Abstract
Infectious bronchitis virus (IBV) replicates in the epithelial cells of trachea and lungs of chicken, however the mechanism of generation of innate immune response against IBV infection in these tissues has not been fully characterized. Our objective was to study innate responses induced early following IBV infection in chickens. Initiation of the transcription of selected innate immune genes such as TLR3, TLR7, MyD88, IL-1β and IFN-β, as well as recruitment of macrophages, were evident following an initial down regulation of some of the observed genes (TLR3, IL-1β, and IFN-γ) in trachea and lung. This initial down-regulation followed by the induction of innate immune response to IBV infection appears to be inadequate for the control of IBV genome accumulation and consequent histopathological changes in these tissues. Potential induction of innate immunity before infection occurs may be necessary to reduce the consequences since vaccine induced immunity is slow to develop.
Keywords: Infectious bronchitis virus, Macrophage, Avian, Toll-like receptor, Cytokine
Highlights
-
•
Innate immune response against avian infectious bronchitis virus (IBV) studied.
-
•
IBV induces toll-like receptor mRNA in lungs and trachea.
-
•
IBV induces pro-inflammatory and antiviral cytokine mRNA expression.
-
•
IBV increases macrophages in respiratory tissues.
Introduction
Infectious bronchitis virus (IBV) is a positive sense RNA virus that belongs to the Family Coronaviridae. IBV primarily targets the epithelial cells of the respiratory, urinary and reproductive tracts in the domestic chicken (Gallus gallus domesticus) (Cavanagh, 2007, Cavanagh et al., 1997, Cook et al., 2012), results pathogenic processes with mortality rates as high as 30% in chicks less than 4 weeks old and egg production loss in layers. The ability of IBV to infect different organ systems depends on the strain of the virus. For example, infection with the Connecticut (Conn) A5968 strain of IBV is limited to replication in the respiratory tract of chickens (Cavanagh, 2005, Uenaka et al., 1998) and the virus is transmitted between individuals through the shedding of viral particles in the nasal and ocular discharge of infected birds (Cook et al., 2012).
For the control of disease caused by IBV, live attenuated viral vaccines are available and had so far been very reliable (Cavanagh, 2003, Cavanagh, 2007). These vaccines use IBV strains such as Massachusetts, Connecticut and Arkansas and combinations thereof providing protection against almost all field strains of IBV. However, emergence of new variant more heterogeneous IBV strains (Cavanagh, 2003), leads to infectious bronchitis outbreaks in vaccinated flocks leading to significant production losses (Shimazaki et al., 2009, Xu et al., 2007). Therefore a novel approach that could be used as an alternative or additional to the existing means of control is urgently needed. One such approach may be the use of innate immune mediators to empower the innate immune system.
The innate and adaptive immune responses to viral infection in chickens are interconnected, with the innate or non-specific response being the more rapid of the two. Immune cells and cells on the mucosal surface are involved in innate immune responses and recognize pathogen associated molecular patterns (PAMPs). PAMPs are generally conserved between the different types of pathogens, but are not expressed by the host cells, and can be recognized by the host cell through membrane associated and intra-cellular toll-like receptors (TLRs) (Akira, 2001). Among TLRs, TLR3 and TLR7 are well known for recognition of RNA virus encoded PAMPs (Akira, 2001). In chickens, TLR3 and TLR7 are orthhologous to their mammalian counterparts (Kannaki et al., 2010). TLR3 recognizes and binds to the double-stranded (ds)RNA intermediates produced during viral replication (Alexopoulou et al., 2001, Higgs et al., 2006, Iqbal et al., 2005) Stimulation of TLR3 leads to activation of TIR-domain-containing adapter-inducing interferon-β (TRIF) adaptor protein-mediated pathway, whereas TLR7 responds to single-stranded (ss)RNA produced during intracellular viral replication, activating the myeloid differentiation primary response gene 88 (MyD88) mediated-pathway (Akira, 2001, Watters et al., 2007). The end products of both TLR3 and TLR7 signaling pathways are the production of anti-viral type I interferon (IFN)-α and -β, and pro-inflammatory cytokines, respectively (Guillot et al., 2005). Interleukin (IL)-1β plays an important role in chemotaxis, stimulating the cellular response and recruiting cells, such as macrophages, to the site of infection (Babcock et al., 2008). Macrophages are important cells of the innate immune response; their functional roles include phagocytosis of foreign material, cytokine and chemokine secretion, and the presentation of antigens to help facilitate the development of antigen-specific adaptive immune responses (Mast et al., 1998, Qureshi et al., 2000, Tate et al., 2010). Macrophages are also source of nitric oxide (NO) production, which is the product of the activity of inducible nitric oxide synthase (iNOS) in defence against microbial infections (Ariaans et al., 2008, Ficken et al., 1987, Naqi et al., 2001, Read, 1999).
Host responses in the trachea following intranasal immunization of chickens using IBV vaccine strains such as attenuated or non-attenuated Massachusetts (Mass) IBV have been studied (Guo et al., 2008, Wang et al., 2006). These studies have shown an increase in the mRNA expression of TLR2, TLR3, TLR6, TLR7, IL-1β, and genes involved in IFN signaling among a number of other genes following IBV immunization. It is not known whether the host responses are elicited against any IBV strain in lung, although the lung is also a target organ for IBV. The nature and extent of the innate host responses elicited against virulent IBV strains in the trachea and lung are also not known. It has also been shown that following immunization with the IBV Mass41 strain that the number of macrophages in bronchoalvelar lavage fluid is increased (Fulton et al., 1990, Fulton et al., 1993). However, it is not known whether these macrophages are mobilized from the trachea, lung, or both tissues in response to IBV infection.
Our main hypothesis is that IBV infection will result in increases in macrophage numbers and relative mRNA expression of innate host response genes in respiratory tissues early following infection. Therefore, the objective of our investigation was to characterize the host innate response in terms of the expression of TLRs, type I and II IFNs, and pro-inflammatory cytokine genes, as well as any changes in macrophage numbers within the lungs and trachea early during infection with IBV. In this study, we saw an up-regulation of TLR3 and TLR7 mRNA and increased macrophage numbers in the trachea and lung, as well as an up-regulation of IFNβ, and IL-1β mRNA expression in trachea that indicated the initiation of innate host responses. Conversely, we also observed an initial down regulation of mRNA expression of genes, namely TLR3, IL-1β, and IFN-γ. This early delay in the induction of innate host responses following the infection may be associated with an increase in the IBV genome load and histological changes in trachea and lungs of IBV infected chickens.
Results
Clinical and pathological observations
Uninfected chickens showed no clinical signs, gross or histological lesions. Although all the IBV infected chickens did not develop respiratory signs, they all showed non-specific signs such as huddling together under the lamp and droopy wings. The infected chickens though did not show any gross lesions in the respiratory tract, histological changes were evident in both trachea and lungs. In the trachea, we saw a significant increase in the pathology over time, with the highest scores evident at 72 hpi for IBV infected tissues when compared to all other time points (p<0.0001, data not shown). For lung, there were also significant increases in the histopathological scores overtime, but only when comparing the various IBV infected tissues to the uninfected controls at time points 12, 48, and 72 hpi (p<0.0001, data not shown).
IBV genome load in trachea and lungs
The viral genome load in the trachea and lung was measured using the complementary DNA (cDNA) originating from RNA extracted from IBV-infected chickens by analysis with real-time quantitative polymerase chain reaction (qPCR). The IBV genome load was significantly higher in the trachea at only 72 hpi (p=0.013) when compared to 12 or 48 hpi due to higher variability among individual birds within the group (data not shown). Conversely, there was no significant difference recorded between time points for IBV genome loads measured within the lungs (p=0.176, data not shown).
Expression of mRNA of TLR3 and TLR7 in the trachea and lungs
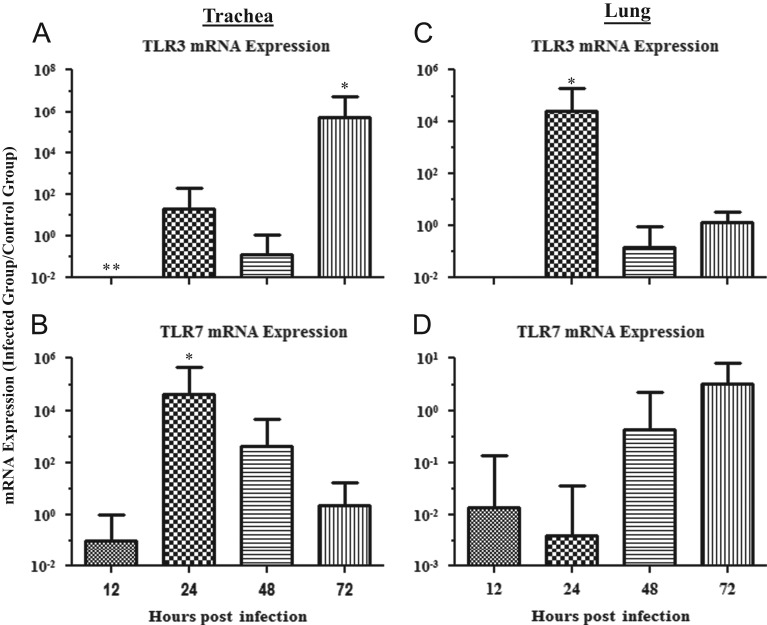
The mRNA expression data for TLR3 and TLR7 in the trachea are illustrated in Fig. 1A and B, respectively. The TLR3 mRNA expression in the trachea was significantly down-regulated at 12 hpi (p=0.033) and significantly up-regulated at 72 hpi (p=0.010) when comparing the IBV infected groups to the uninfected controls. At 24 and 48 hpi, there was no significant difference in the TLR3 mRNA expression in the trachea when comparing IBV infected groups to the control groups (24 hpi, p=0.424; 48 hpi, p=0.565). Conversely, TLR7 mRNA expression in the trachea was significantly up-regulated when compared to the controls at 24 hpi (p=0.001). At 12, 48, and 72 hpi, there was no significant difference in TLR7 mRNA expression in the trachea when comparing IBV infected groups to the control groups (12 hpi, p=0.739; 48 hpi, p=0.217; 72 hpi, p=0.970).
Fig. 1.
Relative TLR3 and TLR7 mRNA expression in trachea and lungs of chickens infected with the Conn A5968 strain of IBV. (A) and (B) represent relative TLR3 and TLR7 mRNA expression in trachea, respectively. (C) and (D) represent TLR3 and TLR7 mRNA expression in the lung, respectively. Chickens were infected intra-tracheally with the Conn A5968 strain of IBV at 6 days of age and trachea and lung tissues were collected at 12, 24, 48, and 72 h post-infection (hpi). There were five IBV-infected chickens at each time point (six animals were sampled at 12 hpi) and five PBS-treated chickens used as controls for each time point (only two animals were sampled at 72 hpi for the control group). Target mRNA expression was normalized via the geometrical mean of Eff.(uninfected control)Cp for all control genes (β-actin and ubiquitin). Error bars represent standard error of the mean (SEM). ⁎=relative mRNA expression is significantly up-regulated when compared to the uninfected controls and ⁎⁎=relative mRNA expression is significantly down-regulated when compared to controls.
The mRNA expression data for TLR3 and TLR7 in lung are illustrated in Fig. 1C and D, respectively. The TLR3 mRNA expression in lung was significantly up-regulated at 24 hpi (p=0.017). In comparison, at 12, 48, and 72 hpi there was no significant difference in TLR3 mRNA levels in trachea when comparing IBV infected groups to the controls (12 hpi, p=0.237; 48 hpi, p=0.606; 72 hpi, p=0.917). There were no significant changes in TLR7 mRNA expression in the lung at any of the time points when comparing IBV infected groups to the control group (12 hpi, p=0.563; 24 hpi, p=0.205; 48 hpi, p=0.641; 72 hpi, p=0.730).
Expression of mRNA of MyD88 and TRIF in lung and trachea
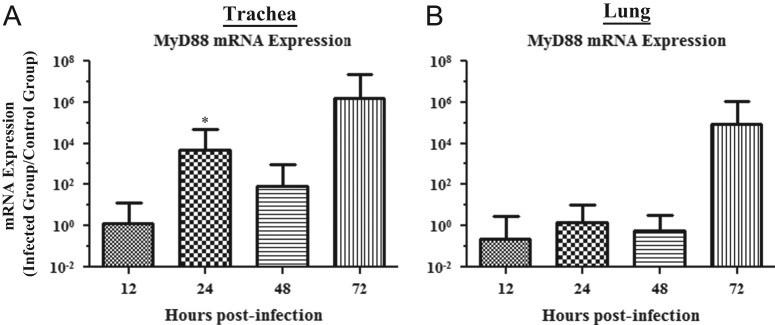
The mRNA expression data for MyD88 in the trachea and lungs are illustrated in Fig. 2A and B, respectively. The MyD88 mRNA expression in trachea was significantly up-regulated at 24 hpi (p=0.022), but there was no significant change in expression levels at 12, 48, or 72 hpi (12 hpi, p=0.922; 48 hpi, p=0.337; 72 hpi, p=0.535). There was no significant change in MyD88 mRNA expression in lung for any of the time points (12 hpi, p=0.652; 24 hpi, p=0.896; 48 hpi, p=0.774; 72 hpi, p=0.893).
Fig. 2.
Relative MyD88 mRNA expression in trachea and lungs of chickens infected with the Conn A5968 strain of IBV. (A) and (B) represent relative MyD88 mRNA expression in trachea and lung, respectively. Experimental design was as indicated in the legend of Fig. 1. Target mRNA expression was normalized via the geometrical mean of Eff.(uninfected control)Cp for all control genes (β-actin and ubiquitin). Error bars represent SEM. ⁎=relative mRNA expression is significantly up-regulated when compared to the uninfected controls.
There was no significant change in TRIF mRNA expression in the trachea and lung at any of the time points (data not shown).
Expression of mRNA of genes of interferons, pro-inflammatory cytokines, and iNOS in trachea and lung
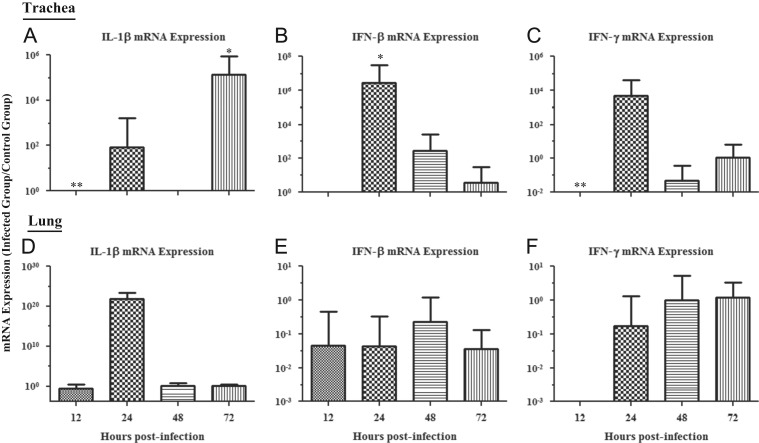
The mRNA expression data for IL-1β, IFN-β, and IFN-γ in trachea are illustrated in Fig. 3A–C, respectively. A significant down-regulation of IL-1β mRNA expression was observed at 12 hpi (p=0.013), as well as a significant up-regulation at 72 hpi (p=0.006). No significant change in IL-1β mRNA expression at 24 or 48 hpi was observed (24 hpi, p=0.905; 48 hpi, p=0.297). For IFN-β mRNA expression, a significant up-regulation was observed at 24 hpi (p=0.004), with no other significant changes observed at the other time points (12 hpi, p=0.230; 48 hpi, p=0.151; 72 hpi, p=0.869). Expression of IFN-γ mRNA was also seen to have a significant down-regulation at 12 hpi (p=0.009) and a trend towards up-regulation by 24 hpi. However, there were no significant changes at any of the other time points (24 hpi, p=0.052; 48 hpi, p=0.220; 72 hpi, p=0.923).
Fig. 3.
Relative IL-1β, IFN-β and -γ mRNA expression in trachea and lungs of chickens infected with the Conn A5968 strain of IBV. (A–C) represents relative IL-1β, IFN-β, and -γ mRNA expression in trachea, respectively. (D–F) represents relative IL-1β, IFN-β, and -γ mRNA expression in the lung, respectively. Experimental design was as indicated in the legend of Fig. 1. Target mRNA expression was normalized via the geometrical mean of Eff.(uninfected control)Cp for all control genes (β-actin and ubiquitin). Error bars represent SEM. ⁎=relative mRNA expression is significantly up-regulated when compared to the uninfected controls, and ⁎⁎=relative mRNA expression is significantly down-regulated when compared to uninfected controls.
The mRNA expression data for IL-1β, IFN-β, and IFN-γ in lung are illustrated in Fig. 3D–F, respectively. There was no significant change in IL-1β mRNA expression in lung at any of the time points (12 hpi, p=0.666; 24 hpi, p=0.439; 48 hpi, p=0.964; 72 hpi, p=0.803). IFN-β mRNA expression was found to have no significant changes at any of the time points as well (12 hpi, p=0.802; 24 hpi, p=0.283; 48 hpi, p=0.444; 72 hpi, p=0.209). Finally, IFN-γ mRNA expression was seen to have the same pattern as the other preceding genes measured in the lung, as there was no significant change at any of the time points (12 hpi, p=0.267; 24 hpi, p=0.446; 48 hpi, p=0.996; 72 hpi, p=0.821).
There was no significant change in iNOS and IFN-α mRNA expression in trachea and lung at any of the time points (data not shown).
Quantification of macrophages present in trachea and lungs
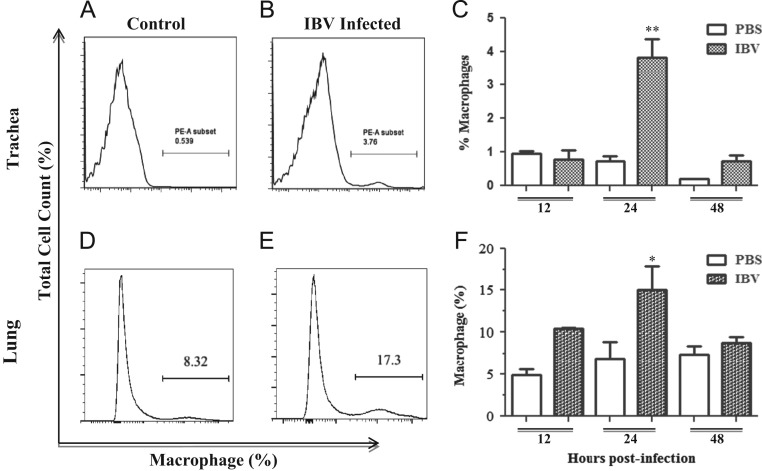
Fig. 4 illustrates the difference in the percentage of macrophages present in the trachea and lungs of control and IBV infected chickens. The percentage of macrophages present in the representative FACS plots from tracheal samples collected from control and IBV infected chickens at 24 hpi are illustrated in Fig. 4A and B respectively. It was observed that macrophage numbers in the trachea only significantly increased in IBV infected chickens at 24 hpi when compared to control chickens (p<0.0001). The percentage of macrophages present in the representative FACS plots from lungs of control and IBV infected chickens at 24 hpi are illustrated in Fig. 4D and E respectively. The only significant increase in the percentage of macrophages in IBV infected lung tissues when compared to the control was seen at 24 hpi (p=0.002). Fig. 4C and F provides quantitative data to show the difference between the IBV infected groups and the uninfected control groups in terms of the percentage of macrophages at each time point for the trachea and lung, respectively.
Fig. 4.
Quantification of macrophages present in trachea and lung. Chickens were infected intra-tracheally with Conn A5968 at 6 days of age and trachea and lung tissues were collected at 12, 24, and 48 hpi. There were three IBV-infected and three PBS-treated chickens used at each time point for the lung macrophage quantification, and in a separate experiment with the same experimental design, four IBV-infected and five PBS-treated chickens used at each time point for quantification of macrophages in trachea. (A) and (B) represent the percent of macrophages in control and IBV infected trachea respectively at 24 hpi. (D) and (E) represent the percent of macrophages in IBV infected and control lung respectively for 24 hpi. (C) and (F) are graphical representations of the percentage of macrophage numbers at each time point for trachea and lungs, respectively. Error bars represent SEM. ⁎=significant increase when compared to uninfected controls at 12, 24, and 48 hpi in the lungs and ⁎⁎=significant increase when compared to all other time points in the trachea.
Discussion
In this study we investigated the innate immune response following infection with a virulent strain of IBV Conn A5968 in the trachea and lungs of chickens. Our findings in this study are four-fold. Firstly, we saw that the increased expression of tested TLRs and downstream signaling molecules that indicates initiation of the innate host response. Secondly, we saw that the macrophage numbers are increased in the trachea and lungs, peaking at 24 h following IBV infection. Finally, we also observed a down-regulation in the expression of some innate immune genes, TLR3, IL-1β and IFN-γ, which occurred in the early phase of viral replication (12 hpi). Altogether, we observed that although the innate response was initiated early following IBV infection with some genes being down-regulated, it may not be adequate for the control of viral replication and associated pathology in the respiratory mucosa.
We observed a significant increase of TLR3 relative mRNA expression in both trachea and lung and TLR7 mRNA expression only in trachea in IBV infected chickens when compared to the uninfected controls. Due to the intercalating of the gene encoding the MyD88 adaptor protein within the pathway activated by TLR7, we were not surprised to see a similar increase in the expression of relative MyD88 mRNA as well following IBV infection when compared to uninfected controls. In avian models such as the Quingyuan goose (Anser domesticus), correlation between increased TLR7 and relative MyD88 gene-expression has been observed (Wei et al., 2013). Surprisingly, there was no significant change in relative TRIF mRNA expression despite the increase in the relative TLR3 gene expression, which is difficult to explain. However, a significant increase in relative IFN-β gene expression was observed in trachea at 24 hpi, supporting the previous claims that the activation of the TLR3 pathway works towards an up-regulation of IFN-β production in chickens after 12 hpi (Karpala et al., 2008, Otsuki et al., 1979, Parvizi et al., 2012).
We also noted a significant down-regulation in the relative expression of TLR3, IFN-γ, and IL-1β mRNA during the initial phase of IBV infection within the trachea when compared to uninfected controls. Although we did not investigate the viral proteins involved and the mechanism of down-regulation of host innate immune molecules in our study, various types of coronaviruses have been known to encode proteins that disrupt the downstream signaling cascades used during the innate immune response to infections (Zhong et al., 2012). The nucelocapsid (N) protein has been shown to interfere with the 2',5'-oligoadenylate synthetase/RNaseL (2'–5' OAS) activation, which is responsible for Type I IFN induction and can also inhibit the production of various pro-inflammatory cytokines and chemokines via global translational shutdown (Ye et al., 2007, Zhong et al., 2012). The non-structural protein 3 (Nsp3) expressed by IBV has also been implicated in de-ubiquitinating activity, which prevents nuclear translocation and/or production of the interferon regulatory factor 3 (IRF3) and sequential synthesis of Type I IFNs (Clementz et al., 2010). In vitro experiments involving the infection of cell cultures with severe acute respiratory syndrome coronavirus (SARS-CoV) and IBV indicate that the interaction of the spike protein found in both coronaviruses and host eukaryotic initiation factor 3 (eIF3) is responsible for the modulation of host immune gene expression (Xiao et al., 2008). However, our study indicated that this initial down-regulation of innate immune genes is associated with the increase in IBV replication and the virus induced histological changes in trachea and lung. Neither Guo et al. nor Wang et al. observed a down-regulation in expression of the these genes (Guo et al., 2008, Wang et al., 2006), which could be due to the use of the less virulent vaccine strains in their studies.
Also, since production of IFN-γ is controlled by cytokines secreted by both antigen-presenting cells and from the adaptive arm of immune system, a slight delay in expression is expected in response to IBV infection (Frucht et al., 2001, Gessani and Belardelli, 1998, Golab et al., 2000). IL-1β mRNA also showed a similar down-regulation when compared to uninfected controls in trachea, but conversely also shows a sharp increase in mRNA expression as the IBV infection progresses. This is further substantiated by the observations of Wang et al. and Guo et al., where both studies found a similar increase in IL-1β expression levels around 72 hpi with Mass IBV immunization (Guo et al., 2008, Wang et al., 2006). In contrast, mammalian studies of IL-1β response to viral infections have been found to be more rapid than in chickens, with up-regulated gene expression observed as early as 6 hpi (Lawrence et al., 2013, Poeck et al., 2010).
Macrophage numbers within the lungs and trachea of IBV infected chickens were found to significantly increase when compared to uninfected controls. In chickens, macrophages are present within the tissue of the lung itself, lining the atria and infundibulae and providing a direct line of defence against respiratory infection (Abdul-Careem et al., 2009; Maina, 2002) and can be mobilized to the lumen following viral infections (Cornelissen et al., 2013). Similarly, Fulton and colleagues witnessed macrophage infiltration into the respiratory lumen of the IBV Mass41 infected chickens when collecting respiratory lavage fluid between 24 and 96 hpi (Fulton et al., 1993). Their study did not identify the source of these macrophages as trachea and lung tissues, while our investigation indirectly indicates that macrophages may have been mobilised from the parenchyma of both the trachea and lung following IBV infection.
Conclusions
In conclusion, the results reported here suggest that IBV infection induces an innate immune response within the respiratory tissues through increased mRNA expression of TLR3 and TLR7, pro-inflammatory cytokines and anti-viral IFNs, and an increase in the number of infiltrating macrophages to both the lungs and trachea, with some of the innate genes being down-regulated immediately following IBV infection.
Materials and methods
Animals
All procedures have been approved by the University of Calgary’s Veterinary Sciences Animal Care Committee. Day-old unsexed specific pathogen free (SPF) layer chicks (White Leghorn) were obtained from Canadian Food Inspection Agency, Ottawa, and raised to 6 days old for use in experiments. The chickens were not immunized against any diseases. The chickens were housed in high containment poultry isolators at the University of Calgary’s Spyhill campus, with ample access to food and water that was nutritionally complete and appropriate for the age of the chickens.
IBV infection in chickens
Conn A5968 strain of IBV was obtained from ATCC (Manassas, Virginia, United States). Six day old SPF chickens were infected with Conn strain of IBV intra-tracheally (2.75×104 EID50 per bird) with controls receiving phosphate buffered saline (PBS). At 12, 24, 48, and 72 h post-infection (hpi), 5–6 IBV infected and 2–5 control chickens were necropsied and portions of the trachea and lungs were stored in RNAlater (Qiagen Inc., Mississauga, ON, Canada) at −20 °C for RNA extraction. At the same time points, lung and trachea were also collected in 10% formol saline (VWR, Edmonton, AB, Canada) for the analysis of changes in histology, with IBV (4–5 from each time point) or PBS (2–5 from each time point) treated groups.
Clinical and pathological observations
The chickens were observed daily for specific (respiratory signs) and non-specific (huddling together, droopy wings and ruffled feathers) clinical signs. Lung and trachea of IBV infected and control chickens that were preserved in 10% formol saline at 12, 24, 48, and 72 hpi and then sent to the Histopathology Diagnostic Services Unit at the University of Calgary Faculty of Veterinary Medicine. The tissues were embedded in paraffin, sectioned at 5 µm, and stained with haematoxylin and eosin. The histological changes observed in the trachea were scored as described in (Grgic et al., 2008). The histological changes observed in lungs were scored based on visible changes in the para-bronchioles of the lung such as mononuclear cell infiltration and extent of loss of air exchange areas of the lung.
Quantification of macrophages from chicken lung and trachea.
Six day old SPF chicks were infected with the Conn IBV strain (2.75×104 EID50 per bird) or treated with PBS. At 12, 24, and 48 hpi the chicks were euthanized and both lungs were collected in Hank’s balanced salt solution (HBSS) for mononuclear cell isolation for each of the IBV (n=3 each time point) and PBS (n=3 at each time point) groups. At the same time points, the tracheas were collected in 0.5 mM EDTA (Ethylenediaminetetraacetic acid) in PBS supplemented with 5% heat inactivated fetal bovine serum (FBS) on ice, for the isolation of cells as described by Booth and O’Shea (2002), for each of the IBV (n=5 at each time point) and PBS (n=5 at each time point) groups.
RNA extraction and cDNA conversion
RNA was extracted from the trachea and lungs of infected and uninfected chickens by a single-step method using Trizol (Invitrogen Canada Inc., Burlington, ON, Canada) according to the manufacturer’s protocol. RNA concentration was quantified using Nanodrop 1000 spectrophotometer at 260 nm wavelength (Thermo Scientific, Wilmington, DE, USA). Reverse transcription of extracted RNA (2000ng) was carried out using 10× RT random primers (High Capacity cDNA Reverse Transcription Kit, Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. The cDNA product was then diluted with 80 µL of RNAse-free water to a final concentration of 20 ng/µL.
Conventional PCR technique
For absolute and relative quantification of the target gene expression, target and reference genes were PCR-amplified from cDNA preparations using primers listed in Table 1, cloned, and used to generate standard curves. For the preparation of the standards, relevant fragments were amplified using standard PCR conditions.
Table 1.
PCR primers used in conventional and real time PCR techniques.
| Primer name | Sequence (5'–3') | Fragment size (Bps) | Reference |
|---|---|---|---|
| IBV N | F- GACGGAGGACCTGATGGTAAR- CCCTTCTTCTGCTGATCCTG | 206 | This study |
| IFN-α | F- ATCCTGCTGCTCACGCTCCTTCTR- GGTGTTGCTGGTGTCCAGGATG | 198 | (Villanueva et al., 2011) |
| IFN-β | F- AGCAAGGACAAGAAGCAAGCR- CGTGCCTTGGTTTACGAAGC | 177 | (Esnault et al., 2011) |
| IFN-γ | F- ACACTGACAAGTCAAAGCCGCACA R- AGTCGTTCATCGGGACCTTGGC | 129 | (Villanueva et al., 2011) |
| TLR3 | F- TCAGTACATTTGTAACACCCCGCCR- GGCGTCATAATCAAACACTCC | 256 | (Villanueva et al., 2011) |
| TLR7 | F- TTCTGGCCACAGATGTGACCR- CCTTCAACTTGGCAGTGCAG | 219 | (Villanueva et al., 2011) |
| IL-1β | F- GTGAGGCTCAACATTGCGCTGTAR- TGTCCAGGCGGTAGAAGATGAAG | 214 | (Villanueva et al., 2011) |
| iNOS | F- GGCAGCAGCGTCTCTATGACTTGR- GACTTTAGGCTGCCCAGGTTG | 185 | (St Paul et al., 2013) |
| MyD88 | F- AGCGTGGAGGAGGACTGCAAGAAG R- CCGATCAAACACACACAGCTTCAG | 264 | (Villanueva et al., 2011) |
| TRIF | F- GCTCACCAAGAACTTCCTGTGR- AGAGTTCTCATCCAAGGCCAC | 181 | (Villanueva et al., 2011) |
| β-actin | F- CAACACAGTGCTGTCTGGTGGTAR- ATCGTACTCCTGCTTGCTGATCC | 205 | (Villanueva et al., 2011) |
| Ubiquitin | F- GGGATGCAGATCTTCGTGAAAR- CTTGCCAGCAAAGATCAACCTT | 147 | (De Boever et al., 2008) |
Preparation of constructs as standards
PCR products were ran at 100 V for 1 h and 15 min on a 1.5% agarose gel and extracted using QIAquick Gel Extraction Kit (Qiagen Inc.) according to the manufacturer’s instructions. The extracted DNA was then cloned into the pCR®2.1-TOPO® vector and amplified through transformation of One Shot® Escherichia (E.) coli, followed by blue/white colony screening (TOPO TA Cloning kit Top 10, Invitrogen, Burlington, ON, Canada). The subsequently positive white cloned colonies had their plasmids extracted using the QIAprep spin miniprep kit (Qiagen Inc.), as per the manufacturer’s instructions. The extracted plasmids were screened using EcoR1 restriction enzyme (Invitrogen, Burlington, ON, Canada) and the positive clones were submitted to the University of Calgary’s Automated DNA Sequencing Services to be sequenced. The Basic Local Alignment Search Tool (BLAST) program (NCBI, Bethesda, MD, USA) was used to determine the accuracy of the target gene insert.
Real-time PCR technique
Real-time PCR technique was used for quantification of IBV genomic RNA and mRNA of host response genes. All the cDNA preparations originated from IBV infected and control chicken trachea and lungs were analyzed using qPCR assays, alongside a dilution series of the plasmids used to generate a standard curve. Fast SYBR® Green Master Mix (Invitrogen, Burlington, ON, Canada) containing AmpliTaq® Fast DNA Polymerase was used for this assay according to the manufacture’s recommendation. In addition, 5 nM of each of the gene-specific primers and 9 μL of a 1:10 dilution series of plasmid DNA, or 20 ng of cDNA extracted from each sample and RNAse-free water were used in the reaction. The optimum thermal cycling parameters for the IBV genome, TLRs, interferon, and pro-inflammatory cytokines included pre-incubation at 95 °C for 20 s; 40 cycles of amplification/extension at 95 °C for 3 s, and 60 °C for 30 s; melting curve analysis at 95 °C for 10 s (Segment 1), 65 °C for 5 s (Segment 2) and 9 °C for 5 s (Segment 3). Fluorescent acquisition was done at 60 °C for 30 s.
Lung mononuclear cell isolation and trachea total cell isolation
The lungs were rinsed multiple times in HBSS to ensure that they were free of blood. Using a sterile scalpel and forceps, the lungs were minced to approximately 5 mm fragments and soaked in 400 U/mL collagenase type I solution (Sigma-Aldrich, Oakville, ON, Canada) for 30 min at 37 °C. The trachea was cut into approximately 5 mm fragments with sterile scissors, suspended in 0.5 mM EDTA in a 50 mL conical tube, and shook for 20 min at 0.28 g (100 RPM) (41 °C). The dispersed cells and tissue fragments of both lung and trachea were separated from larger pieces using a 40 µm cell strainer. The trachea required a further processing of the tissue by grinding with the end of a 3 mL syringe plunger, followed by washing with 0.5 mM EDTA, for three repetitions in order to further separate the cells from cartilage and fatty tissue. The filtered lung cells were pelleted at 233 g for 10 min (4 °C), followed by re-suspension in HBSS and carefully layered onto 4 mL Histopaque 1077 (GE Health Care, Mississauga, ON, Canada) in a 15 mL conical tube at room temperature (with a 1:1 ratio of cell suspension). The layered cells were spun 40 min at 400 g at 20 °C. The cloudy layer, rich in mononuclear cells, was collected and pelleted, washed with HBSS. The filtered trachea cells were spun for 5 min at 500 g at 21 °C, and both the lung and trachea cells were suspended in complete medium (RPMI medium 1640 supplemented with 2 mM/l l-glutamine, 1% penicillin–streptomycin and 10% heat inactivated FBS) and the cells were counted.
Flow cytometry technique
Standard flow cytometry procedures were used in the experiments. The cells were stained with 0.5 ug/mL (100 uL) phycoerythrin (PE)-labeled mouse anti-chicken KUL01 (Mast et al., 1998), with an isotype control (SouthernBiotech, Birmingham, Alabama, USA) or kept as unstained control. The stained samples were analyzed with a BD LSR II (BD Biosciences, Mississauga, ON, Canada). Excitation was performed with a 488 nm argon-ion laser and the emission collected using a 585/42 nm BP filter for PE conjugates.
Data analyses
Quantification of IBV genome loads by real-time PCR was done by calculating the absolute number of IBV copies per 20 ng of trachea or lung cDNA, using the standard curve generated by a serial dilution of plasmids as described previously. The expression of mRNA of TLR3, TLR7, MyD88, TRIF, iNOS, IL-1β, and IFN-α, -β, and -γ were calculated using REST 384© (Relative Expression Software Tool) (Pfaffl, 2001, Pfaffl et al., 2009, Pfaffl et al., 2002, Pfaffl et al., 2004, Vandesompele et al., 2009). The calculation uses the geometrical mean of Efficiency(Eff.)(control)Crossing point (Cp) for all control genes (β-actin and ubiquitin). FlowJo version 7.6.4 (Ashland, OR, USA) was used to complete the flow cytometry data visualization and analysis. Except for the REST 384© results, all data was analyzed using analysis of variance (ANOVA) followed by Tukey's test to identify differences between observations and groups using the statistical package, MINITAB® release 15 (Minitab Inc. State College, Pennsylvania, USA). Comparisons were considered significant at P≤0.05.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada Discovery and the Research Tools and Instruments grant programs, and the University of Calgary. Funding agencies were not involved in the designing, conduction of the experiments, or writing of the manuscript. We would like to acknowledge the staff, especially Brenda Roszell and Dr. Greg Muench of the Veterinary Science Research Station at Spy Hill, University of Calgary for experimental animal management and support in performing animal procedures respectively.
References
- Abdul-Careem M.F., Haq K., Shanmuganathan S., Read L.R., Schat K.A., Heidari M., Sharif S. Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus. Virology. 2009;393:250–257. doi: 10.1016/j.virol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Ariaans M.P., Matthijs M.G., van Haarlem D., van de Haar P., van Eck J.H., Hensen E.J., Vervelde L. The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after Infectious Bronchitis Virus infection. Vet. Immunol. Immunopathol. 2008;123:240–250. doi: 10.1016/j.vetimm.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock A.A., Toft-Hansen H., Owens T. Signaling through MyD88 regulates leukocyte recruitment after brain injury. J. Immunol. 2008;181:6481–6490. doi: 10.4049/jimmunol.181.9.6481. [DOI] [PubMed] [Google Scholar]
- Booth, C., and O’Shea, J.A., 2002. Isolation and culture of intestinal epithelial cells. In: Ian Freshney, R., Freshney, M.G. (Eds.), Culture of Epithelial Cells. Wiley-Liss, Inc., Mississauga, Ontario, pp. 303–335.
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Elus M.M., Cook J.K. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- Clementz M.A., Chen Z., Banach B.S., Wang Y., Sun L., Ratia K., Baez-Santos Y.M., Wang J., Takayama J., Ghosh A.K., Li K., Mesecar A.D., Baker S.C. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J. Virol. 2010;84:4619–4629. doi: 10.1128/JVI.02406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Cornelissen J.B., Vervelde L., Post J., Rebel J.M. Differences in highly pathogenic avian influenza viral pathogenesis and associated early inflammatory response in chickens and ducks. Avian Pathol. 2013;42:347–364. doi: 10.1080/03079457.2013.807325. [DOI] [PubMed] [Google Scholar]
- De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Ficken M.D., Edwards J.F., Lay J.C. Effects of Newcastle disease virus infection on the binding, phagocytic, and bactericidal activities of respiratory macrophages of the turkey. Avian Dis. 1987;31:888–894. [PubMed] [Google Scholar]
- Frucht D.M., Fukao T., Bogdan C., Schindler H., O'Shea J.J., Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- Fulton R.M., Reed W.M., DeNicola D.B. Light microscopic and ultrastructural characterization of cells recovered by respiratory-tract lavage of 2- and 6-week-old chickens. Avian Dis. 1990;34:87–98. [PubMed] [Google Scholar]
- Fulton R.M., Reed W.M., Thacker H.L. Cellular response of the respiratory tract of chickens to infection with Massachusetts 41 and Australian T infectious bronchitis viruses. Avian Dis. 1993;37:951–960. [PubMed] [Google Scholar]
- Gessani S., Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Golab J., Zagozdzon, Stoklosal T., Kaminski R., Kozar K., Jakobisiak M. Direct stimulation of macrophages by IL-12 and IL-18--a bridge too far? Immunol. Lett. 2000;72:153–157. doi: 10.1016/s0165-2478(00)00178-4. [DOI] [PubMed] [Google Scholar]
- Grgic H., Hunter D.B., Hunton P., Nagy E. Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Can. J. Vet. Res. 2008;72:403–410. [PMC free article] [PubMed] [Google Scholar]
- Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M., Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Guo X., Rosa A.J., Chen D.G., Wang X. Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet. Immunol. Immunopathol. 2008;121:332–343. doi: 10.1016/j.vetimm.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs R., Cormican P., Cahalane S., Allan B., Lloyd A.T., Meade K., James T., Lynn D.J., Babiuk L.A., O'Farrelly C. Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006;74:1692–1698. doi: 10.1128/IAI.74.3.1692-1698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M., Philbin V.J., Withanage G.S., Wigley P., Beal R.K., Goodchild M.J., Barrow P., McConnell I., Maskell D.J., Young J., Bumstead N., Boyd Y., Smith A.L. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect. Immun. 2005;73:2344–2350. doi: 10.1128/IAI.73.4.2344-2350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannaki T.R., Reddy M.R., Shanmugam M., Verma P.C., Sharma R.P. Chicken toll-like receptors and their role in immunity. World's Poult. Sci. J. 2010;66:727–738. [Google Scholar]
- Karpala A.J., Lowenthal J.W., Bean A.G. Activation of the TLR3 pathway regulates IFNbeta production in chickens. Dev. Comp. Immunol. 2008;32:435–444. doi: 10.1016/j.dci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Lawrence T.M., Hudacek A.W., de Zoete M.R., Flavell R.A., Schnell M.J. Rabies virus is recognized by the NLRP3 inflammasome and activates interleukin-1beta release in murine dendritic cells. J. Virol. 2013;87:5848–5857. doi: 10.1128/JVI.00203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina J.N. Some recent advances on the study and understanding of the functional design of the avian lung: morphological and morphometric perspectives. Biol. Rev. Camb. Philos. Soc. 2002;77:97–152. doi: 10.1017/s1464793101005838. [DOI] [PubMed] [Google Scholar]
- Mast J., Goddeeris B.M., Peeters K., Vandesande F., Berghman L.R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 1998;61:343–357. doi: 10.1016/s0165-2427(97)00152-9. [DOI] [PubMed] [Google Scholar]
- Naqi S., Thompson G., Bauman B., Mohammed H. The exacerbating effect of infectious bronchitis virus infection on the infectious bursal disease virus-induced suppression of opsonization by Escherichia coil antibody in chickens. Avian Dis. 2001;45:52–60. [PubMed] [Google Scholar]
- Otsuki K., Maeda J., Yamamoto H., Tsubokura M. Studies on avian infectious bronchitis virus (IBV). III. Interferon induction by and sensitivity to interferon of IBV. Arch. Virol. 1979;60:249–255. doi: 10.1007/BF01317496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi P., Mallick A.I., Haq K., Schlegel B., Sharif S. A Toll-like receptor 3 agonist (polyI:C) elicits innate host responses in the spleen and lungs of chickens. Can. J. Vet. Res. 2012;76:230–234. [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Pfaffl, M.W., Vandesompele, J., Kubista, M., 2009. Data Analysis Software. In: Logan, J., Edwards, K., Saunders, N. (Ed.), Real-Time PCR: Current Technology and Applications. Caister Academic Press, Applied and Functional Genomics, Health Protection Agency, London.
- Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschlager N., Schlee M., Rothenfusser S., Barchet W., Kato H., Akira S., Inoue S., Endres S., Peschel C., Hartmann G., Hornung V., Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Heggen C.L., Hussain I. Avian macrophage: effector functions in health and disease. Dev. Comp. Immunol. 2000;24:103–119. doi: 10.1016/s0145-305x(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Read R.C. Evidence-based medicine: empiric antibiotic therapy in community-acquired pneumonia. J. Infect. 1999;39:171–178. doi: 10.1016/s0163-4453(99)90043-9. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y., Watanabe Y., Harada M., Seki Y., Kuroda Y., Fukuda M., Honda E., Suzuki S., Nakamura S. Genetic analysis of the S1 gene of 4/91 type infectious bronchitis virus isolated in Japan. J. Vet. Med. Sci. 2009;71:583–588. doi: 10.1292/jvms.71.583. [DOI] [PubMed] [Google Scholar]
- St Paul M., Paolucci S., Barjesteh N., Wood R.D., Sharif S. Chicken erythrocytes respond to Toll-like receptor ligands by up-regulating cytokine transcripts. Res. Vet. Sci. 2013;95:87–91. doi: 10.1016/j.rvsc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Tate M.D., Pickett D.L., van Rooijen N., Brooks A.G., Reading P.C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J. Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenaka T., Kishimoto I., Uemura T., Ito T., Umemura T., Otsuki K. Cloacal inoculation with the Connecticut strain of avian infectious bronchitis virus: an attempt to produce nephropathogenic virus by in vivo passage using cloacal inoculation. J. Vet. Med. Sci. 1998;60:495–502. doi: 10.1292/jvms.60.495. [DOI] [PubMed] [Google Scholar]
- Vandesompele, J., Kubista, M., Pfaffl, M.W., 2009. Reference Gene Validation Software for Improved Normalization. In: Logan, J., Edwards, K., Saunders, N. (Ed.), Real-Time PCR: Current Technology and Applications. Caister Academic Press, Applied and Functional Genomics, Health Protection Agency, London.
- Villanueva A,I., Kulkarni R.R., Sharif S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev. Comp. Immunol. 2011;35:28–34. doi: 10.1016/j.dci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Rosa A.J., Oliverira H.N., Rosa G.J., Guo X., Travnicek M., Girshick T. Transcriptome of local innate and adaptive immunity during early phase of infectious bronchitis viral infection. Viral Immunol. 2006;19:768–774. doi: 10.1089/vim.2006.19.768. [DOI] [PubMed] [Google Scholar]
- Watters T.M., Kenny E.F., O'Neill L.A. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol. Cell Biol. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- Wei L., Jiao P., Yuan R., Song Y., Cui P., Guo X., Zheng B., Jia W., Qi W., Ren T., Liao M. Goose Toll-like receptor 7 (TLR7), myeloid differentiation factor 88 (MyD88) and antiviral molecules involved in anti-H5N1 highly pathogenic avian influenza virus response. Vet. Immunol. Immunopathol. 2013;153:99–106. doi: 10.1016/j.vetimm.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Xiao H., Xu L.H., Yamada Y., Liu D.X. Coronavirus spike protein inhibits host cell translation by interaction with eIF3f. PLoS One. 2008;3:e1494. doi: 10.1371/journal.pone.0001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhao J., Hu X., Zhang G. Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene. Vet. Microbiol. 2007;122:61–71. doi: 10.1016/j.vetmic.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ye Y., Hauns K., Langland J.O., Jacobs B.L., Hogue B.G. Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist. J. Virol. 2007;81:2554–2563. doi: 10.1128/JVI.01634-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Tan Y.W., Liu D.X. Recent progress in studies of arterivirus- and coronavirus-host interactions. Viruses. 2012;4:980–1010. doi: 10.3390/v4060980. [DOI] [PMC free article] [PubMed] [Google Scholar]