Abstract
The avian influenza viruses (AIVs) can be highly contagious to poultry and a zoonotic threat to humans. Since the memory CD8+ T lymphocyte responses in chickens to AIV proteins have not been defined, these responses to H5N9 AIV hemagglutinin (HA) and nucleocapsid (NP) proteins were evaluated by ex vivo stimulation with virus infected non-professional antigen presenting cells. Secretion of IFNγ by activated T lymphocytes was evaluated through macrophage induction of nitric oxide. AIV specific, MHC-I restricted memory CD8+ T lymphocyte responses to NP and HA were observed 3 to 9 weeks post-inoculation (p.i.). The responses specific to NP were greater than those to HA with maximum responses being observed at 5 weeks p.i. followed by a decline to weakly detectable levels by 9 weeks p.i. The cross-reaction of T lymphocytes to a heterologous H7N2 AIV strain demonstrated their ability to respond to a broader range of AIV.
Keywords: AIV, Chicken, T lymphocyte, Memory immune responses, Hemagglutinin protein, Nucleocapsid protein
Introduction
Avian influenza viruses (AIV) within the Orthomyxoviridae family have segmented, negative sense RNA genomes. These viruses, natural infectious agents of waterfowl and shorebirds, are classified according to their transmembrane hemagglutinin (HA) and neuraminidase (NA) glycoproteins (Alexander, 2000, Krauss et al., 2007, Olsen et al., 2006, Webby and Webster, 2001). Due to their incredibly broad avian host range, AIV strains have been isolated from many different species of birds including ducks, gulls, geese, psittacines and poultry (Alexander, 2000, Olsen et al., 2006). AIV strains with all 16 hemagglutinin (HA) and 9 neuraminidase (NA) types have been isolated from waterfowl or shore birds (Fouchier et al., 2005, Krauss et al., 2007). Depending on the virulence of the virus in poultry, isolates from poultry are classified as either low pathogenic (LP) or highly pathogenic (HP) (Alexander, 2000, Collisson et al., 2008). LPAIV strains cause asymptomatic to mild respiratory and enteric tract infections while the highly pathogenic strains cause clinical illness and systemic infections. Infections of poultry, especially with the highly pathogenic strains, result in severe economic losses (Capua and Marangon, 2003, Tollis and Di Trani, 2002). Human influenza viruses, including those causing high morbidity and significant mortality, such as the H1N1 from 1918, H2N2 from 1957 and H3N2 from 1968 have been shown to have avian origins (Capua and Alexander, 2002, Taubenberger et al., 2001). Even the currently circulating swine origin H1N1 human influenza virus encodes two genes of AIV origin (Babakir-Mina et al., 2009, Garten et al., 2009). Since 1996, highly pathogenic H5N1 AIV strains isolated in Hong Kong have been infecting and subsequently causing deaths in humans, although person-to-person transmission is apparently rare (Capua and Alexander, 2002, Perdue and Swayne, 2005, Ungchusak et al., 2005). Poultry is a logical intermediate host for adaptation of viral strains from wild birds to humans and other mammals, such as swine (Webby and Webster, 2001, Webster, 1997). Indeed, human adapted strains have been shown to consist of genome segments of avian, swine and human origin (Webby and Webster, 2001, Webster, 1997).
Vaccination efficacy is traditionally determined by the demonstration of protective humoral immunity, especially targeting AIV HA and by putative neutralization of viruses (Collisson et al., 2008, Suarez et al., 2006, Swayne and Kapczynski, 2008). While sterile immunity may depend on humoral responses to homologous HA, effector and memory CD8+ T cell immunity in mice has been shown to diminish disease preventing mortality, and even morbidity (Rimmelzwaan et al., 2007, Swain et al., 2004). Humoral immunity of chickens to AIV is well characterized but little information is available regarding the more difficult to evaluate, viral specific T cell immune responses (Kwon et al., 2008, Seo and Webster, 2001, Swayne and Kapczynski, 2008). Because mice are not natural hosts of AIV, all the immunological characterization in mice is based only on mouse adapted viruses. It is relevant to define the T lymphocyte mediated immune responses in chickens since AIVs are established pathogens of poultry and can be transmitted directly from chickens to humans.
Avian T lymphocytes have been stimulated ex vivo with MHC matched chicken kidney cells (CKC) serving as non-professional antigen presenting cells (APCs) and by the adoptive transfer of MHC matched T lymphocytes to naïve chicks prior to viral challenge (Pei et al., 2003, Seo et al., 2000). The availability of a number of poultry lines with defined MHC (located within the chicken B locus) greatly facilitates the evaluation of the adaptive T lymphocyte responses in chickens (Miller et al., 2004). Studies targeting acute infections with a strain of infectious bronchitis virus (IBV), an avian coronavirus, have identified specific CD8+ T cell responses (Seo and Collisson, 1997). Adoptive transfer of either effector T cells prepared from birds 10 days post-infection (p.i.) or of memory T lymphocytes prepared from birds 3 weeks after infection with IBV, provided protection against acute disease after viral challenge (Pei et al., 2003, Seo et al., 2000). Following infection with H9N2 AIV, Seo et al. (2002) described CD8+ T cell responses that correlated with cross-protection to an H5N1 strain. Protection by effector CD8+ T lymphocytes prepared at 7 to 10 days p.i. with AIV was demonstrated following adoptive transfer 1 day prior to AIV challenge (Seo and Webster, 2001). However, none of these studies with AIV identified the individual AIV proteins housing T lymphocytes epitopes or described the kinetics of the T lymphocyte mediated memory responses to AIV.
This current study describes the memory responses of peripheral blood T lymphocytes from chicks to AIV HA and/or NP protein. DNA plasmid vectors expressing AIV proteins were used to delineate the responses induced individually by either HA or NP proteins. Responses were evaluated following ex vivo stimulation with MHC-I matched or mismatched APCs. Both NP and HA induced AIV specific memory T lymphocyte responses between 3 and 9 weeks p.i. Although the T lymphocyte response induced by NP was greater than the response induced by HA until 7 weeks p.i., no differences between the responses to the two proteins were detected by 9 weeks p.i. The maximum response of memory T lymphocytes against either AIV NP or HA protein was observed at 5 weeks p.i.
Results
In vitro expression of AIV proteins
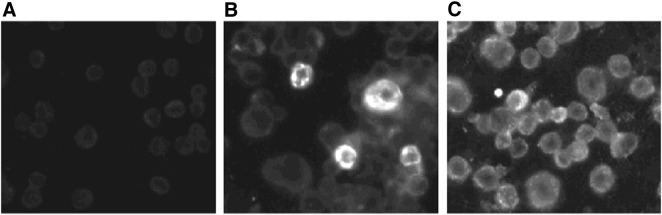
In order to determine the T lymphocyte responses to individual AIV proteins, the NP and HA genes of the low pathogenic H5N9 (Turkey/Wis/68) strain were cloned into the pcDNA3.1/V5-His-TOPO TA vector (Invitrogen, Carlsbad, CA). The eukaryotic expression of the proteins encoded by the plasmids was determined by IFA in CHO-K1 cells 48-h post-transfection with plasmids expressing either NP or HA (Fig. 1 ). No fluorescence was observed in cells transfected with plasmid encoding for LacZ.
Fig. 1.
In vitro expression of pcDNA3.1/V5-His-TOPO TA vectored AIV proteins in transfected CHO-K1 cells (magnification, 200 ×). Expression was detected by an IFA using AIV positive reference serum as the source of primary antibodies. Cells were transfected with plasmids expressing (A) LacZ, (B) HA, and (C) NP.
Humoral immune response to AIV proteins
The in vivo expression of AIV HA and NP and the antibody response to these proteins in the chickens was confirmed by detecting the presence of antibodies specific for HA and NP at 3 weeks p.i. with the plasmids. HI titers of sera from 6 chickens inoculated with the HA expression plasmid using the homologous strain ranged from 5 to 6.5 log2 (GMT). Sera from chickens inoculated with HA plasmid failed to inhibit hemagglutination by a heterologous H7N2 AIV. No HI activity was detected in sera of the 4 chickens inoculated with PBS. ELISA titers for antibodies against NP in 6 NP plasmid inoculated chickens ranged between 2.5 and 3.0 log10 (GMT) at 3 weeks p.i. (Fig. 2 ).
Fig. 2.
Antibody titers induced in individual birds (n = 6) by NP and HA expressing plasmids at 3 weeks p.i. (A) Serum HI antibody titers from chickens inoculated with HA expressing plasmid against the homologous H5N9 AIV strain and heterologous H7N2 AIV strain. (B) Serum anti-NP antibody ELISA titers from chickens inoculated with the NP expressing plasmid. Neither HI nor anti-NP antibodies were detectable in the sera of 4 control birds inoculated with PBS. Each symbol represents the response of an individual chicken.
Memory T cell responses were detected from weeks 3 to 9 p.i. with HA and NP plasmids
Because the T cell specificity for individual AIV proteins have not previously been reported in chickens, T lymphocyte responses to HA, NP or a combination of both HA and NP (HN) were determined following inoculation of B19/B19 chickens with plasmids expressing the HA or NP ORF. In the absence of well-established ELISA or intracellular cytokine staining methods to examine avian T lymphocyte mediated responses an indirect IFNγ assay (NO production from HD11 cells, a chicken macrophage cell line) was precisely standardized for evaluating the ex vivo activation of T lymphocytes by APCs. CKC infected with AIV were used as non-professional APCs for the stimulation of T lymphocytes. As demonstrated in other studies, the primary effector T lymphocyte response observed at 10 days p.i. had declined to basal level by 16th day p.i. (data not shown).
Considering previously conducted adoptive transfer studies, which identified specific memory T cells to IBV by 3 weeks p.i. with maximal responses occurring between weeks 5 and 6 p.i. (Pei et al., 2003), the memory AIV response of peripheral blood memory T lymphocytes were also evaluated between 3 and 9 weeks p.i. with HA, NP, or HN plasmids. The kinetics of the T lymphocyte mediated responses was determined on the same birds during the course of the study. All data represent the response from individual birds.
Following ex vivo co-culture with MHC matched B19/B19 APCs infected with H5N9 virus, memory responses were detected in T cell preparations obtained from all chickens receiving plasmids expressing either AIV protein by 3 weeks p.i. Since neither supernatants from the T cells cultured with uninfected APCs nor T cells from PBS inoculated birds cultured with infected MHC matched B19/B19 birds produced IFNγ (data not shown), the memory T lymphocyte activity was considered AIV specific. The ex vivo stimulation of the T cells from plasmid inoculated birds with MHC mismatched B2/B2 APCs could only induce basal levels of NO (Fig. 3 ), indicating the memory T lymphocyte responses from each group of chickens receiving AIV plasmids was highly MHC restricted.
Fig. 3.
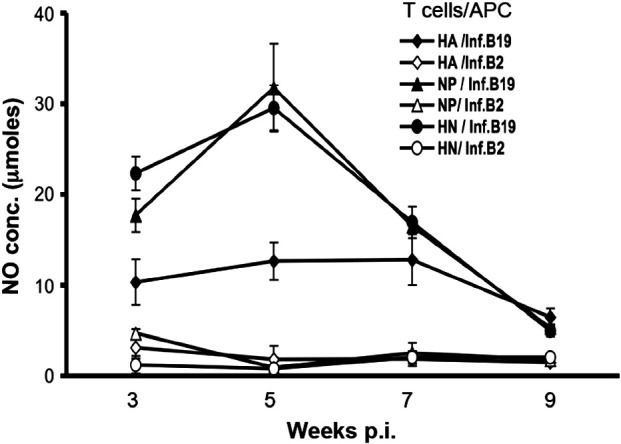
Chicken memory T lymphocyte responses to AIV HA and NP proteins between 3 and 9 weeks p.i. with NP and/or HA expression plasmids. Chickens of the B19/B19 MHC haplotype were inoculated with DNA plasmids expressing AIV HA, NP or both HA and NP (HN). Memory T lymphocytes were stimulated ex vivo with H5N9 AIV infected MHC matched B19/B19 and mismatched B2/B2 APCs. Production of NO by HD11 macrophage cells induced by the secretion of IFNγ from stimulated T lymphocytes was used to quantify lymphocyte activation. Results represent the average (± S.E.) of two separate experiments. Each ex vivo stimulation assay is denoted by the source of T lymphocytes and MHC haplotype of the APCs infected with the virus. The differences in stimulation by matched and mismatched APCs were significant (p ≤ 0.003 to 0.02) for each inoculated antigen and time point. The responses to HN (p ≤ 0.03) at 3 weeks and NP (p ≤ 0.03) and HN (p ≤ 0.007) at 5 weeks p.i. were significantly greater than the responses to HA at the same time points. The p value for the difference between responses to NP and HA at 3 weeks p.i. was 0.07.
The maximum memory T cell responses to NP were detected at 5 weeks p.i. However, while still detectable, memory T cells responses at 9 weeks p.i, were dramatically diminished for all birds receiving the AIV expression plasmids (HA, NP or HN). During weeks 3 through 7, the activity of T cells from the HA plasmid inoculated birds was significantly less than that of T cells isolated from birds receiving either NP or NP plus HA plasmids (Fig. 3). In addition to weaker APC induced stimulation, the levels of HA specific memory T lymphocyte responses were similar at weeks 3, 5 and 7 p.i. These results were observed for two independent experiments.
CD8+ T lymphocyte populations increase with ex vivo stimulation
The phenotype of activated subpopulations of T lymphocytes following co-culture with APCs expressing AIV antigens was determined using flow cytometric analyses (Table 1 ). In order to evaluate the type of T lymphocyte population expanding in response to infected APCs, the proliferation of the lymphocytes from chickens inoculated with both NP and HA (HN) plasmids was determined at 5 weeks p.i. Lymphocyte populations were gated using a chicken pan lymphocyte CD44 specific MAb and MAbs specific for either CD4 or CD8 T cell antigens (Seo and Collisson, 1997). The relative increases in the populations of CD8+ T lymphocytes harvested from each HA and NP (HN) plasmid inoculated chicken were between 62% and 91% following ex vivo stimulation with AIV expressing APCs, in contrast to the increase of 31% to 37% in the CD4+ T lymphocyte populations. The increase in the population of T lymphocytes harvested from PBS inoculated birds was 7% and 1% for CD8+ and CD4+ T lymphocyte subpopulations, respectively, following co-culture with AIV infected APCs. The preferential increase in CD8+ cells correlates with their anticipated expansion following exposure to non-professional APCs endogenously expressing AIV and expressing surface MHC-I.
Table 1.
Proliferation of T lymphocytes from birds 5 weeks p.i. with plasmids expressing HA and NP using flow cytometry.a
| Bird no. | Inoculab | CD4+ T lymphocytes (% of T cells) |
CD8+ T lymphocytes (% of T cells) |
||||
|---|---|---|---|---|---|---|---|
| Uninfectedc | Infectedd | % Increasee | Uninfected | Infected | % Increase | ||
| 1 | HA+NP | 47.8 | 65.1 | 36 | 20.0 | 36.4 | 81 |
| 2 | HA+NP | 44.0 | 59.5 | 35 | 24.7 | 47.1 | 91 |
| 4 | HA+NP | 41.3 | 56.6 | 37 | 27.0 | 43.7 | 62 |
| 5 | HA+NP | 46.0 | 60.5 | 31 | 22.6 | 40.4 | 79 |
| 7 | PBS | 53.8 | 60.8 | 1 | 22.4 | 24.0 | 7 |
Data representative of 2 experiments.
HA + NP-Birds inoculated with 500 μg plasmids expressing HA and NP (HN) or PBS only.
Population of T lymphocytes after stimulation with uninfected APCs.
Population of T lymphocytes after stimulation with infected APCs.
Increase in the population of T lymphocytes following stimulation by APCs expressing AIV.
Responding T cells cross react with a heterologous H7N2 AIV strain
A rationale for targeting cellular immunity is the potential for cross-reactivity between vaccine and heterologous virus. Since the availability of the peripheral T lymphocytes was limited and the same birds were being used throughout the study, all aspects of the memory T lymphocyte mediated responses could not be evaluated at same time points. At 8 weeks p.i. the capacity for memory T lymphocytes specific for the NP and HA proteins of the H5N9 strain to be stimulated with an H7N2 (A/Turkey/Virgina/158512/02) strain of AIV was determined following co-culture with MHC matched APCs infected with either AIV strain (Fig. 4 ). Both heterologous H7N2 and homologous H5N9 AIV infected APCs significantly stimulated IFNγ production from memory T lymphocytes isolated from either HA or NP inoculated chickens (p ≤ 0.01) compared with PBS inoculated chickens. Regardless of the strain used to infect APCs, the observed memory responses generated by T cells obtained from chickens receiving the NP plasmid were again statistically greater (p ≤ 0.008) than that generated by T cells harvested from HA plasmid inoculated chickens.
Fig. 4.
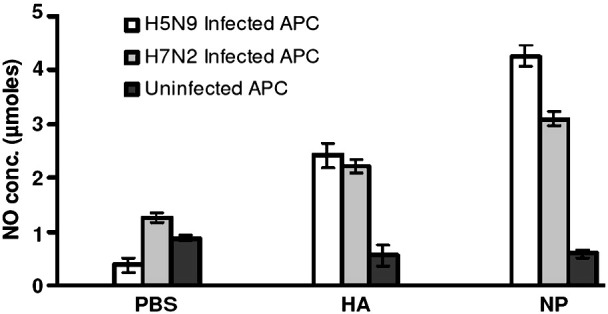
T lymphocytes from B19 birds inoculated with either H5N9 derived HA or NP expression plasmids respond to a heterologous (H7N2) virus. At 8 weeks p.i., T lymphocytes from chickens receiving either HA or NP cloned from the H5N9 strain were co-cultured with APCs infected with H5N9 or H7N2 viruses. The T cell responses are expressed as the average (± S.E.) of NO production for each treatment group. T lymphocytes from plasmid-inoculated chickens had significantly greater responses to H7N2 AIV strain than those from the PBS control group (p ≤ 0.01). PBMC were prepared from three individual chickens for each stimulation assay.
Similar memory T lymphocyte responses were observed with low pathogenic AIV infection
Memory T cells could be readily detected between 3 and 7 weeks p.i. with plasmids expressing either the NP or the HA proteins. In order to evaluate the memory T lymphocyte responses of chickens inoculated with infectious AIV, chicks with the B19/B19 haplotype were inoculated with the low pathogenic H5N9/Turkey/Wis/68 strain and blood was collected at 5 weeks p.i. The ex vivo activation of T lymphocytes by AIV infected APCs was determined by the indirect IFNγ assay (Fig. 5 ). The mean average NO production induced by the ex vivo stimulation of T lymphocytes from infected birds with B19/B19 APCs was specific compared with the uninfected, MHC matched APCs (p ≤ 0.005). The responses to AIV infected APCs were MHC restricted as demonstrated by only basal level activation by B2/B2 APCs.
Fig. 5.

In vivo infection of B19 chickens with the low path H5N9/Tur/Wis/68 AIV generates AIV specific, MHC matched memory T lymphocytes. The potential for infectious AIV to also produce a memory T lymphocyte response was determined 5 weeks p.i. with H5N9. Mean (±S.E.) NO production by each treatment group is represented by the bars. APCs used for ex vivo stimulation were either uninfected (Uninf) or virus-infected (Inf). Significantly lower (p ≤ 0.01) NO production by AIV infected mismatched APCs derived from the CKC of homozygous B2 chicks compared to matched APCs derived from CKC of homozygous B19 chicks indicate MHC restriction. T lymphocytes from three individual birds were used for each ex vivo stimulation assay.
Flow cytometric analysis was used to determine the phenotype of the T lymphocyte subpopulations from the infected chickens responding to ex vivo stimulation. The relative increase in the population of CD8+ T lymphocytes from H5N9 infected chickens was 46% to 95% while the increase in the CD4+ T lymphocyte population ranged from 6 to 28% following co-culture with MHC-I matched, H5N9 AIV infected APCs (Table 2 ). The increase in the population of the lymphocytes from uninfected chickens was only 1 to 14% and 10 to 19% for CD8+ and CD4+ T lymphocytes, respectively. Although increases were observed in the CD4+ lymphocytes, the greater increased proliferation of CD8+ lymphocytes from birds infected with the low pathogenic virus was consistent with detection of a preferential MHC-I restricted AIV specific, CD8+ memory T cell response.
Table 2.
Proliferation of T lymphocytes from birds 5 weeks p.i. with AIV using flow cytometry.
| Bird no. | CD4+ T lymphocytes (% of T cells) |
CD8+ T lymphocytes (% of T cells) |
||||
|---|---|---|---|---|---|---|
| Uninfecteda | Infectedb | % Increasec | Uninfected | Infected | % Increase | |
| I-1d | 40.7 | 52.1 | 28 | 16.3 | 31.7 | 95 |
| I-2 | 48.3 | 58.4 | 21 | 12.1 | 20.5 | 69 |
| I-3 | 49.6 | 60.9 | 23 | 17.8 | 25.9 | 46 |
| I-4 | 55.9 | 59.5 | 6 | 18.5 | 31.7 | 71 |
| I-5 | 43.9 | 53.6 | 22 | 9.55 | 18.5 | 94 |
| C-1e | 42.5 | 50.7 | 19 | 27.9 | 28.1 | 1 |
| C-2 | 39.2 | 43.1 | 10 | 10.8 | 12.3 | 14 |
Population of T lymphocytes after stimulation with uninfected APCs.
Population of T lymphocytes after stimulation with infected APCs.
Increase in the population of T lymphocytes following stimulation with APCs expressing AIV.
I—Chickens infected with H5N9/Turkey/Wis/68 AIV.
C—Control chickens inoculated with PBS.
Discussion
This is the first study delineating the response of chicken memory CD8+ T lymphocytes to specific AIV proteins. Studies evaluating the CD8+ T lymphocyte response to influenza virus in mice have identified NP as housing the dominant CD8+ T cell epitopes (Flynn et al., 1998, Kreijtz et al., 2007, O'Neill et al., 2000, Townsend et al., 1984, Yewdell et al., 1985). In contrast, human cytotoxic T lymphocytes (CTL) have a broader repertoire and the response is directed to multiple influenza viral proteins, including HA (Gianfrani et al., 2000, Gotch et al., 1987, Jameson et al., 1998, Kedzierska et al., 2006, Kreijtz et al., 2008). This study has shown that similar to the responses in humans, memory CD8+ T lymphocytes in chickens are directed against both AIV HA and NP proteins (Gianfrani et al., 2000, Jameson et al., 1998, Kreijtz et al., 2008, McMichael et al., 1986b). Significantly greater responses were induced by NP than by HA at 3 and 5 weeks p.i. The AIV specific T cell responses were primarily MHC-I restricted as non-professional APCs of B19 and B2 haplotypes were used for ex vivo stimulation of T cells and the APCs of B2 haplotype chickens either failed to stimulate or could only weakly stimulate the T lymphocytes derived from the B19 line. Therefore, responding T cells were primarily of the CD8+ phenotype, which also showed significantly greater proliferation than CD4+ T lymphocytes in response to ex vivo APC mediated stimulation. Similar to the MHC-I restricted T lymphocyte responses demonstrated following infection with IBV, CD8+ T cell memory responses to AIV HA and/or NP were detected by 3 weeks p.i. (Pei et al., 2003). Preliminary studies in our lab have also demonstrated an elevation in the levels of expression of CD44 molecule on the surface of the responding T lymphocytes. Greater CD44 expression has been described to be a characteristic of mouse and human memory T lymphocytes (Curtsinger et al., 1998).
The current studies quantified the protein specific T lymphocyte responses of the same birds until 9 weeks p.i. The responses increased from 3 weeks p.i. until 5 weeks p.i. However, by 9 weeks p.i. with plasmids expressing either NP or HA AIV protein, the memory T cell activity had declined to significantly lower levels. The decline in the more vigorous CD8+ T lymphocyte response to NP was more rapid after 5 weeks than the CD8+ T cell response stimulated by HA, such that by 9 weeks p.i. the responses to both proteins individually or in combination were similar. A decline in the CD8+ T lymphocyte mediated protection at 10 weeks after challenge of H9N2- infected chicks with H5N1 had been observed by Seo and Webster (2001). Similarly, a decline in the memory T lymphocyte response specific to influenza virus infection has also been reported in humans (McMichael et al., 1983).
Furthermore, our study also proved the efficacy of the plasmid delivery approach in providing a mechanism to evaluate the T cell response to AIV HA and NP proteins, either individually or in combination. Protection studies were not included following inoculation of the AIV plasmids because of increased biosafety requirements for AIV. However, hemagglutinating antibodies which can be correlated with protection were demonstrated (Toro et al., 2007). The antibodies specific for the HA cloned from a H5N9 virus failed to prevent H7N2 virus-mediated hemagglutination.
Since each ex vivo stimulation assay required specific numbers of necessary controls for the validity of the results of the assay and availability of limited numbers of T lymphocytes for each time, all assays could not be conducted at the same time point. Although at 8 weeks p.i. the response of the memory T lymphocytes was significantly lower than the response at 5 weeks p.i., it remained detectable and the APCs infected with H7N2 AIV were able to effectively ex vivo stimulate the T lymphocytes from H5 inoculated chickens. These observations indicate that despite the absence of shared HI antibody epitopes, HA does have at least one CD8+ epitope that is shared between both strains of the virus.
Adoptive transfer studies of CD8+ T lymphocytes specific for IBV and AIV have demonstrated their importance in protection against heterologous viruses (Pei et al., 2003, Seo et al., 2000, Seo and Webster, 2001). In mice, adoptive transfer of memory CD8+ T lymphocytes specific for influenza virus NP has also been shown to be protective against viral challenge (Lukacher et al., 1984, Taylor and Askonas, 1986). In vivo inoculation of chicks with a DNA plasmid expressing the IBV nucleocapsid protein was shown to provide CTL mediated protection against acute respiratory disease (Seo et al., 1997). Consistent with the greater response to NP from the homologous virus, T lymphocytes of chicks inoculated with NP exhibited greater cross reactivity with the heterologous H7N2 virus than T cells from the birds inoculated with HA. The amino acid sequences in HA from the two AIV strains were 41% identical while the amino acid sequences of NP were 97% identical. The more conserved nature of NP, in comparison to HA could be responsible for the greater cross reactive response (Portela and Digard, 2002). Although the variation could also be attributed to the differences in the amount of antigen generated and presented by the infected APCs (Busch and Pamer, 1998, Crowe et al., 2003, Deng et al., 1997, Doherty et al., 1994), both NP and HA responses were similar by 9 weeks p.i. using the same standardized cell assay.
The differences in the repertoire of T cell epitopes along with the variations in the binding affinity of T cell epitopes with MHC-I can also contribute to the differences in the CD8+ T cell mediated response to influenza viral proteins (Cao et al., 1996, McMichael et al., 1986a). Chickens have 27 distinct defined MHC haplotypes (Miller et al., 2004). Further studies aimed at determining the response to AIV proteins in different MHC lines of chickens is important for identification of immunodominant proteins which can elicit conserved CD8+ T lymphocyte responses amongst various MHC haplotypes.
Although the cross reactivity of memory CD8+ T lymphocytes may not prevent the infection of chickens with a heterotypic strain of AIV, it could contribute to the more rapid clearance of the virally infected cells and augment the protection against clinical illness. This study establishes that chickens CD8+ T lymphocytes respond to AIV NP and HA proteins. The ability of the other AIV proteins to stimulate CD8+ T lymphocytes of chickens needs to be evaluated. Future experiments would also analyze the expression of various cytokines by the activated T lymphocytes. Inclusion of AIV protein targets that can induce cross reactive CD8+ T lymphocyte responses in addition to humoral immunity in chickens is critical for the development of efficacious vaccines which can provide protective immunity against a broader range of AIV types.
Materials and methods
Viruses
Low pathogenic AIVs, H5N9 (A/Turkey/Wis/68) and H7N2 (A/Turkey/Virginia/158512/02), were propagated in the allantoic sacs of 10-day-old embryonated chicken eggs (ECE). The allantoic fluid was harvested 48 h p.i. and the presence of virus was verified by an hemagglutination activity (HA) test according to the OIE guidelines (http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04_AI.pdf). Viruses were quantified by titrating in ECE and expressed as embryo infectious dose 50 (EID50) (Beard, 1989).
Experimental animals
Embryonated eggs of MHC defined B19/B19 and B2/B2 lines of chickens were obtained from Dr. Briles' laboratory at Northern Illinois University (DeKalb, IL). After hatching, chicks were housed in a specific pathogen-free environment at the vivarium facility, Western University of Health Sciences, Pomona, CA. Viral infection studies in chickens were conducted at the biosafety level 2 Lab Animal Research Resource animal facility, Texas A&M University, College Station, TX. All procedures involving the use of chickens were approved by and conducted according to guidelines established by the Institutional Animal Care and Use Committees of Western University of Health Sciences and/or Texas A&M University.
Cloning of NP and HA into a eukaryotic expression plasmid
RNA from H5N9 (A/Turkey/Wis/68) was extracted from allantoic fluid using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. First strand cDNA was synthesized with ImProm-II™ Reverse Transcriptase enzyme (Promega, Madison, WI) using AIV specific Universal 12 primer (5′AGCA/GAAAGCAGG 3′)(Urabe et al., 1993). PFU polymerase (Stratagene, La Jolla, CA) was used to amplify the open reading frames (ORF) of HA and NP using specific primer pairs HA-Forward 5′-ACCATGGAAAGAATAGTGATT-3′and HA-Reverse 5′-GATGCAAATTCTGCA-3′ and NP-Forward 5′-ACCATGGCGTCTCAAGGCACC-3′ and NP-Reverse 5′-ATTGTCATACTCCTCTGC-3′, respectively. Taq DNA polymerase (New England Biolabs, Ipswich, MA) was then used to add TA overhangs on the PFU amplified PCR product. The amplified cDNA products were cloned into the eukaryotic expression vector pcDNA3.1/V5-His-TOPO TA (Invitrogen, Carlsbad, CA). Big Dye terminator v1.1cycle sequencing kit (Applied Biosystems, Foster City, CA) was used to sequence the cloned gene segments and the sequence analysis was performed at GenoSeq, UCLA, Los Angeles, CA. Following confirmation of the sequence of the ORFs, in vitro expression of NP and HA proteins were determined using an indirect immunofluorescence assay (IFA) on CHO-K1 cells transfected with the plasmids expressing AIV proteins. Known chicken serum, positive for AIV (NVSL, Ames, IA) was used at a dilution of 1:100 as primary antibody. Mouse anti-chicken IgG FITC at a dilution of 1:500 (Southern Biotech, Birmingham, AL) was used as the secondary antibody.
Generation of APCs
Primary CKC lines were established from 10-day-old chicks of B19/B19 and B2/B2 MHC haplotype lines as described previously (Seo and Collisson, 1997). CKC of the tenth passage were infected with AIV and used as non-professional APCs for the stimulation of the CD8+ T lymphocytes. The presence of MHC-I on CKC lines and the absence of MHC-II was confirmed by flow cytometric analysis, using anti-chicken MHC-I or anti-chicken MHC-II R-phycoerythrin conjugated monoclonal antibodies (MAbs) (Southern Biotech, Birmingham, AL) (data not shown).
Inoculation of birds
Three-week-old specific, pathogen-free chickens of the B19/B19 MHC haplotype were inoculated, intramuscularly (i.m.), with 500 μg of cDNA expressing HA alone or NP alone, or of 500 μg of each HA and NP (HN) combined. Control birds were inoculated with either pcDNA 3.1 vector expressing LacZ (LacZ) or PBS. For viral inoculations, B19/B19 chicks were inoculated at 3 weeks of age, intranasally, with 108 ELD50 of the low pathogenic H5N9/Tur/Wis/68 AIV strain.
AIV-specific antibody titrations
Serum samples were prepared from blood collected from the jugular vein of chickens at 3 weeks p.i. to evaluate the humoral responses against AIV HA and NP. Hemagglutination inhibition (HI) assays, according to OIE guideline (http://www.oie.int/eng/normes/mmanual/2008/pdf/2.03.04_AI.pdf), were used to identify antibodies specific to H5N9 virus (A/Turkey/Wis/68) HA. HI mediated by the anti-H5 antibodies against H7N2 AIV was also evaluated. Titers were expressed as geometric mean titers (GMT). Titers of ≤ 1 log2were assigned a titer of 1 log2. NP specific antibodies were determined using the AIV Plus ELISA kit (Synbiotics, Kansas City, MO) as described by the manufacturer.
Effector cell preparation
Effector T lymphocytes used for ex vivo stimulation were prepared from peripheral blood mononuclear cells (PBMC) from 2 to 4 individual chickens per group (Pei et al., 2003). Briefly, blood was collected from the jugular vein at 3, 5, 7 and 9 weeks p.i. and diluted 1:2 in Alsever's solution (Sigma-Aldrich, St. Louis, MO). PBMC were prepared by Ficoll-histopaque (Histopaque-1077, Sigma-Aldrich, St. Louis, MO) density gradient centrifugation (Seo and Collisson, 1997). Viable cells were collected from the interface and washed twice with phosphate buffered saline (PBS, pH 7.4). Cells were resuspended in 3 ml of RPMI 1640 (Invitrogen, La Jolla, CA) supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA), 2 mM l-glutamine, and 0.1 mM MEM non-essential amino acids. B lymphocytes were removed by passing the cell suspension through complete RPMI equilibrated nylon wool column and adherent cells were removed by incubating the cell preparation in 25 cm2 tissue culture flasks as described previously (Seo and Collisson, 1997). Flow cytometric analysis of cells labeled with anti-chicken CD3 FITC MAbs (Southern Biotech, Birmingham, AL) as described previously determined that the lymphocyte population recovered after nylon wool separation and removal of adherent cells was 97% positive for the expression of the T lymphocyte marker, CD3 (Bohls et al., 2006).
Ex vivo stimulation of T lymphocytes
T lymphocytes from PBMC were stimulated ex vivo with MHC B19/B19 (matched) and B2/B2 (mismatched) APCs. APCs at a concentration of 1 × 105 cells/ml in 96-well tissue culture plates were incubated for 8 h at 39 °C, 5% CO2. APCs were infected with 1x105 ELD50 of H5N9 (A/Turkey/Wis/68) virus for 1 h followed by removal of the virus and cells before washing 3 times with DMEM supplemented with 10% FBS. One × 106 T lymphocytes in complete RPMI were added to each well. Cells were co-cultured for 24 h, before the media was collected and centrifuged. The clarified supernatants were used to quantify IFNγ production by activated T lymphocytes using a nitric oxide detection assay. At 5 weeks p.i. the pelleted T lymphocytes were collected for FACS analysis to measure the lymphocyte proliferation. Each ex vivo stimulation assay was conducted in duplicate.
Nitric oxide induction assay
A modified indirect IFNγ assay based on NO production (Crippen et al., 2003, Karaca et al., 1996, Pei et al., 2003) from HD11 cells (a chicken macrophage cell line) was used to quantify the IFNγ secreted from ex vivo activated T lymphocytes by APCs. Briefly, cells were incubated in individual wells of 96-well plates at a concentration of 1 × 105 cells/well in complete RPMI media for 2 h at 39 °C, 5% CO2 prior to the addition of 150 μl supernatants from T lymphocyte-APCs cultures. After 24 h of incubation, the accumulation of nitrite in supernatant from stimulated HD11 cells was measured using the Griess reagent assay according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO). To ensure that the data represent the nitric oxide produced specifically by the IFNγ mediated stimulation of HD11 cells and not due to any other soluble inducing factors, nitrite concentration in each sample was normalized by subtracting the nitrite concentration of supernatants from control APCs cultured without T lymphocytes. The concentration of nitrite produced was determined using sodium nitrite solutions with a concentration of 1 to 20 μmol as standards.
FACS analysis
After ex vivo stimulation with AIV infected APCs, T lymphocytes were collected and dual labeled with phycoerythrin-conjugated MAbs specific for CD44 and fluorescein labeled MAbs specific for either CD8 or CD4 (Southern Biotech Birmingham, AL) as previously described (Seo and Collisson, 1997). Flow cytometric analysis was used to determine the concentration of T lymphocyte subpopulations. A minimum of 104 events were collected for each sample. The percentage of CD44+ lymphocytes expressing either CD4 or CD8 surface antigen was determined using FlowJo™ (TreeStar, Inc., Ashland, OR). Cell proliferation was calculated as the percent increase in the population of CD4+ or CD8+ T lymphocytes cultured with uninfected APCs after in vitro stimulation by virus-infected APCs for 24 h.
Statistical significance of differences
The nitric oxide concentrations were expressed as the average of 4 to 6 birds per group. ANOVA (analysis of variance) with significance of p ≤ 0.05 was used to determine statistical differences.
Acknowledgments
This work was supported by USDA-CSREES-NRI grant 2006-35204-16560 and USDA-AICAP grant 2007-35203-18070. We thank Victoria Hampton, Lisa Griggs, Omar Alvarado and Vinayak Brahmakshatriya for technical assistance. Special thanks to Dr. Twani Crippen for her generous gift of HD11 cells and advice with the assays. We are grateful to Dr. Roger Smith for help with the flow cytometry. We acknowledge Dr. Maisie Dawes, Dr. Ghida Banat, Dr. Miguel Saggese, and Dr. Yvonne Drechsler for their help and advice with the manuscript.
References
- Alexander D.J. A review of avian influenza in different bird species. Vet. Microbiol. 2000;74(1-2):3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- Babakir-Mina M., Dimonte S., Perno C.F., Ciotti M. Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch. Virol. 2009;154(8):1349–1352. doi: 10.1007/s00705-009-0438-1. [DOI] [PubMed] [Google Scholar]
- Beard C.W. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. In: Purchase L.H.A.H.G., Domermuth C.H., Pearson J.E., editors. 3rd ed. Kendall/Hunt Publishing Co; Dubuque, IA: 1989. [Google Scholar]
- Bohls R.L., Smith R., Ferro P.J., Silvy N.J., Li Z., Collisson E.W. The use of flow cytometry to discriminate avian lymphocytes from contaminating thrombocytes. Dev. Comp. Immunol. 2006;30(9):843–850. doi: 10.1016/j.dci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Busch D.H., Pamer E.G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J. Immunol. 1998;160(9):4441–4448. [PubMed] [Google Scholar]
- Cao W., Myers-Powell B.A., Braciale T.J. The weak CD8+ CTL response to an influenza hemagglutinin epitope reflects limited T cell availability. J. Immunol. 1996;157(2):505–511. [PubMed] [Google Scholar]
- Capua I., Alexander D.J. Avian influenza and human health. Acta. Trop. 2002;83(1):1–6. doi: 10.1016/s0001-706x(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Capua I., Marangon S. The use of vaccination as an option for the control of avian influenza. Avian. Pathol. 2003;32(4):335–343. doi: 10.1080/0307945031000121077. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Singh S., Drechsler Y. Evolving vaccine strategies for the continuously evolving avian influenza viruses. CAB Rev: Perspectives in Vet. Med. Agric. Nutr. and Nat. Resources. 2008;3:1–17. [Google Scholar]
- Crippen T.L., Sheffield C.L., He H., Lowry V.K., Kogut M.H. Differential nitric oxide production by chicken immune cells. Dev. Comp. Immunol. 2003;27(6-7):603–610. doi: 10.1016/s0145-305x(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Crowe S.R., Turner S.J., Miller S.C., Roberts A.D., Rappolo R.A., Doherty P.C., Ely K.H., Woodland D.L. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 2003;198(3):399–410. doi: 10.1084/jem.20022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Lins D.C., Mescher M.F. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J. Immunol. 1998;160(7):3236–3243. [PubMed] [Google Scholar]
- Deng Y., Yewdell J.W., Eisenlohr L.C., Bennink J.R. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 1997;158(4):1507–1515. [PubMed] [Google Scholar]
- Doherty P.C., Hou S., Tripp R.A. CD8+ T-cell memory to viruses. Curr. Opin. Immunol. 1994;6(4):545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Munster V., Wallensten A., Bestebroer T.M., Herfst S., Smith D., Rimmelzwaan G.F., Olsen B., Osterhaus A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005;79(5):2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten R.J., Davis C.T., Russell C.A., Shu B., Lindstrom S., Balish A., Sessions W.M., Xu X., Skepner E., Deyde V., Okomo-Adhiambo M., Gubareva L., Barnes J., Smith C.B., Emery S.L., Hillman M.J., Rivailler P., Smagala J., de Graaf M., Burke D.F., Fouchier R.A., Pappas C., Alpuche-Aranda C.M., Lopez-Gatell H., Olivera H., Lopez I., Myers C.A., Faix D., Blair P.J., Yu C., Keene K.M., Dotson P.D., Jr., Boxrud D., Sambol A.R., Abid S.H., St George K., Bannerman T., Moore A.L., Stringer D.J., Blevins P., Demmler-Harrison G.J., Ginsberg M., Kriner P., Waterman S., Smole S., Guevara H.F., Belongia E.A., Clark P.A., Beatrice S.T., Donis R., Katz J., Finelli L., Bridges C.B., Shaw M., Jernigan D.B., Uyeki T.M., Smith D.J., Klimov A.I., Cox N.J. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfrani C., Oseroff C., Sidney J., Chesnut R.W., Sette A. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum. Immunol. 2000;61(5):438–452. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Gotch F., McMichael A., Smith G., Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 1987;165(2):408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J., Cruz J., Ennis F.A. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J. Virol. 1998;72(11):8682–8689. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca K., Kim I.J., Reddy S.K., Sharma J.M. Nitric oxide inducing factor as a measure of antigen and mitogen-specific T cell responses in chickens. J. Immunol. Methods. 1996;192(1-2):97–103. doi: 10.1016/0022-1759(96)00026-9. [DOI] [PubMed] [Google Scholar]
- Kedzierska K., Day E.B., Pi J., Heard S.B., Doherty P.C., Turner S.J., Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J. Immunol. 2006;177(10):6705–6712. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- Krauss S., Obert C.A., Franks J., Walker D., Jones K., Seiler P., Niles L., Pryor S.P., Obenauer J.C., Naeve C.W., Widjaja L., Webby R.J., Webster R.G. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007;3(11):e167. doi: 10.1371/journal.ppat.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz J.H., Bodewes R., van Amerongen G., Kuiken T., Fouchier R.A., Osterhaus A.D., Rimmelzwaan G.F. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25(4):612–620. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Kreijtz J.H., de Mutsert G., van Baalen C.A., Fouchier R.A., Osterhaus A.D., Rimmelzwaan G.F. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J. Virol. 2008;82(11):5161–5166. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J.S., Lee H.J., Lee D.H., Lee Y.J., Mo I.P., Nahm S.S., Kim M.J., Lee J.B., Park S.Y., Choi I.S., Song C.S. Immune responses and pathogenesis in immunocompromised chickens in response to infection with the H9N2 low pathogenic avian influenza virus. Virus Res. 2008;133(2):187–194. doi: 10.1016/j.virusres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Lukacher A.E., Braciale V.L., Braciale T.J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 1984;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J., Gotch F.M., Dongworth D.W., Clark A., Potter C.W. Declining T-cell immunity to influenza, 1977-82. Lancet. 1983;2(8353):762–764. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- McMichael A.J., Gotch F.M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J. Exp. Med. 1986;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J., Michie C.A., Gotch F.M., Smith G.L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J. Gen. Virol. 1986;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Miller M.M., Bacon L.D., Hala K., Hunt H.D., Ewald S.J., Kaufman J., Zoorob R., Briles W.E. 2004 Nomenclature for the chicken major histocompatibility (B and Y) complex. Immunogenetics. 2004;56(4):261–279. doi: 10.1007/s00251-004-0682-1. [DOI] [PubMed] [Google Scholar]
- Olsen B., Munster V.J., Wallensten A., Waldenstrom J., Osterhaus A.D., Fouchier R.A. Global patterns of influenza a virus in wild birds. Science. 2006;312(5772):384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- O'Neill E., Krauss S.L., Riberdy J.M., Webster R.G., Woodland D.L. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 2000;81(Pt. 11):2689–2696. doi: 10.1099/0022-1317-81-11-2689. [DOI] [PubMed] [Google Scholar]
- Pei J., Briles W.E., Collisson E.W. Memory T cells protect chicks from acute infectious bronchitis virus infection. Virology. 2003;306(2):376–384. doi: 10.1016/s0042-6822(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Perdue M.L., Swayne D.E. Public health risk from avian influenza viruses. Avian Dis. 2005;49(3):317–327. doi: 10.1637/7390-060305R.1. [DOI] [PubMed] [Google Scholar]
- Portela A., Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002;83(Pt. 4):723–734. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Fouchier R.A., Osterhaus A.D. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 2007;18(6):529–536. doi: 10.1016/j.copbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71(7):5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Webster R.G. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 2001;75(6):2516–2525. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxyl-terminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71(10):7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269(1):183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Peiris M., Webster R.G. Protective cross-reactive cellular immunity to lethal A/Goose/Guangdong/1/96-like H5N1 influenza virus is correlated with the proportion of pulmonary CD8(+) T cells expressing gamma interferon. J. Virol. 2002;76(10):4886–4890. doi: 10.1128/JVI.76.10.4886-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez D.L., Lee C.W., Swayne D.E. Avian influenza vaccination in North America: strategies and difficulties. Dev. Biol. (Basel) 2006;124:117–124. [PubMed] [Google Scholar]
- Swain S.L., Dutton R.W., Woodland D.L. T cell responses to influenza virus infection: effector and memory cells. Viral. Immunol. 2004;17(2):197–209. doi: 10.1089/0882824041310577. [DOI] [PubMed] [Google Scholar]
- Swayne D.E., Kapczynski D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008;225:314–331. doi: 10.1111/j.1600-065X.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Taubenberger J.K., Reid A.H., Janczewski T.A., Fanning T.G. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356(1416):1829–1839. doi: 10.1098/rstb.2001.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.M., Askonas B.A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Tollis M., Di Trani L. Recent developments in avian influenza research: epidemiology and immunoprophylaxis. Vet. J. 2002;164(3):202–215. doi: 10.1053/tvjl.2002.0716. [DOI] [PubMed] [Google Scholar]
- Toro H., Tang D.C., Suarez D.L., Sylte M.J., Pfeiffer J., Van Kampen K.R. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine. 2007;25(15):2886–2891. doi: 10.1016/j.vaccine.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A.R., McMichael A.J., Carter N.P., Huddleston J.A., Brownlee G.G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Ungchusak K., Auewarakul P., Dowell S.F., Kitphati R., Auwanit W., Puthavathana P., Uiprasertkul M., Boonnak K., Pittayawonganon C., Cox N.J., Zaki S.R., Thawatsupha P., Chittaganpitch M., Khontong R., Simmerman J.M., Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1) N. Engl. J. Med. 2005;352(4):333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- Urabe M., Tanaka T., Tobita K. MDBK cells which survived infection with a mutant of influenza virus A/WSN and subsequently received many passages contained viral M and NS genes in full length in the absence of virus production. Arch. Virol. 1993;130(3-4):457–462. doi: 10.1007/BF01309673. [DOI] [PubMed] [Google Scholar]
- Webby R.J., Webster R.G. Emergence of influenza A viruses. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356(1416):1817–1828. doi: 10.1098/rstb.2001.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Arch. Virol. Suppl. 1997;13:105–113. doi: 10.1007/978-3-7091-6534-8_11. [DOI] [PubMed] [Google Scholar]
- Yewdell J.W., Bennink J.R., Smith G.L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1985;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]