Abstract
Porcine aminopeptidase N (pAPN) is a cellular receptor of transmissible gastroenteritis virus (TGEV), a porcine coronavirus. Interaction between the spike (S) protein of TGEV and pAPN initiates cell infection. Small molecules, especially peptides are an expanding area for therapy or diagnostic assays for viral diseases. Here, the peptides capable of binding the pAPN were, for the first time, identified by biopanning using a random 12-mer peptide library to the immobilized protein. Three chemically synthesized peptides recognizing the pAPN showed effective inhibition ability to TGEV infection in vitro. A putative TxxF motif was identified in the S protein of TGEV. Phages bearing the specific peptides interacted with the pAPN in ELISA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays confirmed the protective effect of the peptides on cell infection by TGEV. Moreover, the excellent immune responses in mice induced by the identified phages provided the possibility to develop novel phage-based vaccines.
Keywords: Coronavirus, TGEV, Peptides, Phage display, Vaccine
Introduction
Transmissible gastroenteritis virus (TGEV) is the causative agent of transmissible gastroenteritis (TGE), a highly contagious enteric disease in swine. The typical clinical symptoms of TGE consist of vomiting, acute diarrhea, and dehydration. Older animals usually recover, but in newborn piglets mortality rates may reach 100% (Saif and Wesley, 1992, Laude et al., 1993, Schwegmann-Wessels et al., 2002, Ren et al., 2008). TGE prevalence causes enormous economic losses in pig industry. TGEV is an enveloped, single-stranded, positive-sense RNA virus that belongs to the family Coronaviridae and order Nidovirales (Enjuanes et al., 2000, Yin et al., 2010). They own the largest RNA viral genome, about 30 kb. TGEV produces at least eight subgenomic mRNAs during viral replication and each mRNA consists of 3′ co-terminal nested sets (Laude et al., 1993, Vaughn et al., 1995).
Three major structural proteins of coronavirus: the spike (S), the integral membrane (M) glycoprotein, and the nucleocapsid (N) protein are translated from mRNAs 2, 5 and 6, respectively (Spaan et al., 1988, Laude et al., 1993, Almazán et al., 2000). The M protein is an abundant component of coronaviruses (Rottier, 1995, Ren et al., 2010b). The M protein as the major interferon inducing component has been proposed to play a role in innate immune response to coronaviruses (Charley and Laude, 1988, Laude et al., 1992). Roughly one-third of TGEV M protein assumes a topology in which part of the endodomain constitutes a fourth transmembrane segment, thereby positioning the carboxy terminus of the molecule on the exterior of the virion (Masters, 2006, Risco et al., 1995). TGEV N phosphoprotein complexes with the genomic RNA in a beads-on-a-string fashion to form the nucleocapsid (Suñé et al., 1990, Cavanagh and The Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 1994). The S protein of coronaviruses is a large transmembrane protein with the amino terminus exposed to the virus surface and the carboxy terminus inside the virus particle and it assembles into trimers to form the distinctive viral surface spikes (Delmas and Laude, 1990). Coronavirus S protein plays an important role in inducing neutralizing antibodies (Garwes et al., 1978, Jiménez et al., 1986, Laude et al., 1987, Suñé et al., 1990) and it is also related to host cell tropism (Jacobs et al., 1986, Schwegmann-Wessels et al., 2003, Schwegmann-wessels et al., 2009, Ren et al., 2006), pathogenicity (Siddell, 1995, Krempl et al., 1997), fusion (Collins et al., 1982, Spaan et al., 1988), hemagglutination activity (Krempl et al., 2000, Krempl and Herrler, 2001) and interaction with its cellular receptors such as porcine aminopeptidase N (pAPN) (Delmas et al., 1992, Liu et al., 2009, Ren et al., 2010a).
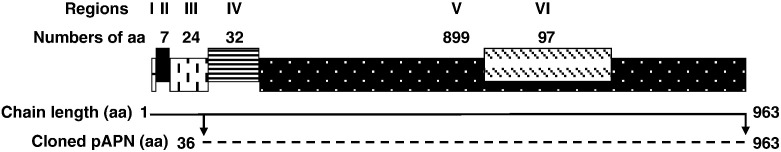
APN, also called CD13 in human is a type II transmembrane ectopeptidase of 150 kDa that contains a zinc-binding motif (HEIAH) and forms a noncovalently bound homodimer on the cellular membrane (Liu et al., 2009). APN was extensively expressed on various cell lines such as hematopoietic cells of myeloid origin, fibroblasts, brain cells, and epithelial cells of the liver, kidney, and intestine, etc. (Tsukamoto et al., 2008, Zhang et al., 2008). It is reported that aminopeptidase N (pAPN) is a cellular receptor for most of group 1 coronaviruses including human coronavirus 229E (HCoV-229E), TGEV, feline coronavirus (FCoV) and canine coronavirus (CCoV) (Delmas et al., 1992, Tresnan et al., 1996, Yeager et al., 1992). Generally, APN as receptors for many coronaviruses are species-specific (Delmas et al., 1994, Kolb et al., 1997), although the feline APN (fAPN) can also serve as a receptor for canine coronavirus, TGEV and human coronavirus 229E in addition to feline infectious peritonitis virus (Tresnan et al., 1996). The pAPN consists of several identified regions, which include the initiator methionine, cytoplasmic topological domain, transmembrane region, cytosolic Ser/Thr-rich junction region, metalloprotease region, and TGEV spike glycoprotein-interacting region, respectively (Fig. 1 ).
Fig. 1.
Schematic drawing of porcine aminopeptidase N. The linear structure of porcine aminopeptidase N (pAPN) was classified into six regions. The feature key of regions I to VI consists of initiator methionine, cytoplasmic topological domain, transmembrane region, cytosolic Ser/Thr-rich junction region, metalloprotease region, and TGEV spike glycoprotein-interacting region, respectively. The number of amino acids in each region is indicated. Region VI (from 717–813 aa) is overlaid within region V (from 65–963 aa). The bold line shows the chain of pAPN and the broken line shows the length of the cloned pAPN used for expression. It should be noted that the sizes of the boxes or the lines are not proportional to the length of the amino acid chain.
Peptide ligands that target a specific protein surface own broad applications as therapeutics by interfering protein–protein interactions. Phage display libraries provide a powerful and inexpensive way to identify such peptides. Phage random peptide library consists of a pool of billions of peptides that can be produced by the fusion of random nucleic acid sequences to the N terminus of one of the capsid protein genes (pVIII or pIII) of a filamentous bacteriophage (Cwirla et al., 1990, Devlin et al., 1990, Scott and Smith, 1990). By optimal biopanning, a single clone of phages with a desired binding specificity from a random phage library can be identified. This approach has been applied successfully in numerous aspects, including antibody engineering (Hayden et al., 1997), peptide and protein drug discovery and manufacture (Kay et al., 1998), diagnostic analysis (Ren et al., 2010c), and vaccine development (Lesinski and Westerink, 2001).
At present, the precise localization of receptor-binding domains (RBD) in TGEV S protein remains unclear. At the same time, the RBD-containing peptides may be useful small molecules for diagnosis and therapy in viral infection. In this study, we identified, for the first time, the specific peptides recognizing pAPN that blocks the binding to TGEV, based on phage display technology. The peptides competitively blocked TGEV infection in vitro. They, therefore, can be used as specific antiviral inhibitors. A putative TxxF motif was identified in the S protein. Moreover, the peptide-containing phages elicited effective immune response in vivo, demonstrating a potential perspective in development of phage-based vaccines.
Results
Expression of pET-apn
The gene encoding a truncated pAPN with the deletion of cytoplasmic topological domain and transmembrane region (Fig. 1) was amplified using PCR and then cloned into pET30a vector. The authenticity of the recombinant plasmid pET-apn was confirmed by DNA sequencing. The pAPN-bearing bacteria were induced with IPTG to express the protein of interest, after pET-apn was transformed into host bacteria. SDS-PAGE indicated that the fusion protein was about 112 kDa and the immunoreactivity of this protein was confirmed by Western blot and immunofluorescence (data not shown).
Enrichment efficacy and binding activity of phages after biopanning
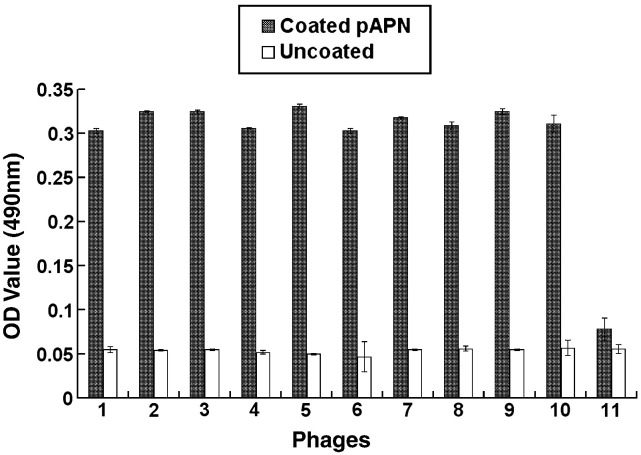
After four rounds of biopanning using the pAPN-protein as an immobilized target, the enrichment efficacy of phages for each round was analyzed. The titer of phages in elution buffer and the amplified phages was increased with the increased panning times (Table 1 ). Ten phage clones were numbered 1 to 10 and picked from the last round panning and amplified in host cells. The binding activity of the selected phages to the target protein was assayed using ELISA. Our results showed that they had a specific binding activity to the pAPN (Fig. 2 ).
Table 1.
Titration of the phages post-panning.
| Round | First | Second | Third | Fourth |
|---|---|---|---|---|
| Elute | 4.6 × 105 | 5.54 × 105 | 5.92 × 105 | 3.6 × 106 |
| Amplification | 8.2 × 1013 | 1.14 × 1014 | 2.38 × 1014 | N/A |
The phages at a density of 2 × 1011 pfu/ml were incubated with the pAPN proteins for each round of panning. The titer of phages in elution buffer (Elute) and in the amplification in the host cells calculated is shown. The phages were selected from the elute in the fourth round of panning and the phage titer of that time was not measured (N/A).
Fig. 2.
Binding of the selected phages to pAPN in ELISA. Ten selected phages (numbers 1 to 10) and an unrelevant phage (number 11) were incubated with coated pAPN or bovine serum albumin control (uncoated) in ELISA plates followed by successive incubation with anti-M13 antibody and HRP-conjugated secondary antibody. OD490 value of individual phage from three parallel wells is shown in y axis; the number of the phages is shown in x axis.
Analysis of the peptide sequences
The DNA of the phages was extracted and then the heterologous genes encoding the peptides on the surface of the recombinant phages were amplified by primer-specific PCR. The DNA sequencing reports showed that three identical peptides were identified among the ten phages (Table 2 ). Phage numbers 1, 4 and 6 had a consensus peptide sequence SVVPSKATWGFA named S. Phages 2, 7, 8 and 10 had a consensus sequence HVTTTFAPPPPR named H. The consensus sequence derived from Phages 3, 5 and 9 was FKPSSPPSITLW named F. The results showed that that the three peptides have sequence homologies and that a TxxF(AK)PxxP overlapping motif could be identified. These identified motifs were compared with TGEV S protein sequence. Several TxxF motifs were identified in the S protein (Supplementary Fig. 1).
Table 2.
Deduced amino acid sequences of phage clones.
| PCR product number | Sequences of deduced amino acids of each peptide |
|---|---|
| 1, 4, 6 | S V V P S K A T W G F A (S) |
| 2, 7, 8, 10 | H V T TT F A P P P P R (H) |
| 3, 5, 9 | F K P S S P P S I T L W (F) |
Ten selected phages (phages 1 to 10) were subjected to DNA extraction and PCR. The deduced amino acid sequences are shown. Three peptide sequences identified are shown and designated as S, H and F, respectively (in parenthesis).
Binding activity of peptides to pAPN and its inhibitory effect on cell infection
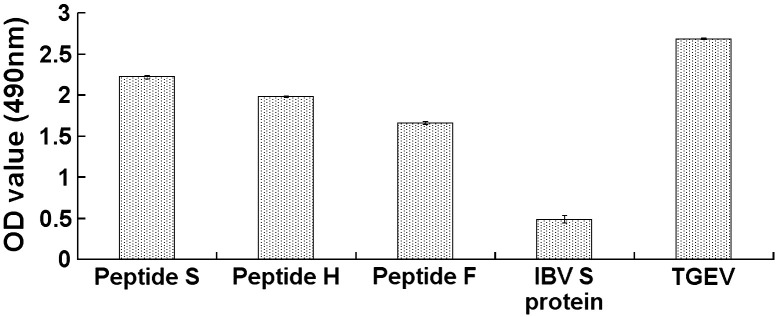
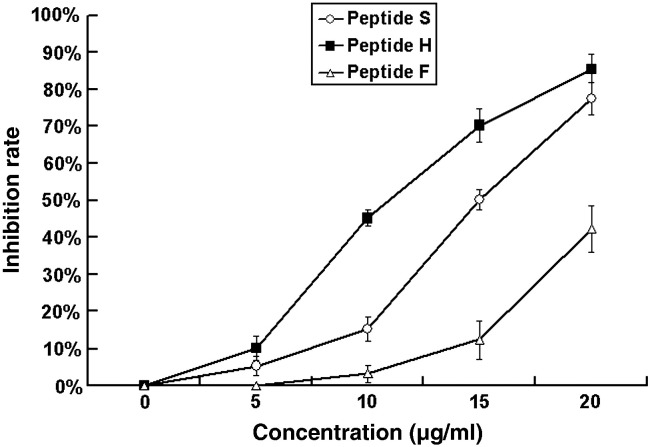
ELISA results showed that the three peptides were capable of binding the pAPN. TGEV coated plates have a higher binding activity than these peptides. There was no significant binding between the IBV S protein and the anti-pAPN antibody (Fig. 3 ). Virus infection inhibition assay showed that ST cells infected by TGEV produced significant cytopathic effect (CPE) at 48 h post infection. In contrast, the CPE number of peptide-treated ST cells infected by TGEV decreased in a dose-dependent manner, and the maximum inhibitory activity of TGEV infection in vitro was achieved at a concentration of 20 μg/ml of each peptides (Fig. 4 ).
Fig. 3.
Binding of the peptides to pAPN. The binding ability of the identified peptides to pAPN was investigated in ELISA by coating the pAPN as target protein. The TGE virion and IBV S protein produced from the same expression system were used as positive and negative controls, respectively. The OD490 value is shown in y axis.
Fig. 4.
Inhibitory effect of peptides on TGEV infection. The infectivity of TGEV was analyzed after ST cells were pre-treated with the identified peptides. The virus infection and mock-treated cells were included as controls. The inhibition rate of the peptides at indicated concentrations is shown in y axis. The 100% infectivity value corresponds to an average plaque number of 170.
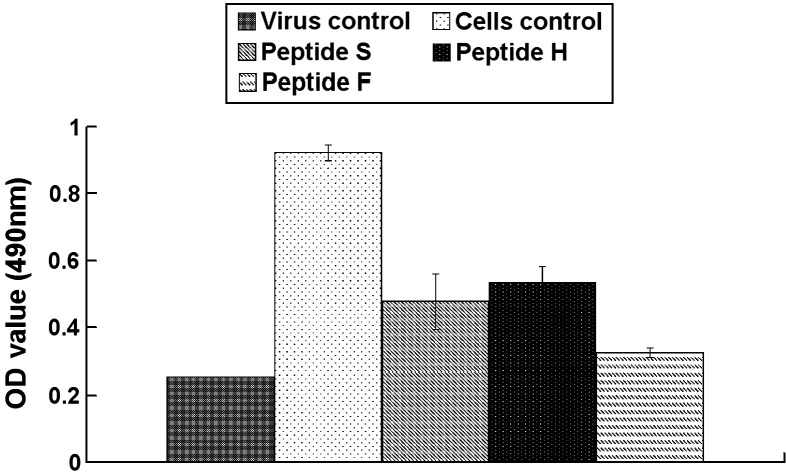
Conventional MTT assays were used to analyze the effect of the peptides on the proliferation of ST cells infected by TGEV. Our results showed that OD490 value of peptide-treated ST cells infected by TGEV was higher than virus infection control, indicating that the proliferation of cells infected by TGEV was enhanced by the peptides (Fig. 5 ). The pre-treatment of the peptides led to increasing number of viable cells, also confirming the antiviral ability of the biologically active peptides.
Fig. 5.
MTT assays analyzing the effect of peptides to cell proliferation. The effect of the peptides on the proliferation of cells infected by TGEV was analyzed by conventional MTT assays. The OD490 value of the peptides at the maximum non-toxic concentration (20 μg/ml) is provided.
Antibody responses elicited by the specific phages
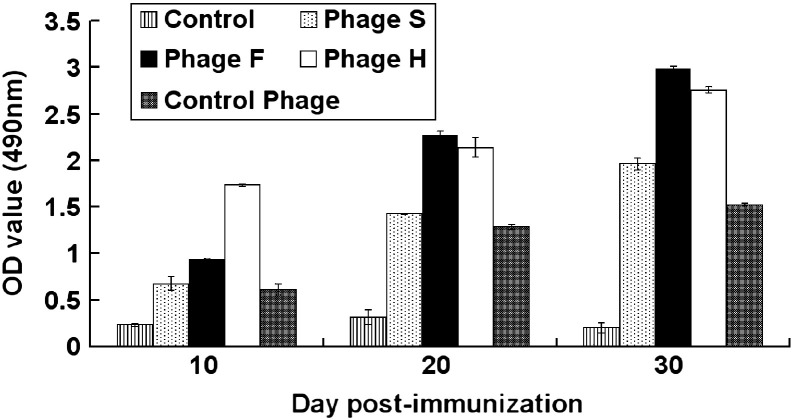
The phages bearing the identified peptides were used to immunize mice. At indicated time points, the antibody from the peripheral blood in the immunized mice was used as primary antibody to detect TGEV in ELISA. The results showed that the peptides displayed on filamentous phages triggered an immune response in mice against the TGE virions. The pAPN-recognizing peptides elicited stronger humoral immune response than the control phages bearing complex peptides, although the control serum might also react with the TGE virions to certain extent (Fig. 6 ).
Fig. 6.
Phages bearing specific peptides trigger antibody responses against TGEV. After the mice were immunized with the peptide-bearing phages, the sera isolated from the animal at indicated time points were used as primary antibody to detect TGEV coated in ELISA plates. The mock-immunized mice and the mice immunized with the phages from the random peptide library were used as controls. The OD490 value is shown.
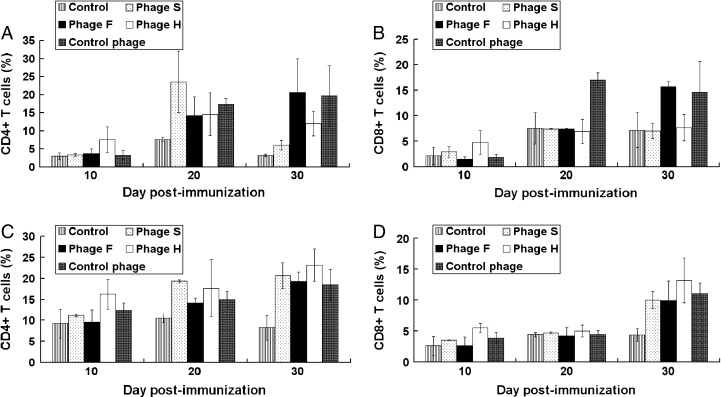
Change of T lymphocyte number from peripheral blood and spleens in immunized mice
The number of T lymphocytes from peripheral blood and spleens in immunized mice was quantified by flow cytometry. The number of CD4+ T cells increased at 20 days post-immunization in the mice immunized with the phages compared with the mock-immunized mice. At 30 days post-immunization, the difference in the CD8+ T cell number between the immunization group and control became significant (Fig. 7 ).
Fig. 7.
Changes of T lymphocytes from peripheral blood and spleens of the immunized mice. The T lymphocytes from the peripheral blood and spleens in immunized mice were isolated. The number of T cell subsets was quantified by flow cytometry. The number of CD4+ and CD8+ T cells in the peripheral blood is shown in A and B, respectively. The number of CD4+ and CD8+ T cells in the spleens is shown in C and D, respectively.
Discussion
The APN serves as a cellular receptor for several coronaviruses. In this study, we aimed at using this protein as target protein for identifying its ligands and investigating the role of the small molecules. The prokaryotic system is an optimal choice for achieving a high-level expression of heterologous protein (Yin et al., 2007). In our previous studies, we expressed the same protein in Escherichia coli by cloning the gene into a different vector, pGEX 6p-1 (Liu et al., 2009). The fused protein has a glutathione-S-transferase (GST) tag with high molecular weight of 26 kDa which might have cross-reaction with other unrelated proteins. Therefore, we cloned the truncated pAPN gene into another prokaryotic vector pET-30a that owns T7 lac promoter and has a small tag (6*Histidine) protein. Rosetta host strain used in this study is designed to enhance the expression of eukaryotic proteins that contain rare codons. It supplies tRNAs for AGG, AGA, AUA, CUA, CCC, and GGA codons on a compatible chloramphenicol-resistant plasmid named pRARE, which makes the Rosetta strain a “universal” translation where translation would otherwise be limited by the codon usage of E. coli (Novy et al., 2001). Many heterologous proteins have been expressed in this system (Li et al., 2010a, Li et al., 2010b, Meng et al., 2010, Ren et al., 2010a, Ren et al., 2010d). In our study, the high level expression of pAPN was achieved again in this expression system. The pAPN is a glycoprotein and its biological activity is the main concern due to the inability of modification post-translation in E. coli. Previously, we generated an anti-pAPN antibody deriving from a rabbit immunized with pGEX-pAPN. In this study, the bacterially expressed pET-apn protein was detected in Western blot by using the anti-pAPN antibody. At the same time, we confirmed that an antibody against pET-apn protein reacted with transiently expressed pAPN on the eukaryotic cells in immunofluorescence assays and pre-incubation of the antibody with cells significantly decreased cell infection by TGEV (data not shown). Taken together, the data indicate that the pAPN used in this study is biologically active. Interestingly, the expression of pAPN was achieved only if the transmembrane domain located at its N-terminus was deleted. The hydrophilicity and antigen index were analyzed using DNASTAR software, and the results showed that the first 35 amino acids in the N terminus of pAPN are a strong hydrophilicity region and low antigen index (data not shown). The results suggest that the transmembrane domain in the pAPN may affect subsequent translation of pAPN in E. coli.
The application of vaccines is still the major prevention measure for TGEV infection, although pAPN has been identified as a cellular receptor for TGEV (Delmas et al., 1992). The importance of effective peptides in the drug discovery has been noticed due to their apparent ability to “home in” on active or biological sites on target proteins (Yi et al., 2003). It has been found that phage-displayed peptides selected from combinatorial libraries that interacting with viral proteins or receptors were capable of blocking infection by hepatitis B virus (Dyson and Murray, 1995), adenovirus type 2 (Ad2) (Hong and Boulanger, 1995), Andes Virus (ANDV) (Hall et al., 2009), Sin Nombre Virus and Hantaan Virus (Larson et al., 2005). In our study, the immobilized pAPN was used as a target for incubation with the phages from the 12-mer phage display library. The binding phages were eluted after four rounds of panning. The increased binding ability between the phages and the pAPN was indicated by the gradually increased phage titration following each panning. Three peptide sequences were obtained from the ten selected phages. The binding of the peptides to the pAPN protein was somewhat lower than the binding between the pAPN and TGEV, although it was much higher than the control, indicating that there are more epitopes on TGEV necessary for interaction between virion and pAPN. Since the peptides did not react with IBV S protein expressed in the same system, the phages do not have cross-reaction with the His-tag protein. Plaque assays showed that the infectivity of TGEV on the native pAPN-expressing ST cells was decreased by the pre-treatment of the individual peptides. There was a slight difference in the abilities of the peptides to inhibit TGEV infection. Interestingly, the antiviral ability of each of the three peptides was positively correlated with their binding ability to pAPN. In our study, MTT assays confirmed the inhibitory effect of the peptides on TGEV infection. Nonetheless, the proliferation rate of the infected cells was lower than the mock-infected cell, although it was improved by pre-treatment of the peptides. The discrepant results between plaque assays and MTT assays may be partially attributed to: 1) the latter is often used to determine the mitochondrial dehydrogenase activities of cells, which cannot reflect the inhibition of cell death or cell respiration totally; 2) cell apoptosis induced by TGEV may interfere with the MTT results to a certain extent (Eleouet et al., 1998).
It has been documented that four major antigenic sites of TGEV are located in the N-terminal half of the S protein (designated S1) (Delmas et al., 1990). In our study, sequence alignment between the peptides and TGEV S protein indicated that the three peptides have the most homology identity in different regions in the S1 protein. We suppose that the peptide sequences or the counterparts in the S protein should be important receptor binding domains (RBDs) that may interact with its cellular receptor of pAPN. The binding of TGEV S protein to pAPN is required for the initial stage of infection (Delmas et al., 1992). The interaction between the peptides and pAPN was investigated using ELISA and virus plaque assays. Interestingly, the peptides showed an effective binding to pAPN and inhibition to TGEV infection, although their sequences are not completely identical to the homologous regions in the S1 protein. The results confirm that short peptides from combinatorial libraries can act as ‘surrogate’ ligands of proteins that interact with other proteins if they possess the required critical residues (Geysen et al., 1985, Ruoslahti and Pierschbacher, 1986, Wells, 1996). The peptides can be used as lead compounds for further optimization and consequent enhancement of antiviral activity. In addition to binding to pAPN, TGEV virions treated with neuraminidase can bind via the S protein to N-acetyl neuraminic acid or N-glycolyl neuraminic acid moieties on the cell surface (Krempl et al., 2000, Schultze et al., 1995). It has been proposed that binding to a surface sialoglycoprotein is required for TGEV as a primary attachment site to initiate infection of intestinal cells (Schwegmann-Wessels et al., 2002). Therefore, it would be interesting to screen the ligands of sialoglycoprotein and analyze their roles in the context of TGEV infection. There are no structural data and only limited functional information regarding the spike glycoproteins of coronaviruses in serogroup 1, which includes HCoV-229E, TGEV, FCoV, and CCoV, although they all use APN as a receptor to enter cells (Benbacer et al., 1997, Bonavia et al., 2003, Delmas et al., 1992, Hegyi and Kolb, 1998, Yeager et al., 1992). Therefore, the peptides identified in this study or the high homologous regions in the S proteins could be major RBDs of viral S proteins. The peptides are also expected to be viable alternatives or peptide-based drugs to antibodies in prevention of TGEV infection. In addition, the binding domain of the S protein on its receptor has been mapped starting at amino acid 506 and shown to correspond to the domain recognized by neutralizing antibodies (Godet et al., 1994). In our study, we identified a TxxF by phage display and four TxxF motifs were found in the domain. Interestingly, three such motifs also existed in the other part of the S protein. Further work will be done to analyze the biological role of the identified motif in the S protein.
In Europe, the appearance of porcine respiratory coronavirus (PRCV), a respiratory mutant of TGEV, has decreased the risk of TGE to some extent, because neutralizing antibodies elicited by the avirulent PRCV can provide cross-protection against TGEV infection (Schwegmann-Wessels and Herrler, 2006). Nevertheless, TGE prevalence is often reported and some TGEVs are being isolated in different regions in China, indicating that TGEV infection is still threatening to the Chinese pig industry (Ao et al., 2006, Bai, 2005). Currently, vaccination is the major prophylaxis measure against TGEV infection. Many studies have provided direct or indirect evidence for the involvement of S protein in inducing a protective immune response or in viral pathogenicity (Daniel and Talbot, 1990, Torres et al., 1996, Krempl et al., 1997, Wang et al., 1992).
TGEV S protein derives from a 1447-amino acid-long precursor polypeptide of which a 16-residue signal peptide is removed (Rasschaert and Laude, 1987). S is the only protein that induces antibodies that neutralize in the absence of complement (Godet et al., 1991, Jiménez et al., 1986, Pulford and Britton, 1991, Suñé et al., 1990). Investigations of its antigenic structure have led to the identification of four to five major groups of B-cell dependent epitopes, all located in the S1 part of the polypeptide chain (Correa et al., 1990, Delmas et al., 1986, Delmas et al., 1990, Gebauer et al., 1991, Enjuanes et al., 1992, Godet et al., 1994). Another significant achievement of this work is the demonstration that the identified phages bearing the specific peptides in their N-terminal region of major coat protein PVIII may induce specific humoral immune responses and an enhanced cell-medicated immunity. The serum antibody of the immunized mice can react with TGE virions, indicating that the phage displaying specific peptides can mimic the natural epitopes of the S protein. In this study, we included the mixed phages from a 12-mer phage library as control for immunizing the mice. In our binding assays, a relative high background was observed between TGEV and the unrelevant phages. On one hand, we speculate that there are TGEV ligands in the library that we did not identify. On the other hand, the heterologous genes were expressed in the PVIII of the phages. It might be possible that other components of the phages may have binding activity to TGEV. More work is needed to confirm our hypotheses.
The number of CD4+ and CD8+ T lymphocytes in the spleens and peripheral blood of the immunized mice increased at 10 days post-immunization. Our findings are interesting, and it seems that the phages bearing heterologous peptides may stimulate T lymphocyte-mediated immune response and phage-based vaccines can be used as excellent heterologous antigens recognized by specific antigen-presenting cells, initiating host immune responses. Since the global abundance of phages and our increasing ability to exploit them, further elucidation of mechanisms of phage-mediated immunization may facilitate the rational design of novel phage-based vaccines. In addition, compared with peptide-based vaccines, the phage-based vaccine owns advantages in production cost and efficiency.
In conclusion, we have obtained the peptide sequences that recognize the pAPN protein using phage display library. The chemically synthesized peptides block cell infection by TGEV through competition binding the viral cellular receptor. The putative RBDs are localized by sequence alignment between the peptides and TGEV S protein. Since many coronaviruses in group I use APN as receptor, the peptides selected in this study may be candidates for their molecule inhibitors. The phages bearing the specific peptides may elicit both humoral and cellular immune responses, indicating that the present methodology has the potential to develop novel phage-based novel vaccines.
Materials and methods
Expression of recombinant plasmid bearing pAPN
Recombinant plasmid, pcDNA3.1-apn containing full-length pAPN (a gift from Dr. Georg Herrler of Institute for Virology, University of Veterinary Medicine Hannover, Germany) was used as template for PCR amplifying a truncated pAPN gene starting from amino acid 36 using sense primer P1–5′ GGGGGGATCCGAGAAGAACAAGAATGCC 3′, and antisense primer P2–5′ CCCCCTCGAGTGCTGTGCTCTATGAACCA 3′. Underscored parts contain BamHI and XhoI restriction sites, respectively. The PCR profile included 95 °C for 5 min, 30 cycles of 95 °C for 1 min, 57 °C for 30 s, 72 °C for 3 min, and then a final extension of 72 °C for 10 min. The purified PCR product was inserted in the same sites of pET-30a vector by conventional cloning techniques, resulting in a recombinant plasmid designated pET-apn. After pET-apn was transformed into E. coli strain BL21(DE3) pLysS (Novagen, Germany), expression of the gene of interest was induced by isopropyl β-d-thiogalactoside (IPTG) at a final concentration of 1 mM at 37 °C. The bacterially expressed protein was identified in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), purified by gel-purification method and used to generate a rabbit anti-pAPN antibody as previously described (Liu et al., 2009).
Virus binding blocking assay
Transmissible gastroenteritis virus (TGEV, Purdue 46-MAD strain, a gift from Dr. Luis Enjuanes, Centro Nacional de Biotecnología, CSIC, Madrid, Spain) was used for virus infection assays. To analyze the blockade of the anti-pAPN antibody to TGEV infection, ST cells in 6-well plates were incubated with the antibody achieved in this study at 37 °C for 1 h. After complete washing, TGEV at an MOI (multiplicity of infection) of 10 was used to infect the cells for 1 h. The cells were washed three times with PBS and the inoculums were replaced with 1% (w/v) methylcellulose in EMEM (1 ml/well) followed by incubation for 48–72 h. Unrelated control rabbit sera were included as control. The wells were subjected to virus-plaque reduction assay using crystal violet staining (Li et al., 2009, Ren et al., 2008).
Biopanning and enrichment analysis
The purified pAPN was used as the target protein and subjected to biopanning using a Ph.D.-12 Phage Display Peptide Library Kit. The procedure was performed according to the manufacturer's instructions with modification (New England Biolabs, USA). Briefly, the protein of interest at a concentration of 10 μg/well in 0.1 M NaHCO3 (pH 8.6) buffer was coated onto 96-well plates overnight at 4 °C. Then the plates were blocked for 1 h at 4 °C with PBS containing 1% BSA followed by six times washing with TBST (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% [vol/vol] Tween 20). The phage library diluted in TBST was added into the plate at a final concentration of 2 × 1011 pfu/ml (100 μl/well) at room temperature for 30 min with gentle rocking. Unbound phage was removed by 10 times washing with TBST. Bound phage was eluted using 100 μl elution buffer (0.2 M glycine–HCl, pH 2.2). The eluates neutralized with 15 μl 1 M Tris–HCl (pH 9.1) were amplified in E. coli ER2738 bacteria. The phages were purified by polyethylene glycol precipitation according to the manufacturer's instructions. Four rounds of panning were performed and the phage concentrations in the elution buffer and after amplification in host cells were tittered.
Binding activity analyzed by ELISA
The pAPN protein was coated onto ELISA plates as above (10 μg/well). In parallel, BSA was used as control. The following day, the plates were blocked for 1 h at room temperature with TBSB (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% BSA) at room temperature for 2 h. The plates were incubated with the selected phages and an unrelevant control phage at a concentration of 1.5 × 1011 pfu/well for 1 h at 37 °C after TBST washing. Subsequently, M13 polyclonal antibody (Abcam, 1:1000 diluted in TBSB) was added into the wells for another 1 h at 37 °C, after washing six times with TBST. The wells were incubated with HRP-conjugated anti-rabbit IgG antibody (Sigma, dilution 1:1000). The color was developed using o-phenylenediamine (OPD) and the optical density (OD) value was read using an ELISA reader at a wavelength of 490 nm. Each experiment was performed in triplicate.
Sequencing and peptide synthesis
Ten positive phage clones were amplified and precipitated with polyethylene glycol/NaCl. DNA of individual phage was purified with a plasmid extraction kit (Nanjing Keygen Biotech. Co., Ltd, China). The genes encoding the exogenous peptides of M13 were amplified with the DNA templates using primers: sense primer 5′-TCACCTCGAAAGCAAGCTGA-3′ and anti-sense primer 5′-CCCTCATAGTTAGCG TAACG-3′. The PCR products were sequenced and the deduced amino acid sequences were compared with transmissible gastroenteritis virus TGEV S protein (GenBank accession number M94101). Peptides derived from the biopanning were synthesized in the Tianma Peptide Engineering Company, Suzhou, China.
Specificity and antiviral activity of selected peptides
The individual peptide was diluted in NaHCO3 (pH 8.6) buffer at a final concentration of 100 μg/ml. Purified TGEV and infectious bronchitis virus (IBV) S protein at the same concentration were used as controls. All the proteins were coated in ELISA plates at 37 °C overnight. Purified pAPN protein (60 μg/ml) was added into the wells (100 μl/well) at 37 °C for 1 h. After rinsing, the wells were incubated with anti-pAPN antibody (dilution 1:1000) for another 1 h. The wells were incubated with HRP-conjugated goat anti-rabbit IgG antibody at room temperature for 1 h, after a complete washing. The ELISA results were recorded at a wavelength of 490 nm as above.
The non-toxic concentration of the peptides to cells was determined using trypan blue staining and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assays (Sui et al., 2010). ST cells in 6-well plates were incubated with serially diluted peptides at 37 °C for 1 h. The cells were washed three times with PBS and infected with TGEV at an MOI of 10 at 37 °C for 1 h. The cells were then overlaid with 1% (w/v in serum-free DMEM) methyl-cellulose and subjected to plaque analysis as above. Mock treated and virus infected cells were used as controls.
MTT assay
ST cells in 96-well plates were incubated with serially diluted peptides at 37 °C for 1 h. TGEV at an MOI of 10 was used to infect the cells at 37 °C for 48–72 h. Subsequently, the wells were washed three times with PBS and MTT (5 mg/ml) was added (10 μl/each well) at 37 °C for 4 h. The supernatant was discarded and dimethyl sulfoxide was added (100 μl/well) into each well for 10 min with gentle shaking. The OD490 of the wells was determined using a plate reader. In parallel, mock-infected and virus-infected cells were included as control.
Animal vaccination and immune response analysis
Healthy Kunming mice (6–7 weeks age) were purchased from Harbin Veterinary Research Institute, China. The mice were divided into five groups (15 mice/group). They were mock-immunized group (M), phages expressing F peptide group (F), phages expressing S peptide group (S), phages expressing H peptide group (H) and a mixed phage from the phage library used as control. For the first vaccination, 200 μl peptide-expressing phages (at a titer of 1.5 × 1011 pfu/ml) were mixed with equal volume of Freund's complete adjuvant and the immunogen was injected to the mice (200 μl/mouse) intramuscularly. Ten days later, the mice received the same antigen mixed with equal volume of Freund's incomplete adjuvant. Ten days later, the mice were injected with the phages alone. Each 10 days post-immunization, the lymphocytes of peripheral blood collected from the eye artery and the spleens of the immunized mice (5 mice/group) were isolated with lymphocyte separation solution (Invitrogen, USA) according to the manufacturer's instruction. The cells were suspended in PBS at a density of 1 × 107 cells/ml. The cells (500 μl/each sample) were centrifuged at 3000 g for 5 min and then washed two times with cold PBS. They were incubated with FITC-conjugated anti-CD4+ T cell antibody (Zhongshan, China) and PE-conjugated anti-CD8+ T cell antibody (Zhongshan, China) at 4 °C for 30 min. The antibodies were 1:1000 diluted in PBS. The cells were washed, re-suspended with PBS and subjected to flow cytometry analysis. At the same time, TGE virions (10 μg/well) were coated in ELISA plates and the serum isolated from the immunized mice was used as primary antibody followed by incubation with HRP-conjugated second antibody at each 10 days post-immunization. The OD490 value was read using an ELISA plate reader.
Statistical analysis
The data were analyzed using Student's t-test, and were expressed as mean value ± standard error of the mean (S.E.M.). The P value < 0.05 was considered statistically significant.
Acknowledgments
We acknowledge the National Natural Science Foundation of China (No. 30700590; 30700591; 30972195), Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (NO706019), funding supported by Program for New Century Excellent Talents in Heilongjiang Provincial University (1155-NCET-005), Heilongjiang Provincial Science and Technology Department, China (ZJN0702-01) and Northeast Agricultural University, China (CXZ008-1). We thank Drs. Georg Herrler and Luis Enjuanes for kindly providing pAPN clone and TGEV Purdue 46-MAD strain, respectively.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.virol.2010.11.014.
Contributor Information
Xiaofeng Ren, Email: rxfemail@yahoo.com.cn, renxf@neau.edu.cn.
Guangxing Li, Email: lgxemail@yahoo.com.cn.
Supplementary data
Supplementary material
References
- Almazán F., González J.M., Pénzes Z., Izeta A., Calvo E., Plana-Durán J., Enjuanes L. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5516–5521. doi: 10.1073/pnas.97.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Z., Zhao Y., Lin S. Diagnosis and therapy of a case of transmissible gastroenteritis. Livest. Poult. Ind. 2006;191:50–51. (in Chinese) [Google Scholar]
- Bai W. Report on the diagnosis and therapy of porcine transmissible gastroenteritis virus. Fujian J. Anim. Husbandry Vet. 2005;27:34. (in Chinese) [Google Scholar]
- Benbacer L., Kut E., Besnardeau L., Laude H., Delmas B. Interspecies aminopeptidase-N chimeras reveal species-specific receptor recognition by canine coronavirus, feline infectious peritonitis virus, and transmissible gastroenteritis virus. J. Virol. 1997;71:734–737. doi: 10.1128/jvi.71.1.734-737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia A., Zelus B.D., Wentworth D.E., Talbot P.J., Holmes K.V. Identification of a receptor-binding domain of the spike glycoprotein of human coronavirus HCoV-229E. J. Virol. 2003;77:2530–2538. doi: 10.1128/JVI.77.4.2530-2538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., The Coronaviridae Study Group of the International Committee on Taxonomy of Viruses Revision of the taxonomy of the Coronavirus, Torovirus, and Arterivirus genera. Arch. Virol. 1994;135:226–237. doi: 10.1007/BF01309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Laude Induction of interferon alpha by transmissible gastroenteritis coronavirus: role of transmembrane glycoprotein E1. J. Virol. 1988;62:8–11. doi: 10.1128/jvi.62.1.8-11.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R., Knobler R.L., Powell H., Buchmeier M.J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell–cell fusion. Virology. 1982;119:358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa I., Gebauer F., Bullido M.J., Sune C., Baay M.F.D., Zwaagstra K.A., Posthumus W.P.A., Lenstra J.A., Enjuanes L. Localization of antigenic sites of the E2 glycoprotein of transmissible gastroenteritis coronavirus. J. Gen. Virol. 1990;71:271–279. doi: 10.1099/0022-1317-71-2-271. [DOI] [PubMed] [Google Scholar]
- Cwirla S.E., Peters E.A., Barrett R.W., Dower W.J. Peptides on phage: a vast library of peptides for identify ligands. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Talbot P.J. Protection from lethal coronavirus infection by affinity-purified spike glycoprotein of murine hepatitis virus, strain A59. Virology. 1990;174:87–94. doi: 10.1016/0042-6822(90)90057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Laude H. Antigenic structure of transmissible gastroenteritis virus. II. Domains in the peplomer glycoprotein. J. Gen. Virol. 1986;67:1405–1418. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- Delmas B., Rasschaert D., Godet M., Gelfi J., Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J. Gen. Virol. 1990;71:1313–1323. doi: 10.1099/0022-1317-71-6-1313. [DOI] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Kut E., Sjöström H., Noren O., Laude H. Determinants essential for the transmissible gastroenteritis virus–receptor interaction reside within a domain of aminopeptidase-n that is distinct from the enzymatic site. J. Virol. 1994;68:5216–5224. doi: 10.1128/jvi.68.8.5216-5224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J.J., Panganiban L.C., Devlin P.E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Dyson M., Murray K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2194–2198. doi: 10.1073/pnas.92.6.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleouet J.F., Chilmonczyk S., Besnardeau L., Laude H. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J. Virol. 1998;72:4918–4924. doi: 10.1128/jvi.72.6.4918-4924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Suñé C., Gebauer F., Smerdou C., Camacho A., Antón I.M., González S., Talamillo A., Méndez A., Ballesteros M.L. Antigen selection and presentation to protect against transmissible gastroenteritis coronavirus. Vet. Microbiol. 1992;33:249–262. doi: 10.1016/0378-1135(92)90053-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Spaan W., Snijder E., Cavanagh D. Nidovirales. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carsten E.B., Estes M.K., Lemon S.M., McGeoch D.J., Maniloff J., Mayo M.A., Pringle C.R., Wickner R.B., editors. Virus Taxonomy. Classification and Nomenclature of Viruses. Academic Press; New York, N.Y: 2000. pp. 827–834. [Google Scholar]
- Garwes D.J., Lucas M.H., Higgins D.A., Pike B.V., Cartwright S.F. Antigenicity of structural components from porcine transmissible gastroenteritis virus. Vet. Microbiol. 1978;3:179–190. [Google Scholar]
- Gebauer F., Posthumus W.P., Correa I., Suñé C., Smerdou C., Sánchez C.M., Lenstra J.A., Meloen R.H., Enjuanes L. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H.M., Barteling S.J., Meloen R.H. Small peptides induce antibodies with a sequence and structural requirement for binding antigen comparable to antibodies raised against the native protein. Proc. Natl. Acad. Sci. U. S. A. 1985;82:178–182. doi: 10.1073/pnas.82.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Rasschaert D., Laude H. Processing and antigenicity of entire and anchor-free spike glycoprotein S of coronavirus TGEV expressed by recombinant baculovirus. Virology. 1991;185:732–740. doi: 10.1016/0042-6822(91)90544-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Grosclaude J., Delmas B., Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994;68:8008–8016. doi: 10.1128/jvi.68.12.8008-8016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P.R., Hjelle B., Njus H., Ye C., Bondu-Hawkins V., Brown D.C., Kilpatrick K.A., Larson R.S. Phage display selection of cyclic peptides that inhibit Andes virus infection. J. Virol. 2009;83:8965–8969. doi: 10.1128/JVI.00606-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M.S., Gillilandt L.K., Ledbetter J.A. Antibody engineering. Curr. Opin. Immunol. 1997;9:201–212. doi: 10.1016/s0952-7915(97)80136-7. [DOI] [PubMed] [Google Scholar]
- Hegyi A., Kolb A.F. Characterization of determinants involved in the feline infectious peritonitis virus receptor function of feline aminopeptidase N. J. Gen. Virol. 1998;79:1387–1391. doi: 10.1099/0022-1317-79-6-1387. [DOI] [PubMed] [Google Scholar]
- Hong S.S., Boulanger P. Protein ligands of the human adenovirus type 2 outer capsid identified by biopanning of a phagedisplayed peptide library on separate domains of wild-type and mutant penton capsomers. EMBO J. 1995;14:4714–4727. doi: 10.1002/j.1460-2075.1995.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L., van der Zeijst B.A., Horzinek M.C. Characterization and translation of transmissible gastroenteritis virus mRNAs. J. Virol. 1986;57:1010–1015. doi: 10.1128/jvi.57.3.1010-1015.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Correa I., Melgosa M.P., Bullido M.J., Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J. Virol. 1986;60:131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B.K., Kurakin A.V., Hyde-DeRuyscher R. From peptides to drugs via phage display. Drug Discov. Today. 1998;3:370–378. [Google Scholar]
- Kolb A.F., Hegyi A., Siddell S.G. Identification of residues critical for the human coronavirus 229E receptor function of human aminopeptidase N. J. Gen. Virol. 1997;78:2795–2802. doi: 10.1099/0022-1317-78-11-2795. [DOI] [PubMed] [Google Scholar]
- Krempl C., Herrler G. Sialic acid binding activity of transmissible gastroenteritis coronavirus affects sedimentation behavior of virions and solubilized glycoproteins. J. Virol. 2001;75:844–849. doi: 10.1128/JVI.75.2.844-849.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl C., Ballesteros M.L., Zimmer G., Enjuanes L., Klenk H.D., Herrler G. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 2000;81:489–496. doi: 10.1099/0022-1317-81-2-489. [DOI] [PubMed] [Google Scholar]
- Larson R.S., Brown D.C., Ye C., Hjelle B. Peptide antagonists that inhibit Sin Nombre virus and hantaan virus entry through the beta3-integrin receptor. J. Virol. 2005;79:7319–7326. doi: 10.1128/JVI.79.12.7319-7326.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Rasschaert D., Huet J.C. Sequence and N-terminal processing of the transmembrane protein El of the coronavirus transmissible gastroenteritis virus. J. Gen. Virol. 1987;68:1687–1693. doi: 10.1099/0022-1317-68-6-1687. [DOI] [PubMed] [Google Scholar]
- Laude H., Gelfi J., Lavenant L., Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 1992;66:743–749. doi: 10.1128/jvi.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Reeth K.V., Pensaert M. Porcine respiratory coronavirus: molecular features and virus–host interactions. Vet. Res. 1993;24:125–150. [PubMed] [Google Scholar]
- Lesinski G.B., Westerink J. Novel vaccine strategies to T-independent antigens. J. Microbiol. Meth. 2001;47:135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Li J., Yin J., Sui X., Li G., Ren X. Comparative analysis on the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- Li G., Hong J., Ren X., Yin J., Feng S., Huo G. Prokaryotic expression of Stx1B subunit of Escherichia coli O157:H7 used to generate monoclonal antibody. Hybridoma. 2010;29:283–289. doi: 10.1089/hyb.2010.0021. [DOI] [PubMed] [Google Scholar]
- Li G., Zeng Y., Yin J., Lillehoj H.S., Ren X. Cloning, prokaryotic expression, and biological analysis of recombinant chicken IFN-γ. Hybridoma. 2010;29:1–6. doi: 10.1089/hyb.2009.0053. [DOI] [PubMed] [Google Scholar]
- Liu B., Li G., Sui X., Yin J., Wang H., Ren X. Expression and functional analysis of porcine aminopeptidase N produced in prokaryotic expression system. J. Biotechnol. 2009;141:91–96. doi: 10.1016/j.jbiotec.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Yin J., Li X., Yang W., Li G., Ren X. Production and characterization of a monoclonal antibody against spike protein of transmissible gastroenteritis virus. Hybridoma. 2010;29:345–350. doi: 10.1089/hyb.2010.0009. [DOI] [PubMed] [Google Scholar]
- Novy R., Drott D., Yaeger K., Mierendorf R. Overcoming the codon bias of E. coli for enhanced protein expression. Innovations. 2001;12:1–3. [Google Scholar]
- Pulford D.J., Britton P. Intracellular processing of the porcine coronavirus transmissible gastroenteritis virus spike protein expressed by recombinant vaccinia virus. Virology. 1991;182:765–773. doi: 10.1016/0042-6822(91)90617-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J. Gen. Virol. 1987;68:1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Ren X., Glende J., Al-Falah M., de Vries V., Schwegmann-Wessels C., Qu X., Tan L., Tschernig T., Deng H., Naim H.Y., Herrler G. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006;87:1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- Ren X., Glende J., Yin J., Schwegmann-Wessels C., Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Li G., Liu B. Binding characterization of determinants in porcine aminopeptidease N, the cellular receptor for transmissible gastroenteritis virus. J. Biotechnol. 2010;150:202–206. doi: 10.1016/j.jbiotec.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Suo S., Jang Y.S. Development of a porcine epidemic diarrhea virus M protein-based ELISA for virus detection. Biotechnol. Lett. 2010;33:215–220. doi: 10.1007/s10529-010-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Wang M., Yin J., Li G. Phages harboring specific peptides that recognize the N protein of the porcine reproductive and respiratory syndrome virus distinguish the virus from other viruses. J. Clin. Microbiol. 2010;48:1875–1881. doi: 10.1128/JCM.01707-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Wang M., Yin J., Ren Y., Li G. Heterologous expression of fused genes encoding the glycoprotein 5 from PRRSV: a way for producing functional protein in prokaryotic microorganism. J. Biotechnol. 2010;147:130–135. doi: 10.1016/j.jbiotec.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco C., Antón I.M., Suñé C., Pedregosa A.M., Martín-Alonso J.M., Parra F., Carrascosa J.L., Enjuanes L. Membrane protein molecules of transmissible gastroenteritis coronavirus also expose the carboxy-terminal region on the external surface of the virion. J. Virol. 1995;69:5269–5277. doi: 10.1128/jvi.69.9.5269-5277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P.J.M. The coronavirus membrane glycoprotein. In: Siddell S.G., editor. The Coronaviridae. Plenum Press; New York, N.Y: 1995. pp. 115–139. [Google Scholar]
- Ruoslahti E., Pierschbacher M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Saif L.J., Wesley R.D. Transmissible gastroenteritis. In: Leman A.D., Straw B., Mengeling W.L., D' Allaire S., Taylor D.J., editors. Diseases of Swine. Iowa State University Press; Ames, Iowa: 1992. pp. 362–386. [Google Scholar]
- Schultze B., Enjuanes L., Herrler G. Analysis of the sialic acid-binding activity of the transmissible gastroenteritis virus. Adv. Exp. Med. Biol. 1995;380:367–370. doi: 10.1007/978-1-4615-1899-0_59. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Herrler G. Transmissible gastroenteritis virus infection: a vanishing specter. Dtsch Tierärztl. Wochenschr. 2006;113:157–159. [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Zimmer G., Laude H., Enjuanes L., Herrler G. Binding of transmissible gastroenteritis coronavirus to cell surface sialoglycoproteins. J. Virol. 2002;76:6037–6043. doi: 10.1128/JVI.76.12.6037-6043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Zimmer G., Schröder B., Breves G., Herrler G. Binding of transmissible gastroenteritis coronavirus to brush border membrane sialoglycoproteins. J. Virol. 2003;77:11846–11848. doi: 10.1128/JVI.77.21.11846-11848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-wessels C., Glende J., Ren X., Qu X., Deng H., Enjuanes L., Herrler G. Comparison of vesicular stomatitis virus pseudotyped with the S proteins from a porcine and a human coronavirus. J. Gen. Virol. 2009;90:1724–1729. doi: 10.1099/vir.0.009704-0. [DOI] [PubMed] [Google Scholar]
- Scott J.K., Smith G.P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Siddell S.G. The Coronaviridae. An introduction. In: Siddell S.G., editor. The Coronaviridae. Plenum Press; New York: 1995. pp. 1–10. [Google Scholar]
- Spaan W., Cavanagh D., Horzinek C. Coronaviruses: structure and genome expression. J. Gen. Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Sui X., Yin J., Ren X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res. 2010;85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suñé C., Jiménez G., Correa I., Bullido M.J., Gebauer F., Smerdou C., Enjuanes L. Mechanisms of transmissible gastroenteritis coronavirus neutralization. Virology. 1990;177:559–569. doi: 10.1016/0042-6822(90)90521-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J.M., Alonso C., Ortega A., Mittal S., Graham F., Enjuanes L. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. Virology. 1996;70:3770–3780. doi: 10.1128/jvi.70.6.3770-3780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase Nserves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996;70:8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H., Shibata K., Kajiyama H., Terauchi M., Nawa A., Kikkawa F. Aminopeptidase N (APN)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer. 2008;8:74. doi: 10.1186/1471-2407-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn E.M., Halbur P.G., Paul P.S. Sequence comparison of porcine respiratory coronavirus isolate reveals heterogenecity in the S, 3 and 3-1 genes. J. Virol. 1995;69:3176–3184. doi: 10.1128/jvi.69.5.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.I., Fleming J.O., Lai M.M.C. Sequence analysis of the spike protein gene of murine coronavirus variants: study of genetic sites affecting neuropathogenicity. Virology. 1992;186:742–749. doi: 10.1016/0042-6822(92)90041-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.A. Binding in the growth hormone receptor complex. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1–6. doi: 10.1073/pnas.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G., Qian J., Wang Z., Qi Y. A phage-displayed peptide can inhibit infection by white spot syndrome virus of shrimp. J. Gen. Virol. 2003;84:2545–2553. doi: 10.1099/vir.0.19001-0. [DOI] [PubMed] [Google Scholar]
- Yin J., Li G., Ren X., Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol. 2007;127:335–347. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Yin J., Glende J., Schwegmann-Wessels C., Enjuanes L., Herrler G., Ren X. Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus. Antiviral Res. 2010;88:311–316. doi: 10.1016/j.antiviral.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Hua G., Andacht T.M., Adang M.J. A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry. 2008;47:11263–11272. doi: 10.1021/bi801181g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Existence of TxxF motifs in the S protein of TGEV. The identified TxxF motifs were matched with the S protein of TGEV strain PUR46-MAD (GenBank accession number M94101) and boxed. The underscored bold S is the amino acid 506 in the S protein.