Abstract
In this first proteomic analysis of an invertebrate iridovirus, 46 viral proteins were detected in the virions of Chilo iridescent virus (CIV) based on the detection of 2 or more distinct peptides; an additional 8 proteins were found based on a single peptide. Thirty-six of the 54 identified proteins have homologs in another invertebrate and/or in one or more vertebrate iridoviruses. The genes for 5 of the identified proteins, 22L (putative helicase), 118L, 142R (putative RNaseIII), 274L (major capsid protein) and 295L, are shared by all iridoviruses for which the complete nucleotide sequence is known and may therefore be considered as iridovirus core genes. Three identified proteins have homologs only in ascoviruses. The remaining 15 identified proteins are so far unique to CIV. In addition to broadening our insight in the structure and assembly of CIV virions, this knowledge is pivotal to unravel the initial steps in the infection process.
Keywords: Chilo iridescent virus, Invertebrate iridovirus 6, Proteomics, LC-MS/MS
Introduction
Chilo iridescent virus (CIV), also known as Invertebrate iridescent virus 6, belongs to the family Iridoviridae and is the type species of the genus Iridovirus (Fauquet et al., 2005, Williams, 1996, Williams et al., 2005, Willis, 1990). Iridoviruses are large, cytoplasmic, icosahedral viruses with a linear double-stranded DNA genome, which is both circularly permuted and terminally redundant (Darai et al., 1983, Goorha & Murti, 1982). The CIV virion consists of an unusual three layer structure containing an outer proteinaceous capsid, an intermediate lipid membrane, and a core DNA–protein complex containing the 212, 482 bp genome (Jakob et al., 2001, Williams, 1996, Williams et al., 2005). Up to now, thirteen complete sequences of iridovirus genomes have been published, including CIV (Huang et al., 2009, Williams et al., 2005). The availability of the CIV sequence facilitates the identification and functional analysis of the proteome of CIV virions. Replication of CIV occurs in the nucleus of infected cells and the assembly takes place in the cytoplasm (Goorha and Murti, 1982).
Many questions remain to be answered concerning the structure and scaffolding of the virus particles, the nature of virus–host interactions and the initial steps in virus infection, including the mechanism behind the onset of transcription of CIV genes. Viral structural proteins are likely to play crucial roles in these processes. Initiation of viral transcription for instance requires one or more virion proteins, since CIV DNA alone is not infectious, similar to what has been shown for the vertebrate iridovirus Frog virus 3 (Willis and Granoff, 1985). In previous studies, efforts have been made to characterize the polypeptides in CIV virions by one- or two-dimensional SDS-PAGE. The presence of 21–28 polypeptides was revealed by one-dimensional SDS-PAGE, while 35 polypeptides were observed in two-dimensional SDS-PAGE (Barray & Devauchelle, 1979, Barray & Devauchelle, 1985, Cerutti & Devauchelle, 1985, Kelly & Tinsley, 1972, Orange & Devauchelle, 1987). The size of these polypeptides ranged from 11 to 300 kDa. However, most of these proteins were not further characterized and it is unknown, except for the major capsid protein MCP, by which CIV genes they are encoded.
In the current study we identified the CIV virion proteins by a proteomic approach, based on a combination of one-dimensional SDS-PAGE and Liquid Chromatography/Mass Spectrometry/Mass Spectrometry (LC-MS/MS). The data obtained were analyzed by searches against a CIV ORF database. This provided a fast and highly sensitive method for the identification of genes through the sequences of the encoded proteins (Pandey and Mann, 2000).
Results
To identify the virion proteins of CIV, the proteins of purified virion particles were separated by one-dimensional SDS-PAGE. Staining of the gel with colloidal blue revealed at least 21 proteins ranging from 10 to 250 kDa (Fig. 1 ) much in line to what has been found previously (Barray & Devauchelle, 1979, Barray & Devauchelle, 1985, Cerutti & Devauchelle, 1985, Kelly & Tinsley, 1972, Orange & Devauchelle, 1987). The gel lane was divided into 6 slices containing proteins with a molecular mass lower than 26 kDa, ranging from 26–34 kDa, 34–43 kDa, 43–55 kDa or 55–95 kDa and higher than 95 kDa, respectively. The proteins were digested with trypsin and analyzed by LC-MS/MS. A decoy database strategy (Elias and Gygi, 2007) was used which, after applying the appropriate filters, resulted in 89 protein hits: 54 CIV proteins, 34 contaminants and 1 decoy hit giving a False Discovery Rate of 1.1%. Out of the 54 CIV proteins, 46 of the more abundant proteins were identified with 2 or more peptides (Table 1 ), while relatively small proteins like ORFs 342R, 227L or 104L as well as some less abundant proteins could be identified with one peptide only (Table 2 ). The proteins with one hit were manually verified to correlate well to the theoretical b+y ion spectrum and to be unique for one protein only (see also Supplementary Material S1).
Fig. 1.
SDS-PAGE profile and LC-MS/MS identification results of purified CIV virion proteins. CIV proteins were separated by 12% one-dimensional SDS-PAGE and stained with colloidal blue. The SDS-PAGE gel was divided into 6 slices, which, based on comparison to a molecular marker, ranged from higher than 95 kDa, 55–95 kDa, 43–55 kDa, 34–43 kDa, 26–34 kDa and lower than 26 kDa. Proteins were in-gel-digested with trypsin, extracted and subjected to LC-MS/MS. The column on the right indicates the relative abundance of the proteins as visualized by SDS-PAGE. The boxes on the left give the ORF numbers of the identified proteins in a particular gel slice in order of the predicted mass (see Table 1, Table 2). Underlined numbers represent single peptide hits. Indications R and L point towards the direction of transcription from the CIV genome (see Fig. 2).
Table 1.
Structural proteins of CIV identified by LC-MS/MS with 2 or more distinct peptides.. The ORFs are ordered by the mass of the encoded proteins (column 3).
| ORF | NCBI accession No | Mol. mass (kDa) | Protein coverage (% by amino acids) | Peptide hits on first rank | Relative abundancea (% peak area) | Predicted domains/function |
|---|---|---|---|---|---|---|
| 443R | AAK82303 | 237.22 | 8.10 | 15 | 0.30 | |
| 295L | AAK82156 | 156.42 | 24.70 | 43 | 0.22 | Bipartite nuclear localization signal |
| 179R | AAB94478 | 137.93 | 14.70 | 24 | 0.06 | CAP10, Putative lipopolysaccharide-modifying enzyme, tyrosine protein kinase |
| 022L | AAD48148 | 135.34 | 32.20 | 34 | 0.20 | Putative nucleoside triphosphatase I; DEXDc; DEAD-like helicase superfamily |
| 261R | AAK82122 | 129.06 | 2.70 | 30 | 2.20 | Potential repetitive protein |
| 209R | AAK82071 | 118.34 | 39.20 | 52 | 0.69 | Serine/threonine protein kinase |
| 396L | AAK82256 | 111.28 | 21.90 | 29 | 0.16 | Potential repetitive protein |
| 268L | AAK82129 | 83.22 | 46.10 | 74 | 2.06 | |
| 149L | AAB94464 | 76.41 | 36.60 | 72 | 0.91 | |
| 232R | AAK82093 | 75.56 | 49.00 | 75 | 1.63 | DNA polymerase (viral) N terminal domain, 2-cysteine adaptor domain, OTU like cysteine protease |
| 439L | AAK82299 | 63.45 | 12.10 | 8 | 0.02 | Protein kinase domain |
| 361L | AAK82221 | 60.58 | 50.70 | 55 | 1.06 | Peptidase_C1A_CathepsinB |
| 380R | AAK82240 | 59.91 | 54.50 | 73 | 2.05 | S_TKc, Serine or threonine-specific kinase subfamily |
| 213R | AAK82075 | 58.42 | 29.70 | 22 | 0.16 | Putative peptidoglycan bound protein |
| 118L | AAB94444 | 55.29 | 55.10 | 65 | 1.77 | Putative envelope protein |
| 198R | AAK82060 | 52.15 | 42.60 | 26 | 0.32 | |
| 274L | AAK82135 | 51.29 | 63.20 | 157 | 17.97 | Major capsid protein |
| 229L | AAK82090 | 50.64 | 22.30 | 15 | 0.25 | |
| 337L | AAK82199 | 46.13 | 27.20 | 25 | 0.21 | Poxvirus protein of unknown function |
| 159L | AAB94468 | 45.76 | 34.90 | 58 | 3.98 | |
| 329R | AAK82190 | 42.74 | 28.80 | 16 | 0.29 | |
| 219L | AAK82081 | 34.64 | 19.00 | 5 | 0.01 | |
| 142R | AAB94459 | 33.64 | 33.60 | 16 | 0.25 | dsRNA-specific ribonuclease |
| 457L | AAK82317 | 33.13 | 25.90 | 47 | 2.97 | |
| 155L | AAB94465 | 29.81 | 39.20 | 23 | 0.40 | |
| 401R | AAK82261 | 28.23 | 25.50 | 11 | 0.04 | HMG-box superfamily of DNA-binding proteins |
| 117L | AAB94443 | 27.45 | 29.70 | 43 | 0.93 | |
| 415R | AAK82275 | 26.66 | 63.20 | 70 | 1.34 | |
| 309L | AAK82170 | 24.83 | 70.00 | 12 | 0.10 | |
| 422L | AAK82282 | 22.73 | 49.50 | 19 | 0.20 | Cydia pomonella granulovirus ORF34 |
| 378R | AAK82238 | 22.21 | 47.70 | 12 | 0.10 | 2-cysteine adaptor domain |
| 355R | AAK82216 | 22.01 | 52.70 | 10 | 0.02 | Catalytic domain of ctd-like phosphatases |
| 234R | AAK82095 | 21.09 | 62.70 | 63 | 3.07 | |
| 111R | AAB94438 | 20.01 | 35.40 | 9 | 0.05 | |
| 096L | AAB94430 | 19.69 | 33.30 | 11 | 0.05 | Fasciclin domain |
| 374R | AAK82234 | 19.12 | 22.40 | 3 | 0.00 | Bat coronavirus spike protein |
| 325L | AAK82186 | 18.91 | 24.50 | 5 | 0.08 | |
| 203L | AAK82065 | 18.53 | 18.80 | 7 | 0.02 | |
| 084L | AAB94426 | 18.40 | 25.50 | 15 | 0.04 | |
| 061R | AAB94416 | 17.91 | 31.60 | 17 | 0.01 | Lysosome associate membrane glycoproteins |
| 123R | AAB94448 | 16.38 | 7.70 | 3 | 0.00 | Dual specificity phosphatases |
| 453L | AAK82313 | 15.91 | 26.10 | 12 | 0.05 | Protein disulfide isomerase |
| 034Rb | AAK81969 | 14.63 | 16.40 | 5 | 0.03 | |
| 366R | AAK82226 | 13.66 | 17.50 | 2 | 0.01 | |
| 138R | AAB94455 | 13.03 | 16.70 | 7 | 0.02 | |
| 312R | AAK82173 | 10.60 | 20.70 | 3 | 0.01 |
The relative abundance was calculated by Bioworks as % peak area over all peaks (including contaminants observed) shown after applying the following filter settings: ΔCn > 0.08, Xcorr > 1.5 for charge state 2+, Xcorr > 3.3 for charge state 3+ and Xcorr > 3.5 for charge state 4+, Sf > 0.6.
This protein was identified in the 34–43 kDa gel piece.
Table 2.
Structural proteins of CIV identified by LC-MS/MS with 1 peptide. The ORFs are ordered by the mass of the encoded proteins (column 3).
| ORF | NCBI Accession No | Molecular mass (kDa) | Peptide sequence | Protein coverage (% by amino acids) | MH+ | Delta m/z (ppm) | z | Xcorr |
|---|---|---|---|---|---|---|---|---|
| 317L | AAK82178 | 43.95 | IVNLIPQGQFQAK | 3.11 | 1455.832 | −0.30 | 2 | 1.77 |
| 130R | AAB94451 | 23.18 | ICFSEQPLLDDFSNK | 7.46 | 1812.847 | 1.04 | 2 | 2.86 |
| 307L | AAK82168 | 22.86 | LKPLGYLNSLQ | 5.58 | 1245.720 | 0.33 | 2 | 1.81 |
| 395R | AAK82255 | 17.28 | YAINNENQYR | 6.62 | 1284.597 | −0.72 | 2 | 2.54 |
| 010R | AAK81948 | 12.84 | TGSMVCSSTR | 8.33 | 1085.471 | 3.19 | 2 | 2.34 |
| 342R | AAK82203 | 9.33 | IQAQNYATMGIYN-QGSQIR* | 21.59 | 2156.055 | 2.74 | 2 | 3.73 |
| 227L | AAK82088 | 7.72 | TFAYEVPIRa | 14.30 | 1095.583 | 1.49 | 2 | 2.61 |
| 104L | AAB94434 | 7.05 | RVACSPR* | 12.30 | 845.441 | 2.01 | 2 | 2.78 |
The same peptide was measured multiple times in different gel slices.
The proteins identified are indicated in Fig. 1. A genomic map of CIV ORFs that encode polypeptides represented in the proteome of CIV particles is shown in Fig. 2 . For individual CIV virion proteins, 2.7% to 70% of the amino acid sequence was covered with peptides retrieved from the analysis. The major capsid protein (MCP) encoded by ORF 274L is one of the most abundant CIV proteins (Barray & Devauchelle, 1979, Barray & Devauchelle, 1985, Cerutti & Devauchelle, 1985, Kelly & Tinsley, 1972) and this is clearly reflected by its relative abundance in the current analysis compared to all other CIV proteins (Table 1). The nature of the other major band is not clear at this moment.
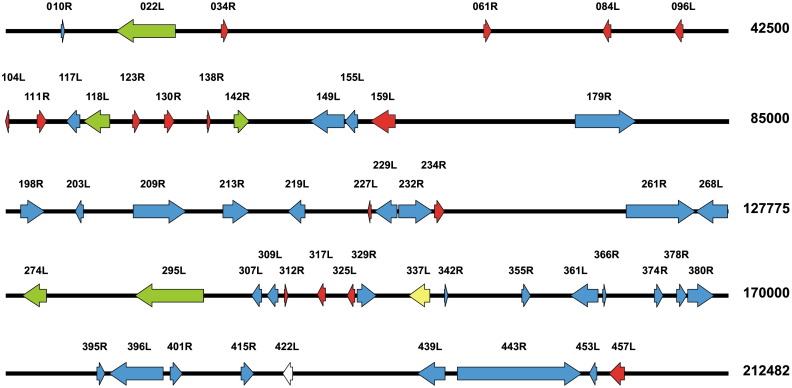
Fig. 2.
Linearized genomic presentation of the 54 CIV structural protein ORFs determined by LC-MS/MS. Arrows indicate the positions and the direction of gene transcription (R or L). Red arrows are ORFs unique to CIV, green arrows represent ORFs present in all sequenced iridovirus genomes. The yellow and the white ORFs, have an entomopox- and baculovirus homolog, respectively. The remaining ORFs are indicated in blue. Genomic positions are indicated on the right in base pair number.
Functional domains alluding to possible functions were found in fifteen other identified virion proteins, including three putative serine/threonine kinases (ORFs 209R, 380R and 439R), one dual specificity phosphatase (123R), a protein with homology to the N-terminal domain of viral DNA polymerases (232R), carboxy-terminal domain (CTD) phosphatase (355R), nucleoside triphosphatase (NTP I) (22L), fasciclin (96L), ribonuclease III (142R), tyrosine protein kinase (179R), cathepsin (361L), DNA binding protein (401R), protein disulfide isomerase (453L), lysosome associate membrane glycoprotein (061R), and a ranavirus envelope protein homolog (118L). For the 38 remaining proteins in the virion, we have no clear idea about their specific function at this moment (Table 1, Table 2). Some of these show partial homology to viral proteins of poxvirus, coronavirus or baculovirus origin.
Recent cryoelectron microscopy studies on the capsid of CIV revealed, in addition to MCP, a group of relatively less abundant capsid proteins (Yan et al., 2009). These proteins form a complex which contains a “finger” protein, a “zip” protein, a pentameric complex and an anchor protein. The molecular mass estimations for the finger and zip proteins, the anchor protein and the monomer of the pentameric complex were estimated to be 19.7, 11.9, 32.4 and 39.3 kDa, respectively. For the finger protein the standard deviation was 1.5 kDa, giving a size range of 18.2–21.2 kDa (Yan et al., 2009). Based on this range, seven candidate genes for the finger protein were found in the CIV proteome: ORFs 234R, 111R, 096L, 374L, 325L, 203L, and 084L from large to small (Table 1). The zip protein with an expected size range of 10.5 to 13.3 kDa (1.4 kDa standard deviation) may correspond to three candidate ORFs represented in the proteome: 010R, 138R and 321R. The monomer of the pentameric complex estimated at 39.3 kDa corresponds most closely in size to ORFs 329R and 219L. Anchor protein candidate genes in the CIV proteome could be 457L or 142R, with sizes close to 32.4 kDa (Table 1).
Discussion
The CIV proteome revealed 54 proteins. The genes encoding these virion proteins are scattered over the genome (Fig. 2). It is not known which of the identified proteins are engaged in the scaffolding and assembly of CIV virions, and which are not essential for building the virion structure, but may be important for other aspects, such as the initial stages of the infection process and the regulation of gene expression. It is possible that one of these additional proteins is involved in chaperoning the viral DNA into the nucleus to initiate DNA replication (Willis and Granoff, 1985). To get a better clue about their importance, the conservation of the CIV virion protein genes in the complete genomes of members of the family Iridoviridae as well as Ascoviridae was assessed. The latter family was included since a common ancestry between iridoviruses and ascoviruses has been inferred from phylogenetic analysis (Stasiak et al., 2000).
Of the 54 ORFs encoding CIV virion proteins identified in the current study, thirty-four have homologs in Invertebrate iridovirus 3 (IIV3), which belongs to the genus Chloriridovirus (Table 3 , column 2) (Chen et al., 2008, Song et al., 2004). Fifteen of the 34 ORFs with homologs in IIV3, also have homologs in one or more vertebrate iridoviruses. The CIV proteome shares five ORFs with all iridoviruses: 022L, 118L, 142L, 274L (MCP) and 295L, and these may be considered to belong to the iridovirus core genes. The Rana gryliovirus (RGV) ORF 53R, which is a homolog of the putative core gene 118L, has been shown to encode a novel iridovirus envelope protein (Zhao et al., 2008). The CIV proteome shares thirteen viral protein homologs with Singapore grouper iridovirus (SGIV) virion proteins identified by two independent mass spectrometric approaches (Chen et al., 2008, Song et al., 2004).
Table 3.
List of CIV virion proteins identified by LC-MS/MS ordered by mass with homolog in other iridoviruses and/or ascoviruses.⁎
| Invertebrate |
Vertebrate |
Ascoviridae‡ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irido-virus |
Chlorirido-virus |
Ranavirus |
Lymphocystivirus |
Megalocytivirus |
|||||||||
| CIV | IIV3 | ATV | TFV | FV3 | SGIV | GIV | STIV | LCDV-C | LCDV-1 | ISKNV | RBIV | OSGIV | |
| 443R | 91L | ||||||||||||
| 295L | 16R | 72R | 45R | 41R | 57L | 29L | 45R | 234R | 92R | 76L | 72L | 75L | a144R,b43R,c84L |
| 179R | 35R | 60R | 29R | 27R | 78L | 44R | 31R | 172R | 110R | a129L, b58R, c90R | |||
| 022L | 87L | 7L | 9L | 9L | 60R | 30L | 11L | 75L | 70L | 63L | 59L | 63L | a15R,b161R,c9R |
| 261R | 91L | ||||||||||||
| 209R | a76R, b115L, c64R | ||||||||||||
| 396L | 91L, 8L | ||||||||||||
| 268L | 74L | ||||||||||||
| 149L | 113L | ||||||||||||
| 232R | 84L | 84L | 21R | b141R | |||||||||
| 439L | 35R | 110R | 114L | 111L | c90R | ||||||||
| 38R | |||||||||||||
| 98L | |||||||||||||
| 361L | 24R | 223L | 23R | a101L, b102R, c114R | |||||||||
| 380R | 10L | 84L | 19R | 19R | 39L | 17L | 21R | 13L, 45R, 149R, 165L 174R,184R, 200L | 5L, 42R, 47R, 50R, 51R, 88L | ||||
| 11L | 150L+ | 83L | |||||||||||
| 213R | 51L | ||||||||||||
| 118L | 6R | 53L | 55R | 53R | 88L | 49L | 55R | 157R | 35L | 7L | 8L | 8L | b157L,d5L |
| 198R | 69L | ||||||||||||
| 274L | 14L | 14L | 96R | 90R | 72R | 39R | 96R | 43L | 80L | 6L | 7L | 7L | a55R,b153R,c41R,d19R |
| 229L | 46R | 3R | 4R | 229L | 16L | 2L | 5R | ||||||
| 337L | 47R | 1L | 2L | 2L | 19R | 4R | 2L | 38R | 89L | 85L | b129L, c54R | ||
| 329R | 99R | ||||||||||||
| 219L | 36R, 91L | ||||||||||||
| 142R | 101R | 25R | 85L | 80L | 84L | 46L | 87L | 186R | 74R | 87R | 83R | 85R | a26R,b8R,c22R,d18L |
| 155L | 113L | ||||||||||||
| 401R | 68R | ||||||||||||
| 117L | 107R | 83L | 20R | 20R | 038L | 16L | 23R | 73R | 109R | ||||
| 415R | 18L | ||||||||||||
| 309L | 63R | ||||||||||||
| 422L | d8R, d9R, d14L | ||||||||||||
| 307L | 33L | 11R | 100L | 94L | 98R | 56R | 152L | 9R | 86R | a142R, c86L, d4R | |||
| 378R | 100L | 84L | 19R | 19R | 39L | 17L | 21R | 13L | 50R | b141R | |||
| 355R | 104L | 67R | 40R | 37R | 61R | 31L | 41R | 147L | 43L | 5L | 6L | a108R, b93L, c109L | |
| 374R | b1R | ||||||||||||
| 203L | 85L | ||||||||||||
| 395R | 1R | ||||||||||||
| 453L | 41R | ||||||||||||
| 366R | 63R | 33R | 32R | 35R | |||||||||
| 010R | 43R | ||||||||||||
| 342R | 115R | ||||||||||||
⁎ORFs in bold are conserved in all analyzed iridio- and ascovirus genomes.
‡ The a-d indices for the ascovirus ORFs refer to the following species: a HvAV3e, Heliothis virescens ascovirus 3e (Asgari et al., 2007), b TnAV2c, Trichoplusia ni ascovirus 2c (Wang et al., 2006), c SfAV1a, Spodoptera frugiperda ascovirus 1a (Bideshi et al., 2006), d DpAV4a, Diadromus pulchellus ascovirus 4a (Bigot et al., 2008, Stasiak et al., 2000). The names of the other viruses are abbreviated as follows; CIV, Chilo iridescent virus (Jakob et al., 2001); IV3, Aedes taeniorhynchus iridescent virus (Delhon et al., 2006); ATV, Ambystoma tigrinum stebbensi virus (Jancovich et al., 2003); TFV, Tiger frog virus (He et al., 2002); FV3. Frog virus 3 (Tan et al., 2004); SGIV, Singapore grouper iridovirus (Song et al., 2004); GIV, Grouper iridovirus (Tsai et al., 2005); STIV, Soft-shelled turtle iridovirus (Huang et al., 2009); LCDV-C, Lymphocystis disease virus - isolate China (Zhang et al., 2004); LCDV-1, Lymphocystis disease virus 1 (Tidona and Darai, 1997); ISKNV, Infectious spleen and kidney necrosis virus (He et al., 2001); RBIV, Rock bream iridovirus (Do et al., 2004); OSGIV, Orange-spotted grouper iridovirus (Lü et al., 2005).
Previous phylogenetic studies on ascoviruses were based on comparative analyses of the capsid protein, DNA polymerase, thymidine kinase, and ATPase III, and led to the hypothesis that ascoviruses may have evolved from invertebrate iridoviruses (Stasiak et al., 2003). The proteomic analysis of CIV performed here showed that 16 ORFs encoding CIV virion proteins have homologs in one or more ascoviruses (Asgari et al., 2007, Bideshi et al., 2006, Bigot et al., 2008, Stasiak et al., 2000, Wang et al., 2006). Nine CIV structural proteins have homologs in Heliothis virescens ascovirus 3e (HvAV3e), thirteen have homologs in Trichoplusia ni ascovirus 2c (TnAV2c), eleven in Spodoptera frugiperda ascovirus (SfAV1a) and six in Diadromus pulchellus ascovirus 4a (DpAV4a). The gene products of six of the eleven SfAV1a homologs were also found in the proteome of SfAV1a virions (Tan et al., 2009a). A homolog of the SfAV1a virion protein P64, which was recently shown to be a major DNA binding protein with proposed DNA condensing activity (Tan et al., 2009b) is not encoded in the CIV genome.
Three of the identified CIV virion ORFs are found in one or more ascoviruses, but not in other iridoviruses (209T, 422L and 374R). One of these (422L) is the only CIV virion ORF with a baculovirus homolog (Cydia pomonella granulovirus ORF34; genus Betabaculovirus). ORF 337L has homology to an entomopoxvirus gene (Table 1, Fig. 2). These results underscore the evolutionary distance of iridoviruses from both baculoviruses and entomopoxviruses and the closer relation to ascoviruses. Despite the proposed close evolutionary relation between the symbiotic ascovirus DpAV4a and Chilo iridescent virus (Bigot et al., 2009) the number of CIV virion proteins with homologs in DpAv4a is limited in comparison to the other ascoviruses.
Although the morphology of the virions of members of the family Ascoviridae differs considerably from that of viruses of the family Iridoviridae, evidence is mounting that the ascoviruses and iridoviruses shared a common ancestor. Phylogenetic analyses based on proteins found in most enveloped dsDNA viruses provide strong evidence that ascoviruses evolved from iridoviruses, despite the marked differences in the characteristics of the virions belonging to these two families and differences in their cytopathology (Bigot et al., 2008). The conservation of structural proteins between CIV and ascoviruses further supports the hypothesis of common ancestry.
In conclusion, this is the first detailed study towards the determination of the virion proteins of an invertebrate iridovirus. This study will contribute to a better understanding of the molecular mechanisms underlying CIV virion assembly, CIV entry into cells, the initial steps of early iridovirus gene expression and the cell to cell movement of this virus.
Materials and methods
Preparation of virus particles and gel electrophoresis
CIV was propagated in larvae of the wax moth, Galleria mellonella, isolated as described by Marina et al. (1999) and further purified by 25–65% sucrose density gradient centrifugation. The purified CIV particles were checked for quality by transmission electron microscopy and quantified by UV spectroscopy. The purified particles were denatured and the proteins were separated by 12% one-dimensional SDS-PAGE. The gel was stained with colloidal blue and the gel lane containing the virion proteins was cut into six segments based on a comparison with molecular markers. Each gel piece was sliced and dehydrated with acetonitrile (100%) (ACN). After vacuum drying, the gel segments were incubated in 10 mM dithiothreitol in 50 mM ammonium bicarbonate (ABC buffer) at 57 °C for 1 h and subsequently in 55 mM iodoacetamide (Sigma) in ABC buffer at room temperature for 1 h. After a final wash step with ABC buffer the gel material was dried.
Trypsin digestion and LC-MS/MS
In-gel protein digestions were performed using sequencing grade modified porcine trypsin (Promega, Madison, WI) in ABC buffer at 37 °C for 15 h, after which the digests were centrifuged at 6000 g. The supernatants were collected, and the remaining gel pieces were extracted with 5% triflouroacetic acid (TFA) and then with 15% ACN /1% TFA. The extracts were combined with the supernatants of the original digests, vacuum-dried, and the dried material was dissolved in 20 μl 0.1% formic acid in water. The peptides resulting from this digestion were analyzed by LC-MS/MS. To this aim, 18 μl of the samples were concentrated over a 0.10 × 32 mm Prontosil 300-5-C18H (Bischoff, Germany) pre-concentration column at a flow of 6 μl/min for 5 min. Peptides were eluted from the pre-concentration column and loaded onto a 0.10 × 200 mm Prontosil 300-3-C18H analytical column with a gradient of 10% to 35% ACN in 0.1% formic acid at a flow of 0.5 μl/min for 50 min. After that the percentage of ACN was increased to 80% (with 0.1% formic acid) in 3 min as a column cleaning step. Between the pre-concentration and analytical column, an electrospray potential of 3.5 kV was applied directly to the eluent via a solid 0.5 mm platina electrode fitted into a P875 Upchurch microT. Full scan positive mode Fourier transform mass spectra (FTMS) were measured between mass-to-charge ratios of 380 and 1400 with a LTQ-Orbitrap spectrometer (Thermo electron, San Jose, CA, USA). MS/MS scans of the four most abundant doubly and triply charged peaks in the FTMS scan were recorded in a data dependent mode in the linear trap (MS/MS threshold = 10.000). All MS/MS spectra obtained with each run were analyzed with Biowork 3.1.1 software (Thermo Fisher Scientific, Inc.). A maximum of 1 allowed differential modification per peptide was set for oxidation of methionines and de-amidation of asparagine and glutamine residues. Carboxamidomethylation of cysteines was set as a fixed modification. Trypsin specificity was set to fully enzymatic and a maximum of 3 missed cleavages with monoisotopic precursor and fragment ions. The mass tolerance for peptide precursor ions was set to 10 parts per million (10 ppm = 0.01 op m/z 1000 amu) and for MS/MS fragment ions to 0.5 Da. An Invertebrate iridescent virus 6 protein database was used for the analysis (AF303741; created July 31, 2001; downloaded from www.ncbi.nlm.nih.gov/sites/entrez) after adding a list of commonly observed contaminants like: BSA (P02769, bovine serum albumin precursor), trypsin (P00760, bovine), trypsin (P00761, porcin), keratin K22E (P35908, human), keratin K1C9 (P35527, human), keratin K2C1 (P04264, human) and keratin K1CI (P35527, human). A decoy database was created by adding the reversed sequences using the program SequenceReverser from the MaxQuant package (Cox and Mann, 2008), resulting in a total of 1058 proteins in the database. To identify the proteins in the CIV virions, the MS/MS spectra obtained from the LC-MS/MS were searched against the CIV ORF database using Bioworks 3.3.1 (Table 1). The peptide identifications obtained were filtered in Bioworks with the following filter criteria: ΔCn > 0.08, Xcorr > 1.5 for charge state 2+, Xcorr > 3.3 for charge state 3+ and Xcorr > 3.5 for charge state 4+ (Peng et al., 2003). Only those proteins that showed a Bioworks Score factor (Sf) larger then 0.6 were considered.
Acknowledgments
This research was supported by a grant from the Scientific and Technological Research Council of Turkey and a Research Project Grant from the Graduate School for Production Ecology and Resource Conservation of Wageningen University, the Netherlands, to İkbal Agah İnce. Monique M. van Oers was supported by a MEERVOUD grant from the Research Council of Earth and Life Sciences (ALW) with financial aid from the Netherlands Organization for Scientific Research (NWO). All proteomic LC-MS/MS measurements were done at Biqualys Wageningen (www.biqualys.nl).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2010.05.038.
Appendix A. Supplementary data
Spectra of single hit peptides, arranged according to predicted molecular mass of corresponding proteins.
References
- Asgari S., Davis J., Wood D., Wilson P., McGrath A. Sequence and organization of the Heliothis virescens ascovirus genome. J. Gen. Virol. 2007;88:1120–1132. doi: 10.1099/vir.0.82651-0. [DOI] [PubMed] [Google Scholar]
- Barray S., Devauchelle G. Étude des polypeptides de structure du virus iridescent de Chilo suppressalis (Iridovirus type 6) Can. J. Microbiol. 1979;25:841–849. doi: 10.1139/m79-124. [DOI] [PubMed] [Google Scholar]
- Barray S., Devauchelle G. Protein synthesis in cells infected by Chilo iridescent virus (Iridovirus, type 6) Arch. Virol. 1985;86:315–326. doi: 10.1007/BF01309835. [DOI] [PubMed] [Google Scholar]
- Bideshi D.K., Demattei M.-V., Rouleux-Bonnin F., Stasiak K., Tan Y., Bigot S., Bigot Y., Federici B.A. Genomic sequence of Spodoptera frugiperda ascovirus 1a, an enveloped, double-stranded DNA insect virus that manipulates apoptosis for viral reproduction. J. Virol. 2006;80:11791–11805. doi: 10.1128/JVI.01639-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot Y., Samain S., Auge-Gouillou C., Federici B. Molecular evidence for the evolution of ichnoviruses from ascoviruses by symbiogenesis. BMC Evol. Biol. 2008;8:253. doi: 10.1186/1471-2148-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot Y., Renault S., Nicolas J., Moundras C., Demattei M.V., Samain S., Bideshi D.K., Federici B.A. Symbiotic virus at the evolutionary intersection of three types of large DNA viruses; iridoviruses, ascoviruses, and ichnoviruses. PLoS One. 2009;4:e6397. doi: 10.1371/journal.pone.0006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti M., Devauchelle G. Characterization and localization of CIV polypeptides. Virology. 1985;145:123. doi: 10.1016/0042-6822(85)90207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.M., Tran B.N., Lin Q., Lim T.K., Wang F., Hew C.-L. iTRAQ analysis of Singapore grouper iridovirus infection in a grouper embryonic cell line. J. Gen. Virol. 2008;89:2869–2876. doi: 10.1099/vir.0.2008/003681-0. [DOI] [PubMed] [Google Scholar]
- Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b. range mass accuracies and proteome-wide protein quantification. Nat. Biotech. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Darai G., Anders K., Koch H.G., Delius H., Gelderblom H., Samalecos C., Flügel R.M. Analysis of the genome of fish lymphocystis disease virus isolated directly from epidermal tumours of Pleuronectes. Virology. 1983;126:466–479. doi: 10.1016/s0042-6822(83)80005-1. [DOI] [PubMed] [Google Scholar]
- Delhon G., Tulman E.R., Afonso C.L., Lu Z., Becnel J.J., Moser B.A., Kutish G.F., Rock D.L. Genome of invertebrate iridescent virus type 3 (Mosquito Iridescent Virus) J. Virol. 2006;80:8439–8449. doi: 10.1128/JVI.00464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J.W., Moon C.H., Kim H.J., Ko M.S., Kim S.B., Son J.H., Kim J.S., An E.J., Kim M.K., Lee S.K., Han M.S., Cha S.J., Park M.S., Park M.A., Kim Y.C., Kim J.W., Park J.W. Complete genomic DNA sequence of rock bream iridovirus. Virology. 2004;325:351–363. doi: 10.1016/j.virol.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Elias J.E., Gygi S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Meth. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Elsevier Academic Press; San Diego, CA: 2005. Virus taxonomy: eighth report of the international committee for virus taxonomy. [Google Scholar]
- Goorha R., Murti K.G. The genome of frog virus 3, an animal DNA virus, is circularly permuted and terminally redundant. Proc. Natl. Acad. Sci. U. S. A. 1982;79:248–252. doi: 10.1073/pnas.79.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.G., Deng M., Weng S.P., Li Z., Zhou S.Y., Long Q.X., Wang X.Z., Chan S.-M. Complete genome analysis of the Mandarin fish infectious spleen and kidney necrosis iridovirus. Virology. 2001;291:126–139. doi: 10.1006/viro.2001.1208. [DOI] [PubMed] [Google Scholar]
- He J.G., Lü L., Deng M., He H.H., Weng S.P., Wang X.H., Zhou S.Y., Long Q.X., Wang X.Z., Chan S.M. Sequence analysis of the complete genome of an iridovirus isolated from the Tiger frog. Virology. 2002;292:185–197. doi: 10.1006/viro.2001.1245. [DOI] [PubMed] [Google Scholar]
- Huang Y., Huang X., Liu H., Gong J., Ouyang Z., Cui H., Cao J., Zhao Y., Wang X., Jiang Y., Qin Q. Complete sequence determination of a novel reptile iridovirus isolated from soft-shelled turtle and evolutionary analysis of Iridoviridae. BMC Genomics. 2009;10:224. doi: 10.1186/1471-2164-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob N.-J., Mueller K., Bahr U., Darai G. Analysis of the first complete DNA sequence of an invertebrate Iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology. 2001;286:182–196. doi: 10.1006/viro.2001.0963. [DOI] [PubMed] [Google Scholar]
- Jancovich J.K., Mao J., Chinchar V.G., Wyatt C., Case S.T., Kumar S., Valente G., Subramanian S., Davidson E.W., Collins J.P., Jacobs B.L. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology. 2003;316:90–103. doi: 10.1016/j.virol.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kelly D.C., Tinsley T.W. The proteins of iridescent virus type 2 and 6. J. Invertebr. Pathol. 1972;19:273–274. [Google Scholar]
- Lü L., Zhou S.Y., Chen C., Weng S.P., Chan S.-M., He J.G. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology. 2005;339:81–100. doi: 10.1016/j.virol.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Marina C.F., Arredondo-Jimenez J.I., Castillo A., Williams T. Sublethal effects of iridovirus disease in a mosquito. Oecologia. 1999;119:383–388. doi: 10.1007/s004420050799. [DOI] [PubMed] [Google Scholar]
- Orange N., Devauchelle G. Lipophilic polypeptides of Chilo iridescent virus (CIV, type 6) membrane. FEMS Microbiol. Lett. 1987;48:59–64. [Google Scholar]
- Pandey A., Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–864. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- Peng J., Elias J.E., Thoreen C.C., Licklider L.J., Gygi S.P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: The yeast proteome. J. Proteome Res. 2003;2:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- Song W.J., Qin Q.W., Qiu J., Huang C.H., Wang F., Hew C.L. Functional genomics analysis of Singapore grouper iridovirus: complete sequence determination and proteomic analysis. J. Virol. 2004;78:12576–12590. doi: 10.1128/JVI.78.22.12576-12590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak K., Demattei M.-V., Federici B.A., Bigot Y. Phylogenetic position of the Diadromus pulchellus ascovirus DNA polymerase among viruses with large double-stranded DNA genomes. J. Gen. Virol. 2000;81:3059–3072. doi: 10.1099/0022-1317-81-12-3059. [DOI] [PubMed] [Google Scholar]
- Stasiak K., Renault S., Demattei M.-V., Bigot Y., Federici B.-A. Evidence for the evolution of ascoviruses from iridoviruses. J. Gen. Virol. 2003;84:2999–3009. doi: 10.1099/vir.0.19290-0. [DOI] [PubMed] [Google Scholar]
- Tan W.G.H., Barkman T.J., Gregory Chinchar V., Essani K. Comparative genomic analyses of frog virus 3, type species of the genus Ranavirus (family Iridoviridae) Virology. 2004;323:70–84. doi: 10.1016/j.virol.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Tan Y., Bideshi D.K., Johnson J.J., Bigot Y., Federici B.A. Proteomic analysis of the Spodoptera frugiperda ascovirus 1a virion reveals 21 proteins. J. Gen. Virol. 2009;90:359–365. doi: 10.1099/vir.0.005934-0. [DOI] [PubMed] [Google Scholar]
- Tan Y., Spears T., Bideshi D.K., Johnson J.J., Hice R., Bigot Y., Federici B.A. P64, a novel major virion DNA-binding protein potentially involved in condensing the Spodoptera frugiperda Ascovirus 1a genome. J. Virol. 2009;83:2708–2714. doi: 10.1128/JVI.01610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidona C.A., Darai G. The complete DNA sequence of Lymphocystis disease virus. Virology. 1997;230:207–216. doi: 10.1006/viro.1997.8456. [DOI] [PubMed] [Google Scholar]
- Tsai C.-T., Ting J.-W., Wu M.-H., Wu M.-F., Guo I.-C., Chang C.-Y. Complete genome sequence of the Grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 2005;79:2010–2023. doi: 10.1128/JVI.79.4.2010-2023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xue J., Seaborn C.P., Arif B.M., Cheng X.W. Sequence and organization of the Trichoplusia ni ascovirus 2c (Ascoviridae) genome. Virology. 2006;354:167–177. doi: 10.1016/j.virol.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Williams T. The iridoviruses. Adv. Virus Res. 1996;46:345–412. doi: 10.1016/s0065-3527(08)60076-7. [DOI] [PubMed] [Google Scholar]
- Williams T., Barbosa-Solomieu V., Chinchar V.G. A decade of advances in iridovirus research. Adv. Virus Res. 2005;65:173–248. doi: 10.1016/S0065-3527(05)65006-3. [DOI] [PubMed] [Google Scholar]
- Willis D.B. Taxonomy of Iridoviruses. In: Darai G., editor. Molecular biology of iridescent viruses. Kluwer; Boston: 1990. [Google Scholar]
- Willis D.B., Granoff A. Trans activation of an immediate-early frog virus 3 promoter by a virion protein. J. Virol. 1985;56:495–501. doi: 10.1128/jvi.56.2.495-501.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Yu Z., Zhang P., Battisti A.J., Holdaway H.A., Chipman P.R., Bajaj C., Bergoin M., Rossmann M.G., Baker T.S. The capsid proteins of a large, icosahedral dsDNA virus. J. Mol. Biol. 2009;385:1287–1299. doi: 10.1016/j.jmb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.-Y., Xiao F., Xie J., Li Z.-Q., Gui J.-F. Complete genome sequence of Lymphocystis disease virus isolated from China. J. Virol. 2004;78:6982–6994. doi: 10.1128/JVI.78.13.6982-6994.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Ke F., Huang Y.-H., Zhao J.-G., Gui J.-F., Zhang Q.-Y. Identification and characterization of a novel envelope protein in Rana grylio virus. J. Gen. Virol. 2008;89:1866–1872. doi: 10.1099/vir.0.2008/000810-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectra of single hit peptides, arranged according to predicted molecular mass of corresponding proteins.