), 7.35 (2H, t, J 7.8 Hz), 10.38 (1H, s, amidic N–H), 11.04 (1H, s, N–H); δ C (75.47 MHz, DMSO) 75.6 (C-3), 110.9 (d, 3 J C–F 7.9 Hz, C-7), 112.0, 112.7 (d, 2 J C–F 25.0 Hz), 115.2, 115.7 (d, 2 J 23.0 Hz), 119.0, 120.6, 121.6, 124.0, 125.2, 135.6 (d, 3 J C–F 7.6 Hz, C-3a), 137.2, 138.2, 156.9 (d, 1 J C–F 24.8 Hz, C-5), 178.8 (C O); m/z (EI) 282 (24, M+), 253 (14, M+−[HC O]), 237 (100, M+−[OH+C O]), 144 (14), 117 (45), 89 (13).
4.3.6. 5-Fluoro-3-hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one (3f)
White solid (0.269 g, 91%), mp 212–213 °C, lit.44 mp 212–214 °C; ν max (KBr) 3340, 3199, 1713, 1625, 1485, 1461, 1188 cm−1; δ H (300 MHz, DMSO) 2.42 (3H, s, CH 3), 6.43 (1H, br s, OH), 6.75 (1H, t, J 7.2 Hz, 5′-H), 6.90–7.00 (4H, m), 7.08 (1H, t, J 8.1 Hz, 6-H), 7.20 (1H, d, J 7.8 Hz, 4-H), 10.40 (1H, s, amidic N–H), 10.93 (1H, s, N–H of indole); δ C (75.47 MHz, DMSO) 13.8 (CH3), 76.6 (C-3), 109.2, 110.8, 111.0 (d, 3 J C–F 7.8 Hz, C-7), 112.8 (d, 2 J C–F 24.0 Hz), 115.7 (d, 2 J C–F 22.7 Hz), 118.8, 119.3, 120.4, 126.8, 134.1, 135.3, 136.4 (d, 3 J C–F 7.7 Hz, C-3a), 138.2, 158.6 (d, 1 J C–F 252 Hz, C-5), 179.1 (C O); m/z (EI) 296 (15, M+), 288 (18), 251 (46, M+−[OH+C O]), 237 (58, 251−[CH2]), 130 (100, N-methylindole), 109 (60), 82 (60).
4.4. Synthesis of symmetrical 3,3-di(indol-3-yl)indolin-2-ones (4a–e)
A mixture of the indole 1 (1 mmol) and an isatin derivative 2 (0.5 mmol) was added to a vial containing a magnetic stir bar and the ionic liquid (TMGT, 1 mL). The reaction mixture was sealed and stirred at room temperature until the isatin derivative was completely consumed (about 1 h, as monitored by TLC on silica gel using a 4:3 mixture of ethyl acetate/n-hexane). After completion of the reaction, the resulting paste was washed with 2×15 mL of cold water to extract the ionic liquid. The remained crude products 4a–e were crystallized from ethanol (95.5%). To recover the ionic liquid, the aqueous filtrate was evaporated under reduced pressure. TMGT, which remained as nonvolatile oil in the evaporating vessel was collected and reused. All the novel products were characterized by their IR, 1H NMR, 13C NMR, and mass spectral data, as well as elemental analysis. Partial assignments of the spectral data are given in the following sections.
4.4.1. 3,3-Di(1H-indol-3-yl)indolin-2-one (4a)
White solid (0.338 g, 93%), mp>300 °C, lit.39 mp 311–313 °C; ν max (KBr) 3420, 3300, 1704, 1610, 1105, 737 cm−1; δ H (500 MHz, DMSO) 6.79 (2H, t, J 7.7 Hz), 6.84 (2H, d, J 2.2 Hz, 2′-H of indoles), 6.92 (1H, t, J 7.5 Hz), 6.97–7.02 (3H, m), 7.22 (4H, d, J 7.8 Hz), 7.35 (2H, d, J 8.1 Hz), 10.58 (1H, s, amidic N–H), 10.94 (2H, br s, N–H); δ C (125.8 MHz, DMSO) 53.4 (C-3), 110.4, 112.4, 115.2, 119.1, 121.6, 121.8, 122.3, 125.1, 125.8, 126.6, 128.7, 135.5, 137.8, 142.2, 179.6 (C O); m/z (EI) 363 (57, M+), 334 (69, M+−[HC O]), 248 (64, M+−[indole]), 219 (95, 334−[indole]), 117 (64), 86 (100).
4.4.2. 5-Methoxy-3,3-di(1-methyl-1H-indol-3-yl)indolin-2-one (4b)
Pale pink solid (0.383 g, 91%), mp 279–281 °C. Found: C, 77.02; H, 5.59; N, 9.89. C27H23N3O2 requires C, 76.94; H, 5.50; N, 9.97%; ν max (KBr) 3200, 3100, 1702, 1603, 1486, 1200, 740 cm−1; δ H (500 MHz, DMSO) 3.61 (3H, s, OCH 3), 3.70 (6H, s, N–CH 3), 6.81–6.87 (4H, m), 6.92 (1H, d, J 7.8 Hz, 6-H), 6.93 (2H, s, 2′-H of indoles), 7.09 (2H, t, J 7.4 Hz), 7.28 (2H, d, J 8.0 Hz), 7.38 (2H, d, J 8.3 Hz), 10.47 (1H, s, amidic N–H); δ C (125.8 MHz, DMSO) 33.2 (N–CH3), 53.7 (C-3), 56.2 (O–CH3), 110.6, 110.8, 113.0, 114.3, 119.3, 121.8, 122, 126.9, 129.4, 135.5, 136.7, 138.2, 155.6 (C-5), 179.3 (C O); m/z (EI) 421 (78, M+), 407 (7, M+−[CH2]), 392 (100, M+−[HC O]), 348 (14), 291 (12, M+−[N-methylindole]), 263 (16, 392−[N-methylindole]), 211 (10), 131 (10).
4.4.3. 5-Bromo-3,3-di(1-methyl-1H-indol-3-yl)indolin-2-one (4c)
Pale pink solid (0.405 g, 86%), mp>300 °C. Found: C, 66.52; H, 4.36; N, 8.86. C26H20BrN3O requires C, 66.40; H, 4.28; N, 8.93%; ν max (KBr) 3390, 3120, 3050, 1738, 1610, 1480, 1337, 1075, 739 cm−1; δ H (500 MHz, DMSO) 3.72 (6H, s, N–CH 3), 6.88 (2H, t, J 7.4 Hz), 7.01 (2H, s, 2′-H of indoles), 7.11 (2H, t, J 7.4 Hz), 7.20 (1H, d, J 8.6 Hz, 7-H), 7.25 (2H, d, J 8.0 Hz), 7.41 (2H, d, J 8.2 Hz), 7.99 (1H, s, 4-H), 8.24 (1H, d, J 8.6 Hz, 6-H), 11.35 (1H, s, amidic N–H); δ C (125.8 MHz, DMSO) 33.2 (CH3), 53.5 (C-3), 110.8, 112.6, 113.5, 114.1, 119.5, 121.6, 122.1, 126.7, 128.2, 129.4, 131.6, 137.7, 138.2, 141.5, 178.9 (C O); m/z (EI) 471 (2, M+, 81Br), 470 (4), 469 (2, M+, 79Br), 468 (4), 442 (10), 411 (26), 285 (26), 223 (35), 149 (73), 57 (100).
4.4.4. 3,3-Di(2-methyl-1H-indol-3-yl)indolin-2-one (4d)
White solid (0.352 g, 90%), mp>300 °C, lit.39 mp 300–301 °C; ν max (KBr) 3370, 3119, 3050, 1705, 1615, 740 cm−1; δ H (500 MHz, DMSO) 1.93 (3H, s, CH 3), 2.06 (3H, s, CH 3), 6.45 (1H, d, J 8.2 Hz), 6.60 (1H, t, J 7.1 Hz), 6.63 (1H, d, J 7.8 Hz), 6.69 (1H, d, J 8.0 Hz), 6.85 (1H, t, J 7.5 Hz), 6.88 (1H, t, J 7.1 Hz), 6.89 (1H, t, J 7.2 Hz), 6.94 (1H, d, J 7.7 Hz), 7.14 (1H, d, J 7.3 Hz), 7.20–7.23 (3H, m), 10.50 (1H, s, amidic N–H), 10.82 (1H, br s, N–H), 10.85 (1H, br s, N–H).
4.4.5. 5-Nitro-3,3-di(1-methyl-1H-indol-3-yl)indolin-2-one (4e)
Pale brown solid (0.384 g, 88%), mp>300 °C. Found: C, 71.64; H, 4.73; N, 12.70. C26H20N4O3 requires C, 71.56; H, 4.62; N, 12.83%; ν max (KBr) 3230, 3105, 3055, 1715, 1608, 1465, 1325, 1158, 730; δ H (500 MHz, DMSO) 3.72 (6H, s, 2×CH 3), 6.89 (2H, t, J 7.5 Hz, 5′-H of indoles), 7.02 (2H, s, 2′-H of indoles), 7.11 (2H, t, J 7.5 Hz, 6′-H of indoles), 7.21 (1H, d, J 8.6 Hz, 7-H), 7.26 (2H, d, J 8.1 Hz), 7.40 (2H, d, J 8.2 Hz), 8.00 (H, s, 4-H), 8.25 (1H, d, J 8.6 Hz, 6-H), 11.36 (1H, s, amidic N–H); δ C (125.8 MHz, DMSO) 33.2 (2×CH3), 53.2 (C-3), 110.8, 110.9, 112.8, 119.6, 121.0, 121.4, 122.2, 126.4, 126.6, 129.6, 136.0, 138.3, 143.1, 148.6, 179.6 (C O); m/z (EI) 436 (86, M+), 422 (22, M+−[CH2]), 407 (100, M+−[HC O]), 393 (25, 422−[CH2]), 361 (28), 306 (21, M+−[N-methylindole]), 278 (22, 306−[HC O]), 232 (18), 131 (33).
4.5. Synthesis of unsymmetrical 3,3-di(indol-3-yl)indolin-2-ones (5a–e)
A mixture of oxindoles 3a–f (1 mmol) and an indole derivative (1 mmol) was added to a vial containing a magnetic stir bar and TMGT (1 mL). The reaction mixture was sealed and stirred at room temperature until the disappearance of the starting materials (1 h, monitored by TLC on silica gel using 4:3 mixture of ethyl acetate/n-hexane). After completion of the reactions, the residues were washed with 2×15 mL of cold water to extract TMGT. The remained crude products 5a–e were recrystallized from ethanol (95.5%). TMGT was recovered from the aqueous extracts by evaporation of water in reduced pressure and reused in the next cycles. Partial assignment of spectral data to the structure of previously unreported products 5 is given in the following sections.
4.5.1. 3,3-Di(1H-indol-3-yl)indolin-2-one (5a)
Compound 5a is identical with 4a (0.327 g, 90%).
4.5.2. 3-(1H-Indol-3-yl)-3-(5-cyano-1H-indol-3-yl)indolin-2-one (5b)
Light pink solid (0.341 g, 88%), mp>300 °C. Found: C, 77.25; H, 4.17; N, 14.41. C25H16N4O requires C, 77.31; H, 4.15; N, 14.42%. ν max (KBr) 3360, 3254, 3100, 2220, 1675, 1618, 1467, 1200, 740 cm−1; δ H (500 MHz, DMSO) 6.82 (1H, t, J 7.4 Hz), 6.91 (1H, d, J 1.8 Hz, 2′-H), 6.97 (1H, t, J 7.6 Hz), 7.02–7.04 (2H, m), 7.07 (1H, d, J 1.8 Hz, 2″-H), 7.15 (1H, d, J 8 Hz), 7.26 (2H, d, J 7.4 Hz), 7.35–7.42 (2H, m), 7.56 (2H, d, J 8.5 Hz), 7.72 (1H, s, 4′-H), 10.72 (1H, s, amidic N–H), 11.02 (1H, br s, N–H), 11.62 (1H, br s, N–H); δ C (125.8 MHz, DMSO) 53.2 (C-3), 101.2, 110.7, 112.7, 114.1, 114.9, 116.3, 119.4, 120.8, 121.6, 122, 122.6, 124.5, 125.2, 125.8, 126.3, 126.4, 127.6, 127.7, 129.0, 134.7, 137.8, 139.7, 142.1, 179.4 (C O); m/z (EI) 388 (44, M+), 359 (57, M+−[HC O]), 334 (24), 273 (63, M+−[indole]), 244 (75, 359−[indole]), 219 (45, 359−[5-cyanoindole]), 142 (44), 117 (100), 94 (66), 71 (42).
4.5.3. 3-(1H-Indol-3-yl)-3-(5-bromo-1H-indol-3-yl)indolin-2-one (5c)
Pale brown solid (0.389 g, 88%), mp 284–286 °C. Found: C, 65.07; H, 3.73; N, 9.42. C24H16BrN3O requires C, 65.18; H, 3.64; N, 9.50%; ν max (KBr) 3390, 3310, 3104, 3050, 1680, 1660, 1468, 1450, 1330, 1100, 740 cm−1; δ H (500 MHz, DMSO) 6.83 (1H, t, J 7.6 Hz), 6.90 (1H, d, J 1.6 Hz), 6.91 (1H, d, J 1.5 Hz), 6.96 (1H, t, J 7.6 Hz), 7.01–7.05 (2H, m), 7.16 (1H, d, J 8.6 Hz), 7.21 (2H, t, J 7.9 Hz), 7.25 (1H, t, J 7.6 Hz), 7.37 (2H, t, J 8.8 Hz), 7.48 (1H, s, 4′-H), 10.68 (1H, s, amidic N–H), 10.97 (1H, br s, N–H), 11.22 (1H, br s, N–H); δ C (125.8 MHz, DMSO) 53.3 (C-3), 110.6, 111.8, 112.6, 114.6, 114.9, 115, 119.3, 121.1, 121.9, 122.5, 124.2, 124.4, 125.2, 125.8, 126.4, 126.7, 128.4, 128.9, 135, 136.6, 137.8, 142.2, 179.5 (C O); m/z (EI) 443 (40, M+, 81Br), 441 (40, M+, 79Br), 414 (43, 443−[HC O]), 412 (42, 441−[HC O]), 363 (49), 334 (62), 299 (25, 414−[indole]), 297 (20, 412−[indole]), 248 (74), 219 (100, 414−[5-bromoindole]), 195 (55), 193 (55), 117 (80).
4.5.4. 1-Methyl-3-(1H-indol-3-yl)-3-(5-bromo-1H-indol-3-yl)indolin-2-one (5d)
Pale brown solid (0.397 g, 87%), mp>300 °C. Found: C, 65.67; H, 4.05; N, 9.28. C25H18BrN3O requires C, 65.81; H, 3.98; N, 9.20%; ν max (KBr) 3350, 3106, 1662, 1604, 1460, 1445, 1087, 740 cm−1; δ H (500 MHz, DMSO) 3.27 (3H, s, N–CH 3), 6.81 (1H, t, J 7.5 Hz), 6.89 (1H, d, J 2.1 Hz), 6.90 (1H, d, J 2.1 Hz), 7.01–7.09 (3H, m), 7.14–7.20 (2H, m), 7.27 (1H, d, J 7.3 Hz), 7.30–7.37 (4H, m), 7.47 (1H, s, 4′-H), 11.00 (1H, br s, N–H), 11.24 (1H, br s, N–H); δ C (125.8 MHz, DMSO) 27.1 (CH3), 52.8 (C-3), 109.7, 111.8, 112.6, 114.6, 114.8, 119.4, 120.8, 122, 123.2, 123.5, 124.3, 124.4, 125.3, 125.4, 126.3, 126.7, 128.3, 129.1, 134.1, 136.6, 137.8, 143.6, 177.6 (C O); m/z (EI) 457 (19, M+, 81Br), 456 (66), 455 (18, M+, 79Br), 454 (60), 429 (5, M+−[C O]), 428 (18, 456−[C O]), 426 (17, 454−[C O]), 414 (18, 428−[CH2]), 392 (100), 377 (15, M+−[Br]), 364 (75), 348 (23), 214 (22), 115 (36).
4.5.5. 3-(1H-Indol-3-yl)-3-(1-methyl-1H-indol-3-yl)indolin-2-one (5e)
White solid (0.344 g, 91%), mp 299–301 °C, lit.39 mp 298–300 °C; ν max (KBr) 3310, 3190, 1683, 1615, 1469, 1330, 1100, 740 cm−1; δ H (500 MHz, DMSO) 3.70 (3H, s, CH 3), 6.80 (1H, t, J 7.5 Hz), 6.84 (1H, t, J 7.7 Hz), 6.87 (2H, s, 2-H of indoles), 6.93 (1H, t, J 7.4 Hz), 6.98–7.03 (2H, m), 7.08 (1H, t, J 7.4 Hz), 7.22–7.26 (4H, m), 7.36 (2H, t, J 8.4 Hz), 10.61 (1H, s, amidic N–H), 10.97 (1H, br s, N–H); δ C (125.8 MHz, DMSO) 33.2 (CH3), 53.4 (C-3), 110.4, 110.6, 112.5, 114.4, 115.1, 119.1, 119.2, 121.5, 121.8, 121.9, 122.0, 122.4, 125.2, 125.8, 126.5, 126.9, 128.7, 129.3, 135.4, 137.8, 138.2, 142.2, 179.5 (C O); m/z (EI) 379 (4, M+), 377 (90, M+−2), 348 (100), 332 (13), 261 (13), 219 (21).
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of University of Guilan.
References and notes
- 1.Martins M.A.P., Frizzo C.P., Moreira D.N., Zanatta N., Bonacorso H. Chem. Rev. 2008;108:2015–2050. doi: 10.1021/cr078399y. [DOI] [PubMed] [Google Scholar]
- 2.Holbrey J.D., Turner M.B., Reichert W.M., Rogers R.D. Green Chem. 2003;5:731–736. [Google Scholar]
- 3.Stracke M.P., Migliorini M.V., Lissner E., Schrekker H.S., Dupont J., Gonçalves R.S. Appl. Energ. 2009;86:1512–1516. [Google Scholar]
- 4.Chowdhury Sh., Mohan R.S., Scott J.L. Tetrahedron. 2007;63:2363–2389. [Google Scholar]
- 5.Hajipour A.R., Ghayeb Y., Sheikhan N., Ruoho A. Tetrahedron Lett. 2009;50:5649–5651. [Google Scholar]
- 6.Yadav J.S., Reddy B.V.S., Baishya G., Reddy K.V., Narsaiah A.V. Tetrahedron. 2005;61:9541–9544. [Google Scholar]
- 7.Mi X., Luo S., He J., Cheng J.-P. Tetrahedron Lett. 2004;45:4567–4570. [Google Scholar]
- 8.Torriero A.A.J., Siriwardana A.I., Bond A.M., Burgar I.M., Dunlop N.F., Deacon G.B., MacFarlane D.R. J. Phys. Chem. B. 2009;113:11222–11231. doi: 10.1021/jp9046769. [DOI] [PubMed] [Google Scholar]
- 9.Yan N., Yang X., Fei Zh., Li Y., Kou Y., Dyson P.J. Organometallics. 2009;28:937–939. [Google Scholar]
- 10.Lee J.S., Wang X., Luo H., Baker G.A., Dai Sh. J. Am. Chem. Soc. 2009;131:4596–4597. doi: 10.1021/ja900686d. [DOI] [PubMed] [Google Scholar]
- 11.Dupont J., de souza R.F., Suarez P.A.Z. Chem. Rev. 2002;102:3667–3692. doi: 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen P., Karhu H., Toth G., Kordas K., Mikkola J.-P. J. Catal. 2009;263:209–219. [Google Scholar]
- 13.Rad-Moghadam K., Sharifi-Kiasaraie M. Tetrahedron. 2009;65:8816–8820. doi: 10.1016/j.tet.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratan Bal T., Anand B., Yogeeswari P., Sriram Dh. Bioorg. Med. Chem. Lett. 2005;15:4451–4455. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 15.Jiang T., Kuhen K.L., Wolff K., Yin H., Bieza K., Caldwell J., Bursulaya B., Tuntland T., Zhang K., Karanewsky D., He Y. Bioorg. Med. Chem. Lett. 2006;16:2109–2112. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 16.Tripathy R., Reiboldt A., Messina P.A., Iqbal M., Singh J., Bacon E.R., Angeles Th.S., Yang Sh.X., Albom M.S., Robinson C., Chang H., Ruggeri B.A., Mallamo J.P. Bioorg. Med. Chem. Lett. 2006;16:2158–2162. doi: 10.1016/j.bmcl.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Cane A., Tournaire M.-C., Barritault D., Crumeyrolle-Arias M. Biochem. Biophys. Res. Commun. 2000;276:379–384. doi: 10.1006/bbrc.2000.3477. [DOI] [PubMed] [Google Scholar]
- 18.Silveira V.Ch., Luz J.S., Oliveira C.C., Graziani I., Ciriolo M.R., Costa Ferreira A.M. J. Inorg. Biochem. 2008;102:1090–1103. doi: 10.1016/j.jinorgbio.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Amal Raj A., Raghunathan R., Sridevikumaria M.R., Raman N. Bioorg. Med. Chem. 2003;11:407–419. doi: 10.1016/s0968-0896(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Arguelles M.C., Mosquera-Vazaquez S., Touron-Touceda P., Sanmartin-Matalobos J., Garcia-Deibe A.M., Belicchi-Ferraris M., Pelosi G., Pelizzi C., Zani F. J. Inorg. Biochem. 2007;101:138–147. doi: 10.1016/j.jinorgbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Maskell L., Blanche E.A., Colucci M.A., Whatmore J.L., Moody Ch.J. Bioorg. Med. Chem. Lett. 2007;17:1575–1578. doi: 10.1016/j.bmcl.2006.12.108. [DOI] [PubMed] [Google Scholar]
- 22.Verma M., Nath Pandeya S., Nand Singh K., Stables J.P. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 23.Igosheva N., Lorz C., O'Conner E., Glover V., Mehmet H. Neurochem. Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen L.-R., Wang Y.-Ch., Lin Y.W., Chou Sh.-Y., Chen Sh.-F., Liu L.T., Wu Y.-T., Kuo Ch.-J., Chen T.Sh.-Sh., Juang Sh.-H. Bioorg. Med. Chem. Lett. 2005;15:3058–3062. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimazawa R., Kuriyama M., Shirai R. Bioorg. Med. Chem. Lett. 2008;18:3350–3353. doi: 10.1016/j.bmcl.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Abadi A.H., Abou-Seri S.M., Abdel-Rahman D.E., Klein Ch., Lozach O., Meijer L. Eur. J. Med. Chem. 2006;41:296–305. doi: 10.1016/j.ejmech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y., Kitajima M., Kogure N., Takayama H. Tetrahedron. 2008;64:7690–7694. [Google Scholar]
- 28.Kogure N., Kobayashi H., Ishii N., Kitajima M., Wongseripipatana S., Takayama H. Tetrahedron Lett. 2008;49:3638–3642. [Google Scholar]
- 29.Zhang Zh., Di Y.-T., Wang Y.-H., Zhang Zh., Mu Sh.-Zh., Fang X., Zhang Y., Tan Ch.-J., zhang Q., Yan X.-H., Guo J., Li Ch.-sh., Hao X.-J. Tetrahedron. 2009;65:4551–4556. [Google Scholar]
- 30.Kam T.-S., Choo Y.-M. Tetrahedron. 2000;56:6143–6150. [Google Scholar]
- 31.Wu Y., Kitajima M., Kogure N., Zhang R., Takayama H. Tetrahedron Lett. 2008;49:5935–5938. [Google Scholar]
- 32.Jursic B.S., Stevens E.D. Tetrahedron Lett. 2002;43:5681–5683. [Google Scholar]
- 33.Klumpp D.A., Yeung K.Y., Prakash G.K.S., Olah G.A. J. Org. Chem. 1998;63:4481–4484. [Google Scholar]
- 34.Sharma I., Saxena A., Ojha C.K., Paradasani C.K.P., Paradasani R.T., Mukherjee T. Proc. Indian Acad. Sci. (Chem. Sci.) 2002;114:523–531. [Google Scholar]
- 35.Marsden S.P., Watson E.L., Raw S.A. Org. Lett. 2008;10:2905–2908. doi: 10.1021/ol801028e. [DOI] [PubMed] [Google Scholar]
- 36.Felpin F.-X., Ibarguren O., Nassar-Hardy L., Fouquet E. J. Org. Chem. 2009;74:1349–1352. doi: 10.1021/jo802467s. [DOI] [PubMed] [Google Scholar]
- 37.Luppi G., Cozzi P.G., Monari M., Kaptein B., Broxterman Q.B., Tomasini C. J. Org. Chem. 2005;70:7418–7421. doi: 10.1021/jo050257l. [DOI] [PubMed] [Google Scholar]
- 38.Malkov A.V., Kabeshov M.A., Bella M., Kysilka O., Malyshev D.A., Pluhackova K., Kocovsky P. Org. Lett. 2007;9:5473–5476. doi: 10.1021/ol7023983. [DOI] [PubMed] [Google Scholar]
- 39.Azizian J., Mohammadi A.A., Karimi N., Mohammadizadeh M.R., Karimi A.R. Catal. Commun. 2006;7:752–755. [Google Scholar]
- 40.Wang Sh.-Y., Ji Sh.-J. Tetrahedron. 2006;62:1527–1535. [Google Scholar]
- 41.Ganachaud Ch., Garfagnoli V., Tron Th., Iacazio G. Tetrahedron Lett. 2008;49:2476–2478. [Google Scholar]
- 42.Frechard A., Fabre N., Pean Ch., Mantaut S., Fauvel M.-T., Rollin P., Fouraste I. Tetrahedron Lett. 2001;42:9015–9017. [Google Scholar]
- 43.Tang Y.-Q., Sattler I., Thiericke R., Grabley S., Feng X.-Z. Eur. J. Org. Chem. 2001:261–267. [Google Scholar]
- 44.Kumar V.P., Reddy V.P., Sridhar R., Srinivas B., Narender M., Rao K.R. J. Org. Chem. 2008;73:1646–1648. doi: 10.1021/jo702496s. [DOI] [PubMed] [Google Scholar]
- 45.Sano D., Nagata K., Itoh T. Org. Lett. 2008;10:1593–1595. doi: 10.1021/ol800260r. [DOI] [PubMed] [Google Scholar]


