Abstract
Transmissible gastroenteritis virus (TGEV) causes transmissible gastroenteritis (TGE), especially in newborn piglets, which severely threatens the worldwide pig industry. In this study, (+)-catechin was evaluated for its antiviral effect against TGEV in vitro. Viability assays revealed that (+)-catechin treatment exerted a dose-dependent rescue effect in TGEV-infected ST cells, and this result was only obtained with the post-treatment application of (+)-catechin. The viral yields in (+)-catechin-treated cultures were reduced by almost three log10 units. Quantitative real-time PCR analysis of the TGEV genome revealed that TGEV RNA replication was restricted after (+)-catechin treatment. Intracellular reactive oxygen species (ROS) detection showed that (+)-catechin alleviated ROS conditions induced by TGEV infection. Our results showed that (+)-catechin exerts an inhibitory effect on TGEV proliferation in vitro and is involved its antioxidation.
Keywords: (+)-Catechin, Transmissible gastroenteritis coronavirus (TGEV), Antiviral, Antioxidation
Highlights
-
•
(+)-Catechin has anti-TGEV effect.
-
•
Anti-TGEV effect was working after TGEV infection.
-
•
(+)-Catechin could relieve CPE caused by TGEV in ST cells.
-
•
TGEV proliferation was decreased after (+)-catechin incubation.
-
•
Antioxidation of (+)-catechin is related to its antiviral effect.
1. Introduction
Transmissible gastroenteritis virus (TGEV) can infect enteric and respiratory tissues and cause transmissible gastroenteritis (TGE), which is characterized by vomiting, severe diarrhoea and dehydration. At present, TGE continues to be considered internationally as a highly contagious disease in swine, which results in a mortality rate close to 100% in newborn piglets (Cavanagh, 1996, Sola et al., 1998). A common vaccine likely cannot induce a local immune response in the small intestine and provide solid protection to piglets (Tuboly et al., 2000). Therefore, the development of effective anti-TGEV agents for controlling TGEV infection remains an important goal.
Green tea is derived from the dried leaves of Camellia sinensis and is one of the most commonly consumed beverages around the world (Ning et al., 2011, Pengbo et al., 2010). Approximately 70% of green tea extracts is catechins (monomeric flavonoids) (Sang et al., 2011). (+)-Catechin is one of the catechins in green tea. Many studies have shown that the catechins of green tea possess multiple pharmacological activities (Chang et al., 2003, Friedman, 2007, Song et al., 2005). Previous antiviral studies have indicated that catechins exert inhibitory effects on different types of viruses in different ways (Friedman, 2007). Typically, epigallocatechin gallate (EGCG), a subclass of catechins, can efficiently inhibit the entry of hepatitis B virus (HBV) into immortalized human primary hepatocytes by inducing clathrin-dependent endocytosis (Huang et al., 2014). The antiviral effect of EGCG on human immunodeficiency virus type 1 (HIV-1) has been shown for several steps in the HIV-1 life cycle, including a destructive effect on viral particles, post-adsorption entry and reverse transcription in acutely infected monocytoid cells (Yamaguchi et al., 2002). The antioxidant and anti-inflammatory activities of (+)-catechin have been previously observed in several studies (Bragança de Moraes et al., 2014). However, the impact of (+)-catechin on viral infections has not been previously investigated in detail.
The present study aimed to investigate the antiviral potential of (+)-catechin. We used a swine testicle (ST) cell line to assess the protective effects of (+)-catechin on TGEV infection in terms of viral replication and cell survival. In addition, the antiviral mechanism of (+)-catechin was preliminarily studied.
2. Materials and methods
2.1. Compounds
(+)-Catechin was purchased from Shanghai Winherb Medical Technology Co., Ltd. (China). It was dissolved in dimethyl sulfoxide (DMSO) and stored at − 20 °C. MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide), 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and dihydroethidium (DHE) were obtained from Beyotime Institute of Biotechnology (Jiangsu, China).
2.2. Cells and virus
Swine testicle (ST) cells were grown in minimal essential medium (MEM; GIBCO, UK) with 10% foetal calf serum (HyClone, China). The TGEV H16 strain was purchased from the National Control Institute of Veterinary Bioproducts and Pharmaceuticals (Beijing, China) and grown in ST cells.
2.3. MTT assay
The cytotoxicity and antiviral effects were tested using the MTT assay. Different concentrations of compounds were added to ST cell monolayers in 96-well culture plates with or without TGEV absorption. Untreated cells served as the control. All of the cells were maintained at 37 °C in 5% CO2. The supernatant was carefully removed from the plate without disturbing the attached cells, and 50 μL of MTT (2 μg/mL) was added to each well. After the plate was incubated at 37 °C for 4 h, the excess MTT was removed, and 200 μL of DMSO was added to each well. The 96-well culture plate was placed in an electronic oscillator for 10 min to dissolve the formazan crystals. The light absorbance of each well was measured at 490 nm in a microplate reader (Thermo).
2.4. Assays of antiviral activity
To investigate the effect of (+)-catechin on TGEV reproduction, three different assays were performed. First, TGEV and (+)-catechin were mixed and incubated at 4 °C for 1 h, and the mixture was then added to ST cell monolayers in culture plates at 37 °C in 5% CO2 for 1 h. After 1 h of adsorption, the unadsorbed virus and compounds were replaced with fresh medium. Second, (+)-catechin was added to ST cells 4 h before TGEV adsorption. The remaining steps were the same as those used in the first assay. Third, TGEV was adsorbed for 1 h at 37 °C in 5% CO2. The unadsorbed viruses were removed, and media containing different concentrations of (+)-catechin were added. Mock-treated cells and cells treated with only TGEV served as controls in all of the assays. The cell morphology was observed, and the antiviral effect was analysed after 48 h using the MTT assay. All of the tests were performed in triplicate.
2.5. Virus titration
The culture supernatants were collected for virus titration. The supernatants were serially diluted 10-fold from 10–1.0 to 10–11.0 and added to ST cell monolayers in 96-well culture plates. Each dilution was added to eight wells. The TCID50 was calculated by the Karber method after 48 h of infection.
2.6. RNA extraction and quantitative real-time PCR
ST cells were cultured in six-well culture plates. TGEV was added to the ST cells at 70–80% confluence in the plates, with the exception of the negative control. After 1 h of incubation at 37 °C in 5% CO2, the medium was removed, and fresh medium containing (+)-catechin was added. Fresh medium with the same concentration of EDTA was added to the positive-control and negative-control wells. TGEV-infected cells were collected 40 h after viral infection to evaluate the inhibitory effects of (+)-catechin on TGEV replication. RNA extraction, cDNA synthesis and quantitative real-time PCR were performed according to a previous report (He et al., 2012b). The Ct method was employed to analyse the data, and the amount of RNA in the samples was normalized to that of β-actin.
2.7. Detection of intracellular reactive oxygen species (ROS)
The intracellular ROS was measured using an oxidation-sensitive fluorescent probe (DCFH-DA). After TGEV infection and (+)-catechin treatment for 24 h, ST cells were washed with PBS and incubated with 10 μM DCFH-DA for 20 min at 37 °C. H2O2 served as a control. The extracellular DCFH-DA was washed three times with PBS. The fluorescence was observed and recorded using a fluorescence microscope (Nikon, Japan).
The intracellular superoxide anion was measured using a fluorescent dye DHE. TGEV infection and (+)-catechin treatment were the same as those used in the DCFH-DA assay. ST cells were washed with PBS and incubated with 2.5 μM DHE for 30 min at 37 °C. The fluorescence was measured using a luminescence plate reader (Perkin Elmer, America). The light absorbance of each well was measured at 610 nm.
3. Results
3.1. Measurement of cytotoxic (+)-catechin concentrations
Before (+)-catechin could be used for antiviral studies, a non-toxic dose was determined by adding different concentrations to ST cells. (+)-Catechin was serially diluted two-fold from 1280 μM to 10 μM. At (+)-catechin concentrations less than 320 μM, there was no difference in the cellular morphology and density between the (+)-catechin-treated and control cells. ST cells treated with 640 μM (+)-catechin presented evident morphological changes, including cell shrinkage, cell size reduction, turning round and shedding.
The (+)-catechin cytotoxic concentration was determined by the MTT assay. Consistent with the morphological observations, no significant difference was obtained at (+)-catechin concentrations less than 320 μM. However, the cell viability observed with 640 μM (+)-catechin was decreased compared with that of the cells treated with (+)-catechin at concentrations less than 320 μM (Fig. 1 ).
Fig. 1.
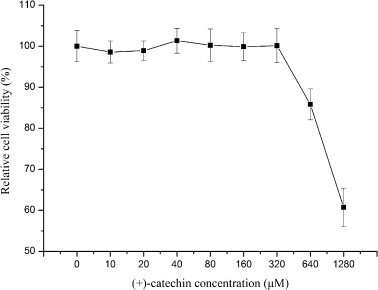
(+)-Catechin cytotoxicity. Different concentrations of (+)-catechin were added to ST cell monolayers in 96-well plates. The tested concentrations were between 20 and 1280 μM. The MTT assay was used to test the cell viability after 48 h of incubation at 37 °C. The relative cell viability was calculated by comparing the O.D.490 values of the (+)-catechin-treated cells with those of the non-treated control cells (set to 100%). The relative cell viability obtained after incubation with different concentrations of (+)-catechin is provided. The cell viability of the cells treated with (+)-catechin at concentrations less than 320 μM was not different from that of the non-treated control cells. The results represent the means ± SD.
According to the test results, we ascertained that a dose of (+)-catechin less than 320 μM was safe.
3.2. Antiviral effect of (+)-catechin
After the cytotoxicity test, we tested the antiviral effect of (+)-catechin. To determine which steps in the viral life cycle were affected by (+)-catechin, the antiviral effect was tested through three different approaches. (+)-Catechin was added before, during and after the incubation of ST cells with TGEV. As shown in Fig. 2A, no difference was found between the virus controls and the samples in which (+)-catechin was added to ST cells before and during TGEV inoculation. However, the cell viability was markedly increased by the addition of (+)-catechin after TGEV incubation. Approximately 93% of ST cells retained a normal viability after (+)-catechin treatment, but 60% of the untreated ST cells were damaged after TGEV infection.
Fig. 2.

(+)-Catechin cytoprotection. (A) The cell viability of TGEV-infected ST cells with different (+)-catechin treatments were measured by the MTT assay. Pretreatment: TGEV were treated with 80 μM (+)-catechin at 4 °C for 1 h prior to viral infection; Competition: ST cells were treated with 80 μM (+)-catechin at 37 °C for 4 h prior to TGEV infection; Post-infection treatment: 80 μM (+)-catechin was added after TGEV infection. The cell protection effect was determined by comparing the O.D.490 values of the (+)-catechin-treated cells with those of the non-treated control cells (set to 100%). (B) Different concentrations of (+)-catechin were added to ST cells after TGEV infection. Concentrations less than 320 μM were used. After 48 h of incubation, the cell viability was measured by the MTT assay. The relative cell viabilities were calculated by comparing the O.D.490 values of the (+)-catechin-treated TGEV-infected cells with those of the uninfected control cells (set to 100%). The results represent the means ± SD.
3.3. Dose-dependence of antiviral effect of (+)-catechin
Different concentrations of (+)-catechin were added to ST cells after TGEV infection. The cell viability was tested by the MTT assay. As shown in Fig. 2B, 10 μM (+)-catechin exerted an obviously protective effect. At drug concentrations in the range of 20 to 40 μM, the cell survival rate reached almost 90%, whereas at drug concentrations greater than 80 μM, the cell survival rate reached almost 95%. However, the protective effect could not be increased with a further increase in concentration.
3.4. Cytopathic effect of (+)-catechin in TGEV-infected ST cells
TGEV exerts a cytopathic effect (CPE) on ST cells (Fig. 3A). This CPE was observed by the addition of 20 μM, 40 μM or 80 μM (+)-catechin after TGEV infection. In agreement with the cytopathic observations, (+)-catechin distinctly delayed and alleviated the CPE. ST cells remained attached to the wall and presented a paving stone sample (Fig. 3C, E and G). In particular, at a concentration of (+)-catechin of 80 μM, the morphology of the (+)-catechin-treated ST cells was almost the same as that of the negative control 48 h after viral inoculation.
Fig. 3.

(+)-Catechin inhibits the cytopathic effect of TGEV (200 ×). (+)-Catechin was added after TGEV absorption for 1 h at 37 °C. Photographic images were collected after 48 h of incubation. (A) TGEV-infected ST cells; (B) ST cells; (C) ST cells treated with 20 μM (+)-catechin after TGEV infection; (D) ST cells treated with 20 μM (+)-catechin; (E) ST cells treated with 40 μM (+)-catechin after TGEV infection; (F) ST cells treated with 40 μM (+)-catechin; (G) ST cells treated with 80 μM (+)-catechin after TGEV infection; and (H) ST cells treated with 80 μM (+)-catechin.
3.5. Inhibition of TGEV replication by (+)-catechin
3.5.1. Effect of (+)-catechin on viral yield in TGEV-infected ST cells
A TCID50 assay was used to confirm the anti-TGEV activity of (+)-catechin by measuring the released virus in the medium. (+)-Catechin was added to ST cells after TGEV infection, and the supernatants after 48 h of incubation were collected. As shown in Fig. 4A, the TCID50 of the control was approximately 106.0, whereas the TCID50s of the groups treated with 20 μM, 40 μM and 80 μM (+)-catechin were 104.5, 104.3 and 103.9, respectively. The TGEV viral burden in the supernatants treated with 80 μM (+)-catechin was reduced by approximately 237 fold.
Fig. 4.

(+)-Catechin inhibits TGEV proliferation. (A) Less viral production was observed in (+)-catechin-treated cells infected with TGEV. The cells were treated with 20 μM, 40 μM or 80 μM (+)-catechin after TGEV infection. Cell culture supernatants were collected after 48 h of incubation at 37 °C, and the viral titre was determined by TCID50. (B) The effect of (+)-catechin on TGEV RNA synthesis was assessed. Cells were treated with 20 μM, 40 μM or 80 μM (+)-catechin after TGEV infection, and cell monolayers were collected by TRIzol after 40 h of incubation. The expression levels of TGEV RNA were measured by quantitative RT-PCR. The (+)-catechin-treated cells presented significant decreases in the TGEV RNA load. The data represent the means ± SD.
3.5.2. Effect of (+)-catechin on viral RNA synthesis
To test the inhibitory effect of (+)-catechin on viral RNA synthesis, real-time quantitative RT-PCR was performed. As shown in Fig. 4B, TGEV RNA synthesis was greatly inhibited by (+)-catechin. The addition of 20 μM (+)-catechin decreased the TGEV RNA levels by more than 3 fold compared with the positive control. The TGEV RNA synthesis was reduced by almost 10 fold by the addition of 80 μM (+)-catechin.
3.6. Alleviation of TGEV infection-induced intracellular ROS by (+)-catechin
TGEV infection can cause a ROS explosion (Fig. 5E), and the ROS levels were approximately the same as the levels observed in cells treated with 100 μM H2O2 for 24 h (Fig. 5A). ROS induction by TGEV infection or H2O2 treatment was greatly down regulated after (+)-catechin treatment (Fig. 5B–D and F–H). The intracellular ROS levels decreased after treatment with 20 μM (+)-catechin. However, the ROS levels were almost completely suppressed with 80 μM (+)-catechin in both the infected cells and H2O2-treated cells. No ROS was detected in the non-infected and non-H2O2-treated cells (Fig. 5I–L).
Fig. 5.

(+)-Catechin alleviates intracellular ROS (100 ×). The intracellular ROS levels were measured by the fluorescent probe DCFH-DA. After 100 TCID50 TGEV infection and (+)-catechin treatment for 24 h, the fluorescence was observed and recorded using a fluorescence microscope. H2O2 was used as a positive control. (A) ST cells treated with 100 μM H2O2; (B) ST cells treated with 20 μM (+)-catechin after 100 μM H2O2 stimulation; (C) ST cells treated with 40 μM (+)-catechin after 100 μM H2O2 stimulation; (D)ST cells treated with 80 μM (+)-catechin after 100 μM H2O2 stimulation; (E) TGEV-infected ST cells; (F) ST cells treated with 20 μM (+)-catechin after TGEV infection; (G) ST cells treated with 40 μM (+)-catechin after TGEV infection; (H) ST cells treated with 80 μM (+)-catechin after TGEV infection; (I) ST cells; (J) ST cells treated with 20 μM (+)-catechin; (K) ST cells treated with 40 μM (+)-catechin; and (L) ST cells treated with 80 μM (+)-catechin.
The intracellular superoxide anion induced by TGEV infection was measured using DHE. As shown in Fig. 6 , TGEV infection can cause an obvious up-regulation of intracellular superoxide anion. As expected, superoxide anion induction by TGEV infection was down regulated after (+)-catechin treatment, especially with 80 μM (+)-catechin. No superoxide anion was induced in the non-infected cells.
Fig. 6.
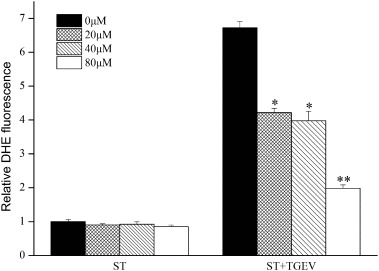
(+)-Catechin alleviates intracellular superoxide anion. The intracellular superoxide anion levels were measured by the fluorescent probe DHE. After 100 TCID50 TGEV infection and (+)-catechin treatment for 24 h, the fluorescence was measured using a luminescence plate reader. The relative DHE fluorescence was calculated by comparing the O.D.610 values of each treated cells with the non-treated control cells (set to 1). The results represent the means ± SD.
4. Discussion
Catechins, a main ingredient of green tea extracts, have multiple bioactivities. The health care and therapeutic effects of catechins have received great attention, and previous studies have proven that these effects are beneficial (Kim et al., 2014, Schramm, 2013). TGEV is one of the major pathogens that causes severe diarrhoea in newborn piglets (Zhang et al., 2013). This study was designed to explore the antiviral effect of catechins in TGEV infection.
Viral infection is a multi-step process involving a number of different cell and viral factors. All viruses rely on host cell proteins and their associated mechanisms to complete the viral life cycle. Viral proliferation can be inhibited regardless of which step in the viral life cycle is been blocked (Fénéant et al., 2015, Ishida et al., 2014). Many studied have proven that catechins can block viral infectivity by inhibiting viral adsorption and entry (Huang et al., 2014, Nakayama et al., 1993, Yamaguchi et al., 2002). However, our results showed that (+)-catechin exerts no inhibitory effect on TGEV infection in TGEV or ST cells pretreated with (+)-catechin, suggesting that (+)-catechin has no effect on viral particle integrity and the entry process.
Interestingly, TGEV proliferation can be suppressed by the addition of (+)-catechin after TGEV infection. As shown by our results, the viral RNA loading in infected cells and the viral titre in the supernatant were markedly decreased after (+)-catechin incubation. This result indicates that (+)-catechin may inhibit TGEV replication and/or viral assembly and release, which is consistent with the results obtained in other antiviral studies of green tea catechins. EGCG can down regulate HBV DNA loading in HepG2.2.2.15 cells and the levels of HBsAg and HBeAg secreted from infected cells into the supernatant (Pang et al., 2014). Epstein–Barr virus spontaneous lytic infection can be restricted at the DNA, gene transcription and protein levels by suppressing the activation of the MEK/ERK1/2 and PI3-K/AKT signalling pathways (Liu et al., 2013). Hepatitis C virus (HCV) can also be inhibited by EGCG at the RNA replication steps in the HCV life cycle (Chen et al., 2012). In our study, (+)-catechin showed the same inhibitory effect on viral replication as other green tea catechins.
Oxidative stress is an important pathogenic mechanism that has been implicated in many viral infections, including HCV (Okuda et al., 2002), human immunodeficiency virus (HIV) (Mollace et al., 2001) and classical swine fever virus (CSFV) (He et al., 2012a). TGEV induces a CPE in ST cells, and this effect is associated with TGEV-induced apoptosis (Eleouet et al., 1998). Furthermore, the ROS response is up regulated in TGEV-infected cells, and ROS accumulation causes oxidative stress, which plays an important role in the TGEV-induced apoptosis pathway (Ding et al., 2013). Antiviral studies have shown that EGCG decreases the ROS levels in MDCK cells infected by influenza A virus and inhibits influenza A replication (Ling et al., 2012). In addition, the replication of enterovirus 71 is inhibited by EGCG, which is consistent with the down regulation of ROS generation in infected Vero cells (Ho et al., 2009). As a natural antioxidant, (+)-catechin can protect human hepatoma cells (HepG2) from oxidative DNA damage induced by heterocyclic amines (Haza and Morales, 2011). Additionally, the apoptosis induced by oxidative stress in dermal fibroblasts can be protected by (+)-catechin (Tanigawa et al., 2014). As shown by our results, (+)-catechin can protect ST cells from TGEV-induced CPE and greatly alleviated the ROS induced by TGEV. In conjunction with the improved intracellular ROS conditions, TGEV RNA replication and the viral titre in the supernatant were clearly down regulated. Hence, the antioxidative activity of (+)-catechin is a key factor in cytoprotection and antiviral activity.
In summary, we demonstrated that (+)-catechin can inhibit TGEV replication in ST cells and that this antiviral activity is related to its antioxidation. This study provides novel findings concerning the antiviral effect of (+)-catechin.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the planning innovation project of S&T in Shaanxi Province (2014KTCQ02-02).
References
- Bragança de Moraes C.M., Bitencourt S., de Mesquita F.C., Mello D., de Oliveira L.P., da Silva G.V. (+)-Catechin attenuates activation of hepatic stellate cells. Cell Biol. Int. 2014;38:526–530. doi: 10.1002/cbin.10228. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising coronaviridae and arteriviridae. Arch. Virol. 1996;142:629–633. [PubMed] [Google Scholar]
- Chang L.K., Wei T.T., Chiu Y.F., Tung C.P., Chuang J.Y., Hung S.K. Inhibition of Epstein–Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003;301:1062–1068. doi: 10.1016/s0006-291x(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Chen C., Qiu H., Gong J., Liu Q., Xiao H., Chen X.W. (−)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 2012;157:1301–1312. doi: 10.1007/s00705-012-1304-0. [DOI] [PubMed] [Google Scholar]
- Ding L., Zhao X., Huang Y., Du Q., Dong F., Zhang H. Regulation of ROS in transmissible gastroenteritis virus-activated apoptotic signaling. Biochem. Biophys. Res. Commun. 2013;442:33–37. doi: 10.1016/j.bbrc.2013.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleouet J.F., Chilmonczyk S., Besnardeau L., Laude H. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J. Virol. 1998;72:4918–4924. doi: 10.1128/jvi.72.6.4918-4924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénéant L., Potel J., François C., Sané F., Douam F., Belouzard S. New insights into the understanding of hepatitis C virus entry and cell-to-cell transmission by using the ionophore Monensin A. J. Virol. 2015;89:8346–8364. doi: 10.1128/JVI.00192-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haza A.I., Morales P. Effects of (+) catechin and (−) epicatechin on heterocyclic amines-induced oxidative DNA damage. J. Appl. Toxicol. 2011;31:53–62. doi: 10.1002/jat.1559. [DOI] [PubMed] [Google Scholar]
- He L., Zhang Y., Lin Z., Li W., Wang J., Li H.L. Classical swine fever virus NS5A protein localizes to endoplasmic reticulum and induces oxidative stress in vascular endothelial cells. Virus Genes. 2012;45:274–282. doi: 10.1007/s11262-012-0773-2. [DOI] [PubMed] [Google Scholar]
- He L., Zhang Y., Dong L., Cheng M., Wang J., Tang Q. In vitro inhibition of transmissible gastroenteritis coronavirus replication in swine testicular cells by short hairpin RNAs targeting the ORF 7 gene. Virol. J. 2012;9:176–184. doi: 10.1186/1743-422X-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.Y., Cheng M.L., Weng S.F., Leu Y.L., Chiu D.T.Y. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- Huang H.C., Tao M.H., Hung T.M., Chen J.C., Lin Z.J., Huang C. (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antiviral Res. 2014;111:100–111. doi: 10.1016/j.antiviral.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Takeshita M., Kataoka H. Functional foods effective for hepatitis C: identification of oligomeric proanthocyanidin and its action mechanism. World J. Hepatol. 2014;6:870–879. doi: 10.4254/wjh.v6.i12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Quon M.J., Kim J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J., Wei F., Li N., Li J., Chen L., Liu Y. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol. Sin. 2012;33:1533–1541. doi: 10.1038/aps.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Li H., Chen L., Yang L., Li L., Tao Y. (−)-Epigallocatechin-3-gallate inhibition of Epstein–Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis. 2013;34:627–637. doi: 10.1093/carcin/bgs364. [DOI] [PubMed] [Google Scholar]
- Mollace V., Nottet H.S., Clayette P., Turco M.C., Muscoli C., Salvemini D. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Nakayama M., Suzuki K., Toda M., Okubo S., Hara Y., Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- Ning P., Gong C., Zhang Y., Guo K., Bai J. Lead, cadmium, arsenic, mercury and copper levels in Chinese Yunnan Pu'er tea. Food Addit. Contam. 2011;4:28–33. doi: 10.1080/19393210.2011.551945. [DOI] [PubMed] [Google Scholar]
- Okuda M., Li K., Beard M.R., Showalter L.A., Scholle F., Lemon S.M. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- Pang J., Zhao K., Wang J., Ma Z., Xiao X. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J. Zhejiang Univ. Sci. B. 2014;15:533–539. doi: 10.1631/jzus.B1300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengbo N., Chunmei G., Zhang Y., Kangkang G. La, Ce, Pr, Nd and Sm concentrations in Pu'er tea of Yunnan, China. J. Rare Earths. 2010;28:636–640. [Google Scholar]
- Sang S., Lambert J.D., Ho C.T., Yang C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Schramm L. Going green: the role of the green tea component EGCG in chemoprevention. Journal of Carcinogenesis & Mutagenesis. 2013;4:1000142. doi: 10.4172/2157-2518.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola I., Castilla J., Pintado B., Sánchez-Morgado J.M., Whitelaw C.B.A., Clark A.J. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J. Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.M., Lee K.H., Seong B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Tanigawa T., Kanazawa S., Ichibori R., Fujiwara T., Magome T., Shingaki K. (+)-Catechin protects dermal fibroblasts against oxidative stress-induced apoptosis. BMC Complement. Altern. Med. 2014;14:133. doi: 10.1186/1472-6882-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuboly T., Yu W., Bailey A., Degrandis S., Du S., Erickson L. Immunogenicity of porcine transmissible gastroenteritis virus spike protein expressed in plants. Vaccine. 2000;18:2023–2028. doi: 10.1016/s0264-410x(99)00525-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Honda M., Ikigai H., Hara Y., Shimamura T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002;53:19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Hu R., Tang X., Wu C., He Q., Zhao Z. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch. Virol. 2013;158:1631–1636. doi: 10.1007/s00705-013-1659-x. [DOI] [PubMed] [Google Scholar]


