Abstract
The porcine reproductive and respiratory syndrome virus nsp1 is predicted to be auto-cleaved from the replicase polyprotein into nsp1α and nsp1β subunits. In infected cells, we detected the actual existence of nsp1α and nsp1β. Cleavage sites between nsp1α/nsp1β and nsp1β/nsp2 were identified by protein microsequencing analysis. Time course study showed that nsp1α and nsp1β mainly localize into the cell nucleus after 10 h post infection. Further analysis revealed that both proteins dramatically inhibited IFN-β expression. The nsp1β was observed to significantly inhibit expression from an interferon-stimulated response element promoter after Sendai virus infection or interferon treatment. It was further determined to inhibit nuclear translocation of STAT1 in the JAK–STAT signaling pathway. These results demonstrated that nsp1β has ability to inhibit both interferon synthesis and signaling, while nsp1α alone strongly inhibits interferon synthesis. These findings provide important insights into mechanisms of nsp1 in PRRSV pathogenesis and its impact in vaccine development.
Keywords: PRRSV, nsp1, Proteolytic cleavage, Interferon antagonist
Introduction
Porcine reproductive and respiratory syndrome (PRRS), a disease initially described in the United States in 1987 (Keffaber, 1989) and in Europe in 1990 (Wensvoort et al., 1991), has caused tremendous economic losses to the swine industry worldwide, with recent costs in the United States of at least $600 million annually (Neumann, 2005). Hallmark symptoms of PRRS are mild to severe respiratory disease in infected newborn and growing swine, and reproductive failure in pregnant sows. The etiologic agent, PRRSV, was first discovered in the Netherlands in 1991 (Wensvoort et al., 1991). In the United States, PRRSV was first isolated in 1992 (Benfield et al., 1992, Collins et al., 1992). Nucleotide sequence comparisons have shown that PRRSV can be divided into distinct European (Type I) and North American (Type II) genotypes (Allende et al., 1999, Nelsen et al., 1999).
The host innate immune response is the first defense line to prevent viral infection. A key aspect of the antiviral innate immune response is the synthesis and secretion of type I interferons (IFN) such as IFN-α and IFN-β, which exhibit antiviral, anti-proliferative and immunomodulatory functions (reviewed by Samuel, 2001, Haller and Weber, 2007, Randall and Goodbourn, 2008). Two events required to trigger an effective antiviral innate immune response are: (a) detection of the invading virus by immune system receptors; and (b) initiation of protein signaling cascades that regulate the synthesis of IFNs. Initially, the pathogen-associated molecular pattern in dsRNA is recognized by host cell receptors, including Toll-like receptor 3 (TLR3), retinoic acid-inducible protein I (RIG-I) or melanoma differentiation-associated gene 5 (MDA5). In one dsRNA-signaling pathway, RIG-1 caspase recruitment domains associate with IFN-β promoter stimulator 1 (IPS-1) to activate the downstream kinases, such as TBK1 and IKKɛ, resulting in the phosphorylation and activation of transcription factors, including IRF3 and NF-kB. The coordinate activation of these transcription factors results in the formation of a transcriptionally competent enhanceosome that induces type I IFN production (Thanos and Maniatis, 1995). After being secreted, type I IFN binds to their receptors on adjacent cell surfaces to activate the so-called JAK–STAT signaling pathway. The coupling of receptor–ligand activates JAKs (Janus activated kinases), leading to phosphorylation of STATs (signal transducers and activators of transcription). The phosphorylated STAT1 and STAT2, in association with IRF9, form the heterotrimeric complex ISGF3. ISGF3 translocates to the nucleus where it binds to IFN-stimulated response elements (ISRE) in the promoter and induces the transcription of IFN-stimulated genes (ISGs). Activation of these genes enables the cell to fight the infection and inhibit virus replication (Weber et al., 2004).
Previous studies have demonstrated the important role of interferon-mediated innate immune responses against PRRSV infection. In an earlier study, inoculation of pigs with porcine respiratory coronavirus, a potent inducer of type I interferon, provided protection from a subsequent PRRSV infection (Buddaert et al., 1998). Overend et al. (2007) showed that recombinant swine IFN-β protects swine alveolar macrophages and MARC-145 cells (a PRRSV permissive cell line) from infection with PRRSV. Royaee et al. (2004) showed that utilizing an expression plasmid encoding porcine IFN-α as an adjuvant resulted in a temporary increase in the frequency of PRRSV-specific IFN-γ secreting cells in vaccinated animals, which demonstrated an important role of type I interferon as a link between the innate and adaptive immune responses. However, PRRSV infection appears to elicit a poor innate antiviral type I interferon response, which is postulated to result in a weak adaptive immune response as demonstrated by cell-mediated immune responses of short duration, and slow development of virus-specific IFN-γ secreting cells leading to a prolonged viremia (Meier et al., 2003, Royaee et al., 2004). Miller et al. (2004) showed that stimulation of MARC-145 cells by exogenous double-stranded RNA resulted in significant increases in type I IFN mRNA expression, but the double-stranded RNA induction of type I IFN activation was significantly inhibited by dual-exposure with PRRSV. Results from Luo et al (2008) showed that PRRSV infection significantly blocked dsRNA-induced IFN-β production. However, little is known about the molecular mechanism of PRRSV proteins in the regulation of interferon activity.
PRRSV contains a single positive-stranded RNA genome, encoding nine open reading frames. The replicase-associated genes, ORF1a and ORF1b, are situated at the 5′ end of the genome and represent nearly 75% of the viral genome. According to the studies of the closely related equine arteritis virus (EAV), the ORF1a-encoded replicase polyprotein pp1a is predicted to be proteolytically cleaved into eight nonstructural products, nsp1 to nsp8 (Snijder and Meulenberg, 1998, Allende et al., 1999, Nelsen et al., 1999). The nsp1 contains two putative cysteine protease domains, PCP1α and PCP1β. PCP1α was predicted to auto-cleave between nsp1α/nsp1β, and release nsp1α from pp1a, while PCP1β was predicted to auto-cleave between nsp1β/nsp2, and release nsp1β from the pp1a (den Boon et al., 1995, Snijder and Meulenberg, 1998). In a previous study, den Boon et al. (1995) identified nsp1 auto-cleaved into nsp1α and nsp1β using an in vitro translation system. Johnson et al. (2007) showed that recombinant nsp1 protein could be auto-catalytically processed into nsp1α and nsp1β. However, these two cleavage products have not been identified in virus-infected cells. More importantly, our recent study suggested that nsp1 is the main protein antagonizing cellular production of type I interferon. However, the detailed mechanism of how nsp1 is involved in the inhibition of interferon production is unknown. In the present study, we identified the nsp1α and nsp1β in virus-infected cells and determined the role of nsp1α and nsp1β in the inhibition of type I interferon synthesis and signaling. The capability of PRRSV nsp1α and nsp1β to interfere with the establishment of the innate antiviral state suggests that these two proteins are critical virulence determinants of PRRSV, which provides important insight into the mechanism of PRRSV pathogenesis and future PRRSV vaccine development.
Results
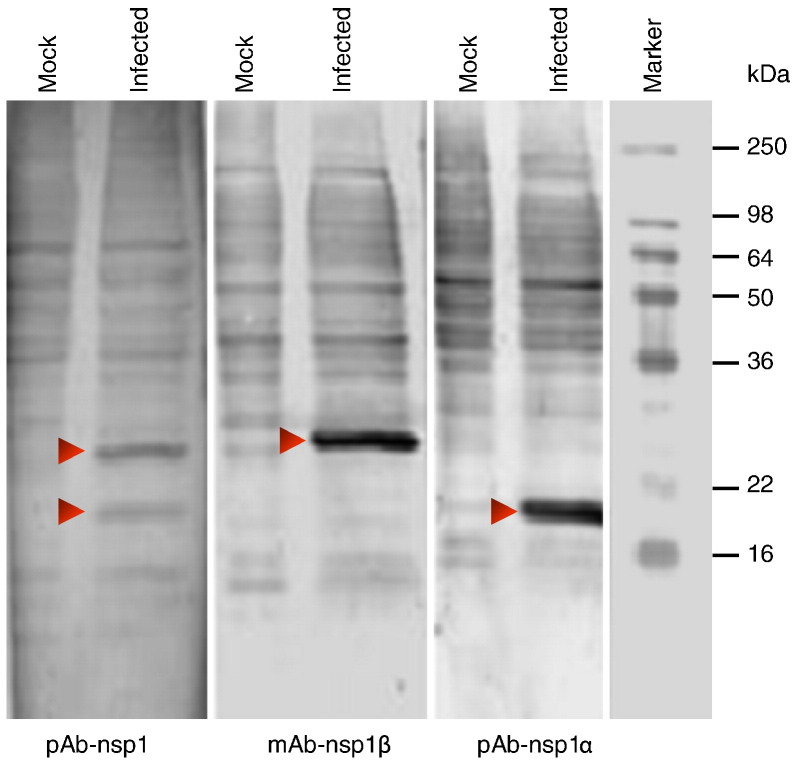
Identification of nsp1α and nsp1β in PRRSV-infected cells
Based on the study of EAV, the PRRSV nsp1 is predicted to be cleaved into nsp1α and nsp1β subunits. Using an in vitro translation system, den Boon et al. (1995) showed that PRRSV nsp1 was auto-cleaved into α and β subunits of approximately 20 and 27 kDa, respectively. These two auto-cleavage products were also obtained when the nsp1 recombinant protein was purified from expression in E. coli (Johnson et al., 2007). To determine if these two putative subunits actually exist in virus-infected cells, we generated rabbit polyclonal antiserum (pAb-nsp1, pAb-nsp1α) and a monoclonal antibody (mAb-nsp1β) directed against the nsp1, nsp1α and nsp1β using purified recombinant nsp1α and nsp1β proteins that were self-cleaved products from nsp1 expression in E. coli (Johnson et al., 2007). These antibodies were used to probe mock-infected and PRRSV SD23983 infected MARC-145 cells by Western blot. As shown in Fig. 1 , from PRRSV infected cell lysate, pAb-nsp1α recognized a protein band slightly larger than the molecular weight marker 16 kDa, which corresponds to the predicted size of nsp1α at 20 kDa. The mAb-nsp1β detected a sharp protein band above 22 kDa molecular weight marker, which corresponds to the predicted size of nsp1β at 27 kDa. Both protein bands were detected using pAb-nsp1. These specific protein bands were not detected in cell lysates from mock-infected cells or in cell lysates from infected cells with pre-immune serum or negative control mAb. This result demonstrated that the two auto-cleavage products, nsp1α and nsp1β, actually exist in PRRSV-infected cells.
Fig. 1.
Identification of PRRSV nsp1 auto-cleavage products nsp1α and nsp1β in infected cells by Western blot. MARC-145 cells were infected with SD23983 virus or mock-infected. Viral proteins from cell lysate were separated by 15% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was probed with rabbit polyclonal anti-sera against nsp1 (pAb-nsp1) or nsp1α (pAb-nsp1α), or monoclonal antibody against nsp1β (mAb-nsp1β). Arrows point to specific PRRSV nsp1 proteins.
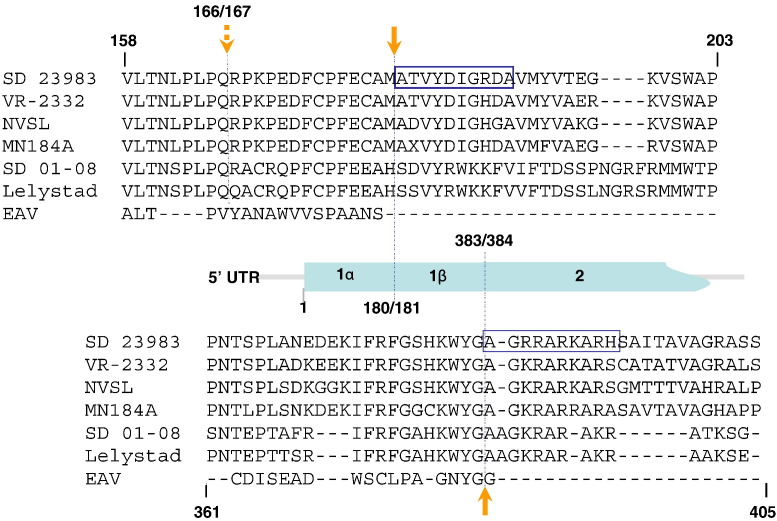
The nsp1 is located at the N-terminal of the pp1a polyprotein that is translated with “ATG” start codon. Based on the homologous sequence comparison between PRRSV and EAV, the Type II PRRSV nsp1α/1β was predicted to be cleaved between 166Q↓R167, while nsp1β/nsp2 was predicted to be cleaved between 383G↓A384 (den Boon et al., 1995, Allende et al., 1999, Nelsen et al., 1999, Wootton et al., 2000, Ziebuhr et al., 2000). For subsequent functional study of each individual nsp1 protein, it is critical to elucidate the actual cleavage sites. Therefore, we performed protein N-terminal sequencing analysis on these cleavage sites. Each individual nsp1β and nsp2 protein was immunoprecipitated from SD23983 infected cells using specific anti-nsp1β and anti-nsp2 monoclonal antibodies. These precipitated proteins were separated by SDS-PAGE and transferred to a PVDF membrane. Proteins corresponding to the predicted size of nsp1β and nsp2 were excised from PVDF membrane and subjected to protein identification by N-terminal sequential Edman degradation. For nsp2 N-terminal sequence, the primary (10) major signals of the sequence were AGRRARKARH, which is consistent with the predicted N-terminal sequence of nsp2 at the cleavage site of 383G↓A384 (Ziebuhr et al., 2000). This cleavage site is well conserved between different PRRSV isolates (Fig. 2 ). Interestingly, the Edman degradation of nsp1β generated a major signal of N-terminal sequence as ATVYDIGRDA, which indicated the cleavage site between nsp1α/nsp1β is at 180M↓A181 that is 14 amino acids downstream of the predicted cleavage site at 166Q↓R167. Sequence analysis showed that this cleavage site is conserved among different PRRSV Type II strains (Fig. 2). While this manuscript was in preparation, a recent report from Sun et al. (2009) identified crystal structure of nsp1α from another Type II PRRSV strain, XH-GD, and the same nsp1α/nsp1β cleavage site was determined in their study, which further confirmed that the actual cleavage site between nsp1α/nsp1β of Type II PRRSV is at 180M↓A181. The corresponding cleavage site on Type I PRRSV is at 180H↓S181 based on our sequence analysis (Fig. 2). Whether this is the actual cleavage site needs to be further identified in Type I PRRSV.
Fig. 2.
Comparative sequence analysis of the nsp1α/nsp1β and nsp1β/nsp2 cleavage sites. Partial ORF1a amino acid sequence of six PRRSV strains corresponding to SD23983 ORF1a amino acid position 160–204 and 361–409 was aligned with EAV nsp1. The alignment was generated by ALIGNX program of Vector NTI Suite software (InforMax, Inc.). Two boxes depicted in the map of amino acid sequence comparison represent the result of nsp1β and nsp2 protein N-terminal sequencing, which was determined by sequential Edman degradation of 10 cycles for each viral protein. The upward and downward solid arrows point to the identified cleavage site of nsp1β/nsp2 and nsp1α/nsp1β, respectively. The downward dashed arrow points to the predicted nsp1α/nsp1β cleavage site.
Subcellular localization of nsp1α and nsp1β
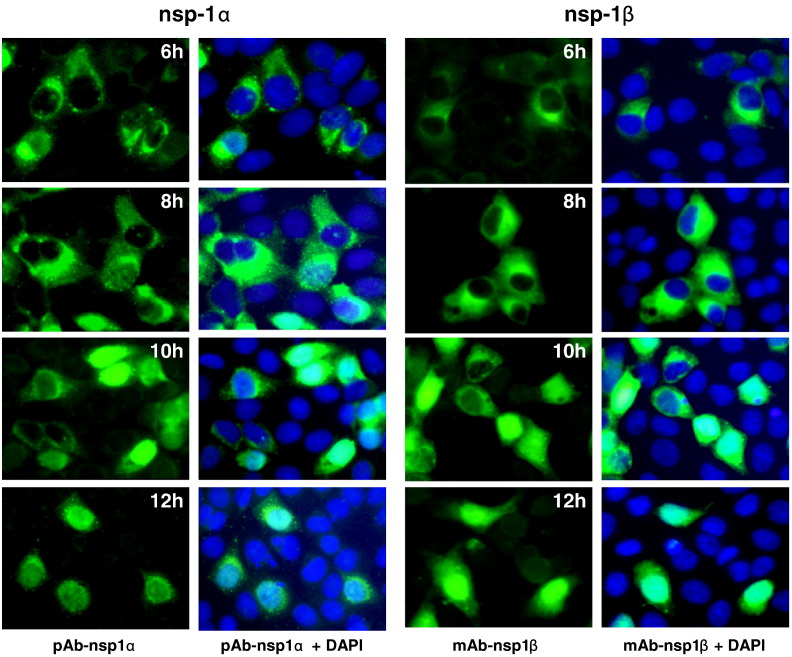
In EAV, nsp1 is expressed as a single protein that is actively imported into the nucleus during the early stage of infection (Tijms et al., 2002). To determine whether PRRSV nsp1 follows the same cellular distribution pattern as that of EAV, we detected the subcellular localization of nsp1α and nsp1β following the time course of viral infection in MARC-145 cells. PRRSV SD23983 infected MARC-145 cells were fixed at 4, 6, 8, 10 and 12 hours post infection (hpi), and stained with pAb-nsp1α or mAb-nsp1β antibodies (Fig. 3 ). Viral protein specific fluorescence can be detected as early as 6 hpi. All PRRSV infected MARC-145 cells stained with anti-nsp1α or anti-nsp1β antibody showed small dot-like bright punctate fluorescent foci, mostly concentrated on one side of the perinuclear region in the cell cytoplasm. At 8 hpi, more intense and widespread fluorescence was observed for both proteins. Small punctate dot-like fluorescent staining pattern was observed for nsp1α, while the nsp1β was stained as bright fluorescence mostly around the perinuclear region in cytoplasm. At 10 hpi, we found that in some infected cells, nsp1α or nsp1β localized into cell nucleus. To determine the percentage of cells showing nuclear localization for nsp1α or nsp1β, we counted infected cells in five fields of view under fluorescent microscopy. For nsp1α, 49% of infected cells showed nuclear localization, while more infected cells (82%) showed nsp1β nuclear localization. At 12 hpi, almost all of the infected cells (98%) showed nuclear localization for nsp1β, and about 83% of infected cells showed nuclear localization for nsp1α. Although both proteins were localized into the nucleus, the localization pattern was quite different. For the nsp1α proteins that retained in the cytoplasm, a scattered punctate dot-like fluorescent staining pattern was consistently observed. In contrast, nsp1β expression showed a predominant nuclear and cytoplasmic staining pattern, and the fluorescent staining in the cytoplasm was diffused through the cytoplasm.
Fig. 3.
Detection of nsp1α and nsp1β expression in virus-infected cells by indirect immunofluorescence assay. MARC-145 cells were infected with PRRSV SD23983 and fixed at 6, 8, 10 and 12 hpi. Cells were stained with a PRRSV protein specific primary antibody, pAb-nsp1α or mAb-nsp1β. FITC-conjugated goat anti-rabbit or anti-mouse antibody was used as secondary antibody. Cell nucleus was stained with DAPI. Images were obtained by fluorescence and phase-contrast microscopy using a 40× objective.
The PRRSV nsp1α and nsp1β inhibit IFN-β activation
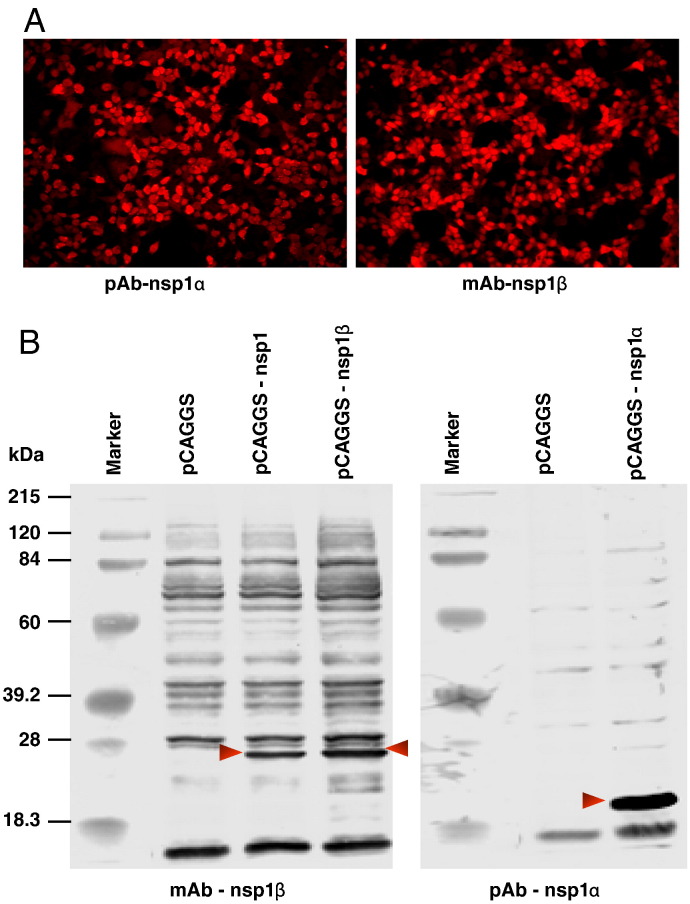
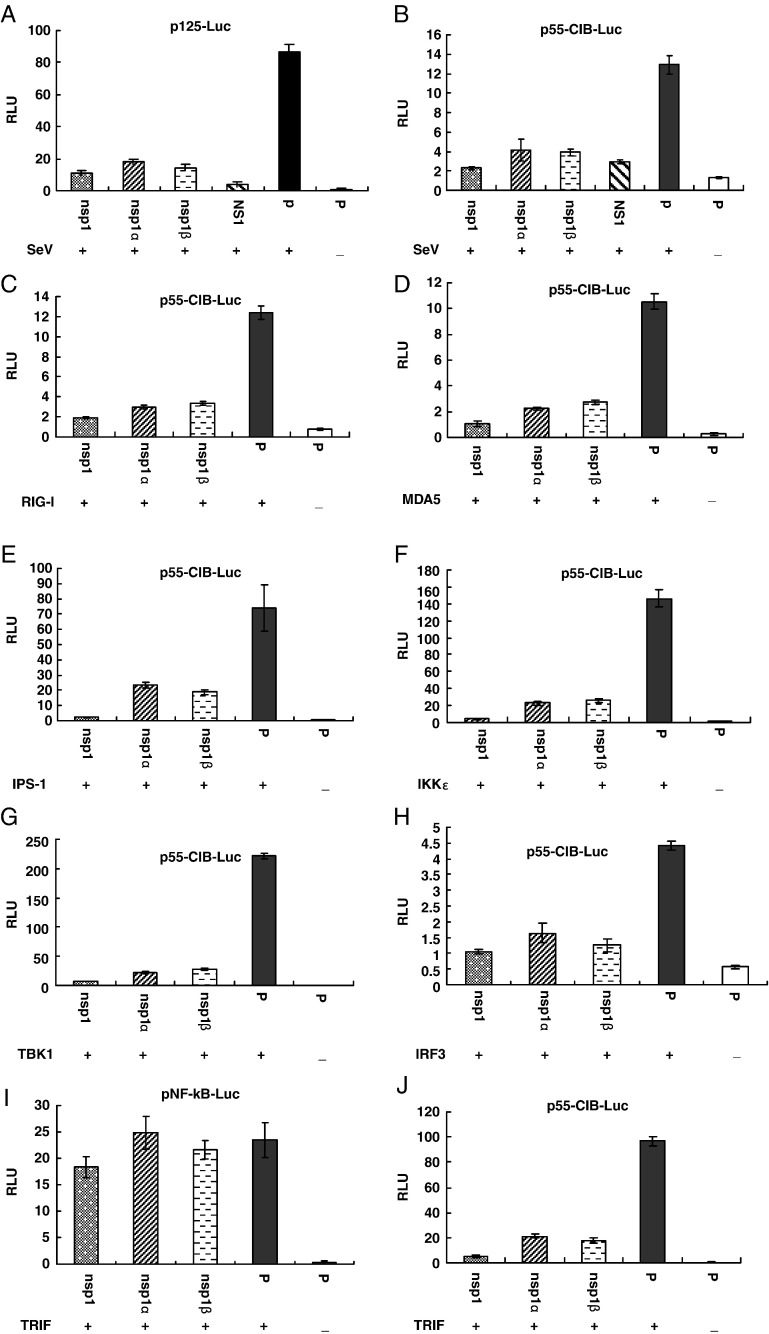
Previous studies showed that stimulation of MARC-145 cells by exogenous double-stranded RNA resulted in significant increases in type I IFN mRNA expression measured by real-time PCR. However, the double-stranded RNA induction of type I IFN activation was significantly inhibited by exposure with PRRSV (Miller et al., 2004, Luo et al., 2008). Our preliminary result showed that PRRSV nsp1 is the main protein responsible for the inhibition of type 1 IFN activation. Since the two auto-cleaved products of nsp1, nsp1α and nsp1β, showed different subcellular localization patterns, we speculated that these two proteins may inhibit the interferon response by different mechanisms. In this study, we used an IFN-β promoter-luciferase reporter system to determine which of the nsp1 auto-cleaved products had an effect on IFN-β activation. Based on our N-terminal protein sequencing analysis, the SD23983 virus nsp1 contains amino acid (AA) 1–383 of pp1a, nsp1α contains AA 1–180, and nsp1β contains AA 181–383 of pp1a. Each of these protein-encoding regions was cloned into the pCAGGS plasmid to generate plasmids pCAGGS-nsp1, pCAGGS-nsp1α or pCAGGS-nsp1β. Protein expression from these plasmids was confirmed by IFA and Western blot using pAb-nsp1α or mAb-nsp1β antibodies (Fig. 4 ). HEK293T cells were co-transfected with each of these plasmids, a reporter plasmid, p125-Luc that contains IFN-β promoter driving the expression of the luciferase reporter gene, and a Renilla luciferase expression plasmid (pRL-SV40) to normalize expression levels of samples. As a positive control, influenza nsp1 (Solórzano et al., 2005) was used to co-transfect the cells with the reporter plasmid. Twenty-four hours after transfection, cells were infected with SeV to induce luciferase production. As shown in Fig. 5A, expression of either nsp1, nsp1α or nsp1β strongly suppressed the expression of the IFN-β promoter-driven luciferase. As we expected, the expression of influenza nsp1 also significantly inhibited the luciferase synthesis. In contrast, a strong reporter signal was observed in cells transfected with empty plasmid, pCAGGS after infection by SeV.
Fig. 4.
Identification of nsp1α and nsp1β expression in transfected cells. HEK293T cells were transfected with either control plasmid, pCAGGS or plasmids expressing PRRSV nsp1 proteins, including pCAGGS, pCAGGS-nsp1α or pCAGGS-nsp1β. At 24 h post-transfection, cells were fixed for immunofluorescence assay or harvested for Western blot analysis. (A) Immunofluorescent detection of nsp1α and nsp1β expression in HEK293T cells. Cells were stained with a specific antibody as indicated at the bottom of the picture, and DyLight 549-conjugated goat anti-rabbit or anti-mouse antibody was used as the secondary antibody. Images were taken by fluorescence and phase-contrast microscopy using a 20× objective. (B) Western blotting analysis nsp1, nsp1α and nsp1β expression in 293T cells. Membranes were probed with an nsp1 protein specific antibody as indicated at the bottom of each membrane. Arrows point to the specific PRRSV proteins.
Fig. 5.
PRRSV nsp1 proteins inhibit IFN-β production. (A, B) HEK293T cells cultured in 24-well plates were cotransfected with a plasmid expressing nsp1 proteins, a plasmid expressing influenza virus NS1, or pCAGGS empty vector (P), pRL-SV40, and a luciferase reporter plasmid p125-Luc (A) or pCIB-55-Luc (B). At 20 h post transfection, cells were infected with Sendai virus (SeV) for 16 h to stimulate the production of interferon. (C–H, J) HEK293T cells in 24-well plates were cotransfected with the plasmid pEFneo-RIG-I (C), pEFneo-MDA5 (D), pEGFP-IPS-1 (E), pEFneo-TBK1 (F), pEFneo-IKKɛ (G), or pCAGGS-IRF3 (H), or pcDNA3-TRIF (J), along with pRL-SV40, pCAGGS expressing nsp1 proteins, and pCIB-55 plasmid for 20–24 h. (I) HEK293T cells were cotransfected with pNF-kB-luc, pcDNA3-TRIF, pRL-SV40 and pCAGGS expressing nsp1 proteins or pCAGGS empty vector (P) for 20 h. Cells were harvested and measured for firefly and Renilla luciferase activities. Relative luciferase activity is defined as a ratio of firefly luciferase reporter activity to Renilla luciferase activity. Each data point shown represents a mean value from three experiments. Error bars show standard deviations of the normalized data.
The IFN-β promoter contains four positive regulatory domains (PRDs), including the binding site for three different transcription factors, interferon regulatory factor 3 (IRF3) (PRDs I and III), nuclear factor-kB (NF-kB) (PRDII) and activating protein 1 (AP1) (PRD IV). Maximal activation of the IFN-β promoter requires the binding of transcription factors to the promoter and forming a so-called enhanceosome on the PRDs (reviewed in Randall and Goodbourn, 2008). Previous study from Luo et al. (2008) demonstrated that PRRSV suppresses IFN-β transcription by interfering with IRF3 activity but not NF-kB and AP-1 activities, and its effect is through interrupting the IPS-1 activity in the upstream of the IRF3 signaling pathway. We investigated whether the PRRSV nsp1 associated proteins were the proteins to block the IRF3 signaling pathway. Cells were cotransfected with control plasmids or with plasmids expressing the PRRSV nsp1 proteins, the plasmid pRL-SV40, and a plasmid containing a firefly luciferase gene under the control of a promoter with three IRF3 binding site (p55-CIB-Luc). As shown in Fig. 5B, after infection with SeV, expression of either nsp1, nsp1α or nsp1β effectively blocked the IRF3 dependent reporter gene expression.
We further determined whether the nsp1α or nsp1β was the protein interfering with the IPS-1 activity. Since MDA5 or RIG-1 is associated with IPS-1 caspase, we cotransfected cells with a plasmid expressing MDA5, RIG-1 or IPS-1 protein, a plasmid expressing individual PRRSV proteins, the plasmid pRL-SV40, and the p55-CIB-Luc reporter plasmid. Previous study showed that overexpression of RIG-1 or IPS-1 in cells led to activation of transcription from the reporter plasmid (Childs et al., 2007). As shown in Figs. 5C–E, this activity was suppressed by co-expression of the nsp1α or nsp1β. This result suggests that PRRSV nsp1α and nsp1β may block the IPS-1 mediated IFN-β induction. Another possibility is that the downstream portion of the signaling pathway was being blocked by these proteins. TBK1 and IKKɛ are essential components downstream of RIG-1/IPS-1 caspase. We further tested the effect of PRRSV nsp1α and nsp1β on the TBK1 and IKKɛ mediated IFN-β induction. Interestingly, either nsp1α or nsp1β had the ability to suppress TBK1 and IKKɛ mediated reporter gene expression (Figs. 5F–G). Both TBK1 and IKKɛ expression should induce the activation of downstream transcription factor IRF3, and overexpression of IRF3 itself should lead to activation of transcription from the reporter plasmid. Again, nsp1α and nsp1β blocked this effect (Fig. 5H). To confirm that the PRRSV nsp1 proteins were specifically affect on the IRF3 signaling pathway, activation of NF-kB was examined using a reporter plasmid containing a firefly luciferase gene under the control of an NF-kB responsive promoter with two NF-kB binding sites (pNF-kB-Luc). HEK293T cells were co-transfected with PRRSV nsp1 proteins expression plasmids, pNF-kB-Luc (or p55-CIB-Luc), pRL-SV40, and a plasmid expressing TRIF, a key component upstream of NF-kB and IRF3 signaling pathways. As we expected, PRRSV nsp1 proteins did not affect the NF-kB dependent reporter gene expression (Fig. 5I). In contrast, PRRSV nsp1 blocked the IRF3 dependent reporter gene expression (Fig. 5J). Taken together with the observations in Fig. 5, the data suggest that the PRRSV nsp1 proteins (α and β) might act to block processes downstream of the activation of IRF-3, possibly somewhere in the nucleus.
We further studied the activation and nuclear translocation of IRF3 in transfected cells. In response to cellular stimulation, the IRF3 is activated by forming a phosphorylated dimer, which subsequently translocates into the cell nucleus. Interestingly, when cells were transfected with plasmid expressing PRRSV nsp1, nsp1α or nsp1β, and infected with SeV as an activator of IRF3, we did not observe any effect of PRRSV proteins on the phosphorylation and translocation of IRF3 in transfected cells. These data suggest that the PRRSV nsp1 proteins have ability to block induction of IFN-β at a point downstream of activation of IRF-3, since nsp1α and nsp1β expression inhibit the activation of the IFN-β promoter but do not block the activation of IRF-3. The observation that both nsp1α and nsp1β are largely nuclear-located is in agreement with the hypothesis that they may have a direct effect on the formation of the transcription enhanceosome on the IFN-β promoter inside the nucleus.
The PRRSV nsp1β strongly inhibits gene expression from an ISRE promoter
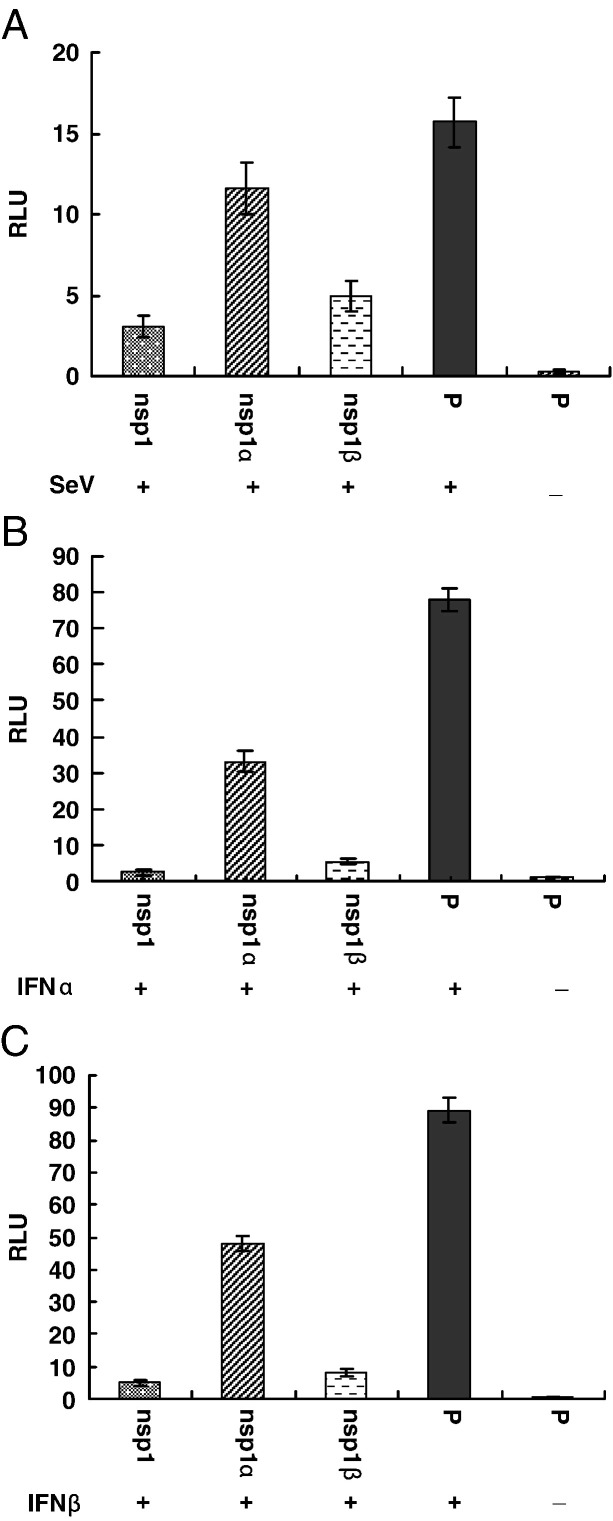
While our results showed that PRRSV nsp1α and nsp1β strongly inhibit IFN-β synthesis, we further tested whether PRRSV nsp1α or nsp1β could inhibit the cellular response to interferon (interferon signaling). When interferon binds to a receptor, the signaling process through the JAK-STAT pathway results the activation of genes with an ISRE promoter. To determine whether PRRSV nsp1α or nsp1β has ability to inhibit the activation of genes with an ISRE promoter, cells were co-transfected with a plasmid expressing individual PRRSV proteins (nsp1, nsp1α or nsp1β), and a reporter plasmid expressing luciferase under the control of the ISRE promoter. As shown in Fig. 6 , after stimulating with SeV, IFN-α or IFN-β, the nsp1β and nsp1 significantly inhibit the expression of luciferase from the ISRE promoter, which indicates that nsp1β not only inhibits interferon synthesis, but also inhibits interferon signaling. Some level of inhibition on luciferase expression was observed in cells transfected with nsp1α, but the luciferase expression level is consistently higher in nsp1α transfected cells than those cells transfected with nsp1β.
Fig. 6.
PRRSV nsp1 proteins inhibit expression from an ISRE promoter. HEK293T cells were cotransfected with pISRE-luc, pRL-SV40 and pCAGGS expressing nsp1 proteins or pCAGGS empty vector (P) for 20 h. Cells were then infected with Sendai virus (A) or treated with IFN-α (B) and IFN-β (C) for 20 h. The cells were harvested and measured for firefly and Renilla luciferase activities. Relative luciferase activity is defined as a ratio of firefly luciferase reporter activity to Renilla luciferase activity. Each data point shown represents a mean value from three experiments. Error bars show standard deviations of the normalized data.
The PRRSV nsp1β but not nsp1α inhibit the translocation of STAT1
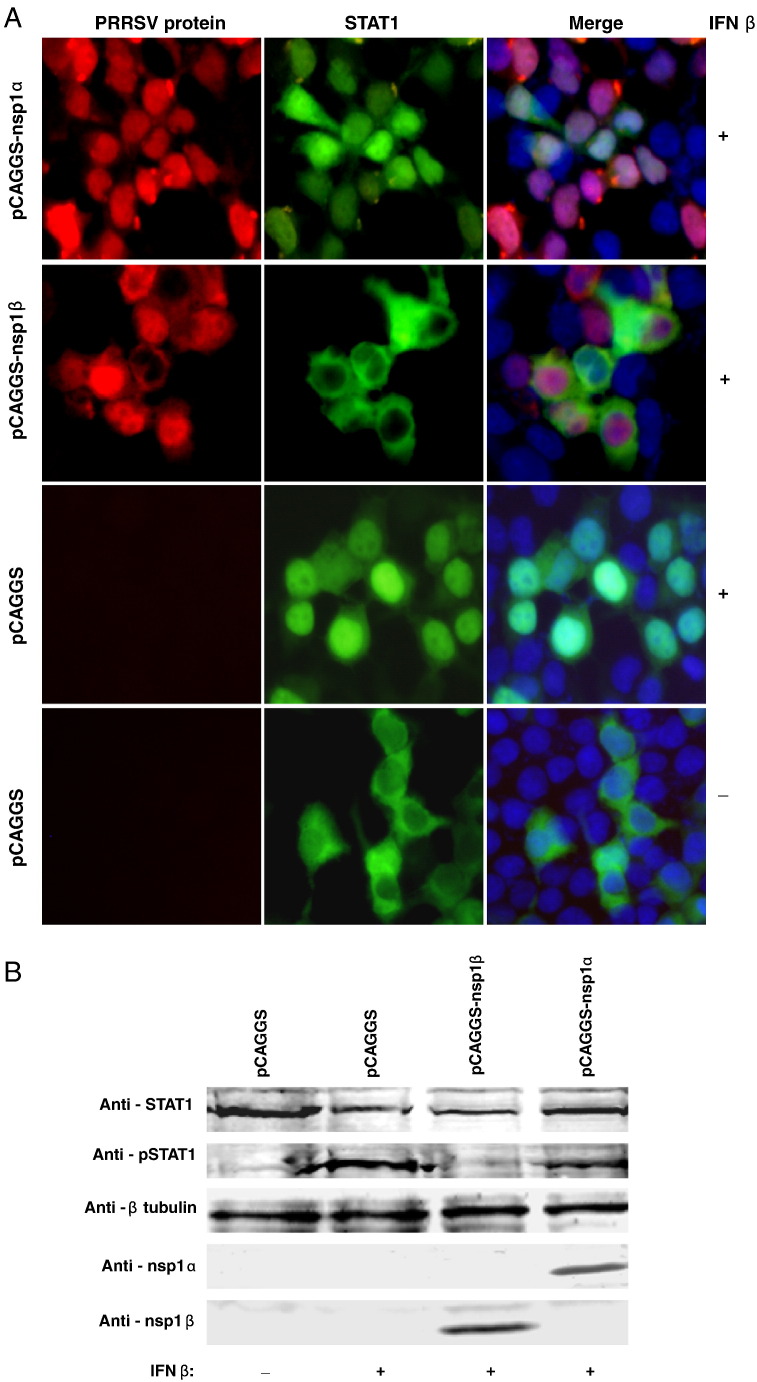
One of the mechanisms of viral proteins inhibiting interferon signaling is to interfere with the function of a key transcription factor, STAT1. Upon interferon signaling, the STAT1 is phosphorylated and translocated from the cell cytoplasm into the nucleus, where it binds to the ISRE promoter to activate the expression of ISG genes. The STAT1 translocation was analyzed in cells cotransfected with a plasmid expression of STAT1-GFP fusion protein, and a plasmid expression of nsp1α or nsp1β. Twenty-four hours post transfection, cells were stimulated with IFN-β. After 2 h, the STAT1-GFP localization was observed by fluorescent microscopy. In cells transfected with nsp1α or negative control empty plasmid, treatment of IFN-β caused STAT1-GFP to translocate into the nucleus (Fig. 7A). Interestingly, in cells transfected with nsp1β, STAT1-GFP is retained in the cell cytoplasm after treatment with IFN-β (Fig. 7A). This result indicates that PRRSV nsp1β has the ability to inhibit the translocation of STAT1-GFP into the cell nucleus.
Fig. 7.
Analysis of PRRSV nsp1 protein's effect on STAT1 translocation and phosphorylation. (A) HEK293T Cells were transfected with the indicated plasmid and STAT1-GFP for 20 h and then treated with IFN-β for 2 h. Cells were fixed and stained with pAb-nsp1α or mAb-nsp1β. DyLight 549-conjugated goat anti-rabbit or anti-mouse antibody (red fluorescence) was used as the secondary antibody. Cell nucleus was stained with DAPI (blue fluorescence). The protein localization was analyzed by fluorescence phase-contrast microscopy using a 40× objective. (B) HEK293T cells were transfected with the indicated plasmid for 24 h and then treated with IFN-β for 2 h. Cells were harvested, and lysates were analyzed by Western blot using antibodies recognizing total and phosphorylated forms of STAT1, pAb-nsp1α, mAb-nsp1β and anti-β-tubulin as a loading control.
To further analyze the mechanism of STAT1 activation, phosphorylation of the STAT1 was determined in cells cotransfected with plasmids expressing PRRSV proteins and STAT1. Transfected cells were treated with IFN-β for 2 h. Cells were harvested and analyzed by Western blot for STAT1 phosphorylation using an antibody specific to STAT1 or the phosphorylated form of STAT1. The Western blot result showed that similar levels of total STAT1 protein were expressed in cells transfected with different PRRSV proteins. However, the phospho-STAT1 was barely detected in cells transfected with nsp1β (Fig. 7B). These results indicate that nsp1β inhibits phosphorylation and activation of the STAT1 in the IFN-β signaling pathway.
Discussion
Since the first isolation of PRRSV, a wealth of information has been produced on the structural proteins. However, little is known about the structure and function of PRRSV nonstructural proteins (nsp), which account for 75% of the viral genome. Individual PRRSV nsp proteolytic processing products were mainly predicted based on the homologous genome sequence comparison between PRRSV and EAV (Snijder and Meulenberg, 1998, Allende et al., 1999, Nelsen et al., 1999, Ziebuhr et al., 2000). This study is the first to identify and characterize individual nsp processing products in PRRSV-infected cells. Our results demonstrated the actual existence of two auto-cleaved products of nsp1, nsp1α and nsp1β in PRRSV infected cells. This result is consistent with previous findings in the in vitro system (den Boon et al., 1995, Johnson et al., 2007). More importantly, we identified the actual cleavage site between nsp1α/nsp1β and nsp1β/nsp2 of Type II PRRSV. This data is further supported by the recent identification of the crystal structure of nsp1α (Sun et al., 2009). Identification of the actual cleavage sites between nsp1α/nsp1β and nsp1β/nsp2 has clarified the confusion previously reported in the PRRSV literature, especially for the nsp1α/nsp1β site. Further analysis of the dynamic movement of nsp1 proteins in PRRSV infected cells revealed the interesting localization patterns of nsp1α and nsp1β. Our results showed that during the early stage of infection (6 and 8 hpi), the nsp1α and nsp1β were mainly retained in the cell cytoplasm. At this stage, especially at 6 hpi, we observed that both nsp1α and nsp1β co-localized with most of the PRRSV ORF1a encoded nsps, including nsp2, nsp4, nsp7 and nsp8, into a perinuclear site as a distinct fluorescent spot (data not shown), which is assumed to be the site of the replication complex (Snijder and Meulenberg, 1998). At the later stages of infection (10 and 12 hpi), these two proteins are mainly localized into the cell nucleus. One simple explanation of this phenomenon is that during the early stage of infection, nsp1 is synthesized as part of a large pp1a polyprotein staying in the cytoplasm, and the subunits are subsequently liberated as individual proteins capable of translocating to the nucleus. Another possibility is that there may be two forms of nsp1α (and nsp1β): The cytoplasmic form participates in the replication complex formation, and the nuclear form interacts with the host protein(s). We have noticed that certain lots of rabbit anti-nsp1α serum stained nsp1α predominantly in cytoplasm by immunofluorescence assay.
Another important finding from this study is that PRRSV nsp1α and nsp1β proteins were determined to be involved in blockage of the type I interferon synthesis and signaling pathway. As we indicated above, each of these two proteins encode a papain-like cysteine protease (PCP) (nsp1α encodes PCPα; nsp1β encodes PCPβ). Thus, the nsp1α and nsp1β are multifunctional proteins. They not only play an important role in the proteolytic processing of replicase polyproteins, but are also involved in suppressing of the host innate antiviral response. Previous studies showed that proteases of positive-stranded RNA viruses are commonly multifunctional, and they are actively involved in antagonizing the host cell antiviral response. For example, hepatitis C virus NS3 and NS4A proteins associated to form an active enzyme, which possesses RNA helicase and serine protease activities in the polyprotein processing and HCV replication. The NS3/4A also has an ability to block the type I interferon gene expression by targeting the toll-like receptor 3 adaptor protein TRIF and interfering with the RIG-I-dependent signaling pathway (Karayiannis, 2005, Li et al., 2005). The N (pro) of pestiviruses is the first protein encoded by the single large open reading frame of the positive-sense RNA genome. It is a cysteine protease, which has an autoproteolytic activity to cleave itself off from the polyprotein. The N (pro) subverts host cell antiviral responses by targeting IRF3 and promoting its proteasomal degradation, a process that is independent of the proteolytic activity of N (pro) (Hilton et al., 2006, Bauhofer et al., 2007, Chen et al., 2007, Seago et al., 2007).
It remains to be determined where in the IFN induction pathway PRRSV nsp1 proteins (α and β) are having their effect. Overexpression of components of the pathway by which IRF-3 is activated in response to cytoplasmic dsRNA stimulation showed that PRRSV nsp1 proteins act downstream of all of them. We hypothesize that the PRRSV nsp1 proteins may be acting downstream of IRF-3 activation, which may have a direct effect on the formation of the transcription enhanceosome on the IFN-β promoter inside the nucleus. This hypothesis is supported by our observation that the nsp1α and nsp1β were predominantly localized into the cell nucleus during the later time of infection, and by the fact that IRF3 appears to be activated and transported into the nucleus normally after SeV infection of nsp1, nsp1α or nsp1β transfected cells. An example of this case is the Thogoto virus ML protein. The ML protein prevents the IRF3 dimerization and binding to c-AMP response element binding protein (CBP), which in turn prevents the formation of the transcription enhanceosome on the IFN-β promoter (Jennings et al., 2005). Further studies are required to map the exact point(s) on the IFN induction pathway at which PRRSV nsp1 proteins act. It will be interesting to determine the requirement for nuclear localization of the PRRSV nsp1 proteins, which relates to their interferon antagonist function. PRRSV nsp1 does not contain the traditional nuclear localization signal (NLS), a similar feature as the EAV nsp1 (Tijms et al., 2002), which suggests that these proteins may be bound with cellular protein(s) and shuttled into the nucleus.
Besides their inhibition in IFN-β synthesis, the PRRSV nsp1, especially nsp1β, was determined to be able to inhibit interferon signaling pathway. The nsp1β demonstrated strong inhibition effect on the expression of reporter gene from an ISRE promoter after IFN-β stimulation. Its action on this pathway appears on the blockage of the STAT1 phosphorylation and preventing the STAT1 nuclear localization. Limited inhibition effect of nsp1α on this pathway was observed. Under natural infection, both nsp1α and nsp1β exist in the infected cells. It was consistently shown that cells transfected with nsp1 (nsp1α plus nsp1β) had lower levels of luciferase reporter signal than those cells transfected with nsp1β or nsp1α alone. Based on our results, it seems that the sum of effect by nsp1α and nsp1β alone are equivalent to the effect of nsp1. Another interesting observation is that nsp1β alone appears to suppress STAT1 phosphorylation, whereas nsp1α alone appears to lead to disappearance of STAT1 from the cytoplasm. Fig. 7A shows nsp1α concentrated in dots on the edge of nuclei and almost no cytoplasmic STAT1, whereas nsp1β does not show intense perinuclear fluorescence and has no apparent effect on the cytoplasmic fluorescence signal of STAT1, only on exclusion of STAT1 from nuclei. The detailed mechanism involved in this pathway needs to be further elucidated in the future. The fact that PRRSV nsp1 is also a functional antagonist in IFN signaling pathway opens a wide range of possibilities towards analyzing the pathways involved. There is no previous evidence for the ability of arteriviruses to interfere with IFN signaling pathways. In contrast, coronaviruses, a family of viruses closely related to arteriviruses, have been well studied on their function of antagonizing interferon signaling. The SARS-CoV nsp1 not only inhibits IFN production, but also inhibits IFN-dependent signaling pathways (Wathelet et al., 2007). This study demonstrated an initial step toward understanding the effect of PRRSV proteins on the IFN signaling pathway. Future studies are required to elucidate the detailed mechanisms that PRRSV involve in this pathway. It will be interesting to determine the effect of PRRSV nsp1 proteins on other components of IFN signaling.
Like the SARS-CoV nsp1, PRRSV nsp1 proteins are able to target multiple steps of the interferon response. Viral proteins that target multiple steps of interferon activation increase the likelihood that the virus can completely inhibit the interferon response. The observation that nsp1a and nsp1β can act independently to antagonize the host cell interferon response does not exclude the possibility that additional PRRSV proteins might exert a similar function or act synergistically to antagonize interferon activity. Preliminary studies from our laboratory and others suggest that PRRSV most likely expresses a number of proteins to suppress host antiviral innate immune response. A recent study from Frias-Staheli et al. (2007) reported that the cysteine protease encoded by PRRSV nsp2 is capable of inhibiting Ub- and ISG15-dependent innate immune responses. Under natural viral infection conditions, the IFN antagonist activity involves entire viral proteins that function simultaneously. The function of a single protein may be enhanced or diminished in the context of the multi-protein system. Therefore, future studies are needed to identify the other PRRSV proteins that function as interferon antagonists, and study the synergist effect of the PRRSV nsp1 proteins with other proteins. It is possible that other PRRSV proteins target different parts of innate immune response from those of nsp1 proteins, such as SARS-CoV N, ORF3b and ORF6, which interact in different steps of the interferon synthesis and signaling pathway (Kopecky-Bromberg et al., 2007). Viruses encoding multiple proteins that are able to target more than one step of the interferon response are most likely to cause a severe inhibition of interferon. The data from this study demonstrated that PRRSV nsp1 proteins, especially nsp1β, are virulence factors that can inhibit multiple steps of the host interferon response. Their synergistic effect with other PRRSV proteins, such as nsp2, during the course of infection could be able to shut down the host cell innate immune response completely. This may explain why the induction of interferon and some innate cytokines is inhibited during PRRSV infection.
In summary, PRRSV has evolved strategies to fight the interferon system by blocking the IFN synthesis and signaling. Results from this study demonstrated that the PRRSV nsp1α and nsp1β are key players in this context. This work provides further insight into the immune evasion strategies utilized by PRRSV. Identification of the specific viral protein(s) responsible for the antagonizing of IFN response is an important initial step in the development of vaccine and therapeutics aimed at disrupting this critical aspect of viral pathogenesis. Future study should be directed towards identifying the region or amino acids on the PRRSV nsp1 that can be altered in order to remove (or decrease) the interferon antagonist function.
Materials and methods
Cells and viruses
HEK293T cells and MARC-145 cells (a PRRSV permissive cell line) were cultured in modified Eagle medium (Invitrogen) containing 10% fetal bovine serum. North American Type II PRRSV isolate SD23983 was used to infect MARC-145 cells for subsequent experiments. The Sendai virus (SeV) Cantell strain was grown in embryonated chicken eggs. Virus titer was determined by hemagglutination assay using chicken red blood cells as described previously (Yonemitsu and Kaneda, 1999).
Monoclonal and polyclonal antibody production
For production of antibodies to nsp1α, nsp1β, nsp1, and nsp2, recombinant proteins were expressed in E. coli and purified as described previously (Johnson et al., 2007, Brown et al., 2009). Monoclonal antibodies (mAb-nsp1β, mAb-nsp2) were produced by immunizing BALB/c mice with 50 μg of nsp1β or nsp2 antigen mixed with Freund's incomplete adjuvant at 2-week intervals for 8 weeks. Mouse splenocytes were fused with NS-1 myeloma cells. An immunofluorescent assay was used to screen for specific anti-PRRSV mAbs as we described previously (Fang et al., 2006). Hybridomas secreting PRRSV-specific mAbs were sub-cloned, and mAbs were obtained from cell culture supernatant or mouse ascites fluid. MAbs were isotyped using an IsoStrip Kit (Serotec, Inc.) following the manufacturer's instructions. Polyclonal antibodies (pAb-nsp1, pAb-nsp1α) were raised in New Zealand white rabbits by using recombinant proteins nsp1 and nsp1α. For primary immunizations, 100 μg of nsp1 or nsp1α antigen was mixed with an equal volume of Freund's incomplete adjuvant, and injected subcutaneously at six different locations. Rabbits were boosted twice at 2-week intervals.
Indirect immunofluorescence assay (IFA)
Virus infected or plasmid DNA transfected cells were fixed with 3.7% formaldehyde in PBS (pH 7.4) for 10 min, and then permeabilized with 0.1% Triton X-100 plus 2% BSA in PBS for 30 min at room temperature. Fixed cells were incubated for 1 h with the primary antibodies (mouse ascites at dilution of 1:100 or rabbit polyclonal antibody at dilution of 1:50) at 37 °C. FITC- or DyLight 549-conjugated goat anti-mouse or anti-rabbit antibody (1:100 dilution; ICN Biomedicals, Inc.; KPL, Inc.) was used as the secondary antibody. Nuclear staining with 4, 6-diamidino-2-phenylindole-dihydrochloride (DAPI) was performed as recommended by the manufacturer (Molecular Probes). Cell preparations were imaged under an inverted fluorescent microscope (Olympus). Images were taken with a 40× or 20× objective. Images were processed with DP-BSW (Version 03.02, Olympus) and Adobe Photoshop 6.0 software.
Western blot
Virus infected or plasmid DNA transfected cells were harvested and lysed in lyses buffer (1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1 mM EDTA in PBS) supplemented with protease inhibitor cocktail (Roche). Lysates were frozen at − 80 °C, thawed, and centrifuged to remove the insoluble pellet. The protein concentration in the supernatant was determined by a Bradford assay (Bio-Rad). Equal amounts of proteins were separated by electrophoresis on a polyacrylamide gel. The separated proteins were blotted onto a nitrocellulose membrane as we described previously (Wu et al., 2001). After blotting, membranes were blocked with 5% nonfat dry milk in PBST (0.05% Tween 20 in 1× PBS). The membrane was then incubated with primary antibodies (1:1000 dilution of mAb; 1:200 dilution for pAb) for 1 h at room temperature. For detecting the expression of PRRSV proteins, rabbit polyclonal antibodies against nsp1 or nsp1α, or mouse monoclonal antibody against nsp1β were used. For detection of STAT1 expression, a nitrocellulose membrane was probed with rabbit anti-total STAT1, or rabbit anti-phospho-STAT1 recognizing phosphorylation at tyrosine 701 (Santa Cruz Biotechnology). The mouse anti-β-tubulin (Lamda Biotech) antibody was used as a control. After incubation with primary antibody, the membrane was washed with PBST. The secondary antibody, goat anti-mouse or anti-rabbit (Kirkegaard & Perry Laboratories, Gaithersburg, MD), was added and incubated for 60 min. The membrane was developed using the method we described previously (Wu et al., 2001).
Protein N-terminal sequencing
The PRRSV nsp1β and nsp2 protein N-terminal sequencing was carried out at the Harvard Microchemistry and Proteomics Analysis Facility (Cambridge, MA). The PRRSV proteins were immunoprecipitated from the SD23983 virus infected cell lysate using anti-nsp1β and anti-nsp2 monoclonal antibodies. The immunoprecipitated PRRSV proteins were separated by electrophoresis on a polyacrylamide gel. After gel electrophoresis, gels were soaked in transfer buffer (10 mM 3-[cyclohexylamino]-1-propanesulfonic acid, 10% methanol, pH 11.0) for 5 min. At the same time, the PVDF membrane was rinsed with 100% methanol and stored in transfer buffer. The gel, sandwiched between a sheet of PVDF membrane and blotting papers, was assembled into a blotting apparatus for 1 h at 0.24 A in transfer buffer. The transferred PVDF membrane was washed in deionized H2O and stained with 0.5% Ponceau S in 1% acetic acid. The membrane was finally rinsed in deionized H2O and air dried. Corresponding protein bands were cut from the membrane and were subjected to automatic sequential Edman degradation for 10 cycles using the PE/ABD Procise 494 HT Protein Sequencing System.
Plasmids
The nsp1α, nsp1β and nsp1 regions of SD23983 were RT-PCR amplified from genomic RNA, and PCR products were cloned into a eukaryotic expression vector, pCAGGS [a generous gift from Dr. Adolfo Garcia-Sastre (Muñoz-Jordan et al., 2003)], designated as pCAGGS-nsp1α, pCAGGS-nsp1β and pCAGGS-nsp1. Reporter plasmids expressing the firefly luciferase under the control of either the IFN-β promoter (p125-Luc) or an artificial promoter containing three IRF3 binding sites (p55-CIB-Luc) were kindly provided by Dr. Takashi Fujita (Yoneyama et al., 1996). The pNF-kB-Luc or pISRE-Luc reporter plasmids expressing the firefly luciferase under the control of a promoter with NF-kB-response element or the interferon-stimulated response element (ISRE) was purchased from Stratagene (La Jolla, CA). The pRL-SV40 plasmid that expresses a Renilla luciferase under the control of a simian virus (SV) 40 promoter was purchased from Promega (Madison, WI). The pEFneo-RIG-I, pEFneo-MDA5, pEFneo-TBK1, and pEFneo-IKKɛ were kindly provided by Dr. Bin Gotoh (Komatsu et al., 2007). The pcDNA3-TRIF was purchased from Addgene. The pEGFP-STAT1 and pN1-IPS-1 plasmids were constructed by PCR amplification of STAT1 and IPS-1 genes from the full-length cDNA clones (ATCC IMAGE clone ID 3627218 and ID 5751684, respectively), and subsequently cloned into pEGFP-N1 vector. The pEGFP-IRF3 plasmid was kindly provided by Dr. John Hiscott (Hiscott et al., 1999), and was used as a template for cloning of IRF3 gene into pCAGGS vector to construct pCAGGS-IRF3 plasmid. The pCAGGS-NS1 plasmid was constructed by RT-PCR amplification of NS1 gene from influenza A/swine/Texas/4199-2/98 (TX/98, H3N2 subtype, a generous gift from Dr. Jurgen Richt; Solórzano et al., 2005), and subsequently cloned into pCAGGS vector.
Cell transfection and luciferase reporter assay
HEK293T cells were seeded in 24-well plates and transfected with various combinations of plasmids DNA: the pEFneo-RIG-I, pEFneo-MDA5, pN1-IPS-1, pEFneo-TBK1, pEFneo-IKKɛ, pcDNA3-TRIF, or pCAGGS-IRF3 was mixed with a plasmid encoding the PRRSV protein (or empty pCAGGS), luciferase reporter plasmid and pSV40-RL. Transfection was performed using HD-FuGENE 6 transfection reagent followed the manufacturer's instruction (Roche Molecular Biochemicals). For the Sendai virus or IFN stimulation, HEK293T cells were transfected with a plasmid encoding the PRRSV proteins (or empty pCAGGS, pCAGGS-NS1), reporter plasmids and pSV40-RL. At 20 h post-transfection, cells were infected with Sendai virus at 5000 HA unit/0.5 ml/well for 12–16 h, or induced by treatment with 2000 IU/0.5 ml/well of IFN α or IFN β for 16 h. Cells were harvested at the indicated time points. Cell lysates were prepared and subjected to reporter gene assay using the dual luciferase reporter system (Promega) according to manufacturer's instruction. Firefly and Renilla luciferase activities were measured in a luminometer (Bethold). Values for each sample were normalized using the Renilla luciferase values.
Acknowledgments
We thank Dr. Bin Gotoh (University of Fukui, Fukui) for providing the pEFneo-RIG-I, pEFneo-MDA5, pEFneo-TBK1, and pEFneo-IKKɛ plasmids; Dr. Adolfo Garcia-Sastre (Mount Sinai School of Medicine, New York) for providing pCAGGS plasmid; Dr. Takashi Fujita (Kyoto University, Tokyo) for providing the p125Luc and p55-CIB-Luc plasmid; and Dr. John Hiscott (McGill University, Montreal) for providing pEGFP-IRF3 plasmid. This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service grant number 2004-35605 and 2007-01745, South Dakota Center for Infectious Disease Research and Vaccinology 2010 program.
References
- Allende R., Lewis T.L., Lu Z., Rock D.L., Kutish G.F., Ali A., Doster A.R., Osorio F.A. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- Bauhofer O., Summerfield A., Sakoda Y., Tratschin J.D., Hofmann M.A., Ruggli N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J. Virol. 2007;81:3087–3096. doi: 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Brown E., Lawson S., Welbon C., Murtaugh M.P., Nelson E.A., Zimmerman J.J., Rowland R.R.R., Fang Y. Antibody response of nonstructural proteins: implication for diagnostic detection and differentiation of Type I and Type II porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2009;16:628–635. doi: 10.1128/CVI.00483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddaert W., Van Reeth K., Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv. Exp. Med. Biol. 1998;440:461–467. doi: 10.1007/978-1-4615-5331-1_59. [DOI] [PubMed] [Google Scholar]
- Chen Z., Rijnbrand R., Jangra R.K., Devaraj S.G., Qu L., Ma Y. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology. 2007;366:277–292. doi: 10.1016/j.virol.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs K., Stock N., Ross C., Andrejeva J., Hilton L., Skinner M., Randall R., Goodbourn S. Mda-5, but not RIG-1, is a common target for paramixovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., Gorcyca D., Chladek D., McCullough S., Morrison R.B., Joo H.S. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diag. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- den Boon J.A., Faaberg K.S., Meulenberg J.J.M., Wassenaar A.L.M., Plagemann P.G.W., Gorbelenya A.E., Snijder E.J. Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papainlike cysteine proteases. J. Virol. 1995;69:4500–4505. doi: 10.1128/jvi.69.7.4500-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Pekosz A., Haynes L., Nelson E., Rowland R.R.R. Production and characterization of monoclonal antibodies against the nucleocapsid protein of severe acute respiratory syndrome coronavirus (SARS-CoV) Adv. Exp. Med. Biol. 2006;581:153–156. doi: 10.1007/978-0-387-33012-9_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Staheli N., Glannakopoulos N.V., Klkkert M., Taylor S.L., Bridgen A., Paragas J., Richt J.A., Rowland R.R.R., Schmaljohn C.S., Lenschow D.J., Snijder E.J., Garcia-Sastre A., Virgin H.W. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbes. 2007;2:404–416. doi: 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller, O., Weber, F., 2007. Pathogenic viruses: smart manipulators of the interferon system. CTMI 316, 315–334. [DOI] [PMC free article] [PubMed]
- Hilton, L., Moganeradj, K., Zhang, G., Chen, Y.H., Randall, R.E., McCauley, J.W., Goodbourn, S., 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 80, 11723–11732. [DOI] [PMC free article] [PubMed]
- Hiscott, J., Pitha, P., Genin, P., Nguyen, H., Heylbroeck, C., Mamane, Y., Algarte, M., Lin, R., 1999. Triggering the interferon response: the role of IRF-3 transcription factor. J. Interferon Cytokine Res. 19, 1–13. [DOI] [PubMed]
- Jennings S., Martinez-Sobrido L., Garcia-Sastre A., Weber F., Kochs G. Thogoto virus ML protein suppresses IRF3 function. Virology. 2005;331:63–72. doi: 10.1016/j.virol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Johnson C.R., Yu W., Murtaugh M. Cross-reactive antibody responses to nsp1 and nsp2 of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007;88:1184–1195. doi: 10.1099/vir.0.82587-0. [DOI] [PubMed] [Google Scholar]
- Karayiannis P. The hepatitis C virus NS3/4A protease complex interferes with pathways of the innate immune response. Journal of Hepatology. 2005;43:743–745. doi: 10.1016/j.jhep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keffaber K.K. Reproductive failure of unknown etiology. Am. Assoc. Swine Prac. Newsletter. 1989;1:1–10. [Google Scholar]
- Komatsu T., Takeuchi K., Gotoh B. Bovine parainfluenza virus type 3 accessory proteins that suppress beta interferon production. Microbes Infect. 2007;9:954–962. doi: 10.1016/j.micinf.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K., Foy, E., Ferreon, J.C., Nakamura, M., Ferreon, A.C., Ikeda, M., Ray, S.C., Gale, M. Jr, Lemon, S.M., 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. 102, 2992–2997. [DOI] [PMC free article] [PubMed]
- Luo R., Xiao S., Jiang Y., Jin H., Wang D., Liu M., Chen H., Fang L. Porcine reproductive and respiratory syndrome virus (PRRSV) suppresses interferon-β production by interfering with the RIG-I signaling pathway. Mol. Immunol. 2008;45:2839–2846. doi: 10.1016/j.molimm.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier W.A., Galeota J., Osorio F.A, Husmann R.J., Schnitzlein W.M., Zuckermann F.A. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller L.C., Laegreid W.W., Bono J.L., Chitko-Mckown C.G., Fox J.M. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol. 2004;149:2453–2463. doi: 10.1007/s00705-004-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Jordan J.L., Sánchez-Burgos G.G., Laurent-Rolle M., García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C.J., Murtaugh M.P., Faaberg K.S. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. JAVMA. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- Overend C., Mitchell R., He D., Rompato G., Grubman M.J., Garmendia A.E. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2007;88:925–931. doi: 10.1099/vir.0.82585-0. [DOI] [PubMed] [Google Scholar]
- Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signaling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Royaee A.R., Husmann R., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F., Lunney J.K. Deciphering the involvement of innate immune factors in the development of the host responses to PRRSV vaccination. Vet. Immunol. Immunopathol. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seago J., Hilton L., Reid E., Doceul V., Jeyatheesan J., Moganeradj K. The Npro product of classical swine fever virus and bovine viral diarrhea virus uses a conserved mechanism to target interferon regulatory factor-3. J. Gen. Virol. 2007;88:3002–3006. doi: 10.1099/vir.0.82934-0. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Solórzano A., Webby R.J., Lager K.M., Janke B.H., García-Sastre A., Richt J.A. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol. 2005;79:7535–7543. doi: 10.1128/JVI.79.12.7535-7543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Xue F., Guo Y., Ma M., Hao N., Zhang X.C., Lou Z., Li X., Rao Z. Crystal structure of porcine reproductive and respiratory syndrome virus (PRRSV) leader protease Nsp1{alpha} J. Virol. 2009;83:10931–10940. doi: 10.1128/JVI.02579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Tijms M.A., van der Meer Y., Snijder E.J. Nuclear localization of non-structural protein 1 and nucleocapsid protein of equine arteritis virus. J. Gen. Virol. 2002;83:795–800. doi: 10.1099/0022-1317-83-4-795. [DOI] [PubMed] [Google Scholar]
- Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J. Virol. 2007;81:11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Kochs G., Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemrad M., deKluyer E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F., Broekhuijsen J.M., Moonen P.L.J.M., Zetstra T., de Boer E.A., Tibben H.J., de Jong M.F., van't Veld P., Groenland G.J.R., van Gennep J.A., Voets M.T.H., Verheijden J.H.M., Braamskamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet. Quarterly. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wootton S., Yoo D., Rogan D. Full-length sequence of a Canadian porcine reproductive and respiratory syndrome virus (PRRSV) isolate. Arch. Virol. 2000;145:2297–2323. doi: 10.1007/s007050070022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.H., Fang Y., Farwell R., Steffen-Bien M., Rowland R.R.R., Christopher-Hennings J., Nelson E.A. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF 2b. Virology. 2001;287:183–191. doi: 10.1006/viro.2001.1034. [DOI] [PubMed] [Google Scholar]
- Yonemitsu Y, Kaneda Y. Hemagglutinating virus of Japanliposome mediated gene delivery to vascular cells. In: Baker A.H., editor. Molecular Biology of Vascular Diseases. Methods in Molecular Medicine. Humana Press; Clifton: 1999. pp. 295–306. [DOI] [PubMed] [Google Scholar]
- Yoneyama M., Suhara W., Fukuhara Y., Sato M., Ozato K., Fujita T. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3) J. Biochem. 1996;120:160–169. doi: 10.1093/oxfordjournals.jbchem.a021379. [DOI] [PubMed] [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]