Abstract
The severe acute respiratory syndrome (SARS) epidemic started in November 2002 and spread worldwide. The pathological changes in several human organs of patients with SARS have been extensively described. However, to date, little has been reported about the effects of this infection on the thyroid gland. Femoral head necrosis and low serum triiodothyronine and thyroxine levels, commonly found in patients with SARS, raise the possibility of thyroid dysfunction. We have undertaken this study to evaluate for any potential injury to the thyroid gland caused by SARS on tissue samples obtained from 5 SARS autopsies. The terminal deoxynucleotidyl transferase-mediated dUPT nick end–labeling assay was performed to identify apoptotic cells. The follicular epithelium was found to be damaged with large numbers of cells exfoliated into the follicle. The terminal deoxynucleotidyl transferase-mediated dUPT nick end–labeling assay demonstrated many cells undergoing apoptosis. Follicular architecture was altered and showed distortion, dilatation, and collapse. No distinct calcitonin-positive cells were detectable in the SARS thyroids. In conclusion, both parafollicular and follicular cells were injured. This may provide an explanation both for low serum triiodothyronine and thyroxine levels and the osteonecrosis of the femoral head associated with patients with SARS. Apoptosis may play a role in the pathogenesis of SARS associated coronavirus infection in the thyroid gland.
Keywords: SARS, Thyroid, Calcitonin, TUNEL
1. Introduction
In November 2002, a new infectious disease now known as severe acute respiratory syndrome (SARS) broke out in Guangdong Province, China, followed by rapid spread of this disease to other parts of China, other Asian countries, and the rest of the world. When the epidemic was over in late 2003, more than 8000 people had been infected, and close to 800 had died. In the summer of 2004, there was another, small-scale outbreak of SARS in Asian countries, but this time, it was quickly brought under control. However, the threat of yet another outbreak of SARS and other new infectious viral diseases continues to exist, and the need to understand all aspects of such diseases is urgent. Following the first outbreak, the tissue pathology of several organs was described extensively [1], [2], [3], [4], [5] and has thus contributed to the understanding of the probable pathogenesis of SARS. However, to date, the effects of SARS on the thyroid gland are unknown because no detailed histopathological investigation of the thyroid gland in patients with SARS has been reported. As SARS is a disease known to cause multiple organ injury [2], [3], [4], the possibility of thyroid gland injury must be taken into consideration. The thyroid gland is an organ whose neuroendocrine functions affect the entire body including the immune system [6], [7], [8]. The hormones thyroxine (T4) and triiodothyronine (T3) produced in the follicular cells of the thyroid gland are responsible for regulating normal metabolic and neural activity. Calcitonin produced by the parafollicular cells of the thyroid gland plays an important role in modulating calcium homeostasis.
A substantial number of patients with SARS have shown abnormalities in either of these 2 functions, which lends support to the supposition that SARS has a harmful effect on the thyroid gland. In some cases, decreased T3 and T4 serum levels were detected suggesting follicular cell dysfunction [9], [10]. In other cases, osteonecrosis of the femoral head was found in recovered patients with SARS, which may have been caused by a decrease of inhibition of osteoclasts resulting from calcitonin deficiency [11], [12]. Therefore, in addition to steroid therapy, calcitonin deficiency caused by injury to the parafollicular cells may also have contributed to the pathogenesis of this common complication of SARS.
In a previous publication [4], [13], we reported the effects of SARS on several organs and focused particularly on SARS in relation to the respiratory tract and the immune system. To further investigate the effects of SARS associated coronavirus (CoV) on the thyroid, we have undertaken a detailed study of the thyroid gland with special attention to the pattern of cellular and architectural alterations on parafollicular and follicular cells. Because overexpression of certain nonstructural proteins of the SARS-CoV has shown the ability to induce apoptosis in several cell types in vitro, we made a working assumption that apoptosis among other factors might play a role in the pathogenesis of SARS [14], [15], [16], [17]. Therefore, we also used the terminal deoxynucleotidyl transferase-mediated dUPT nick end–labeling (TUNEL) assay in this study.
2. Materials and methods
The use of the human specimens and related ethical issues were approved by the Research Administration Committee of Peking University and the related hospitals.
2.1. Materials
Five thyroid samples were obtained from autopsies on 4 men and a woman with SARS. Clinical data are presented in Table 1 . The patients' ages ranged from 24 to 50 years. From 7 men and 3 women of comparable ages, 10 normal thyroid samples were used as controls.
Table 1.
Clinical data of the SARS cases
| Case no. | Age/Sex | Course (d) | Clinical symptoms | Chest radiograph | Lymphopenia | SARS pathogen (real-time PCR) | History | Steroid treatment | Diagnosis | Postmortem interval (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5814 | 51/male | 45 | Fever and cough | Infiltrates in the right lower portion of the lung | Yes | Yes | Healthy | + | SARS | 8 |
| 5816 | 50/male | 33 | Fever, cough and shortness of breath | Bilateral ground glass changes | Yes | Yes | Coronary heart disease, primary hypertension | − | SARS | 12 |
| 5818 | 31/male | 35 | Fever and nonproductive cough | Ground glass changes in the periphery of the left lower lobe and the right middle lobe | Yes | Yes | Non-Hodgkin lymphoma | + | SARS | 23 |
| 5819 | 49/female | 32 | Fever and shortness of breath | Interstitial infiltrates | Yes | Yes | No notable medical history | + | SARS | 20 |
| 5821 | 24/male | 21 | Fever and cough | Bilateral patchy exudation in the lower lobes | Yes | Yes | Psychosis | + | SARS | 1 |
Abbreviation: PCR, polymerase chain reaction.
2.2. Methods
The thyroid samples from patients with SARS and normal controls were processed in an identical manner. They were fixed in 4% paraformaldehyde, dehydrated, and then embedded in paraffin. Sections (5 μm thick) were cut and stained with hematoxylin-eosin, and the TUNEL assay and immunohistochemistry studies were performed.
2.3. TUNEL assay
This assay was performed using a TdT-FragEL DNA fragmentation detection kit (CalBiochem, San Diego, CA) to detect apoptosis. In brief, after deparaffinizing and rehydration, thyroid sections were first covered with 1 × TdT balance buffer and were incubated at room temperature for 15 to 30 minutes. Balance buffer was removed by blotting, followed by immediate addition of TdT-labeling reaction mixture, covering of the tissue with parafilm, and incubation at 37°C for a 1-hour period. Tris buffered saline (TBS) was substituted for TdT enzyme for negative control slides. Sections were rinsed with TBS buffer in between incubations at room temperature, covered with stop solution for 5 minutes, with 3% H2O2 for 5 minutes, and with blocking buffer for 10 minutes. Finally, horseradish peroxidase conjugate was added and incubated at room temperature for 30 minutes. After another rinse with TBS, sections were developed with 3,3′-diaminobenzidine (DAB) solution for colorization at room temperature for 10 to 15 minutes. The sections were then dehydrated in 100% ethanol, cleared with xylene, and coverslipped.
2.4. Immunohistochemistry
Sections were stained with antibodies to calcitonin (calcitonin Ab-2; NeoMarkers, Inc, Fremont, CA). In brief, sections of thyroid were deparaffinized, rehydrated, treated with 3% H2O2 for 30 minutes at 37°C, and then subjected to antigen retrieval using the microwave method with sodium citrate buffer at pH 6. Sections were then blocked with 1:20 normal goat serum, incubated at 37°C for 30 minutes, and covered with anticalcitonin polyclonal primary antibodies (diluted 1:200) for overnight incubation at 4°C. Biotinalated goat antirabbit secondary antibody (Zymed, South San Francisco, CA) diluted 1:100 was applied for 30 minutes at 37°C, and strepavidin-peroxidase complex (Zymed) diluted 1:100 was next applied for another 30 minutes at 37°C. Finally, sections were rinsed with phosphate buffered saline (PBS) incubated with DAB solution for color reaction, and dehydrated with increasing concentrations of alcohol, cleared with xylene, and then coverslipped.
To validate the specificity of the immunoreaction, PBS was substituted for calcitonin antibody on additional tissue sections as a negative control.
3. Results
3.1. Histomorphology
In contrast to normal thyroid, the thyroid glands from patients with SARS consistently showed destruction of the follicular epithelium and exfoliation of epithelial cells into the follicle. The remaining numbers of epithelial cells on the follicular scaffold were sparse or were absent. In some areas, the follicular epithelial cells formed clusters without colloid. Some follicles were dilated, others were collapsed and distorted with an irregular outline, and still others showed a microfollicle configuration. Parafollicular cells could not be identified morphologically on routine hematoxylin-eosin sections. In one SARS case, the capillaries in the connective tissues between follicles were markedly congested, and the follicular epithelium showed severe damage (Fig. 1 ). Neither neutrophilic nor lymphoid infiltration was present in the interfollicular connective tissue. In the thyroid gland of the female patient with SARS, in addition to the changes described above, fibrosis was present in the interfollicular connective tissue (Fig. 2 ).
Fig. 1.
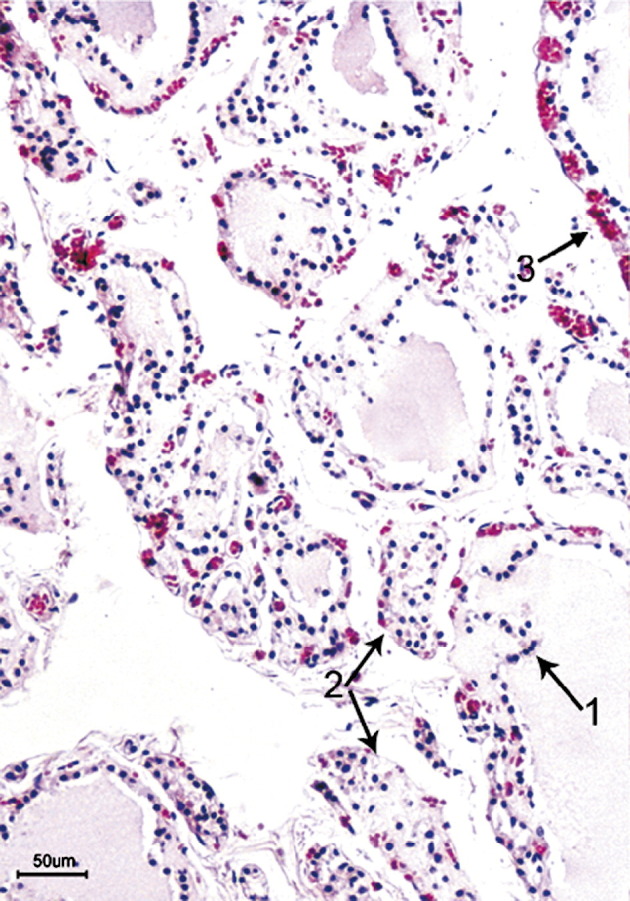
Thyroid of a patient with SARS showing disruption of the lining epithelium with extensive exfoliation into the luminal cavity of a dilated follicle (arrow 1), formation of epithelial cell clusters with no lumen or colloid (arrow 2), and with markedly congested capillaries in the connective tissues between follicles (arrow 3) (hematoxylin-eosin stain; bar, 50 μm).
Fig. 2.
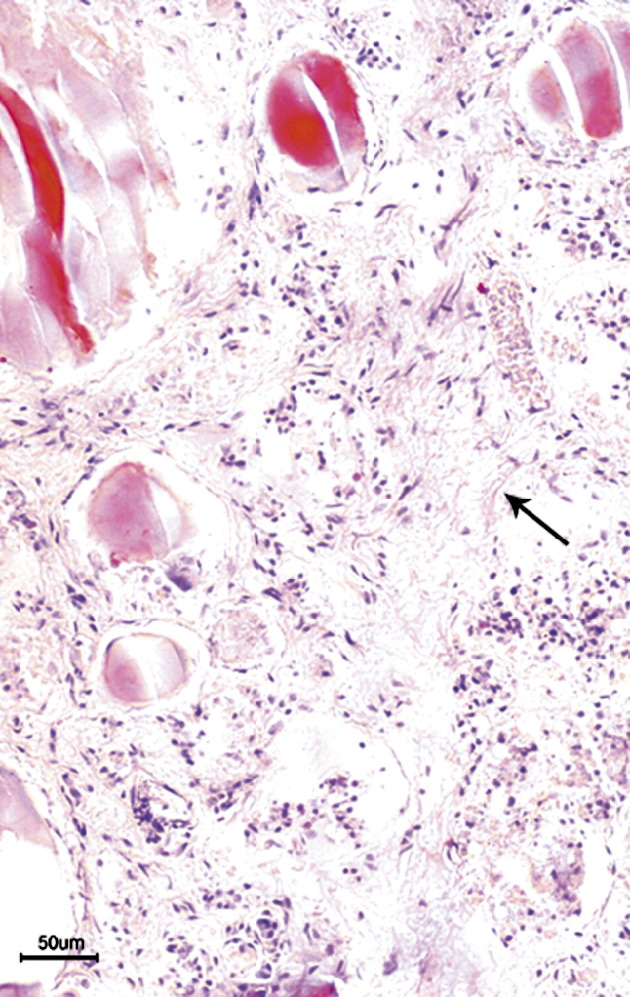
Loss of follicles with increased fibrous tissue (arrow) in interfollicular region in a patient with SARS (hematoxylin-eosin stain; bar, 50 μm).
There was no definite correlation of the above features with the duration of disease before death or with the postmortem interval.
3.2. Apoptosis with TUNEL assay
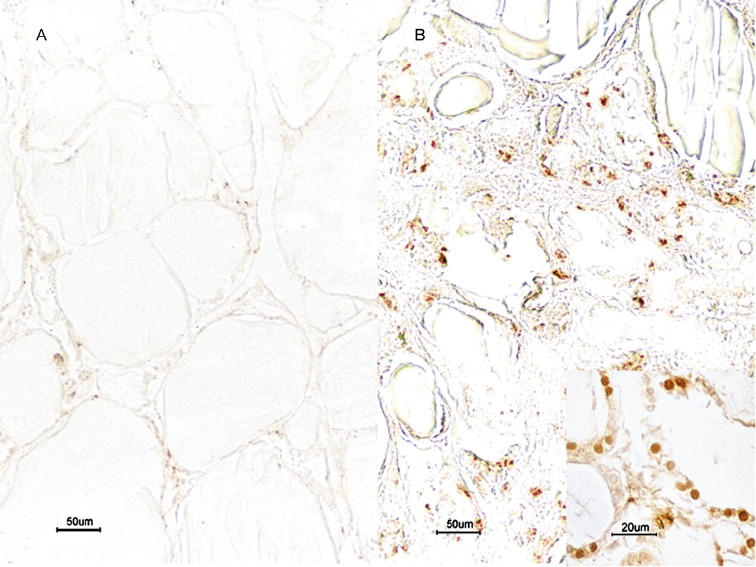
There were few apoptotic cells present in the thyroid tissue sections of the normal controls (Fig. 3A). However, apoptosis was observed in both the follicular epithelium and the interfollicular region of all patients with SARS (Fig. 3B). The cell type undergoing apoptosis in the interfollicular stroma could not be definitively ascertained because of loss of normal cellular morphology.
Fig. 3.
TUNEL stain of normal (A) and SARS (B) thyroid samples. (A) Few TUNEL-positive cells in a normal thyroid (bar, 50 μm). (B) Distinct apoptosis in the follicular epithelium and the interfollicular region in a patient with SARS (insert figure, apoptosis in follicular epithelium; bar, 20 μm) (bar, 50 μm).
3.3. Calcitonin expression
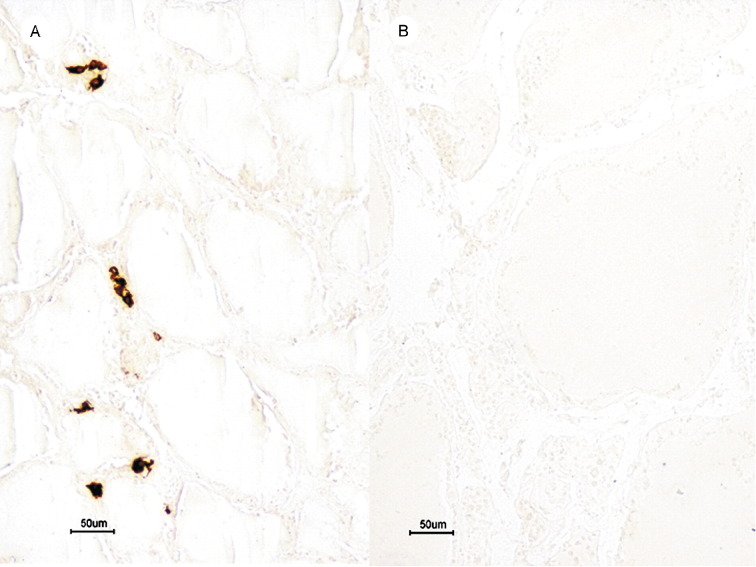
Calcitonin-positive cells were present in the normal control thyroid tissue and were located predominantly among follicles and between follicular epithelial cells. Positive immunocytochemical signals were strong and distinct (Fig. 4A). These cells displayed the characteristic morphologic features of parafollicular cells, including large cell size, an oval to polygonal shape with pale cytoplasm, and oval nuclei. In the thyroid glands of patients with SARS, however, calcitonin-positive cells were entirely absent (Fig. 4B). The specificity of the immunostaining was validated by the control results.
Fig. 4.
Calcitonin immunostaining. (A) Calcitonin-positive cells present predominantly in interfollicular region of a normal thyroid (bar, 50 μm). (B) Absence of calcitonin-positive cells in the thyroid of a patient with SARS (bar, 50 μm).
4. Discussion
Our study has demonstrated that thyroid glands in patients with SARS were significantly affected by the disease with extensive injury to the follicular epithelial cells and the parafollicular cells.
The damage to follicular cells was characterized by destruction of the follicular epithelium and desquamation of the epithelial cells into the follicular lumen. The presence of apoptosis was confirmed by the TUNEL assay. A previous study reported that serum levels of T3 and T4 in patients with SARS were significantly lower than those in the control group, and the more severe the disease, the lower the level of T3 in these patients. Serum T3 and T4 levels were decreased, respectively, in 94% and 46% of 48 patients with SARS during the acute phase and in 90% and 38% during the convalescent phase of the disease [9]. Serum thyroid stimulating hormone (TSH) concentration in patients with SARS was also significantly decreased compared with that of the control group [9]. The extent of morphological injury and the large quantity of cells undergoing apoptosis that we observed in the thyroid follicular epithelium provide an explanation for the diminished serum T3 and T4 levels in patients with SARS. In contrast, the reduced TSH level reported in patients with SARS cannot be readily explained by the destruction of follicular epithelium, as under normal circumstances, low serum levels of T3 and T4 would lead to an increased TSH level provided that the hypothalamus-pituitary function is intact. Therefore, the hypothalamus-pituitary dysfunction may be the underlying cause of low serum TSH. Leow et al [10] reported that 4 of 61 SARS survivors (6.7%) were hypothyroid including 3 cases with central hypothyroidism and 1 with primary hypothyroidism with positive autoimmune antibodies. In addition to hypothyroidism, they reported a more frequent occurrence of central hypocortisolism in a substantial number of patients with SARS. Hypocortisolism and hypothyroidism together may explain the frequent occurrence of the wide range of nonspecific symptoms found in recovered patients with SARS, which has been referred to as post-SARS sickness syndrome [10], [18]. Leow et al [10] postulated that SARS caused central hypocortisolism and hypothyroidism by inducing hypophysitis or by directly affecting the hypothalamus. Such a potential effect on the hypothalamus is consistent with the presence of edema and neuronal degeneration together with the identification of viral genome sequences in the hypothalamus and cortex of the brain of patients with SARS, as described in a previous report [4]. Some authors have suggested another explanation for hypothalamic-pituitary-adrenal (HPA) axis dysfunction, in which the temporary dysfunction of the HPA axis in recovered patients with SARS was ascribed to an adaptive physiological response to prolonged activation of the HPA axis during the acute phase of the disease [18]. With respect to hypothalamic-pituitary-thyroid axis deficiency, our results seem to indicate that in addition to such hypothalamic-pituitary dysfunction as is described above, primary injury to the thyroid gland itself may have a key role in the pathogenesis of hypothyroidism in patients with SARS.
In the present study, strong granular cytoplasmic staining was identified in calcitonin-positive cells of the normal thyroid glands of the control group, and these cells showed morphologic features consistent with normal parafollicular cells. In sharp contrast, there were no calcitonin-positive cells detected in the thyroids of any of the patients with SARS. This strongly suggests that in addition to injury to the follicular cells, SARS infection also damages the structure and function of cells in the interfollicular tissue including parafollicular cells, to the extent that calcitonin production may be adversely affected. This is also supported by the results obtained from the TUNEL assay, which demonstrated extensive apoptosis in both follicular cells and cells in the interfollicular regions, including parafollicular cells in patients with SARS but not in the control subjects.
The specificity of the TUNEL assay is sometimes questioned because both apoptosis and necrosis cause DNA degradation, which in case of necrosis may lead to a false-positive assay result. However, cellular necrosis in vivo is usually associated with an intense inflammatory response. Furthermore, necrotic cells are morphologically characterized by cytoplasmic swelling and total cell lysis [19], [20]. Because in this study microscopic examination detected neither inflammatory infiltrates nor morphological cellular features of necrosis, the observed changes in these SARS thyroid glands appear to be consistent with apoptosis.
Osteonecrosis of the femoral head is frequently observed in recovered patients with SARS [11], [12]. As most patients receive high-dose glucocorticoids during the course of their illness, this complication has been thought to be related to glucocorticoid administration. However, it is possible that there is an alternative explanation for osteonecrosis related to loss of calcitonin-producing cells in SARS thyroid glands. The mechanism by which glucocorticoids induce osteoporosis through decrease of ossification by osteoblast inhibition and by stimulating the action of osteoclasts, which in turn accelerate bone resorption, is well known [21]. This may in turn result in fracture and collapse of trabeculae of spongy bone, leading to compression of capillaries and ischemia, resulting in ischemic necrosis of the femoral head.
However, the observation of loss of calcitonin-producing cells in SARS thyroid glands in this study may furnish an alternative or additional explanation for bone necrosis in this disease, that is, through decrease of inhibition of bone resorption due to loss of calcitonin release. Calcitonin is known to inhibit osteolysis and increase calcium deposition in the bone [22], [23], [24], [25], and decreased production of calcitonin in the thyroid glands of patients with SARS may lead to increased osteolysis and necrosis of the bone.
No significant difference in serum PTH level was observed between the 48 patients with SARS and the control group, neither during the acute nor the convalescent phase of the illness [9]. There has been no report on the serum levels of calcitonin hormone in patients with SARS. However, it has been noted that the serum calcium level is decreased in patients with SARS [26], [27], [28]. It appears that with prolongation of steroid administration, the degree of hypocalcemia increases [27]. Under normal circumstances, reduction of calcitonin would lead to elevation of serum calcium. However, serum calcium is regulated by a number of other factors including parathyroid hormone and 1,25-dihydroxycholecalciferol (vitamin D3) [22], [29], as well as absorption by the intestines and secretion by the kidneys [22]. Most of the patients with SARS in this cohort (and most patients with SARS in general) received large dosages of glucocorticoids that would inhibit the production of 1,25-dihydroxycholecalciferol and lead to hypocalcemia. Because SARS-CoV infects multiple organs and can cause severe damage to the function of the kidneys and intestines [5], [30], it is likely that this may also contribute to the reduced serum calcium level in SARS.
The injury to the thyroid in patients with SARS is most likely caused by SARS-CoV itself rather than by glucocorticoids, as glucocorticoid damage to the thyroid and/or the parafollicular cells has, to our knowledge, never been reported. Moreover, glucocorticoids were not given to 1 of the 5 patients in this study, and that the patient's thyroid showed pathology identical to those of the other patients.
It is not clear how the SARS-CoV induces organ injury in general or injury to the thyroid gland in particular. Several mechanisms have been proposed to explain the severe organ damage caused by SARS-CoV infection. The most important of these mechanisms include host immune overreaction, immune deficiency related to infection and destruction of lymphocytes, inhibition of the innate immune response (in particular the type 1 interferon response), and direct cellular destruction [4], [31], [32], [33]. In addition, there is evidence that apoptosis plays a role in the pathogenesis of SARS infection [15]. In vitro experiments have shown that overexpression of certain nonstructural SARS-CoV proteins can induce apoptosis [14], [15], [16], [17] in several cell types. In a study of liver pathology, apoptosis was detected in the tissue specimens of 3 patients with SARS [34]. Our results also suggest that apoptosis may partially contribute to injury of the thyroid gland.
Previous studies have not detected any SARS viral genomic sequences in the thyroid gland itself, but such sequences were found in monocytes and lymphocytes in blood perfusing the thyroid [4], [35]. As such, further investigation is necessary to ascertain if injury to the thyroid is caused directly by the virus or indirectly via other mechanisms associated with the disease process.
Our findings on the thyroid and the previous observation of low serum T4 and T3 levels also have implications for the care of patients with SARS in the acute phase and of recovered patients with SARS. It appears that monitoring of thyroid hormones is necessary, with proper replacement therapy as indicated. In most cases, hypothyroidism was reversible, and thyroid hormones normalized within 3 to 9 months [10]. Further investigation will be needed to determine the relationship between osteonecrosis and calcitonin deficiency.
In conclusion, in this study, we found evidence of thyroid gland damage in patients with SARS, which may partially be the result of apoptosis. The injury to follicular cells may provide an explanation for the low serum T3 and T4 levels frequently found in patients with SARS. The damage to the parafollicular cells that was identified may result in calcitonin deficiency, which most likely contributes to the pathogenesis of femoral head necrosis, a common complication of SARS. However, the exact mechanism by which SARS causes injury to the thyroid gland remains unclear and warrants further investigation.
Footnotes
This study was supported by grants from Peking University and the Ministry of Science and Technology, People's Republic of China, entitled “Histopathology, tissue collection and the pathogenesis of SARS” (2003AA208105) and “Pathogenesis and clinical pathology of SARS” (2003AA208107). The support of the Lifu Foundation is also gratefully acknowledged.
References
- 1.Nicholls J.M., Poon Leo L.M., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang Z.W., Zhang L.J., Zhang S.J. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35:526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. JEM. 2005;202:417–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X., Gong E., Gao D. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol. 2005;100:169–176. doi: 10.1111/j.1572-0241.2005.40377.x. [DOI] [PubMed] [Google Scholar]
- 6.Furugaki K., Shirasawa S., Ishikawa N. Association of the T-cell regulatory gene CTLA4 with Graves' disease and autoimmune thyroid disease in the Japanese. J Hum Genet. 2004;49:166–168. doi: 10.1007/s10038-003-0120-5. [DOI] [PubMed] [Google Scholar]
- 7.Klecha A.J., Genaro A.M., Lysionek A.E., Caro R.A., Coluccia A.G., Cremaschi G.A. Experimental evidence pointing to the bi-directional interaction between the immune system and the thyroid axis. Int J Immunopharmacol. 2000;22:491–500. doi: 10.1016/s0192-0561(00)00012-6. [DOI] [PubMed] [Google Scholar]
- 8.Davis S.L. Environmental modulation of the immune system via the endocrine system. Domest Anim Endocrinol. 1998;15:283–289. doi: 10.1016/s0739-7240(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang W., Ye Y.X., Yao H. Evaluation and observation of serum thyroid hormone and parathyroid hormone in patients with severe acute respiratory syndrome. J Chin Antituberculous Assoc. 2003;25:232–234. [Google Scholar]
- 10.Leow M.K., Kwek D.S., Ng A.W., Ong K.C., Kaw G.J., Lee L.S. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong N., Du X.K. Avascular necrosis of bone in severe acute respiratory syndrome. Clin Radiol. 2004;59:602–608. doi: 10.1016/j.crad.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith J.F., Antonio G.E., Kumta S.M., Hui D.S., Wong J.K., Joynt G.M. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235:168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 13.Zhan J., Deng R., Tang J., Zhang B., Tang Y., Wang J.K. The spleen as a target in Severe Acute Respiratory Syndrome (SARS) FASEB J. 2006 doi: 10.1096/fj.06-6324com. [in press] [DOI] [PubMed] [Google Scholar]
- 14.Law P.T., Wong C.H., Au T.C. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J Gen Virol. 2005;86:1921–1930. doi: 10.1099/vir.0.80813-0. [DOI] [PubMed] [Google Scholar]
- 15.Tan Y.J., Fielding B.C., Goh P.Y. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78:14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X., Shan Y., Zhao Z., Chen J., Cong Y. G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells. J Virol. 2005;2:66. doi: 10.1186/1743-422X-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fielding B.C., Tan Y.J., Shuo S. Characterization of a unique group-specific protein (U122) of the severe acute respiratory syndrome coronavirus. J Virol. 2004;78:7311–7318. doi: 10.1128/JVI.78.14.7311-7318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrousos G.P., Kaltsas G. Post-SARS sickness syndrome manifestations and endocrinopathy: how, why, and so what? Clin Endocrinol (Oxf) 2005;63:363–365. doi: 10.1111/j.1365-2265.2005.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majno G., Joris I. Apoptosis, oncosis and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Wyllie A.H., Kerr J.F., Currie A.R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda S., Kogawa M., Wada S. Glucocorticoid-induced osteoporosis. Nippon Rinsho. 2003;61:280–286. [PubMed] [Google Scholar]
- 22.Guyton A.C., Hall J.E. Textbook of Medical Physiology. 9th ed. W. B Saunders Company; Philadelphia (Pa): 1996. Parathyroid hormone, calcitonin, calcium and phosphate metabolism, vitamin D, bone, and teeth; pp. 985–1002. [Google Scholar]
- 23.Foster G.V., Doyle F.H., Bordier P., Matrajt H. Effect of thyrocalcitonin on bone. Lancet. 1966;2:1428–1431. doi: 10.1016/s0140-6736(66)90593-9. [DOI] [PubMed] [Google Scholar]
- 24.Wase A.W., Peterson A., Rickes E., Solewski J. Some effects of thyrocalcitonin on the calcium metabolism of the rat. Endocrinology. 1966;79:687–691. doi: 10.1210/endo-79-4-687. [DOI] [PubMed] [Google Scholar]
- 25.Khan B.T., Ewald F.C., Tachdjian M.O. In vivo effect of thyrocalcitonin on bone solubility. Endocrinology. 1967;81:398–399. doi: 10.1210/endo-81-2-398. [DOI] [PubMed] [Google Scholar]
- 26.Booth C.M., Matuka L.M., Tomlinson G.A. Clinical features and short-term outcome of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 27.Li N., Wang G.F., Wu Y.F. Side effects of glucocorticosteroids in the management of 1291 patients of SARS. J oF Peking University (Health Sciences) 2004;36:519–524. [PubMed] [Google Scholar]
- 28.Song S.Z., Liu H.Y., Shen H. Comparison of serum biochemical features between SARS and other viral pneumonias. Chinese Critical Care Medicine. 2004;16:664–666. [PubMed] [Google Scholar]
- 29.Pechet M.M., Bobadilla E., Carroll E.L., Hesse R.H. Regulation of bone resorption and formation. Influences of thyrocalcitonin, parathyroid hormone, neutral phosphate and vitamin D3. Am J Med. 1967;43:696–710. doi: 10.1016/0002-9343(67)90112-x. [DOI] [PubMed] [Google Scholar]
- 30.Gong E., Gao Z., Zheng J. Pathological changes and pathogenesis of fatal severe acute respiratory syndrome. Chin J Infect Dis. 2003;21:390–395. [Google Scholar]
- 31.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau Y.L., Peiris J.S. Pathogenesis of severe acute respiratory syndrome. Curr Opin Immunol. 2005;17:404–410. doi: 10.1016/j.coi.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo A.W., Tang N.L., To K.F. How the SARS coronavirus causes disease: host or organism? J Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau T.N., Lee K.C., Yao H. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding Y., He L., Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]