Abstract
Two experiments were conducted to study the effects of lignocellulose supplementation on immune function in layer pullets at different stages of growth. Four-wk-old pullets (Experiment 1) were fed a control, diet (Diet C); Diet C plus 1% mixed soluble/insoluble fiber (Diet MF), or plus 1% insoluble fiber (Diet IF). At 7.5 wk-of-age, heterophil phagocytosis, and oxidative burst in Groups MF (328.5 beads/100 cells; 4,330.0 ΔRFU; relative fluorescent units) and IF (350.3; 5,264.4) were greater (P < 0.05) than Controls (303.4; 3,509.0). At 8 wk-of-age, Group MF and IF relative weights of bursa of Fabricius (0.57 g/100 g BW; 0.58 g /100 g BW), thymus glands (0.77; 0.78), and areas of Peyer's patches (PP) (2.7 cm2; 2.9 cm2) were higher (P < 0.05) than Controls (bursa, 0.50 g; thymus, 0.70 g; PP area, 1.8 cm2). In Experiment 2, 10-wk-old pullets were fed a control diet or diets containing 1.5% MF or IF for 8 wk. At 14 wk-of-age IF pullets had higher (P < 0.05) heterophil phagocytosis efficiency (447.9 beads/100 cells) than Controls (376.4) and MF and IF had greater (P < 0.05) oxidative burst (1,302.9 and 1,857.7 ΔRFU) than Controls (744.1). At 17 wk-of-age MF and IF had increased (P < 0.05) proliferation of T-lymphocytes (ConcanavalinA-stimulated) (100.4 and 103.1% of unstimulated cells) and B-lymphocytes (lipopolysaccharide-stimulated) (122.4 and 129.0) than Controls (ConA, 79.4; lipopolysaccharide, 106.6). At 18 wk-of-age, IF pullets were heavier (1,607.5 g, P < 0.05) than Controls (1,506.5 g), had heavier (P < 0.05) bursa of Fabricius (1.12 g) than MF and Control groups (0.98 g; 0.92 g) and cecal tonsils of MF (0.38 g) and IF (0.39 g) weighted more (P < 0.05) than Controls (0.33 g). Number of jejunal and ileal PP (10.0) in IF pullets was higher (P < 0.05) than Controls (7.1). These results indicate that both MF and IF can improve development of the immune system of young and grower pullets during periods of maturation and involution of lymphoid organs.
Keywords: layer pullet, fiber, heterophil function, lymphocyte proliferation, Peyer's patches
INTRODUCTION
Body weight at the onset of egg production and uniformity in the growing phase are important in determining the quality of point of lay pullets and profitability during the laying period (Lacin et al., 2008). However, growth of pullets can be reduced by infectious diseases (Yegani and Korver, 2008) and all ages and physiological stages require a strong, functional immune system to protect against pathogens. At hatch, the immune system is not well developed and it matures with age: the lymphoid organs and tissues increase in size reaching their maxima before or soon after pullets become sexually mature (Ciriaco et al., 2003; Rodríguez-Méndez et al., 2010; Oláh et al., 2014). However, there is variation in the maximum sizes and rates of development and involution of the immune organs among different strains and breeds of poultry (Zhang et al., 2006).
The innate and acquired immune systems protect poultry from invading pathogens (Genovese et al., 2013). However, the effectiveness of the immune system can vary depending on age, physiological state, and the virulence of pathogens. Prophylactic in-feed antibiotics or antibiotic growth promoters have until recently been used for improvement of growth and health status (Dibner et al., 2007; Chowdhury et al., 2009). However, the European Union prohibited the use of antibiotics as non-therapeutic growth promoters for poultry in 2006 and in other countries their use has been restricted (Laxminarayan et al., 2015). As a result, efforts have been focused on finding alternative growth promoters and immunomodulators (Mateos et al., 2002).
Prebiotics and dietary fibers have been shown to influence the growth, health, and various immune responses of poultry (Siri et al., 1992; Lowry et al., 2005). Such research has focused more commonly on meat-type chickens (Guo et al., 2003; Huang et al., 2007; Sadeghi et al., 2015) rather than slower growing, layer strains of poultry. Dietary fiber intakes can be increased by selective incorporation of high fiber feed ingredients into poultry diets or by supplementation with more purified products.
Not many studies have compared the effects of the more purified lignocellulose fiber sources of insoluble fiber and mixtures of soluble (SF) and insoluble (IF) dietary fibers on the immune function of grower layer pullets after the post-hatch period and up to the point of lay. During this period the immune system and organs mature and lymphoid organ involution occurs (Ciriaco et al., 2003; Rodrigues-Mendez et al., 2010; Oláh et al., 2014). Therefore, two experiments were carried out with Hy-Line Brown layer pullets. In the first experiment the effect of two different commercial lignocellulose fiber supplements, a mixed soluble/insoluble fiber (MF) product and an IF product were used to determine their separate effects on the growth and immune function of young pullets when the immune system and organs are still developing. In the second experiment, the effects of the 2 fiber sources on growth and immune function were determined in older pullets up to point of lay when involution of lymphoid organs is starting to occur.
MATERIALS AND METHODS
Birds, Diets, and Husbandry
For each experiment healthy, vaccinated pullets were randomly selected from a single house on a grower farm. Different farms supplied the pullets used for Experiments 1 and 2.
In Experiment 1, four-wk-old Hy-Line Brown pullets obtained from a local commercial pullet grower farm in Victoria, Australia, were kept in a house containing 9 pens, 0.9 × 1.8 × 1.8 m, width × length × height, with slatted floors. The ambient temperature in the house was 20 to 23°C and light period was 12 h from 07:30 to 19:30. Three experimental diets were used: Control diet (Diet C) − from 4 to 6 wk-of-age an un-supplemented commercial starter diet was used as the control diet and from 6 to 8 wk-of-age a grower pullet diet (Table 1, Ridley AgriProducts Pty Ltd., Pakenham, 3810, Australia), Diet MF − the Control diets supplemented with 1% MF (OptiCell C5, Agromed Austria GmbH, Kremsmünster, Austria containing (manufacturer's analysis) ∼59% crude fiber, ∼85% mixed soluble and insoluble fiber, ∼30% lignin), and Diet IF − the Control diets supplemented with 1% IF (Arbocel RC Fine, JRS Co. Inc., Rosenberg, Germany containing (manufacturer's analysis) 65 to 70% crude fiber high in insoluble cellulose and >20% lignin). Fresh water was available at all times.
Table 1.
Chemical composition (%) of starter and grower diets used in Experiment 1 and grower diet used in Experiment 2 used as the control experimental diet (manufacture's information1).
| Experiment 1 | Experiment 2 | ||
|---|---|---|---|
| Chemical Composition2 | Starter | Grower | Grower |
| Dry matter | 89.7 | 89.4 | 86.0 |
| Moisture | 10.3 | 10.6 | 14.0 |
| Protein | 20.5 | 18.0 | 15.5 |
| Fat | 5.0 | 3.0 | 2.5 |
| Maximum fiber3 | 3.7 | 4.4 | 8.0 |
| ME kcal/kg | 2,943 | 2,796 | 2,700 |
| Calcium | 1.0 | 1.0 | 1.0 |
| Phosphorus | 0.64 | 0.56 | 0.64 |
| Available Phosphorus | 0.5 | 0.40 | 0.5 |
1Ridley AgriProducts Pty Ltd., Pakenham, 3810, Australia.
2Feed analysis and ingredients were obtained from manufacturer but for confidentiality reasons the quantities used were not able to be obtained.
Ingredients: wheat, barley, field peas, meat and bone meal, beef tallow, canola meal expeller, soybean meal, canola seed, Millrun mix, oat hulls, limestone, MDCP, salt, sodium bicarb, choline chloride, methionine, lysine, vitamin and mineral premix, phytase and xylanase.
3Analyzed crude fiber – Experiment 1 starter diet 3% and grower diet 4.36%; Experiment 2 grower diet 4.2% (FeedTest, Agrifood Technology Pty Ltd, Werribee, Vic., Australia).
Crude fiber concentrations analyzed by FeedTest (Agrifood Technology Pty Ltd., Werribee, Victoria, Australia) in the 3 starter diets were Control 3%, MF 3.8%, IF 3.6% and in the three grower diets were Control 4.36%, MF 4.94%, IF 4.99%.
In Experiment 2, Hy-Line Brown layer grower pullets aged 10 wk obtained from a commercial supplier in Victoria, Australia, were kept in a house containing 18 pens as in Experiment 1. Temperature and light interval were the same as Experiment 1. The 3 experimental diets were: Control diet a commercial grower diet (Table 1, Ridley AgriProducts Pty Ltd., Pakenham, 3810, Australia) with no fiber added (C diet), the MF diet − the Control diet supplemented with 1.5% MF (OptiCell C5), and the IF diet − the Control diet supplemented with 1.5% IF (Arbocel RC Fine). Fresh water was available at all times.
The crude fiber concentration in the 3 diets were Control 4.2%, MF 5.1%, IF 5.2% (FeedTest, Agrifood Technology Pty Ltd., Victoria, Australia).
Experimental Design and Sample Collection
Experiment 1.
On arrival pullets were again checked for abnormalities and signs of ill-health, leg-banded, weighed, and 36 pullets randomly placed in 9 pens, 4 pullets/pen and 3 pens/treatment. Pen treatments were randomly distributed within the house. After 3.5 wk on the diets 8 pullets/treatment were randomly taken, 2 to 3 pullets/pen, and 1 to 2 mL blood samples taken from the brachial vein and immediately transferred from the syringe into sterile EDTA coated tubes (Vacuette, Greiner Bio-One, Thailand Ltd) and kept on ice. Heterophils isolated from blood were used to assess innate immune function.
After 4 wk on the diets, all pullets (12/treatment) were weighed and killed with an intravenous overdose of pentobarbitone sodium solution (Lethabarb, Virbac Animal Health, Milperra, NSW, Australia, containing 325 mg/mL of pentobarbital sodium). From each pullet the bursa of Fabricius, all lobes of both left and right thymus glands and spleen were collected and weighed. The small intestines (8 samples/treatment), from the start of the jejunum to the junction with the ceca, were washed with 0.9% normal saline to remove digesta and stored at −20°C for later staining to visualize Peyer's patches (PP).
Experiment 2.
On the day after arrival, 54 Hy-Line Brown pullets were health checked, leg-banded, weighed, and randomly allocated to 3 treatments of 3 pullets/pen, 6 pens/treatment. Treatment pens were randomized within the house. Four wk after placing the pullets on the experimental diets, 1 to 2 mL blood samples were taken from 8 pullets/treatment (1 to 2 pullets/pen), kept on ice and were used for isolation of heterophils and the assessment of innate immune function by phagocytosis and oxidative burst assays.
Seven weeks after putting pullets on the experimental diets, 2.5 to 3 mL blood samples were obtained from 8 pullets/treatment (1 to 2 pullets/pen) and immediately transferred in as sterile a manner as possible into sterile EDTA coated Vacuette tubes and kept on ice for isolation of lymphocytes and the assessment of cell mediated immune function by a lymphocyte proliferation assay. At 8 wk after starting the experiment, all pullets (18 pullets/treatment) were weighed, killed, and lymphoid organs and tissues including cecal tonsils were collected from each pullet and weighed as in Experiment 1. Samples of small intestines (8 samples/treatment), from the start of the jejunum to the junction with the ceca, were collected and stored as in Experiment 1 for PP measurements. Tissues samples from the middle of the jejunum of 5 pullets from each treatment were taken and stored at −20oC in RNAlater (Cat. No. R0901, Sigma-Aldrich, Castle Hill, NSW, 1765, Australia) for measurement of mRNA expression of the gene MUC2.
The experimental procedures were approved by Animal Ethics Committee at La Trobe University under the approved numbers LTU AEC12-68 (Experiment 1) and LTU AEC14-16 (Experiment 2).
Methods of Analysis of Tissue Samples
Peyer's Patches.
After opening the jejunum and ileum longitudinally, they were soaked in 5% acetic acid for 24 h, washed with tap water, stained with 0.5% polychrome methylene blue (Amber Scientific, Midvale, WA, Australia) and rinsed in tap water (Cornes, 1965). Whole intestines were photographed and the images examined with AutoCAD computer software (Autodesk AutoCAD Design Suite, 2014, San Rafael, CA). The numbers of PP (of areas greater than 1 mm) were counted and surface areas of PP and the combined areas of jejunum and ileum were measured. For each sample, the relative area of PP was calculated as total area of PP as a percentage of the area of the jejunum and ileum.
Jejunal MUC2 mRNA Expression.
Total mRNA was extracted from jejunal tissues with TRIzol reagent (Invitrogen, ThermoFisher Scientific, Scoresby, Victoria, Australia), further purified with Ambion 10× TURBO DNase Buffer (Catalog No. AM 1097, ThermoFisher Scientific) and cDNA synthesized with a DyNAmo cDNA synthesis kit (Cat. No. F-470 L, ThermoFisher Scientific) (Yokhana et al., 2016). A thermal cycler (Stratagene Mx3000P QPCR System, Agilent Technologies, Mulgrave, Victoria, Australia), was used to convert the mRNA into cDNA. Primers for target and reference genes were β-Actin (NM_205518) forward ATGGCTCCGGTATGTGCAA, reverse TGTCTTTCTGGCCCATACCAA (Grommen et al., 2008); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward GCCATCACAGCCACACAGA and reverse TTTCCCCACAGCCTTAGCA (Azzam et al., 2011). MUC2 forward TCACCCTGCATGGATACTTGCTCA and reverse TGTCCATCTGCCTGAATGACAGGT (Azzam et al., 2011). Simplex real-time qPCR analysis (RT-qPCR) was conducted using SensiMix SYBR Low-ROX kit (Cat. No. QT625-02, Bioline Australia Pty. Ltd., Redfern, NSW, Austraila) in a Stratagene Mx3000P QPCR System (Agilent Technologies). Agarose gel electrophoresis was carried out to confirm the purity and size of the bands of the PCR products (Qiagen-Australia, Chadstone, Victoria, Australia). Efficiency of PCR was determined for both the target and endogenous control genes from the standard curves generated with different dilutions of the cDNA synthesized from the chicken jejunum using the formula PCR Efficiency = 10 (−1/slope). The slope values were calculated from standard curves and used in the following formula (Harrison et al., 2007; Gopinath et al., 2011) to obtain the corrected cycle time (Corrected Ct):
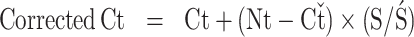 |
where Ct is the mean cycle time of the target gene Ct, Nt is the mean Ct of housekeeping genes of all the experimental samples, Cť is the mean Ct of housekeeping gene of the particular sample, S is the target gene slope, and Ś is the endogenous control slope.
Isolation and Analysis of Heterophils
Isolation.
Heterophils were isolated using a modification of the discontinuous gradient method described by Andreasen and Latimer (1989). Briefly, 1 mL of uncoagulated blood was diluted in a 1:1 ratio with RPMI-1640 medium (product number: R8755, Sigma-Aldrich) and this mixture was layered on top of a discontinuous gradient of 3 mL Histopaque-1077 (product number: 10771, Sigma-Aldrich) over 3 mL of Histopaque-1119 (product number: 11191, Sigma-Aldrich). Centrifugation, washing of the samples, and hypotonic removal of erythrocytes were similar to those described by Andreasen and Latimer (1989). The final pellet was suspended in 1 mL RPMI-1640 medium and viability of cells was determined using the trypan blue (product number: T8154, Sigma-Aldrich) dye exclusion method (Farnell et al., 2006). The concentration was adjusted to 5 × 105 heterophils/mL RPMI-1640 for phagocytosis and 2 × 106 heterophils/mL RPMI-1640 for oxidative burst assays which were both carried out on the same day. For each batch of heterophils isolated, smears were made, stained, and examined under light microscopy to check on the purity of the samples. Purity of heterophil samples was greater than 95%. At all stages from collection of blood samples to completion of isolation, samples were kept on ice and ice cold reagents used.
Phagocytosis.
The protocol for ring doves (Rodríguez et al., 1999; Paredes et al., 2007) with slight modification was used. Heterophils and latex beads, polystyrene (1.1 μm mean particle size, product number: LB11, Sigma-Aldrich) were placed, in duplicate, in sterile cover glass slides (Lab-Tek II CC2 four-chamber slides, ThermoFisher Scientific) and incubated for 30 min in a humidified incubator (Galaxy S Series, HD Scientific Supplies Pty Ltd, Wetherill Park, NSW, 2164, Australia) at 37°C in the presence of 5% CO2 atmosphere. After washing to remove excess beads, the cells on the slides were fixed with absolute methanol, dried, and stained using Wright's stain (Amber Scientific, Midvale, WA, Australia). Two methods for expressing phagocytosis were used. In the first, phagocytosis index, was calculated as the total number of latex beads phagocytized by the first 100 heterophils counted. For the second method, phagocytosis percentage (%P) was calculated as the percentage of heterophils that phagocytized at least one latex bead. Phagocytosis efficiency − the ratio PI:%P which indicates the overall efficiency of phagocytosis by heterophils was then calculated.
Oxidative Burst Assay.
A modification of the methods of Wan et al. (1993) and He et al. (2003) was used. Production of reactive oxygen species by heterophils was measured by oxidation of 2΄, 7΄-dichlorofluorescin diacetate (product number: D6883, Sigma-Aldrich) to fluorescent 2΄, 7΄-dichlorofluorescein after incubation for 1 h, with or without the agonist phorbol 12-myristate 13-acetate (PMA, product number: P8139, Sigma-Aldrich), in the humidified incubator used above. The dichlorofluorescein produced, expressed in relative fluorescent units (RFU), was determined using a fluorescence microplate reader (EnSpire, PerkinElmer Co, Waltham, MA) at wavelengths of 485 nm excitation and 530 nm emission. The results were then calculated as ΔRFU: the change in RFU of PMA stimulated cells when compared to the RFU of non-stimulated cells (He et al., 2003).
Isolation of Lymphocytes and Measurement of Proliferation
Isolation.
Lymphocytes were separated from peripheral blood using Histopaque-1.077 (Lavoie and Grasman, 2005; Mabuchi, 2014). All procedures were undertaken in a sterile environment using sterile reagents and equipment. Briefly, blood samples were diluted 1:1 with Dulbecco's phosphate buffered saline (DPBS (1×), Gibco, ThermoFisher Scientific) and gently pipetted over 2 mL Histopaque-1077. After centrifuging at 650 × g for 25 min at room temperature, the mononuclear cell layer (lymphocytes) was collected, washed with DPBS, centrifuged at 270 × g for 4 min at 4°C and the lymphocyte pellet suspended in DPBS. Viability of isolated cells was determined by trypan blue exclusion and the concentration adjusted to 5 × 106 cells/mL DPBS for measurement of proliferation, on the same day. In order to reduce contamination of isolated cell preparations with thrombocytes blood was diluted prior to layering on Histopaque and a slow centrifugation speed was used as recommended by Lavoie and Grasman (2005). At all stages from collection of blood samples to completion of isolation, samples were kept on ice and ice cold reagents used.
Lymphocyte Proliferation Assay.
Proliferation of lymphocytes was measured using 3 mitogens (Gogal et al., 1997; Lavoie and Grasman, 2005; Mabuchi, 2014). Concanavalin A (ConA, 2.5 μg/mL) from Canavalia ensiformis (Type: IV-S, product no. C5275, Sigma-Aldrich) was used as a T lymphocyte agonist, lipopolysaccharide (LPS, 3.125 μg/mL) from Escherichia coli 055:B5 (product no. L6529, Sigma-Aldrich) as a B lymphocyte stimulating agonist, and PMA (1.25 μg/mL) as T and B lymphocyte stimulating agonist (Palacios et al., 2007). After incubating lymphocytes for 2.5 d at 37°C, alamarBlue (BUF012B, AbD Serotec, Oxford, UK) was added and 8 h later, following its reduction by released cell metabolites (Zhi-Jun et al., 1997), absorbance was measured at wavelengths of 570 nm and 600 nm. Proliferation was expressed as a percentage increase of mitogen stimulated lymphocytes relative to non-stimulated lymphocytes.
Statistical Analyses
In both experiments there was no significant difference between mean BW within the same treatment on a pen basis (3 pens/treatment, Experiment 1; 6 pens/treatment, Experiment 2), therefore all data were analyzed on an individual pullet basis. All data for Experiments 1 and 2 were tested for homogeneity of variances and normality using IBM SPSS Statistics, Version 23.0 for Windows, Corporation, Armonk, NY). Data of both experiments were analyzed by one-way analysis of variance (ANOVA) and the treatment means were compared with the Turkey's multiple range test. The data of relative weight of bursa of Fabricius in Experiment 1 were analyzed by a non-parametric ANOVA and the treatment means were compared with the Mann–Whitney U test (Gibbons and Chakraborti, 2011) using (SPSS 23.0 for Windows).
RESULTS AND DISCUSSION
Live Body Weight
In Experiment 1, the 4 wk old pullets given diets MF or IF for 4 wk were heavier (P < 0.05) than pullets given the un-supplemented Control diet (Table 2). The mean BW of Control and MF pullets were within the normal ranges for Hy-Line Brown pullets, however, that for IF pullets was above the upper weight for the range (Hy-Line International, 2014). In Experiment 2, after feeding diets for 8 wk (18 wk-of-age), live BW of pullets fed diet IF were heavier (P < 0.05) than Controls (Table 3). Again, the mean BW of pullets in both Control and MF treatment groups (Table 3) were within the normal range for the strain and that of the IF pullets was above the upper weight (Hy-Line International, 2014).
Table 2.
Experiment 1. Live body weights (g), weights of immune organs, and measurements of jejunal and ileal area and Peyer's patches (PP) and concentration of MUC2 mRNA expression of 8 wk old Hy-Line Brown layer strain pullets given diets containing different types of fiber for 4 wk (Mean, pooled SEM, n = 12 for live body and immune organ weights, n = 8 for jejunal and ileal area and PP measurements, n = 5 for mRNA expression of MUC2).
| Treatment | C1 | MF2 | IF3 | Pooled SEM |
|---|---|---|---|---|
| Initial body weight† | 350.5 | 357.8 | 351.9 | 2.41 |
| Body weight (8 wk age)† | 647.9a | 696.3b | 717.5b | 8.5 |
| Absolute weights (g) | ||||
| Bursa of Fabricius | 3.2a | 4.0b | 4.1b | 0.1 |
| Thymus glands | 4.6a | 5.4b | 5.6b | 0.1 |
| Spleen | 2.6a | 2.9a | 3.4b | 0.1 |
| Relative weights (%) | ||||
| Bursa of Fabricius | 0.50a | 0.57b | 0.58b | 0.01 |
| Thymus glands | 0.70a | 0.77b | 0.78b | 0.01 |
| Spleen | 0.40a | 0.42a,b | 0.47b | 0.01 |
| Jejunum + ileum area (cm2) | 109.6a | 119.2a,b | 125.3b | 2.7 |
| Number of PP | 8.9a | 10.9a,b | 11.6b | 0.5 |
| Area of PP (cm2) | 1.8a | 2.7b | 2.9b | 0.1 |
| relative area of PP (% of area of jejunum + ileum) | 1.6a | 2.2b | 2.4b | 0.1 |
| MUC2 mRNA expression | 14.6a | 15.5a,b | 17.9b | 0.6 |
a,bMeans within the same row with different superscripts differ significantly (P < 0.05).
1= control diet (no added MF or IF).
2= mixed soluble and insoluble fiber (MF) diet containing 1% OptiCell C5.
3= insoluble fiber (IF) diet containing 1% Arbocel RC fine.
†Standard range of body weights at 4 wk (initial body weight) = 257 to 273 g and at 8 wk = 650 to 690 g (Hy-Line International, 2014).
Table 3.
Experiment 2. Live body weights (g), weights of immune organs, and measurements of jejunal and ileal area and Peyer's patches (PP) of 18 wk old Hy-Line Brown layer strain pullets given diets containing different types of fiber for 8 wk (Mean, pooled SEM, n = 18 for live body and immune organ weights, n = 8 for jejunal and ileal area and PP measurements).
| Treatment | C1 | MF2 | IF3 | Pooled SEM |
|---|---|---|---|---|
| Initial body weight† | 924.8 | 930.3 | 929.2 | 8.4 |
| Body weight (18 wk age)† | 1,506.5a | 1,552.6a,b | 1,607.5b | 11.1 |
| Absolute weights (g) | ||||
| Bursa of Fabricius | 0.92a | 0.98a | 1.12b | 0.02 |
| Thymus glands | 3.9 | 4.1 | 4.3 | 0.1 |
| Spleen | 3.2 | 3.3 | 3.5 | 0.1 |
| Cecal tonsils | 0.33a | 0.38b | 0.39b | 0.01 |
| Relative weights (%) | ||||
| Bursa of Fabricius | 0.06 | 0.06 | 0.07 | 0.002 |
| Thymus glands | 0.26 | 0.26 | 0.27 | 0.007 |
| Spleen | 0.21 | 0.22 | 0.22 | 0.003 |
| Cecal tonsils | 0.02 | 0.02 | 0.03 | 0.001 |
| Jejunum + ileum) area (cm2) | 180.1a,b | 199.9b | 176.2a | 3.8 |
| Number of PP | 7.1a | 8.0a,b | 10.0b | 0.5 |
| Area of PP (cm2) | 2.8a | 3.5a,b | 4.2b | 0.2 |
| relative area of PP (% of area of jejunum + ileum) | 1.6a | 1.8a | 2.4b | 0.1 |
a,bMeans within the same row with different superscripts differ significantly (P < 0.05).
1= control diet (no added MF or IF).
2= mixed soluble and insoluble fiber (MF) diet containing 1.5% OptiCell C5.
3= insoluble fiber (IF) diet containing 1.5% Arbocel RC fine.
†Standard range of body weights at 10 wk (initial body weight) = 863 to 917 g and at 18 wk = 1470 to 1570 g (Hy-Line International, 2014).
In a previous study, Yokhana et al. (2016) showed that supplementing the diet of layer pullets aged 8 to 18 wk with a lower concentration (1 g/100 g diet) of the same IF product as used here (Arbocel RC Fine), did not result in an increase in live BW either after 5 or 10 wk of feeding the supplemented diet. Thus, the age at which dietary supplements are given and the dietary fiber concentration and chemical composition can be important if BW is to be improved. Other researchers have found that fiber supplements can improve BW gain in layer strain chickens. Guzmán et al. (2015) found, in younger layer pullets than used here (from hatching to 5 wk-of-age), that inclusion in the diet of 2 or 4% cereal straw as IF or sugar beet pulp as MF, slightly increased average daily gain. Incharoen and Maneechote (2013) showed that BW of similar aged (4 to 8 wk) layer pullets of a different strain, H and N Brown Nick, was significantly increased as a result of including 3 or 6% whole rice hulls as IF in the feed. Fiber has also been shown to have beneficial effects on weight gain of meat-type poultry (eg Bogusławska-Tryk, 2005; González-Alvarado et al., 2010; Sarikhan et al., 2010).
Lymphoid Organs and Tissues
In both experiments increases in weights were observed in primary and secondary lymphoid organs (Tables 2 and 3). Feeding the MF and IF diets to pullets for 4 wk in Experiment 1 resulted in an increase (P < 0.05) in the absolute and relative weights of the primary lymphoid organs (the bursa of Fabricius and thymus glands) compared to Controls. However, for the spleen, a secondary lymphoid organ, only weights in pullets in the IF group were higher (P < 0.05) than those in Control and MF groups: for relative splenic weights, those of IF pullets were higher (P < 0.05) only when compared to Control pullets (Table 2). The development of the spleen can be influenced by both the bursa (Glick and Dreesen, 1967; Heller and Perek, 1973) and the thymus glands (Hoshi and Mori, 1973) and it is possible that the larger size in the young pullets in Experiment 1 could be associated with the significantly larger bursal and thymus weights (Table 2). The lower weights and relative weights of bursa and thymus at 18 wk-of-age (Experiment 2, Table 3), compared with those at 8 wk (Experiments 1, Table 2), are consistent with published patterns of growth and involution of lymphoid glands (Hedge et al., 1982; Oláh et al., 2014).
Despite the growth rate of the bursa of Fabricius being reported to slow at about 6 to 8 wk-of-age and involution to have started by about 18 wk (Ciriaco et al., 2003; Rodríguez-Méndez et al., 2010; Oláh et al., 2014), supplementing the diets of 8 wk old pullets for 10 wk with IF (Experiment 2) resulted in heavier (P < 0.05) bursa at 18 wk-of-age than in Controls and MF supplemented pullets (Table 3). A slowing of growth rate and involution at about 12 to 16 wk-of-age occurs in the thymus gland (Ciriaco et al., 2003; Oláh et al., 2014) and in the older pullets in Experiment 2, the thymus glands in Group IF were not significantly heavier than those of Control or MF group pullets (Table 3). Thus supplementing diets of younger pullets with IF and MF during the growth period of immune organs can increase primary lymphoid organ weight and, in older pullets, IF may reduce the rate of involution of the bursa but does not appear to affect the thymus glands. The effects on lymphoid organs may help to improve lifetime immunity in laying hens.
The effects of fiber supplementation on lymphoid organ weights have been reported in young broilers up to about 7 wk-of-age (Guo et al., 2003; Dong et al., 2007; Zhang et al., 2008; Akhtar et al., 2012) and ducks (Shi-bin and Hong, 2012) but there have not been any reports in older layer poultry such as used here. However, the responses recorded are not consistent across experiments. Zhang et al. (2008) reported that supplementing the diet of broiler chickens with IF as β-1,3/1,6-glucan, significantly increased the relative weights of the thymus and spleen at 3 and 6 wk-of-age when compared with control chickens. Guo et al. (2003) reported that in younger broiler chicks given β-1,3/1,6-glucan from day old to 2 wk-of-age there was an increase in relative weights of the bursa of Fabricius and spleen but not thymus glands. Addition of wheat bran arabinoxylan, mainly composed of IF, from 1 to 49 days-of-age significantly increased thymus gland weight (Akhtar et al., 2012).
Cecal tonsils form the largest mass of secondary lymphoid tissues in the intestinal tract (Gómez del Morel et al., 1998) and are located in the lumen of the ceca near the ileo-cecal junction. In Experiment 2, the absolute weights of the cecal tonsils (Table 3) were heavier (P < 0.05) in the 18 wk old MF and IF pullets than those of the Controls but relative weights though heavier in MF and IF Groups than the Controls, were not significantly different. Rezaian and Hamedi (2007) observed that in White Leghorn pullets, the cecal tonsil weights peaked in size at about 20 wk-of-age. The development and size of the cecal tonsils is influenced by the bursa of Fabricius and the thymus (Hoshi and Mori, 1973; Befus et al., 1980; Gómez del Morel et al., 1998) and microbial antigens in the gastrointestinal tract (GIT) are needed for their development (Hedge et al., 1982; Jeurissen et al., 1989; Friedman et al., 2003). It is possible therefore that changes in microbial numbers or species within the lumen of the gut as a result of feeding MF or IF, also contributed to influence the size of the cecal tonsils.
In addition to cecal tonsils, the GIT also contains other lymphoid tissues such as PP, discrete lymphoid tissue aggregates found mainly in the small intestine (Jeurissen et al., 1989; Oláh et al., 2014). After hatching, the PP increase in size and number up to about 12 wk-of-age then they decrease in number and size as poultry age (Befus et al., 1980). In Experiment 1 (Table 2) the total number of PP in the jejunum plus ileum of the IF Group was greater (P < 0.05) than in the Control Group and, although there were more PP in pullets of Group IF than MF and Group MF than Control, the differences were not significant. Total areas of PP and relative areas were greater (P < 0.05) in Groups MF and IF than Controls but the difference between MF and IF Groups was not significant (Table 2). In Experiment 2 the number of PP in the jejunum plus ileum was higher (P < 0.05) in Group IF pullets compared to the Control Group (Table 3). The total area of PP and relative areas were greater (P < 0.05) between IF and Control Groups and although areas were greater in IF than MF and MF than Controls, the differences were not significant.
The increase in size or number of PP in both Experiments (Tables 2 and 3) using diets supplemented with IF at 1% or 1.5% of the diet suggests there could be an improvement in gut immunity from some types of fiber supplements. The positive contribution of IF on the growth of PP could have resulted from the mechanical effects of the fiber on intestinal walls, the production of fermentable substrates such as fructo-oligosaccharides (FOS) through microbial action; reductions in pathogens or changes in the microflora population and increases in numbers of beneficial bacteria in the gut (Cao et al., 2003; Friedman et al., 2003; McReynolds et al., 2009). The positive effects of dietary fibers on the growth and development of PP seen in both of these experiments support the reports of a significant increase in numbers of intestinal lymphoid nodules in CS7BL/6J-Min/+ mice (Min mice) given FOS (Pierre et al., 1997) and size of the PP in BALB/c mice given diets supplemented with FOS (Hosono et al., 2003).
Besides the cecal tonsils and PP providing protection in poultry against pathogens entering the gut, goblet cells in the mucosal layer produce mucins that have antimicrobial functions (Dibner et al., 2007; Forder et al., 2007). Activity of the gene MUC2 is important for maintaining the gel layer on the mucosal surface of the intestines and in preventing pathogens from infecting an animal (Johansson et al., 2013). In Experiment 1, MUC2 mRNA expression was higher (P < 0.05) in the jejunal tissue of IF pullets than in the other 2 groups (Table 2). This increase, plus the increase (P < 0.05) in area of the jejunum and ileum (Table 2), suggests that IF supplementation might be able to improve the amount of mucin2 produced, either through increasing the numbers of goblet cells or by increasing the production by each cell. Thus a more effective antimicrobial gel barrier might be produced in the small intestine by supplementing diets with IF. Diet composition has been shown to alter the chemical composition of mucins produced and the number of goblet cells (Dibner et al., 2007; Cheled-Shoval et al., 2014) and the IF, β-glucan, has been shown to increase mucin2 production (Cox et al., 2010) as have dietary mannan-oligosaccharides (Cheled-Shoval et al., 2014).
Heterophil Functions
The heterophil functions of phagocytosis and oxidative burst were both improved by fiber supplementation. In Experiment 1 after 3.5 wk on the experimental diets, the phagocytosis index was greater (P < 0.05) in the pullets in Group IF than Controls (Table 4). The higher %P of Group IF than MF and Control pullets were not significant (Table 4). Phagocytosis efficiency was higher (P < 0.05) in heterophils of Groups MF and IF pullets compared to Controls (Table 4). In Experiment 2, 4 wk after the start of feeding the experimental diets (14 wk-of-age), the phagocytosis index of heterophils (Table 4) of pullets given diet IF was higher (P < 0.05) than that of pullets given the Control diet and only the heterophils of pullets fed the IF supplemented diet were more efficient (P < 0.05) at phagocytizing latex beads than pullets of the Control Group (phagocytosis efficiency, Table 4).
Table 4.
Heterophil phagocytosis and oxidative burst1 in 7.5 wk old Hy-Line Brown pullets fed for 3.5 wk (Experiment 1) or 14 wk old pullets fed for 4 wk (Experiment 2) on diets containing different types of fiber (Mean, pooled SEM, n = 8).
| Treatment | C2 | MF3 | IF4 | Pooled SEM |
|---|---|---|---|---|
| Experiment 1 | ||||
| PI (latex beads/100 cells) | 303.4a | 328.5a,b | 350.3b | 7.0 |
| %P (% cells containing 1 or more beads) | 81.4 | 82.1 | 83.8 | 1.0 |
| PE (PI:%P) | 3.7a | 4.0b | 4.2b | 0.1 |
| Oxidative burst (ΔRFU)5 | 3,509.0a | 4,330.0b | 5,264.4c | 184.8 |
| Experiment 2 | ||||
| PI (latex beads/100 cells) | 376.4a | 403.5a,b | 447.9b | 10.8 |
| %P (% cells containing 1 or more beads) | 75.4 | 79.4 | 82.3 | 1.5 |
| PE (PI:%P) | 5.0a | 5.1a,b | 5.5b | 0.1 |
| Oxidative burst (ΔRFU)5 | 744.1a | 1,302.9b | 1,857.7b | 135.6 |
a–cMeans within the same row with different superscripts differ significantly (P < 0.05).
1After 1 h incubation with phorbol 12-myristate 13-acetate.
2Control diet - see Table 1 for diets used in Experiment 1 and Experiment 2.
3MF mixed soluble and insoluble fiber Control diet plus 1.0% (Experiment 1) or 1.5% (Experiment 2) OptiCell C5.
4IF insoluble fiber diet Control diet plus 1.0% (Experiment 1) or 1.5% (Experiment 2) Arbocel RC fine.
PE, phagocytosis efficiency; PI, phagocytosis index; %P, phagocytosis percentage.
5ΔRFU Change in relative fluorescent unit, see methods for calculation.
Heterophil oxidative burst activity in young pullets in Experiment 1 was greater (P < 0.05) in both MF and IF Groups after 1 h incubation with PMA compared to the Control Group and higher (P < 0.05) in Group IF pullets than in Group MF pullets (Table 4). In Experiment 2, oxidative burst was higher (P < 0.05) in pullets given the MF or IF diets than those fed the Control diet (Table 4). This improvement in PMA stimulated oxidative burst (P < 0.05) in both Group MF and IF pullets in both experiments (Table 4) suggests that the ability of poultry to destroy phagocytized bacteria or pathogens would has been improved by fiber supplementation
Similar effects of an IF supplement on heterophil phagocytosis and oxidative burst was described by Lowry et al. (2005) who showed that feeding purified β-glucan to day old White Leghorn male chicks for 4 days significantly increased phagocytic ability of heterophils as well as oxidative burst. Similarly, McReynolds et al. (2009) found that alfalfa, which is mainly composed of IF (Chen et al., 2013), enhanced oxidative burst production and degranulation of heterophils in laying hens compared to those in an unfed group of hens during the molting period. The authors suggested that this enhancement might be due to the long transit time of alfalfa in the gastrointestinal tract stimulating bacterial degradation of alfalfa cellulose into FOS and thereafter into fermentable substrates such as short chain fatty acids. These compounds could then increase mucosal structure and functionality of the small and large intestines as well beneficially modifying bacterial content of the GIT. The results of the current studies are consistent with outcomes of studies in broiler chicks. Guo et al. (2003) showed that supplementing broiler chickens with β-1,3/1,6-glucan as IF for 4 wk improved macrophage (a component of innate immune function) phagocytic activity. In a study by Vetvicka and Oliveira (2014) supplementing starter diets for 28 days with β-1,3/1,6-D-glucan significantly enhanced the ability of neutrophils (heterophils) of 28 day old Leghorn chickens to phagocytose synthetic particles when compared to an un-supplemented control group.
Beneficial effects of feeding both MF and IF were observed in the current experiments and the different responses to MF and IF in the 2 experiments may have been due to the dietary concentrations used in the 2 experiments or the ages of the pullets. It is not possible to determine, from the current experiments, the mechanisms through which heterophil function was improved but it might have resulted from, for example; enhancement of gut associated lymphoid tissue function and improvement of gut health (Kalmendal et al., 2011); decreased gut pathogens and increased beneficial microorganisms (Cao et al., 2003); improved nutrient availability from improved digestibility and absorption of nutrients (Jørgensen et al., 1996; Jiménez-Moreno et al., 2009; Yokhana et al., 2016).
Lymphocyte Proliferation
In Experiment 2, the proliferation of ConA-stimulated lymphocytes (T lymphocytes) in the MF and IF group pullets was higher (P < 0.05) 8 h after adding the indicator dye, alamarBlue, compared to the Control Group (Table 5). Proliferation of lymphocytes stimulated with LPS (B lymphocytes) was greater (P < 0.05) in MF and IF Groups compared to Controls (Table 5). The enhancement of lymphocyte proliferation in both MF and IF pullets in this experiment might have resulted from the improvement in the growth of bursa of Fabricius as the site of B lymphocytes differentiation (Oláh et al., 2014) and of the cecal tonsils and PP which are aggregates of lymphoid tissue containing T and B lymphocytes (Del Moral et al., 1998; Smith et al., 2014).
Table 5.
Experiment 2. Mitogen stimulated proliferation of lymphocytes1 from Hy-Line Brown pullets fed from 10 wk-of-age for 7 wk with diets containing different types of fiber. (Mean, pooled SEM, n = 8).
| Mitogen2 | C3 | MF3 | IF3 | Pooled SEM |
|---|---|---|---|---|
| ConA | 79.4a | 100.4b | 103.1b | 3.7 |
| LPS | 106.6a | 122.4b | 129.0b | 3.1 |
| PMA | 119.3 | 124.3 | 125.5 | 4.8 |
a,bMeans within the same row with different superscripts differ significantly (P < 0.05).
1Proliferation measured 8 h after adding alamarBlue and expressed as a percentage of the increase of mitogen stimulated lymphocytes relative to non-stimulated lymphocytes.
2Mitogens - ConA - 2.5 μg/mL Concanavalin A; LPS - 3.125 μg/mL lipopolysaccharide from Escherichia coli; PMA - 1.25 μg/mL phorbol 12-myristate 13-acetate.
3See Table 3 for details of diets.
Fiber supplements have been used in poultry to improve cellular mediated immunity measured by lymphocyte proliferation. Sato et al. (2012) supplementing a broiler diet with arabinoxylan from 14 to 28 days-of-age, significantly increased splenic T lymphocyte ConA-stimulated proliferation. Jiang et al. (2014) supplemented diets of 7 wk old egg-type ducks for 8 wk with alfalfa meal as an IF. Their results showed a significant improvement in T and B lymphocyte proliferation compared with the un-supplemented controls and they suggested that the positive effect of the alfalfa meal was possibly due to beneficial changes in intestinal microbial populations: signals from gut microorganisms are suggested by Cerf-Bensussan and Gaboriau-Routhiau (2010) to influence interactions between immune cells of the intestine.
In the commercial diets used here, xylanase (added by the manufacturer to the wheat based diets) can break down arabinoxylans to smaller fractions such as arabinose and xylose in the small intestines of the pullets, and it could have contributed to the degradation of some fractions of the dietary fiber used here. The products of degradation could in turn contribute to decreasing digesta viscosity, accelerating small intestinal fermentation and increasing the digestibility and availability of nutrients (Zhang et al., 2014). Increasing the viscosity of digesta as a result of xylanase degradation of arabinoxylans will slow down its passage rate and subsequently change the balance of microorganisms by increasing of the fermentative microflora thus producing great amounts of short chain fatty acids and lactic acid (Gao et al., 2007). Gao et al. (2007) also showed that xylanase use resulted in enhanced growth, digestibility, and absorption of nutrients and improved humoral immunity and proliferation of lymphocytes stimulated with the mitogen phytohaemagglutinin.
In conclusion, both MF and IF supplements significantly influenced immune function of growing pullets; however, their effects on immune organs and heterophil and lymphocyte responses varied. The variation may have been due to the differences in the chemical structures of the compounds or the proportions of the compounds making up these 2 lignocellulose fiber supplements. Further study would be required to determine possible modes of action of the different fiber types in eliciting beneficial effects on the immune system. Because of the wide variety of ingredients used experimentally to increase dietary fiber content across published experiments, and the variation in chemical structure of fibers in those diets it is not possible to derive specific recommendations regarding effective concentrations of different dietary fibers that will improve growth and immune functions. However, from the positive results described here, both types of commercial dietary lignocellulose fibers (MF and IF) and especially IF, might be useful alternatives to antibiotics for enhancement of growth and the innate, cell-mediated and gut associated immune functions of 4 to 8 wk and 10 to 18 wk old pullets prior to egg laying.
Acknowledgments
The authors thank the Ministry of Higher Education and Scientific Research-Iraq for providing a scholarship to Sherzad Mustafa Hussein and the Ministry of Higher Education and Scientific Research-Kurdistan Regional Government and the University of Duhok, Kurdistan Region, Iraq for giving him study leave. Sherzad Hussein was supported with a La Trobe University, School of Life Sciences a Postgraduate Publication Award. We also thank Chris Rowell from Hy-Line Company and Peter Cransberg and Elise Davine from Ridley AgriProducts Pty Ltd. for providing information on diet formulations. Thanks to my colleague Yuko Mabuchi for showing me how to carry out immune function assays, to Rob Evans and the LARTF staff for help with care of pullets and to Greg Parkinson for advice.
REFERENCES
- Akhtar M., Tariq A. F., Awais M. M., Iqbal Z., Muhammad F., Shahid M., Hiszczynska-Sawicka E.. 2012. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohydr. Polym. 90:333–339. [DOI] [PubMed] [Google Scholar]
- Andreasen C. B., Latimer K. S.. 1989. Separation of avian heterophils from blood using Ficoll-Hypaque discontinuous gradients. Avian Dis. 33:163–167. [PubMed] [Google Scholar]
- Azzam M., Zou X., Dong X., Xie P.. 2011. Effect of supplemental L-threonine on mucin 2 gene expression and intestine mucosal immune and digestive enzymes activities of laying hens in environments with high temperature and humidity. Poult. Sci. 90:2251–2256. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Johnston N., Leslie G. A., Bienenstock J.. 1980. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer's patches. J. Immunol. 125:2626–2632. [PubMed] [Google Scholar]
- Bogusławska-Tryk M. 2005. Effect of different levels of cellulose in the diet on the proteolytic activity of the pancreas in broiler chickens. Folia Biol. 53(Suppl. 1):19–23. [Google Scholar]
- Cao B., Zhang X., Guo Y., Karasawa Y., Kumao T.. 2003. Effects of dietary cellulose levels on growth, nitrogen utilization, retention time of diets in digestive tract and caecal microflora of chickens. Asian Australas. J. Anim. Sci. 16:863–866. [Google Scholar]
- Cerf-Bensussan N., Gaboriau-Routhiau V.. 2010. The immune system and the gut microbiota: friends or foes? Nat. Rev. Immunol. 10:735–744. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S. L., Gamage N. W., Amit-Romach E., Forder R., Marshal J., Van Kessel A., Uni Z.. 2014. Differences in intestinal mucin dynamics between germ-free and conventionally reared chickens after mannan-oligosaccharide supplementation. Poult. Sci. 93:636–644. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang H., Gao L., Zhao F., Lu Q., Sa R.. 2013. Effect of graded levels of fiber from alfalfa meal on intestinal nutrient and energy flow, and hindgut fermentation in growing pigs. J. Anim. Sci. 91:4757–4764. [DOI] [PubMed] [Google Scholar]
- Chowdhury L., Islam K., Khan M., Karim M., Haque M., Khatun M., Pesti G.. 2009. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult. Sci. 88:1616–1622. [DOI] [PubMed] [Google Scholar]
- Ciriaco E., Pinera P. P., Diaz-Esnal B., Laura R.. 2003. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius). Microsc. Res. Tech. 62:482–487. [DOI] [PubMed] [Google Scholar]
- Cornes J. 1965. Number, size, and distribution of Peyer's patches in the human small intestine. Gut, 6:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. M., Sumners L. H., Kim S., McElroy A. P., Bedford M. R., Dalloul R. A.. 2010. Immune responses to dietary β-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 89:2597–2607. [DOI] [PubMed] [Google Scholar]
- Del Moral M. G., Fonfria J., Varas A., Jimenez E., Moreno J., Zapata A.. 1998. Appearance and development of lymphoid cells in the chicken (Gallus gallus) caecal tonsil. Anat. Rec. 250:182–189. [DOI] [PubMed] [Google Scholar]
- Dibner J., Knight C., Yi G., Richards J.. 2007. Gut development and health in the absence of antibiotic growth promoters. Asian-Australas. J. Anim. Sci. 20:1007–1014. [Google Scholar]
- Dong X., Gao W., Tong J., Jia H., Sa R., Zhang Q.. 2007. Effect of polysavone (Alfalfa extract) on abdominal fat deposition and immunity in broiler chickens. Poult. Sci. 86:1955–1959. [DOI] [PubMed] [Google Scholar]
- Farnell M., Donoghue A., De Los Santos F. S., Blore P., Hargis B., Tellez G., Donoghue D.. 2006. Upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. Poult. Sci. 85:1900–1906. [DOI] [PubMed] [Google Scholar]
- Forder R. E. A., Howarth G. S., Tivey D. R., Hughes R. J.. 2007. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult. Sci. 86:2396–2403. [DOI] [PubMed] [Google Scholar]
- Friedman A., Bar-Shira E., Sklan D.. 2003. Ontogeny of gut associated immune competence in the chick. World's Poult. Sci. J. 59:209–219. [Google Scholar]
- Gao F., Jiang Y., Zhou G., Han Z.. 2007. The effects of xylanase supplementation on growth, digestion, circulating hormone and metabolite levels, immunity and gut microflora in cockerels fed on wheat-based diets. Br. Poult. Sci. 48:480–488. [DOI] [PubMed] [Google Scholar]
- Genovese K. J., He H., Swaggerty C. L., Kogut M. H.. 2013. The avian heterophil. Develop. Comp. Immunl. 41:334–340. [DOI] [PubMed] [Google Scholar]
- Gibbons J. D., Chakraborti S.. 2011. Nonparametric statistical inference. Springer, Berlin Heidelberg. [Google Scholar]
- Glick B., Dreesen L. J.. 1967. The influence of selecting for large and small bursa size on adrenal, spleen, and thymus weights. Poult. Sci. 46:396–402. [Google Scholar]
- Gómez del Moral M., Fonfriía J., Varas A., Jimínez E., Moreno J., Zapata A. G.. 1998. Appearance and development of lymphoid cells in the chicken caecal tonsil. The Anat. Rec. 250:182–189. [DOI] [PubMed] [Google Scholar]
- Gogal R. Jr, Ahmed S. A., Larsen C.. 1997. Analysis of avian lymphocyte proliferation by a new, simple, nonradioactive assay (lympho-pro). Avian Dis. 41:714–725. [PubMed] [Google Scholar]
- González-Alvarado J., Jiménez-Moreno E., González-Sánchez D., Lázaro R., Mateos G.. 2010. Effect of inclusion of oat hulls and sugar beet pulp in the diet on productive performance and digestive traits of broilers from 1 to 42 days of age. Anim. Feed Sci. Tech. 162:37–46. [Google Scholar]
- Gopinath V., Raj G. D., Raja A., Kumanan K., Elankumaran S.. 2011. Rapid detection of Newcastle disease virus replication in embryonated chicken eggs using quantitative realtime polymerase chain reaction. J. Virol. Methods. 171:98–101. [DOI] [PubMed] [Google Scholar]
- Grommen S. V., Taniuchi S., Darras V. M., Takahashi S., Takeuchi S., Groef B. D.. 2008. Identification of unique thyrotropin receptor (TSHR) splice variants in the chicken: The chicken TSHR gene revisited. Gen. Comp. Endocr. 156:460–463. [DOI] [PubMed] [Google Scholar]
- Guo Y. M., Ali R. A., Qureshi M. A.. 2003. The influence of beta-glucan on immune responses in broiler chicks. Immunopharm. Immunot. 25:461–472. [DOI] [PubMed] [Google Scholar]
- Guzmán P., Saldaña B., Mandalawi H., Pérez-Bonilla A., Lázaro R., Mateos G.. 2015. Productive performance of brown-egg laying pullets from hatching to 5 weeks of age as affected by fiber inclusion, feed form, and energy concentration of the diet. Poult. Sci. 94:249–261. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Tarpey I., Rothwell L., Kaiser P., Hiscox J. A.. 2007. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian. Pathol. 36:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Farnell M. B., Kogut M. H.. 2003. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 135:177–184. [DOI] [PubMed] [Google Scholar]
- Hedge S. N., Rolls B. A., Turvey A., Coates M. E.. 1982. Influence of gut microflora on the lymphoid tissue in the chicken (Gallus domesticus) and Japanese quail (Coturnix coturnix Japonica). Comp. Biochem. Physiol. 72:205–209. [Google Scholar]
- Heller E. D., Perek M.. 1973. The effect of bursa Fabricius removal in day-old chicks upon growth performance, blood and spleen characteristics. Poult. Sci. 52:1065–1068. [DOI] [PubMed] [Google Scholar]
- Hoshi H., Mori T.. 1973. Identification of the bursa-dependent and thymus dependent areas in the Tonsilla Caecalis of chickens. Tohoku J. Exp. Med. 111:309–322. [DOI] [PubMed] [Google Scholar]
- Hosono A., Ozawa A., Kato R., Ohnishi Y., Nakanishi Y., Kimura T., Nakamura R.. 2003. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer's patch cells. Biosci. Biotechnol. Biochem. 67:758–764. [DOI] [PubMed] [Google Scholar]
- Huang R. L., Deng Z. Y., Yang C. B., Yin Y. L., Xie M. Y., Wu G. Y., Li T.-J., Li L.-L., Tang Z.-R., Kang P.. 2007. Dietary oligochitosan supplementation enhances immune status of broilers. J. Sci. Food Argic. 87:153–159. [Google Scholar]
- Hy-Line International 2014. Hy-Line Brown management guide – Commercial layers. 1– 40Hy-Line International, Iowa, USA: Retrieved in January 2014 from http://www.hyline.com.au/wp-content/uploads/2015/06/HL-Brown-Commercial-Manual-BRN-COM-ENG.pdf. [Google Scholar]
- Incharoen T., Maneechote P.. 2013. The effects of dietary whole rice hull as insoluble fiber on the flock uniformity of pullets and on the egg performance and intestinal mucosa of laying hens. Am. J. Agric. Biol. Sci. 8:323–329. [Google Scholar]
- Jeurissen S. H. M., Janse E. M., Kok G. L., De Boer G. F.. 1989. Distribution and function of non-lymphoid cells positive for monoclonal antibody CVI-ChNL-68.2 in healthy chickens and those infected with Marek's disease virus. Vet. Immunol. Immunop. 22:123–133. [DOI] [PubMed] [Google Scholar]
- Jiang J., Song X., Wu J., Jiang Y.. 2014. Effects of alfalfa meal on the intestinal microbial diversity and immunity of growing ducks. J. Anim. Physiol. Anim. Nutr. 98:1039–1046. [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J., González-Serrano A., Lázaro R., Mateos G.. 2009. Effect of dietary fiber and fat on performance and digestive traits of broilers from one to twenty-one days of age. Poult. Sci. 88:2562–2574. [DOI] [PubMed] [Google Scholar]
- Johansson M. E., Sjövall H., Hansson G. C.. 2013. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H., Zhao X. -Q., Knudsen K. E. B., Eggum B. O.. 1996. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 75:379–395. [DOI] [PubMed] [Google Scholar]
- Kalmendal R., Elwinger K., Holm L., Tauson R.. 2011. High-fibre sunflower cake affects small intestinal digestion and health in broiler chickens. Br. Poult. Sci. 52:86–96. [DOI] [PubMed] [Google Scholar]
- Lacin E., Yildiz A., Esenbuga N., Macit M.. 2008. Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech J. Anim. Sci. 53:466–471. [Google Scholar]
- Lavoie E., Grasman K.. 2005. Isolation, cryopreservation, and mitogenesis of peripheral blood lymphocytes from chickens (Gallus domesticus) and wild herring gulls (Larus argentatus). Arch. Environ. Con. Tox. 48:552–558. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Van Boeckel T., Teillant A.. 2015. The economic costs of withdrawing antimicrobial growth promoters from the livestock sector. OECD Food, Agriculture and Fisheries Papers, No. 78, 41. Retrieved from 10.1787/5js64kst5wvl-en. [DOI]
- Lowry V. K., Farnell M. B., Ferro P. J., Swaggerty C. L., Bahl A., Kogut M. H.. 2005. Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar enteritidis. Intern. J. Food Micro. 98:309–318. [DOI] [PubMed] [Google Scholar]
- Mabuchi Y. 2014. Effects of protein intake on immunity of two Columbidae species. PhD Diss Univ. La Trobe, Melbourne. [Google Scholar]
- Mateos G., Lázaro R., Gracia M.. 2002. The feasibility of using nutritional modifications to replace drugs in poultry feeds. J. Appl. Poult. Res. 11:437–452. [Google Scholar]
- McReynolds J., Genovese K., He H., Swaggerty C., Byrd J., Ricke S., Nisbet D., Kogut M.. 2009. Alfalfa as a nutritive modulator in maintaining the innate immune response during the molting process. J. Appl. Poult. Res. 18:410–417. [Google Scholar]
- Oláh I., Nágy N., Vervelde L.. 2014. Structure of the Avian Lymphoid System. 11– 14 in Avian Immunology. Schat K. A., Kaspers B., Kaiser P., eds Academic Press, Boston, USA. [Google Scholar]
- Palacios M. G., Cunnick J. E., Winkler D. W., Vleck C. M.. 2007. Immunosenescence in some but not all immune components in a free-living vertebrate, the tree swallow. Proc. R. Soc. Lond. [Biol]. 274:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes S. D., Terrón M. P., Marchena A. M., Barriga C., Pariente J. A., Reiter R. J., Rodríguez A. B.. 2007. Tryptophan modulates cell viability, phagocytosis and oxidative metabolism in old ringdoves. Basic Clin. Pharmacol. Toxicol. 101:56–62. [DOI] [PubMed] [Google Scholar]
- Pierre F., Perrin P., Champ M., Bornet F., Meflah K., Menanteau J.. 1997. Short-chain fructo-oligosaccharides reduce the occurrence of colon tumors and develop gut-associated lymphoid tissue in Min mice. Cancer Res. 57:225–228. [PubMed] [Google Scholar]
- Rezaian M., Hamedi S.. 2007. Histological study of the caecal tonsil in the cecum of 4–6 months old white leghorn chicks. Am. J. Anim. Vet. Sci. 2:50–54. [Google Scholar]
- Rodríguez A., Marchena J., Nogales G., Duran J., Barriga C.. 1999. Correlation between the circadian rhythm of melatonin, phagocytosis, and superoxide anion levels in ring dove heterophils. J. Pineal Res. 26:35–42. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Méndez A. J., Luna-Acosta J. L., Carranza M., Harvey S., Arámburo C., Luna M.. 2010. Growth hormone expression in stromal and non-stromal cells in the bursa of Fabricius during bursal development and involution: Causal relationships? Gen. Comp. Endocrinol. 167:297–307. [DOI] [PubMed] [Google Scholar]
- Sadeghi A., Toghyani M., Gheisari A.. 2015. Effect of various fiber types and choice feeding of fiber on performance, gut development, humoral immunity, and fiber preference in broiler chicks. Poult. Sci. 94:2734–2743. [DOI] [PubMed] [Google Scholar]
- Sarikhan M., Shahryar H., Gholizadeh B., Hosseinzadeh M., Beheshti B., Mahmoodnejad A.. 2010. Effects of insoluble fiber on growth performance, carcass traits and ileum morphological parameters on broiler chick males. Int. J. Agr. Biol. 12:531–536. [Google Scholar]
- Sato K., Takahashi K., Aoki M., Kamada T., Yagyu S.. 2012. Dietary supplementation with modified arabinoxylan rice bran (MGN-3) modulates inflammatory responses in broiler chickens. J. Poult. Sci. 49:86–93. [DOI] [PubMed] [Google Scholar]
- Shi-bin Y., Hong C.. 2012. Effects of dietary supplementation of chitosan on growth performance and immune index in ducks. Afr. J. Biotechnol. 14:3490–3495. [Google Scholar]
- Siri S., Tobioka H., Tasaki I.. 1992. Effects of dietary fibers on growth performance, development of internal organs, protein and energy utilization, and lipid content of growing chicks. Jap. Poult. Sci. Assoc. 29:106–114. [Google Scholar]
- Smith A. L., Powers C., Beal R. K.. 2014. The Avian Enteric Immune System. 227– 250 in Avian Immunology. Schat K. A., Kaspers B., Kaiser P., eds Academic Press, Boston, the United States of America. [Google Scholar]
- Vetvicka V., Oliveira C.. 2014. β (1–3)(1–6)-D-glucan with strong effects on immune status in chicken: potential importance for efficiency of commercial farming. J. Nutr. Health Sci. 1:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C. P., Myung E., Lau B. H.. 1993. An automated micro-fluorometric assay for monitoring oxidative burst activity of phagocytes. J. Immunol. Methods. 159:131–138. [DOI] [PubMed] [Google Scholar]
- Yegani M., Korver D. R.. 2008. Factors affecting intestinal health in poultry. Poult. Sci. 87:2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokhana J., Parkinson G., Frankel T.. 2016. Effect of insoluble fiber supplementation applied at different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 95:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Guo Y., Wang Z.. 2008. The modulating effect of beta-1, 3/1, 6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian-Australas. J. Anim. Sci. 21:237–244. [Google Scholar]
- Zhang H., Hunt H., Kulkarni G., Palmquist D., Bacon L.. 2006. Lymphoid organ size varies among inbred lines 63 and 72 and their thirteen recombinant congenic strains of chickens with the same major histocompatibility complex. Poult. Sci. 85:844–853. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu J., Lei L., Jiang Y., Gao F., Zhou G.. 2014. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different sections of the gastrointestinal tract of broilers fed wheat-based diets. Asian-Australas J. Anim. Sci. 27:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi-Jun Y., Sriranganathan N., Vaught T., Arastu S., Ahmed S. A.. 1997. A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J. Immunol. Methods. 210:25–39. [DOI] [PubMed] [Google Scholar]


