Overview
Antibodies have been used for more than a century to prevent and treat illness, neutralize drugs and poisons, and accentuate or depress the immune system. Their specificity and diversity and their relative safety make them potent therapy in antibody deficiencies, certain infections and several autoimmune/inflammatory disorders.
This article discusses 3 principal uses of immunoglobulins: for infectious diseases, for immunodeficiency, and for immunomodulation. These subjects are discussed in that order, because antibody was first used for infections (since the 1890s), next used for immunodeficiency (since the 1950s), and then used for immunomodulation (since the 1970s, after the introduction of intravenous immunoglobulin [IVIG]). The last use, for a great variety of disorders, is now the largest consumer for immunoglobulin products.
This article does not discuss the use of therapeutic monoclonal antibodies, of which 18 are now licensed in the United States, and more are in the pipeline. The therapeutic use of monoclonals for infections and for immunomodulation is in its neonatal period, and, like infants, great expectations have been bestowed upon them.
Immunoglobulins for prevention and treatment of infectious diseases
Emil von Behring was awarded the first Nobel Prize in Medicine in 1901 for development of equine antiserum for the treatment of diphtheria and tetanus. His citation stated “For his work on serum therapy, especially its application against diphtheria, by which he has opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and death.”
Since then antibodies in multiple forms (animal and human serums, immune globulins and monoclonal antibodies) have been developed, primarily for prevention of infectious diseases, and less commonly for their treatment. These antibodies are presented in Table 1 . This section reviews their uses, with an emphasis on their value in the treatment of human infections, as summarized in Table 2 .
Table 1.
Antibody preparations available for passive immunity in the United States
| Product | Abbreviation(s)/brand name(s) | Principal use |
|---|---|---|
| Standard Human Immune Serum Globulins (HISG, γ-Globulin) | ||
| Immune globulin, intravenous | IVIG, IGIV | Treatment of antibody deficiency, immune thrombocytopenic purpura, Kawasaki disease, other immunoregulatory and inflammatory diseases |
| Immune globulin, intramuscular | Immunoglobulin, IGIM | Treatment of antibody deficiency; prevention of measles, hepatitis A |
| Immune globulin, subcutaneous | SCIG | Treatment of antibody deficiency |
| Special Human Immune Serum Globulins for Intramuscular or Subcutaneous Use | ||
| Hepatitis B immune globulin | HBIG | Prevention of hepatitis B |
| Varicella-zoster immune globulin | VZIG | Prevention or modification of chickenpox |
| Rabies immune globulin | RIG | Prevention of rabies |
| Tetanus immune globulin | TIG | Prevention or treatment of tetanus |
| Vaccinia immune globulin | VIG | Prevention or treatment of vaccinia, prevention of smallpox |
| Rho(D) immune globulin | RhoGAM | Prevention of Rh hemolytic disease |
| Special Human Intravenous Immune Globulins | ||
| Cytomegalovirus immune globulin | CMV-IVIG, CMVIG, CytoGam | Prevention or treatment of cytomegalovirus infection |
| Hepatitis B immune globulin, intravenous | HepaGam B | Prevention of hepatitis B (including liver transplantation) |
| Vaccinia immune globulin, intravenous | VIG-IVIG | Prevention or treatment of vaccinia, prevention of smallpox |
| Rho(D) immune globulin intravenous | WinRho SDF | Treatment of immune thrombocytopenic purpura |
| Botulinum immune globulin | BIG, Baby BIG | Treatment of newborn botulism |
| Animal Serums and Globulins | ||
| Tetanus antitoxin (equine) | TAT | Prevention or treatment of tetanus (when TIG unavailable) |
| Diphtheria antitoxin (equine) | DAT | Treatment of diphtheria |
| Botulinum antitoxins (equine heptavalent)a | HBAT | Treatment of botulism |
| Latrodectus mactans antivenin (equine) | Treatment of black widow spider bites | |
| Crotalidae polyvalent antivenin (equine) | Treatment of most snake bites | |
| Crotalidae polyvalent immune Fab (ovine)a | Treatment of most snake bites | |
| Micrurus fulvius antivenin (equine) | Treatment of coral snake bites | |
| Digoxin immune Fab fragments (ovine)a | Digibind, DigiFab | Treatment of digoxin or digitoxin overdose |
| Lymphocyte/thymocyte immune globulin (equine) | Equine ATG, Atgam | Immunosuppression |
| Lymphocyte/thymocyte immune globulin (rabbit) | Rabbit ATG, thymoglobulin | Immunosuppression |
Fab fragment.
Table 2.
Summary of the efficacy of antibody in the prevention and treatment of infectious diseases
| Infection | Prophylaxis | Treatment |
|---|---|---|
| Bacterial Infections | ||
| Respiratory infections (streptococcal, Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae) | Proved (NR)a | Proved (NR) |
| Diphtheria | Unproved (NR) | Proved |
| Pertussis | Unproved (NR) | Unproved (NR) |
| Tetanus | Proved | Proved |
| Other clostridial infections | ||
| Clostridium botulinum | Proved | Proved |
| Newborn botulism | Unproved | Proved |
| Clostridium difficile | Unproved | Probable benefit |
| Staphylococcal infections | ||
| Toxic shock syndrome | Unproved (NR) | Probable benefit |
| Antibiotic resistance | Unproved | Possible benefit (NR) |
| Staphylococcus epidermidis in newborns | Unproved | Possible benefit |
| Toxic shock | Unproved (NR) | Probable benefit |
| Newborn sepsis | Possible benefit (NR) | Probable benefit |
| Shock, intensive care, and trauma | Unproved | Possible benefit (NR) |
| Pseudomonas infections | ||
| Cystic fibrosis | Unproved (NR) | Unproved (NR) |
| Burns | Unproved (NR) | Unproved (NR) |
| Viral Diseases | ||
| Hepatitis A | Proved | No benefit |
| Hepatitis B | Proved | No benefit |
| Hepatitis C | Unproved (NR) | No benefit |
| HIV infection | Unproved (NR) | Unproved (NR) |
| RSV infection | Proved | Unproved (NR) |
| Herpesvirus infections | ||
| CMV | Proved | Possible benefit |
| EBV | Unproved (NR) | Unproved (NR) |
| HSV | Unproved (NR) | Unproved (NR) |
| VZV | Proved | Unproved (NR) |
| Parvovirus | Possible benefit | Proved (NR) |
| Enterovirus infections | ||
| In newborns | Unproved | Possible benefit |
| Encephalomyelitis | Possible benefit | Probable benefit (NR)a |
| Poliovirus | Proved (NR) | Unproved (NR) |
| Ebola | Unproved | Unproved |
| Rabies | Proved | No benefit |
| Measles | Proved | No benefit |
| Rubella | Unproved (NR) | No benefit |
| Mumps | Unproved (NR) | No benefit |
| Tick-borne encephalitis | Possible benefit | No benefit |
| Vaccinia | Proved | Proved |
| Variola | Proved | Unproved |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; NR, not recommended; RSV, respiratory syncytial virus; VZV, varicella-zoster virus.
Recommended for immunodeficient patients.
Modified from Stiehm ER, Keller MA. Passive immunization. In: Feigen RD, Cherry JD, Demmler-Harrison GJ, et al, editors. Textbook of pediatric infectious diseases. 6th edition. Philadelphia: Saunders/Elsevier; 2009. p. 3447–79.
Antibody works by several mechanisms. It can neutralize viruses and bacterial toxins, lyse bacteria with the aid of complement, prevent the spread of microbes to adjacent cells or along nerve roots, coat bacteria for opsonization by phagocytes, block microbial attachment by saturating microbial receptors, and facilitate lysis of infected cells by binding them to cytotoxic cells with an Fc receptor.
Bacterial Infections
Antibody is particularly valuable in bacterial diseases associated with toxin production because much of the tissue damage results from action of the toxin; these can be neutralized rapidly by antibody before antibiotics kill the bacterium.
Anthrax (Bacillus anthracis)
Anthrax is a rare but serious infection, predominantly of ruminant animals, caused by an aerobic gram-positive rod [1]. Humans are infected through the skin (cutaneous anthrax), by ingestion (gastrointestinal anthrax), or by inhalation of anthrax spores (inhalational anthrax) [1]. The last often results from prolonged exposure to animal hides or carcasses or infected soil, and rarely by deliberate spore exposure in the bioterrorism setting. After inhalation the spores are ingested by alveolar macrophages and transported to regional nodes, where the spores germinate and release potent exotoxins. These toxins damage cell membranes, increase capillary permeability, cause pulmonary damage, and lead to shock and cardiovascular collapse.
A vaccine is available for individuals at high risk for exposure and for the military.
Before the antibiotic era and as early as 1903, anthrax antitoxin (usually equine) was used in therapy [2]. An antitoxin is of value in a bioterrorism attack, both before and after exposure. The US Government is collecting plasma from immunized donors to develop a human high-titer IGIV [3]. A human monoclonal antibody is being tested in animals and humans [4].
Clostridial infections
Diphtheria (Corynebacterium diphtheriae)
Many of the adverse effects of diphtheria result from the action of its potent toxin on the heart, central nervous system, and other organs [5]. Thus the prompt use of antitoxin is indicated, in addition to antibiotics [6]. The dose used depends on the localization and severity of infection, ranging from 20,000 units for mild infection of short duration to 120,000 units for severe illness with neck edema. The equine antitoxin is given intravenously, so must be preceded by skin testing for hypersensitivity and possible desensitization. The antitoxin is available through the US Centers for Disease Control (CDC).
A smaller dose of antitoxin can be used in asymptomatic, exposed, susceptible individuals. Before the availability of diphtheria vaccine, antitoxins were given to health care workers caring for patients with diphtheria [7].
Tetanus (Clostridium tetani)
Equine antitoxin for the treatment of tetanus was initiated by von Behring in the 1890s for toxin neutralization. Extensive studies have been carried out to determine the optimal dose of antitoxin and the possible benefit of intrathecal antitoxin, particularly in tetanus neonatorum, a common problem in developing countries [8]. Since the 1960s a human tetanus immune globulin (TIG) has been available, but in some areas of the world equine antitoxin is still used.
TIG is given to unimmunized or incompletely immunized patients who sustain contaminated or deep puncture wounds [8]. The recommended dose of TIG is 250 IU, along with initiation of active immunization. If TIG is unavailable, human IVIG can also be used; it contains variable titers of tetanus antitoxin but a minimal dose of 200–400 mg/kg is suggested for tetanus prophylaxis [8], [9].
Clostridium difficile gastroenteritis
Clostridium difficile infection of the gastrointestinal tract is usually associated with antibiotic-associated diarrhea, often with pseudomembranous colitis and sometimes toxic megacolon [10] Toxic strains of Clostridium difficile release 2 distinct toxins, both of which have potent cytotoxic and inflammatory properties [11]. Infection generally leads to an antibody response to the toxin, and most individuals older than 2 years have such antibodies. High levels of these antibodies acquired after colonization may result in the asymptomatic carrier state [12].
Some patients with symptomatic infection, many of whom are immunodeficient or immunosuppressed, develop antibiotic-resistant diarrhea; many have low or absent IgG antibodies to toxin A. Such patients may respond to IVIG given 300 to 500 mg/kg every 1 to 3 weeks [13]. Such therapy increases antitoxin levels, controls the diarrhea, and prevents relapses [14], [15]. Controlled trials have not been performed.
Botulism (Clostridium botulinum)
Botulism is a severe paralytic poisoning resulting for the ingestion or absorption of neurotoxin or spores of Clostridium botulinum. Several variants are recognized: food poisoning from ingestion of contaminated canned food, wound botulism from a contaminated soft-tissue infection, inhalational botulism among individuals working with the toxin or in a bioterrorist event, infantile botulism (see next section), and adult-type infant botulism in adults with preexisting gastrointestinal disease [16], [17], [18]. In the last 2 types, ingested spores multiply in the gastrointestinal tract to elaborate toxin; the absorbed toxin results in a paralytic disorder.
A few cases of botulism have been associated with use of botulism toxin for cosmetic use [19], [20].
An heptavalent fab fragment equine antitoxin (HBAT) to types A, B, C, D, E, F and G is available in the United States through the CDC [21], [22]. Sensitivity testing must be conducted before their use. Antitoxin to all 3 types is given unless the toxin type is known. Additional doses may be needed in severe wound botulism. Antitoxin can also by used prophylactically in individuals known to have ingested contaminated food. It is not used for infantile botulism.
Infantile botulism (Clostridium botulinum)
This severe paralytic disorder of infants results from the ingestion of Clostridium botulinum spores in baby formulae or food, resulting in slow onset of constipation, abdominal bloating, poor feeding, and respiratory paralysis [22]. Such infants must be hospitalized for prolonged periods for tube feeding and respiratory support, often for as long as 6 to 9 months. Human IV botulism immune globulin is available for treatment of infantile botulism [23]. Despite its high cost ($50,000 per vial) it is cost-effective because of the shortened hospital stay needed.
Gas gangrene (Clostridium perfringens)
There is no antitoxin for gas gangrene.
Bacterial respiratory infections
Respiratory infections with Streptoccocci, Streptococcus pneumonia, Haemophilus influenzae, and Neisseria meningitides are reduced in immunodeficient patients receiving immunoglobulin therapy. These patients include young infants with poor antibody responses to polysaccharide antigens, patients infected with the human immunodeficiency virus (HIV), and patients with primary antibody immunodeficiencies. Before antibiotics, immune serum or animal serum was used as therapy for severe bacterial infection [24], [25].
Other studies suggest that a large dose of IVIG decreases the frequency of otitis in patients with recurrent otitis and normal immunity [26].
Thus regular use of IVIG in antibody-deficient patients in doses of 400 to 600 mg/kg every 3 to 4 weeks or an equivalent amount given subcutaneously decreases the frequency and severity of otitis and other respiratory tract infections [27], [28].
Streptococcal infection
Circulating antibody may play a role in the prevention and treatment of invasive group A streptococcal infection [29]. Newborns with transplacental antibody and patients on IVIG rarely develop streptococcal illnesses. Equine antitoxin was used with some success in the treatment of erysipelas and scarlet fever in the 1920s and 1930s [30]. A preventive vaccine against the streptococcal M protein has been contemplated but is not yet unavailable.
Treatment with IVIG, in addition to antibiotics, is probably beneficial [25], [31]. Streptococcal pyrogenic exotoxins types A, B, and C and mitogenic factor elaborated by certain strains of streptococci may be responsible for these complications. These exotoxins are potent superantigens that activate certain T lymphocytes directly, leading to synthesis and/or release of multiple cytokines with resultant shock, fever, and organ failure.
IVIG contains neutralizing antibodies to these antigens of varying titers from batch to batch [32]. Despite this variability IVIG is recommended, in addition to antibiotics, in the management of these infections, not only to neutralize pyrogenic toxins but to dampen cytokine storm and release [33]. Controlled trials are unavailable but case reports and large series compared with historical controls are encouraging [34]. Large doses of IVIG are recommended (eg, 1–2 g/kg over several days).
Staphylococcal infections
Staphylococcal infections are ubiquitous and of varying severity, ranging from superficial skin infections to deep-seated cellulitis, osteomyelitis, and overwhelming shock [35], [36]. These severe infections occur when the organism is resistant to antibiotics or is a strain associated with toxin production.
One well-recognized syndrome is toxic shock associated with tampon use in menstruating women [36]. This syndrome results from release of the toxic shock syndrome toxin-1, a potent superantigen that initiates the release of multiple cytokines and a clinical picture of rapidly progressive fever, shock, and organ failure. Most authorities recommend a high dose of IVIG to neutralize the toxin and dampen cytokine storm [35], [37].
A second situation in which IVIG may be of value is in neonatal staphylococcal infection, usually coagulase-negative Staphylococcus epidermidis. This is the most common cause of sepsis in premature infants and is aggravated in part by the use of catheters and central lines [38], [39].
One controlled study indicated that IVIG was of value in decreasing the incidence of this infection [40]. Other studies were not confirmatory, possibly because of differences in titer for the protective antibodies [39].
Immunoglobulin is also used in the treatment of antibiotic-resistant staphylococcal infection. Older studies from Waisbren [41] and current studies from Russia suggest clinical benefit [42]. Animal studies support such a combined approach [43].
Infection in high-risk newborns
Newborns, particularly premature newborns with birth weight less than 2000 g are potential candidates for immunoglobulin therapy in view of the frequency and severity of infections. All newborns have low levels of IgM and IgA, and, if premature, a deficiency of transplacental maternal IgG, the deficiency of which is proportional to the degree of immaturity [44]. Premature infants also have defects in antibody synthesis, complement levels, opsonic activity, neutrophil mobilization and killing, and cellular immune responses [44].
Accordingly several studies sought to determine the value of IGIV in the prevention or early treatment of infection in premature infants. These studies differ in terms of entry criteria, immunoglobulin dose and duration, and end points (eg, type and severity of infection, survival). Meta-analyses of prospective, randomized, placebo-controlled prevention studies suggest a slight reduction (3%) in the frequency of sepsis but no difference in mortality, length of nursery stay, or other complications of prematurity [45], [46], [47].
By contrast meta-analysis of 6 controlled studies for the treatment of proven sepsis, involving 262 premature infants, showed that IGIV therapy reduced mortality from 20% to 11%, a significant difference [48]. There was a suggestive benefit for infants with suspected sepsis also. Infants with neutropenia may particularly benefit.
Because a common cause of neonatal sepsis is Staphylococcus epidermidis, a hyperimmune staphylococcal IVIG may be of particular benefit in the prevention of neonatal sepsis. Two recent studies of IGIV from either immunized donors (Altastaph) [49] or selected donors with high titers to a fibrinogen-binding protein (Veronate) [50] did not show a significantly decreased incidence of infection. Studies of monoclonal antibodies to staphylococcal antigens are in progress.
Thus the 1990 National Institutes of Health consensus statement that IGIV should not be given routinely to infants of low birth weight but that it may be of value in selected premature newborns with proven or suspected infection remains valid [51].
Shock, intensive care, and trauma
Patients undergoing severe stress associated with trauma, extensive surgery, or intensive care have profound exposure to and susceptibility to infection, usually as a result of enteric gram-negative infections [52], [53]. Monoclonal antibodies, IgM-enriched IGIV, and regular IGIV have been studied in these situations with inconclusive results [5]. Laupland and colleagues [54] reviewed 14 randomized trials of IGIV and found suggestive benefit in terms of length of stay in the intensive care unit (ICU) and mortality. Similar studies in pediatric patients in the ICU have not been performed.
Despite the lack of controlled trials, IGIV is often used in critically ill patients, particularly neutropenic patients, because of possible benefit and rare side effects.
Viral Diseases
Although many viral diseases are prevented by immunoglobulin, just a few are amenable to antibody therapy, as presented in Table 2. This section focuses on some viral diseases in which antibodies can be used in therapy.
Vaccinia and smallpox (variola)
Although smallpox (variola) has been eradicated from the world since 1977, immunization with live vaccinia virus (cowpox virus) is still used by the military and by certain laboratory personnel working with vaccinia [5]. Further, smallpox is a potential bioterrorism weapon so a supply of vaccinia immune globulin (VIG) is being stockpiled by the US Government for complications of smallpox vaccine and for a response to biological warfare.
Kempe [55] used immune globulin from vaccinated individuals (VIG) to prevent the spread in a 1953 outbreak of smallpox in Madras, India. He also showed that VIG could be used to treat the not infrequent complications of smallpox vaccine including vaccinia eczematum, generalized vaccinia, autoinoculation, and prevention of spread to high-risk individuals exposed to a recently vaccinated individual.
VIG, both for IV and intramuscular (IM) use, is prepared from vaccinated donors and is commercially available. The usual dose is 100 mg/kg [56].
Parvovirus B19
Parvovirus is a DNA virus that causes fifth disease (slapped cheek syndrome, a common exanthem of childhood that usually provides lifelong immunity to subsequent exposure [57]). Parvovirus infects erythroid progenitors (its receptor is the common red cell P antigen) to cause red cell aplasia in patients with congenital or acquired immunodeficiencies including HIV, immunosuppressed organ transplant recipients, and patients with sickle cell disease [57], [58], [59].
IGIV contains neutralizing antibody to parvovirus such that prolonged high-dose therapy can eradicate the infection. Parvovirus infection during pregnancy can also cause fetal hydrops [60]. Arthritis and chronic fatigue syndrome are uncommon manifestations of chronic parvovirus infections [60], [61].
The IVIG dose needed to eradicate parvovirus in not established but is large (1–2 g/kg) and should be repeated until the virus is eradicated as indicated by serum polymerase chain reaction analysis [62], [63].
Cytomegalovirus
Antibodies to cytomegalovirus (CMV) either in the form of hyperimmune IV CMV immune globulin (CMVIG-Cytogam) or regular IGIV have been used for more than a decade to prevent CMV infection in recipients of bone marrow and solid organ transplant [64]. CMVIG is prepared from donors with high anti-CMV titers but regular IGIV also contains CMV antibodies at lower titers. Testing of donor and recipient for CMV infection, the use of CMV antibody-negative blood donors, and the use of antiviral drugs have greatly reduced the indications for CMV antibody [65]. CMVIG is still used in heart and heart-lung transplants (along with antivirals) if either the donor or the recipient is CMV-seropositive [66]. CMVIG is also of suggestive benefit in severe CMV pneumonitis along with antiviral treatment [67].
CMVIG may also be of value for in utero CMV infection; infusions of CMVIG were given intraperitoneally at 28 and 29 weeks to a CMV-infected fetus, with possible benefit [68]. Nigro and colleagues [69] gave 31 pregnant women with primary CMV infection CMVIG during pregnancy; some women received additional CMVIG into the amniotic sac or umbilical cord. Only one woman gave birth to an infant with CMV infection compared with CMV infection in 7 of 14 infants of control women who did not receive antibody therapy. These data are encouraging but are not from well-controlled studies.
Thus the use of CMVIG in recipients of organ transplant, severe CMV infections, or in utero CMV infections is unproved but of suggestive therapeutic benefit.
Herpes simplex
Transplacental maternal antibody has a proven preventive effect in herpes simplex virus (HSV) infection in the newborn period: mothers with a reactivated herpex infection (ie, preexisting infection) during delivery are 10-fold less likely to transmit HSV to their newborn infants during vaginal delivery than are mothers with primary HSV infection acquired during late pregnancy [70].
Masci and colleagues [71] used IVIG to prevent recurrent genital HSV infection with suggestive benefit. The value of HSV monoclonal antibody or IVIG is being evaluated for treatment of disseminated neonatal disease.
Epstein-Barr virus infection
Epstein-Barr virus (EBV) antibodies are present in variable titers in IVIG, particularly in CMVIG, because donors with high titers of CMV often have high titers of EBV. A few patients with posttransplant EBV-induced lymphoproliferative syndrome or hepatitis have been treated successfully with a combination of IGIV or CMVIG, antiviral therapy and interferon-α [72], [73], [74]. Similar results have been achieved in EBV infection in X-linked lymphoproliferative syndrome: such patients have a hereditary predisposition to overwhelming EBV infection [75].
Varicella-zoster infection
Varicella-zoster immune globulin (VZIG), available since 1978, is prepared from plasma with high titers to VZ virus [76]. The commercial product VariZIG is used for the prevention or modification of susceptible high-risk immunodeficient or immunosuppressed children exposed to chickenpox or shingles. It is also used in susceptible women during late pregnancy, newborn infants whose mother develops chickenpox perinatally, and exposed premature infants of less than 28 weeks’ gestation. It is not of benefit in established chickenpox or zoster infection [77].
Enteroviral infections
Encephalomyelitis
Before poliovirus vaccine was introduced, immunoglobulin was used in the prevention of poliomyelitis [78]. Immunodeficient individuals are susceptible to chronic enteroviral encephalitis, usually echovirus or coxsackievirus or less commonly, attenuated poliovirus vaccine strains [79], [80], [81], [82]. Regular doses of IGIV given to antibody-deficient patients have markedly reduced the frequency of enterovirus encephalitis in these patients. Attenuated poliovirus has been replaced in many countries by inactivated (Salk) vaccine.
High-dose IVIG (sufficient to increase the serum IgG levels to 1000 mg/mL) has been used successfully in immunodeficient patients with enteroviral encephalomyelitis [80], [81], [82], [83]. Some patients have been given intrathecal infusions [80], [81]. Not all IVIG-treated patients are cured: some may have viral strains for which the IVIG has no neutralizing antibody. For these instances typing of the cerebrospinal fluid and treatment with selective IVIG units with antibodies to the infecting serotype may be necessary. Antiviral therapy with pleconoril has also been used [83].
Neonatal enteroviral infection
Severe and sometimes fatal disseminated enterovirus infection can develop in neonates [84], [85], [86]. High-dose IVIG has been used in such infants with suggested benefit in decreasing the severity of the illness [84]. Maternal plasma may also be used in the likelihood that the mother has antibody to the organism involved [85].
IVIG has also been used to prevent spread to unaffected infants in a nursery [86]. Unless the titer in the IVIG is known, large doses are recommended.
Hepatitis B immune globulin in recipients of liver transplant
An increasingly important use of hyperimmune hepatitis B immune globulin (HBIG) is to prevent hepatitis B recurrence in hepatitis B-seropositive recipients of liver transplant, many of whom are transplanted because of complications of hepatitis B [87], [88]. Hepatitis B reoccurs in half of the patients in 3 years [89].
Such recurrences can be reduced significantly by giving large doses of HBIG for a prolonged period beginning at the time of transplantation and continuing indefinitely after transplantation [89]. Antiviral agents such as lamivudine are also given simultaneously. The dose of HBIG after transplantation is varied so as to maintain a continuous serum anti-HbS titer. Hepatitis B vaccine can also be given to induce active immunity.
The 2 types of HBIG available include the 16% IGIM used for prophylaxis in newborns of hepatic B-positive mothers and for unimmunized exposed susceptibles and a 5% HBIG for IV use in liver transplantation. The use of the latter adds a considerable cost to liver transplantation. The University of California at Los Angeles Medical Center spends $500,000 per year on HBIG, nearly all for the liver transplant program.
A hyperimmune hepatitis C immune globulin for hepatitis C liver transplantation is also under study. Monoclonal antibodies to hepatitis B and C are under development.
Regional viral infections
West Nile fever
West Nile fever, caused by the West Nile virus, is common in many tropical regions where Culex mosquitoes are endemic. It has spread to Europe and the United States, and can also be transmitted by infected blood and organ transplantation. Several case reports and animal studies suggest that IVIG prepared from seropositive donors modifies the severity and mortality [90], [91].
Ebola
Ebola virus, a filivirus, causes severe and often fatal hemorrhagic fever in tropical Africa. There is no effective antiviral agent. Goat hyperimmune serum protected guinea pigs from experimental infection if given within 72 hours of exposure. This product was used for emergency prophylaxis in 4 patients exposed by a laboratory accident. Only one developed mild infection [92].
Equine serum has protected monkeys against low-dose virus challenge but not high-dose virus challenge [93]. Blood from convalescing patients has also been used with promising results [94]. Other animal antisera have been developed, as have monoclonal antibodies.
Tick-borne encephalitis
Tick-borne encephalitis caused by a flavivirus is endemic in central Europe. A vaccine is available as is a hyperimmune immune globulin. A combination has been also used [95], [96].
Argentine hemorrhagic fever
Argentine hemorrhagic fever caused by the Junin virus has a high mortality from vascular or neurologic complications. Maiztegui and colleagues [97] found that immune plasma given before the ninth day of illness reduced mortality to 1% among 91 patients given immune plasma compared with 16.5% mortality among 97 patient given normal plasma.
Severe acute respiratory distress syndrome
Convalescent plasma and IVIG have been used in the treatment of severe acute respiratory distress syndrome caused by a corona virus. Studies were inconclusive [98].
Summary of Antibody Use in Infectious Diseases
Antibody is a time-honored way to prevent viral infection after exposure, and has a crucial role in the treatment of bacterial diseases associated with toxin production. It is also of value in prevention of certain viral infections as well as in the treatment of parvovius, enterovirus infection, and certain regional viral infections.
Immunoglobulins in primary immunodeficiencies
Polyclonal immunoglobulin, now used in scores of diverse disorders [99], [100], [101], was first used in the prevention of infectious diseases. In 1952, Ogden Bruton [102] reported a child with agammaglobulinemia and initiated the first use of repeat injections of immunoglobulin as replacement therapy. In his report γ-globulin fractionated from human plasma was administered subcutaneously to an 8-year-old boy who had no known γ-globulin in a serum protein electorophoresis. This child had multiple infections, including 19 episodes of septicemia, which were ameliorated by chronic treatment with the immunoglobulin. This experience represented the dawn of immunoglobulin therapy for primary immunodeficiency and defined its use in a disease for which no therapeutic alterative was available.
Since then, the study of primary immunodeficiency has expanded markedly. There are now more than 140 distinct diagnoses, most of which have defects of humoral immunity [103]. Approximately 1 in 2000 people are living with a primary immunodeficiency in the United States, of whom greater than 50% have an antibody deficiency potentially requiring immunoglobulin replacement therapy [104]. Other primary immunodeficiency registries confirm that greater than 50% have an antibody deficiency [105], [106], [107], [108]. Treatment with immunoglobulin remains the best therapeutic option for most of these patients.
Primary Antibody Deficiencies
Characteristics of antibody immunodeficiencies appropriate for replacement therapy are presented in Table 3 . The clearest indications for immunoglobulin therapy are those associated with an absence of B cells (category I). These patients are unable to make antibodies or immunoglobulin I. Examples include agammaglobulinemia and certain types of severe combined immunodeficiency. Several gene defects may be responsible for these illnesses [109], but all need immunoglobulin replacement therapy.
Table 3.
Conceptual classification of the primary antibody immunodeficiencies
| Category | B cells | IgG quantity | IgG quality (antigen-specific antibody) | Diagnostic examples | Immunoglobulin replacement therapy | Cessation of therapy for reevaluation |
|---|---|---|---|---|---|---|
| I | Absent | Absent | Absent | Agammaglobulinemia Severe combined deficiency disease |
Absolute indication, provide immediately | Inappropriate |
| II | Present | Low | Low | Hyper IgM CVID NEMO deficiency (subset) |
Absolute indication, provide after firm diagnosis | Inappropriate |
| III | Present | Normal | Low | Specific antibody deficiency NEMO deficiency (subset) Subclass deficiency with specific antibody defect |
Provide if diagnosis is firm | Single trial appropriate only if diagnosis is not related to a specific genetic defect |
| IV | Present | Low | Normal | Transient hypogammaglobulinemia of infancy Primary hypogammaglobulinemia |
Provide when clinically indicated | Reassess if indicated with a single trail |
| V | Present | Normal, but IgG subclass deficient | Normal | IgG1, IgG2, or IgG3 subclass deficiency | Provide when clinically indicated | Reassess if indicated with a single trail |
| VI | Present | Normal | Normal | Recurrent infection | As adjunct therapy only where indicated | As appropriate |
The next category (II) of patients needing immunoglobulin are those who have B cells but cannot make IgG and generate specific IgG antibodies. Because IgG represents the major defense of humoral immunity against infection, these patients also require immunoglobulin replacement therapy. This diagnostic category includes the hyper IgM syndrome (HIGM) and common variable immunodeficiency (CVID). HIGM is caused by several specific gene mutations [110], but most CVID cases have no identifiable genetic lesions [111].
Diagnosis can be made by either identifying a specific gene mutation, or by defining the quantitative and qualitative deficit of IgG [112]. As in patients in category I, continuous and uninterrupted replacement therapy with immunoglobulin is warranted. If the diagnosis is confirmed molecularly, immunoglobulin therapy must be continued. In a few cases, it may be clinically appropriate to stop immunoglobulin therapy once during a lifetime to determine if the defect is fixed [1]. This strategy should not be repeated if the single trial indicates a persistent deficit. If a trial off immunoglobulin therapy is considered, this should be performed in late spring or summer, when respiratory infections are less prevalent.
A third diagnostic category (III) of antibody deficiencies is those associated with qualitative defects in humoral immunity [113]. These patients have B cells and produce normal quantities of IgG but the quality of IgG is diminished. These individuals are unable to respond appropriately to specific antigenic challenges such as vaccinations or infections. This category includes those with specific antibody deficiency with normal immunoglobulins [113] and certain patients with NEMO (NF-kappa;-B essential modulator) deficiency [114], [115]. Diagnosis is made after documentation of an ineffective vaccination response, a failed humoral response to an infection, or a specific molecular/genetic diagnosis linked to this category [112].
A fourth category (IV) includes patients with lower than expected levels of IgG but who are able to mount effective antibody responses. This category forms a subset of individuals referred to as having “isolated hypogammaglobulinemia” when only the IgG level is low. Although hypogammaglobulinemia can be a component of many immunologic defects, in isolated hypogammaglobulinemia antibody quality is adequate, with normal responses to vaccination or infection.
Because the normal age-specific ranges of IgG define the lower limit at the 2.5th percentile, one of 40 individuals has low levels of IgG. The question becomes, when there is no deficit of antibody quality, is isolated hypogammaglobulinemia clinically a problem? It is also important to discern when hypogammaglobulinemia represents a primary versus a secondary problem with increased loss of IgG. Examples of the latter include draining chylothorax [116] or intestinal lymphangiectasia [117]. In these individuals, the hypogammaglobulinemia is less likely to cause a problem because antibody synthesis is intact and often accelerated.
In patients with primary hypogammaglobulinemia, the level of IgG that is associated with a definitive risk for infection is not defined, especially when antibody quality is intact [112]. Some insurance companies recommend replacement therapy for patients who have an IgG level less than 400 mg/dL and a history of recurrent infection. Although that situation may be reasonable, questions still exist about how to manage the patient recognized as having primary hypogammaglobulinemia with low IgG levels (ie, <150) but no history of infection.
Diagnostic examples include transient hypogammaglobulinemia of infancy (THI) [118], [119], [120] or otherwise unexplained primary hypogammaglobulinemia [121]. The former diagnosis is established in retrospect, as the IgG level normalizes with age. Thus, in select cases of THI immunoglobulin replacement may be considered as a temporizing measure. However, primary hypogammaglobulinemia remains a difficult diagnostic and therapeutic dilemma.
Other patients (a fifth diagnostic category [V]) have a deficiency of one of the 3 major IgG subclasses, IgG1, IgG2, or IgG3. IgG4 deficiency is common and should not be considered an abnormality [99]. Although a deficiency of one of the major IgG subclasses indicates some immunologic deviation, most of these patients have a normal total IgG level, intact responses to specific antigens, and are not candidates for immunoglobulin replacement therapy. Those with impaired antibody specificity do not fall in to this category, but into the third category. However, even without impairment in antibody quality, immunoglobulin replacement in some patients in a deficiency subclass does reduce the incidence of infections [122], [123]. Nevertheless, most insurers in the United States have additional criteria for justifying therapy in patients with IgG in deficiency subclasses.
A final diagnostic category is patients with recurrent infection who do not have hypogammaglobulinemia subclass deficiency or deficits of antibody quality. Thus, they have infectious susceptibility without evidence of identifiable immune abnormality. The infectious burden in these individuals can be high and most certainly has an explanation, so nonhumoral diagnoses should be aggressively sought. There are also patients an explanation of whose infectious susceptibility presently evades clinical science. immunoglobulin replacement therapy has been considered in these individuals under certain circumstances.
Immunoglobulin Preparations for Antibody Immunodeficiencies
Although Bruton [102] gave immunoglobulin to his patient by the subcutaneous (SC) route, subsequent patients until 1970 received immunoglobulin by weekly IM injections [124]. This strategy was necessary because the immunoglobulin preparations were not purified to the degree required for IV administration. In the early 1970s, immunoglobulin preparations with low quantities of immunoglobulin aggregates were developed for IV administration. IVIG and IGIV have numerous advantages, including achieving high peak and trough IgG levels and convenient monthly dosing regimens. Although limited studies have compared IVIG with IMIG, the IV route has become the preferred route of immunoglobulin administration worldwide [125].
Seven IVIG preparations are currently approved by the US Food and Drug Administration (FDA) for replacement therapy in primary immunodeficiency (Table 4 ). Each has been studied in a licensing trial in patients with primary immunodeficiency and found to be safe and effective. The primary end point in most of these clinical trials has been the prevention of serious bacterial infection compared with the expected frequency of such infections before diagnosis [126]. The rate of infection can be surprisingly high, as shown by Bruton’s [102] first patient mentioned earlier. The early diagnosis and treatment of primary immunodeficiency with immunoglobulin products has reduced morbidity and mortality and considerable savings of health care expenditures [127], [128].
Table 4.
IVIG products for replacement therapy in primary immunodeficiency available in the United States in 2010
| Product | Form | Stabilizer/Sugar | IgA (μg/ml) | Osm (mOsm/kg or L) | Sodium (mg/ml) | Storage | Manufacturer |
|---|---|---|---|---|---|---|---|
| Carimune | Lyophilized | Sucrose | Trace | 768 (12%) | <2.4 | RT (24 m) | CSL |
| Flebogamma | 5% liquid | Sorbitol | <50 | 240–370 | ? | RT (24 m) | Griffols |
| Gammagard liquid | 10% liquid | Glycine | 37 | 240–300 | None added | RT (6 m) 4° (36 m) |
Baxter |
| Gammagard SD | Lyophilized | Glucose | <2.2 | 1250 (10%) | 8.5 | RT (24 m) | Baxter |
| Gammunex | 10% liquid | Glycine | 46 | 258 | Trace | RT (9 m) 4° (36 m) |
Talecris |
| Octagam | 5% liquid | Maltose | <200 | 310–380 | <0.7 | RT (24 m) | Octapharma |
| Privigen | 10% liquid | Proline | <25 | 240–440 | Trace | RT (24 m) | CSL |
Abbreviation: RT, room temperature.
All IVIG products are purified from human plasma pools under strict manufacturing guidelines. Although each manufacturer has its own process there are more similarities than differences in the various methods. All processes remove non-IgG impurities and IgG aggregates and add stabilizers to prevent in vitro aggregate formation. Despite these efforts, adverse reactions during IVIG administration are not uncommon [129]. All immunoglobulin manufacturers have robust measures to screen donors and to inactivate blood-borne pathogens; the safety of immunoglobulin preparations in the last decade has been superb [130].
There are subtle differences among different IVIG products from different companies; several companies have more than one product on the market [131]. This situation can lead to confusion about which IVIG to administer to which patient.
In general, most IVIG products are tolerated by most patients. The characteristics of the individual IVIG preparations, as outlined in Table 2, may help in selecting the best product for each patient. They differ as to concentration, stabilizers, sugar content, IgA content, sodium content, and osmality. The volume of individual vials, storage requirements, need to reconstitute a lyophilized product before use, local availability, and price are also variable. Many patients may tolerate one product more effectively than another. Thus, when a patient tolerates a particular immunoglobulin product it is advisable to continue with that product whenever possible [99].
Three other preparations of immunoglobulin are approved by the US FDA (Table 5 ). One is approved for IM administration and two for SC administration. Few patients receive their immunoglobulin by the IM route.
Table 5.
Other immunoglobulin products for replacement therapy in primary immunodeficiency available in the United States in 2010
| Product | Approved route |
Form | Stabilizer/ Sugar |
IgA (μg/ml) |
Sodium (mg/ml) |
Storage | Manufacturer |
|---|---|---|---|---|---|---|---|
| Gammastan | IM | ∼16% liquid | Glycine | ? | 3.0 | 4° | Talecris |
| Vivaglobin | SC | 16% liquid | Glycine | 1700 | <3.2 | 4° | CSL |
| Hizentra | SC | 20% liquid | Proline | <50 | Trace | RT | CSL |
Subcutaneous Immunoglobulin
The SC administration of immunoglobulin resurfaced in 1980 in the United States [132]. Subcutaneous immunoglobulin (SCIG) is usually given in the abdominal wall or thigh with a thin bored needle and an infusion pump, delivered over several hours. Although its initial use in the United States was limited, the SC route gained popularity in Europe; extensive clinical experience indicated that it was equivalent to IVIG therapy [133], [134]. A crossover trial with IVIG and a US FDA licensing trial showed that SCIG was equivalent to IVIG in preventing infection in primary immunodeficiency [135], [136].
SCIG has advantages and disadvantages compared with IVIG therapy (many related to patient preferences) and these have been reviewed extensively [137], [138], [139]. One advantage of SCIG over IVIG is the markedly decreased incidence of systemic reactions [134], [135]. Another is eliminating the need for IV access or indwelling IV access devices. The most serious disadvantage is the need for more frequent administration (at least weekly) to administer sufficient immunoglobulin [140]. Another disadvantage is less frequent physician encounters because most SCIG infusions are given at home by caretakers or home infusion companies.
Dose and Administration of IVIG and SCIG
The dose and frequency of immunoglobulin therapy is a complex topic and draws on both evidence- and experience-based sources. These recommendations are presented in a several reviews and consensus statements [99], [112], [138], [139], [141]. The recommendations include starting doses of 400 to 600 mg/kg/mo. After several months this dose can be altered depending on the trough level and the clinical response. Patients vary as to their requirement to maintain reasonable resistance to infection [141], [142].
SCIG is typically used after the patient has been on IVIG for several months. The weekly SCIG dose is usually one-fourth of the previous monthly IVIG dose. Some immunoglobulin-naive patients are started on immunoglobulin therapy with SCIG so the number of initial doses may need to be increased.
The amount of SCIG given at a single site for an adult is usually 20 mL of the 16% solution (ie, 3.2 g). More than one site can be used simultaneously to deliver the target dose. This procedure has been facilitated by the availability of special tubing, needle sets, catheters, and pumps. Infusion site reactions are not uncommon but are rarely severe [136].
IVIG is usually administered monthly and SCIG is usually administered weekly, but other schedules are often used. These schedules include shorter or longer intervals between infusions of IVIG to achieve a satisfactory clinical response. SCIG can be given biweekly, or divided into more frequent injections, even small daily doses. The latter is generally self-administered at home, well tolerated, and preferred by some patients because of the small daily dose needed [143], [144].
Trough levels of immunoglobulin achieved must be considered. Several studies have correlated resistance to infection with specific IgG trough levels. Targeting a specific trough level may be feasible for patients with agammaglobulinemia who have a profound deficiency of IgG [126] but more difficult for other antibody deficiencies [145], [146], [147]. In agammaglobulinemia, a trough level of 500 mg/dL is a minimally acceptable level and 800 mg/dL a more desirable trough level [99], [126]. These recommendations may not be appropriate in other disorders in which baseline IgG levels and antibody titers are variable; in these cases the clinical response must be considered.
Summary of Immunoglobulin Use in Primary Immunodeficiency
Polyclonal immunoglobulin is essential therapy for the primary antibody immunodeficiency diseases. The different disorders in which immunoglobulin therapy are used are reviewed. Several immunoglobulin products are available for their treatment; they have similar therapeutic properties but there are individual differences among the available products. immunoglobulin can be given either intravenously (IVIG) or subcutaneously (SCIG). Dosage, frequency of infusions, achieved trough levels, and advantages and disadvantages of IVIG and SCIG are discussed.
IVIG in autoimmune and inflammatory diseases
The early 1980s witnessed an increase in the use of IVIG as an immunomodulator for inflammatory and autoimmune disorders. More than 70% of the IVIG prescribed is for patients with autoimmune and inflammatory diseases, despite the fact that IVIG is approved for just a handful of indications (Box 1 ). In the late 1990s, this situation led to an IVIG shortage, compromising those patients who depend on IgG replacement therapy to correct their underlying antibody deficiency.
Box 1. FDA-approved indications for IVIG.
-
•
Primary immunodeficiency
-
•
Idiopathic thrombocytopenic purpura
-
•
Kawasaki disease
-
•
B-cell chronic lymphocytic leukemia
-
•
Pediatric HIV
-
•Bone marrow transplantation
- Graft-versus-host disease (GVHD)
- Interstitial pneumonia
- Infections
-
•
Chronic inflammatory demyelinating polyneuropathy
In 2006, the American Academy of Allergy, Asthma and Immunology’s Committee on Primary Immunodeficiency evaluated the use of IVIG for multiple disorders. The strength of the evidence for a beneficial effect and the basis for this recommendation were classified (Box 2 ). This section reviews the use of IVIG for the autoimmune and inflammatory conditions in this report (Box 3 ), in the context of a review of the mechanisms of action of IVIG in these conditions.
Box 2. Levels of evidence-based medical decisions.
-
•Categorization of evidence and basis of recommendation
-
Ia.From meta-analysis of randomized controlled studies
-
Ib.From at least one randomized controlled study
-
IIa.From at least one controlled trial without randomization
-
IIb.From at least one other type of quasiexperimental study
-
III.From nonexperimental descriptive studies, such as comparative, correlation, or case-control studies
-
IV.From expert committee reports or opinions or clinical experience of respected authorities or both
-
Ia.
-
•Strength of recommendation
-
A.Based on category I evidence
-
B.Based on category II evidence or extrapolated from category I evidence
-
C.Based on category III evidence or extrapolated from category I or II evidence
-
D.Based on category IV evidence or extrapolated from category I, II, or III evidence
-
A.
Box 3. Strength of the evidence for the effectiveness of IVIG in autoimmune/inflammatory diseases.
- Autoimmune cytopenias
-
•Definitely beneficial:
- Idiopathic thrombocytopenic purpura (Ia-A)
-
•Might provide benefit
- Autoimmune neutropenia (III-D)
- Autoimmune hemolytic anemia (III-D)
- Fetomaternal alloimmune thrombocytopenia (III-D)
- Neonatal isoimmune hemolytic anemia (III-D)
- Posttransfusion purpura (III-D)
-
•
- Inflammatory neuropathies
-
•Definitely beneficial:
- Guillain-Barré syndrome (Ia-A)
- Chronic inflammatory demyelinating polyneuropathy (Ia-A)
- Multifocal motor neuropathy (Ia-A)
-
•Probably beneficial:
- Myasthenia gravis (Ib-IIa-B)
- Lambert-Eaton myasthenic syndrome (Ib-A)
- IgM antimyelin-associated glycoprotein paraprotein-associated peripheral neuropathy (Ib-A)
- Stiff man syndrome (Ib-A)
-
•Might provide benefit:
- Relapsing-remitting multiple sclerosis (Ia-A)
- Intractable childhood seizures (Ia-A)
- Rasmussen syndrome (IIB-B)
- Acute disseminated encephalomyelitis (III-C)
- Lumbosacral or brachial plexitis (III-C)
- Human T-lymphotropic virus-1–associated myelopathy (III-C)
- Postinfectious cerebellar ataxia (III-D)
- Acute idiopathic dysautonomia (III-D)
-
•Unlikely to be beneficial:
- Demyelinating neuropathy associated with monoclonal IgM (Ib-A)
- Amyotrophic lateral sclerosis (III-C)
- POEMS syndrome (III-C)
- Paraneoplastic neuropathies (III-C)
-
•
- Rheumatologic and organ-specific autoimmune diseases
-
•Definitely beneficial:
- Graves ophthalmopathy (Ib-A)
-
•Probably beneficial:
- Autoimmune uveitis (IIA-B)
-
•Might provide benefit:
- Severe rheumatoid arthritis (IIb-B)
- Autoimmune diabetes mellitus (IIb-B)
- Vasculitides and antineutrophil antibody syndromes (III-D)
- Systemic lupus erythematosus (III-D)
-
•Unlikely to be beneficial:
- Antiphospholipid antibody syndrome (III-D)
-
•
- IVIG in other inflammatory disorders
-
•Definitely beneficial:
-
•Probably beneficial:
- Toxic epidermal necrolysis/ Stevens-Johnson syndrome (IIa-B)
-
•Might provide benefit:
- Steroid-dependent asthma (Ib-A)
- Prevention of acute humoral rejection in renal transplants (Ib-A)
- Treatment of acute humoral rejection in renal transplants (III-C)
- Pediatric autoimmune neuropsychiatric disorder associated with streptococcus (PANDAS) (IIb-B)
- Delayed pressure urticaria (IIb-B)
- Chronic urticaria (III-C)
- Acute myocarditis (III-C)
- Autoimmune blistering diseases (III-C)
- Autoimmune liver disease (III-D)
- Prevention of pregnancy loss in a subset of women (repeat second-trimester loss) with spontaneous recurrent abortions (Ia-A)
-
•Unlikely to be beneficial:
- Nonsteroid-dependent asthma (Ib-A)
- Prevention of chronic GVHD after bone marrow transplantation (Ib-A)
- Chronic fatigue syndrome (Ib-A)
- Atopic dermatitis (IIa-B)
- Autism (III-C)
-
•
Adapted from Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2006; 117:S525–53.
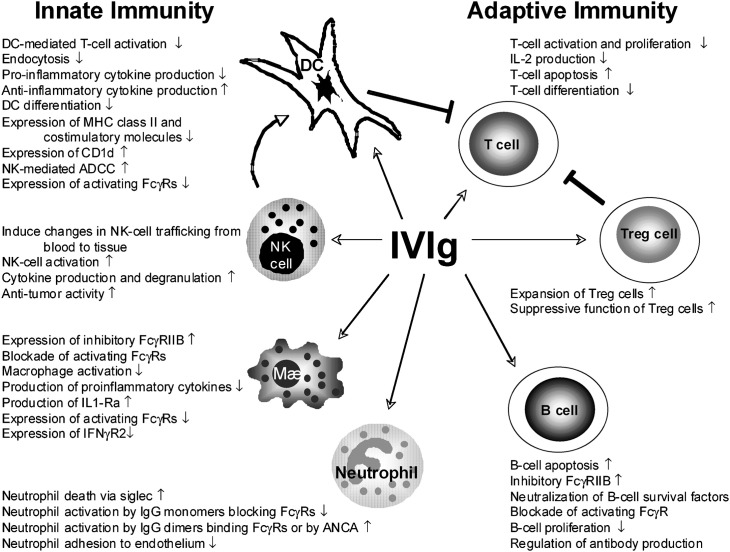
The multiple effects of IVIG on the innate and adaptive immune system are illustrated in Fig. 1 .
Fig. 1.
Multiple effects of IVIG on the innate and adaptive immune system.
(Adapted from Tha-In T, Bayry J, Metselaar HJ, et al. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol 2008;29:613; with permission.)
Historical Note: IVIG in Immune Thrombocytopenic Purpura
The first use of IVIG for an autoimmune process was in children with immune thrombocytopenic purpura (ITP). Imbach and colleagues [148] observed that antibody-deficient patients receiving IVIG who also had ITP had a marked increase in platelet count after IVIG infusions. Subsequently, these investigators examined the therapeutic effects of IVIG in children with a primary diagnosis of ITP; they used high-dose IVIG (400 mg/kg) for 4 consecutive days. The investigators reported a dramatic increase in platelet count within hours of the administration of IVIG. In some patients, the increase in platelet count was sustained; in others, repeat IVIG treatments were necessary.
Fc Receptor Blockade
Box 3 presents the indications for IVIG in autoimmune cytopenias as well its likely benefit. Several hypotheses have been proposed to explain the rapid increase in platelet count (or other antibody-coated cells) after IVIG administration. The most accepted hypothesis is that high-dose IVIG induces an Fc receptor blockade of reticuloendothelial cells in the liver and spleen, preventing them from removing antibody-sensitized cells.
Debre and colleagues [149] provided evidence for this hypothesis when they infused Fcγ fragments in children with ITP, and showed an increase in platelet count after the infusion. The Fc receptor blockade theory may account for the rapid increase in platelet count after the IVIG infusion, but not for the long-term benefits of IVIG. Thus additional mechanisms have been sought.
Fc Receptor Modulation
One such mechanism, supported by animal studies, is that IVIG stimulates inhibitory FcγRIIB receptors found on a variety of cell types including B cells that in turn inhibit antibody and immune function [150]. Samuelsson and colleagues [151] showed in a mouse model of ITP that IVIG suppresses or inhibits antiplatelet antibody production through this FcγRIIB receptor.
Subsequently Ravetch and colleagues [152], [153] identified distinct motifs in the IVIG that have a propensity to engage and activate the FcγRIIB inhibitor receptor that inhibits antibody synthesis. These distinct properties were attributed to the carbohydrate moiety in the IVIG molecule, representing about 5% of the total IgG molecule. More than 30 different covalently attached carbohydrate glycans in the IgG molecule have been identified. Glycosylation of the IgG is essential for binding to all Fcγ receptors. The important glycan moiety in the IgG molecule is attached to the asparagine (Asn297) in the second domain of the constant region of the IgG molecule.
Using a K/BxN serum-induced arthritis model in mice, Kaneko [152] showed that IgG at 1 g/kg inhibited the inflammatory arthritic process. Deglycosylated or neuraminidase-treated IVIGs were unable to inhibit this inflammation. Kaneko then showed that IVIG enriched for the sialylated glycan moiety had comparable inhibitory effects on the inflammatory process at one-tenth of the dosage used with intact IVIG. This investigator showed that this inhibitory activity resided in the IgG Fc fragment, and was dependent on FcγRIIB expression on effector macrophages.
Anthony and colleagues [154] have engineered a recombinant/sialylated human IgG1 Fc protein that had the same immune modulating activity as native IVIG. These investigators showed that the action of sialylated Fc in the rheumatoid arthritis mouse model is mediated through the interaction of sialylated Fc with the SIGN-R1 receptor on macrophages [154]. The investigators propose that the interaction between sialylated Fc and SIGN-R1 produces an antiinflammatory state that upregulates inhibitory FcγRIIB receptors on effector cells, making these cells more resistant to triggering by immune complexes. They suggest that DC-SIGN, the human homolog of SIGN-R1, has a comparable role for the antiinflammatory effects of IgG Fc fragments.
Acceleration of IgG Catabolism
Another mechanism proposed by Yu and Lennon [155] suggested that the administration of high-dose IVIG augments the catabolism of endogenous serum IgG. IgG catabolism occurs through a process by which the IgG molecule binds to a specialized Fc receptor found on endothelial cells (eg, FcRn), which protects the IgG molecule from normal catabolism and its removal from the plasma. This process accounts for the long serum IgG half-life (21 days). High-dose IVIG saturates the FcRn receptor, resulting in the accelerated catabolism of autoantibodies [156], [157]. Hansen and Balthasar [157] have supporting data in a rat model of immune thrombocytopenia using monoclonal antibodies.
Presence of Antiidiotypic Antibodies
The uses of IVIG in several autoimmune inflammatory neuropathies are presented in Box 3. The FDA has recently approved the use of IVIG in chronic inflammatory demyelinating polyneuropathy. This table also shows the evidence-based efficacy of IVIG in rheumatic disorders. Aside from the mechanisms involving the FcγRIIB inhibitory receptor and the accelerated catabolism of autoimmune antibodies through the FcRn receptor, it has also been proposed that the administration of IVIG can regulate autoreactive B cells by restoring the idiotypic-antiidiotypic network.
Other autoimmune diseases may be associated with a deficiency of these antiidiotypic antibodies, which are believed to regulate the production and activity of these autoantibodies (Box 4 ). Kazatchkine and colleagues [158] showed that F(ab′)2 fragments prepared from IVIG could bind to several autoantibodies (eg, antifactor VIII, antithyroglobulin, anti-DNA, antiintrinsic factor, neutrophil cytoplasmic antigens), and thus lead to increased catabolism of these autoimmune antibodies and prevent them from inducing tissue injury [159]. These investigators postulated that IVIG may work, at least in part, in certain autoimmune diseases by neutralizing the functional activity of various autoantibodies or inhibiting their binding to their respective autoantigens [160].
Box 4. Diseases that may be associated with deficiencies of antiidiotypic antibodies.
-
•
Myasthenia gravis
-
•
Autoimmune neuropathies
-
•
Guillain-Barré syndrome
-
•
Antifactor VIII autoimmune disease
-
•
Autoimmune thyroiditis
-
•
Systemic lupus erythematosus
Inhibition of Complement Activation
Another mechanism by which IVIG may benefit autoimmune disease is by preventing the uptake of complement on target tissues. Berger and colleagues [161] showed that high concentrations of IgG inhibit the uptake of C3 on antibody-sensitized erythrocytes. Thus, any inflammatory or autoimmune process that involves a C3b-or C4b-dependent process could be modulated by IVIG therapy. This situation is best exemplified in patients with dermatomyositis in whom the disease is mediated by activation of C3 and deposition of the membrane attack complex on the endomysial capillaries [162]. Treatment with IVIG inhibits complement-induced inflammation by decreasing complement deposition on the endomysial capillaries of muscle tissues [163], [164]. This mechanism of IVIG is relevant not only in dermatomyositis but also in Guillain-Barré syndrome and myasthenia gravis [165], [166].
Fas Ligand Inhibition
As shown in Box 3, IVIG is used in many other inflammatory diseases. However, the evidence-based data for several of these diseases are not so strong as some of the autoimmune disorders discussed earlier. Nevertheless, one inflammatory disease in which IVIG may be beneficial is toxic epidermal necrolysis or Stevens-Johnson syndrome. Patients with toxic epidermal necrolysis have high levels of serum-soluble Fas ligand that bind to Fas receptors on keratinocytes to induce apoptosis (cell death). Viard and colleagues [167] showed that the anti-Fas antibodies in IVIG block the interaction of Fas ligand with Fas receptors on the keratinocytes, preventing destruction of the epithelium.
Inhibition of Neutrophil Adhesion
IVIG contains antibodies to several cell-surface molecules [168] including antibodies to a 10-peptide sequence containing the (Arg-Gly-Asp) motif that is expressed on cell surfaces and matrix proteins that are part of the integrin adhesion system. IVIG inhibits the adhesion of B cells to fibronectin and inhibits platelet aggregation [169]. Turhan and colleagues [170] and Chang and colleagues [171] investigated the effect of IVIG on a mouse model of sickle cell acute vasoocclusive crisis, in which the adhesion of sickled red blood cells to leukocytes causes the vasoocclusive disease. In this model, high-dose IVIG given after the onset of a crisis resulted in improved blood flow and prolonged survival. These investigators showed that IVIG reverses acute vasoocclusive crisis in sickle cell mice by inhibiting neutrophil adhesion to the capillary endothelial cells.
Summary of IVIG Use in Inflammatory/Autoimmune Disorders
The various mechanisms of the antiinflammatory and immunomodulatory properties of IVIG are reviewed. The first use of IVIG was in the treatment of immune thrombocytopenia, presumably because of Fc receptor blockade. Other mechanisms are reviewed as well as the evidence for the value of IVIG in multiple disorders. IVIG may have yet undiscovered immunomodulating properties on both the innate and adaptive immune systems. Future advances will include a better understanding of its mechanisms of action and modification of the IgG molecule to enhance its immunomodulating properties.
References
- 1.Lucey D. Anthrax. In: Mandel G., Bennett J.E., Donin R., editors. Principles and practice of infectious diseases. 6th edition. Elsevier/Churchill Livingston; Philadelphia: 2005. pp. 3618–3624. 2485–91. [Google Scholar]
- 2.Gold H., Chester P.A. Studies on anthrax: clinical report of ten human cases. J Lab Clin Med. 1935;21:134–152. [Google Scholar]
- 3.Cidrap HHS to buy 20,000 courses of anthrax antitoxin. cidrap.umn.edu/cidrap/content/bt/anthrax/news/jun2006anthrax.html Available at: Accessed June 20, 2006.
- 4.Migone T., Subramanian M., Zhong J. Raxibacumab or the treatment of inhalational anthrax. N Engl J Med. 2009;361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 5.Stiehm E.R., Keller M.A. Passive immunization. In: Feigen R.D., Cherry J.D., Demmler-Harrison G.J., editors. Textbook of pediatric infectious diseases. 6th edition. Saunders/Elsevier; Philadelphia: 2009. pp. 3447–3479. [Google Scholar]
- 6.American Academy of Pediatrics . Diphtheria. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 280–283. [Google Scholar]
- 7.Faber H.K., McIntosh R. McGraw-Hill; New York: 1966. History of the American Pediatric Society 1887–1965. p. 35–6. [Google Scholar]
- 8.American Academy of Pediatrics . Tetanus. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 655–660. [Google Scholar]
- 9.Lee D.C., Lederman H.M. Anti-tetanus toxoid antibodies in intravenous gamma globulin: an alternative to tetanus immune globulin. J Infect Dis. 1992;166:642–645. doi: 10.1093/infdis/166.3.642. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox M.H. Treatment of Clostridium difficile infection. J Antimicrob Chemother. 1998;41(Suppl C):41–46. doi: 10.1093/jac/41.suppl_3.41. [DOI] [PubMed] [Google Scholar]
- 11.Cleary R. Clostridium difficile-associated diarrhea and colitis: clinical manifestations, diagnosis, and treatment. Dis Colon Rectum. 1998;41:1435–1449. doi: 10.1007/BF02237064. [DOI] [PubMed] [Google Scholar]
- 12.Kyne L., Warny M., Qamar A. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 13.McPherson S., Rees C.J., Ellis R. Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea. Dis Colon Rectum. 2006;49:640–645. doi: 10.1007/s10350-006-0511-8. [DOI] [PubMed] [Google Scholar]
- 14.Leung D., Kelly Y.M., Boguniewicz C.P. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633–637. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 15.Salcedo J., Keates S., Pothoulakis C. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gastroenterology. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnon S.S., Schechter R., Inglesby T.V. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1071. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 17.Berg B.O. Syndrome of infant botulism. Pediatrics. 1977;59:321–322. [PubMed] [Google Scholar]
- 18.Centers for Disease Control . Centers for Disease Control; Atlanta (GA): 1979. Botulism in the United States, 1899–1977. Handbook for epidemiologists, clinicians and laboratory workers. [Google Scholar]
- 19.Chertow D.S., Tan E.T., Maslanka S.E. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. JAMA. 2006:2476–2479. doi: 10.1001/jama.296.20.2476. [DOI] [PubMed] [Google Scholar]
- 20.Souayah N., Karim H., Kamin S.S. Severe botulism after focal injection of botulinum toxin. Neurology. 2006;67:1855–1856. doi: 10.1212/01.wnl.0000244417.34846.b6. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. MMWR Morb Mortal Wkly Report. 2010;59(10):299. [PubMed] [Google Scholar]
- 22.American Academy of Pediatrics . Botulism and infant botulism. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 259–262. [Google Scholar]
- 23.Arnon S.S., Schechter R., Maslanka S.E. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;345:462–471. doi: 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 24.Alexander H.E. Treatment of Haemophilus influenzae infection and of meningococcic and pneumococcic meningitis. Am J Dis Child. 1943;66:172–187. [Google Scholar]
- 25.Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simoes E.A.F., Groothuis J.R., Tristram D.A. Respiratory syncytial virus-enriched globulin for the prevention of acute otitis media in high risk children. J Pediatr. 1996;129:214–219. doi: 10.1016/s0022-3476(96)70245-7. [DOI] [PubMed] [Google Scholar]
- 27.Mofenson L.M., Moye J., Jr., Bethel J. Prophylactic intravenous immunoglobulin in HIV-infected children with CD4+ counts of 0.20 × 109/L or more: effect on viral, opportunistic, and bacterial infections. JAMA. 1992;268:483–488. doi: 10.1001/jama.268.4.483. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Child Health and Human Development (NICHHD) Intravenous Immunoglobulin Study Group Intravenous immune globulin for the prevention of bacterial infections in children with symptomatic human immunodeficiency virus infection. N Engl J Med. 1991;325:73–80. doi: 10.1056/NEJM199107113250201. [DOI] [PubMed] [Google Scholar]
- 29.Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- 30.Lucchesi P.F., Bowman J.E. Antitoxin versus no antitoxin in scarlet fever. JAMA. 1930;103:1049–1051. [Google Scholar]
- 31.Perez C.M., Kubak B.M., Cryer H.G. Adjunctive treatment of streptococcal toxic shock syndrome using intravenous immunoglobulin: case report and review. Am J Med. 1997;102:111–113. [PubMed] [Google Scholar]
- 32.Schrage B., Duan G., Yang L.P. Different preparations of intravenous immunoglobulin vary in their efficacy to neutralize streptococcal superantigens: implications for treatment of streptococcal toxic shock syndrome. Clin Infect Dis. 2006;43:743–746. doi: 10.1086/507037. [DOI] [PubMed] [Google Scholar]
- 33.Lamothe F., D’Amico P., Ghosen P. Clinical usefulness of intravenous human immunoglobulins in invasive group A streptococcal infections: case report and review. Clin Infect Dis. 1995;21:1469–1470. doi: 10.1093/clinids/21.6.1469. [DOI] [PubMed] [Google Scholar]
- 34.Kaul R., McGeer A., Norrby-Tegllund A. Intravenous immunoglobulin for streptococcal toxic shock syndrome–a comparative observational study. Clin Infect Dis. 1999;28:800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Pediatrics . Staphylococcal infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 601–615. [Google Scholar]
- 36.Melish M.E., Murata S., Fukunaga C. Vaginal tampon model for toxic shock syndrome. Rev Infect Dis. 1989;11(Suppl 1):219–228. doi: 10.1093/clinids/11.supplement_1.s238. 238–46. [DOI] [PubMed] [Google Scholar]
- 37.Suen J., Chesney P.J., Davis J.P. Toxic shock syndrome. In: Feigen R.D., Cherry J.D., Demmler-Harrison G.J., editors. Textbook of pediatric infectious diseases. 6th edition. Saunders/Elsevier; Philadelphia: 2009. pp. 862–884. [Google Scholar]
- 38.Fischer G.W., Cieslak T.J., Wilson S.R. Opsonic antibodies to Staphylococcus epidermidis: in vitro and in vivo studies using human intravenous immune globulin. J Infect Dis. 1994;169:324–329. doi: 10.1093/infdis/169.2.324. [DOI] [PubMed] [Google Scholar]
- 39.Jenson H.B., Pollock B.H. The role of intravenous immunoglobulin for the prevention and treatment of neonatal sepsis. Semin Perinatol. 1998;22:50–63. doi: 10.1016/s0146-0005(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 40.Baker C.J., Melish M.E., Hall R.T. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. N Engl J Med. 1992;327:213–219. doi: 10.1056/NEJM199207233270401. [DOI] [PubMed] [Google Scholar]
- 41.Waisbren B.A. The treatment of bacterial infections with the combination of antibiotics and gamma globulin. Antibiot Chemother. 1957;7:322–332. [PubMed] [Google Scholar]
- 42.Kelly J. Immunotherapy against antibiotic-resistant bacteria: the Russian experience with an antistaphyloccocal hyperimmune plasma and immunoglobulin. Microbes Infect. 2000;2:1383–1392. doi: 10.1016/s1286-4579(00)01292-2. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M.W. Synergism between human gamma globulin and chloramphenicol in the treatment of experimental bacterial infections. Antibiot Chemother. 1956;7:315–321. [PubMed] [Google Scholar]
- 44.Lewis D.B., Tu W. The physiologic immunodeficiency of immaturity. In: Stiehm E.R., Ochs H.D., Winkelstein J.W., editors. Immunologic disorders in infants and children. 5th edition. Elsevier/Saunders; Philadelphia: 2004. pp. 687–760. [Google Scholar]
- 45.Jenson H.B., Pollock B.H. Meta-analyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics. 1997;99:E2. doi: 10.1542/peds.99.2.e2. [DOI] [PubMed] [Google Scholar]
- 46.Lacy J.B., Ohlsson A. Administration of intravenous immunoglobulins for prophylaxis or treatment of infection in preterm infants: meta-analysis. Arch Dis Child. 1995;72:F151–F155. doi: 10.1136/fn.72.3.f151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth-weight infants (Cochrane Review). The Cochrane Library. Oxford. Issue 1. 2001. [DOI] [PubMed]
- 48.Ohlsson A., Lacy J.B. Intravenous immunoglobulin for suspected or subsequently proven infection in neonates. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD001239.pub2. CD001239. [DOI] [PubMed] [Google Scholar]
- 49.Benjamin D.K., Schelonka R., White R. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol. 2006;26:290–295. doi: 10.1038/sj.jp.7211496. [DOI] [PubMed] [Google Scholar]
- 50.Bloom B., Schelonka R., Kueser T. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr Infect Dis J. 2005;24:858–866. doi: 10.1097/01.inf.0000180504.66437.1f. [DOI] [PubMed] [Google Scholar]
- 51.NIH Consensus Development Conference: diseases, doses, recommendations for intravenous immunoglobulin. HLB Newsletter. Natl Inst Heart Lung Blood Dis. 1990;6:73–78. [Google Scholar]
- 52.Glinz P.W., Nydegger U.E., Ricklin T. Polyvalent immunoglobulins for prophylaxis of bacterial infections in patients following multiple trauma. Intensive Care Med. 1985;11:288–294. doi: 10.1007/BF00273538. [DOI] [PubMed] [Google Scholar]
- 53.Sandberg E.T., Kline M.W., Shearer W.T. The secondary immunodeficiencies. In: Stiehm E.R., editor. Immunologic disorders in infants and children. 4th edition. WB Saunders; Philadelphia: 1996. pp. 553–601. [Google Scholar]
- 54.Laupland K.B., Kirkpatrick A.W., Delaney A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: a systematic review and meta-analysis. Crit Care Med. 2007;35:2686–2692. [PubMed] [Google Scholar]
- 55.Kempe C.H. Studies on smallpox and complications of smallpox vaccination. Pediatrics. 1960;26:176–189. [PubMed] [Google Scholar]
- 56.American Academy of Pediatrics . Smallpox (variola) In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 596–598. [Google Scholar]
- 57.Brown K.E. Parvovirus B19. In: Mandell G.L., Bennett J.E., Doling R., editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 5th edition. Churchill Livingstone; Philadelphia: 2000. pp. 1685–1693. [Google Scholar]
- 58.Inoue S., Kinra N.K., Mukkamala S.R. Parvovirus B19 infection: aplastic crisis, erythema infectiosum and idiopathic thrombocytopenic purpura. Pediatr Infect Dis J. 1991;10:251–253. [PubMed] [Google Scholar]
- 59.Young N.S. Parvovirus infection and its treatment. Clin Exp Immunol. 1996;104(Suppl 1):26–30. [PubMed] [Google Scholar]
- 60.American Academy of Pediatrics . Parvovirus B19. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 491–494. [Google Scholar]
- 61.McGhee S.A., Kaska B., Liebhaber M. Persistent parvovirus-associated chronic fatigue treated with high dose intravenous immunoglobulin. Pediatr Infect Dis J. 2005;24:272–274. doi: 10.1097/01.inf.0000155194.66797.20. [DOI] [PubMed] [Google Scholar]
- 62.Frickhofen N.J., Abkowitz L., Safford M. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia with AIDS. Ann Intern Med. 1990;113:926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 63.Koduri P.R., Kumapley R.J., Valladares J. Chronic pure red cell aplasia caused by parvovirus B19 in AIDS: use of intravenous immunoglobulin: a report of eight patients. Am J Hematol. 1999;61:16–20. doi: 10.1002/(sici)1096-8652(199905)61:1<16::aid-ajh4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 64.Bulinski P., Toledo-Pereyra L.H., Dalal S. Cytomegalovirus infection in kidney transplantation: prophylaxis and management. Transplant Proc. 1996;28:3310–3311. [PubMed] [Google Scholar]
- 65.Jassal S.V., Roscoe J.M., Zaltzman J.S. Clinical practice guidelines: prevention of cytomegalovirus disease after renal transplantation. J Am Soc Nephrol. 1998;9:1697–1708. doi: 10.1681/ASN.V991697. [DOI] [PubMed] [Google Scholar]
- 66.Bonaros N.E., Kocher A., Dunkler D. Comparison of combined prophylaxis of cytomegalovirus hyperimmune globulin plus ganciclovir versus cytomegalovirus hyperimmune globulin alone in high-risk heart transplant recipients. Transplantation. 2004;77:890–897. doi: 10.1097/01.tp.0000119722.37337.dc. [DOI] [PubMed] [Google Scholar]
- 67.Paar D.P., Pollard R.B. Immunotherapy of CMV infections. Adv Exp Med Biol. 1996;394:145–151. doi: 10.1007/978-1-4757-9209-6_15. [DOI] [PubMed] [Google Scholar]
- 68.Negishi H., Yamada H., Hirayama E. Intraperitoneal administration of cytomegalovirus hyperimmunoglobulin to the cytomegalovirus-infected fetus. J Perinatol. 1998;18:466–469. [PubMed] [Google Scholar]
- 69.Nigro G., Adler S.P., La Torre R. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 70.American Academy of Pediatrics . Herpes simplex. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 363–373. [Google Scholar]
- 71.Masci S., De Simone C., Famularo G. Intravenous immunoglobulins suppress the recurrences of genital herpes simplex virus: a clinical and immunological study. Immunopharmacol Immunotoxicol. 1995;17:33–47. doi: 10.3109/08923979509052718. [DOI] [PubMed] [Google Scholar]
- 72.Delone P.J., Corkill J., Jordan M. Successful treatment of Epstein-Barr virus infection with ganciclovir and cytomegalovirus hyperimmune globulin following kidney transplantation. Transplant Proc. 1995;27(Suppl 1):58–59. [PubMed] [Google Scholar]
- 73.Oettle H., Wilborn F., Schmidt C.A. Treatment with ganciclovir and Ig for acute Epstein-Barr virus infection after allogeneic bone marrow transplantation. Blood. 1993;82:2257–2262. [PubMed] [Google Scholar]
- 74.Taguchi Y., Purtilo D.T., Okano M. The effect of intravenous immunoglobulin and interferon-alpha on Epstein-Barr virus-induced lymphoproliferative disorder in a liver transplant recipient. Transplantation. 1994;57:1813–1815. [PubMed] [Google Scholar]
- 75.Filipovich A.H., Gross T., Jyonouchi H. Immune-mediated hematologic and oncologic disorders, including Epstein-Barr virus infection. In: Stiehm E.R., editor. Immunologic disorders in infants and children. 4th edition. WB Saunders; Philadelphia: 1996. pp. 855–888. [Google Scholar]
- 76.Brunell P.A., Gershon A.A. Passive immunization against varicella zoster infections. J Infect Dis. 1973;127:415–423. doi: 10.1093/infdis/127.4.415. [DOI] [PubMed] [Google Scholar]
- 77.American Academy of Pediatrics . Varicella-zoster infections. In: Pickering L.K., Baker C.J., Kimberlin D.W., editors. Red Book: 2009 Report of the Committee on infectious diseases. 28th edition. American Academy of Pediatrics; Elk Grove Village (IL): 2009. pp. 714–727. [Google Scholar]
- 78.Bodian D. Experimental studies on passive immunization against poliomyelitis: I. Protection with human gamma globulin against intramuscular inoculation and combined passive and active immunization. Am J Hyg. 1951;54:132–143. doi: 10.1093/oxfordjournals.aje.a119463. [DOI] [PubMed] [Google Scholar]
- 79.McKinney R.E., Katz S.L., Wilfert C.M. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 80.Dwyer J.M., Erlendsson K. Intraventricular gamma-globulin for the management of enterovirus encephalitis. Pediatr Infect Dis J. 1988;7:S30–S33. [PubMed] [Google Scholar]
- 81.Kondoh H.K., Kobayashi Y., Sugio K. Successful treatment of echovirus meningoencephalitis in sex-linked agammaglobulinaemia by intrathecal and intravenous injection of high titer gammaglobulin. Eur J Pediatr. 1987;146:610–612. doi: 10.1007/BF02467368. [DOI] [PubMed] [Google Scholar]
- 82.Misbah S.A., Spickett G.P., Ryba C.J. Chronic enteroviral meningoencephalitis, in agammaglobulinemia: case report and literature review. J Clin Immunol. 1992;12:266–270. doi: 10.1007/BF00918150. [DOI] [PubMed] [Google Scholar]
- 83.Desmond R.A., Accortt N.A., Talley L. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother. 2006;50:2409–2414. doi: 10.1128/AAC.00227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abzug M.J., Keyserling H.L., Lee M.L. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201–1206. doi: 10.1093/clinids/20.5.1201. [DOI] [PubMed] [Google Scholar]
- 85.Rentz A.C., Libbey J.E., Fujinami R.S. Investigation of treatment failure in neonatal echovirus 7 infection. Pediatr Infect Dis. 2006;25:259–262. doi: 10.1097/01.inf.0000202071.38484.93. [DOI] [PubMed] [Google Scholar]
- 86.Nagington J., Gandy G., Walker J. Use of normal immunoglobulin in an echovirus 11 outbreak in a special-care baby unit. Lancet. 1983;ii:443–446. doi: 10.1016/s0140-6736(83)90402-6. [DOI] [PubMed] [Google Scholar]
- 87.Terrault N.A., Zhou S., Combs C. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24:1327–1333. doi: 10.1002/hep.510240601. [DOI] [PubMed] [Google Scholar]
- 88.Grazi G.L., Mazziotti A., Sama C. Liver transplantation in HBsAg-positive HBV-DNA-negative cirrhotics: immunoprophylaxis and long term outcome. Liver Transpl Surg. 1996;2:418–425. doi: 10.1002/lt.500020603. [DOI] [PubMed] [Google Scholar]
- 89.Samuel D., Bismuth A., Serres C. HBV-infection in liver transplantation in HBsAg positive patients: experience with long-term immunoprophylaxis. Transplant Proc. 1991;23:1492–1494. [PubMed] [Google Scholar]
- 90.Makhoul B., Braun E., Herskovitz M. Hyperimmune gammaglobulin for the treatment of West Nile virus encephalitis. Isr Med Assoc J. 2009;11:151–153. [PubMed] [Google Scholar]
- 91.Ben-Nathan D, Gershoni-Yahalom O, Samina I. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. http://www.biomedcentral.com/1471-2334/9/18 Available at: [DOI] [PMC free article] [PubMed]
- 92.Kudoyarova-Zubavichene N.M., Sergeyev N.N., Chepurnov A.A. Preparation and use of hyperimmune serum for prophylaxis and therapy of Ebola virus infections. J Infect Dis. 1999;179(Suppl 1):218–223. doi: 10.1086/514294. [DOI] [PubMed] [Google Scholar]
- 93.Jahrling P.B., Geisbert T.W., Geisbert J.B. Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(Suppl):224–234. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 94.Mupapa K., Massamba M., Kibadi K. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J Infect Dis. 1999;179(Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 95.Dumpis U., Crook D., Oksi J. Tick-borne encephalitis. Clin Infect Dis. 1999;28:882–890. doi: 10.1086/515195. [DOI] [PubMed] [Google Scholar]
- 96.Von Hedenström M., Heberle U., Theobald K. Vaccination against tick-borne encephalitis (TBE): influence of simultaneous application of TBE immunoglobulin on seroconversion and rate of adverse events. Vaccine. 1995;13:759–762. doi: 10.1016/0264-410x(94)00032-i. [DOI] [PubMed] [Google Scholar]
- 97.Maiztegui J.I., Fernandez N.J., De Damilano A.J. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;ii:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 98.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orange J.S., Hossny E.M., Weiler C.R. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 100.Constantine M.M., Thomas W., Whitman L. Intravenous immunoglobulin utilization in the Canadian Atlantic provinces: a report of the Atlantic Collaborative Intravenous Immune Globulin Utilization Working Group. Transfusion. 2007;47:2072–2080. doi: 10.1111/j.1537-2995.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 101.Provan D., Chapel H.M., Sewell W.A. Prescribing intravenous immunoglobulin: summary of Department of Health guidelines. BMJ. 2008;337:a1831. doi: 10.1136/bmj.a1831. [DOI] [PubMed] [Google Scholar]
- 102.Bruton O.C. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [PubMed] [Google Scholar]
- 103.Geha R.S., Notarangelo L.D., Casanova J.L. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120:776–794. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyle J.M., Buckley R.H. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27:497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- 105.Leiva L.E., Zelazco M., Oleastro M. Primary immunodeficiency diseases in Latin America: the second report of the LAGID registry. J Clin Immunol. 2007;27:101–108. doi: 10.1007/s10875-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 106.Kirkpatrick P., Riminton S. Primary immunodeficiency diseases in Australia and New Zealand. J Clin Immunol. 2007;27:517–524. doi: 10.1007/s10875-007-9105-z. [DOI] [PubMed] [Google Scholar]
- 107.Luzi G., Businco L., Aiuti F. Primary immunodeficiency syndromes in Italy: a report of the national register in children and adults. J Clin Immunol. 1983;3:316–320. doi: 10.1007/BF00915792. [DOI] [PubMed] [Google Scholar]
- 108.Stray-Pedersen A., Abrahamsen T.G., Froland S.S. Primary immunodeficiency diseases in Norway. J Clin Immunol. 2000;20:477–485. doi: 10.1023/a:1026416017763. [DOI] [PubMed] [Google Scholar]
- 109.Conley M.E., Dobbs A.K., Farmer D.M. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 110.Notarangelo L.D., Lanzi G., Peron S. Defects of class-switch recombination. J Allergy Clin Immunol. 2006;117:855–864. doi: 10.1016/j.jaci.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 111.Yong P.F., Tarzi M., Chua I. Common variable immunodeficiency: an update on etiology and management. Immunol Allergy Clin North Am. 2008;28:367–386. doi: 10.1016/j.iac.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Bonilla F.A., Bernstein I.L., Khan D.A. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94:S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 113.Wolpert J., Knutsen A. Natural history of selective antibody deficiency to bacterial polysaccharide antigens in children. Pediatr Asthma Allergy Immunol. 1998;12:183–191. [Google Scholar]
- 114.Hanson E.P., Monaco-Shawver L., Solt L.A. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Orange J.S., Levy O., Brodeur S.R. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 116.Orange J.S., Geha R.S., Bonilla F.A. Acute chylothorax in children: selective retention of memory T cells and natural killer cells. J Pediatr. 2003;143:243–249. doi: 10.1067/S0022-3476(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 117.Strober W., Wochner R.D., Carbone P.P. Intestinal lymphangiectasia: a protein-losing enteropathy with hypogammaglobulinemia, lymphocytopenia and impaired homograft rejection. J Clin Invest. 1967;46:1643–1656. doi: 10.1172/JCI105656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalal I., Reid B., Nisbet-Brown E. The outcome of patients with hypogammaglobulinemia in infancy and early childhood. J Pediatr. 1998;133:144–146. doi: 10.1016/s0022-3476(98)70195-7. [DOI] [PubMed] [Google Scholar]
- 119.Dorsey M.J., Orange J.S. Impaired specific antibody response and increased B-cell population in transient hypogammaglobulinemia of infancy. Ann Allergy Asthma Immunol. 2006;97:590–595. doi: 10.1016/S1081-1206(10)61085-X. [DOI] [PubMed] [Google Scholar]
- 120.Whelan M.A., Hwan W.H., Beausoleil J. Infants presenting with recurrent infections and low immunoglobulins: characteristics and analysis of normalization. J Clin Immunol. 2006;26:7–11. doi: 10.1007/s10875-006-8144-1. [DOI] [PubMed] [Google Scholar]
- 121.Yong P.F., Chee R., Grimbacher B. Hypogammaglobulinaemia. Immunol Allergy Clin North Am. 2008;28:691–713. doi: 10.1016/j.iac.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 122.Barlan I.B., Geha R.S., Schneider L.C. Therapy for patients with recurrent infections and low serum IgG3 levels. J Allergy Clin Immunol. 1993;92:353–355. doi: 10.1016/0091-6749(93)90179-j. [DOI] [PubMed] [Google Scholar]
- 123.Abdou N.I., Greenwell C.A., Mehta R. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int Arch Allergy Immunol. 2009;149:267–274. doi: 10.1159/000199723. [DOI] [PubMed] [Google Scholar]
- 124.Janeway C.A., Rosen F.S. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med. 1966;275:826–831. doi: 10.1056/NEJM196610132751508. [DOI] [PubMed] [Google Scholar]
- 125.Cunningham-Rundles C., Siegal F.P., Smithwick E.M. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann Intern Med. 1984;101:435–439. doi: 10.7326/0003-4819-101-4-435. [DOI] [PubMed] [Google Scholar]
- 126.Quartier P., Debre M., De Blic J. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134:589–596. doi: 10.1016/s0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 127.Pickett D., Modell V., Leighton I. Impact of a physician education and patient awareness campaign on the diagnosis and management of primary immunodeficiencies. Immunol Res. 2008;40:93–94. doi: 10.1007/s12026-007-8013-x. [DOI] [PubMed] [Google Scholar]
- 128.Simoens S. Pharmacoeconomics of immunoglobulins in primary immunodeficiency. Expert Rev Pharmacoecon Outcomes Res. 2009;9:375–386. doi: 10.1586/erp.09.37. [DOI] [PubMed] [Google Scholar]
- 129.Carbone J. Adverse reactions and pathogen safety of intravenous immunoglobulin. Curr Drug Saf. 2007;2:9–18. doi: 10.2174/157488607779315480. [DOI] [PubMed] [Google Scholar]
- 130.Quinti I., Pierdominici M., Marziali M. European surveillance of immunoglobulin safety–results of initial survey of 1243 patients with primary immunodeficiencies in 16 countries. Clin Immunol. 2002;104:231–236. doi: 10.1006/clim.2002.5239. [DOI] [PubMed] [Google Scholar]
- 131.Gelfand E.W. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6:592–599. doi: 10.1016/j.intimp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 132.Berger M., Cupps T.R., Fauci A.S. Immunoglobulin replacement therapy by slow subcutaneous infusion. Ann Intern Med. 1980;93:55–56. doi: 10.7326/0003-4819-93-1-55. [DOI] [PubMed] [Google Scholar]
- 133.Gardulf A., Andersen V., Bjorkander J. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345:365–369. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 134.Gardulf A., Hammarstrom L., Smith C.I. Home treatment of hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet. 1991;338:162–166. doi: 10.1016/0140-6736(91)90147-h. [DOI] [PubMed] [Google Scholar]
- 135.Chapel H.M., Spickett G.P., Ericson D. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. doi: 10.1023/a:1006678312925. [DOI] [PubMed] [Google Scholar]
- 136.Ochs H.D., Gupta S., Kiessling P. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–273. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 137.Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112:1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 138.Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28:779–802. doi: 10.1016/j.iac.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 139.Moore M.L., Quinn J.M. Subcutaneous immunoglobulin replacement therapy for primary antibody deficiency: advancements into the 21st century. Ann Allergy Asthma Immunol. 2008;101:114–121. doi: 10.1016/S1081-1206(10)60197-4. [DOI] [PubMed] [Google Scholar]
- 140.Bonilla F.A. Pharmacokinetics of immunoglobulin administered via intravenous of subcutaneous routes. Immunol Allergy Clin North Am. 2008;28:803–819. doi: 10.1016/j.iac.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 141.Schiff R.I. Individualizing the dose of intravenous immune serum globulin for therapy of patients with primary humoral immunodeficiency. Vox Sang. 1985;49(Suppl 1):15–24. doi: 10.1111/j.1423-0410.1985.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 142.Bonagura V.R., Marchlewski R., Cox A. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122:210–212. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 143.Gardulf A., Nicolay U. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:434–442. doi: 10.1097/01.all.0000246619.49494.41. [DOI] [PubMed] [Google Scholar]
- 144.Fasth A., Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28:370–378. doi: 10.1007/s10875-008-9180-9. [DOI] [PubMed] [Google Scholar]
- 145.Eijkhout H.W., van Der Meer J.W., Kallenberg C.G. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165–174. doi: 10.7326/0003-4819-135-3-200108070-00008. [DOI] [PubMed] [Google Scholar]
- 146.Roifman C.M., Schroeder H., Berger M. Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol. 2003;3:1325–1333. doi: 10.1016/s1567-5769(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 147.Busse P.J., Razvi S., Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 148.Imbach P., Barandun S., d’Apuzzo V. High dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;i:1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 149.Debre M., Bonnet M.C., Fridman W.H. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–949. doi: 10.1016/0140-6736(93)92000-j. [DOI] [PubMed] [Google Scholar]
- 150.Nimmerjahn F., Ravetch J. Fcg receptor as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 151.Samuelsson A., Towers T.L., Ravetch J.V. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 152.Kaneko Y., Nimmerjahn F., Ravetch J. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 153.Anthony R., Nimmerjahn F., Ashline D. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Anthony R.M., Wermeling F., Karlsson M.C. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2009;10:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu Z., Lennon V.A. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340:227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 156.Bleeker W., Teeling J., Hack C. Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood. 2001;98:3136–3142. doi: 10.1182/blood.v98.10.3136. [DOI] [PubMed] [Google Scholar]
- 157.Hansen R.J., Balthasar J.P. Effects of intravenous immunoglobulin on platelet count and antiplatelet antibody disposition in a rat model of immune thrombycytopenia. Blood. 2002;100:2087–2093. [PubMed] [Google Scholar]
- 158.Kazatchkine M., Dietrich G., Ronda N. V region-mediated selection of autoreactive repertoires by intravenous immunoglobulin (IVIG) Immunol Rev. 1994;139:79–107. doi: 10.1111/j.1600-065x.1994.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 159.Nydegger U., Sultan Y., Kazatchkine M. The concept of anti-idiotypic regulation of selected autoimmune diseases by intravenous immunoglobulin. Clin Immunol Immunopathol. 1989;53:S72–S82. doi: 10.1016/0090-1229(89)90072-x. [DOI] [PubMed] [Google Scholar]
- 160.Sultan Y., Kazatchkine M.D., Maisonneuve P. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet. 1984;ii:765–768. doi: 10.1016/s0140-6736(84)90701-3. [DOI] [PubMed] [Google Scholar]
- 161.Berger M., Rosenkranz P., Brown C.Y. Intravenous and standard immune serum globulin preparations interfere with uptake of 125I-C3 onto sensitized erythrocytes and inhibit hemolytic complement activity. Clin Immunol Immunopathol. 1985;34:227–236. doi: 10.1016/0090-1229(85)90027-3. [DOI] [PubMed] [Google Scholar]
- 162.Basta M. Modulation of complement-mediated immune damage by intravenous immune globulin. Clin Exp Immunol. 1996;104:21–25. [PubMed] [Google Scholar]
- 163.Dalakas M.C. Intravenous immune globulin for dermatomyositis. N Engl J Med. 1994;330:1392–1393. doi: 10.1056/nejm199405123301918. [DOI] [PubMed] [Google Scholar]
- 164.Dalakas M.C. Controlled studies with high-dose intravenous immunoglobulin in the treatment of dermatomyositis, inclusion body myositis, and polymyositis. Neurology. 1998;51:S37–S45. doi: 10.1212/wnl.51.6_suppl_5.s37. [DOI] [PubMed] [Google Scholar]
- 165.Basta M., Illa I., Dalaskas M. Increased in vitro uptake of the complement C3b in the serum of patients with Guillain-Barré syndrome, myasthenia gravis and dermatomyositis. J Neuroimmunol. 1996;71:227–229. doi: 10.1016/s0165-5728(96)00133-6. [DOI] [PubMed] [Google Scholar]
- 166.Dalakas M.C. Intravenous immune globulin therapy for neurologic diseases. Ann Intern Med. 1997;126:721–730. doi: 10.7326/0003-4819-126-9-199705010-00008. [DOI] [PubMed] [Google Scholar]
- 167.Viard I., Wehrli P., Bullanim R. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 168.Negi V., Elluru S., Siberil S. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–245. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- 169.Vassilev T., Kazatchkine M., Van Huyen J. Inhibition of cell adhesion by antibodies to Arg-Gly-Asp (RGD) in normal immunoglobulin for therapeutic use. Blood. 1999;93:3624–3631. [PubMed] [Google Scholar]
- 170.Turhan A., Jenab P., Bruhns P. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103:2397–2400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 171.Chang J., Shi P.A., Chiang E.Y. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]