Abstract
Cyclophilins (Cyps) belong to the family of peptidyl-prolyl isomerases (PPIases). The PPIase activity of most Cyps is inhibited by the immunosuppressive drug cyclosporin A and several of its non-immunosuppressive analogs, which can also block the replication of nidoviruses (arteriviruses and coronaviruses). Cyclophilins have been reported to play an essential role in the replication of several other RNA viruses, including human immunodeficiency virus-1, hepatitis C virus, and influenza A virus. Likewise, the replication of various nidoviruses was reported to depend on Cyps or other PPIases. This review summarizes our current understanding of this class of nidovirus-host interactions, including the potential function of in particular CypA and the inhibitory effect of Cyp inhibitors. Also the involvement of the FK-506-binding proteins and parvulins is discussed. The nidovirus data are placed in a broader perspective by summarizing the most relevant data on Cyp interactions and Cyp inhibitors for other RNA viruses.
Keywords: Cyclophilin A, FK-506-binding proteins, Parvulins, Coronavirus, Arterivirus, Cyclosporin A, Alisporivir, NIM-811, RNA virus
Highlights
-
•
Nidovirus replication is inhibited by cyclophilin inhibitors.
-
•
Arterivirus replication depends on cyclophilin A.
-
•
Cyclosporin A blocks arterivirus RNA synthesis.
-
•
Using cyclophilin inhibitors against nidoviruses in vivo needs more investigation.
1. Nidoviruses, an introduction
The order Nidovirales currently comprises four families – the Coronaviridae, Arteriviridae, Roniviridae, and Mesoniviridae - that span across a wide range of hosts, including mammalian, avian, reptile, fish, and invertebrate species (https://talk.ictvonline.org/taxonomy/). Within this order, the coronaviruses and arteriviruses have been studied in most detail, due to the societal and economic impact of some family members, unusual features of their pathogenesis, and the complexity of their molecular biology. The latter includes having large to very large polycistronic positive-strand RNA genomes, with sizes ranging from 13 to 16 kb for arteriviruses, via ~ 20 kb for mesoniviruses, to 26–34 kb for roni- and coronaviruses (Gorbalenya et al., 2006, Nga et al., 2011). The best-known members of the arterivirus family are porcine reproductive and respiratory syndrome virus (PRRSV) and equine arteritis virus (EAV). The coronaviruses (CoVs) are classified into two subfamilies: the Torovirinae and the Coronavirinae, the latter being subdivided into the genera Alpha-, Beta-, Gamma-, and Deltacoronavirus. Most mammalian CoVs are alpha- or betacoronaviruses and these genera include the four ‘established’ human coronaviruses (HCoVs 229E, OC43, NL63 and HKU1), the zoonotic coronaviruses causing severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (Vijay and Perlman, 2016), and related viruses from bats (Hu et al., 2017, Li et al., 2005) and camels (Sabir et al., 2016). Thus far, gamma- and deltacoronaviruses have been discovered mostly in avian species (Woo et al., 2012).
1.1. Societal and economic impact of nidoviruses
In the past 15 years, nidovirus research has been driven forward in particular by the emergence of two life-threatening CoVs in humans, the betacoronaviruses SARS-CoV and MERS-CoV, which most likely originate from bats and were introduced by zoonotic transfer from intermediate hosts, civet cats and dromedary camels for SARS-CoV and MERS-CoV, respectively (Ge et al., 2013, Hu et al., 2015, Menachery et al., 2015). Of note, the presence of neutralizing antibodies in camels suggests that MERS-CoV or related viruses may have been present in this reservoir for decades (Hu et al., 2015). The short-lived SARS-CoV outbreak in 2002–2003 resulted in 8098 reported cases leading to 774 deaths, while affecting 29 countries (http://www.who.int/csr/sars/en/). Between its emergence in 2012 and March 2018, MERS-CoV has caused > 2100 laboratory-confirmed human infections and at least 750 deaths (http://www.who.int/emergencies/mers-cov/en/). The clinical presentation of SARS-CoV and MERS-CoV ranges from asymptomatic or mild symptoms to acute respiratory disease, in the case of SARS originally described as an “atypical pneumonia” accompanied by fever and severe respiratory distress (Hui et al., 2014). The SARS-CoV and MERS-CoV outbreaks greatly augmented the interest in the CoV family, although human CoVs had already been known since the 1960's, when HCoV-OC43 and HCoV-229E were identified. These two viruses are known to cause mild respiratory disease and, after rhinoviruses, are a leading cause of common colds (10–15% of the cases; reviewed in Wat, 2004). More recently, two additional HCoVs were discovered, HCoV-NL63 and HCoV-HKU1, which again are associated with respiratory disease (reviewed in Pyrc et al., 2007).
The potential impact of nidoviruses as veterinary pathogens is exemplified by the porcine epidemic diarrhea coronavirus (PEDV) and also by the arterivirus PRRSV, which both continue to cause major economic losses in the swine industry worldwide (Holtkamp et al., 2013, Lin et al., 2016). Likewise, ronivirus infections have done significant damage in the Asian shrimp farming industry (Flegel, 2012). Such outbreaks and the threat of additional emerging (zoonotic) nidoviruses, in combination with the lack of effective antiviral strategies, highlight the importance of advancing our knowledge of the replication of the members of this diverse virus order and their interactions with the host.
1.2. Nidovirus molecular biology
The conserved genome organization and expression strategy of nidoviruses includes the translation of two large replicase open reading frames (ORFs 1a and 1b) from the genomic RNA. This yields the replicase polyprotein (pp) 1a and, following a −1 ribosomal frameshift, the C-terminally extended pp1ab. The two polyproteins are proteolytically processed by multiple internal proteases to liberate (in the case of arteri- and coronaviruses) at least 13–16 nonstructural proteins (nsps). Among these nsps are subunits containing RNA-dependent RNA-polymerase and helicase functions, key players in the enzyme complex responsible for viral RNA synthesis. Together with recruited host cell proteins, nidovirus nsps form membrane-associated replication and transcription complexes (Gosert et al., 2002, Hagemeijer et al., 2012, Pedersen et al., 1999, van Hemert et al., 2008a, van Hemert et al., 2008b) that localize to a network of virus-induced structures, typically including double-membrane vesicles, in the perinuclear region of the infected cell (reviewed in de Wilde et al., 2017b; Romero-Brey and Bartenschlager, 2016; van der Hoeven et al., 2016). A nested set of subgenomic (sg) mRNAs is produced to express the structural and accessory proteins that are encoded downstream of the nidovirus replicase gene (Pasternak et al., 2006, Sawicki et al., 2007, Snijder et al., 2013, Sola et al., 2011). Despite these common features of viruses in the order Nidovirales, the various nidovirus taxa differ strikingly in the type, number, and size of their structural proteins, which also explains the observed variation in virion structure and morphology.
The recent outbreaks of emerging nidoviruses inspired extensive studies of their epidemiology and pathogenesis, and underlined the importance of developing prophylactic and therapeutic options, including vaccines and drugs targeting either viral functions or host factors recruited to support nidovirus replication. In this context, the inhibition of a range of RNA viruses by cyclophilin (Cyp) inhibitors (Hopkins and Gallay, 2015) prompted several research teams to investigate their impact on nidovirus replication, mainly for coronaviruses and - to a lesser extent – for arteriviruses. Below we will first describe the key features of members of the Cyp family and then summarize our current knowledge regarding their involvement in nidovirus replication. This includes the anti-nidoviral effect of cyclosporin A (CsA), the best known Cyp inhibitor (Borel et al., 1976, Handschumacher et al., 1984), and several of its non-immunosuppressive analogs. Finally, our current knowledge on the involvement of Cyps in the replication of other RNA viral pathogens is summarized, to illustrate the wide variety of mechanisms by which this common host factor can be involved in supporting viral replication.
2. Cyclophilins and cyclophilin inhibitors
The peptidyl/prolyl isomerases (PPIases) comprise the immunophilin superfamily, to which the Cyps and the FK506-binding proteins (FKBPs) families belong, and the parvulin protein family (Schiene-Fischer, 2006). Cyps and FKBPs are ubiquitous in both eukaryotes and prokaryotes, and both protein families were initially identified on the basis of their ability to bind the immunosuppressive drugs CsA (Handschumacher et al., 1984), and FK506 or rapamycin (Lane et al., 1991), respectively. The parvulins were initially discovered in the cytoplasm of E. coli (Rahfeld et al., 1994) and are the smallest proteins known to have PPIase activity. This activity is essential for catalyzing the cis-trans isomerization of the peptide bond upstream of proline residues, which is a rate-limiting step in protein folding (Lang et al., 1987, Schmid, 1993). The identification of the first protein with PPIase activity (Fischer et al., 1984) coincided with the purification from bovine thymocytes of a cellular protein with high affinity for the immunosuppressant CsA: cyclosporin-binding protein A (CypA) (Handschumacher et al., 1984). Five years later, it was discovered that both proteins were one and the same (Fischer et al., 1989, Takahashi et al., 1989). Cyps are involved in a wide range of cellular processes, including protein folding, protein trafficking, and cell signaling (Naoumov, 2014). Despite the fact that all members of the PPIase superfamily share the same enzymatic activity, protein sequences and structures differ enormously between the three families (Barik, 2006, Davis et al., 2010, Hanes, 2015). The human genome is currently believed to encode 19 cyclophilins, 18 FKBPs, and three parvulins (Pin1, Par14, and Par17) (Gray et al., 2015).
Cyclophilins have been identified in a range of organisms, including mammals, plants, insects, fungi, and bacteria (Barik, 2006, Wang and Heitman, 2005). Not all Cyps catalyze the cis-trans isomerization of proline-preceding peptide bonds; in fact in vitro PPIase activity has been demonstrated for only seven of the human Cyps (Davis et al., 2010). Some Cyps, like CypA, consist of solely a PPIase domain, while in other Cyps this domain is flanked by additional sequences or modular domains, which control their subcellular localization and/or are thought to be specific for cellular functions (Barik, 2006, Schiene-Fischer, 2015). Despite the fact that Cyps have been implicated in a range of cellular processes, the function of many Cyps is unknown. Also, it has proven to be difficult to identify the natural substrates of the PPIase activity (reviewed in Hopkins and Gallay, 2015). Besides their role in specific cellular functions (reviewed in Naoumov, 2014), CypA, CypB, and CypD have been shown to also function in the replication of certain groups of RNA viruses. Below we will briefly summarize the cellular function of these Cyps.
2.1. Cyclophilin A
The 18-kDa cytosolic CypA is also referred to as peptidyl-prolyl isomerase A (PPIA) or Cyp18. It is one of the most abundant proteins in the cytoplasm (0.1–0.4% of total protein content) and is expressed in all tissues (Harding et al., 1986, Ryffel et al., 1991). In the cytosol, CypA plays a role in a broad range of cellular functions, like facilitating protein folding, protein trafficking, T-cell activation, and cell signaling (reviewed in Naoumov, 2014; Nigro et al., 2013). Although CypA normally is an intracellular protein, inflammatory stimuli like infections, hypoxia, or oxidative stress can elicit CypA secretion via a vesicular transport mechanism that depends on Rho kinase activation (reviewed in Bukrinsky, 2015). CypA proved to be non-essential for cell growth as depletion of CypA in cells or in PPIA-/- knockout mice did not affect survival and/or growth kinetics (Chatterji et al., 2009, Colgan et al., 2005, de Wilde et al., 2017c).
2.2. Cyclophilin B
The 22-kDa CypB essentially consists of a PPIase domain that is equipped with a cleavable N-terminal signal sequence to target the protein to the lumen of the endoplasmic reticulum (ER) (Price et al., 1991, Spik et al., 1991). N-terminally truncated CypB is secreted in response to inflammatory stimuli, although the mechanism by which CypB is cleaved is currently unclear (reviewed in Bukrinsky, 2015). In addition, binding of CsA to the CsA-binding site in CypB induces the release of CypB from the ER via the secretory pathway. It has been hypothesized that this binding of CsA interferes with a functional interaction of CypB with other ER-associated proteins that normally retains CypB ER-associated (Fearon et al., 2011, Price et al., 1994).
2.3. Cyclophilin D
The mitochondrially targeted CypD regulates the mitochondrial permeability transition pore (MPTP) that controls the Ca2+ transport from mitochondrion to the cytosol. Upon sensitization to calcium overload and oxidative stress, CypD binds the MPTP to induce opening of the pore to release Ca2+. These processes ultimately lead to necrosis (Elrod and Molkentin, 2013, Rasola and Bernardi, 2011) or necroptosis, a programmed form of necrosis that depends on CypD (Linkermann and Green, 2014). CsA-mediated CypD inhibition or CypD knockout studies in cell culture have been shown to prevent necrosis and CypD-/- mice were resistant to necrotic (Baines et al., 2005, Nakagawa et al., 2005b) and necroptotic cell death (reviewed in Linkermann and Green, 2014).
2.4. Cyclosporin A and FK-506
In the 1970s and 1980s, the immunosuppressive properties of CsA and FK-506 were discovered and revolutionized the field of organ transplantation by reducing the risk of organ rejection (reviewed in Tedesco and Haragsim, 2012). CsA, isolated from the soil fungus Tolypcladium inflatum, is a lipophilic, neutral, cyclic endecapeptide (Borel et al., 1976). FK-506 (or Tacrolimus) was isolated from Streptomyces tsukubaensis and is functionally but not structurally related to CsA (Goto et al., 1987). The immunosuppressive properties of CsA and FK-506 depend on complex formation with Cyps and FKBP12, respectively. In turn, these complexes sequester and inhibit calcineurin, a serine/threonine protein phosphatase that is a key player in T cell activation (Liu et al., 1991). The interaction with calcineurin inhibits the phosphatase activity of the latter enzyme, which prevents the nuclear translocation of its substrate nuclear factor of activated T cells (NF-AT) and consequently the expression of immune genes like IL-2 and IL-4 (Liu et al., 1991). In this manner, CsA and FK-506 suppress the activation of cytotoxic and helper T cells (Ho et al., 1996, Schreiber and Crabtree, 1992, reviewed in Martinez-Martinez and Redondo, 2004). These drugs proved to be suitable immunosuppressive agents due to the absence of the toxicity commonly associated with other fungus-derived immunosuppressive pharmaceuticals. Subsequently, CsA also revolutionized the treatment of autoimmune diseases such as rheumatoid arthritis, Crohn's disease, and vernal keratoconjunctivitis, as well as various dermatological diseases (reviewed in Hopkins and Gallay, 2015).
2.5. Non-immunosuppressive CsA analogs
CsA has be shown to inhibit the replication of a variety of DNA and RNA viruses in cell culture (see also chapters 3 and 4), but in the context of antiviral therapy, immunosuppression by compounds like CsA would obviously be undesirable. To address this issue, a variety of CsA analogs that do bind Cyps without inducing immunosuppression have been engineered. These second-generation Cyp inhibitors include Alisporivir (ALV; or Debio-025) (Landrieu et al., 2010, Paeshuyse et al., 2006), NIM811 (Ciechomska et al., 2005), and SCY-635 (Hopkins et al., 2010). These compounds have been modified to abolish their affinity for calcineurin, while increasing their affinity for Cyps. As a result, these non-immunosuppressive CsA analogs are more potent inhibitors of the interactions of Cyps with (viral) proteins, while their administration does no longer lead to inhibition of NF-AT signaling, as calcineurin's phosphatase activity is not inhibited. Therefore, these drugs have become critical tools to study Cyp functions, cellular signaling pathways, and/or interactions of Cyps that are relevant for viral replication (as discussed in this review). Also, most of these CsA analogs have been explored as antiviral drugs (reviewed in Frausto et al., 2013; Hopkins and Gallay, 2015).
3. Cyps and Cyp inhibitors in nidovirus replication
3.1. Cyclophilin inhibitors can block coronavirus replication
Various laboratories have shown that, in cell culture, the replication of members of different CoV genera can be inhibited by CsA treatment. Low-micromolar levels of CsA inhibit the replication of alphacoronaviruses (HCoV-229E, transmissible gastroenteritis virus (TGEV), feline coronavirus (FCoV), and PEDV) (de Wilde et al., 2011, Kim and Lee, 2014, Pfefferle et al., 2011, Tanaka et al., 2012) and betacoronaviruses (mouse hepatitis virus (MHV), SARS-CoV, and MERS-CoV) (de Wilde et al., 2013b, de Wilde et al., 2011, Pfefferle et al., 2011). Collectively, these reports established the broad-spectrum anti-coronavirus activity of CsA in cell culture-based infection models (for a complete overview of nidoviruses known to be inhibited by treatment with CsA and/or its analogs, see Table 1).
Table 1.
Overview of the effect of Cyp inhibitors and of Cyps reported to be involved in nidovirus replication.
| Virus(family) |
Cyclophilin inhibitors |
Depletion of cyclophilins |
||||||
|---|---|---|---|---|---|---|---|---|
| Inhibitor | Readout | EC50 (µM) | Reference | Cyps | Assay | Observed effect | Reference | |
| Arteriviruses | ||||||||
| EAV | CsA, Debio-064 | Virus-driven GFP expression, virus yields | 0.3–1.0 µM | (de Wilde et al., 2013a) | CypA | siRNA, shRNA, knockout | Inhibition of virus replication | (de Wilde et al., 2013a, de Wilde et al., 2017c) |
| PRRSV | CsA, Debio-064 | Virus-driven GFP expression, virus yields | 5.1–5.2 µM | (de Wilde et al., 2013a) | –d | – | – | – |
| Alphacoronaviruses | ||||||||
| HCoV-OC43 | CsA | Virus-induced cell death | 2.5 µMc | (Favreau et al., 2012) | CypD | CsA treatment | Increased cell survival by interfering with virus-induced apoptosis | (Favreau et al., 2012) |
| HCoV-NL63 | CsA, ALV, NIM-811, other CsA analogs, FK-506 | qPCR, virus yields | 0.8–6.6 µM | (Carbajo-Lozoya et al., 2014, Carbajo-Lozoya et al., 2012, Pfefferle et al., 2011) | CypA | shRNA | Inhibition of virus replication | (Carbajo-Lozoya et al., 2014) |
| HCoV-229E | CsA, ALV, FK-506 | Virus-driven GFP or luciferase expression, virus yields | 2.3–12.2 µM | (Carbajo-Lozoya et al., 2012, de Wilde et al., 2017a, de Wilde et al., 2011, Pfefferle et al., 2011, von Brunn et al., 2015) | CypA? | shRNA, knockout | Inhibition of virus replication in Huh7.5 cells, no effect in Huh7 | (de Wilde et al., 2017c, von Brunn et al., 2015) |
| PEDV | CsA | Virus yields | 5 µMc | (Kim and Lee, 2014) | – | – | – | – |
| FCoV | CsA, DTMa | Virus yields | 2.7–4.1 µM | (Pfefferle et al., 2011, Tanaka et al., 2015, Tanaka et al., 2012, Tanaka et al., 2017) | CypA, CypB, Pin1b | shRNA, knockout | Inhibition of virus production | (Pfefferle et al., 2011, Tanaka et al., 2015, Tanaka et al., 2017) |
| Betacoronaviruses | ||||||||
| MHV | CsA, ALV | Virus-driven GFP expression, virus yields | 6.3–12 µM | (de Wilde et al., 2017a, de Wilde et al., 2011) | – | – | – | – |
| TGEV | CsA | Virus yields | 10 µM | (Pfefferle et al., 2011) | – | – | – | – |
| SARS-CoV | CsA, ALV, FK-506 | Virus-driven GFP expression, virus yields | 8.3–12 µM | (Carbajo-Lozoya et al., 2012, de Wilde et al., 2017a, de Wilde et al., 2011, Pfefferle et al., 2011) | CypA, CypB | siRNA, shRNA | None | (de Wilde et al., 2011; de Wilde et al., unpublished observations) |
| MERS-CoV | CsA, ALV | Virus-induced cell death, virus yields | 3.4–7.5 µM | (de Wilde et al., 2017a, de Wilde et al., 2013b) | CypA | Knockout | Minor reduction in virus replication | (de Wilde et al., 2017a, 2017c) |
| Gammacoronaviruses | ||||||||
| IBV (Beaudette) | CsA | Virus yields | 5.5 µM | (Pfefferle et al., 2011) | – | – | – | – |
DTM is an inhibitor of the parvulin Pin-1.
Pin-1 is member of the parvulin family and not a cyclophilin protein family member.
Only one inhibitor concentration used, no EC50 value was reported.
Not reported.
Coronavirus infection in cell culture can also be inhibited by non-immunosuppressive CsA analogs, with EC50 values similar to those of CsA. Treatment with ALV led to a two- to five-log reduction of MERS-CoV, HCoV-229E, and SARS-CoV progeny titers (EC50 values ranging from 1.3 to 8 µM) (de Wilde et al., 2017a). In addition, similar low-micromolar concentrations of ALV, NIM-811, and other CsA analogs effectively blocked HCoV-NL63 infection (Carbajo-Lozoya et al., 2014). Notably, for some CoVs variable antiviral effects were observed in different cell types, including Huh7 cells and various Vero cell subtypes, with e.g. the antiviral effect of ALV against SARS-CoV being less pronounced in Vero-E6 cells than in another Vero cell subclone (de Wilde et al., 2017a). The reasons for these variations in antiviral effects are poorly understood, but may include variations in drug uptake, Cyp expression levels, and/or the overall kinetics of virus replication in a particular cell line.
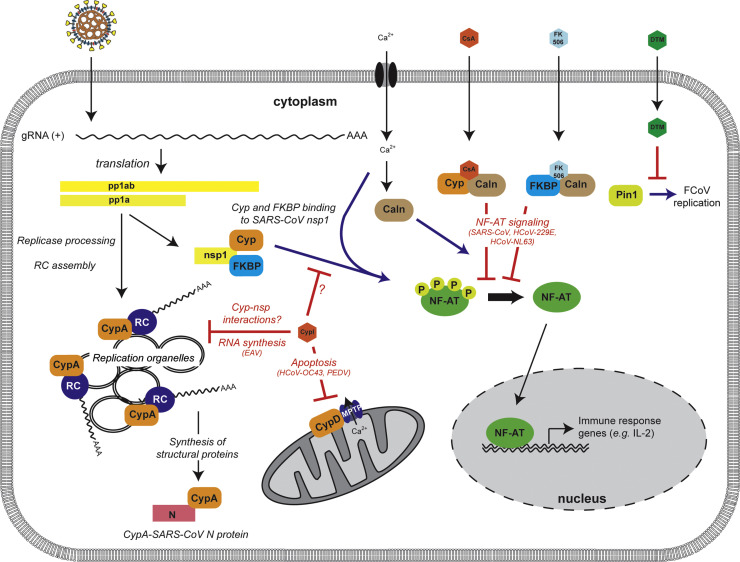
Unfortunately, ALV treatment did not protect mice from a challenge infection with mouse-adapted SARS-CoV, highlighting that inhibition in cell culture infection models does not necessarily translate into an antiviral effect in vivo (de Wilde et al., 2017a). This failure could be taken as a first sign that Cyp inhibitors may not be useful for the clinical treatment of CoV infections. It is clear, however, that this conclusion first needs to be corroborated in further studies, also including other compounds and other coronavirus (or nidovirus) models. This might more rigorously establish whether currently used Cyp inhibitors are worth pursuing, either directly or as leads for the development of other host-directed antiviral drugs. Furthermore, the development of non-immunosuppressive Cyp inhibitors with increased potency, solubility, or drug plasma levels may enhance the antiviral activity in vivo. In any case, the use of Cyp inhibitors in fundamental research may still help to uncover the putative role of Cyps in the replication of CoVs and other nidoviruses, as will be discussed below. Also, a summary of the (putative) roles of Cyps in nidovirus replication is depicted in Fig. 1.
Fig. 1.
Schematic overview of the presumed role of cyclophilins, FK-506 binding proteins and parvulins in nidovirus replication, as well as the effect of related inhibitors. Blue arrows indicate processes or interactions that positively affect virus replication. In red, inhibitory effects on infection are indicated that are induced by chemical inhibitors, protein-inhibitor complexes, or protein-protein complexes. See chapter 2 and chapter 3 for more details. Abbreviations: gRNA (+), positive-stranded genomic nidovirus RNA; pp1a, polyprotein 1a; pp1ab, polyprotein 1ab; RC, replication complex; Caln, calcineurin; CsA, cyclosporin A; Cyp, cyclophilin; FKBP, FK-506 binding protein; Pin-1, Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1; CypI, cyclophilin inhibitors; MPTP, mitochondrial permeability transition pore; nsp, nonstructural protein; IL-2, interleukin-2; DTM, dipentamethylene thiuram monosulfide; P, phosphate; NF-AT, nuclear factor of activated T-cells.
3.2. The potential role of Cyps in coronavirus infection
The inhibitory effect of treatment with CsA or its analogs (see above) suggested a role for one or more Cyps as host factor(s) in CoV replication. In 2004, seven years before the first reports on the inhibition of nidovirus replication by Cyp inhibitors, Luo et al. (2004) already suggested CypA to be an interaction partner of the SARS-CoV nucleocapsid (N) protein after using surface plasmon resonance to measure the binding affinity of purified SARS-CoV N protein and CypA. In a follow-up study, co-immunoprecipitation of purified CypA with SARS-CoV N protein confirmed the interaction of the two proteins (Chen et al., 2005). Using a similar co-immunoprecipitation approach, CypA was also shown to bind to cellular CD147, which is present on the plasma membrane. It was postulated that a CD147-CypA-N complex could promote SARS-CoV entry (Chen et al., 2005), although the formation of this tri-partite protein complex was not demonstrated. Moreover, a conceptual difficulty is that, during virus entry, the putative virion-associated CypA-N protein complex would be physically separated from CD147 by the viral envelope, and consequently it is difficult to envisage an interaction between these two proteins. Using a mass-spectrometry-based approach, Neuman et al. (2008) confirmed the presence of CypA in SARS-CoV virions, although no function was proposed for virion-associated CypA.
A yeast two-hybrid (Y2H) screen identified potential interactions between SARS-CoV nsp1 and several cyclophilins (CypA, CypB, CypH, CypG) and FK506-binding proteins (FKBP1A and FKBP1B) (Pfefferle et al., 2011). Subsequently, the importance of CypA as a host factor in CoV replication was further substantiated using knockdown or knockout of CypA expression, which was achieved using a variety of approaches. In Caco-2 and Huh7.5 cells, shRNA-mediated knockdown of CypA mRNA expression to below 3% of the normal levels was reported to nearly completely block HCoV-NL63 replication (Carbajo-Lozoya et al., 2014) and reduce HCoV-229E-driven luciferase expression by 10- to 20-fold (von Brunn et al., 2015). CypA- or CypB-knockout in feline fcwf-4 cells resulted in a near-complete block of FCoV replication. Interestingly, in the parental fcwf-4 cells shRNA-mediated knockdown of CypA and CypB only marginally reduced FCoV replication, suggesting that relatively small amounts of CypA and CypB may suffice to support normal levels of FCoV replication. Transient overexpression of PPIase-defective CypA or CypB mutants in parental fcwf-4 cells (still expressing endogenous CypA and CypB) slightly reduced replication, suggesting that PPIase activity of CypA and CypB is relevant for FCoV replication (Tanaka et al., 2017).
The importance of CypA was further highlighted in infection experiments using Huh7.5-CypA-knockdown cells transiently expressing CypA variants derived from non-synonymous single-nucleotide polymorphism (SNP)-containing PPIA genes. HCoV-229E replication was reduced in cells expressing different CypA variants displaying reduced stability (von Brunn et al., 2015). The involvement of CypA as host factor in coronavirus replication was further analyzed using MERS-CoV and HCoV-229E infection in CypA-knockout Huh7 cells generated via CRISPR-Cas9 technology. In these cells, compared to control cells, a 3-fold reduction in MERS-CoV titers was observed (de Wilde et al., 2017c), but, interestingly, HCoV-229E replication was unchanged compared to parental Huh7 cells. For HCoV-229E, these results appear to be at odds with the study summarized above in which CypA depletion led to a 10- to 20-fold reduction of HCoV-229E-driven luciferase expression (von Brunn et al., 2015). Explanations for these contradictory results may include the use of wild-type versus reporter gene-expressing viruses, different readouts of virus replication, and the use of Huh7 instead of Huh7.5 cells.
For SARS-CoV, the role of CypA and/or other Cyps in infection remains unclear. siRNA-mediated CypA depletion (de Wilde et al., 2011) nor shRNA-mediated depletion of CypA in Vero cells (6% remaining CypA mRNA levels; de Wilde et al., unpublished observations) did not lead to a reduction in SARS-CoV replication. However, since low levels of CypA may suffice to facilitate normal replication of other coronaviruses (as described above for FCoV), the dependence of SARS-CoV on CypA and other Cyps will require the generation of gene knockouts in a cell line permissive to SARS-CoV infection.
CypD has been reported to play a role in PEDV and HCoV-OC43 infection. CypD is part of the mitochondrial permeabilization transition pore and is involved in the caspase-independent cell death induced by PEDV and HCoV-OC43 (Favreau et al., 2012, Kim and Lee, 2014), an event that is inhibited by CsA. Upon HCoV-OC43 infection of differentiated LA-N-5 cells, the pro-apoptotic protein BAX re-localizes to mitochondria, where it may interact with CypD. Like CsA treatment, shRNA-mediated knockdown of CypD protected differentiated LA-N-5 cells from HCoV-OC43-induced programmed cell death, suggesting that CypD is involved in programmed cell death, most likely necroptosis, in HCoV-OC43-infected cells (Favreau et al., 2012).
The role in CoV infection of Cyps in general and CypA in particular remains elusive. Although Cyps differ in their cellular localization and function (see chapter 2), it is conceivable that other Cyps can substitute for CypA as a host factor in coronavirus replication. One candidate is the ER-associated CypB that may be in close proximity to the site of coronavirus replication, which is also associated with ER-derived membrane structures. However, it should be noted that CypB is located in the lumen of the ER and CypB may be therefore physically separated from the replication complexes on the cytosolic side of the ER membrane. Nevertheless, a CypA/CypB double-knockout cell line, which would prevent one Cyp from substituting for the other, may shed more light on the importance of both these Cyps in CoV replication.
3.3. NF-AT signaling and FKBPs in coronavirus infection
Pfefferle et al. (2011) showed that SARS-CoV nsp1 binds to Cyps and FKBPs and also influenced NF-AT-regulated gene activation (Fig. 1). This activation was blocked by CsA treatment (Pfefferle et al., 2011). The authors showed that, in cell culture, SARS-CoV infection in itself is a poor activator of NF-AT signaling, but in the presence of the NF-AT activators phorbol 12-myristate 13-acetate (PMA) and ionomycin SARS-CoV infection further upregulated NF-AT-dependent gene expression compared to cells treated with PMA and ionomycin only. The importance of the NF-AT signaling pathway in coronavirus replication was further investigated using the drug FK-506. Indeed, the replication of SARS-CoV, HCoV-229E, and HCoV-NL63 (EC50 of 6.9 µM, 5.4 µM, and 5.1 µM, respectively) was found to be inhibited by FK-506, with a > 2 log reduction of virus yields at doses above 20 µM (Carbajo-Lozoya et al., 2012). However, in contrast to the broad-spectrum inhibitory effect of CsA, the replication of some other coronaviruses (TGEV, IBV, and FCoV) was not affected by FK-506 treatment in cell culture (Tanaka et al., 2012, von Brunn et al., 2015).
3.4. Parvulins are involved in FCoV replication
The work of Tanaka et al. (2015) implicated a member of the third group of proteins with PPIase activity, parvulins, in CoV infection (Fig. 1). While FKBP inhibitors did not block FCoV replication in feline fcwf-4 cells, chemical inhibition of Pin1 by dipentamethylene thiuram monosulfide (DTM) reduced it approximately 10-fold. In Pin1-knockout cells, FCoV replication was only marginally affected. Pin1 has been shown to be an important mediator of cell cycle progression (Lin et al., 2015), and has also been implicated as a proviral factor in HCV and HIV-1 infection (Lim et al., 2011, Watashi et al., 2008). Pin1 depletion led to a 5-fold reduction in HCV replication, which could be compensated by transient expression of wild-type Pin1, but not by expression of PPIase-defective mutant Pin1 (Lim et al., 2011). The involvement of Pin1 in HIV-1 infection is distinct from its role in HCV replication: HIV-1 infection induces Pin1 phosphorylation that in turn reduced expression and virion association of APOBEC3G, a known HIV-1 restriction factor (Watashi et al., 2008). At the moment, the role of Pin1 has been investigated for FCoV only, and whether this protein has a general role in CoV infection remains to be investigated further.
3.5. CypA is involved in arterivirus replication
Research on the role of Cyps and Cyp inhibitors in nidovirus infection was extended to the arterivirus family. In line with the inhibitory effect of CsA treatment on CoV replication in cell culture, the replication of the arteriviruses EAV and PRRSV was found to be inhibited in the presence of low-micromolar concentrations of CsA and Debio-064, another non-immunosuppressive CsA analog. Interestingly, with EC50 values below 1 µM, replication of EAV was the most sensitive among all nidoviruses tested so far (de Wilde et al., 2013a), suggesting that EAV depends most strongly on Cyps for its replication. Both drugs prevented viral protein expression in arterivirus-infected cells and reduced virus yields by 4 logs (de Wilde et al., 2013a). Likewise, both compounds blocked in vitro RNA synthesis in assays using semi-purified replication structures from EAV-infected cells, pointing towards direct inhibition of viral RNA synthesis. In CsA-resistant EAV mutants, selected by serial passaging in the presence of the compound, mutations primarily mapped to nsp5, a multispanning membrane-associated subunit of the viral replicase. In addition, in vitro RNA synthesis activity of replication complexes prepared from cells infected with CsA-resistant EAV is indeed insensitive to CsA treatment (de Wilde et al., manuscript in preparation). The role of nsp5 in arterivirus replication is poorly understood, but the protein has been suggested to play a role in fine-tuning the conversion of intracellular membranes into viral replication organelles (van der Hoeven et al., 2016).
Of note, we previously postulated CypA to be associated with EAV replication organelles as the protein cosediments with membrane-associated EAV replication complexes in density gradients and pre-treatment of these samples with CsA prevents this cosedimentation. The presence of CypA, but not its PPIase activity (de Wilde et al., manuscript in preparation), was indeed shown to be pivotal for EAV replication (de Wilde et al., 2013a) as knockout of CypA expression resulted in a 3-log reduction of EAV progeny yields. Replication was affected much less strongly in cells expressing reduced levels of CypA (de Wilde et al., 2013a, de Wilde et al., 2017c), again highlighting that small amounts of CypA may suffice to support efficient nidovirus replication (see above). Collectively, these observations indicate that CypA is an essential host factor that binds to arterivirus replication organelles and supports RNA synthesis (Fig. 1). The inhibition of EAV RNA synthesis by CsA treatment suggests that CypA has a direct role in this process, rather than being involved in the formation of replication organelles, which is in contrast to what has been reported for HCV (Chatterji et al., 2015, Madan et al., 2014) (see below).
4. Cyps and their inhibitors in the replication of other RNA viruses
The importance of Cyps in RNA virus replication and the antiviral effects of Cyp inhibitors have been documented quite extensively. In this chapter, we will briefly summarize the most relevant findings regarding the involvement of Cyps in the replication of other groups of RNA viruses. In this respect, human immunodeficiency virus 1 (HIV-1), hepatitis C virus (HCV), and influenza virus have been studied in most detail, and these data have also been summarized previously in a number of excellent reviews (Baugh and Gallay, 2012, Campbell and Hope, 2015, Hopkins and Gallay, 2015, Liu et al., 2013).
4.1. HIV-1
During HIV-1 infection, CypA plays a role in the disassembly stage of the HIV-1 capsid core and the nuclear import of the reverse-transcribed HIV-1 genome. However, the molecular details of this process remain poorly understood. CypA binds the capsid core by interacting with capsid protein (CA) monomers that enclose the viral genome. In general, it is believed that in this way CypA ensures that the HIV-1 genome, which following entry is reverse-transcribed into cDNA inside these capsid cores, reaches the nucleus before it is sensed by the immune system (reviewed in Campbell and Hope, 2015; Hopkins and Gallay, 2015). CsA and NIM-811 were shown to inhibit HIV-1 replication in human T cells by disrupting this CA-CypA interaction (Billich et al., 1995, Franke and Luban, 1996). The hydrophobic pocket of CypA interacts with CA to catalyze the cis/trans isomerization of the Gly89–Pro90 peptide bond, which induces a conformational change in HIV-1 CA (Bosco et al., 2002, Franke et al., 1994, Gamble et al., 1996, Thali et al., 1994). This event is thought to trigger the disassembly of the HIV-1 capsid core, although the available data is conflicting. Some researchers indeed showed that CypA binds CA to destabilize the capsid core (Fricke et al., 2013), while others found that CypA stabilizes the capsid core (Shah et al., 2013). A third study suggests that this discrepancy may be caused by concentration differences, with low amounts of CypA stabilizing the core and high levels leading to its disassembly (Liu et al., 2016).
The interaction with CA may also prevent CA from binding to a HIV-1 restriction factor, presumably TRIM5α, which was shown to restrict retrovirus replication in Old-World monkey cells (Towers et al., 2003, and reviewed in Hopkins and Gallay, 2015; Luban, 2007). This hypothesis is strengthened by the observation that (New-World) owl monkey cells express a TRIM5-CypA fusion protein, in which the TRIM5α SPRY domain has been replaced with CypA. The CypA domain of this fusion protein directs the TRIM5α domain to the HIV-1 capsid core to restrict HIV-1 replication. Also, in contrast to the inhibition of HIV-1 infection by CsA in human T cells, treatment of owl monkey cells with this Cyp inhibitor enhanced HIV-1 replication. This increased replication efficiency is presumably mediated by a block of the TRIM5-CypA interaction with the HIV-1 CA (Nisole et al., 2004, Sayah et al., 2004).
4.2. HCV
After reports on the potent inhibition of HCV replication in hepatocytes by CsA (Nakagawa et al., 2004, Watashi et al., 2003), CypA was identified as an essential host factor for HCV replication (Chatterji et al., 2009, Kaul et al., 2009, Nakagawa et al., 2005a, Yang et al., 2008). Its importance was further substantiated by the observation that HCV infection was restricted in CypA-knockout mice (Dorner et al., 2013). Subsequently, several key observations were made during studies aiming to elucidate the mechanism(s) by which CypA regulates HCV replication (for excellent reviews, see Hopkins and Gallay, 2015; Lin and Gallay, 2013):
-
i.
Inactivation of CypA's PPIase activity by mutagenesis revealed that this enzymatic activity is pivotal for efficient HCV replication in cell culture (Chatterji et al., 2009, Liu et al., 2009b);
-
ii.
CypA interacts directly with the HCV replication complex by binding to NS5A. Multiple proline residues located in NS5A domains II and III are determinants of CypA binding and are substrates for its PPIase activity (Fernandes et al., 2010, Foster et al., 2011, Hanoulle et al., 2009, Shirota et al., 2002). Binding of CypA to NS5A domain II induces conformational changes in this domain that favor RNA binding and NS5A-NS5B interactions (Foster et al., 2011, Ngure et al., 2016);
-
iii.
Resistance to Cyp inhibitors requires mutations in NS5A (Coelmont et al., 2010, Garcia-Rivera et al., 2012, Kaul et al., 2009). The NS5A D320E mutation is assumed to contribute most to resistance and facilitates CypA-independent replication of HCV (Coelmont et al., 2010);
-
iv.
There is growing evidence that the CypA-NS5A interaction is not directly linked to the RNA-synthesizing activity of the HCV replication complex, but merely is involved in the formation and stabilization of the HCV-induced membranous web (replication organelle) with which the replication complex is associated (Chatterji et al., 2015, Madan et al., 2014).
The efficacy of CsA and its non-immunosuppressive analogs ALV, NIM811, and SCY-365 in blocking HCV infection in cell culture models (Ciesek et al., 2009, Coelmont et al., 2010, Hopkins et al., 2010, Ishii et al., 2006, Ma et al., 2006, Paeshuyse et al., 2006, Watashi et al., 2003) ultimately led to the evaluation of these drugs in clinical trials, of which ALV reached the phase III clinical trial. Overall, these trials showed that ALV - when combined with direct-acting antivirals – can contribute to an IFN-free pan-genotypic HCV therapy, although until date none of the Cyp inhibitors have been approved for HCV treatment. The outcome of these trials has been reviewed in detail in (Hopkins and Gallay, 2012, Membreno et al., 2013, Naoumov, 2014).
4.3. Flaviviruses
Besides HCV, CsA also inhibits the replication of other flaviviruses, as established for West Nile virus (WNV), dengue virus (DENV), and yellow fever virus (YFV) (Qing et al., 2009). The latter study showed an essential role of CypA's PPIase activity in DENV and WNV replication. In the case of WNV and Japanese encephalitis virus (JEV), the inhibitory effect of CsA was attributed to blocking a functional CypA-NS5 interaction (Qing et al., 2009), while CypA was shown to interact with NS4B during YFV infection (Vidotto et al., 2017). Interestingly, JEV seems to depend mainly on enzymatically active CypB for its replication, mostly via an interaction with NS4A (Kambara et al., 2011).
4.4. Influenza viruses
In contrast to CypA's proviral role in e.g. HIV-1 and HCV replication, CypA acts as a restriction factor for influenza virus replication (reviewed in Liu et al., 2013). The multifunctional matrix (M1) protein is involved in various stages of the virus replication cycle. CypA interacts with the M1 protein to accelerate its degradation through the ubiquitin/proteasome-dependent pathway (Liu et al., 2009a, Liu et al., 2012b). Overexpression of CypA in transgenic mice reduced influenza virus replication (Li et al., 2016). Furthermore, influenza A virus infection of macrophages isolated from these CypA-overexpressing mice showed an upregulation of host antiviral genes compared to macrophages from wild-type C57BL/6 mice. This upregulation may likely contribute to resistance of influenza virus infection in mice (Li et al., 2016).
Interestingly, the Cyp inhibitor CsA and other Cyp inhibitors exhibit broad-spectrum influenza virus A and B antiviral activity (Hamamoto et al., 2013, Ma et al., 2016). In vivo studies showed survival of CsA-treated mice that were infected with a lethal influenza virus dose (Schiltknecht and Ada, 1985). Furthermore, Liu et al. claimed that CsA limits influenza A virus replication in a CypA-dependent and -independent manner: CsA increased CypA's binding affinity for M1 and at the same time decreased nuclear import of the influenza virus genome in CypA-depleted cells (Liu et al., 2012a).
5. Discussion and outlook
Efficient viral replication depends on the concerted interactions between viral proteins and host cell factors. Cyps were demonstrated to play a multifaceted role in the replication of a range of RNA viruses, which was investigated in most detail for HIV-1 and HCV. The key role of Cyps in the viral life cycle has been demonstrated by a wide variety of techniques, including the use of cells or mice partially or completely depleted for specific Cyps, by complementation studies with PPIase-deficient Cyp proteins or mutant viral proteins that are unable to interact with Cyps, using (non-)immunosuppressive Cyp inhibitors, and by the analysis of drug-resistant viruses.
For most viruses, CypA is believed to be the main Cyp involved in virus replication, but its specific mode of action in virus replication appears to differ greatly among viruses. For instance, CypA is believed to bind the HIV-1 CA to ensure that the reverse-transcribed HIV-1 genome reaches the nucleus before it is sensed by the immune system, while during HCV infection CypA is involved in the generation of virus-induced replication organelles and/or the proper folding of HCV proteins that interact with viral RNA or other viral proteins. Current data from others and our laboratory suggest a diverse role of Cyps, and especially CypA, in nidovirus replication. For example, CypA has been hypothesized to be incorporated into SARS-CoV virions (Neuman et al., 2008), which may suggest – for reasons that are not fully understood at the moment – that CypA's chaperone function enhances the infectivity of SARS-CoV. Pfefferle et al. (2011) reported that a variety of Cyps, including CypA, is able to modulate NF-AT signaling in SARS-CoV-infected cells by interacting with SARS-CoV nsp1. However, the relevance of these results in the context of the viral replication cycle is currently unclear, as non-immunosuppressive analogs do not interfere with NF-AT signaling but do inhibit SARS-CoV replication.
In the case of the arterivirus EAV, CypA plays a role in viral RNA synthesis, albeit that its exact function is poorly understood. Whether CypA is also involved in the RNA synthesis activity of other nidoviruses warrants further investigation, e.g. by testing Cyp inhibitors on the activity of isolated replication complexes from nidovirus-infected cells. In any case, the mechanistic details of the interactions of nidoviral proteins and Cyps remain to be resolved, which may also provide a basis for new therapeutic approaches for other (nido)viruses that recruit Cyps as a host factor in their replication.
References
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Barik S. Immunophilins: for the love of proteins. Cell. Mol. Life Sci. 2006;63:2889–2900. doi: 10.1007/s00018-006-6215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh J., Gallay P. Cyclophilin involvement in the replication of hepatitis C virus and other viruses. Biol. Chem. 2012;393:579–587. doi: 10.1515/hsz-2012-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A., Hammerschmid F., Peichl P., Wenger R., Zenke G., Quesniaux V., Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J. Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J.F., Feurer C., Gubler H.U., Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Bosco D.A., Eisenmesser E.Z., Pochapsky S., Sundquist W.I., Kern D. Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A. Proc. Natl. Acad. Sci. USA. 2002;99:5247–5252. doi: 10.1073/pnas.082100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brunn A., Ciesek S., von Brunn B., Carbajo-Lozoya J. Genetic deficiency and polymorphisms of cyclophilin A reveal its essential role for Human Coronavirus 229E replication. Curr. Opin. Virol. 2015;14:56–61. doi: 10.1016/j.coviro.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. Extracellular cyclophilins in health and disease. Biochim. Biophys. Acta. 2015;1850:2087–2095. doi: 10.1016/j.bbagen.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E.M., Hope T.J. HIV-1 capsid: the multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015;13:471–483. doi: 10.1038/nrmicro3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajo-Lozoya J., Muller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajo-Lozoya J., Ma-Lauer Y., Malesevic M., Theuerkorn M., Kahlert V., Prell E., von Brunn B., Muth D., Baumert T.F., Drosten C., Fischer G., von Brunn A. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014;184C:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterji U., Bobardt M., Selvarajah S., Yang F., Tang H., Sakamoto N., Vuagniaux G., Parkinson T., Gallay P. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. J. Biol. Chem. 2009;284:16998–17005. doi: 10.1074/jbc.M109.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterji U., Bobardt M., Tai A., Wood M., Gallay P.A. Cyclophilin and NS5A inhibitors, but not other anti-hepatitis C virus (HCV) agents, preclude HCV-mediated formation of double-membrane-vesicle viral factories. Antimicrob. Agents Chemother. 2015;59:2496–2507. doi: 10.1128/AAC.04958-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J., Xing J., Shang P., Qian A., Li Y., Shaw P.X., Wang J., Duan S., Ding J., Fan C., Zhang Y., Yang Y., Yu X., Feng Q., Li B., Yao X., Zhang Z., Li L., Xue X., Zhu P. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechomska I., Legat M., Golab J., Wesolowska A., Kurzaj Z., Mackiewicz A., Kaminska B. Cyclosporine A and its non-immunosuppressive derivative NIM811 induce apoptosis of malignant melanoma cells in in vitro and in vivo studies. Int. J. Cancer. 2005;117:59–67. doi: 10.1002/ijc.21153. [DOI] [PubMed] [Google Scholar]
- Ciesek S., Steinmann E., Wedemeyer H., Manns M.P., Neyts J., Tautz N., Madan V., Bartenschlager R., von Hahn T., Pietschmann T. Cyclosporine A inhibits hepatitis C virus nonstructural protein 2 through cyclophilin A. Hepatology. 2009;50:1638–1645. doi: 10.1002/hep.23281. [DOI] [PubMed] [Google Scholar]
- Coelmont L., Hanoulle X., Chatterji U., Berger C., Snoeck J., Bobardt M., Lim P., Vliegen I., Paeshuyse J., Vuagniaux G., Vandamme A.M., Bartenschlager R., Gallay P., Lippens G., Neyts J. DEB025 (Alisporivir) inhibits hepatitis C virus replication by preventing a cyclophilin A induced cis-trans isomerisation in domain II of NS5A. PLoS One. 2010;5:e13687. doi: 10.1371/journal.pone.0013687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan J., Asmal M., Yu B., Luban J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. J. Immunol. 2005;174:6030–6038. doi: 10.4049/jimmunol.174.10.6030. [DOI] [PubMed] [Google Scholar]
- Davis T.L., Walker J.R., Campagna-Slater V., Finerty P.J., Paramanathan R., Bernstein G., MacKenzie F., Tempel W., Ouyang H., Lee W.H., Eisenmesser E.Z., Dhe-Paganon S. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010;8:e1000439. doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner M., Horwitz J.A., Donovan B.M., Labitt R.N., Budell W.C., Friling T., Vogt A., Catanese M.T., Satoh T., Kawai T., Akira S., Law M., Rice C.M., Ploss A. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod J.W., Molkentin J.D. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ. J. 2013;77:1111–1122. doi: 10.1253/circj.cj-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau D.J., Meessen-Pinard M., Desforges M., Talbot P.J. Human coronavirus-induced neuronal programmed cell death is cyclophilin d dependent and potentially caspase dispensable. J. Virol. 2012;86:81–93. doi: 10.1128/JVI.06062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon P., Lonsdale-Eccles A.A., Ross O.K., Todd C., Sinha A., Allain F., Reynolds N.J. Keratinocyte secretion of cyclophilin B via the constitutive pathway is regulated through its cyclosporin-binding site. J. Invest. Dermatol. 2011;131:1085–1094. doi: 10.1038/jid.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes F., Ansari I.U., Striker R. Cyclosporine inhibits a direct interaction between cyclophilins and hepatitis C NS5A. PLoS One. 2010;5:e9815. doi: 10.1371/journal.pone.0009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Bang H., Mech C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta. 1984;43:1101–1111. [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Flegel T.W. Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 2012;110:166–173. doi: 10.1016/j.jip.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Foster T.L., Gallay P., Stonehouse N.J., Harris M. Cyclophilin A interacts with domain II of hepatitis C virus NS5A and stimulates RNA binding in an isomerase-dependent manner. J. Virol. 2011;85:7460–7464. doi: 10.1128/JVI.00393-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke E.K., Luban J. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology. 1996;222:279–282. doi: 10.1006/viro.1996.0421. [DOI] [PubMed] [Google Scholar]
- Franke E.K., Yuan H.E., Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- Frausto S.D., Lee E., Tang H. Cyclophilins as modulators of viral replication. Viruses. 2013;5:1684–1701. doi: 10.3390/v5071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke T., Brandariz-Nunez A., Wang X., Smith A.B., Diaz-Griffero F., 3rd Human cytosolic extracts stabilize the HIV-1 core. J. Virol. 2013;87:10587–10597. doi: 10.1128/JVI.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble T.R., Vajdos F.F., Yoo S., Worthylake D.K., Houseweart M., Sundquist W.I., Hill C.P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivera J.A., Bobardt M., Chatterji U., Hopkins S., Gregory M.A., Wilkinson B., Lin K., Gallay P.A. Multiple mutations in hepatitis C virus NS5A domain II are required to confer a significant level of resistance to alisporivir. Antimicrob. Agents Chemother. 2012;56:5113–5121. doi: 10.1128/AAC.00919-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Enjuanes L., Ziebuhr J., Snijder E.J. Nidovirales: evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Kino T., Hatanaka H., Nishiyama M., Okuhara M., Kohsaka M., Aoki H., Imanaka H. Discovery of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant. Proc. 1987;19:4–8. [PubMed] [Google Scholar]
- Gray K.A., Yates B., Seal R.L., Wright M.W., Bruford E.A. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 2015;43:D1079–D1085. doi: 10.1093/nar/gku1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeijer M.C., Vonk A.M., Monastyrska I., Rottier P.J., de Haan C.A. Visualizing coronavirus RNA synthesis in time by using click chemistry. J. Virol. 2012;86:5808–5816. doi: 10.1128/JVI.07207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto I., Harazaki K., Inase N., Takaku H., Tashiro M., Yamamoto N. Cyclosporin A inhibits the propagation of influenza virus by interfering with a late event in the virus life cycle. Jpn. J. Infect. Dis. 2013;66:276–283. doi: 10.7883/yoken.66.276. [DOI] [PubMed] [Google Scholar]
- Handschumacher R.E., Harding M.W., Rice J., Drugge R.J., Speicher D.W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Hanes S.D. Prolyl isomerases in gene transcription. Biochim. Biophys. Acta. 2015;1850:2017–2034. doi: 10.1016/j.bbagen.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoulle X., Badillo A., Wieruszeski J.M., Verdegem D., Landrieu I., Bartenschlager R., Penin F., Lippens G. Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B. J. Biol. Chem. 2009;284:13589–13601. doi: 10.1074/jbc.M809244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding M.W., Handschumacher R.E., Speicher D.W. Isolation and amino acid sequence of cyclophilin. J. Biol. Chem. 1986;261:8547–8555. [PubMed] [Google Scholar]
- Ho S., Clipstone N., Timmermann L., Northrop J., Graef I., Fiorentino D., Nourse J., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Holtkamp D.J., Kliebenstein J.B., Neumann E.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. JSHAP. 2013;21:72–84. [Google Scholar]
- Hopkins S., Gallay P. Cyclophilin inhibitors: an emerging class of therapeutics for the treatment of chronic hepatitis C infection. Viruses. 2012;4:2558–2577. doi: 10.3390/v4112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S., Gallay P.A. The role of immunophilins in viral infection. Biochim. Biophys. Acta. 2015;1850:2103–2110. doi: 10.1016/j.bbagen.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S., Scorneaux B., Huang Z., Murray M.G., Wring S., Smitley C., Harris R., Erdmann F., Fischer G., Ribeill Y. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob. Agents Chemother. 2010;54:660–672. doi: 10.1128/AAC.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Ge X., Wang L.F., Shi Z. Bat origin of human coronaviruses. Virol. J. 2015;12:221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N., Luo D.S., Zheng X.S., Wang M.N., Daszak P., Wang L.F., Cui J., Shi Z.L. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13:e1006698. doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr. Opin. Pulm. Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Ishii N., Watashi K., Hishiki T., Goto K., Inoue D., Hijikata M., Wakita T., Kato N., Shimotohno K. Diverse effects of cyclosporine on hepatitis C virus strain replication. J. Virol. 2006;80:4510–4520. doi: 10.1128/JVI.80.9.4510-4520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H., Tani H., Mori Y., Abe T., Katoh H., Fukuhara T., Taguwa S., Moriishi K., Matsuura Y. Involvement of cyclophilin B in the replication of Japanese encephalitis virus. Virology. 2011;412:211–219. doi: 10.1016/j.virol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Kaul A., Stauffer S., Berger C., Pertel T., Schmitt J., Kallis S., Zayas M., Lohmann V., Luban J., Bartenschlager R. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog. 2009;5:e1000546. doi: 10.1371/journal.ppat.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lee C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology. 2014;460–461:180–193. doi: 10.1016/j.virol.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrieu I., Hanoulle X., Bonachera F., Hamel A., Sibille N., Yin Y., Wieruszeski J.M., Horvath D., Wei Q., Vuagniaux G., Lippens G. Structural basis for the non-immunosuppressive character of the cyclosporin A analogue Debio 025. Biochemistry. 2010;49:4679–4686. doi: 10.1021/bi1003266. [DOI] [PubMed] [Google Scholar]
- Lane W.S., Galat A., Harding M.W., Schreiber S.L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J. Protein Chem. 1991;10:151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F.X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987;329:268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Li J., Chen C., Wong G., Dong W., Zheng W., Li Y., Sun L., Zhang L., Gao G.F., Bi Y., Liu W. Cyclophilin A protects mice against infection by influenza A virus. Sci. Rep. 2016;6:28978. doi: 10.1038/srep28978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lim Y.S., Tran H.T., Park S.J., Yim S.A., Hwang S.B. Peptidyl-prolyl isomerase Pin1 is a cellular factor required for hepatitis C virus propagation. J. Virol. 2011;85:8777–8788. doi: 10.1128/JVI.02533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Li H.Y., Lee Y.C., Calkins M.J., Lee K.H., Yang C.N., Lu P.J. Landscape of Pin1 in the cell cycle. Exp. Biol. Med. 2015;240:403–408. doi: 10.1177/1535370215570829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Gallay P. Curing a viral infection by targeting the host: the example of cyclophilin inhibitors. Antivir. Res. 2013;99:68–77. doi: 10.1016/j.antiviral.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., Green D.R. Necroptosis. New Engl. J. Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Perilla J.R., Ning J., Lu M., Hou G., Ramalho R., Himes B.A., Zhao G., Bedwell G.J., Byeon I.J., Ahn J., Gronenborn A.M., Prevelige P.E., Rousso I., Aiken C., Polenova T., Schulten K., Zhang P. Cyclophilin A stabilizes the HIV-1 capsid through a novel non-canonical binding site. Nat. Commun. 2016;7:10714. doi: 10.1038/ncomms10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J.D., Jr., Lane W.S., Friedman J., Weissman I., Schreiber S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun L., Yu M., Wang Z., Xu C., Xue Q., Zhang K., Ye X., Kitamura Y., Liu W. Cyclophilin A interacts with influenza A virus M1 protein and impairs the early stage of the viral replication. Cell. Microbiol. 2009;11:730–741. doi: 10.1111/j.1462-5822.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhao Z., Li Z., Xu C., Sun L., Chen J., Liu W. Cyclosporin A inhibits the influenza virus replication through cyclophilin A-dependent and -independent pathways. PLoS One. 2012;7:e37277. doi: 10.1371/journal.pone.0037277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhao Z., Xu C., Sun L., Chen J., Zhang L., Liu W. Cyclophilin A restricts influenza A virus replication through degradation of the M1 protein. PLoS One. 2012;7:e31063. doi: 10.1371/journal.pone.0031063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhao Z., Liu W. Insights into the roles of cyclophilin A during influenza virus infection. Viruses. 2013;5:182–191. doi: 10.3390/v5010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Yang F., Robotham J.M., Tang H. Critical role of cyclophilin A and its prolyl-peptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J. Virol. 2009;83:6554–6565. doi: 10.1128/JVI.02550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J. Cyclophilin A, TRIM5, and resistance to human immunodeficiency virus type 1 infection. J. Virol. 2007;81:1054–1061. doi: 10.1128/JVI.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Luo H., Zheng S., Gui C., Yue L., Yu C., Sun T., He P., Chen J., Shen J., Luo X., Li Y., Liu H., Bai D., Yang Y., Li F., Zuo J., Hilgenfeld R., Pei G., Chen K., Shen X., Jiang H. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem. Biophys. Res. Commun. 2004;321:557–565. doi: 10.1016/j.bbrc.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Li F., Musharrafieh R.G., Wang J. Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antivir. Res. 2016;133:62–72. doi: 10.1016/j.antiviral.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Boerner J.E., TiongYip C., Weidmann B., Ryder N.S., Cooreman M.P., Lin K. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob. Agents Chemother. 2006;50:2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan V., Paul D., Lohmann V., Bartenschlager R. Inhibition of HCV replication by cyclophilin antagonists is linked to replication fitness and occurs by inhibition of membranous web formation. Gastroenterology. 2014;146:1361–1372. doi: 10.1053/j.gastro.2014.01.055. (e1361-e1369) [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez S., Redondo J.M. Inhibitors of the calcineurin/NFAT pathway. Curr. Med. Chem. 2004;11:997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- Membreno F.E., Espinales J.C., Lawitz E.J. Cyclophilin inhibitors for hepatitis C therapy. Clin. Liver Dis. 2013;17:129–139. doi: 10.1016/j.cld.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Jr., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.Y., Donaldson E.F., Randell S.H., Lanzavecchia A., Marasco W.A., Shi Z.L., Baric R.S. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Sakamoto N., Enomoto N., Tanabe Y., Kanazawa N., Koyama T., Kurosaki M., Maekawa S., Yamashiro T., Chen C.H., Itsui Y., Kakinuma S., Watanabe M. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem. Biophys. Res. Commun. 2004;313:42–47. doi: 10.1016/j.bbrc.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Sakamoto N., Tanabe Y., Koyama T., Itsui Y., Takeda Y., Chen C.H., Kakinuma S., Oooka S., Maekawa S., Enomoto N., Watanabe M. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031–1041. doi: 10.1053/j.gastro.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Naoumov N.V. Cyclophilin inhibition as potential therapy for liver diseases. J. Hepatol. 2014;61:1166–1174. doi: 10.1016/j.jhep.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Neuman B.W., Joseph J.S., Saikatendu K.S., Serrano P., Chatterjee A., Johnson M.A., Liao L., Klaus J.P., Yates J.R., 3rd, Wuthrich K., Stevens R.C., Buchmeier M.J., Kuhn P. Proteomics analysis unravels the functional repertoire of coronavirus nonstructural protein 3. J. Virol. 2008;82:5279–5294. doi: 10.1128/JVI.02631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga P.T., Parquet Mdel C., Lauber C., Parida M., Nabeshima T., Yu F., Thuy N.T., Inoue S., Ito T., Okamoto K., Ichinose A., Snijder E.J., Morita K., Gorbalenya A.E. Discovery of the first insect nidovirus, a missing evolutionary link in the emergence of the largest RNA virus genomes. PLoS Pathog. 2011;7:e1002215. doi: 10.1371/journal.ppat.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngure M., Issur M., Shkriabai N., Liu H.W., Cosa G., Kvaratskhelia M., Gotte M. Vol. 2. 2016. Interactions of the disordered domain II of Hepatitis C Virus NS5A with Cyclophilin A, NS5B, and Viral RNA show extensive overlap; pp. 839–851. (ACS Infect Dis.). [DOI] [PubMed] [Google Scholar]
- Nigro P., Pompilio G., Capogrossi M.C. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S., Lynch C., Stoye J.P., Yap M.W. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc. Natl. Acad. Sci. USA. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeshuyse J., Kaul A., De Clercq E., Rosenwirth B., Dumont J.M., Scalfaro P., Bartenschlager R., Neyts J. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;43:761–770. doi: 10.1002/hep.21102. [DOI] [PubMed] [Google Scholar]
- Pasternak A.O., Spaan W.J., Snijder E.J. Nidovirus transcription: how to make sense…? J. Gen. Virol. 2006;87:1403–1421. doi: 10.1099/vir.0.81611-0. [DOI] [PubMed] [Google Scholar]
- Pedersen K.W., van der M.Y., Roos N., Snijder E.J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Schopf J., Kogl M., Friedel C.C., Muller M.A., Carbajo-Lozoya J., Stellberger T., von Dall'armi E., Herzog P., Kallies S., Niemeyer D., Ditt V., Kuri T., Zust R., Pumpor K., Hilgenfeld R., Schwarz F., Zimmer R., Steffen I., Weber F., Thiel V., Herrler G., Thiel H.J., Schwegmann-Wessels C., Pohlmann S., Haas J., Drosten C., von Brunn A. The SARS-coronavirus-host Interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E.R., Zydowsky L.D., Jin M.J., Baker C.H., McKeon F.D., Walsh C.T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc. Natl. Acad. Sci. USA. 1991;88:1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E.R., Jin M., Lim D., Pati S., Walsh C.T., McKeon F.D. Cyclophilin B trafficking through the secretory pathway is altered by binding of cyclosporin A. Proc. Natl. Acad. Sci. USA. 1994;91:3931–3935. doi: 10.1073/pnas.91.9.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J. Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing M., Yang F., Zhang B., Zou G., Robida J.M., Yuan Z., Tang H., Shi P.Y. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob. Agents Chemother. 2009;53:3226–3235. doi: 10.1128/AAC.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahfeld J.U., Schierhorn A., Mann K., Fischer G. A novel peptidyl-prolyl cis/trans isomerase from Escherichia coli. FEBS Lett. 1994;343:65–69. doi: 10.1016/0014-5793(94)80608-x. [DOI] [PubMed] [Google Scholar]
- Rasola A., Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Romero-Brey I., Bartenschlager R. Endoplasmic reticulum: the favorite intracellular niche for viral replication and assembly. Viruses. 2016;8:E160. doi: 10.3390/v8060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel B., Woerly G., Greiner B., Haendler B., Mihatsch M.J., Foxwell B.M. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399–404. [PMC free article] [PubMed] [Google Scholar]
- Sabir J.S., Lam T.T., Ahmed M.M., Li L., Shen Y., Abo-Aba S.E., Qureshi M.I., Abu-Zeid M., Zhang Y., Khiyami M.A., Alharbi N.S., Hajrah N.H., Sabir M.J., Mutwakil M.H., Kabli S.A., Alsulaimany F.A., Obaid A.Y., Zhou B., Smith D.K., Holmes E.C., Zhu H., Guan Y. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351:81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81:20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah D.M., Sokolskaja E., Berthoux L., Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- Schiene-Fischer C. Multidomain peptidyl prolyl cis/trans isomerases. Biochim. Biophys. Acta. 2015;1850:2005–2016. doi: 10.1016/j.bbagen.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Schiene‐Fischer C. Springer Berlin Heidelberg; Berlin, Heidelberg: 2006. Peptidyl Prolyl cis/trans Isomerases, Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine; pp. 1378–1381. [Google Scholar]
- Schiltknecht E., Ada G.L. In vivo effects of cyclosporine on influenza A virus-infected mice. Cell. Immunol. 1985;91:227–239. doi: 10.1016/0008-8749(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Schmid F.X. Prolyl isomerase: enzymatic catalysis of slow protein-folding reactions. Annu Rev. Biophys. Biomol. Struct. 1993;22:123–142. doi: 10.1146/annurev.bb.22.060193.001011. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Shah V.B., Shi J., Hout D.R., Oztop I., Krishnan L., Ahn J., Shotwell M.S., Engelman A., Aiken C. The host proteins transportin SR2/TNPO3 and cyclophilin A exert opposing effects on HIV-1 uncoating. J. Virol. 2013;87:422–432. doi: 10.1128/JVI.07177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y., Luo H., Qin W., Kaneko S., Yamashita T., Kobayashi K., Murakami S. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J. Biol. Chem. 2002;277:11149–11155. doi: 10.1074/jbc.M111392200. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Kikkert M., Fang Y. Arterivirus molecular biology and pathogenesis. J. Gen. Virol. 2013;94:2141–2163. doi: 10.1099/vir.0.056341-0. [DOI] [PubMed] [Google Scholar]
- Sola I., Mateos-Gomez P.A., Almazan F., Zuniga S., Enjuanes L. RNA-RNA and RNA-protein interactions in coronavirus replication and transcription. RNA Biol. 2011;8:237–248. doi: 10.4161/rna.8.2.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Haendler B., Delmas O., Mariller C., Chamoux M., Maes P., Tartar A., Montreuil J., Stedman K., Kocher H.P. A novel secreted cyclophilin-like protein (SCYLP) J. Biol. Chem. 1991;266:10735–10738. [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sato Y., Osawa S., Inoue M., Tanaka S., Sasaki T. Suppression of feline coronavirus replication in vitro by cyclosporin A. Vet. Res. 2012;43:41. doi: 10.1186/1297-9716-43-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Amano A., Morisaki M., Sato Y., Sasaki T. Cellular peptidyl-prolyl cis/trans isomerase Pin1 facilitates replication of feline coronavirus. Antivir. Res. 2015 doi: 10.1016/j.antiviral.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sato Y., Sasaki T. Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J. Gen. Virol. 2017;98:190–200. doi: 10.1099/jgv.0.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco D., Haragsim L. Cyclosporine: a review. J. Transplant. 2012;2012:230386. doi: 10.1155/2012/230386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Bukovsky A., Kondo E., Rosenwirth B., Walsh C.T., Sodroski J., Gottlinger H.G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- Towers G.J., Hatziioannou T., Cowan S., Goff S.P., Luban J., Bieniasz P.D. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 2003;9:1138–1143. doi: 10.1038/nm910. [DOI] [PubMed] [Google Scholar]
- van der Hoeven B., Oudshoorn D., Koster A.J., Snijder E.J., Kikkert M., Barcena M. Biogenesis and architecture of arterivirus replication organelles. Virus Res. 2016;220:70–90. doi: 10.1016/j.virusres.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert M.J., de Wilde A.H., Gorbalenya A.E., Snijder E.J. The in vitro RNA synthesizing activity of the isolated arterivirus replication/transcription complex is dependent on a host factor. J. Biol. Chem. 2008;283:16525–16536. doi: 10.1074/jbc.M708136200. [DOI] [PubMed] [Google Scholar]
- van Hemert M.J., van den Worm S.H., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008;4:e1000054. doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidotto A., Morais A.T., Ribeiro M.R., Pacca C.C., Terzian A.C., Gil L.H., Mohana-Borges R., Gallay P., Nogueira M.L. Systems biology reveals NS4B-Cyclophilin A interaction: a new target to inhibit YFV replication. J. Proteome Res. 2017;16:1542–1555. doi: 10.1021/acs.jproteome.6b00933. [DOI] [PubMed] [Google Scholar]
- Vijay R., Perlman S. Middle East respiratory syndrome and severe acute respiratory syndrome. Curr. Opin. Virol. 2016;16:70–76. doi: 10.1016/j.coviro.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat D. The common cold: a review of the literature. Eur. J. Intern. Med. 2004;15:79–88. doi: 10.1016/j.ejim.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watashi K., Hijikata M., Hosaka M., Yamaji M., Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- Watashi K., Khan M., Yedavalli V.R., Yeung M.L., Strebel K., Jeang K.T. Human immunodeficiency virus type 1 replication and regulation of APOBEC3G by peptidyl prolyl isomerase Pin1. J. Virol. 2008;82:9928–9936. doi: 10.1128/JVI.01017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Li Y., van der Meer Y., Vuagniaux G., Lysek R., Fang Y., Snijder E.J., van Hemert M.J. Cyclophilin inhibitors block arterivirus replication by interfering with viral RNA synthesis. J. Virol. 2013;87:1454–1464. doi: 10.1128/JVI.02078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Raj V.S., Oudshoorn D., Bestebroer T.M., van Nieuwkoop S., Limpens R.W., Posthuma C.C., van der Meer Y., Barcena M., Haagmans B.L., Snijder E.J., van den Hoogen B.G. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-alpha treatment. J. Gen. Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Falzarano D., Zevenhoven-Dobbe J.C., Beugeling C., Fett C., Martellaro C., Posthuma C.C., Feldmann H., Perlman S., Snijder E.J. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. doi: 10.1016/j.virusres.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2017 doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Zevenhoven-Dobbe J.C., Beugeling C., Chatterji U., de Jong D., Gallay P., Szuhai K., Posthuma C.C., Snijder E.J. Coronaviruses and arteriviruses display striking differences in their cyclophilin A-dependence during replication in cell culture. Virology. 2017 doi: 10.1016/j.virol.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Robotham J.M., Nelson H.B., Irsigler A., Kenworthy R., Tang H. Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principal mediator of cyclosporine resistance in vitro. J. Virol. 2008;82:5269–5278. doi: 10.1128/JVI.02614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]