Abstract
As World Health Organization advocates, the global burden of sanitation related disease and access to safely managed sanitation and safely treated wastewater should be monitored strictly. However, the spread of pathogens through various agricultural applications or direct discharge of sewage sludge generated in municipal wastewater treatment plants poses a serious challenge on the environment and public health. Anaerobic digestion (AD), the principal method of stabilizing biosolids, can efficiently and largely deactivate viable pathogens, including parasite, virus, and the pathogens harboring antibiotic resistance genes. This review aims to provide a critical overview regarding the deactivation of sludge-associated pathogens by AD, through which a serious concern on the effectiveness and rationality of AD towards sludge pathogens control was raised. Meanwhile, the underlying deactivation mechanisms and affecting factors were all discussed, with the focus on pathogen-associated modeling, engineering design and technological aspects of AD. Lastly, a matric method incorporating the operating strategy of AD with the risk assessment was proposed for evaluating the reliability of AD-based pathogen deactivation, while the research agenda forward was also outlined.
Keywords: Anaerobic digestion, Pathogens, Biosludge, Biofertilizer, Wastewater treatment plant
Graphical abstract
Highlights
-
•
Data for the pathogens found in AD and their potential health risk was presented.
-
•
Research on the sanitation performance of AD has been reviewed.
-
•
Mechanism and factors affecting biosludge hygienization have been reviewed.
-
•
Hotspots and challenges for future work on biosludge sanitation have been evaluated.
-
•
Suggestions to mitigate the pathogen problems for biosolids were proposed.
1. Introduction
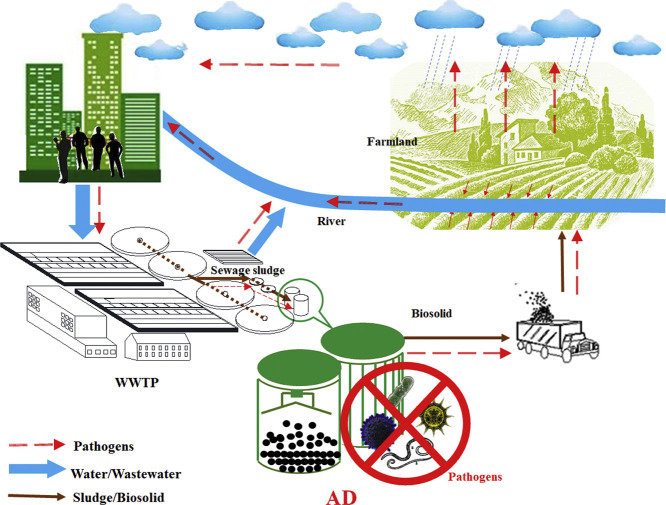
Municipal wastewater may carry various kinds of waterborne infectious pathogens which migrate within municipal wastewater treatment plant (WWTP) between liquid and biosludge. It had been reported that about 6.5 million metric ton (dry weight) of sewage sludge were generated annually, in which waterborne pathogens may be concentrated substantially (Shen et al., 2015). As such, biosolid disposal sites might act as the sources of pathogens, leading to a potential risk of uncontrollable spread into surface water, ground water and soils (Fröschle et al., 2015a, Fröschle et al., 2015b). Without proper control, sludge-associated pathogens could continue to grow, causing potential threats to the human health. In addition to pathogen-associated diseases, sludge pathogen is also a source of antibiotic resistance genes (ARGs). Increasing evidence suggests that ARGs in municipal wastewater are transferrable to biosludge and crops fertilized with human waste, leading to severer and long-lasting influence on public health (Devarajan et al., 2015; Su et al., 2015). Although several natural processes have been employed for inactivation of pathogens in waste sewage sludge (e.g. sun exposure, soil adsorption etc.), anaerobic digestion (AD) of biosludge has been considered as a highly efficient technology for killing pathogenic microorganisms (Fig. 1 ).
Fig. 1.
Scheme of anaerobic digestion as sewage sludge stabilization unit in WWTPs for biofertilizer production.
AD widely used for biosludge treatment with the aims of volume reduction and biogas generation is a complex biological process with the multiple-steps of reactions, i.e. hydrolysis, acidogenesis, acetogenesis and methanogenesis by which organic fraction of bio sludge being mainly converted to biomethane or hydrogen, an emerging source of renewable energy. In many countries, the solid residue produced from AD of sewage sludge has been used as fertilizer for crop production as well as soil stabilizer for improving soil quality. It can also be used as fish/aquaculture food sources after vermicomposting, converting the biosolids into aquatic worms. The worms mainly consist of protein and smaller fractions of fat, sugar and ash but contains low amount of heavy metals, which has a broad application potential as fish feed (Elissen et al., 2010). No matter for agricultural or fish/aquaculture purposes, AD is considered as the preliminary step. On one hand, AD improves the biodegradability of the raw sludge and raises the organic fractions that are more available for earthworms. On the other land, AD, as a pretreatment for vermicomposting step, lowered the pathogen loading rate for the worms. Compare to fish/aquaculture food resource, the public exercises much more due caution for the agricultural use (Wan et al., 2016). However, these applications of such digestate residue should be strictly regulated to ensure that it is hygienically safe.
The relative abundances of pathogenic microorganisms including bacterium, parasite and virus had been demonstrated to significantly decline after AD of sewage sludge. Different pathogenic species might have different susceptibilities to AD, the decay of pathogens was also related to the operating conditions (Ju et al., 2016; Kearney et al., 1993a; Sahlström et al., 2008). So far, the mechanism of pathogens deactivation has not been fully understood. Currently, extensive effort has been devoted to identifying the potential ecological and health risk of viable and infectious pathogens surviving after anaerobic stabilization of sewage sludge, while strong attention has also been given to standardize the quantification methods of viable pathogens (e.g. helminth eggs). Furthermore, with the steadily increasing antibiotic consumption, proliferation and release of ARGs-associated pathogens into the environment via biosolid discharge is of greater concern. Given such a situation, AD has gained increasing interest due to its merit of controlling ARGs, though the mechanism behind is yet clear. These suggest that the life cycle of ARGs after entering into the soil environment deserves more investigations. Otherwise, the digestate fertilizer as a carrier of pathogens with ARGs may pose a potential health risk to farmers during crop production, especially some specific pathogens use the soil as a transmission vector in their life cycles (Manser et al., 2015; Wang et al., 2015).
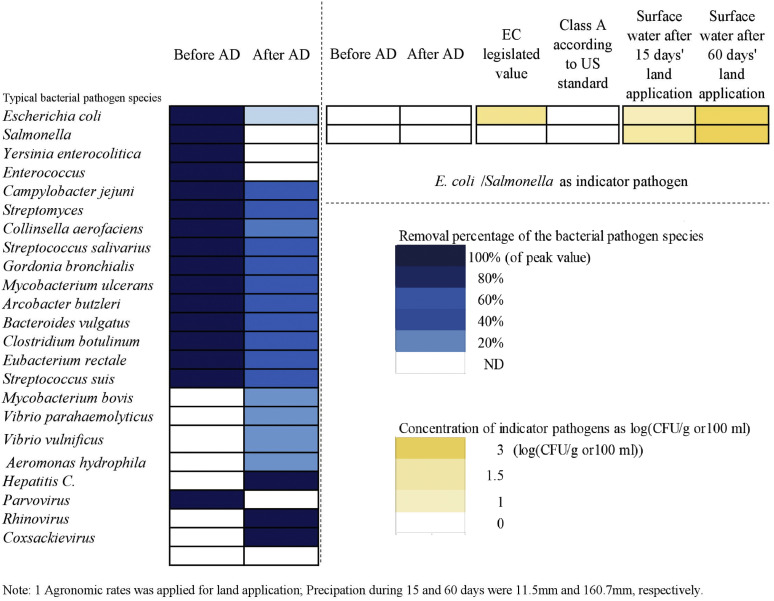
With its increasing sensitivity to environmental and health, digestate fertilizer has been regulated in the zero draft of ministerial outcome document “Towards a Pollution-Free Planet” by 2017 UN Environment Assembly in 2017. The EU animal by-products regulation stipulates that digestate leaving biogas or composting plants are considered acceptable only if Escherichia coli and are both below 1000/g, and Salmonella is not detectable in 5 tested samples of 25 g (Ahn et al., 2007; Commission Regulation, 2011). In the U.S., only the sludge of Class A, i.e. containing undetectable levels of pathogens, can be used for agricultural purposes. However, 75% of the stabilized sewage sludge used for agriculture belonged to Class B biosolids with pathogens (Grübel and Suschka, 2015). For Class A biosolids, of the level of fecal coliforms as an indicator of pathogen should be strictly monitored, while the monitoring of enteric viruses, helminth ova, and Salmonella spp. May not be required as long as process time and temperature are properly controlled at the required levels. It should be stressed that even the level of indicator pathogens is below detection limit, this cannot be directly translated to the absence of potential pathogenic risk due to the occurrence of other pathogens. So far, comprehensive survey of pathogen content and categories in AD digestate has not been undertaken. Moving forward, a fundamental question is how to optimize AD operation for minimizing potential pathogenic risks. Obviously, without a complete risk assessment, it is almost impossible to ensure the safe use of AD digestate. Therefore, this review attempts to offer a systematic overview of pathogens profile in WWTPs, the role of AD in mitigating pathogens, the possible mechanisms of pathogen deactivation in AD, the operating conditions and the principal factors against pathogens. Meanwhile, the potential use of digested sludge as environment-friendly biofertilizer was also discussed.
2. Pathogens in biosludge and their potential threats
2.1. Bacterial pathogens, parasites and virus
Full understanding of the bacterial pathogens profile is the crucial and primary step for the safe disposal and reuse of AD biosolid. With the rapid development of advanced molecular detection methods, more and more viable but nonculturable pathogens have been found in biosludge. As for total prokaryotic pathogenic diversity, there have been reports about accessing the phylogenetic and functional diversity with whole metagenome shotgun sequencing as well as bioinformatics analysis with MetaPhlAn (Metagenomic Phylogenetic Analysis) tool (Lu et al., 2015; Li et al., 2015). However, none has conducted research on the pathogenic communities with amplicon-based sequencing of marker gene which is more cost-effective. And 16S rRNA, such as high-throughput MiSeq sequencing, might be one more cost-effective amplicon-based sequencing methods to predict the functional capabilities of bacterial pathogenic communities after conducting bioinformatics analysis with MEGAN tool or Tax4Fun software package. For the community of fungi and others pathogenic eukaryotes, high-throughput sequencing for nuclear ribosomal internal transcribed spacer (ITS) is probably a method for useful molecule identification and sensitive blast searches and sequence clustering operations for the ITS region in eukaryotes can be made by some advanced software tool such as ITSx. Many of the detected pathogens, such as Aeromonas, Clostridium, Enterococcus, Corynebacterium, Klebsiella, Legionella, Mycobacterium, Salmonella, Streptococcus Vibrio and parasitic geohelminths or helminths have been reported and majority of these pathogens could cause severe morbidity or even mortality for human beings by inflicting respiratory diseases, gastroenteritis, conjunctivitis, cystitis, genital disease, skin and soft tissue infections, etc. (Table 1 ). Among the detected pathogens, Escherichia coli, Salmonella, Yersinia enterocolitica, Enterococcus were vulnerable to AD, while Campylobacter jejuni, and Streptomyces, Collinsella aerofaciens, Streptococcus salivarius and Gordonia bronchialis etc. were reported to be much more resistant to and hardly removed by AD (Ju et al., 2016; Kearney et al., 1993b; Stiborova et al., 2015). It should also be noted that some pathogens can be enriched, and even some become emerging and reemerging after AD (Li et al., 2015). Furthermore, some spore-forming pathogens e.g. Clostridium and Bacillus species with high resistance to acute stresses can survive after mesophilic or even thermophilic anaerobic digestion, therefore creating a hygienic problem when biosolid is spread (Dixit et al., 2005; Guzmán et al., 2007; Lloret et al., 2013). For example, biosolids produced from thermophilic AD could not meet the requirements by Directive of the European Parliament and of the Council for spreading of sludge on land due to the presence of C. perfringens spores (e.g. 9.6 × 104 CFUs mL−1) (Lloret et al., 2013).
Table 1.
Pathogens detected in biosludge and their potential health threats.
| Genus | Strains | Potential disease and illnesses | References | |
|---|---|---|---|---|
| Bacterium | Clostridium | Clostridium perfringens (spore-forming bacteria) | Necrotricentertis, equine colitis, food poisoning, lamb dysentery, and neonatal hemorrhagic necrotic enterotoxemias or gas gangrene | (Kashan et al., 2015; Meer et al., 1997) |
| Bacillus | Bacillus amyloliquefaciens, Bacillus cereus | Emetic or diarrheal syndrome | (Ahn et al., 2007; Govasmark et al., 2011; Marrollo, 2016) | |
| Campylobacter | Campylobacter jejuni | Diarrheal illness | (Wéry et al., 2008) | |
| Escherichia | Escherichia coli | (Shannon et al., 2007) | ||
| Mycobacterium | Mycobacterium tuberculosis, Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium phlei, Mycobacterium bovis and Mycobacterium kansasii | Tuberculosis, paratuberculosis, skin and soft tissue infections, cervical lymphadenitis, fibronodular disease with middle lobe bronchiectasis | (Gautam et al., 2017; Hamilton et al., 2017) | |
| Corynebacteriu | Diphtheria (Corynebacterium diphtheriae), sheep and goat caseous lymphadenitis (Corynebacterium pseudotuberculosis) | (Raynal et al., 2018; Wittchen et al., 2018) | ||
| Enterococcus | Nosocomial infections | (Guzman Prieto et al., 2016) | ||
| Klebsiella | Klebsiella pneumoniae | Nosocomial infections, pulmonary infections | (Holt et al., 2015) | |
| Legionella | Respiratory infections | (Comas, 2016) | ||
| Streptococcus | Streptomyces somaliensis, Streptococcus Salivarius | Mycetoma, meningitis | (Fahal et al., 2015; Ju et al., 2016; Sehu et al., n.d.) | |
| Vibrio | Vibrio cholera, Vibrio parahaemolyticus | Diarrhea (cholera disease), gastrointestinal and wound infection | (Fu et al., 2018; Trinh et al., 2018) | |
| Aeromonas | Aeromonas hydrophila | Diarrhea | (Teunis and Figueras, 2016) | |
| Salmonella | Salmonella typhimurium | Human gastroenteritis | (Cullinan et al., 2017) | |
| Yersinia | Enteric infections | (Shannon et al., 2007) | ||
| Shigella | Acute bloody diarrhea | (Chauret et al., 1999) | ||
| Staphylococcus | Staphylococcus aureus | Dysenteric syndrome | (Börjesson et al., 2009) | |
| Propionibacterium | Propionibacterium acnes | Skin disorder | (Ju et al., 2016; Petersen et al., 2015) | |
| Eggerthella | Eggerthella lenta | Life-threatening infections with gastroenteritis | (Ju et al., 2016; Wong et al., 2014) | |
| Collinsella | Collinsella aerofaciens | Irritable bowel syndrome | (Bag et al., 2017; Ju et al., 2016) | |
| Gordonia | Gordonia bronchialis | Osteomyeliti | (Ju et al., 2016; Siddiqui et al., 2012) | |
| Parasites | Geohelminths/helminths | Ascaris lumbricoides | Parasite | (Alves et al., 2016; Shamma and Al-Adawi, 2002) |
| Virus | DNA virus | Adenovirus, Herpesvirus, Papillomavirus Bocavirus | Respiratory diseases, gastroenteritis, conjunctivitis, cystitis, genital disease (Adenovirus), late-term abortions (Herpesvirus) oropharyngeal and genital cancers (Papillomavirus), severe acute respiratory tract infection (Bocavirus) | (Kang et al., 2018; Moesker et al., 2015; Newcomer et al., 2017; Ponterio and Gnessi, 2015; Shen et al., 2017) |
| RNA viruses | Coronavirus, Klassevirus, Rotavirus, Enterovirus Poliovirus | Severe acute respiratory tract infection (Coronavirus), gastroenteritis and respiratory infection (Klassevirus), neonatal fever (Rotavirus), poliomyelitis (Enterovirus Poliovirus) | ||
The accumulation of viral pathogens was also observed in biosludge, and it might threaten the safety of nearby workers, while causing infectious risks associated with land application (De Serres and Laliberté, 1997). As summarized in Table 1, viruses found in biosludge generally include DNA virus (e.g. Adenovirus, Herpesvirus, Papillomavirus Bocavirus) and RNA viruses (e.g. Coronavirus, Klassevirus, Rotavirus), and some of which were classified as emerging viruses with high abundance. Viruses seemed closely associated with Enterococci, and they were more resistant than coliforms during AD, but less resistant than spore formers, such as Clostridia (Avery et al., 2014; Sahlström, 2003). On the other hand, DNA viruses were found to be more persistent than the sing-stranded RNA phage (Decrey and Kohn, 2017).
Although most of digestate-borne pathogens and their ecological impacts had been reported in the literature, it is difficult to assert which kinds of pathogen would pose higher risks to public health and which should be the primary targets of deactivation during AD. In this regard, it had been proposed that the stable pathogens with high occurrence frequency should be primarily targeted (e.g. Clostridium, Mycobacterium, Collinsella aerofaciens and Streptococcus Salivarius). Meanwhile, the enriched, and emerging-reemerging pathogens should also need to be monitored closely, including Propionibacterium acnes, Gordonia bronchialis, Eggerthella lenta, Mycobacterium bovis, Streptococcus salivarius, Collinsella aerofaciens (Ju et al., 2016; Lahiri et al., 2014; Li et al., 2015). More importantly, the potential environmental risks associated with these infectious pathogens should be fully assessed in a holistic manner.
2.2. Host of ARGs
The risk of pathogens harboring ARGs in biosludge are gaining more and more public concern. Bacterial pathogens can serve as a host of ARGs of multidrugs and macrolide-lincosamide-streptogramin (Ju et al., 2016). It has been known that ARGs can proliferate and spread through mobile genetic elements, such as integron, plasmids, transposon etc., with transferrable genes encoded with pathogenicity factors (Yu et al., 2016). Accumulating evidence suggests that the increased antibiotic resistance in bacterial pathogenesis could be induced and facilitated by the genetic-transferring-derived virulence proteins, which interfered with the signal transduction, while failed to regulate normal cellular metabolism. Thermophilic AD has been adopted as a remedial mean to attenuate integrons and ARGs in sewage sludge, including tetracycline resistance genes (tetA, tetO, tetX) and integron-integrase gene (intI1) (Diehl and LaPara, 2010; Ghosh et al., 2009). It had been reported that thermophilic treatment helped to reduce the accumulation and spread of these pathogens-associated ARGs in soil (Kang et al., 2017). On the contrary, there was evidence showing that most ARGs were hardly reduced by AD. Thus, further investigation should be urgently needed to evaluate the ARGs reduction by AD. This in turn will guide the operation of AD to minimize the spread of ARGs, helping to better manage the overall risk of r digestate residue in agricultural applications.
3. Factors affecting pathogens deactivation in AD and the mechanisms
3.1. Temperature
The abundance of pathogens in sewage sludge generally fluctuates with the process configuration and operation conditions. Temperature had been considered as the primary suppressive factor of pathogens in anaerobic digested sludge (Forbis-Stokes et al., 2016). In fact, lethal effect of high temperature could be primarily attributed to denaturation of inactivating enzymes or capsid protein (Pandey and Soupir, 2011). In fact, mild thermal process operated at moderate temperature was able to inactivate a variety of enzymes, such as indigenous epectin methylesterase, glyceraldehyde-3-phosphate dehydrogenase, creatine kinase, triose phosphate isomerase, acid phosphatase, serum albumin and immunoglobulin G, Lactate dehydrogenase (Veeramuthu et al., 1998). Thermal conditions applied to AD could induce pathogens destruction by break-up of the gel structure and cell lysis (Arora and Kazmi, 2015). According to Arrhenius equation, the inactivation rate of pathogens (e.g. helminth ova and enteric viruses) at 55 °C was 15–17 times higher than those at 25–37 °C (Pandey and Soupir, 2011). Thermophilic digestion could completely kill all Coli-aerogenes and Enterococcus, while viable pathogens were still detected under mesophilic conditions at a sludge retention time of 12–15 days (Iwasaki et al., 2011). Similarly, the multidrug-resistant bacteria were found to survive in dairy waste after 22-day mesophilic anaerobic digestion, but they disappeared after thermophilic digestion at 55 °C (Beneragama et al., 2013; Nilmini et al., 2013). The potential sul-harboring and integrons-harboring pathogens (e.g. Actinomycetales and Corynebacterium) could be completely eradicated through thermophilic digestion, while 0.26% and 0.17% of which could still survive under mesophilic conditions (Sun et al., 2016). Moreover, ARG subtypes showed different responses to mesophilic and thermophilic conditions. Metagenomic analysis further revealed the lower abundance of mobilome (e.g. integrons, insert sequences and plasmids) as well as lower horizontal gene transfer potential under thermophilic conditions compared to mesophilic conditions. However, it should be realized that heat-resistant pathogens including Bacillus cereus, Erysopelotrix rhusiopathiae, Listeria monocytogene, Staphylococcus aureus, Streptococcus faecalis, Yersinia enterocolitica, C. perfringens spores etc. were hardly removed by mesophilic and thermophilic AD or composting (Elmerdahl Olsen and Errebo Larsen, 1987; Kearney et al., 1993a). Therefore, it appears that high temperature should not be always regarded as a guaranty of high ARGs removal.
3.2. Ammonia concentration
The toxicity of ammonia on pathogens is notable and ammonia sanitization was often employed as one simple and low-cost alternative to inactivate bacteria, viruses, protozoa, adenovirus, reovirus, bacteriophages and other pathogens (Fidjeland et al., 2015; Magri et al., 2015). Dosage should not exceed the essential amount for anaerobic microorganism in digester (Rajagopal et al., 2013; Yenigün and Demirel, 2013). Meanwhile, other factors, e.g. substrates, inocula, temperature, pH and acclimation periods should be controlled since they can change the effect of ammonia on pathogens by modifying the equilibrium between toxic-N (Free ammonia) and non-toxic-N fractions among nitrogen compounds (Scaglia et al., 2014). Helminth eggs and Clostridium spp. spores which showed strong resistance to ammonia inactivation at ambient temperature should be inactivated with assisting techniques (Fidjeland et al., 2015).
Furthermore, it has been known that ammonia can effectively deactivate bacterial pathogens by altering the intracellular/extracellular K+/NH3 ratio and exchange reaction (Sprott and Patel, 1986). As for viruses, NH3 may cause the loss in genome integrity, specifically, the cleavage of viral RNA in intact particles (Decrey et al., 2015). As for ascaris eggs, the mechanism of ammonia inactivation remains unclear, but it is widely believed that uncharged free ammonia is able to penetrate through the cell membrane, resulting in higher intracellular pH (Pecson et al., 2007).
3.3. VFAs (volatile fatty acids) and pH
High-concentration volatile fatty acids (VFAs) coupled with acidic pH favors deactivation and elimination of pathogens in sewage sludge. For example, VFAs produced at the acedogenic stage of AD could effectively inactivate Escherichia coli, Salmonella typhimurium, or even Clostridium spp. (Orzi et al., 2015; Sahlström, 2003). In contrast, VFAs were ineffective in deactivating the Ralstonia solanacearum, a soil-born pathogen, while Bacteriophages MS2 was found to be insensitive to pH at 50 °C (Chen et al., 2016). Enterococci and Enterobacteriaceae which are the ubiquitous and potentially opportunistic intestine pathogens could survive and proliferate under stressed conditions due to their high tolerance to solution pH (Fisher and Phillips, 2009). However, it is not practical to adjust or control the level of VFAs or pH in anaerobic digester for deactivation of pathogen because such control would adversely impact on the overall performance of AD, or even caused the operation failure. Currently, significant reduction of fecal coliform, polio virus, ascaris egg and other pathogens had been reported in both two-stage and two-phase AD (Leite et al., 2016; Wahidunnabi and Eskicioglu, 2014; Wan et al., 2018).
It should be noted that the underlying mechanism VFAs-promoted deactivation of pathogens had not yet been reported. According the findings from research of bacterial pathogens in gastrointestinal tract microbiota, high-concentration short chain VFAs and low pH generated during anaerobic hydrolysis and acidogenesis could likely exert adverse effects on electron transfer and proton translocation, leading to pathogen deactivation. However, some VFAs at low concentration (e.g. Salmonella Typhimurium, enterohemorrhagic Escherichia coli) were found to stimulate virulence gene expression, leading to systemic infection (Vogt et al., 2015).
3.4. Solid retention time (SRT)
Prolonged sludge retention time (SRT) in AD offers an alternative for enhancing deactivation of 90% of pathogenic species (e.g. Yersinia enterocolitica) could be deactivated by AD at the SRT of 18.2 d (defined as T90) (Kearney et al., 1993a), while total coliforms could be reduced to below detection limit at the SRT longer than 15 days in AD (Coelho et al., 2011; Iranpour and Cox, 2007). The similar phenomenon was also observed for Escherichia coli and Salmonella spp. which could be removed at a minimum SRT of 15 days (Forster-Carneiro et al., 2010). However, some pathogens could still survive even at a prolonged SRT, e.g. 90% of deactivation of Campylobacter jejuni required an SRT as long as 438.6 day which was far beyond the operational range of SRT for AD (Kearney et al., 1993b).
3.5. Other factors
Table 2 summarizes the effect of temperature, SRT, ammonia, VFAs and pH on the removal efficiency of pathogens in AD. It can be concluded that there is no single factor that has broad-spectrum anti-pathogen effect. Thermal condition maintained for the AD could kill more species of pathogens in comparison. In addition to the factors discussed above, many other factors, such as nutrients availability, embryonated status, operation modes of AD etc., can also affect the growth and accumulation of pathogens. For example, Campylobacter jejuni can use amino acids and vitamins that are not used by other bacteria, hence creating a favorable living environment to counter-count deactivation stress, while Salmonella typhimurium, Listeria monocytogenes and Yersinia enterocolitica need to compete with other bacterial species for carbohydrates. In addition, embryonated or dominant ova showed different viabilities when exposed to AD, e.g. about 35% of fully developed Ascaris suum ova still remained viable after 16-day AD against 65% for unembryonated ova (Manser et al., 2015). Batch or continuous operation modes of AD may also influence pathogenic profile in sewage sludge (Narula et al., 2011). Enteropathogenic Escherichia coli and Salmonella Typhimurium were reported to have the lower T90 values in batch anaerobic digestion than those in semi-continuous or full-continuous operations (Kearney et al., 1993a). To survive or infect host, pathogens have to compete each other or with anaerobic bacteria for nutrients and microsites (Vogt et al., 2015), whereas some compounds in AD had been known to protect pathogens from deactivation (Pandey and Soupir, 2011).
Table 2.
Influence of main factors on the removal efficiency of some typical pathogens.
| Genus/species | Temperature |
NH3 | VFA | SRT | Unrestricted parameters | Refs | |
|---|---|---|---|---|---|---|---|
| Mesophilic | Thermophilic | ||||||
| Clostridium perfringens | × | 乄 | 乄 | (Fidjeland et al., 2015; Kearney et al., 1993a; Lloret et al., 2013; Orzi et al., 2015; Sahlström, 2003) | |||
| Clostridium difficile | 乄 | (Xu et al., 2015) | |||||
| Bacillus cereus | 乄 | 乄 | (Govasmark et al., 2011; Kearney et al., 1993a) | ||||
| Campylobacter jejuni | 438.6d | √/× | (Kearney et al., 1993a; Wagner et al., 2008) | ||||
| Escherichia coli | 乄 | 乄 | <15d | (Forster-Carneiro et al., 2010; Lloret et al., 2013; Orzi et al., 2015; Sahlström, 2003) | |||
| Corynebacteriu | √ | (Sun et al., 2016) | |||||
| Enterococcus | 乄 | (Iwasaki et al., 2011) | |||||
| Enterococci | × | (Fisher and Phillips, 2009) | |||||
| Enterobacteriacea | × | (Fisher and Phillips, 2009) | |||||
| Listeria monocytogenes | 乄/× | 乄 | (Kearney et al., 1993a; Orzi et al., 2015) | ||||
| Streptococcus Salivarius | × | (Ju et al., 2016) | |||||
| Streptococcus faecalis | × | (Kearney et al., 1993b) | |||||
| Actinomycetales | √ | (Sun et al., 2016) | |||||
| Salmonella typhimurium; Salmonella spp. | 乄 | √ | √ | <15d | (Forster-Carneiro et al., 2010; Lloret et al., 2013; Orzi et al., 2015; Sahlström, 2003) | ||
| Yersinia enterocolitica, | 乄/× | × | 乄 | 18.2 d | (Kearney et al., 1993b) | ||
| Ralstonia solanacearum | 乄 | × | (Chen et al., 2016) | ||||
| Phytophthora capsici | 乄 | ||||||
| Staphylococcus aureus | × | 乄 | (Kearney et al., 1993b; Viau and Peccia, 2009) | ||||
| Collinsella aerofaciens | × | (Ju et al., 2016) | |||||
| Erysopelotrix rhusiopathiae | × | (Kearney et al., 1993b) | |||||
| Bacteriophages MS2 | 乄 | (Chen et al., 2016) | |||||
| Gordonia bronchialis | × | (Ju et al., 2016) | |||||
| Cryptosporidium | 20d | √ | (Côté et al., 2006) | ||||
| Giardia | 20d | √ | (Côté et al., 2006) | ||||
| Ascaris suum ova | × | (Manser et al., 2015) | |||||
| Helminths eggs | 乄 | (Fidjeland et al., 2015) | |||||
Note: √: complete eradication; 乄: Partially removed; ×, hardly removed;乄/× or √/×, different viewpoints existed among researchers towards removal efficiency.
Correlation between bacterial community succession and pathogenic population in AD had been established (Luo et al., 2017). As both total organics and nitrogen in sewage sludge were reduced substantially during AD, this in turn led to the shift in the archaea and bacteria communities, including pathogens. In general, less diversified microbial community was observed after AD of sewage sludge (Ennouri et al., 2016). For example, Firmicutes (e.g. Bacilli and Clostridia as representative order) and Actinobacteria (e.g. Actinomycetales as typical member) were found to be the dominant phyla in raw sludge, while their abundances declined during the digestion, along with the enrichment of Bacteroidetes. It was also found that the microbial diversity was further lowered when AD was turned from mesophilic to thermophilic operation with notable shifts towards the higher number of thermotolerant taxa and lower abundances of Bacteroidetes and Proteobacteria (Sun et al., 2016; Stiborova et al., 2015). It is worth to note that Bacteroidetes and Proteobacteria had been considered as potential pathogens that harbored ARGs, while Actinomycetales, Bacilli Corynebacterium were known as sul and integron carrier (Sun et al., 2017). Other operating conditions, such as SRT, VFA, ammonia as discussed above can also influence the community structure. After shifting of operation conditions of AD, positive or negative correlations were observed between bacterial pathogens or between pathogens and integrase genes or ARGs, indicating that the bacterial community shift was one of the key drivers of variation in survival or death of anti-drug-resistant pathogens during AD (Sun et al., 2016; Jang et al., 2019).
For a practical AD system, environmental factors are often correlated and have intertwined effect on the outcome (Decrey and Kohn, 2017; Kumar et al., 1999). For example, temperature can act directly or indirectly on pathogens by enhancing the toxicity of ammonia and VFAs. It also related to time. And pH effects are not significant itself but cannot be separated from both temperature and ammonia concentration (Pecson et al., 2007). Slight change in temperature, ammonia, VFA and SRT would shape distinct bacterial community succession. Thus, the overall hygienic performance of AD should be assessed and optimized based on these parameters on the integrity.
4. Models, engineering designs and technological aspects
Mathematical modeling of anaerobic digestion is useful for improving digester performance. The IWA Anaerobic Digestion Model No. 1 has widely used for describing and predicting AD performance under various scenarios. In this model, the degradation process is described by Monod kinetics, while the disintegration and hydrolysis of sludge is supposed to follow first order kinetics. As such, it has been debated the rational of using simple first order kinetic for highly complicated sophisticated AD. In addition, the model takes into account seven bacterial groups, but does not distinguish among them, therefore undermining the predictive power of the models. So far, pathogens or ARGs removal has not yet considered in the models for AD. As a step forward, pathogenic variables should be included in AD optimization and automation (Appels et al., 2011).
In fact, AD as mature technology has been extensively applied for treating sewage sludge with the aims for volume reduction and biogas generation. However, as elucidated above, the issues associated with pathogens, to some extent, might be overlooked. The current design and operation are primarily driven by maximizing biogas production, while going forward, the engineering trade-off should be considered by including pathogen-associated environmental risks. So far, various engineering designs, such as two-stage AD, two-phase AD, co-digestion, membraned enhanced AD with different configurations etc. have been explored. In sanitary engineering practice, a robust AD project for biosludge treatment that not only fulfills the bio-energy generating functions but also produce clean biosolids requires comprehensive technological measures, including: (a) Reception and inspection area; (b) Mixer equipment; (c) Comminuting drums that screen the raw sludge; (d) Separation of liquid from the digestate and the pumps. (e) Heating equipment; (f) Sufficient volume and long retention time; (g) Biogas combustion or utilization device (for heat, power, or fuel) and the necessary safety precautions. With the development of novel pretreatment or post-treatment to enhance pathogens removal, more work needs to been done to identify optimized management strategies and comprehensive technical measures for engineering practice.
5. Is AD reliable for sludge pathogens control: challenges for the potential use of digested sludge?
Up to now, many large municipalities worldwide have been or are considering options for optimizing and upgrading AD in WWTPs to make biosolids more “RELIABE” for land application. Improved techniques have been developed for AD to enhance the removal efficiency of pathogens by employing pretreatment and post-treatment. Thermal or electrical-thermal pre-treatment is believed to induce hydrolysis of sludge by breaking the gel structure and cell lysis, which involved pathogens destruction as well (Ennouri et al., 2016; Daneshmand et al., 2012). Low frequency pretreatment of ultrasound prior to thermophilic AD was also found to achieve significant logarithmic removal of fecal coliforms, somatic coliphages and other pathogens in sludge. Ultrasound generated the shear stress induced by cavitation while high temperature led to denaturation and loss of function of enzymes, nucleic acids, organelles and other cellular structures (Neumann et al., 2018). Microwave irradiation, UV, or nanoparticle were also reported to successfully improve the AD performance, achieving higher inactivation rate of fecal coliforms than single AD (Hong et al., 2006; Neto et al., 2006; Miller et al., 2013). The biotechnological development was less reported to integrate with AD compared to physiochemical technologies. One notable example is anaerobic digestion pasteurization latrine system developed by researchers in North Carolina of the States – a self-contained and energy neutral on-site sanitation system – using the biogas generated from the sludge to pasteurize the digestate at 65–75 °C to produce a safe fertilizer (Forbis-Stokes et al., 2016). It was essentially the integrated AD with thermal treatment via technical modifications. From my standpoint, modern biosystem developed with AD as a solution to the problem should be built based on the psychological differences between the pathogens and normal anaerobes or predation by higher trophic level in the food chain. But the mild conditions maintained for anaerobes in pure-biosystem might not thoroughly eradicate the pathogens to the required level. Therefore, physiochemical methods were preferred to integrate with AD. And the biological-based digestion and physiochemical-based sanitation were generally separated in two compartments/steps so that the harsh conditions in sanitation stage would not have fatal or harmful effect on the anaerobes and other functional organisms in AD.
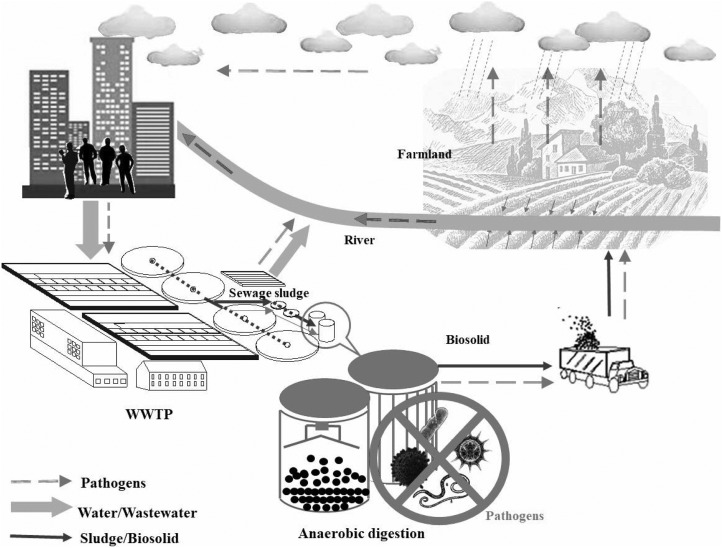
AD with assisted techniques has been tested to achieve good performance. However, is it really reasonable to declare that AD is “RELIABE” or NOT after narrowly focusing on the research of pathogen deactivation within a digester or a reactor? In fact, the outbreak of infectious diseases caused by digestate residue is an extremely complicated event since the disseminating procedures involved a complex matrix of sub-procedures and factors, e.g. levels of pathogens in the sludges, particular combination of chemicals, usage rate at farmland, sun exposure, climate, infection routes and host susceptibility etc. Some pathogenic microbes are easily deactivated via AD, but may be regenerated during the subsequent transmission. On the other hand, some pathogenic microbes, though existed in digestate, could not be removed after exposed to sunlight or plants during fertilization. Meanwhile, antagonistic microbes and antimicrobial substances also need to be taken into account (Cao et al., 2013). As shown in Fig. 2 , even though AD may deactivate a wide range of pathogens, while meeting both EU legislated requirement and U.S. Class A standard for solid residue, pathogens still could be detected in surface water after use of digestates on land (REF). Therefore, there is a concern associated with the use of digestates for agricultural purpose.
Fig. 2.
Variation of typical pathogens in biosludge during anaerobic digestion and land application.
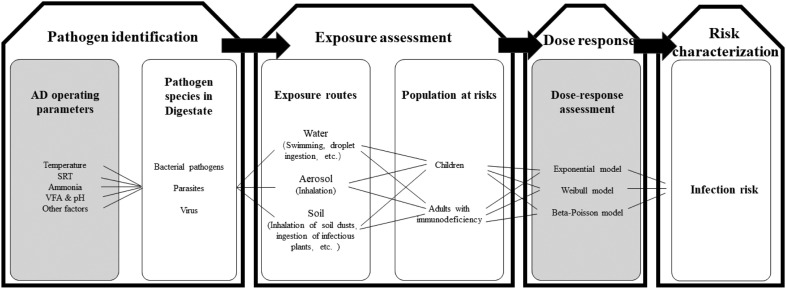
AD can be considered as hygienically reliable only if the subsequent pathogenic migration process is also tracked. Quantitative microbial risk assessment (QMRA) combined with AD is an advisable solution. The QMRA approach is recommended for AD under different sensitive conditions, including temperature, SRT, concentration of free ammonia, pH, VFA, etc. Pathogens species discharged determine whether it is necessary to undertake risk assessment and the extent of mitigation. Then the exposure routes and the population at risks should be estimated when the exposure occurred. After exposure assessment, the risk can be characterized based on quantitative dose response models, which have been or will be developed for the species/subspecies of the pathogens identified. Finally, the infection risk associated with AD digestate can be assessed more precisely by expressing in annual probability of infection and disability-adjusted life years. Now it seems that enrichment of dose response models is top priority for the digestate-relevant pathogens, since the value of parameters in dose response models are varied with different pathogenic microorganisms (Xiao et al., 2018). Sometimes, aerosol transport models for pathogen doses are needed for the receptors downwind of land application sites. Studies of relevant infection outbreaks and epidemiological investigations for digestate-relevant are also highly required. With more complete and accurate models, QMRA is helpful to develop more appropriate AD management strategies, on a physiological hygienical level to evaluate whether an AD of certain SRT, temperature etc. is reasonable and reliable. This is also helpful for risk assessors and environmental regulators to make policy and engineering decisions (Fig. 3 ).
Fig. 3.
QMRA framework for pathogens in biosludge.
6. Concluding remarks
This review sheds light into the role of AD in deactivating sludge-associated pathogens. Variety of pathogens, including bacterium, parasites, virus and pathogenic host of ARGs has been successfully deactivated by AD. The inactivation mechanism could be attributed to many forms of cytopathy, such as enzyme denaturation, intracellular/extracellular ion content alteration, viral RNA cleavage, bacterial community succession, etc., at given temperature, ammonia concentration, VFAs content, pH value, SRT, etc. And AD hygienic performance can be optimized by setting these parameters at reasonable range. Moving forward, in order to be available for agricultural purposes, the digestate residue discharged from AD should inevitably be evaluated and assessed by looking into the integrated ecological and epidemiology system. It appears that future study should look into:
-
1)
There is a need to reveal the diversity and property of more pathogens within digestate, since whole genome sequences of many species have not been obtained and relevant primer have not been designed.
-
2)
Scientific credibility for the restrictions on crop and access to application sites even for Class A biosolids should be reviewed since regrowth of pathogens occur or host of ARGs proliferate;
-
3)
Virus and the ones harboring ARGs should be tracked and more fully described. Relationships between resistant-pathogen concentrations and fecal indicators should also be identified.
-
4)
Better quantification of human exposure routes to pathogens is recommended. Prospective epidemiological studies are indispensable, with dose response models probably required to conduct a comprehensive risk assessment on a quantitative level. In brief, it is required to conduct a comprehensive and system life cycle analysis about pathogens' fate-originally from activated sludge bed, digester, composting handling, exposure on farmland to host characteristics.
-
5)
Incorporating pathogens and ARGs removal into the present anaerobic digestion models is needed.
Finally, pathogen attenuation at the source WWTPs is the basic principle in the science of sewage sludge management. AD process should be operated and maintained with strictly monitoring pathogens of low content, low removal rate and low potential risks for the public health. Only AD that converts sewage sludge to qualified biofertilizer is a robust biological process gaining acceptance within the scientific community and public domain.
Acknowledgment
The authors would like to thank Shandong Key Laboratory of Water Pollution Control and Resource Reuse (grant number 2019KF08, 2019). This work was supported in part by National Natural Science Foundation of China Projects (grant number 51708338, 2017) and Doctoral Research Fund of Shandong Jianzhu University (grant number XNBS1702, 2018).
Editor: Huu Hao Ngo
Contributor Information
Qian Zhao, Email: zhaoqian@sdjzu.edu.cn.
Yu Liu, Email: cyliu@ntu.edu.sg.
References
- Ahn J., Balasubramaniam V.M., Yousef A.E. Inactivation kinetics of selected aerobic and anaerobic bacterial spores by pressure-assisted thermal processing. Int. J. Food Microbiol. 2007;113:321–329. doi: 10.1016/j.ijfoodmicro.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Alves E.B.d.S., Conceição M.J., Leles D. Ascaris lumbricoides, Ascaris suum, or “Ascaris lumbrisuum”? J. Infect. Dis. 2016;213:1355-1355. doi: 10.1093/infdis/jiw027. [DOI] [PubMed] [Google Scholar]
- Appels L., Lauwers J., Degrève J., Helsen L., Lievens B., Willems K., Dewil R. Anaerobic digestion in global bio-energy production: potential and research challenges. Renew. Sust. Energ. Rev. 2011;15:4295–4301. [Google Scholar]
- Arora S., Kazmi A.A. The effect of seasonal temperature on pathogen removal efficacy of vermifilter for wastewater treatment. Water Res. 2015;74:88–99. doi: 10.1016/j.watres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Avery L.M., Anchang K.Y., Tumwesige V., Strachan N., Goude P.J. Potential for pathogen reduction in anaerobic digestion and biogas generation in Sub-Saharan Africa. Biomass Bioenergy. 2014;70:112–124. [Google Scholar]
- Bag S., Ghosh T.S., Das B. Complete Genome Sequence of Collinsella aerofaciens Isolated from the Gut of a Healthy Indian Subject. Genome Announcements. 2017:5. doi: 10.1128/genomeA.01361-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneragama N., Iwasaki M., Lateef S.A., Yamashiro T., Ihara I., Umetsu K. The survival of multidrug-resistant bacteria in thermophilic and mesophilic anaerobic co-digestion of dairy manure and waste milk. Anim. Sci. J. 2013;84:426–433. doi: 10.1111/asj.12017. [DOI] [PubMed] [Google Scholar]
- Börjesson S., Melin S., Matussek A., Lindgren P.-E. A seasonal study of the mecA gene and Staphylococcus aureus including methicillin-resistant S. aureus in a municipal wastewater treatment plant. Water Res. 2009;43:925–932. doi: 10.1016/j.watres.2008.11.036. [DOI] [PubMed] [Google Scholar]
- Cao Y., Chang Z., Wang J., Ma Y., Fu G. The fate of antagonistic microorganisms and antimicrobial substances during anaerobic digestion of pig and dairy manure. Bioresour. Technol. 2013;136:664–671. doi: 10.1016/j.biortech.2013.01.052. [DOI] [PubMed] [Google Scholar]
- Chauret C., Springthorpe S., Sattar S. Fate of Cryptosporidium oocysts, Giardia cysts, and microbial indicators during wastewater treatment and anaerobic sludge digestion. Can. J. Microbiol. 1999;45:257–262. [PubMed] [Google Scholar]
- Chen L., Jian S., Bi J., Li Y., Chang Z., He J., Ye X. Anaerobic digestion in mesophilic and room temperature conditions: digestion performance and soil-borne pathogen survival. JEnvS. 2016;43:224–233. doi: 10.1016/j.jes.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Coelho N.M.G., Droste R.L., Kennedy K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011;45:2822–2834. doi: 10.1016/j.watres.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Comas I. Legionella effectors reflect strength in diversity. Nat. Genet. 2016;48:115. doi: 10.1038/ng.3492. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EU) No. 142/2011.
- Côté C., Massé D.I., Quessy S. Reduction of indicator and pathogenic microorganisms by psychrophilic anaerobic digestion in swine slurries. Bioresour. Technol. 2006;97:686–691. doi: 10.1016/j.biortech.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cullinan M., Clarke M., Dallman T., Peart S., Wilson D., Weiand D. Salmonella typhimurium gastroenteritis leading to chronic prosthetic vascular graft infection. JMM Case Reports. 2017;4 doi: 10.1099/jmmcr.0.005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmand T.N., Beton R., Hill R.J., Gehr R., Frigon D. Inactivation mechanisms of bacterial pathogen indicators during electro-dewatering of activated sludge biosolids. Water Res. 2012;46:3999–4008. doi: 10.1016/j.watres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- De Serres G., Laliberté D. Hepatitis A among workers from a waste water treatment plant during a small community outbreak. Occup. Environ. Med. 1997;54:60–62. doi: 10.1136/oem.54.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrey L., Kohn T. Virus inactivation in stored human urine, sludge and animal manure under typical conditions of storage or mesophilic anaerobic digestion. Environmental Science: Water Research & Technology. 2017;3:492–501. doi: 10.1039/c6ew00311g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrey L., Kazama S., Udert K.M., Kohn T. Ammonia as an in situ sanitizer: inactivation kinetics and mechanisms of the ssRNA virus MS2 by NH3. Environ. Sci. Technol. 2015;49:1060–1067. doi: 10.1021/es5044529. [DOI] [PubMed] [Google Scholar]
- Devarajan N., Laffite A., Graham N.D., Meijer M., Prabakar K., Mubedi J.I., Elongo V., Mpiana P.T., Ibelings B.W., Wildi W., Poté J. Accumulation of clinically relevant antibiotic-resistance genes, bacterial load, and metals in freshwater lake sediments in Central Europe. Environ. Sci. Technol. 2015;49:6528–6537. doi: 10.1021/acs.est.5b01031. [DOI] [PubMed] [Google Scholar]
- Diehl D.L., LaPara T.M. Effect of temperature on the fate of genes encoding tetracycline resistance and the integrase of class 1 integrons within anaerobic and aerobic digesters treating municipal wastewater solids. Environ. Sci. Technol. 2010;44:9128–9133. doi: 10.1021/es102765a. [DOI] [PubMed] [Google Scholar]
- Dixit A., Alam S.I., Dhaked R.K., Singh L. Sporulation and heat resistance of spores from a Clostridium sp. RKD. J. Food Sci. 2005;70:m367–m373. [Google Scholar]
- Elissen H.J.H., Mulder W.J., Hendrickx T.L.G., Elbersen H.W., Beelen B., Temmink H., Buisman C.J.N. Aquatic worms grown on biosolids: biomass composition and potential applications. Bioresour. Technol. 2010;101:804–811. doi: 10.1016/j.biortech.2009.08.060. [DOI] [PubMed] [Google Scholar]
- Elmerdahl Olsen J., Errebo Larsen H. Bacterial decimation times in anaerobic digestions of animal slurries. Biol. Wastes. 1987;21:153–168. [Google Scholar]
- Ennouri H., Miladi B., Diaz S.Z., Güelfo L.A.F., Solera R., Hamdi M., Bouallagui H. Effect of thermal pretreatment on the biogas production and microbial communities balance during anaerobic digestion of urban and industrial waste activated sludge. Bioresour. Technol. 2016;214:184–191. doi: 10.1016/j.biortech.2016.04.076. [DOI] [PubMed] [Google Scholar]
- Fahal A., Mahgoub E.L.S., Hassan A.M.E.L., Abdel-Rahman M.E. Mycetoma in the Sudan: an update from the Mycetoma Research Centre, University of Khartoum. Sudan. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidjeland J., Nordin A., Pecson B.M., Nelson K.L., Vinnerås B. Modeling the inactivation of ascaris eggs as a function of ammonia concentration and temperature. Water Res. 2015;83:153–160. doi: 10.1016/j.watres.2015.06.030. [DOI] [PubMed] [Google Scholar]
- Fisher K., Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- Forbis-Stokes A.A., O'Meara P.F., Mugo W., Simiyu G.M., Deshusses M.A. On-site fecal sludge treatment with the anaerobic digestion pasteurization latrine. Environ. Eng. Sci. 2016;33:898–906. doi: 10.1089/ees.2016.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster-Carneiro T., Riau V., Pérez M. Mesophilic anaerobic digestion of sewage sludge to obtain class B biosolids: microbiological methods development. Biomass Bioenergy. 2010;34:1805–1812. [Google Scholar]
- Fröschle B., Heiermann M., Lebuhn M., Messelhäusser U., Plöchl M. Hygiene and sanitation in biogas plants. In: Guebitz G.M., Bauer A., Bochmann G., Gronauer A., Weiss S., editors. Biogas Science and Technology. Springer International Publishing; Cham: 2015. pp. 63–99. [Google Scholar]
- Fröschle B., Messelhäusser U., Höller C., Lebuhn M. Fate of Clostridium botulinum and incidence of pathogenic clostridia in biogas processes. J. Appl. Microbiol. 2015;119:936–947. doi: 10.1111/jam.12909. [DOI] [PubMed] [Google Scholar]
- Fu Y., Ho B.T., Mekalanos J.J. Tracking vibrio cholerae cell-cell interactions during infection reveals bacterial population dynamics within intestinal microenvironments. Cell Host & Microbe. 2018;23:274–281. doi: 10.1016/j.chom.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S.S., Mac Aogáin M., Bower J.E., Basu I., O'Toole R.F. Differential carriage of virulence-associated loci in the New Zealand Rangipo outbreak strain of Mycobacterium tuberculosis. Infect. Dis. 2017;49:680–688. doi: 10.1080/23744235.2017.1330553. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Ramsden S.J., LaPara T.M. The role of anaerobic digestion in controlling the release of tetracycline resistance genes and class 1 integrons from municipal wastewater treatment plants. Appl. Microbiol. Biotechnol. 2009;84:791–796. doi: 10.1007/s00253-009-2125-2. [DOI] [PubMed] [Google Scholar]
- Govasmark E., Stäb J., Holen B., Hoornstra D., Nesbakk T., Salkinoja-Salonen M. Chemical and microbiological hazards associated with recycling of anaerobic digested residue intended for agricultural use. Waste Manag. 2011;31:2577–2583. doi: 10.1016/j.wasman.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Grübel K., Suschka J. Hybrid alkali-hydrodynamic disintegration of waste-activated sludge before two-stage anaerobic digestion process. Environ. Sci. Pollut. Res. 2015;22:7258–7270. doi: 10.1007/s11356-014-3705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán C., Jofre J., Montemayor M., Lucena F. Occurrence and levels of indicators and selected pathogens in different sludges and biosolids. J. Appl. Microbiol. 2007;103:2420–2429. doi: 10.1111/j.1365-2672.2007.03487.x. [DOI] [PubMed] [Google Scholar]
- Guzman Prieto A.M., van Schaik W., Rogers M.R.C., Coque T.M., Baquero F., Corander J., Willems R.J.L. Global emergence and dissemination of enterococci as nosocomial pathogens: attack of the clones? Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K.A., Weir M.H., Haas C.N. Dose response models and a quantitative microbial risk assessment framework for the Mycobacterium avium complex that account for recent developments in molecular biology, taxonomy, and epidemiology. Water Res. 2017;109:310–326. doi: 10.1016/j.watres.2016.11.053. [DOI] [PubMed] [Google Scholar]
- Holt K.E., Wertheim H., Zadoks R.N., Baker S., Whitehouse C.A., Dance D., Jenney A., Connor T.R., Hsu L.Y., Severin J., Brisse S., Cao H., Wilksch J., Gorrie C., Schultz M.B., Edwards D.J., Nguyen K.V., Nguyen T.V., Dao T.T., Mensink M., Minh V.L., Nhu N.T.K., Schultsz C., Kuntaman K., Newton P.N., Moore C.E., Strugnell R.A., Thomson N.R. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in “Klebsiella pneumoniae”, an urgent threat to public health. Proc. Natl. Acad. Sci. 2015;112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.M., Park J.K., Teeradej N., Lee Y.O., Cho Y.K., Park C.H. Pretreatment of sludge with microwaves for pathogen destruction and improved anaerobic digestion performance. Water Environ. Res. 2006;78:76–83. doi: 10.2175/106143005x84549. [DOI] [PubMed] [Google Scholar]
- Iranpour R., Cox H.H.J. Evaluation of thermophilic anaerobic digestion processes for full-scale class A biosolids disinfection at hyperion treatment plant. Biotechnol. Bioeng. 2007;97:19–39. doi: 10.1002/bit.21176. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Yamashiro T., Beneragama N., Nishida T., Kida K., Ihara I., Takahashi J.-i., Umetsu K. The effect of temperature on survival of pathogenic bacteria in biogas plants. Anim. Sci. J. 2011;82:707–712. doi: 10.1111/j.1740-0929.2011.00887.x. [DOI] [PubMed] [Google Scholar]
- Jang H.M., Choi S., Shin J., Kan E., Kim Y.M. Additional reduction of antibiotic resistance genes and human bacterial pathogens via thermophilic aerobic digestion of anaerobically digested sludge. Bioresour. Technol. 2019;273:259–268. doi: 10.1016/j.biortech.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Ju F., Li B., Ma L., Wang Y., Huang D., Zhang T. Antibiotic resistance genes and human bacterial pathogens: co-occurrence, removal, and enrichment in municipal sewage sludge digesters. Water Res. 2016;91:1–10. doi: 10.1016/j.watres.2015.11.071. [DOI] [PubMed] [Google Scholar]
- Kang H.N., Park H.K., Lee H.J., Moon J.H., Oh J.W., Kim C.R. Rotavirus infection as a frequent cause of neonatal fever. Pediatr. Int. 2018;60:366–371. doi: 10.1111/ped.13504. [DOI] [PubMed] [Google Scholar]
- Kang Y., Li Q., Xia D., Shen M., Mei L., Hu J. Short-term thermophilic treatment cannot remove tetracycline resistance genes in pig manures but exhibits controlling effects on their accumulation and spread in soil. J. Hazard. Mater. 2017;340:213–220. doi: 10.1016/j.jhazmat.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Kashan D., Muthu N., Chaucer B., Davalos F., Bernstein M., Chendrasekhar A. Uterine perforation with intra-abdominal Clostridium perfringens gas gangrene: a rare and fatal infection. J. Gynecol. Surg. 2015;32:182–184. doi: 10.1089/gyn.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney T.E., Larkin M.J., Levett P.N. The effect of slurry storage and anaerobic digestion on survival of pathogenic bacteria. J. Appl. Bacteriol. 1993;74:86–93. doi: 10.1111/j.1365-2672.1993.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Kearney T.E., Larkin M.J., Frost J.P., Levett P.N. Survival of pathogenic bacteria during mesophilic anaerobic digestion of animal waste. J. Appl. Bacteriol. 1993;75:215–219. doi: 10.1111/j.1365-2672.1993.tb02768.x. [DOI] [PubMed] [Google Scholar]
- Kumar R., Gupta M.K., Kanwar S.S. Fate of bacterial pathogens in cattle dung slurry subjected to anaerobic digestion. World J. Microbiol. Biotechnol. 1999;15:335–338. [Google Scholar]
- Lahiri A., Kneisel J., Kloster I., Kamal E., Lewin A. Abundance of Mycobacterium avium ssp. hominissuis in soil and dust in Germany – implications for the infection route. Lett. Appl. Microbiol. 2014;59:65–70. doi: 10.1111/lam.12243. [DOI] [PubMed] [Google Scholar]
- Leite W.R.M., Gottardo M., Pavan P., Belli Filho P., Bolzonella D. Performance and energy aspects of single and two phase thermophilic anaerobic digestion of waste activated sludge. Renew. Energy. 2016;86:1324–1331. [Google Scholar]
- Li B., Ju F., Cai L., Zhang T. Profile and fate of bacterial pathogens in sewage treatment plants revealed by high-throughput metagenomic approach. Environ. Sci. Technol. 2015;49:10492–10502. doi: 10.1021/acs.est.5b02345. [DOI] [PubMed] [Google Scholar]
- Lloret E., Pastor L., Pradas P., Pascual J.A. Semi full-scale thermophilic anaerobic digestion (TAnD) for advanced treatment of sewage sludge: stabilization process and pathogen reduction. Chem. Eng. J. 2013;232:42–50. [Google Scholar]
- Lu X., Zhang X.X., Wang Z., Huang K., Wang Y., Liang W., Tang J. Bacterial pathogens and community composition in advanced sewage treatment systems revealed by metagenomics analysis based on high-throughput sequencing. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Li B., Li L.-G., Zhang T., Angelidaki I. Antibiotic resistance genes and correlations with microbial community and metal resistance genes in full-scale biogas reactors as revealed by metagenomic analysis. Environ. Sci. Technol. 2017;51:4069–4080. doi: 10.1021/acs.est.6b05100. [DOI] [PubMed] [Google Scholar]
- Magri M.E., Fidjeland J., Jönsson H., Albihn A., Vinnerås B. Inactivation of adenovirus, reovirus and bacteriophages in fecal sludge by pH and ammonia. Sci. Total Environ. 2015;520:213–221. doi: 10.1016/j.scitotenv.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Manser N.D., Wald I., Ergas S.J., Izurieta R., Mihelcic J.R. Assessing the fate of Ascaris suum ova during mesophilic anaerobic digestion. Environ. Sci. Technol. 2015;49:3128–3135. doi: 10.1021/es505807a. [DOI] [PubMed] [Google Scholar]
- Marrollo R. The Diverse Faces of Bacillus cereus. Academic Press; 2016. Chapter 5 - Bacillus cereus food-borne disease A2 - Savini, Vincenzo; pp. 61–72. [Google Scholar]
- Meer, R.R., Songer J.G., Park D.L., 1997. Human Disease Associated with Clostridium perfringens Enterotoxin. In: Ware, G.W., editor. Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews. Springer New York, New York, pp. 75–94. [DOI] [PubMed]
- Miller J.H., Novak J.T., Knocke W.R., Young K., Hong Y., Vikesland P.J., Pruden A. Effect of silver nanoparticles and antibiotics on antibiotic resistance genes in anaerobic digestion. Water Environ. Res. 2013;85:411–421. doi: 10.2175/106143012x13373575831394. [DOI] [PubMed] [Google Scholar]
- Moesker, F.M., van Kampen J.J.A., van der Eijk A.A., van Rossum A.M.C., de Hoog M., Schutten M., Smits S.L., Bodewes R., Osterhaus A.D.M.E., Fraaij P.L.A., 2015. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin. Microbiol. Infect. 21, 964.e961-964.e968. [DOI] [PMC free article] [PubMed]
- Narula R.J., Grimberg S., Rogers S., Mondal S. Pathogen reduction and factors responsible for pathogen reduction in dairy farm operations treating dairy manure. Biological Engineering Transactions. 2011;4:115. [Google Scholar]
- Neto R.C., Santos J.U., Franco R.M.B. Evaluation of activated sludge treatment and the efficiency of the disinfection of Giardia species cysts and Cryptosporidium oocysts by UV at a sludge treatment plant in Campinas, south-east Brazil. Water Sci. Technol. 2006;54:89–94. doi: 10.2166/wst.2006.453. [DOI] [PubMed] [Google Scholar]
- Neumann P., Barriga F., Alvarez C., Gonzalez Z., Vidal G. Process performance assessment of advanced anaerobic digestion of sewage sludge including sequential ultrasound–thermal (55° C) pre-treatment. Bioresour. Technol. 2018;262:42–51. doi: 10.1016/j.biortech.2018.03.057. [DOI] [PubMed] [Google Scholar]
- Newcomer B.W., Cofield L.G., Walz P.H., Givens M.D. Prevention of abortion in cattle following vaccination against bovine herpesvirus 1: a meta-analysis. Prev. Vet. Med. 2017;138:1–8. doi: 10.1016/j.prevetmed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Nilmini B., Yusuke M., Takaki Y., Masahiro I., Suraju A.L., Chun Y., Kazutaka U. The survival of cefazolin-resistant bacteria in mesophilic co-digestion of dairy manure and waste milk. Waste Manage. Res. 2013;31:843–848. doi: 10.1177/0734242X13477717. [DOI] [PubMed] [Google Scholar]
- Orzi V., Scaglia B., Lonati S., Riva C., Boccasile G., Alborali G.L., Adani F. The role of biological processes in reducing both odor impact and pathogen content during mesophilic anaerobic digestion. Sci. Total Environ. 2015;526:116–126. doi: 10.1016/j.scitotenv.2015.04.038. [DOI] [PubMed] [Google Scholar]
- Pandey P.K., Soupir M.L. Escherichia coli inactivation kinetics in anaerobic digestion of dairy manure under moderate, mesophilic and thermophilic temperatures. AMB Express. 2011;1:18. doi: 10.1186/2191-0855-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B.M., Barrios J.A., Jiménez B.E., Nelson K.L. The effects of temperature, pH, and ammonia concentration on the inactivation of Ascaris eggs in sewage sludge. Water Res. 2007;41:2893–2902. doi: 10.1016/j.watres.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Petersen R., Lomholt H.B., Scholz C.F.P., Brüggemann H. Draft Genome Sequences of Two Propionibacterium acnes Strains Isolated from Progressive Macular Hypomelanosis Lesions of Human Skin. Genome Announcements. 2015:3. doi: 10.1128/genomeA.01250-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponterio E., Gnessi L. Adenovirus 36 and obesity: an overview. Viruses. 2015;7:2787. doi: 10.3390/v7072787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R., Massé D.I., Singh G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013;143:632–641. doi: 10.1016/j.biortech.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Raynal J.T., Bastos B.L., Vilas-Boas P.C.B., Sousa T.d.J., Costa-Silva M., de Sá M.d.C.A., Portela R.W., Moura-Costa L.F., Azevedo V., Meyer R. Identification of membrane-associated proteins with pathogenic potential expressed by Corynebacterium pseudotuberculosis grown in animal serum. BMC Res. Notes. 2018;11:73. doi: 10.1186/s13104-018-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlström L. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour. Technol. 2003;87:161–166. doi: 10.1016/s0960-8524(02)00168-2. [DOI] [PubMed] [Google Scholar]
- Sahlström L., Bagge E., Emmoth E., Holmqvist A., Danielsson-Tham M.-L., Albihn A. A laboratory study of survival of selected microorganisms after heat treatment of biowaste used in biogas plants. Bioresour. Technol. 2008;99:7859–7865. doi: 10.1016/j.biortech.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Scaglia B., D'Imporzano G., Garuti G., Negri M., Adani F. Sanitation ability of anaerobic digestion performed at different temperature on sewage sludge. Sci. Total Environ. 2014;466-467:888–897. doi: 10.1016/j.scitotenv.2013.07.114. [DOI] [PubMed] [Google Scholar]
- Sehu, M.M., Heney C., Chandra S., Bergh H., Nimmo G. “Streptococcus salivarius” meningitis post spinal procedure: Diagnosis by 16S and a call to better aseptic practices. Int. J. Infect. Dis. 21, 405.
- Shamma M., Al-Adawi M.A. The morphological changes of Ascaris lumbricoides ova in sewage sludge water treated by gamma irradiation. Radiat. Phys. Chem. 2002;65:277–279. [Google Scholar]
- Shannon K.E., Lee D.Y., Trevors J.T., Beaudette L.A. Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Sci. Total Environ. 2007;382:121–129. doi: 10.1016/j.scitotenv.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Shen L., Chen C.Y., Huang D., Wang R., Zhang M., Qian L., Zhu Y., Zhang A.Z., Yang E., Qaqish A., Chumakov K., Kouiavskaia D., Vignuzzi M., Nathanson N., Macadam A.J., Andino R., Kew O., Xu J., Chen Z.W. Pathogenic events in a nonhuman primate model of oral poliovirus infection leading to paralytic poliomyelitis. J. Virol. 2017;91 doi: 10.1128/JVI.02310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Linville J.L., Urgun-Demirtas M., Mintz M.M., Snyder S.W. An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towards energy-neutral WWTPs. Renew. Sust. Energ. Rev. 2015;50:346–362. [Google Scholar]
- Siddiqui N., Toumeh A., Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J. Clin. Microbiol. 2012;50:3119–3121. doi: 10.1128/JCM.00563-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G.D., Patel G.B. Ammonia toxicity in pure cultures of methanogenic bacteria. Syst. Appl. Microbiol. 1986;7:358–363. [Google Scholar]
- Stiborova H., Wolfram J., Demnerova K., Macek T., Uhlik O. Bacterial community structure in treated sewage sludge with mesophilic and thermophilic anaerobic digestion. Folia Microbiol. 2015;60:531–539. doi: 10.1007/s12223-015-0396-9. [DOI] [PubMed] [Google Scholar]
- Su J.-Q., Wei B., Ou-Yang W.-Y., Huang F.-Y., Zhao Y., Xu H.-J., Zhu Y.-G. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ. Sci. Technol. 2015;49:7356–7363. doi: 10.1021/acs.est.5b01012. [DOI] [PubMed] [Google Scholar]
- Sun W., Qian X., Gu J., Wang X.J., Duan M.L. Mechanism and effect of temperature on variations in antibiotic resistance genes during anaerobic digestion of dairy manure. Sci. Rep. 2016;6:30237. doi: 10.1038/srep30237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Qian X., Gu J., Wang X.J., Zhang L., Guo A.Y. Mechanisms and effects of arsanilic acid on antibiotic resistance genes and microbial communities during pig manure digestion. Bioresour. Technol. 2017;234:217–223. doi: 10.1016/j.biortech.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Teunis P., Figueras M.J. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front. Microbiol. 2016;7:1395. doi: 10.3389/fmicb.2016.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh S.A., Leyn S.A., Rodionov I.D., Godzik A., Satchell K.J.F. Draft Genome Sequences of Two Vibrio parahaemolyticus Strains Associated with Gastroenteritis after Raw Seafood Ingestion in Colorado. Genome Announcements. 2018:6. doi: 10.1128/genomeA.01387-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeramuthu G.J., Price J.F., Davis C.E., Booren A.M., Smith D.M. Thermal inactivation of Escherichia coli O157:H7, Salmonella senftenberg, and enzymes with potential as time-temperature indicators in ground Turkey thigh meat. J. Food Prot. 1998;61:171–175. doi: 10.4315/0362-028x-61.2.171. [DOI] [PubMed] [Google Scholar]
- Viau E., Peccia J. Survey of wastewater indicators and human pathogen genomes in biosolids produced by class A and class B stabilization treatments. Appl. Environ. Microbiol. 2009;75:164–174. doi: 10.1128/AEM.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt S.L., Peña-Díaz J., Finlay B.B. Chemical communication in the gut: effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe. 2015;34:106–115. doi: 10.1016/j.anaerobe.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Wagner A.O., Gstraunthaler G., Illmer P. Survival of bacterial pathogens during the thermophilic anaerobic digestion of biowaste: laboratory experiments and in situ validation. Anaerobe. 2008;14:181–183. doi: 10.1016/j.anaerobe.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wahidunnabi A.K., Eskicioglu C. High pressure homogenization and two-phased anaerobic digestion for enhanced biogas conversion from municipal waste sludge. Water Res. 2014;66:430–446. doi: 10.1016/j.watres.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Wan J., Gu J., Zhao Q., Liu Y. COD capture: a feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016;6 doi: 10.1038/srep25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J., Jing Y., Rao Y., Zhang S., Luo G. Thermophilic alkaline fermentation followed by mesophilic anaerobic digestion for efficient hydrogen and methane production from waste activated sludge: focusing on dynamics of bacterial pathogens as revealed by the combination of metagenomic and qPCR analysis. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02632-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-H., Wang X., Huppes G., Heijungs R., Ren N.-Q. Environmental implications of increasingly stringent sewage discharge standards in municipal wastewater treatment plants: case study of a cool area of China. J. Clean. Prod. 2015;94:278–283. [Google Scholar]
- Wéry N., Lhoutellier C., Ducray F., Delgenès J.-P., Godon J.-J. Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res. 2008;42:53–62. doi: 10.1016/j.watres.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Wittchen M., Busche T., Gaspar A.H., Lee J.H., Ton-That H., Kalinowski J., Tauch A. Transcriptome sequencing of the human pathogen Corynebacterium diphtheriae NCTC 13129 provides detailed insights into its transcriptional landscape and into DtxR-mediated transcriptional regulation. BMC Genomics. 2018;19:82. doi: 10.1186/s12864-018-4481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D., Aoki F., Rubinstein E. Bacteremia Caused by Eggerthella lenta in an Elderly Man with a Gastrointestinal Malignancy: A Case Report. Canadian Journal of Infectious Diseases and Medical Microbiology. 2014:25. doi: 10.1155/2014/802481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Yin P., Zhang Y., Zhao X., Sun L., Yuan H., Lu J., Hu S. Occurrence, genotyping, and health risk of Cryptosporidium and Giardia in recreational lakes in Tianjin. China. Water Res. 2018;141:46–56. doi: 10.1016/j.watres.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Xu C., Salsali H., Weese S., Warriner K. Inactivation of Clostridium difficile in sewage sludge by anaerobic thermophilic digestion. Can. J. Microbiol. 2015;62:16–23. doi: 10.1139/cjm-2015-0511. [DOI] [PubMed] [Google Scholar]
- Yenigün O., Demirel B. Ammonia inhibition in anaerobic digestion: a review. Process Biochem. 2013;48:901–911. [Google Scholar]
- Yu Z., He P., Shao L., Zhang H., Lü F. Co-occurrence of mobile genetic elements and antibiotic resistance genes in municipal solid waste landfill leachates: a preliminary insight into the role of landfill age. Water Res. 2016;106:583–592. doi: 10.1016/j.watres.2016.10.042. [DOI] [PubMed] [Google Scholar]