Abstract
A case of fatal systemic coronavirus infection is described in a 53-day-old Pekinese dog. Pathological findings and immunohistochemical identification using a monoclonal anti-canine Coronavirus antibody are included. Visceral lesions consisted of extensive fibrinopurulent bronchopneumonia, multiple renal cortical infarcts, severe coalescing centrilobular hepatic fatty change with minimal random hepatic necrosis, and multifocal splenic haemorrhage with lymphoid depletion. Moderate chronic diffuse enteritis was associated with intraluminal adult ascarids. Identification of type I and type II coronavirus in this subject had been previously confirmed by genotype-specific real-time reverse transcription-polymerase chain reaction (RT-PCR) assays of the intestinal contents, while only Coronavirus type II was detected in visceral organs. This case represents the first description of morphological lesions associated with a type II pantropic fatal coronavirus infection in the dog.
Keywords: Coronavirus, Dog, Immunohistochemistry
Coronavirus (order Nodavirales, family Coronaviridae) are enveloped, single-stranded, positive-sense RNA viruses, classified into three groups according to specific serological cross-reactivity and genomic features (Weiss and Navas-Martin, 2005). Well-known canine coronaviruses (CCoVs) belong to the group I coronaviruses, together with transmissible gastroenteritis virus of swine (TGEV), porcine epidemic diarrhoea virus (PEDV), porcine respiratory coronavirus (PRCoV), feline coronaviruses (FCoVs) including feline infectious peritonitis virus (FIPV) and some human coronavirus (HCoVs 229E and NL63). CCoV infection may be asymptomatic; but clinical signs that occur in young pups include diarrhoea, vomiting, dehydration, loss of appetite and even death (Tennant et al., 1991).
Here we describe the pathological findings and immunohistochemical identification of a highly pathogenic variant of CCoV responsible for systemic visceral lesions and death in a puppy.
A 53-day-old male Pekinese dog was presented with a fever (39.5 °C–40 °C), lethargy, inappetence, haemorrhagic diarrhoea, vomiting and ataxia. The pup had received a single dose of a polyvalent vaccine containing modified-live canine distemper virus, canine adenovirus, canine parvovirus and killed Leptospira icterohaemorrhagica and L. canicola at about 45 days of age. No information regarding other treatments were available. The dog died after 2 days and was necropsied.
Gross lesions included diffuse, moderate, heamorrhagic enteritis, 200 ml of sero-sanguineous fluid in the abdominal cavity and lesions in visceral organs. Some adult nematodes were found within the lumen of small intestine (consistent with ascarids). The lungs had bilateral extensive, coalescing red areas of consolidation in all lobes and peripheral emphysema (Fig. 1 ). Multifocal to coalescing areas of hepatic discoloration were present (partially consistent with post-mortem changes) in association with patchy hyperaemia. The spleen showed diffuse moderate to severe enlargement and discolouration with multifocal subcapsular reddening. Multifocal cortical infarcts were found in both kidneys which also exhibited slight palor. The mediastinal and mesenteric lymph node were enlarged with petecchiae on the surfaces.
Fig. 1.
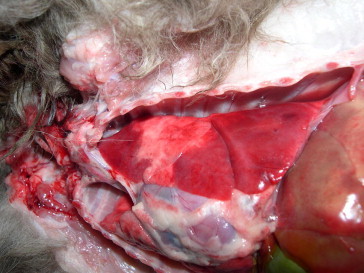
Thoracic cavity and portion of liver, dog, Pekinese. Extensive, coalescing, red areas of lobar consolidation both in the apical, medium and caudal lung lobes are evident. Multifocal to coalescing areas of hepatic discoloration are also observable. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Formalin-fixed samples from lungs, kidneys, spleen, liver, and small intestine were submitted for histology. Sections were cut at 4 μm and stained with H&E for histological examination. In addition Gram, Giemsa, PAS, and PASM histochemical stains were performed. Immunohistochemical analyses were also carried out using a monoclonal antibody (MAb) anti-CCoV recognizing the nucleocapsid (N) structural protein of the virus (kindly provided by Prof. C. Buonavoglia, University of Bari). Briefly, following hydration of the sections, endogenous peroxidases were blocked in a 0.3% hydrogen peroxide methanol solution for 20 min. A standard high temperature unmasking technique was applied (10 min boiling at 750 W in citrate buffer, pH6). Blocking with 5% BSA for 30 min preceded incubation with the MAb anti-CCoV (1:100 in 2.5% BSA PBS) for 1 hour at room temperature. The mouse EnVision system (Dakocytomation) was applied as described by the manufacturer and DAB (3,3 1-diaminobenzidine) (Peroxidase Substrate Kit DAB, Vector Laboratories Inc.) was used as chromogen. Control tissue sections were used omitting either primary or secondary conjugated antibodies.
In a previous study, virology aspects related to this case were investigated. Specifically, fresh visceral tissues from the same dog were used to confirm the presence of CCoV by isolation on A-72 cells (canine fibroma) cell culture and by genotype-specific real-time reverse transcription-polymerase chain reaction (RT-PCR) assays (Buonavoglia et al., 2006, Decaro et al., 2005a). Decaro and co-authors excluded other important canine viruses by molecular diagnostic assay as previously described, particularly real time PCR was performed for parvovirus (Decaro et al., 2005b) and RT-PCR for distemper virus (Elia et al., 2006); additionally, tests for anaerobe and aerobe bacteria were carried out on standard bacteriology media from fresh tissue swabs (Buonavoglia et al., 2006).
On histopathology of the lungs there was an extensive coalescing fibrinopurulent infiltrate that extends from the alveoli into the bronchioles and bronchi. Numerous macrophages that exhibited marked erythrophagocytosis were present within the alveoli (Fig. 2 a and c). Within adjacent areas of the pulmonary parenchyma there was multifocal necrosis, severe haemorrhages, and oedema, with degeneration of the hypertrophic bronchial and bronchiolar epithelium and transmigration of mononuclear cells and neutrophils (Fig. 2b). Monocytes and neutrophils margination and migration through vessel walls (mainly small to medium arteries) were present in association with a marked perivascular serous-fibrinous oedema and mild mural fibrinoid vascular necrosis (Fig. 2b). Less severely affected peripheral regions of the pulmonary parenchyma showed, diffuse emphysema, interstitial oedema, and hyperemia. Gram and Giemsa staining did not reveal any etiological agent. The small intestine had moderate diffuse post-mortem changes affecting the superficial mucosa, but there were also diffuse hyperemia, mild fibrosis and chronic inflammation (lymphocytes and plasma cells) within the lamina propria, and multifocal, moderate crypt ectasia with lumens containing cellular debris (crypt necrosis, “crypt abscesses”). In the liver, multifocal, centrilobular and midzonal, moderate microvascular hepatocellular fatty change were present as well as necrosis of individual hepatocytes or small groups of hepatocytes. Periportal (limiting plate) hepatocytes showed slight hyperplasia with binucleation and mild thickening of cordons. Within the spleen there was multifocal hyperemia and diffuse, severe lymphoid depletion. The remaining lymphoid follicles contained pyknotic and karyorrhectic lymphocytes (leukocytolysis), rare plasma cells and scattered macrophages with intracytoplasmic pigment (haemosiderin) and cellular debris. In the kidney there were large multifocal areas of coagulative necrosis, associated with marked marginal hyperemia that extended from the superficial cortex to the medulla. Thrombi were not detectable within the sections. Routine special stains (PAS and PASM) did not show any other significant diagnostic features.
Fig. 2.
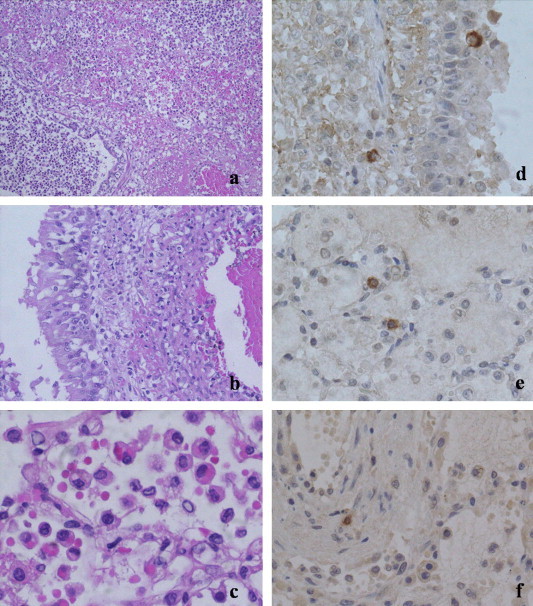
Lung, dog, Pekinese. (a) A severe densely cellular fibrinopurulent infiltrate extends from alveoli to bronchioles, H&E 10×; (b) monocytes and neutrophils margination and migration through oedematous and degenerated blood vessels wall is evident in association with mild mural fibrinous necrosis as well as transmigration of mononuclear cells through hypertrophic bronchial epithelium, H&E 20×; (c) severe intralveolar erythrophagocytosis is detected, H&E 40×; (d–f) immunohistochemical staining with a monoclonal anti-canine coronavirus antibody, DAB revelation system, hematoxylin counterstaining, 40×; (d) intralesional positive cells are migrating both within and around bronchial and bronchiolar epithelium; (e) scattered positive cells are also evident within alveolar septae and lumina; (f) rare stained cells are detected within blood vessels wall.
On immunohistochemistry with MAb anti-CCoV antibody, macrophages/histiocytes had a marked diffuse brown staining within their cytoplasm. In the lungs intralesional positively stained cells were detected both within and around bronchial and bronchiolar epithelium and scattered within alveolar septae and lumina; occasionally, positive cells were found within the arterial walls (Fig. 2a–c). Kupffer cells and some perivascular mononuclear cells stained positive in the liver. Within the spleen occasional less intensively staining cells were primarily identified within the periarteriolar necrotic follicular debris. Rare positive cells were present in the perivascular areas of the kidneys. No positively stained cells were detected within the small intestine. The negative controls failed to show intracytoplasmic staining.
Decaro and co-authors studies observed a virus-induced cytopathic in A-72 cells and CCoV type II strain (CB/05) was isolated from the intestinal contents, lungs, spleen, liver, and kidneys of this dog; CCoV Type I and Type II were identified in the intestinal contents by genotype-specific RT-PCR assays and CCoV type II RNA was also detected in lungs, spleen, liver, kidney and brain (Buonavoglia et al., 2006, Decaro et al., 2005a). Virological and bacteriological investigation did not identify any other pathogens (Buonavoglia et al., 2006, Decaro et al., 2005a, Decaro et al., 2005b).
This case represents therefore a systemic fatal infection by CCoV Type II in a dog.
Apart from the well-known group I viruses, group II and group III are also recognized within the Coronaviridae family. Group II CoVs have a specific structural gene coding for a haemagglutinin-esterase (HE) protein and include some HCoVs (OC43 and HKU1) and some other less common mammalian CoVs (Weiss and Navas-Martin, 2005).
Structural proteins of coronaviruses are fundamental to understand tissue tropism and pathophysiology and their ability to change and become either more pathogenic or pantropic, thereby causing more generalized and severe disease. Genomic mutations in structural (i.e. spike gene) and non-structural genes, for instance, allow the development of systemic and fatal FIP in FeCoV positive cats (Vennema et al., 1998, Rottier et al., 2005). Similarly, some mild respiratory HCoVs (229 and OC43) have been able to give rise to the more severe CoVs infection in humans (NL63 and HKV1) (Weiss and Navas-Martin, 2005). The recent SARS outbreaks caused by a mutant non-human CoVs indicates that some coronaviruses have the ability to shift host-species (Perlman and Dandekar, 2005).
CCoV infection has been always considered an intestinal disease characterized by lesions affecting the apices of the villi and caused by group I CoVs, sub-grouped as type I (genetically related to type I FCoV) and type II (classical mildly pathogenic strains, genetically more related to type II FCoV) (Pratelli, 2006). Interestingly, some respiratory endemic CCoVs infections have been recently identified and assigned to a third CCoV that has been classified within group II on the basis of the genetic similarity with some bovine and HCoV (OC43) and evidence of the HE gene, suggesting a crossing of species barriers (Erles et al., 2003, Erles and Brownlie, 2005). Furthermore, in recent studies a CCoV was associated with mortality in puppies that exhibited both gross pathology and histopathology changes indicative of canine parvovirus type 2 (CPV-2) infection (i.e. crypt necrosis), similarly to what was found in our case (Evermann et al., 2005). No DNA analysis was performed to establish the specific CCoV genotype in these enteric lethal CPV2-like outbreaks. It is therefore not surprising that a highly pathogenic canine coronavirus would present with systemic pathological changes different from the classical enteric disease. The intestinal pattern found in our pup would be more suggestive of a CPV2 infection, despite the presence of autolytic changes that precluded the adequate assessment of the intestine for lesions, but no evidence of CPV was found with molecular tests in this dog (Buonavoglia et al., 2006). Additionally, adult ascarids are frequently detected in puppies and generally are not responsible for specific histological changes but may cause clinical disease by their physical presence (i.e. impaction).
FIP and SARS have some common features despite different pathogenetic mechanisms that are not completely understood (Gu et al., 2005, Perlman and Dandekar, 2005). They both originate from highly pathogenic mutated CoV strains, and they are typically associated with severe alveolar damage within the lungs, marked fibrinous exudation, prominent histiocytic/macrophagic involvement (often in SARS with pronounced evidence of erythrophagocytosis) and lymphopenia (Gu et al., 2005). All these features were evident in our case; specifically, lymphopenia was documented by the splenic lymphocytolysis and subsequently documented in experimental infections performed with this CCoV strain (Buonavoglia et al., 2006). In addition, in this case, positive immunohistochemical staining was detected in mononuclear phagocytes, suggesting a similarity to FIP in its pathogenesis in which virus-positive monocytes migrating through blood vessel walls are responsible for the vasculitis and perivasculitis and associated fibrinous/granulomatous lesions (Kipar et al., 2005). Additional immunohistochemistry needs to be undertaken and other markers should be included for more precisely identify the positive staining cells as macrophages/histiocytes. Evaluation of additional cases may help our understanding of the role or vascular lesions in the pathogenesis of the disease. The latter may have been responsible for the renal infarcts and the abdominal effusion. A disseminated intravascular coagulation (DIC) associated with a respiratory failure can be considered as causes of death. Lack of visceral thrombi might be due either to a hyperacute course of the disease or to predominance of respiratory failure on DIC.
In our case, immunohistochemical investigation of CCoVs antigen within visceral tissues was carried out in collaboration with colleagues studying the molecular aspects of CCoVs (Buonavoglia et al., 2006). Virus isolation in cell cultures confirmed the presence of coronavirus within the tissue and by RT-PCR a type II CCoV could be identified, with some mutations in non-structural genes when compared to other CCoVs (Buonavoglia et al., 2006). Although, several tissues (i.e. brain) were not submitted for histopathology, the submitted fresh tissue was positive for CCoVs and negative for other significant viruses (i.e. morbillivirus, parvovirus, adenovirus) when tested with molecular assays (Buonavoglia et al., 2006, Decaro et al., 2005a). The histopathologic changes found cannot rule out an enteric CPV2 infection in association with more classical canine respiratory diseases (i.e. Bordetella bronchiseptica, Mycoplasma spp, CAV-2). Virological and bacteriological investigations did not detect any other pathogens, and because no data was available about any treatment of this animal, concomitant and/or secondary respiratory bacterial infections cannot therefore be completely excluded. Nevertheless, our findings emphasise that CCoV infection can be systemic and fatal in dogs, but that they may also be mistaken for a different infection (morphological mimicry) and/or secondary lesions.
In conclusion, this study confirms the mutational capability of CoVs in the canine species and describes some of the associated lesions. Screenings and investigation of CCoVs systemic infections will allow a better understanding of the pathology, epidemiology and potential risks of this disease to animals and humans.
Acknowledgement
The authors thank Prof. Canio Buonavoglia from the University of Bari for providing all the molecular data and for his scientific support.
References
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., Di Trani L., Tempesta M., Buonavoglia C. Genotype specific fluorogenic RT-PCR assays for the detection and quantification of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Meth. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Trani L.D., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L., Buonavoglia C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Meth. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Erles K., Brownlie J. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch. Virol. 2005;150:1493–1504. doi: 10.1007/s00705-005-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evermann J.F., Abbot J.R., Han S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J. Vet. Diagn. Invest. 2005;17:610–614. doi: 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.-Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., May H., Menger S., Weber M., Leukert W., Reinacher M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005;42:321–330. doi: 10.1354/vp.42-3-321. [DOI] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A. Genetic evolution of canine coronavirus and recent advances in prophylaxis. Vet. Res. 2006;37:191–200. doi: 10.1051/vetres:2005053. [DOI] [PubMed] [Google Scholar]
- Rottier P.J.M., Nakamura K., Schellen P., Volders H., Haijema B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005;79:14122–14130. doi: 10.1128/JVI.79.22.14122-14130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Kelly D.F., Carter S.D., Gaskell C.J. Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


