Abstract
The recent identification of a novel human coronavirus responsible of a SARS-like illness in the Middle-East a decade after the SARS pandemic, demonstrates that reemergence of a SARS-like coronavirus from an animal reservoir remains a credible threat. Because SARS is contracted by aerosolized contamination of the respiratory tract, a vaccine inducing mucosal long-term protection would be an asset to control new epidemics. To this aim, we generated live attenuated recombinant measles vaccine (MV) candidates expressing either the membrane-anchored SARS-CoV spike (S) protein or its secreted soluble ectodomain (Ssol). In mice susceptible to measles virus, recombinant MV expressing the anchored full-length S induced the highest titers of neutralizing antibodies and fully protected immunized animals from intranasal infectious challenge with SARS-CoV. As compared to immunization with adjuvanted recombinant Ssol protein, recombinant MV induced stronger and Th1-biased responses, a hallmark of live attenuated viruses and a highly desirable feature for an antiviral vaccine.
Keywords: Coronavirus, Severe acute respiratory syndrome, Spike glycoprotein, Measles vaccine
Highlights
-
•
Generation of live recombinant measles vaccine expressing SARS-CoV spike protein.
-
•
Induction of high titers anti-SARS-CoV neutralizing antibodies in mice.
-
•
Protection of immunized mice from intranasal infectious challenge with SARS-CoV.
-
•
Induction of Th1-biased responses and IgA.
Introduction
Severe acute respiratory syndrome (SARS) is a newly emerged, human infectious disease that first appeared in China in late 2002. Between November 2002 and July 2003, the virus spread to 29 different countries on 5 continents and was responsible for 8096 clinical cases, leading to 774 deaths (WHO, 2004). WHO case management guidelines and restricted travel advices allowed to bring SARS under control by July 2003. The etiological agent of SARS was identified as a novel coronavirus, named SARS-associated coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003) that is genetically distinct from previously characterized members of the Coronaviridae family (Rota et al., 2003). During the 2002–2003 outbreak, SARS-CoV has been isolated in Chinese civets and racoon dogs (Guan et al., 2003) from which the virus was likely introduced into the human population (Kan et al., 2005, Song et al., 2005). Other SARS-CoV-like viruses sharing more than 88% nucleotide identities with SARS-CoV have been isolated from Chinese horseshoe bats, which have therefore been proposed to represent a natural reservoir host of SARS-CoV (Li et al., 2005). To date, endemic bat SARS-CoV-like viruses have also been detected in Africa and Europe (for review: Balboni et al., 2012), and reemergence of a SARS-like disease from an animal reservoir remains a credible public health threat. An efficient vaccine would be the most effective way to control a new epidemic.
Similar to other coronaviruses, SARS-CoV is an enveloped, positive-stranded RNA virus whose replication takes place in the cytoplasm of infected cells. Viral particles are composed of four major structural proteins: the nucleoprotein (N), the small envelope protein (E), the membrane protein (M), and the large spike protein (S). The spike protein is a type-I transmembrane glycoprotein of 1255 amino acids. It assembles into homotrimers at the surface of viral particles, and gives the virion its crown-like appearance (Neuman et al., 2006). Each monomer (180 kDa) is composed of a signal sequence (a.a. 1–14), a large ectodomain (a.a. 15–1190) with 23 potential N-glycosylation sites, a transmembrane domain (a.a. 1191–1227), and a short cytoplasmic tail of 28 a.a. (Ksiazek et al., 2003, Rota et al., 2003). The S protein is responsible for viral entry, binds to the cellular receptor ACE2 (Li et al., 2003) and mediates fusion between the viral and cellular membranes (Petit et al., 2005, Simmons et al., 2005). Structurally, the N-terminal globular head (S1 domain, a.a. 1–680) contains the receptor-binding region (Wong et al., 2004), and the membrane-anchored stalk region (S2 domain, a.a. 727–1255) mediates oligomerization and fusion (Petit et al., 2005). Similarly to other coronaviruses, cleavage of the S protein by proteases into its S1 and S2 subunits is required for activation of the membrane fusion domain following binding to target cell receptors (Matsuyama et al., 2010, Simmons et al., 2005).
Due to its critical involvement in receptor recognition, as well as virus attachment and entry, the S protein is the most promising and studied candidate antigen for SARS-CoV vaccine development. It is the major target for neutralizing antibodies in human patients (He et al., 2005, Nie et al., 2004) and in animal models (Buchholz et al., 2004, Tripp et al., 2005). Passive transfer of IgG from convalescent SARS patients enhanced the recovery of acute phase patients when administered within 15 days after the onset of symptoms (Cheng et al., 2005, Yeh et al., 2005). Administration of S-specific antibodies, including monoclonal antibodies, to naïve animals conferred protection against a subsequent SARS-CoV infection, demonstrating that the antibodies alone can protect against SARS in mice (Bisht et al., 2004), hamsters (Roberts et al., 2006), ferrets (ter Meulen et al., 2004) and Rhesus macaques (Miyoshi-Akiyama et al., 2011). Accordingly, several candidate vaccines relying on the induction of spike-specific neutralizing antibodies, including DNA vaccines (Callendret et al., 2007, Yang et al., 2004), live viral vectors (Buchholz et al., 2004, Chen et al., 2005, Kapadia et al., 2005), live attenuated vaccines (Lamirande et al., 2008), subunit vaccines (Bisht et al., 2005, He et al., 2006, Zhou et al., 2006) and inactivated virus vaccine (Stadler et al., 2005, Zhou et al., 2005), have been reported to induce a protective immune response in various animal models. Only a few of them have been evaluated in phase I clinical trials and, lacking a natural challenge, there is no data on efficacy in humans (Roberts et al., 2008, Roper and Rehm, 2009).
An ideal vaccine against SARS should induce long-lasting protective responses after a single administration, be produced at low cost and scaled up to millions of doses. Live attenuated vaccines are particularly appropriate for mass vaccination as they are inexpensive to manufacture and induce a strong immunity and long-term memory after a single injection. To evaluate such a vaccine approach, we previously developed a vector derived from the live-attenuated Schwarz strain of measles virus (MV) (Combredet et al., 2003). MV vaccine is a live-attenuated negative-stranded RNA virus proven to be one of the safest and most effective human vaccines. Produced on a large scale in many countries and distributed at low cost through the Extended Program on Immunization (EPI), this vaccine induces life-long immunity to measles after one or two injections. We previously showed that MV vector stably expressed different proteins from HIV and flaviviruses and induced strong and long-term transgene-specific neutralizing antibodies and cellular immune responses, even in the presence of preexisting immunity to MV (Brandler et al., 2007, Brandler et al., 2013, Despres et al., 2005, Guerbois et al., 2009, Lorin et al., 2004). In the present study, we evaluated the immunogenic potential of recombinant MV-SARS vectors expressing either the full-length or the secreted ectodomain of the spike glycoprotein of SARS-CoV. In a mouse model of MV infection, MV-SARS recombinant viruses induced neutralizing antibodies against SARS-CoV and fully protected immunized animals from intranasal challenge with SARS-CoV. Antibody responses induced by MV-SARS vectors were quantitatively and qualitatively compared to responses induced by a prototype subunit vaccine prepared from alum-adjuvanted recombinant Ssol protein.
Results
Recombinant MVSchw-SARS viruses express the SARS-CoV spike glycoprotein, secrete its soluble ectodomain, and replicate efficiently
We synthesized human codon-optimized genes encoding the full-length, membrane anchored SARS-CoV spike (S) protein and its entire ectodomain (residues 1–1193, hereafter designed as Ssol), which is expressed in mammalian cells as a soluble and secreted polypeptide (Callendret et al., 2007, Callendret et al., unpublished results). Their length respects the “rule of six”, which stipulates that the total number of nucleotides into the MV genome must be a multiple of 6 (Calain and Roux, 1993). MV editing- and polyadenylation-like sequences were mutated (Lamb and Kolakofsky, 2001, Schneider et al., 1997). Both S and Ssol sequences were inserted as an additional transcription unit (ATU) into MV vector (pTM-MVSchw plasmid), which contains an infectious MV cDNA corresponding to the anti-genome of the Schwarz vaccine strain (Combredet et al., 2003) ( Fig. 1A). The resulting pTM-MVSchw-S and pTM-MVSchw-Ssol plasmids were transfected into helper 293-T7-MV cells as previously described (Combredet et al., 2003). The corresponding recombinant measles viruses MV-S and MV-Ssol were successfully rescued as indicated by the formation of syncytia, and then propagated in Vero cell culture. We analyzed the replication of MV-S and MV-Ssol viruses on Vero cells by using the same MOI (0.01) than for standard MV stock production (Fig. 1B). The growth of MV-Ssol was only slightly delayed, compared with that of parental empty Schwarz MV (MVSchwarz). The final yield, routinely obtained at 60 h post-infection, was high and identical to that of parental MVSchwarz (106 TCID50/ml). Viral growth and yield of MV-S were more affected than that of MV-Ssol. This may be due to reduced MV budding because of the insertion of full length S at the surface of the infected cells, as already observed for MV expressing membrane-anchored forms of HIV1 gp160 (Lorin et al., 2004).
Fig. 1.
Characterization of MVSchw-SARS recombinant viruses expressing the full-length transmembrane spike glycoprotein (S) or its secreted ectodomain (Ssol). (A) Schematic representation of the pTM-MVSchw-ATU2 vector containing the Schwarz MV cDNA with a green fluorescent protein (eGFP) gene as an additional transcription unit (ATU). Codon-optimized synthetic genes coding for the full-length S protein or secreted ectodomain (Ssol) were inserted into pTM-MVSchw between the BsiWI and BssHII sites of the ATU, in place of the eGFP gene. MV genes are indicated: N (nucleoprotein), PVC (phoshoprotein and V/C accessory proteins), M (matrix), F (fusion), H (hemaglutinin), L (polymerase). T7: T7 RNA polymerase promoter. hhR: hammerhead ribozyme. T7t: T7 RNA polymerase terminator. h∂vR: hepatitis delta virus (HDV) ribozyme. (B) Growth kinetics of recombinant MVSchw-SARS viruses. Vero cells were infected with the parental MVSchw (open circles) or recombinant MV-S (filled squares) or MV-Ssol (filled triangles) viruses at an M.O.I. of 0.01. At the indicated time points, cells were collected and cell-associated virus titers were determined as described in Materials and methods. (C) Immunostaining of spike polypeptides in syncytia of MVSchw-SARS-infected Vero cells. Cells were fixed 24–48 h after infection with the indicated viruses and permeabilized with triton X-100 (upper panels) or non-permeabilized (lower panels), then labeled as described in Materials and methods with anti-S mouse polyclonal antibodies and Cy3-conjugated anti-mouse IgG antibodies. (D) Lysates and supernatants of Vero-NK cells infected with MVSchw-SARS viruses were analyzed by western blot as described in Materials and methods using rabbit polyclonal antibodies specific for the S protein. As controls, whole cell lysates prepared from uninfected or SARS-CoV infected VeroE6 cells were analyzed. The positions of full-length S and Ssol polypeptides as well as molecular weight markers (kDa) are shown.
We analyzed the expression of SARS-CoV spike antigens by indirect immunofluorescence (IFA) of Vero cells infected by recombinant viruses and by immunoblotting of infected-cell lysates. At 48 h post-infection, IFA performed on permeabilized cells using an anti-S hyperimmune mouse ascitic fluid revealed a strong expression of both full-length S and entire ectodomain Ssol of the spike protein along the compartments of the secretory pathway in measles-induced syncytia (Fig. 1C, upper panels). When infected cells were not permeabilized before labeling, only the cell surface of MV-S induced syncytia was readily stained, indicating that the membrane-anchored S is efficiently transported to the surface (Fig. 1C, lower panels). Western blot analysis of cell lysates and supernatants using rabbit anti-S polyclonal antibodies confirmed the expression of S and Ssol proteins in recombinant MV-infected Vero cells with the expected apparent molecular mass of ~180 kDa (Fig. 1D, left panel). Under reducing SDS-PAGE conditions the full length S protein migrates as a doublet, which was described as two differentially glycosylated forms (Song et al., 2004). The lighter product was suggested to be an ER-resident form of the glycoprotein and the heavier a Golgi-processed form containing complex carbohydrates. Other bands of lower molecular weight were also observed that probably correspond to minor degradation fragments, since they were not present at earlier time points (not shown). Expression levels were similar in lysates of MV-infected Vero-NK cells and SARS-CoV infected VeroE6 cells. As expected, the full-length S protein was only detected in cell lysates. In contrast, Ssol was clearly detected both in lysate and supernatant of MV-Ssol infected Vero cells at 40 h after infection (Fig. 1D, right panel), indicating an efficient secretion. Consistently, the Ssol protein secreted in the cell culture medium was heavier than the Ssol observed within cell lysates, which is in agreement with this glycoprotein being synthesized in an immature form in the ER prior to transfer to the Golgi, from which it is secreted.
MV-S and MV-Ssol induce Th1-type immune response and SARS-CoV neutralizing antibodies in mice
The immunogenicity of the recombinant MVSchwarz-SARS viruses was investigated in genetically modified CD46-IFNAR mice susceptible to MV infection (Mrkic et al., 1998) and compared to the immunogenicity of purified Ssol polypeptide produced in mammalian cells, which constitutes a potential subunit vaccine candidate against SARS (Du et al., 2008). The CD46-IFNAR mice express CD46, the human receptor for vaccine MV strains, and lack the INF-α/β receptor. They have been used previously as a model to evaluate the immunogenicity of recombinant MV (Brandler et al., 2007, Combredet et al., 2003, Despres et al., 2005, Guerbois et al., 2009, Lorin et al., 2004). Eight to twelve-week-old male CD46-IFNAR mice were immunized with two intraperitoneal (i.p.) injections at 4-week interval of 105 TCID50 of MV-S or MV-Ssol recombinant viruses. As controls, a group of mice was injected with empty MV vector (105 TCID50) and another group with 2 µg of purified Ssol protein adjuvanted with 50 µg of aluminum hydroxide (alum), usual doses for small rodents. Mice sera were collected three weeks after each injection. SARS-CoV- and measles-specific antibody responses were evaluated for each individual mouse by indirect ELISA against SARS-CoV and MV native antigens, respectively.
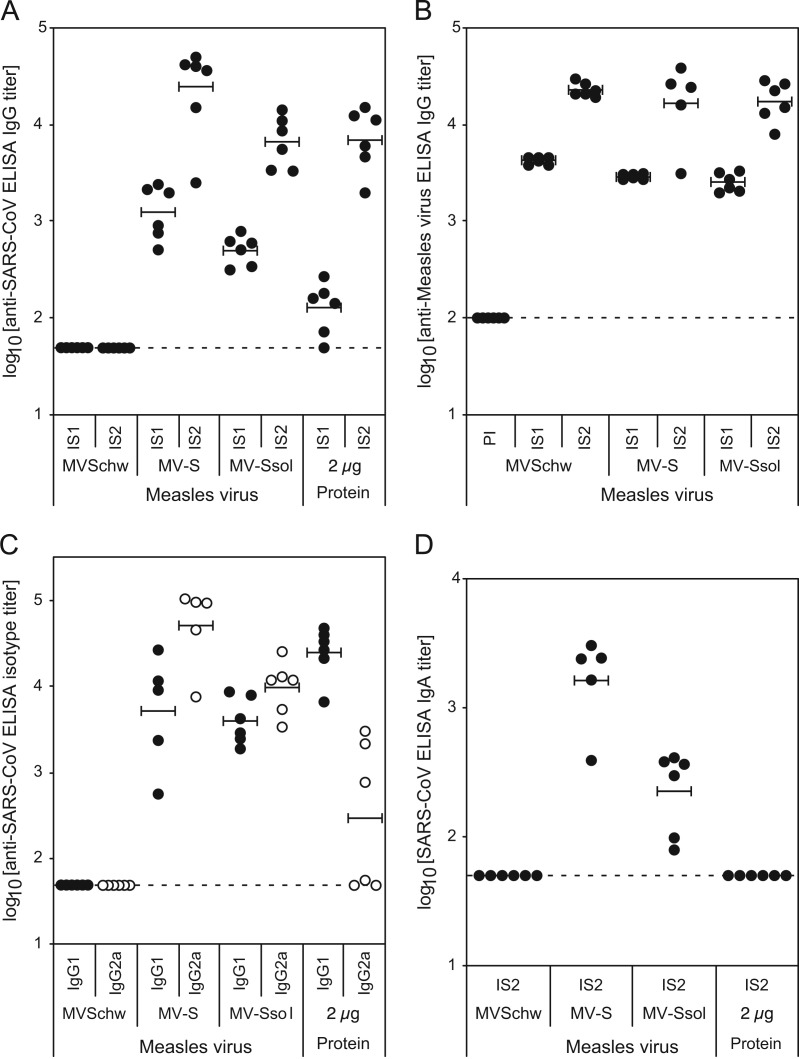
Significant titers of anti-SARS IgG were raised in all mice after the first injection of recombinant MV-SARS viruses, whereas preimmune sera (not shown) and sera from control animals that received empty MVSchw remained negative ( Fig. 2A). These titers were higher for both MV-S and MV-Ssol injected animals (average titer of 3.1±0.3 and 2.7±0.1 log 10, respectively) than for animals immunized with Alum-adjuvanted Ssol protein (2.1±0.3 log 10 titer, p<10−3). After the second injection, titers were boosted 10–20 times for animals immunized with MV vectors. Tallying with the results observed after the first injection, MV-S induced the highest IgG titers (4.4±0.5 log 10, p<0.1). MV-Ssol induced similar titers (3.8±0.2 log 10) than alum-adjuvanted Ssol protein (3.8±0.3 log 10). Interestingly, antibodies to MV were raised at similar levels in all mice that received either MVSchw or MV-SARS viruses (Fig. 2B), indicating that expression of the heterologous S protein by the recombinant viruses did not alter their replication in vivo nor modify their measles-specific immunogenicity.
Fig. 2.
Antibody response in IFN-α⧸βR−/− CD46+/− mice immunized with MVSchw-SARS recombinant viruses. Groups of 6 IFN-α⧸βR−/− CD46+/− mice were injected twice intraperitoneally at four-week interval with 105 TCID50 of the indicated recombinant MVSchw-SARS measles viruses or with parental MVSchw, as control. Another group of mice was immunized with two intramuscular injections of 2 µg of purified Ssol polypeptide adjuvanted with 50 µg Alum. Sera were collected before immunization (PI) or 3 weeks after each injection (IS1 and IS2, respectively). SARS-CoV-specific (A) or MV-specific (B) IgG antibody titers were determined by indirect ELISA, as described in Materials and methods. (C) SARS-CoV-specific, IgG1 (filled circles) and IgG2a (open circles) isotype titers were determined for each IS2 serum by indirect ELISA. (D) SARS-CoV-specific IgA antibody titers were determined for each IS2 serum by indirect ELISA. Values obtained for each individual mouse are represented with circles, with means for each group of mice shown by horizontal bars. Detection limits of the assays are indicated by dotted lines.
Additionally, the quality of the humoral response induced by the various immunogens was studied on sera collected 3 weeks after the second injection by IgG and IgA isotype analysis and neutralization assay. We first determined the specific IgG1 and IgG2a isotype titers to the SARS-CoV antigens by anti-SARS ELISA (Fig. 2C). The immunizations with the alum-adjuvanted Ssol protein almost exclusively induced IgG1, indicating that the induced immune responses are predominantly of Th2-type as we previously observed in BALB/c mice (Callendret et al., unpublished results). Contrariwise, MV-S and MV-Ssol viruses induced significantly higher titers of IgG2a than IgG1 antibodies, particularly for MV-S (average ratio IgG2a over IgG1 of 14.7 and 2.7 respectively), reflecting a predominant Th1-type immune response induced by the live recombinant measles vector. Interestingly, MV-Ssol and MV-S viruses also induced moderate (2.4±0.3 log 10) to high titers (3.2±0.3 log 10) of anti-SARS IgA antibodies respectively (Fig. 2D), whereas sera from animals immunized with alum-adjuvanted Ssol protein remained negative (log 10 titer<1.7).
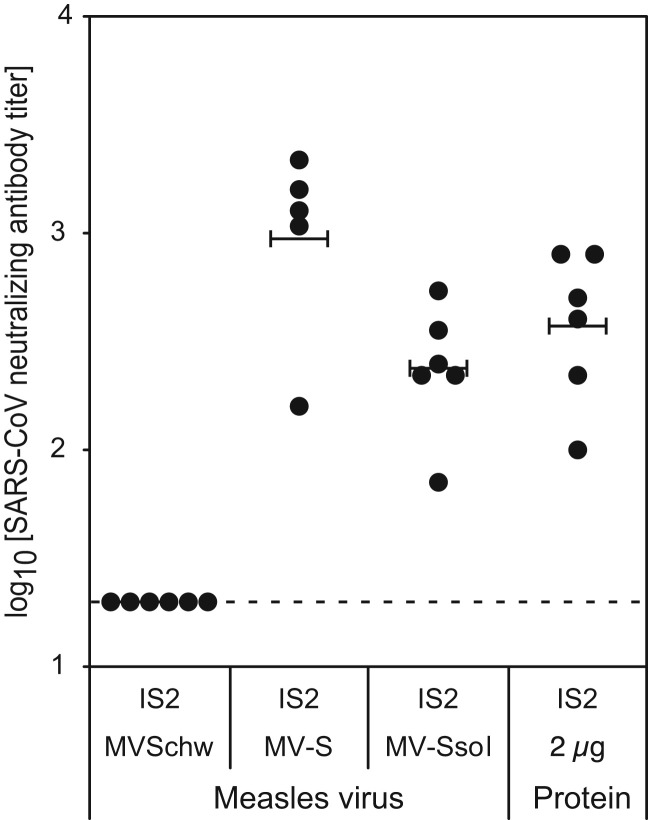
We then further examined whether the antibody response had SARS-CoV neutralizing activity, which represents the unique correlate of protective immunity against SARS (Enjuanes et al., 2008, Roberts et al., 2008). Neutralizing titers were determined by using an infectivity reduction assay as the highest serum dilution that suppressed SARS-CoV cytopathic effect in at least 50% of the inoculated wells of cultured FRhK-4 cells ( Fig. 3). Immunization with MV-Ssol and alum-adjuvanted Ssol protein induced comparable level of neutralizing antibodies (2.4±0.3 and 2.6±0.3 log 10 titer, respectively), whereas MV-S induced significantly three- to four-fold higher neutralizing titers (3.0±0.4 log 10 titer, p<0.1).
Fig. 3.
Neutralizing antibody response in IFN-α⧸βR−/− CD46+/− mice immunized with MVSchw-SARS recombinant viruses. SARS-CoV neutralizing antibody titers were determined for each IS2 serum as the reciprocal of the highest dilution of serum which completely prevented SARS-CoV cytopathic effect in 50% of the wells. Values obtained for each individual mouse are represented with circles, with means for each group of mice shown by horizontal bars. Detection limits of the assays are indicated by dotted lines.
Immunization with recombinant MV-SARS protects from intranasal experimental challenge
To determine whether immunization by recombinant MV-SARS could induce protection from an experimental challenge, mice were inoculated intranasally with 105 pfu of SARS-CoV five weeks after the second immunization. Mice inoculated with empty MVSchw were used as controls. To evaluate the level of protection against viral replication, we quantified the SARS-CoV infectious titers in lung homogenates prepared two days after challenge ( Fig. 4). Control mice immunized with empty MVSchw were all infected except one, SARS-CoV replicating in their lungs at titers up to 106 pfu/lung. This shows that CD46-IFNAR mice are susceptible to intranasal SARS-CoV infection, in agreement with previous observations describing a self-limited bronchiolitis with mild or moderate pneumonitis in 129/Sv mice (Hogan et al., 2004) and no increase in susceptibility, pathogenesis and histological outcomes in IFNAR mice of the same genetic background (Frieman et al., 2010).
Fig. 4.
Protection of MVSchw-SARS-immunized mice from intranasal challenge with SARS-CoV. Five weeks after the second injection of MVSchw-SARS recombinant viruses or Ssol polypeptide, mice were subjected to intranasal challenge with 105 pfu of SARS-CoV. SARS-CoV infectious titers (expressed as log10 pfu/lungs for individual mice) were dermined in lung homogenates collected two days after challenge, using plaque assay on Vero cells. Values for each individual mouse are represented with filled circles, and means for each group by horizontal bars. The detection limits of the assays are indicated by dotted lines. Data shown are from one experiment representative of two.
On the contrary, all mice immunized with MV-S were fully protected from challenge. The absence of SARS-CoV replication was confirmed by quantitative real-time RT-PCR, which evidenced more than a 1000-fold reduction of SARS-CoV RNA levels in the lungs of MV-S-immunized mice (log 10 geq/lungs<3.8, below the detection level) compared to MVSchw-immunized mice (7.6±1.9 log 10 geq/lungs). Mice immunized with MV-Ssol or adjuvanted Ssol were also protected, albeit less efficiently; one mouse in either group had low residual viral titers in the lungs (Fig. 4) and SARS-CoV RNA remained detectable albeit at strongly reduced levels in the lungs of half of the immunized mice (not shown).
Altogether, these experiments demonstrate that recombinant MV-SARS viruses expressing either the full-length or the secreted ectodomain of the spike protein induced neutralizing antibodies to SARS-CoV at titers that were sufficient to protect animals from experimental intranasal infection. Noticeably, the efficiency of protection followed the hierarchy observed for neutralization antibody levels, MV-S conferring complete and better protection from virus replication in mice lungs than both MV-Ssol and the reference adjuvanted subunit vaccine.
Discussion
The objective of this study was to evaluate the proof-of-concept of a new SARS-CoV vaccine strategy based on a standard measles vaccine engineered to express the SARS-CoV spike protein. This strategy might provide a safe recombinant vaccine to protect from SARS-CoV in the regions that might become affected by SARS-CoV re-emergence.
Due to its critical role in viral entry, the S protein is the most promising candidate antigen for SARS-CoV vaccine development (Enjuanes et al., 2008, Roberts et al., 2008, Roper and Rehm, 2009). We designed human codon-optimized genes encoding the full-length SARS-CoV spike (S) protein and its soluble ectodomain (Ssol). These sequences were inserted into MV vector. Recombinant viruses were produced by reverse genetics and grew at standard titers. They expressed high levels of SARS-CoV full-length spike protein at the cell membrane and led to efficient secretion of the soluble ectodomain. Immunization of mice susceptible to MV with recombinant MV-S and MV-Ssol viruses induced specific antibodies that neutralized SARS-CoV infection in vitro. The induction of measles-specific immunity was not altered by the expression of the transgenes. Immunization primed a SARS-CoV-specific memory response that was vigorously boosted by a second injection. The anchored full-length form of the spike protein induced the highest titers of neutralizing antibodies, as we previously observed with HIV gp160 (Lorin et al., 2004), and also a more robust response than a prototype subunit vaccine prepared from adjuvanted recombinant Ssol protein. As compared to immunization with adjuvanted Ssol protein, both recombinant MV-SARS viruses induced higher titers of IgG2a than IgG1 antibodies, indicating a Th1 biased response, a hallmark of live attenuated viruses and a highly desirable feature for an antiviral vaccine. Remarkably, both recombinant MV-SARS vectors also induced SARS-CoV specific IgA, whereas the subunit reference vaccine did not. This raises an interesting point since several clinical studies already evidenced that parenteral administration of live attenuated measles vaccine to children stimulates secretory IgA responses in nasal washes (Bellanti et al., 2004, Simon et al., 2011). Although we did not assess the ability of recombinant MV-SARS to induce secretory IgA in the lungs and upper respiratory tract of immunized mice, this result is strongly indicative of the potential of MV-SARS to induce SARS-CoV specific secretory IgA responses.
To evaluate the efficacy of recombinant MV-SARS vectors, we relied on challenge with wt human SARS-CoV and evaluation of lung virus titers since, unfortunately, mouse-adapted SARS-CoV is not lethal in 129/Sv mice (Frieman et al., 2010). After two successive immunizations with recombinant MV-S virus, all mice were fully protected from intranasal infectious challenge with SARS-CoV and neither infectious virus nor residual viral RNA could be recovered from the lungs of the challenged animals. Interestingly, despite a lower replication rate in cell culture, MV-S conferred a better protection from SARS-CoV replication in mice lungs than MV-Ssol. This may reflect better immunogenicity of the native anchored-form of S or suggest structural dissimilarities between the monomeric soluble Ssol ectodomain and its corresponding membrane-associated trimeric form. In that respect, the absence, or inefficient presentation, of antibody epitopes on truncated soluble immunogens derived from functional virion-associated glycoproteins has already been evidenced for filovirus attachment proteins (Sullivan et al., 2006) and HIV gp160 (Davenport et al., 2011, Kovacs et al., 2012).
Usually given intramuscularly, measles vaccine protects very efficiently against measles disease, which is contracted by aerosolized contamination of the upper respiratory tract, similarly to SARS. Our data demonstrate that parenteral immunization with MV-SARS vector protected mice lungs from SARS-CoV intranasal infection. A similar approach was previously reported by Liniger et al. (2008) that also demonstrated the induction of neutralizing antibodies in mice to SARS-CoV spike expressed by a MV vector. However, this study did not evaluate the efficacy of vaccine preparation to protect immunized mice from SARS-CoV challenge. Our observation that MV-SARS induced a strong Th1 response appears of particular importance given the recently reported safety concerns by Tseng et al., which described Th2-type lung immunopathology upon challenge of mice vaccinated with inactivated whole virus, SARS VLP or alum-adjuvanted S protein (Tseng et al., 2012). They suggested that most previously described vaccine-induced immunopathology might proceed from the same Th2-type immunopathology with prominent eosinophil infiltration upon SARS-CoV challenge. Such effects will likely not occur with live vaccines since they induce potent Th1-biased responses. In agreement with this hypothesis, it was recently reported that a live attenuated SARS-CoV lacking E protein expression provided long-term protection against lethal challenge in an aged-mice model in the absence of any sign of vaccine-induced immunopathology (Fett et al., 2013).
Live attenuated RNA virus vaccines like those against mumps, measles, polio or rubella viruses induce long-term cell-mediated and humoral immunity after one or two injections. Using MV as a vaccination vector presents a number of advantages: this highly efficient and most safe vaccine is easily produced at large scale, vaccine strains are genetically stable, MV does not recombine or integrate genetic material, vaccine does not persist or diffuse. The Schwarz/Moraten strain from which we derived our vector (Combredet et al., 2003) is currently the most attenuated and the most widely used measles vaccine. Measles vector expresses very stably large amounts of heterologous genetic material, likely due to the absence of geometric constraints on the size of helicoidal nucleocapsids in this pleiomorphic virus (Tangy and Naim, 2005). Although the stability of expression of the specific SARS transgene remains to be determined, the remarkable genetic stability of added, expressed ORFs has already been described by us and others for a variety of transgenes inserted into measles vector and other members of the Mononegavirales. The measles vector demonstrated a strong capacity to stimulate both cellular and humoral neutralizing immunity against a number of antigens and provided protection from experimental challenge both in mice and primates (Brandler et al., 2007, Brandler et al., 2012, Despres et al., 2005, Lorin et al., 2004, Tangy and Naim, 2005). A measles-HIV vaccine candidate currently under clinical development has been evaluated in phase I clinical trial, demonstrating the GMP preclinical and clinical safety and immunogenicity of this vector (Lorin et al., 2012, Stebbings et al., 2012).
Recombinant MV vaccines might be used to immunize both the pediatric and adult/adolescent populations in case of SARS-CoV outbreaks. The presence of anti-MV immunity in nearly the entire adult human population might restrict the use of recombinant MV to naïve infants, an already worthy goal in any event. However, numerous studies have shown that revaccinating immunized individuals results in a boost of anti-MV antibodies, suggesting that the attenuated live vaccine replicates and expresses its proteins in spite of preexisting immunity (Dilraj et al., 2000, Rager-Zisman et al., 2003, Sepulveda-Amor et al., 2002). Likewise, the presence of maternal anti-MV antibodies has been shown to limit the induction of anti-measles antibodies but not the induction of specific T-cell responses in infants given measles vaccine during the first year of life (Gans et al., 2003, Gans et al., 2001). Moreover, we previously demonstrated that recombinant MV vectors were immunogenic and induced protection in the presence of MV preexisting immunity in animal models (Brandler et al., 2013, Lorin et al., 2004), which opens the possibility of using recombinant MV vector to immunize adolescents and adults, although this point needs to be evaluated in human trials. Measles appears very difficult to eliminate and still causes 160,000 deaths annually worldwide, mostly in poorly developed countries (Centers for Disease and Prevention, 2009). Europe also recently experienced severe measles outbreaks (Cottrell and Roberts, 2011). Improving and maintaining measles vaccination for decades is essential to contain this most contagious disease. In this context, the use of recombinant MV designed to immunize the pediatric or adult populations appears desirable.
In conclusion, we have produced new recombinant MV-SARS viruses able to induce neutralizing antibodies to SARS-CoV and full protection from intranasal challenge, thus making the proof-of-concept of this strategy for SARS vaccine development and more generally for severe respiratory infectious diseases. These characterized SARS vaccine candidates deserve to be evaluated in a much more adapted non-human-primate model, in which the cross-protective potential of the induced immune responses against zoonotic SARS isolates/variants could be addressed. Indeed, several studies have underlined the need to induce broadly neutralizing antibodies able to protect against heterologous viral variants, which may arise during independent emergence events (Bolles et al., 2011, Sheahan et al., 2011). Such antigenic variants have already been isolated from animals during the 2002–2003 epidemics and from sporadic human cases during the 2003–2004 reemergence (Deming et al., 2006, Yang et al., 2005), and are likely to emerge again from the large heterogeneous zoonotic reservoir of SARS-like viruses, should a new outbreak occur. The recent identification of a novel human betacoronavirus, MERS-CoV, as the agent of a SARS-like illness in the Middle-East, has raised great concerns about the pandemic potential of the disease (Perlman, 2013, WHO, 2013, Zaki et al., 2012). A decade after the SARS pandemic, it has renewed worldwide attention on coronaviruses and stressed the need of continued research and investment in the development of safe and effective vaccines against highly pathogenic coronaviruses.
Materials and methods
Cell lines, viruses and antigens
FRhK-4 (Fetal Rhesus monkey Kidney) and Vero-NK (African Green Monkey Kidney) cells were grown at 37 °C under 5% CO2 in complete DMEM [Dulbecco's modified Eagle medium with 4.5 mg/ml l-glucose, 100 U/ml penicillin and 100 µg/ml streptomycin], supplemented with 5% heat-inactivated fetal calf serum (FCS) (DMEM-5). Helper 293-T7-MV cells stably expressing T7 RNA polymerase and N and P genes from Schwarz MV were grown in complete DMEM supplemented with 10% FCS (DMEM-10).
SARS-CoV FFM-1 strain (Drosten et al., 2003) was kindly provided by Dr. H.W. Doerr (Institute of Medical Virology, Frankfurt University Medical School, Germany). Viral stocks were produced and titrated as described previously (Callendret et al., 2007). All work involving infectious SARS-CoV was performed in an enhanced biosafety level 3 containment laboratory with rigorous safety procedures according to WHO guidelines.
Crude cell lysates prepared from SARS-CoV infected VeroE6 cells and inactivated frozen by gamma irradiation, as described (Callendret et al., 2007), were used as SARS native antigens for ELISA. A soluble monomeric spike protein, termed Ssol and corresponding to the entire S ectodomain (residues 1–1193) fused with the FLAG tag was produced and purified by immunoaffinity chromatography (Sigma-Aldrich) from the supernatant of stable mammalian cell lines (Callendret et al., unpublished results).
Construction and rescue of recombinant MVSchw-SARS viruses
A human codon-optimized gene encoding the SARS-CoV spike (S) protein of the ♯031589 specimen (Callendret et al., 2007) was chemically synthesized by Geneart (Regensburg, Germany) and subcloned into the pCI mammalian expression vector (Promega), yielding plasmid pCI-Ssynth. In addition to codon bias optimization, regions of very high (>80%) or low (<30%) GC content were avoided whenever possible. Furthermore, cis-acting sequence motifs such as internal TATA-boxes, chi-sites, ribosomal entry sites, ARE, INS, and CRS sequence elements, as well as repetitive sequences, RNA secondary structures and splice donor and acceptor sites, were avoided. The resulting optimized gene had an increased GC content (61.7% from 38.9%). The sequence of the codon-optimized S gene is available upon request. A codon-optimized gene encoding the soluble and secreted spike ectodomain (Ssol) was obtained by PCR amplification using the pCI-Ssynth plasmid as a template and oligonucleotides 5'-ACTAGCTAGC GGATCCACCA TGTTCATCTT CCTG-3' containing Nhe-1 restriction site (underlined) and 5'-AGTATCCGGA CTTGATGTAC TGCTCGTACT TGC-3' containing BspE1 restriction site. The PCR fragment was inserted into pCI-Ssol which contains the wild-type non-optimized gene encoding Ssol (Callendret et al., unpublished data), yielding plasmid pCI-Scube.
Measles recombinant vector was derived from plasmid pTM-MVSchw-ATU2 which carries an infectious cDNA corresponding to the anti-genome of the Schwarz MV vaccine strain and an additional transcription unit containing unique restriction sites for the insertion of foreign sequences downstream of the P gene (Combredet et al., 2003) (Fig. 1A). The full-length (S) and secreted (Ssol) spike sequences were amplified by PCR using the pCI-Ssynth or pCI-Scube plasmids as templates and oligonucleotides 5'-ATAGGATCCC GTACGACCAT GTTCATCTTC CTGCTGTTC-3' containing BsiWI restriction site (underlined) and 5'-ATAGGATCCG CGCGCTTATC AGGTGTAGTG CAGCTTCAC-3' or 5'-ATAGGATCCG CGCGCTCATT ATTTATCGTC GTCATCTTTA TAATC-3' containing BssHII restriction site (underlined). After digestion with BsiWI and BssHII restriction enzymes, the resulting DNA fragments were inserted into the corresponding sites of pTM-MVSchw-eGFP. The sequences inserted into the MV vector respect the “rule of six”, which stipulates that the number of nucleotides of the MV genome must be a multiple of 6 (Calain and Roux, 1993, Schneider et al., 1997). The resulting plasmids pTM-MVSchw-S and pTM-MVSchw-Ssol were used to rescue recombinant viruses using a helper-cell-based system as previously described (Combredet et al., 2003). Single viral clones were amplified on Vero-NK cells. All viral stocks were stored at −80 °C and titrated by an endpoint limiting dilution assay on Vero-NK cell monolayers. Growth curves of recombinant and parental viruses were determined on Vero-NK cells infected at an M.O.I. of 0.01, as described (Combredet et al., 2003).
Analysis of spike protein expression in infected cells
For indirect immunofluorescence assays, monolayers of Vero-NK cells plated on 20 mm glass coverslips in a 12-well plate were infected with the recombinant MVSchw-SARS or parental MV-Schw viruses at a multiplicity of infection (M.O.I.) of 0.05. When syncytia were clearly visible but not yet confluent (24–48 h after infection) cells were washed in d-PBS, fixed with PBS-4% paraformaldehyde for 15 min and in some instances permeabilized with PBS-0.2% triton X-100 for 10 min. Coverslips were then incubated for 60 min with anti-Ssol hyperimmune mouse ascitic fluid diluted 1/1000 in PBS-1% donkey serum (DKS). After subsequent incubation with a Cy3-conjugated anti-mouse IgG secondary antibody (Jackson Immunoresearch), the samples were mounted on slides with DAPI-containing Vectashield (Vector laboratory) and analyzed under an Axioplan 2 epifluorescence microscope (Zeiss). Pictures were acquired with an Axiocam MRm camera and processed with the Axiovision software (v 4.2, Zeiss).
For western blot assays, cytosolic cell extracts were prepared from Vero-NK cells infected with the recombinant MVSchw-SARS or parental MV-Schw viruses, essentially as described previously (Lorin et al., 2004). Proteins were separated by 8% SDS-polyacrylamide gels and transferred onto a PVDF membrane prior to immunoblotting with rabbit anti-S antibodies, as described (Callendret et al., 2007).
Mice experiments and characterization of humoral immune responses
All experiments were approved and conducted in accordance to the Pasteur Institute guidelines in compliance with European animal welfare regulations. To obtain CD46+/− IFNα/ßR−/− mice permissive for measles vaccine (Mrkic et al., 1998), FVB mice heterozygous for the measles vaccine CD46 receptor transgene were backcrossed to 129/Sv mice lacking the type IFN (Combredet et al., 2003). After more than 10 generations of backcrossing in our breeding colony, the resulting CD46-IFNAR line acquired a uniform 129/Sv background.
Six- to nine-week-old CD46-IFNAR mice were used to assess the immune response induced by recombinant MVSchw-SARS. Groups of 6 mice were injected intraperitoneally (i.p.) with 105 TCID50 of recombinant MVSchw-SARS or parental MVSchw. Control groups of mice were injected intramuscularly with formulations containing 2 µg of purified Ssol polypeptide and 50 µg aluminum hydroxide (Alu-Gel-S, Serva). Booster injections were administered 4 weeks thereafter. Serum samples were collected one week before immunization and 3 weeks after each injection (PI, IS1 and IS2 sera).
We measured the induction of SARS-CoV specific antibodies in immunized mice by indirect ELISA and neutralization assay, as described previously (Callendret et al., 2007). Briefly, microtiter plates were coated with SARS-CoV infected or mock-infected VeroE6 crude cell lysates and incubated with serial dilutions of the test sera. Bound antibodies were revealed with mouse-specific anti-IgG (1/2000, Amersham) secondary antibody coupled to horseradish peroxidase and TMB (3,3'-5,5'-tetramethylbenzidine, KPL). Readings from wells coated with mock lysates were substracted from wells with SARS-CoV lysates and the SARS-CoV specific IgG titers were calculated as the reciprocal of the highest dilution of individual serum, giving an absorbance of 0.5. The isotype determination of the antibody responses was performed using isotype-specific (IgG1 and IgG2a) secondary antibodies coupled to horseradish peroxidase (Southern Biotech). IgA titers were similarly measured using mouse-specific anti-IgA secondary antibody (1/2000, Southern Biotech) and calculated as the reciprocal of the highest dilution of individual serum, giving an absorbance of 0.2. MV specific antibodies were similarly measured using ELISA plates coated with purified measles antigens (Trinity Biotech, USA). For neutralization, two-fold serial dilution of heat-inactivated serum samples were incubated at 37 °C for 60 min with 100 TCID50 of SARS-CoV and added to a subconfluent monolayer of FRhK-4 cells in a 96-well microtiter plate. Each dilution of serum was tested in quadruplicate and cytopathic effect (CPE) endpoints were read up to 7 days after inoculation. Neutralizing antibody titers were determined according to the Reed and Muench method (Reed and Muench, 1938) as the reciprocal of the highest dilution of each serum, which suppressed CPE in at least 2 out of 4 wells.
Challenge infection of animals with SARS-CoV
Five weeks after the second immunization, animals were lightly anesthetized with Isoflurane (Mundipharma) and inoculated intranasally with 105 pfu of SARS-CoV in 40 µl PBS. Mice were euthanized 48 h after challenge infection. Lung homogenates were prepared in 500 µl DMEM supplemented with 2% FCS and titrated for infectious virus on VeroE6 monolayers, as described (Callendret et al., 2007). Viral RNA was extracted using the QIAamp Viral RNA Mini kit (Qiagen) from 100 µl of tissue homogenates according to the manufacturer's recommendations, eluted in 60 µl of RNAse-free water and analyzed by real-time RT-PCR, as described below. Challenge infection of all animals and subsequent analysis involving infectious materials were done in the “Jean Merieux” biosafety level 4 containment laboratory. Statistical analysis was performed on the log 10 of the viral titers measured for individual mice using the Student's independent t-test, with the assumptions used for small samples (normal distribution of the variables and same variance for the populations to be compared).
SARS-CoV genome RNA quantification by real-time RT-PCR
SARS-CoV genome RNA levels in tissue RNA samples were quantified by real-time RT-PCR with the PRISM 7700 sequence detection system. Briefly, RNA samples (2 µl) were reverse-transcribed and amplified with the TaqMan® One Step PCR Master Mix Reagents Kit (Applied Biosystems) according to the manufacturer's recommendations, in combination with primer set and flurorogenic probe specific for the nucleoprotein (N) gene (Drosten et al., 2004). Reactions were performed in thermo-fast® 96-well reaction plates (Abgene) with 25 µl mixtures containing 2 µl of template RNA, 300 nM concentration (each) of primers SANS1 (5'-TGGACCCAC AGATTCAAC TGA-3') and SANPAs2 (5'-GCTGTGAAC CAAGACGCA GTAT-3') (Eurogentec), and a 100 nM concentration of probe SANP1 (6-FAM-TAACCAGAATGGAGGACGCAATGG-TAMRA) (Eurogentec). The cycling conditions were 45 °C for 15 min, followed by 95 °C for 5 min and then 45 cycles of 95 °C for 15 s and 60 °C for 60 s. Fluorescence was read at the combined annealing-extension step at 60 °C. Absolute quantification of RNA was done by using in vitro transcribed RNA standards prepared from the cloned SARS-CoV N gene and quantified spectrophotometrically. Analytical sensitivity of this real-time N RT-PCR assay was experimentally determined by using limiting serial dilutions of the N RNA standard. Using probit non-linear regression analysis, the 95% detection limit of the RT-PCR was calculated at 40 copies of input RNA per reaction, which corresponded to 3.8 log 10 virus genome-equivalent (geq) per mouse tissue, assuming 100% efficiency in RNA preparation.
Acknowledgments
We thank Prof. Dr. H.W. Doerr (Institute of Medical Virology, Frankfurt University Medical School, Germany) for providing us with the SARS-CoV FFM-1 strain. We also thank Dr. Annette Martin for critical reading of the manuscript and helpful suggestions, Prof. Sylvie van der Werf for continuous support and Philippe Loth for expert technical assistance in performing the SARS-CoV challenge experiment. This work was supported in part by Grants from GlaxoSmithKline Biologicals. B.C. was supported by a fellowship from GlaxoSmithKline Biologicals. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Balboni A., Battilani M., Prosperi S. The SARS-like coronaviruses: the role of bats and evolutionary relationships with SARS coronavirus. N. Microbiol. 2012;35:1–16. [PubMed] [Google Scholar]
- Bellanti J.A., Zeligs B.J., Mendez-Inocencio J., Garcia-Garcia M.L., Islas-Romero R., Omidvar B., Omidvar J., Kim G., Fernandez De Castro J., Sepulveda Amor J., Walls L., Bellini W.J., Valdespino-Gomez J.L. Immunologic studies of specific mucosal and systemic immune responses in Mexican school children after booster aerosol or subcutaneous immunization with measles vaccine. Vaccine. 2004;22:1214–1220. doi: 10.1016/j.vaccine.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R., Subbarao K., Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334:160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler S., Lucas-Hourani M., Moris A., Frenkiel M.P., Combredet C., Fevrier M., Bedouelle H., Schwartz O., Despres P., Tangy F. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl. Trop. Dis. 2007;1:e96. doi: 10.1371/journal.pntd.0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler S., Marianneau P., Loth P., Lacote S., Combredet C., Frenkiel M.P., Despres P., Contamin H., Tangy F. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J. Infect. Dis. 2012;206:212–219. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- Brandler S., Ruffie C., Combredet C., Brault J.B., Najburg V., Prevost M.C., Habel A., Tauber E., Despres P., Tangy F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K., Collins P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calain P., Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callendret B., Lorin V., Charneau P., Marianneau P., Contamin H., Betton J.-M., van der Werf S., Escriou N. Heterologous viral RNA export elements improve expression of severe acute respiratory syndrome (SARS) coronavirus spike protein and protective efficacy of DNA vaccines against SARS. Virology. 2007;363:288–302. doi: 10.1016/j.virol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callendret B., Lorin V., Marianneau P., Contamin H., Rose T., Huerre M., van der Werf S., Charneau P., Escriou N., Efficient protection from SARS coronavirus infection conferred by a recombinant spike glycoprotein expressed as a soluble polypeptide in stable mammalian cell lines, Unpublished results.
- Centers for Disease Control Prevention, 2009. Global measles mortality, 2000–2008. MMWR, Morbidity and Mortality Weekly Report, vol. 58, pp. 1321–1326. [PubMed]
- Chen Z., Zhang L., Qin C., Ba L., Yi C.E., Zhang F., Wei Q., He T., Yu W., Yu J., Gao H., Tu X., Gettie A., Farzan M., Yuen K.Y., Ho D.D. Recombinant modified vaccinia virus ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J. Virol. 2005;79:2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combredet C., Labrousse V., Mollet L., Lorin C., Delebecque F., Hurtrel B., McClure H., Feinberg M.B., Brahic M., Tangy F. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 2003;77:11546–11554. doi: 10.1128/JVI.77.21.11546-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell S., Roberts R.J. Measles outbreak in Europe. BMJ – Br. Med. J. 2011;342:d3724. doi: 10.1136/bmj.d3724. [DOI] [PubMed] [Google Scholar]
- Davenport T.M., Friend D., Ellingson K., Xu H., Caldwell Z., Sellhorn G., Kraft Z., Strong R.K., Stamatatos L. Binding interactions between soluble HIV envelope glycoproteins and quaternary-structure-specific monoclonal antibodies PG9 and PG16. J. Virol. 2011;85:7095–7107. doi: 10.1128/JVI.00411-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R., West A., Donaldson E., Curtis K., Johnston R., Baric R. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres P., Combredet C., Frenkiel M.P., Lorin C., Brahic M., Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J. Infect. Dis. 2005;191:207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- Dilraj A., Cutts F.T., Bennett J.V., Fernandez de Castro J., Cohen B., Coovadia H.M. Persistence of measles antibody two years after revaccination by aerosol or subcutaneous routes. Pediatr. Infect. Dis. J. 2000;19:1211–1213. doi: 10.1097/00006454-200012000-00021. [DOI] [PubMed] [Google Scholar]
- Drosten C., Chiu L.L., Panning M., Leong H.N., Preiser W., Tam J.S., Gunther S., Kramme S., Emmerich P., Ng W.L., Schmitz H., Koay E.S. Evaluation of advanced reverse transcription-PCR assays and an alternative PCR target region for detection of severe acute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 2004;42:2043–2047. doi: 10.1128/JCM.42.5.2043-2047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Du L., He Y., Jiang S., Zheng B.J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today (Barc.) 2008;44:63–73. doi: 10.1358/dot.2008.44.1.1131830. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Dediego M.L., Alvarez E., Deming D., Sheahan T., Baric R. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133:45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett C., DeDiego M.L., Regla-Nava J.A., Enjuanes L., Perlman S. Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein. J. Virol. 2013;87:6551–6559. doi: 10.1128/JVI.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M., Lamirande E.W., Roberts A., Heise M., Subbarao K., Baric R.S. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans H., DeHovitz R., Forghani B., Beeler J., Maldonado Y., Arvin A.M. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- Gans H., Yasukawa L., Rinki M., DeHovitz R., Forghani B., Beeler J., Audet S., Maldonado Y., Arvin A.M. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J. Infect. Dis. 2001;184:817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J., Butt K.M., Wong K.L., Chan K.W., Lim W., Shortridge K.F., Yuen K.Y., Peiris J.S., Poon L.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Guerbois M., Moris A., Combredet C., Najburg V., Ruffie C., Fevrier M., Cayet N., Brandler S., Schwartz O., Tangy F. Live attenuated measles vaccine expressing HIV-1 Gag virus like particles covered with gp160DeltaV1V2 is strongly immunogenic. Virology. 2009;388:191–203. doi: 10.1016/j.virol.2009.02.047. [DOI] [PubMed] [Google Scholar]
- He Y., Li J., Heck S., Lustigman S., Jiang S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J. Virol. 2006;80:5757–5767. doi: 10.1128/JVI.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhu Q., Liu S., Zhou Y., Yang B., Li J., Jiang S. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology. 2005;334:74–82. doi: 10.1016/j.virol.2005.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan R.J., Gao G., Rowe T., Bell P., Flieder D., Paragas J., Kobinger G.P., Wivel N.A., Crystal R.G., Boyer J., Feldmann H., Voss T.G., Wilson J.M. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J. Virol. 2004;78:11416–11421. doi: 10.1128/JVI.78.20.11416-11421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M., Liang W., Zheng H., Wan K., Liu Q., Cui B., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Qi X., Chen K., Du L., Gao K., Zhao Y.T., Zou X.Z., Feng Y.J., Gao Y.F., Hai R., Yu D., Guan Y., Xu J. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 2005;79:11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S.U., Rose J.K., Lamirande E., Vogel L., Subbarao K., Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J.M., Nkolola J.P., Peng H., Cheung A., Perry J., Miller C.A., Seaman M.S., Barouch D.H., Chen B. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. USA. 2012;109:12111–12116. doi: 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lamb R.A., Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- Lamirande E.W., DeDiego M.L., Roberts A., Jackson J.P., Alvarez E., Sheahan T., Shieh W.J., Zaki S.R., Baric R., Enjuanes L., Subbarao K. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden Syrian hamsters. J. Virol. 2008;82:7721–7724. doi: 10.1128/JVI.00304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Liniger M., Zuniga A., Tamin A., Azzouz-Morin T.N., Knuchel M., Marty R.R., Wiegand M., Weibel S., Kelvin D., Rota P.A., Naim H.Y. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine. 2008;26:2164–2174. doi: 10.1016/j.vaccine.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin C., Mollet L., Delebecque F., Combredet C., Hurtrel B., Charneau P., Brahic M., Tangy F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 2004;78:146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin C., Segal L., Mols J., Morelle D., Bourguignon P., Rovira O., Mettens P., Silvano J., Dumey N., Le Goff F., Koutsoukos M., Voss G., Tangy F. Toxicology, biodistribution and shedding profile of a recombinant measles vaccine vector expressing HIV-1 antigens, in cynomolgus macaques. Naunyn-Schmiedeberg's Arch. Pharmacol. 2012;385:1211–1225. doi: 10.1007/s00210-012-0793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi-Akiyama T., Ishida I., Fukushi M., Yamaguchi K., Matsuoka Y., Ishihara T., Tsukahara M., Hatakeyama S., Itoh N., Morisawa A., Yoshinaka Y., Yamamoto N., Lianfeng Z., Chuan Q., Kirikae T., Sasazuki T. Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model. J. Infect. Dis. 2011;203:1574–1581. doi: 10.1093/infdis/jir084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkic B., Pavlovic J., Rulicke T., Volpe P., Buchholz C.J., Hourcade D., Atkinson J.P., Aguzzi A., Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Adair B.D., Yoshioka C., Quispe J.D., Orca G., Kuhn P., Milligan R.A., Yeager M., Buchmeier M.J. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006;80:7918–7928. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y., Wang G., Shi X., Zhang H., Qiu Y., He Z., Wang W., Lian G., Yin X., Du L., Ren L., Wang J., He X., Li T., Deng H., Ding M. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 2004;190:1119–1126. doi: 10.1086/423286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S. The middle East respiratory syndrome – how worried should we be? mBio. 2013;4:e00531-13. doi: 10.1128/mBio.00531-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C.M., Melancon J.M., Chouljenko V.N., Colgrove R., Farzan M., Knipe D.M., Kousoulas K.G. Genetic analysis of the SARS-coronavirus spike glycoprotein functional domains involved in cell-surface expression and cell-to-cell fusion. Virology. 2005;341:215–230. doi: 10.1016/j.virol.2005.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Bazarsky E., Skibin A., Chamney S., Belmaker I., Shai I., Kordysh E., Griffin D.E. The effect of measles–mumps–rubella (MMR) immunization on the immune responses of previously immunized primary school children. Vaccine. 2003;21:2580–2588. doi: 10.1016/s0264-410x(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Roberts A., Lamirande E.W., Vogel L., Jackson J.P., Paddock C.D., Guarner J., Zaki S.R., Sheahan T., Baric R., Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133:20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Thomas W.D., Guarner J., Lamirande E.W., Babcock G.J., Greenough T.C., Vogel L., Hayes N., Sullivan J.L., Zaki S., Subbarao K., Ambrosino D.M. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J. Infect. Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev. Vaccines. 2009;8:887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Schneider H., Kaelin K., Billeter M.A. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology. 1997;227:314–322. doi: 10.1006/viro.1996.8339. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Amor J., Valdespino-Gomez J.L., Garcia-Garcia Mde L., Bennett J., Islas-Romero R., Echaniz-Aviles G., de Castro J.F. A randomized trial demonstrating successful boosting responses following simultaneous aerosols of measles and rubella (MR) vaccines in school age children. Vaccine. 2002;20:2790–2795. doi: 10.1016/s0264-410x(02)00179-2. [DOI] [PubMed] [Google Scholar]
- Sheahan T., Whitmore A., Long K., Ferris M., Rockx B., Funkhouser W., Donaldson E., Gralinski L., Collier M., Heise M., Davis N., Johnston R., Baric R.S. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J. Virol. 2011;85:217–230. doi: 10.1128/JVI.01805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.K., Ramirez K., Cuberos L., Campbell J.D., Viret J.F., Munoz A., Lagos R., Levine M.M., Pasetti M.F. Mucosal IgA responses in healthy adult volunteers following intranasal spray delivery of a live attenuated measles vaccine. Clin. Vaccine Immunol.: CVI. 2011;18:355–361. doi: 10.1128/CVI.00354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.C., Seo M.Y., Stadler K., Yoo B.J., Choo Q.L., Coates S.R., Uematsu Y., Harada T., Greer C.E., Polo J.M., Pileri P., Eickmann M., Rappuoli R., Abrignani S., Houghton M., Han J.H. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2004;78:10328–10335. doi: 10.1128/JVI.78.19.10328-10335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.D., Tu C.C., Zhang G.W., Wang S.Y., Zheng K., Lei L.C., Chen Q.X., Gao Y.W., Zhou H.Q., Xiang H., Zheng H.J., Chern S.W., Cheng F., Pan C.M., Xuan H., Chen S.J., Luo H.M., Zhou D.H., Liu Y.F., He J.F., Qin P.Z., Li L.H., Ren Y.Q., Liang W.J., Yu Y.D., Anderson L., Wang M., Xu R.H., Wu X.W., Zheng H.Y., Chen J.D., Liang G., Gao Y., Liao M., Fang L., Jiang L.Y., Li H., Chen F., Di B., He L.J., Lin J.Y., Tong S., Kong X., Du L., Hao P., Tang H., Bernini A., Yu X.J., Spiga O., Guo Z.M., Pan H.Y., He W.Z., Manuguerra J.C., Fontanet A., Danchin A., Niccolai N., Li Y.X., Wu C.I., Zhao G.P. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. USA. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K., Roberts A., Becker S., Vogel L., Eickmann M., Kolesnikova L., Klenk H.D., Murphy B., Rappuoli R., Abrignani S., Subbarao K. SARS vaccine protective in mice. Emerg. Infect. Dis. 2005;11:1312–1314. doi: 10.3201/eid1108.041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings R., Fevrier M., Li B., Lorin C., Koutsoukos M., Mee E., Rose N., Hall J., Page M., Almond N., Voss G., Tangy F. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PloS One. 2012;7:e50397. doi: 10.1371/journal.pone.0050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Geisbert T.W., Geisbert J.B., Shedlock D.J., Xu L., Lamoreaux L., Custers J.H., Popernack P.M., Yang Z.Y., Pau M.G., Roederer M., Koup R.A., Goudsmit J., Jahrling P.B., Nabel G.J. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3:e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangy F., Naim H.Y. Live attenuated measles vaccine as a potential multivalent pediatric vaccination vector. Viral Immunol. 2005;18:317–326. doi: 10.1089/vim.2005.18.317. [DOI] [PubMed] [Google Scholar]
- ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L., Kuiken T., de Kruif J., Preiser W., Spaan W., Gelderblom H.R., Goudsmit J., Osterhaus A.D. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363:2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp R.A., Haynes L.M., Moore D., Anderson B., Tamin A., Harcourt B.H., Jones L.P., Yilla M., Babcock G.J., Greenough T., Ambrosino D.M., Alvarez R., Callaway J., Cavitt S., Kamrud K., Alterson H., Smith J., Harcourt J.L., Miao C., Razdan R., Comer J.A., Rollin P.E., Ksiazek T.G., Sanchez A., Rota P.A., Bellini W.J., Anderson L.J. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J. Virol. Methods. 2005;128:21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PloS One. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2004. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 〈http://www.who.int/csr/sars/country/table2004_04_21/en/index.html〉 (accessed 29.08.2013).
- WHO, 2013. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update – as of 13 August 2013. 〈http://www.who.int/csr/disease/coronavirus_infections/update_20130813〉 (accessed 29.08.2013).
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Werner H.C., Kong W.P., Leung K., Traggiai E., Lanzavecchia A., Nabel G.J. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. USA. 2005;102:797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K.M., Chiueh T.S., Siu L.K., Lin J.C., Chan P.K., Peng M.Y., Wan H.L., Chen J.H., Hu B.S., Perng C.L., Lu J.J., Chang F.Y. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang W., Zhong Q., Hou W., Yang Z., Xiao S.Y., Zhu R., Tang Z., Wang Y., Xian Q., Tang H., Wen L. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23:3202–3209. doi: 10.1016/j.vaccine.2004.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M., Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]