Abstract
Porcine epidemic diarrhoea virus (PEDV), belongs to the genus Alphacoronavirus in the family Coronaviridae and causes severe diarrhoea, vomiting, dehydration and high mortality in seronegative newborn piglets. Thus, a precise and rapid diagnosis of PEDV infection is important for the application of control measures to limit viral dissemination. In this investigation, a monoclonal antibodies (MAbs)-based competitive enzyme-linked immunosorbent assay (ELISA) for detecting antibodies against PEDV was developed and validated. The diagnostic performance of the test was evaluated by receiver operating characteristic (ROC) analysis using a panel of 829 known sera collected from different commercial pig farms, with or without a history of PED presence and from experimentally infected pigs. The competitive ELISA showed excellent diagnostic performance and discriminatory power with high sensitivity (Se) and specificity (Sp) values (Se = 96.5%, 95% IC 94.1–98.1; Sp = 98.2%, 95% IC 96.3–99.3). Moreover, this competitive ELISA method has other properties, such as high feasibility of testing sera without pre-treatment and automatic and instrument-mediated revealing, that makes it appropriate for large-scale screenings of affected pig farms in endemic regions or for monitoring plans in PEDV-free areas.
Keywords: PEDV, Competitive ELISA, Monoclonal antibodies
Highlights
-
•
A monoclonal antibodies (MAbs)-based competitive ELISA for detecting antibodies against PEDV was developed and validated.
-
•
The diagnostic performance of the test was evaluated by ROC analysis using a panel of 829 known sera.
-
•
The ELISA showed excellent diagnostic performance and discriminatory power.
1. Introduction
Porcine epidemic diarrhoea (PED) is highly contagious enteric viral disease of swine, characterized by diarrhoea, vomiting, and dehydration, followed by high mortality in new born piglets (50–100%). The aetiological agent called Porcine Epidemic Diarrhoea Virus (PEDV) belongs to the genus Alphacoronavirus in the family Coronaviridae of the order Nidovirales (Jung and Saif, 2015). PEDV may infect animals of all ages but older pigs usually show a milder form of the disease with lower mortality rates (Alvarez et al., 2015). PEDV has been firstly detected in the United Kingdom in 1978, and since then has spread worldwide causing significant economic losses in all main swine producing areas, including Asia and North America, (Horie et al., 2016; Lee, 2015; Song and Park, 2012; Sun et al., 2015; Vlasova et al., 2014). In Europe, PEDV spread to most countries between the 1970s and 1990s (Saif et al., 2012); then outbreaks became infrequent (Song and Park, 2012) but the virus persisted in an endemic form in the pig population at a low rate, causing sporadic cases in weaner or feeder pigs in few European countries. In Italy, PEDV re-emerged in a typical epidemic form in 2005 to 2006 (Martelli et al., 2008), but the disease progressively disappeared once more. Recently, following the 2013 epidemic in the US, single, limited or multiple PEDV outbreaks have been diagnosed in several European countries (Dastjerdi et al., 2015; EFSA, 2016; Grasland et al., 2015; Hanke et al., 2015; Hanke et al., 2017; Mesonero-Escuredo et al., 2018; Mesquita et al., 2015; Stadler et al., 2015; Steinrigl et al., 2015; Theuns et al., 2015; Toplak et al., 2016; Van der Wolf et al., 2015), including Italy (Alborali et al., 2014; Boniotti et al., 2016). The disease spread from 2015 onward, causing hundreds of cases, mainly in high-density pig production areas in the Po valley (North Italy), affecting fattening units as well as farrow-to-finish or farrow-to-weaner farms (Boniotti et al., 2018), and characterized by high variability of size and clinical disease. Based on their genome, isolates from these cases were closely related to each other (Boniotti et al., 2016). Following sequencing, they were classified as S INDEL strain, being the German isolate (Stadler et al., 2015) 99.4% identical to the OH851 strain isolated in the US in January 2014 (Wang et al., 2014). The circulating strains responsible for the outbreaks in 2014 in Italy were genetically analysed and he comparison of partial sequences of the RdRp and membrane (M) genes and the total glycoprotein spike (S1) gene showed a high nt identity with the S-INDEL strain USA/OH851/2014 from the USA (98.7%, 99.8%, 99.3–99.5%, respectively) and with the strains detected in Germany (100%, 100%, 99.7%).This situation was totally different from what detected from 2007 to 2012 when the PEDV strains circulating in Italy showed a high genetic variability. In particular, a novel coronavirus generated by recombination between PEDV and TGEV, called Swine Enteric Coronavirus (SeCoV), was identified (EFSA, 2016; Boniotti et al., 2016). Moreover, in May 2016 a PEDV/SeCoV recombinant strain was detected In Italy; this new strain rapidly spread since January 2017 and then overcome the S-INDEL OH851-like strain, representing nowadays over 90% of the circulating strains (Papetti et al., 2017).
Although only a single serotype of PEDV has been described, phylogenetic studies of the S gene have indicated that PEDV can be genetically divided into 2 groups: genogroup 1 (G1; classical) and genogroup 2 (G2; field epidemic or pandemic) (Huang et al., 2013; Lee, 2015). Each genogroup can be further separated into subgroups 1a and 1b, and 2a and 2b, respectively. G1a comprises the prototype PEDV strain, CV777, vaccine strains (e.g., 83P-5 cited by Colin et al., 2015) and other cell culture-adapted strains, whereas G1b includes new variants detected in China, United States and South Korea, and more recently in Europe. G2a and G2b contain global field isolates that were responsible for earlier local epidemics in Asia and recent pandemic outbreaks in North America and Asia, respectively. The US G1b variants were called S INDEL strains because of the presence of insertions and a deletion in the N-terminal domain (NTD) of S1 gene compared to sequences of original US G2b PEDV strains (Huang et al., 2013; Wang et al., 2014; Lee, 2015).
In Europe, the recent re-occurrence of PEDV (closely related to the INDEL OH 851 strain) infections led to necessarily asses the capacity of the existing diagnostic assays to correctly diagnose PED outbreaks.
From acutely infected pigs, PEDV can be identified by reverse transcription polymerase chain reaction (RT-PCR) (Kim et al., 2001; Chen et al., 2014) in faecal or intestinal samples. Low levels of viraemia have been also observed in serum (Jung and Saif, 2015; Lohse et al., 2017). The virus is present and excreted by infected animals for a limited period, typically less than one month (Lee, 2015), while the serological response is expected to last for a much longer period (Crawford et al., 2015). Therefore, it is important that diagnostic laboratories can efficiently and quickly detect PEDV infection when outbreaks of disease occur. Commercial test for the detection of antibodies to PEDV are available but different European laboratories have developed a variety of “in–house” assays.
The main purpose of this study was to describe the development of an in-house competitive enzyme-linked immunosorbent assay (ELISA) based on monoclonal antibodies (MAbs) for the detection of antibodies against PEDV in individual pig sera and to estimate the diagnostic performance of this assay.
2. Materials and methods
2.1. In-house competitive ELISA
The competitive ELISA validated in our study is an immunoassay using the whole virus, grown in cell culture, as a source of antigens. This assay is based on a double antibody sandwich, using a purified MAb as catcher and a horseradish peroxidase (HRP)-conjugated MAb as a tracer. The MAbs employed for the set-up of the competitive ELISA were produced against the CV-777 PEDV strain as described in a previous study (Sozzi et al., 2010). Their characterization (MAbs screening and selection) is described in Supplemental file n. 1.
The competitive ELISA is described as follows: the ELISA microplates were coated with the purified MAb 1F12 at a concentration of 10 μg/mL in a volume of 50 μL/well and incubated overnight at 5 ± 3 °C in ELISA coating buffer (carbonate bicarbonate buffer pH 9.2 ± 0.2). The serum sample was diluted 1:4 and 1:8 on an auxiliary microplate by adding 25 μL in 25 μL of a sample dilution buffer (phosphate-buffered saline [PBS], pH 7.2 ± 0.2), containing Tween 20 [0.05%] (MP Biochemicals LLC, Illkirch, France). Moreover, in order to reduce nonspecific binding, yeast extract [1%] (Biolife Italiana Srl, Milan, Italy) and mouse serum [1%] (IZSLER, Brescia, Italy) were added to the PBS for saturating sites that were still free after adsorption. Internal controls were included in each plate: the positive and negative control sera and the 100% control wells, and all were examined as double repetitions. Control (negative and positive) sera were collected respectively from specific-pathogen-free (SPF) pigs and from animals experimentally infected with a PEDV INDEL-strain identified in Italy on 2015 (strain 10,674/2015). A volume of 50 μL of antigen (whole VERO cell culture-adapted G1a PEDV strain CV777, inactivated with β-propriolactone) optimally diluted (1:3) in the dilution buffer (phosphate-buffered saline [PBS], pH 7.2 ± 0.2, containing Tween 20 [0.05%], yeast extract [1%]) was added to all the samples and controls, in which 50 μL of the dilution buffer was added. At the end of a 60 ± 5 min incubation at 37 ± 2 °C, 50 μL of each sample or control-antigen mixture were transferred onto the previously coated microplate. A volume of 25 μL/well of the HRP-conjugated 4C3 MAb (10 mg/mL, 1:1000 in dilution buffer) was added, and the plates were incubated again by applying the same described conditions. Then, after a cycle of 3 washings with PBS (pH 7.2 ± 0.2, containing Tween 20 [0.05%; washing solution]), 50 μL/well of ortho-phenylenediamine (Sigma-Aldrich, St. Louis, MO) substrate was added. Finally, the reaction was blocked after 10 ± 2 min by adding 50 μL/well of 1 M sulfuric acid (Carlo Erba Reagents Srl, Cornaredo, Milan, Italy). The optical density (OD) of the samples was read at 492 nm, using a microplate spectrophotometer (Multiskan EX, Thermo Fisher Scientific, Waltham, MA). Laboratory grade reagents and filtered water (Milli-Q Academic, EMD Millipore Corp., Billerica, MA) were employed. Furthermore, all instruments were calibrated and maintained within the Quality Assurance System ISO/IEC 17025:2018.
The results were interpreted using the following formula:
Fulfilling of the following criteria, which correspond to those normally applied in previous studies conducted in our laboratory was needed for considering a run valid: mean OD of 100% control wells = 1.5 ± 0.5 and difference between mean OD of negative and positive controls ≥ 0.8.
2.2. Panel of sera employed for the validation of the method
A panel of 829 samples was employed, including 415 negative sera and 414 PEDV-positive sera. They were divided into the following detailed categories:
-
359 field samples without PEDV exposure. Collection of serum samples was performed in 12 selected Italian farms during 2015. Farms were classified as non-exposed to PEDV based on historical data and lack of enteric signs compatible with viral diarrhoea. The observation period in each farm lasted at least four months, during which multiple faecal samples were taken and tested negative for PEDV RNA based on quantitative real-time PCR (qPCR) described by Bertasio et al., 2016. Sera were taken from nursery and grow-finishing pigs;
-
30 negative sera originating from SPF pigs;
-
373 field samples with PEDV exposure. Collection of serum samples was performed in 17 Italian commercial pig farms between 2014 and 2015 after the PEDV outbreaks. The PEDV infection status was first determined by the presence of enteric signs and then confirmed based on identification of both PEDV RNA by qPCR in faecal samples and PEDV antigen by a double antibody sandwich ELISA (Sozzi et al., 2010). PEDV qRT-PCR assay were performed using primers and probes targeting the S1 gene of PEDV as previously described (Bertasio et al., 2016). Positive samples were collected during a period from 2 to 3 weeks to 3 to 4 months after PCR positivity. In these outbreaks G1b PEDV strains with high similarity to the US OH851 “mild INDEL strains” were identified by sequencing analysis of the S1 gene from positive faecal samples (Boniotti et al., 2016).
-
67 collection sera from eight three-weeks old piglets obtained during two previous experimental infections. Five piglets have orally been infected with an US PEDV INDEL strain and sera sampled at 0-7-14-21 and 28 days post infection (dpi). The remaining three piglets have been infected with an Italian PEDV S-INDEL strain identified on January 2015 and sera collected at 0-1-2-4-5-6-7-8-15-18-24-31-41 and 52 dpi. According to the results obtained by using the cELISA test, i.e. positivity was detected starting from 5 to 7 dpi (Supplemental file n.2), 26 sera were considered negative and 41 positive.
To evaluate the Sp of the method, known positive sera for other porcine coronaviruses were tested:
-
24 experimental samples with known TGEV exposure, obtained from three 5-week-old pigs orally inoculated with TGEV strain Purdue, from which sera were collected at 0, 7, 10, 17, 24, 31, 40 and 60 dpi.
-
11 experimental samples with known HEV exposure, obtained from hyperimmunised animals: six rabbits, one guinea pig and four pigs inoculated with HEV 741 VR ATCC, strain 67 N (Mengeling et al., 1972).
2.3. Statistical analysis
A receiver operating characteristic (ROC) curve is a graphical representation of the relative effects of the false negative and false positive rates for every possible cut-off, and it is an effective method of evaluating the quality or performance of diagnostic tests.Therefore, the discriminating power of the competitive ELISA for anti-PEDV antibody detection was evaluated by a ROC curves analysis (Zhou et al., 2002) using MedCalc Statistical Software version 13.1.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). The area under the curve (AUC) is a global statistic summary of diagnostic accuracy and can distinguish between non-informative (AUC = 0.5), less accurate (0.5 < AUC ≤ 0.7), moderately accurate (0.7 < AUC ≤ 0.9), highly accurate (0.9 < AUC < 1) and perfect tests (AUC = 1) (Swets, 1988). The accuracy of a diagnostic test is displayed in an interactive dot diagram where data of the negative and positive groups are displayed as dots on two vertical axes.
3. Results
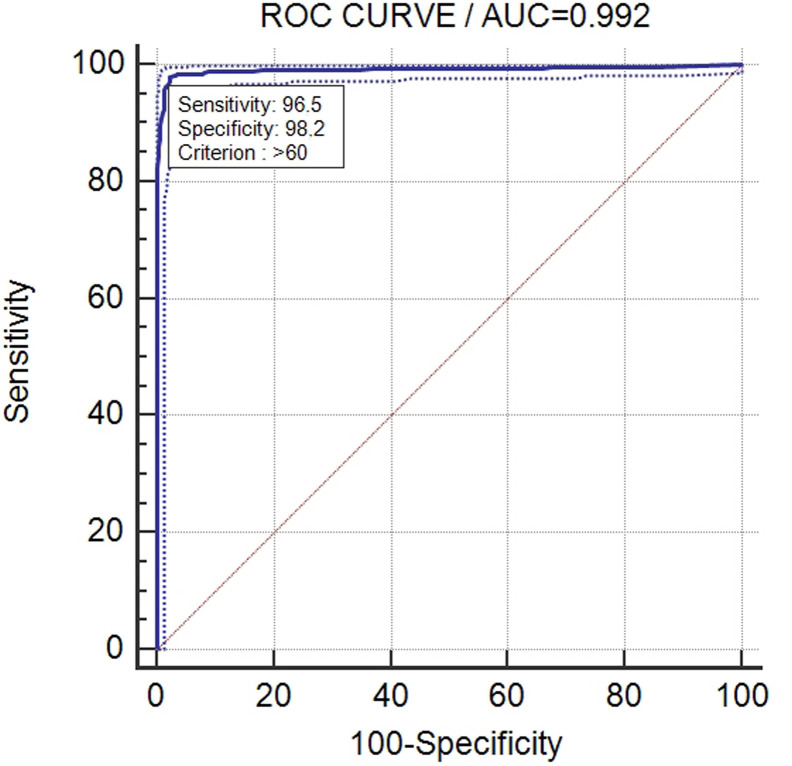
The diagnostic performance of the competitive ELISA for anti-PEDV antibody detection was evaluated by testing 829 swine sera, which were classified as positive or negative according to their known origin. Only when all the validation criteria were fulfilled. The results of ELISA test were considered valid and used for further analysis. Thus, the ROC curves were calculated based on previous classification of the sera into positive and negative groups. The ROC curves permitted both to select the optimal cut-off values and to estimate the diagnostic sensitivities and specificities. The shape and the relevant AUC values demonstrated the highly accuracy (AUC = 0.992 at the 1/4 dilution, 95% IC 0.983–0.997) of the competitive ELISA for anti-PEDV antibody detection, that has nearly 100% sensitivity (Se) and specificity (Sp). By using the first serum dilution, the cut-off value representing the optimal balance of Se and Sp (Se: 97.8–95% CI 95.8–99.1; Sp: 97.69–95% CI 95.7–98.9) was 56% (percentage of inhibition). Considering the test performances, a cut-off value easily applicable in routine activity corresponding to 60% (Se: 96.5–95% CI 94.1–98.1; Sp: 98.2–95% CI 96.3–99.3), that still allows working with high levels of Se and Sp, was selected (Fig. 1 ). The 1/4 serum dilution was selected as the screening one since it provided the best diagnostic Se and Sp values, as well as the highest discrimination window between positive and negative sera. In addition, 1/8 or even more serial dilutions could be used to estimate the antibody level (i.e., the serum titre could be expressed as the dilution closer to the cut-off value).
Fig. 1.
Receiver operating characteristic (ROC) curve based on result for a panel composed of 829 swine sera (415 negative and 414 positive), employed to set the cut-off values for the competitive enzyme-linked immunosorbent assay for serologic detection of PEDV. AUC = area under the curve.
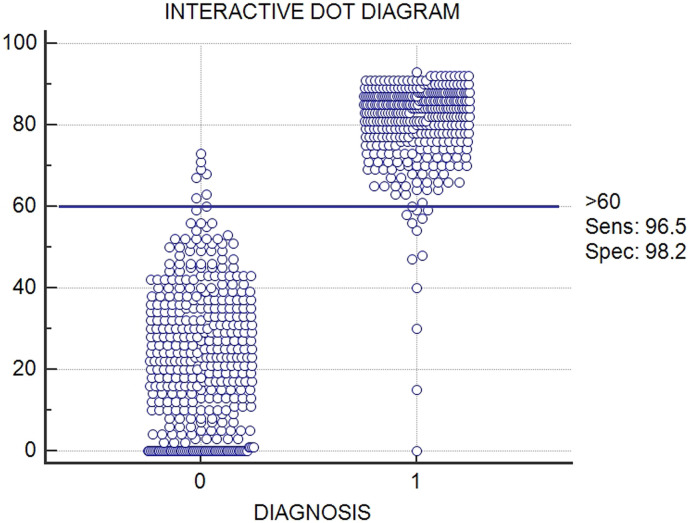
The interactive dot diagram (Fig. 2 ) displayed the accuracy of a diagnostic test. The horizontal line placed in the selected cut-off (60%) showed a very good discriminating power of the test with a best separation (minimal false negative and false positive results) between the two groups (positive-1 and negative-0).
Fig. 2.
Interactive dot diagram (MedCalc Stadistical Software). In the graph, the data of the positive and negative samples are displayed as dots on two vertical axes (0 = negative samples, 1 = positive samples). A horizontal line indicates the cut-off point with the best separation (minimal false negative and false positive results) between the two groups. The corresponding test characteristics i.e. sensitivity and specificity are shown at the right side of the graph.
Both sets of 24 and 11 experimental samples, respectively positive for TGEV and HEV, yielded negative results in the competitive PEDV ELISA. Thus, heterologous positive sera were clearly differentiated from the PEDV-positive sera, suggesting that a satisfactory analytical Sp was achieved.
4. Discussion
Therefore, it is important that diagnostic laboratories can efficiently and quickly detect PEDV infection when outbreaks of disease occur. A precise and rapid diagnosis of PEDV infection is important for the application of effective measures control viral dissemination and severity of the disease outbreaks, and thus reliable virological and serological diagnostic assays are needed. Rapid identification of PEDV and its differentiation from other enteric swine pathological agents are achieved by using virological assays, whereas serological tests provide useful information regarding past exposure to PEDV, prevalence of infection and epidemiology of the disease. The determination of PEDV-specific antibodies may also be used to evaluate sow immunity and/or presence of immunoglobulin in the colostrum, which might help to predict the level of specific protection in piglets.
In this investigation, a MAbs-based competitive ELISA for the detection of PEDV antibodies was developed in house and validated. Ideally, the diagnostic Sp and Se of a such test should be calculated using clearly true negative and true positive samples as defined by a combination of “gold standard” methods. In the present study, a ROC curve approach, considering different groups of pig sera obtained either from farms classified as PED-negative or -positive based on their history, presence of clinical signs and results of virological tests (Real-Time RT-PCR and antigenic ELISA), or from experimentally infected pigs, in which antibodies could be detected starting from 5 to 7 days post infection, was used to estimate the assay accuracy for the PEDV competitive ELISA. In particular, the diagnostic Se and Sp obtained by this validation study confirmed those obtained in a previous study (Sozzi et al. 2014), in which this competitive ELISA was evaluated by comparing it with the immunoperoxidase monolayer assay (IPMA). A total of 296 field serum samples taken from pigs in PEDV-exposed or unexposed Italian farms were used. Field samples were collected from finishing pig farms in Lombardy and Emilia Romagna 4 to 8 weeks after the beginning of the last wave of PED in Italy from 2005 to 2006. In those outbreaks, sequencing analysis of the S1 gene from Real-Time RT-PCR-positive faecal samples identified a G1a PEDV strain. The results obtained by the PEDV competitive ELISA presented a high correlation with those obtained by the IPMA test (i.e., a kappa score = 0.97), which indicates an almost full concordance between the two methods. According to the results obtained, to the cELISA for anti-PEDV antibody detection it could be attributed nearly 100% sensitivity (Se). However, we selected that cut-off value (60%) both easily applicable in routine activity and representing the optimal balance of Se (96.5) and Sp (97.69). The sensitivity of the test is therefore very high also in the case of infection due to the more recent PEDV strains belonging to S-INDEL group and it is likely not affected by genetic differences among circulating strains as already observed by Gerber et al. (2016) who sustained the capacity of the cELISA to correctly identified pigs infected with G1a, G1b and G2b PEDV.
To date, only a single serotype of PEDV has been recognised. Since the results reported here were obtained by testing sera taken after PED outbreaks caused by viruses belonging to different genogroups, we can conclude that this serological assay is able to detect anti-PEDV antibodies against various strains, including both the old European strains and the contemporary S INDEL strains, that have infected pig herds in several European countries, including Italy, in the last three years.
Additionally, the properties of this PEDV competitive ELISA and its use in field conditions have been analysed. In fact, it has been employed in parallel with a variety of “in-house” and commercial assays set up for the detection of anti-PEDV antibodies in two interlaboratory studies (Gerber et al., 2016; Strandbygaard et al., 2016) to test shared panels of pig sera collected both in the field and during experimental studies in some European and North American countries. As described in these two studies, our “in-house” competitive ELISA “performed very well and exhibited both high Se and Sp” (Se: 97–95% CI 90.0–100.0 Sp: 94–95% CI 83.0–100.0) (Strandbygaard et al., 2016) and “had the overall highest detection” levels (Gerber et al., 2016).
5. Conclusions
The PEDV competitive ELISA method here described could be considered a useful test for routine detection of PEDV antibodies due to its Se and Sp. The scope of the current studies is the implementation of this method to acquire data on the incidence of viral enteritis due to PEDV in pig herds by indirect serological investigations. Indeed, this method could likely allow successive confirmation of the effect of targeted immunoprophylactic actions, with regard to the current and evolving epidemiological situation.
Acknowledgements
The authors are grateful to Mrs. Daniela Bresciani, Mrs. Giuliana Botti and Mrs. Michela Fazio for excellent technical support.
This work was funded by the Ministry of Health, Italy (project PED_SURV-E52I14001210001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rvsc.2018.10.011.
Appendix A. Supplementary data
Supplemental file 1
Supplemental file 2
References
- Alborali G.L., Boniotti B., Lavazza A. Proceedings of the International Conference on Swine Enteric Coronavirus Diseases, Chicago, USA. 2014. Surveillance and control of PED coronavirus in pig in Italy; p. 7. [Google Scholar]
- Alvarez J., Sarradell J., Morrison R., Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertasio C., Giacomini E., Lazzaro M., Perulli S., Papetti A., Lavazza A., Lelli D., Alborali G., Boniotti M.B. Porcine epidemic diarrhea virus shedding and antibody response in swine farms: a longitudinal study. Front. Microbiol. 2016;7(2009) doi: 10.3389/fmicb.2016.02009. Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniotti M.B., Papetti A., Lavazza A., Alborali G.L., Sozzi E., Chiapponi C., Faccini S., Bonilauri P., Cordioli P., Marthaler D. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronaviruses, Italy. Emerg. Infect. Dis. 2016;22(1) doi: 10.3201/eid2201.150544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniotti M.B., Papetti A., Bertasio C., Giacomini E., Lazzaro M., Cerioli M., Faccini S., Bonilauri P., Vezzoli F., Lavazza A., Alborali G.L. Porcine epidemic diarrhea virus in Italy: disease spread and the role of transportation. Transbound. Emerg. Dis. 2018 doi: 10.1111/tbed.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shi Y., Deng H., Gu T., Xu J., Ou J., Jiang Z., Jiao Y., Zou T., Wang C. Characterization of the porcine epidemic diarrhea virus codon usage bias. Infect. Genet. Evol. 2014;28(Dec):95–100. doi: 10.1016/j.meegid.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K., Lager K., Miller L., Opriessnig T., Gerber P., Hesse R. Evaluation of porcine epidemic diarrhea virus transmission and the immune response in growing pigs. Vet. Res. 2015;6(46):49. doi: 10.1186/s13567-015-0180-5. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi A., Carr J., Ellis R.J., Steinbach F., Williamson S. Porcine epidemic diarrhea virus among farmed pigs, Ukraine. Emerg. Infect. Dis. 2015;21:2235–2237. doi: 10.3201/eid2112.150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Scientific Report on the collection and review of updated epidemiological data on porcine epidemic diarrhoea. EFSA J. 2016;14(2):4375. doi: 10.2903/j.efsa.2016.4375. 2016. 52 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P.F., Lelli D., Zhang J., Strandbygaard B., Moreno A., Lavazza A., Perulli S., Bøtner A., Comtet L., Roche M., Pourquier P., Wang C., Opriessnig T. Diagnostic evaluation of assays for detection of antibodies against porcine epidemic diarrhea virus (PEDV) in pigs exposed to different PEDV strains. Prev. Vet. Med. 2016;Dec 1(135):87–94. doi: 10.1016/j.prevetmed.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasland B., Bigault L., Bernard C., Quenault H., Toulouse O., Fablet C., Rose N., Touzain F., Blanchard Y. Complete genome sequence of a porcine epidemic diarrhea S gene indel strain isolated in France in December 2014. Genome Announc. 2015;3(3) doi: 10.1128/genomeA.00535-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D., Jenckel M., Petrov A., Ritzmann M., Stadler J., Akimkin V., Blome S., Pohlmann A., Schirrmeier H., Beer M., Höper D. Comparison of porcine epidemic diarrhoea viruses from Germany and the United States. Emerg. Infect. Dis. 2015;21:493–496. doi: 10.3201/eid2103.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke D., Pohlmann A., Sauter-Louis C., Höper D., Stadler J., Ritzmann M., Steinrigl A., Schwarz B.A., Akimkin V., Fux R., Blome S., Beer M. Porcine epidemic diarrhea in Europe: in-detail analyses of disease dynamics and molecular epidemiology. Viruses. 2017;9(7) doi: 10.3390/v9070177. Jul 6. pii: E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Kabemura M., Masatani T., Matsuu A., Ozawa M. Isolation and molecular characterization of porcine epidemic diarrhea viruses collected in Japan in 2014. Arch. Virol. 2016;161(8):2189–2195. doi: 10.1007/s00705-016-2900-1. Aug. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Piñeyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio. 2013;4(5) doi: 10.1128/mBio.00737-13. Oct 15. e00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204(2):134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Song D.S., Park B.K. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J. Vet. Diagn. Investig. 2001;13(6):516–520. doi: 10.1177/104063870101300611. [DOI] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol J. Dec. 2015;22(12):193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse L., Krog J.S., Strandbygaard B., Rasmussen T.B., Kjaer J., Belsham G.J., Bøtner A. Experimental infection of young pigs with an early European strain of porcine epidemic diarrhoea virus and a recent US strain. Transbound. Emerg. Dis. 2017;64(5):1380–1386. doi: 10.1111/tbed.12509. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli P., Lavazza A., Nigrelli A.D., Merialdi G., Alborali L.G., Pensaert M.B. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet. Rec. 2008;162:307–310. doi: 10.1136/vr.162.10.307. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Boothe A.D., Ritchie A.E. Characteristics of a coronavirus (strain 67N) of pigs. Am. J. Vet. Res. 1972;33:297–308. [PubMed] [Google Scholar]
- Mesonero-Escuredo S., Strutzberg-Minder K., Casanovas C., Segalés J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhoea cases in Spain. Porcine Health Manag. 2018;4(5) doi: 10.1186/s40813-018-0083-8. Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J.R., Hakze-Van Der Honing R., Almeida A., Lourenco M., van der Poel H.M., Nascimento M.S.J. Outbreak of porcine epidemic diarrhea virus in Portugal, 2015. Transbound. Emerg. Dis. 2015;62(6):586–588. doi: 10.1111/tbed.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti A., Bertasio C., Cerioli M., Salogni C., Faccini S., Bonilauri P., Vezzoli F., Giovannini S., Lavazza A., Alborali G.L., Boniotti M.B. Proceedings EPIZONE - 11th Annual Meeting, 19–21 September 2017. ANSES; Paris, France: 2017. Identification of a recombinant PEDV/SeCoV strains during a molecular surveillance of Italian epidemic waves between 2015 and 2017; p. 136. [Google Scholar]
- Saif L.J., Pensaert M.B., Sestak K., Yeo S.G., Jung K. Coronaviruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Disease of Swine. John Wiley & Sons, Inc.; 2012. pp. 01–524. [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi E., Luppi A., Lelli D., Martin A.M., Canelli E., Brocchi E., Lavazza A., Cordioli P. Comparison of enzyme-linked immunosorbent assay and RT-PCR for the detection of porcine epidemic diarrhoea virus. Res. Vet. Sci. 2010;88(1):166–168. doi: 10.1016/j.rvsc.2009.05.009. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi, E., Papetti, A., Lelli, D., Boniotti, B., Moreno, A., Brocchi, E., Alborali, L., Lavazza, A., Cordioli, P., Diagnosis and investigations on PED in North Italy. Proceedings 8th Annual Epizone Meeting “Primed for Tomorrow”: 23–25 September 2014 Copenhagen, Denmark.
- Stadler J., Zoels S., Fux R., Hanke D., Pohlmann A., Blome S., Weissenböck H., Weissenbacher-Lang C., Ritzmann M., Ladinig A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015;11 doi: 10.1186/s12917-015-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrigl A., Fernández S.R., Stoiber F., Pikalo J., Sattler T., Schmoll F. First detection, clinical presentation and phylogenetic characterization of Porcine epidemic diarrhea virus in Austria. BMC Vet. Res. 2015;30(11):310. doi: 10.1186/s12917-015-0624-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandbygaard B., Lavazza A., Lelli D., Blanchard Y., Grasland B., Poder S.L., Rose N., Steinbach F., van der Poel W.H., Widén F., Belsham G.J., Bøtner A. Inter-laboratory study to characterize the detection of serum antibodies against porcine epidemic diarrhoea virus. Vet. Microbiol. 2016;197:151–160. doi: 10.1016/j.vetmic.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Ma J., Wang Y., Wang M., Song W., Zhang W., Lu C., Yao H. Genomic and epidemiological characteristics provide new insights into the phylogeographical and spatiotemporal spread of porcine epidemic diarrhea virus in Asia. J. Clin. Microbiol. 2015;53(5):1484–1492. doi: 10.1128/JCM.02898-14. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Theuns S., Conceição-Neto N., Christiaens I., Zeller M., Desmarets L.M., Roukaerts I.D., Acar D.D., Heylen E., Matthijnssens J., Nauwynck H.J. Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in Belgium, January 2015. Genome Announc. 2015;21(May (3)) doi: 10.1128/genomeA.00506-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak I., Ipavec M., Kuhar U., Kušar D., Papić B., Koren S., Toplak N. Complete genome sequence of the porcine epidemic diarrhea virus strain SLO/JH-11/2015. Genome Announc. 2016;4(2) doi: 10.1128/genomeA.01725-15. Apr 7. pii: e01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wolf P.J., Van Walderveen A., Meertens M.N., Van Hout A.J., Duinhof T.F., Geudeke M.J. Proceedings of the 7th European Symposium of Porcine Health Management, Nantes, France. 2015. First case of Porcine Epidemic Diarrhea (PED) caused by a new variant of PED virus in The Netherlands; p. 79. [Google Scholar]
- Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A., Collins J., Saif L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014;20(10):1620–1628. doi: 10.3201/eid2010.140491. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20(5):917–919. doi: 10.3201/eid2005.140195. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.H., Obuchowsky N.A., McClish D.K. Wiley & Sons, Interscience; New York: 2002. Statistical Methods in Diagnostic Medicine. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental file 1
Supplemental file 2