Abstract
Coronaviruses (CoVs) are positive-stranded RNA viruses with potential as immunization vectors, expressing high levels of heterologous genes and eliciting both secretory and systemic immune responses. Nevertheless, its high recombination rate may result in the loss of the full-length foreign gene, limiting their use as vectors. Transmissible gastroenteritis virus (TGEV) was engineered to express porcine reproductive and respiratory syndrome virus (PRRSV) small protein domains, as a strategy to improve heterologous gene stability. After serial passage in tissue cultures, stable expression of small PRRSV protein antigenic domains was achieved. Therefore, size reduction of the heterologous genes inserted in CoV-derived vectors led to the stable expression of antigenic domains. Immunization of piglets with these TGEV vectors led to partial protection against a challenge with a virulent PRRSV strain, as immunized animals showed reduced clinical signs and lung damage. Further improvement of TGEV-derived vectors will require the engineering of vectors with decreased recombination rate.
Keywords: Positive-strand RNA viruses, RNA vector stability, Coronavirus derived vectors, TGEV, PRRSV
Highlights
-
•
Foreign gene stability in TGEV-derived vectors was achieved by decreasing insert size.
-
•
A set of PRRSV antigens was stably expressed by TGEV-derived vectors.
-
•
PRRSV M protein was used as a scaffold for PRRSV antigenic domains expression.
-
•
Piglet immunization with rTGEVs encoding PRRSV antigens led to partial protection.
Introduction
The order Nidovirales comprises enveloped single-stranded, positive-sense RNA viruses. The Nidovirales order includes the Coronaviridae family that contains viruses with the largest known RNA genome, of around 30 Kb (Enjuanes et al., 2008). Coronavirus (CoVs) infect a wide range of mammalian and avian species. The development of efficient CoV reverse genetics systems (Almazan et al., 2006, Almazan et al., 2013, Almazan et al., 2000, Almazan et al., 2014, Casais et al., 2001, Thiel et al., 2001, Yount et al., 2003, Yount et al., 2002) makes them promising expression vectors, with several advantages over other viral expression systems. CoVs replicate in the cytoplasm without a DNA intermediary, making integration of the virus genome into the host cell chromosome unlikely (Lai and Cavanagh, 1997). In addition, these viruses have the largest RNA virus genome and, in principle, have room for the insertion of large foreign genes (Enjuanes et al., 2005, Masters, 1999). As CoVs in general infect both respiratory and enteric mucosal surfaces, they may be used to target the antigen to these areas, stimulating the mucosal immune system to induce a pleiotropic secretory immune response, including lactogenic immunity (Sola et al., 2003). In fact, it has been described that a pleiotropic secretory immune response is best induced by the stimulation of gut associated lymphoid tissues (Saif, 1996). Moreover, the tropism of CoVs may be engineered by modifying the spike (S) gene (Casais et al., 2003, Sanchez et al., 1999), and non-pathogenic CoV strains infecting most species of interest (human, porcine, bovine, canine, feline, and avian) are available and therefore are suitable to develop safe virus vectors (Cavanagh et al., 2007, Ortego et al., 2002).
In fact, several studies have reported the construction of CoV-derived viral vectors expressing high levels of heterologous proteins, including reporter and viral proteins (Bentley et al., 2013, Ribes et al., 2011, Shen et al., 2009, Sola et al., 2003). Foreign gene expression levels can be regulated by the use of different transcription-regulating sequences (TRSs) ranging from intermediate to high gene expression levels (Alonso et al., 2002a). In addition, our group has recently identified an optimized transcription-regulating motif, enhancing by 5-fold the mRNA levels of a viral gene, which can be used in expression vectors based in CoV genomes (Mateos-Gomez et al., 2011). Additionally, a combination of these TRSs could be used to drive the expression of two or three heterologous genes from just one infectious cDNA (i.e., dicistronic or tricistronic vectors).
Genetic stability of a heterologous gene within the viral vector is essential for its development as a live immunization vector. In general, the stability of heterologous genes is high for DNA viruses and negative RNA viruses, in which the low level of recombination contributes to the maintenance of the inserted foreign genes (Bukreyev et al., 2006) In contrast, positive RNA viruses are highly prone to recombination, both homologous and non-homologous (Alejska et al., 2005, Figlerowicz et al., 2003) leading to the loss of the inserted genes and avoiding their expression over a long time period. CoVs are positive RNA genomes with high recombination frequency (Denison et al., 2011, Lai, 1996, Sanchez et al., 1992). Genetic instability leading to the loss of heterologous genes has been frequently reported in CoV-derived vectors, both in vitro (Bentley et al., 2013, Cruz et al., 2010, Sola et al., 2003) and in vivo (Bentley et al., 2013).
In view of the frequent instability of CoV-based vectors expressing proteins of large size, we explored whether the reduction of heterologous gene size was a useful strategy to increase insert stability, by reducing the probability of the presence of toxic domains in the inserted gene or protein. In fact, the expression of small protein domains is a common strategy used to reduce toxicity when toxic proteins are expressed in bacteria (Edwards et al., 2000, Samuelson, 2011).
TGEV infects the enteric and respiratory tissues of newborn piglets resulting in a mortality of nearly 100% (Saif and Wesley, 1992). Interestingly, some non-enteric TGEV variants with alterations in the S protein have a tropism restricted to the respiratory tract, and show attenuated phenotype (Sanchez et al., 1999). TGEV-derived vectors have been successfully engineered for the expression of green fluorescent protein (GFP). The GFP gene was expressed by replacing the non-essential genes 3a and 3b, leading to very stable (>20 passages in tissue culture) high expression levels of the heterologous protein (50 μg/106cells) (Sola et al., 2003). Recombinant TGEV (rTGEV) vectors have been engineered for dicistronic expression of heterologous genes, such as porcine reproductive and respiratory syndrome virus (PRRSV) GP5 and M proteins (Cruz et al., 2010), or rotavirus VP2 and VP6, in which formation of rotavirus virus like particles (VLPs) in the cytoplasm of rTGEV infected cells was observed (Enjuanes et al., 2007).
TGEV has been previously used as an immunization vector to confer partial protection against PRRSV infection (Cruz et al., 2010). In addition, an engineered rTGEV in which the tropism was modified replacing the S protein by the homologous one from mouse hepatitis virus (MHV) was used to confer protection against rotavirus infections (Ribes et al., 2011). The engineered rTGEV expressing rotavirus VP7 protein was then evaluated in the mouse model. The recombinant virus triggered a humoral response via systemic (serum IgG and IgA) and mucosal (intestinal IgA) antibodies. In addition, partial protection against rotavirus-induced diarrhea was observed in 62% of the challenged animals.
Porcine reproductive and respiratory syndrome (PRRS) is the most important infectious disease affecting swineherds worldwide. It is characterized by reproductive failure in sows, as well as severe pneumonia in piglets (Lunney et al., 2010). The causative agent of PRRS is PRRS virus (PRRSV) that is included in Arteriviridae family, in the order Nidovirales. PRRSV is an enveloped, single-stranded positive sense RNA virus of approximately 15 Kb in length that contains 9 open reading frames (ORFs). ORF1a and ORF1b encode the replicase non-structural proteins, while ORFs 2 to 7 encode structural proteins: the small envelope protein (E), the membrane protein (M), nucleocapsid protein (N) and the glycoproteins GP2a, GP3, GP4, GP5, and the recently identified protein 5a (Dokland, 2010, Firth et al., 2011, Johnson et al., 2011). Currently, PRRS causes huge economic losses in the swine industry, but commercially available vaccines are only partially effective (Charerntantanakul, 2012).
PRRSV infection induces a weak innate immune response, probably contributing to the reduced and delayed subsequent humoral and cellular immune responses, and also to virus persistence (Kimman et al., 2009). This is probably due to the limited interferon alpha (IFN-α) elicited by PRRSV (Albina et al., 1998, Calzada-Nova et al., 2010). The knowledge on PRRSV correlates of protection is limited. Neutralizing antibodies against PRRSV are mainly directed to GP5 protein (Kim and Yoon, 2008, Ostrowski et al., 2002), although neutralizing antibodies recognizing GP3 and GP4 have also been described following PRRSV infection (Costers et al., 2010, Oleksiewicz et al., 2002, Vanhee et al., 2011). PRRSV M protein is a potent inducer of T-cell proliferation in piglets infected with PRRSV, and may also play a role in protection (Bautista et al., 1999, Jeong et al., 2010).
Current vaccines against PRRSV have a limited efficacy. Best results have been obtained using modified live vaccines, although they have several problems such as incomplete protection, virus shedding and possible reversion to virulence (Charerntantanakul, 2012). Vector-based vaccines could represent an advantage to stimulate both humoral and cell immune responses against PRRSV (Cruz et al., 2010). Given the potential of CoV-derived vectors, and the requirement of more efficient vaccines against PRRSV, the work presented here is focused on the use of TGEV as a vector for the expression of PRRSV antigenic combinations. The expression of PRRSV small domains containing the epitopes relevant for protection would lead to a significant increase in vector stability.
Previous work from our laboratory has shown that rTGEVs co-expressing full-length PRRSV GP5 (wild type or modified) and M proteins induced partial protection against PRRSV (Cruz et al., 2010). The modest results obtained may be due to the instability of GP5 protein in the rTGEV system, resulting in a significant loss of GP5 expression in 8–10 passages in tissue culture. Expression of full-length PRRSV GP3 or GP4 proteins was also toxic for rTGEV leading to the loss of the heterologous gene sequence (M. Becares, S. Zuñiga and L. Enjuanes, unpublished results). In this work the stability of the expression of small domains of PRRSV GP3, GP4 and GP5, previously described as potentially relevant in the induction of protection against PRRSV has been studied, in comparison with the expression of full-length proteins, using rTGEV vectors. Our results showed that reduction of the heterologous genes size inserted in the CoV-derived vector is a promising strategy to achieve stable expression. Additionally, as PRRSV M protein was stable in rTGEV, several antigenic structures were engineered using this protein as scaffold for the expression of small antigenic domains, resulting in high stability. Furthermore, immunization of piglets with these live attenuated rTGEV vectors partially protected against PRRSV, with reduction of clinical signs and lung damage as well as a faster viremia decrease.
Results
PRRSV M protein is stably expressed by rTGEV
PRRSV M protein is a long non-glycosilated membrane protein of around 170 amino acids, which is the most highly conserved structural protein of PRRSV (Meng et al., 1995) and has been involved in the induction of T-cell response against PRRSV (Bautista et al., 1999). A rTGEV vector expressing PRRSV M protein was generated encoding PRRSV M gene in the location previously occupied by non-essential genes 3a and 3b. PRRSV M gene expression was driven by the transcription-regulating sequence of gene 3a (TRS3a) ( Fig. 1A).
Fig. 1.
Expression of PRRSV M protein by rTGEV-derived vectors. (A) Schematic structure of the rTGEV cDNA encoding the PRRSV M gene. The numbers and letters inside the rectangles indicate the viral genes. TRS, transcription-regulating sequence. (B) RT-PCR analysis of ten clones from plaque-purified passage 16 rTGEV-S7.1-TRS3a-M virus. Genomic RNA (gRNA) and subgenomic mRNA (mRNA) encoding PRRSV M protein were detected. The arrow indicates the expected size of the corresponding PCR product. Numbers on the left indicate the molecular weight markers (Mw) size in base pairs. (C) Immunofluorescence analysis of ST cells infected with the passage 16 rTGEV-S7.1-TRS3a-M at 8 hpi. A polyclonal antibody specific for TGEV and a secondary antibody staining red were used to identify virus-infected cells. Expression of PRRSV M protein was detected with a monoclonal antibody and a secondary antibody staining green.
A rTGEV-S7.1-TRS3a-M was recovered, with a titer of 108 pfu/ml, as expected for rTGEV viruses. In order to test the stability of PRRSV M protein expressed by this vector, cloned viruses were serially passaged in tissue culture and the maintenance of the heterologous gene was evaluated at different passages by the analysis of plaque-purified viral clones. The presence of the heterologous sequence in the viral genome was evaluated by RT-PCR, using specific primers flanking the insertion region. After 8 and 16 serial passages of the rTGEV-S7.1-TRS3a-M, all the isolated viral clones still contained M gene sequence (Fig. 1B), with the expected size and sequence as revealed by PCR product sequencing. In addition, all the isolated clones expressed M protein mRNA (Fig. 1B), confirming M gene stability in rTGEV system.
Moreover, M protein expression was analyzed by immunofluorescence in ST cells infected at moi 0.5. M protein was expressed in 96% of the infected cells (Fig. 1C), with expression levels remaining constant through the passages (data not shown). Altogether, these results indicated that expression of PRRSV M protein in rTGEV was fully stable.
Expression of small domains of PRRSV GP5 protein using rTGEV
Netralizing antibodies recognizing GP5 are considered the most relevant for protection, with the epitope responsible for the neutralization located in the ectodomain of GP5 protein (Kim and Yoon, 2008, Ostrowski et al., 2002). Previous studies from our group indicated that the partial protection observed after immunization with rTGEVs expressing full length GP5 protein, both wild type or glycosylation mutants, was probably due to the toxicity of this protein in rTGEV system, leading to heterologous gene loss with passages (Cruz et al., 2010). As a consequence, we decided to evaluate the expression of small domains containing epitopes potentially relevant in protection, as the reduction of potential toxic domains could increase vector stability.
In a first approach, a rTGEV co-expressing GP5 ectodomain (GP5ecto) and M protein was engineered. GP5ecto transcription was driven by the TRS3a, and that of M protein by an optimized TRS partially derived from gene N TRS (TRS22N) (Alonso et al., 2002a). M protein was included in the rTGEV construct because it was fully stable and we previously observed that it increased GP5 stability, probably by forming the heterodimer observed in the native virus (Cruz et al., 2010). In fact, we postulate that the expressed GP5 domains and M protein could form a heterodimer similar to the one observed in the virus, what may be important for its immunogenicity. GP5ecto consisted in the 68 most N-terminal amino acids of the Olot91 GP5 protein, which according to bioinformatics predictions cover the ectodomain of the protein. This domain included the protein motifs relevant in protection, such as the immunodominant epitope and the epitope critical in neutralization as well as the glycosylation sites ( Fig. 2A). To allow GP5ecto protein detection, a hemaglutinin (HA) tag was fused to GP5 protein domain (Fig. 2A). This tag is small (9 amino acids) and was previously used for CoV protein tagging, without showing any toxicity (Alvarez et al., 2010). rTGEV-S7.1-TRS3a-GP5ecto-TRS22N-M was recovered with titers similar to those of wt virus. The stability of GP5ecto domain with virus passages was analyzed by RT-PCR analysis of isolated clones. After 8 passages in tissue culture, 80% of the isolated clones contained the GP5ecto sequence (Fig. 2B) and expressed the corresponding mRNA (data not shown), representing a modest increase of stability compared with GP5 full-length (60% stable) (Fig. 2B). Unfortunately, in both cases, the heterologous GP5 sequences were lost at passage 16 (data not shown), indicating that GP5ecto long-term stability did not represent a sufficient improvement as compared to full-length GP5. Similar conclusions were extracted from immunofluorescence analysis of protein expression (data not shown).
Fig. 2.
Stability of PRRSV GP5 domains in rTGEV vectors. (A) Schematic representation of PRRSV GP5 constructs: full-length GP5 (GP5), GP5 ectodomain (GP5ecto), and GP5 fragment (GP5fr) that comprises the ectodomain lacking the signal peptide (SP). Immunodominant epitope (IDE) and epitope critical in neutralization (ECN), N-glycosylation sites (yellow), and the cysteine involved in GP5-M heterodimer formation (red) are also shown. GP5ecto and GP5fr included an HA or FLAG tag, respectively, for their detection (TG, blue). (B) RT-PCR analysis of ten clones from plaque-purified passage 8 rTGEV-S7.1-TRS3a-GP5-TRS22N-M (GP5), rTGEV-S7.1-TRS3a-GP5ecto-TRS22N-M (GP5ecto) and rTGEV-S7.1-TRS3a-GP5fr-TRS22N-M (GP5fr) viruses. The arrow indicates the expected size of the corresponding PCR product. Numbers on the left indicate the molecular weight markers (Mw) size in base pairs. Lower size bands (indicated by red asterisks) correspond to deletion products from heterologous gene, meaning genomic instability. Numbers on the right indicate the overall stability of each construct.
In a second step, an additional reduction in GP5 size was designed, by eliminating the predicted signal peptide of GP5, whose cleavage is controversial (Thaa et al., 2013, Wissink et al., 2003). The resulting 34 amino acid fragment of GP5 protein (GP5fr) was inserted in rTGEV, leading to rTGEV-S7.1-TRS3a-GP5fr-TRS22N-M virus. This small domain contained the GP5 epitope critical in neutralization, glycosylation sites and the cysteine residue involved in the GP5-M heterodimer formation. For GP5fr detection a FLAG tag was fused at the carboxi-terminus (Fig. 2A). This FLAG tag has been successfully used in CoV protein tagging (Alvarez et al., 2010). The additional size reduction of the GP5 fragment cloned in rTGEV led to an improvement in heterologous gene stability after 8 passages in tissue culture, with 90% of the independent clones containing GP5fr sequence (Fig. 2B) and expressing GP5fr mRNA (data not shown). Moreover, long-term stability was significantly improved, with up to 50% of the isolated clones stably maintaining GP5fr after 16 passages in tissue culture (data not shown).
PRRSV minor envelope protein domains expressed by rTGEV
Studies on PRRSV immunobiology have revealed that PRRSV neutralizing antibodies recognized epitopes within the minor structural glycoproteins GP2a, GP3 and GP4 (Costers et al., 2010, Oleksiewicz et al., 2002). rTGEVs were engineered expressing these proteins, alone or in various combinations, including the tricistronic expression of GP2a, GP3 and GP4. None of those proteins was stably expressed by rTGEV vectors, with PRRSV GP3 protein resulting extremely toxic for rTGEV system, leading to its expression loss in early stages (M. Becares, S. Zuñiga and L. Enjuanes, unpublished results). The recent identification of antigenic, linear domains in GP3 and GP4 (Costers et al., 2010, Vanhee et al., 2011) allowed the application of the small domain expression strategy to these proteins. Fusion domains including GP3 or GP4 epitopes critical in neutralization, flanked by a few amino acids (Table 1), and preceded by the corresponding signal peptide were designed. These peptidic domains consisting of 55 and 50 amino acids of GP3 (GP3fr) and GP4 (GP4fr), respectively, were fused to the FLAG tag at their C terminus end ( Fig. 3A). The GP3 and GP4 fragments were cloned in TGEV genome in the location previously occupied by non-essential genes 3a and 3b and their transcription was driven by the TRS3a. Recombinant viruses rTGEV-S7.1-TRS3a-GP3fr and rTGEV-S7.1-TRS3a-GP4fr were recovered with titers similar to those of the wt virus. The stability of the recombinant viruses was analyzed after 8 and 16 passages in tissue culture, by studying 10 plaque-purified clones by RT-PCR. All the clones maintained the heterologous gene sequence, and expressed the corresponding mRNA (Fig. 3B), indicating that both GP3fr and GP4fr were fully stable in the rTGEV vector. Protein detection using anti-FLAG antibody failed for GP3fr, GP4fr and GP5fr, both in immunofluorescence and Western blot assays (data not shown).
Table 1.
Structure of PRRSV fusion proteins expressed by rTGEV. Amino acids in each segment forming PRRSV fusion proteins.
| Fusion proteina | Segment 1b | Segment 2 | Segment 3 | Segment 4 |
|---|---|---|---|---|
| GP3fr | GP3 (1–28) | GP3 (51–77) | FLAGc | – |
| GP4fr | GP4 (1–25) | GP4 (51–75) | FLAG | – |
| GP5fr | GP5 (35–68) | FLAG | – | – |
| GP3ep-Mloop | M (1–66) | GP3 (51–77) | FLAG | M (67–173) |
| GP3ep-NtermM | FLAG | GP3(51–77) | M (1–173) | – |
PpuMI (AGGTCCT) and BlpI (GCTCGAGC) restriction sites were introduced at the 5′ and 3′ ends, respectively. An optimized Kozak sequence (GCCACC) was placed immediately upstream the ATG start codon to improve protein translation efficiency.
The numbers in brackets indicate the amino acids included in the construct.
All constructs included the FLAG tag sequence (amino acids DYKDDDDK).
Fig. 3.
Expression of PRRSV minor envelope protein domains by rTGEV vectors. (A) Schematic representation of PRRSV GP3 and GP4 proteins, and corresponding GP3fr and GP4fr expressed by rTGEV. Several motifs are indicated, such as signal peptide (SP), the epitope critical in neutralization (ECN), the transmembrane domain (TM), the glycosylation sites (yellow), and immunodominant epitope (IDE). GP3fr and GP4fr included a FLAG tag (FG) for their detection. (B) RT-PCR analysis of ten clones from plaque-purified passage 16 rTGEV-S7.1-TRS3a-GP3fr (GP3fr) and rTGEV-S7.1-TRS3a-GP4fr (GP4fr) viruses. Genomic RNA (gRNA) and GP3fr and GP4fr subgenomic mRNAs (mRNA) were detected. The arrow indicates the expected size of the corresponding PCR product. Numbers on the left indicate the molecular weight markers (Mw) size in base pairs.
Altogether, these data revealed that heterologous gene size reduction led to a drastic increase in the stability of rTGEV vectors.
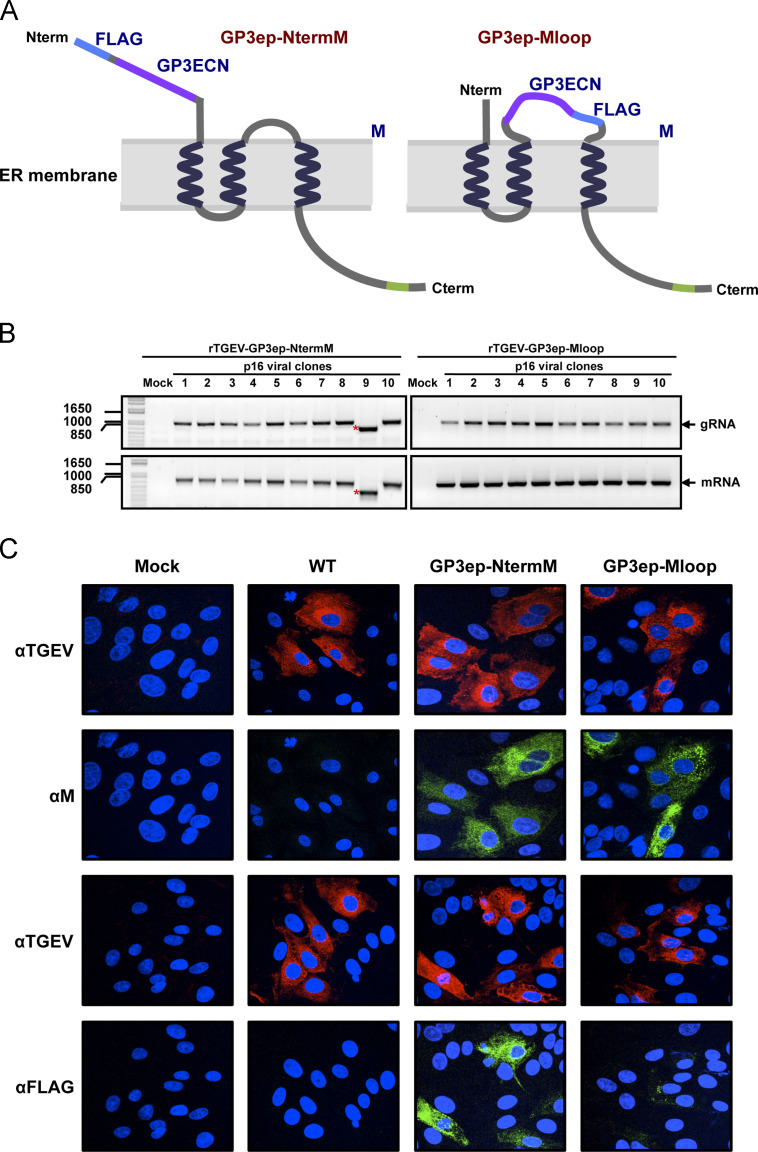
PRRSV M protein as scaffold for PRRSV antigenic domains expression
M protein is the most conserved structural protein among PRRSV strains (Kapur et al., 1996, Murtaugh et al., 1998) and it is the main inducer of virus-specific T-cell response (Bautista et al., 1999, Jeong et al., 2010). Our results indicated that M protein was fully stable in rTGEV (see above). Therefore, we postulated that M protein could be used as a scaffold for the expression of small antigenic domains. As a proof of principle, the GP3 epitope critical in neutralization (ECN) domain was selected for expression fused to M protein. Two exposed locations into M protein were predicted using TMpred transmembrane topology prediction algorithm (Hofmann and Stoffel, 1993): the N-terminus and a loop comprising amino acids 63 to 70. GP3 ECN was inserted at these M protein locations, leading to chimeric structures, GP3ep-NtermM and GP3ep-Mloop, respectively ( Fig. 4A). These chimeric genes were cloned into rTGEV vector, and recombinant viruses rTGEV-S7.1-TRS3a-GP3ep-NtermM and rTGEV-S7.1-TRS3a-GP3ep-Mloop were rescued with titers similar to those of the parental virus. The stability of the recovered viruses was analyzed. After 8 or 16 passages in tissue culture 10 independent clones were screened by RT-PCR. All the independent clones maintained the heterologous gene sequence (data not shown) after 8 passages, whereas after 16 passages 90% and 100% of rTGEV-S7.1-TRS3a-GP3ep-NtermM and rTGEV-S7.1-TRS3a-GP3ep-Mloop, respectively, contained the heterologous gene sequence and expressed the corresponding mRNA (Fig. 4B).
Fig. 4.
PRRSV M protein as an scaffold for antigenic domain expression. (A) Chimeric proteins expressed by rTGEV. Two M protein locations were chosen for the expression of GP3 epitope involved in neutralization (GP3ECN) fused to a FLAG tag (blue), leading respectively to the chimeras GP3ep-NtermM and GP3ep-Mloop. M protein contains in its C-terminal an endoplasmic reticulum retention signal (green). (B) RT-PCR analysis of ten clones from plaque-purified passage 16 rTGEV-S7.1-TRS3a-GP3ep-NtermM (GP3ep-NtermM) and rTGEV-S7.1-TRS3a-GP3ep-Mloop (GP3ep-Mloop) viruses. Genomic RNA (gRNA) and PRRSV M protein subgenomic mRNA (mRNA) were detected. The arrow indicates the expected size of the corresponding PCR product. Numbers on the left indicate the molecular weight markers (Mw) size in base pairs. Lower size bands (indicated by red asterisks) correspond to deletion products from heterologous gene, meaning genomic instability. (C) Immunofluorescence analysis of ST cells infected with passage 16 rTGEV-S7.1-TRS3a-GP3ep-NtermM (GP3ep-NtermM) and rTGEV-S7.1-TRS3a-GP3ep-Mloop (GP3ep-Mloop) at 8 hpi. TGEV specific polyclonal antiserum and a secondary antibody staining red were used to identify infected cells. Expression of chimeric proteins was detected using a monoclonal antibody specific for PRRSV M protein (αM) or the FLAG tag (αFLAG) and a secondary antibody staining green.
In order to evaluate stability and expression levels of the chimeric proteins, double immunofluorescence was performed on cells infected with passage 16 rTGEV-S7.1-TRS3a-GP3ep-NtermM and rTGEV-S7.1-TRS3a-GP3ep-Mloop viruses. M protein scaffold was detected in 95% of infected cells in both cases (Fig. 4C). This detection level was similar to that observed in rTGEV-S7.1-TRS3a-M expressing full-length wt M protein (see above). In contrast, FLAG epitope was detected in 92% and 63% of the rTGEV-S7.1-TRS3a-GP3ep-NtermM and rTGEV-S7.1-TRS3a-GP3ep-Mloop infected cells, respectively (Fig. 4C). These results strongly suggested that M protein N-terminus was a more exposed location, and therefore better to present antigens. This data is in agreement with previous observations demonstrating that arterivirus M protein is tolerant to manipulations of its ectodomain (Verheije et al., 2002).
Evaluation of the protective potential of the rTGEVs expressing PRRSV antigens
In order to evaluate the protection provided by rTGEVs expressing PRRSV antigens, the rTGEVs that showed an increased stability in cell culture were tested in vivo. For that purpose, rTGEV-S7.1-TRS3a-M, rTGEV-S7.1-TRS3a-GP5fr-M, rTGEV-S7.1-TRS3a-GP3fr, rTGEV-S7.1-TRS3a-GP4fr, and rTGEV-S7.1-TRS3a-GP3ep-NtermM were selected for in vivo experiments.
Two groups of twelve days-old piglets were inoculated with 1×108 pfu/animal of each rTGEV-S7.1-TRS3a-M, rTGEV-S7.1-TRS3a-GP5fr-M, rTGEV-S7.1-TRS3a-GP3fr, rTGEV-S7.1-TRS3a-GP4fr, and rTGEV-S7.1-TRS3a-GP3ep-NtermM (immunized group), or rTGEV (non-immunized group), respectively, by three routes: oral, nasal and intragrastic. Previous data from our group indicated that, in these conditions, the virus in the inoculum reached the target organs (respiratory and digestive tracts) and replicated to high titers (Cruz et al., 2011, Sanchez et al., 1999). A boost was performed 2 weeks after inoculation using the same conditions. Two weeks later, a challenge was performed with 1×106 TCID50 of PRRSV Olot91-like virulent strain. A control group was inoculated with 1×108 pfu/animal of rTGEV and boosted two weeks later, but not challenged. Pigs were monitored for clinical signs, focusing on respiratory symptoms such as tachypnoea, and abdominal breathing. PRRSV infection resulted in moderate fever, depression and respiratory signs that persist from days 5 to 25 after challenge ( Fig. 5A). The percentage of animals showing respiratory symptoms was significantly higher in the non-immunized group than in the immunized group (Fig. 5A). Moreover, the average weight gain, which was reduced in challenged animals, was higher in immunized animals than in non-immunized animals between 21 and 28 days post-challenge (data not shown).
Fig. 5.
Protection conferred by rTGEVs expressing PRRSV antigens. (A) Percentage of animals showing respiratory symptoms. Observations were made daily during a time frame of 28 days post-challenge. ⁎⁎, p-value<0.05, ⁎, p-value<0.1. (B) Lung samples collected at 28 days post-challenge were stained with hematoxylin-eosin. Representative pictures obtained with a 10x objective are shown (left panels). Lung damage was scored by observation of 50 random fields per animal (right panel). (C) IL-8 levels in serum. Serum samples were collected at the indicated times post-immunization, mixed in pools of three animals per sample, and cytokine levels were analyzed by a 7-plex fluorescent microsphere immunoassay. Dots represent individual samples and solid lines represent mean titer for each group. ⁎, p-value<0.1.
Lung damage was analyzed by histopathology of lungs from five randomly chosen piglets per group. Lungs from challenged piglets exhibited features that are characteristic of PRRSV infection such as pneumocyte hypertrophy and hyperplasia, and intra- alveolar accumulation of cell debris (Fig. 5B, left panels). The lungs from immunized animals showed a lower degree of lung damage than those from non-immunized piglets (Fig. 5B, right panel), indicating a certain degree of protection. The lower extent of lung inflammation observed in immunized animals was in agreement with the lower levels of proinflamatory cytokine IL-8 observed in vaccinated animals׳ sera (Fig. 5C). Immunized animals showed a moderate increase in IL-8 by 3 days post-challenge (31 dpi, as shown in the figure), but levels rapidly returned to normal, while non-vaccinated animals showed a higher elevation of this cytokine, that continued at elevated levels during the experimental infection. Altogether, these results suggested that rTGEV vectors expressing PRRSV antigens conferred partial protection against PRRSV infection.
To further analyze the protection conferred by rTGEVs expressing PRRSV antigens, PRRSV viremia was analyzed by RT-qPCR at different times post-challenge. Similar virus titers in serum were obtained in all challenged animals at the initial stages post-challenge (28–42 dpi) ( Fig. 6). Interestingly, a significant reduction in virus titer was observed in the immunized group at 21–28 days post-challenge (49–56 dpi).
Fig. 6.
PRRSV titer in serum. PRRSV RNA was isolated from sera at the indicated times post-immunization, and viremia in serum was quantified by RT-qPCR. Dots represent individual animals and solid lines represent mean titer for each group. **, p-value<0.05.
Immune response elicited by rTGEVs expressing PRRVS antigens
In order to evaluate the potential of rTGEVs stably expressing PRRSV antigens as inducers of immunity against PRRSV, the humoral response was analyzed at different times post-inoculation. The antibody response against the TGEV vector, PRRSV virus and PRRSV individual proteins expressed by rTGEVs were determined by ELISA. All the animals elicited a high humoral immune response against TGEV indicating that the vector infected target tissues as expected, even though the piglets presented pre-existing anti-TGEV antibodies (data not shown). Seroconversion against total PRRSV was observed in all challenged animals by day 10 after infection with the virulent virus, while for individual GP3, GP4 and M protein it was detected by day 14 after challenge. In all the above-mentioned cases, no differences were observed between immunized and non-immunized animals (data not shown). The humoral response against PRRSV N protein followed identical pattern to that obtained for anti-PRRSV antibodies (data not shown). These data indicated that anti-PRRSV total antibodies response was most likely directed against N protein and did not play a role in protection, in agreement with previous reports showing an early non-neutralizing antibody response obtained after PRRSV infection (Mateu and Diaz, 2008). Interestingly, a higher and faster antibody response against GP5 protein was found from day 42 post-immunization in immunized animals as compared to the non-immunized ones ( Fig. 7A).
Fig. 7.
Humoral immune response elicited by rTGEV expressing PRRSV antigens. (A) Humoral response specific for GP5. Serum samples were collected at the indicated times post-immunization and analyzed by ELISA using purified full-length GP5. Dots represent individual animals and solid lines represent mean titer of each group. The arrow indicates the time when challenge was performed. ⁎⁎⁎, p-value<0.01; ⁎⁎, p-value<0.05. (B) Neutralizing antibodies induced at the indicated times post-challenge. Neutralization assays were performed with PRRSV Olot91 strain infecting MARC-145 cells. Dots represent individual animals and solid lines represent mean titer of each group. ⁎, p-value<0.1.
The neutralizing antibody response was evaluated in sera from immunized and non-immunized animals at 14, 21 and 28 days post-challenge. Non-immunized animals showed higher levels of PRRSV neutralizing antibodies than the immunized ones (Fig. 7B). This data, suggested a less effective PRRSV infection in the immunized piglets, supporting partial protection against PRRSV infection.
Discussion
In this study rTGEV was engineered for the expression of small protein domains relevant in immune response against PRRSV. Previous results from our group indicated that instability of certain heterologous gene expression by rTGEV might represent an important limitation for its use as an immunogenic vector. The expression of small protein domains was used as a strategy to improve heterologous gene stability. Stable expression of protein antigenic domains contained in highly unstable full-length proteins was achieved. Additionally, as full-length PRRSV M protein was stably expressed by rTGEV, it was used as a scaffold for the generation of chimeric proteins that exposed other PRRSV antigens, resulting in highly stable expression. Therefore, size reduction of the heterologous genes inserted in CoV-derived vectors resulted in a promising strategy to achieve stable expression. Protection experiments showed that rTGEV, stably expressing PRRSV antigenic structures, elicited partial protection against PRRSV, with a reduction of clinical signs and lung damage in immunized piglets.
The potential of CoV-derived vectors as systems for gene delivery has been limited due to its restricted stability. In general, genetic stability is highly dependent on the nature of the foreign gene, with some inserts maintained at least twenty passages whereas others are lost at passage two (de Haan et al., 2005, Enjuanes et al., 2007, Shen et al., 2009, Sola et al., 2003). In addition, other factors affect genetic stability of recombinant CoVs, such as the insert size (de Haan et al., 2005), and the genomic location in which it is inserted (Bentley et al., 2013). The maintenance of the inserted genes will also depend on the recombination rate, conditioned both by the insert size and the presence in the foreign sequence of regions showing homology with the virus genome, favoring homologous recombination (Wang et al., 2003). Furthermore, other heterologous gene or protein characteristics may affect insert stability, leading to loss of the inserts harmful for the infected cell or virus replication. Therefore, it is very difficult to predict the specific insert stability in advance, before a highly effort-consuming process to generate the CoV-derived vectors expressing the heterologous antigen has been accomplished.
PRRSV GP5 and M genes have similar lengths (606 and 522 nucleotides, respectively) and small ectodomains exposed between three transmembrane domains. Nevertheless, M protein was fully stable in rTGEV vectors, while GP5 protein resulted toxic and its expression was lost after 4–5 passages of the virus in cell culture. Interestingly, PRRSV hydrophobic M protein resulted highly toxic in other expression systems, including bacteria and insect cells (Jeong et al., 2010, Plana-Durán et al., 1997). As deletion of the heterologous genes could be due to homologous recombination between the heterologous gene and TGEV genome because of sequence identity, this possibility was analyzed. No statistically significant sequence identity was identified between PRRSV GP5 sequence and TGEV genome. In fact, analysis of the deletions observed in the unstable recovered viral clones did not show a common pattern of recombination. In constrast, random deletions were observed ranging from small deletions affecting TRS, to larger ones covering most PRRSV GP5 gene sequence (data not shown), that in all cases led to the loss of protein expression. Additionally, the GP5 gene, when expressed alone in rTGEV vectors, was lost at very early passages while it was stably maintained until passage 8 when it was co-expressed with M protein, most likely due to the formation of GP5-M heterodimers (Cruz et al., 2010). These data suggested that the instability was caused by protein toxicity affecting host cell viability or viral life cycle, rather than a negative effect of the heterologous gene sequences on virus genomic stability. This toxicity would confer selective advantage to those viral clones that did not express GP5 protein.
In this work we showed that size reduction of the foreign insert significantly improved heterologous gene stability. Even in the case of highly toxic inserts, such as PRRSV GP3 protein, size reduction led to 100% stability. Therefore, heterologous gene size reduction is a promising strategy to achieve stable expression in TGEV-derived vectors and in general in CoVs. This effect could be due to a decrease of the probability of non-homologous recombination in shorter sequences, or to the elimination of protein domains toxically affecting the host cell or rTGEV life cycle. This result is in agreement with previous studies showing that gene size affected foreign gene stability in CoV vectors, as the larger firefly luciferase gene resulted less stable than the shorter Renilla luciferase gene, when expressed by Feline Infectious Peritonitis virus (FIPV) vectors (de Haan et al., 2005).
In this paper we used PRRSV M protein, which we have shown that displays a high stability in rTGEV vectors as a scaffold for the expression of small antigenic domains. This approach may be useful to modify the trafficking and accumulation of small protein domains expressed alone. In fact, protein detection using anti-Flag antibody failed for GP3fr, GP4fr and GP5fr, both in immunofluorescence and Western blot assays (data not shown), probably due to low accumulation of those peptides inside the cell. Interestingly, GP3ep-NtermM was detected at high levels, indicating a higher accumulation in the infected cell of the chimeric protein.
Long-term stability of CoV-derived vectors has not been systematically addressed. Few proteins have been reported to be stably expressed by CoVs. Among these, GFP was stable for more than 6 or 20 passages in tissue culture when expressed by MHV or TGEV, respectively (Sarma et al., 2002, Sola et al., 2003), but it showed instability in IBV-derived vectors (Bentley et al., 2013). PRRSV M protein was also stably expressed by rTGEV for more than 16 passages (this manuscript). Proteins such as luciferase expressed by MHV and IBV-derived vectors (Bentley et al., 2013, de Haan et al., 2005), PRRSV GP5 protein expressed using TGEV virus vectors (Cruz et al., 2010) or GUS when expressed by TGEV minigenomes (Alonso et al., 2002b) were lost at early passages. In our experience, using rTGEV vectors, only around 25% of the heterologous genes were stably expressed for more than 8 passages in tissue culture [(Alonso et al., 2002b, Cruz et al., 2010, Sola et al., 2003), and unpublished results]. This instability is a key limiting factor in the use of CoV-based vectors for the expression of full-length proteins.
To improve the stability and efficacy of CoV-derived vectors it would be essential to understand the factors that control the high recombination frequency of CoVs. To this end, a detailed analysis of CoV proteins involved in genetic recombination is needed. Several enzymes involved in CoV RNA synthesis, such as nsp13 (helicase), nsp15 (endonuclease), nsp14 (exonuclease), nsp7 and nsp8 (RNA processivity components), or N protein could modulate recombination in CoVs. The engineering of recombination defective CoV mutants by knocking-down one or several genes involved in the recombination process could be the first step to achieve stable expression of large heterologous genes.
In this study, three PRRSV protein domains from GP3, GP4 and GP5 proteins, recognized by neutralizing antibodies were expressed by rTGEV and used as immunogens (Costers et al., 2010, Plagemann, 2004, Vanhee et al., 2011, Wissink et al., 2003), and the humoral response elicited by these rTGEVs was measured. After challenge, a faster response against GP5 protein was observed in immunized piglets. In contrast, the response against GP3 and GP4 was similar in immunized and non-immunized piglets. These data suggested that GP5 fragment was immunogenic, while GP3 and GP4 domains were antigenic but had a reduced immunogenicity.
Immunization of piglets with a combination of rTGEV expressing PRRSV antigens led to a clear reduction of clinical symptoms after challenge, a lower degree of lung damage and a faster viremia reduction. These results represent an improvement over previous vaccination experiments using rTGEV vectors expressing PRRSV antigens (Cruz et al., 2010).
PRRSV correlates of protection remain to be identified (Kimman et al., 2009), what represents an additional limitation for the development of new vaccine candidates. Neutralizing antibodies seem relevant for preventing PRRSV infection (Lopez and Osorio, 2004), but not enough to provide full protection (Murtaugh and Genzow, 2011). T-cell responses seem also required in PRRSV clearance (Mateu and Diaz, 2008). In the present study, immunized animals showed a significant faster recall antibody response against GP5 protein, which is generally considered the main target of neutralizing antibodies (Kim and Yoon, 2008, Ostrowski et al., 2002). Non-immunized animals developed a higher neutralizing response after challenge with a virulent PRRSV strain, what is considered as an indication of higher infection, whereas the immunized animals were significantly, although partially, protected against PRRSV infection. With the available data, it is not possible to determine whether the observed protection was due to an undetectable neutralizing antibody response before challenge [even commercial available vaccines have been reported to fail in the induction of detectable levels of neutralizing antibodies before challenge (Geldhof et al., 2012)] to immune cell responses [most likely directed to M protein present in the immunization cocktail], or both. The higher GP5 protein specific antibody response was observed from day 10 post-challenge, while significant differences in neutralizing antibodies between immunized and non-immunized animals were observed between 21 and 28 days post-challenge, correlating with the differences in viremia, what suggests that the observed neutralizing response was due to a higher infection of non-immunized swine. The relative contribution to protection of the humoral and cellular responses has not been determined. When a correlation between protection and induction of specific cytokines was analyzed, IL-8 levels were significantly different between immunized and non-immunized piglets, with levels consistently higher in non-immunized animals. The exacerbated IL-8 response elicited in non-immunized animals correlated with the higher lung damage observed in these animals. This result was in agreement with previous studies showing that piglets with more severe symptoms, including viremia and lung lesions, had a continuous elevation of IL-8 in serum, while in animals with milder symptoms IL-8 levels returned to normal by 7 dpi (Petry et al., 2007). Higher, but not significant, levels of IFN-α were also observed in immunized animals compared to non-immunized animals (data not shown), at day 3 post-PRRSV challenge. This result suggested that immunized animals developed a higher innate immune response, which nevertheless did not seem strong enough to induce a higher adaptative immune response.
The construction of rTGEVs expressing small antigenic domains has considerably improved the stability of the expression vectors. Nevertheless, some of these small antigens may have limited immunogenicity. Therefore, the expression of full-length antigens by engineering CoV vectors with decreased recombination rate deserves further attention to definitely launch CoVs as efficacious vaccine vectors for animal and human health.
Materials and methods
Ethics statement
Experiments involving animals were performed in strict accordance with EU (2010/63/UE) and Spanish (RD 1201/2005 and 32/2007) guidelines. All the protocols were approved by the in site Ethical Review Committee.
Cells and viruses
Baby hamster kidney (BHK-21) cells stably transformed with the gene coding for porcine aminopeptidase N (BHK-pAPN) (Delmas et al., 1994) were grown in Dulbecco׳s modified Eagle׳s medium (DMEM) supplemented with 5% fetal calf serum (FCS) and G418 (1.5 mg/ml) as a selection agent. Recombinant TGEV viruses obtained in this work were grown in swine testis (ST) cells (McClurkin and Norman, 1966). Tissue culture adapted PRRSV Olot91 (GenBank KC862570) strain was grown and titrated in monkey kidney MARC-145 cells (Kim et al., 1993).
Challenge PRRSV strain
Challenge was performed with a virulent PRRSV strain homologous to PRRSV Olot91 (PRRSV-Olot91-like). PRRSV-Olot91-like was propagated in porcine macrophages differentiated from fresh peripheral blood mononuclear cells (PBMCs) as previously described (Enjuanes et al., 1976). Briefly, 6×108 PBMCs isolated from fresh blood by centrifugation were seeded in 90-mm-diameter plates in Roswell Park Memorial Institute medium (RPMI) supplemented with 40% heat-inactivated swine serum. After 48 h, non-adherent cells where removed and attached macrophages, that showed 80% of confluence, were infected with 105 TCID50 of the parental PRRSV-Olot91-like. At 72 h post-infection (hpi), when cytopathic effect was clear, supernatant was collected and centrifuged. Virus was titrated in porcine alveolar macrophages (PAMs) as previously described (Duan et al., 1997).
Plasmid constructs
Fusion products GP3fr, GP4fr, GP5fr GP3ep-Mloop and GP3ep-NtermM were chemically synthesized and purchased from GeneArt (Germany). The PRRSV Olot91 protein sequences forming the fusion products are summarized in Table 1.
GP5ecto sequence was amplified by PCR using the forward primer (5′-GCAGGTCCTATGTACCCCTACGACGTGCCCGACTACGCCATGAGATGTTCTCACAAATTGGGGC-3′) and the reverse primer (5′-GCGCTCAGCTCAGGTCTCGACTGCCCAATCAAAATG-3′), which included PpuMI and BlpI restriction sites (underlined), respectively. M sequence was amplified using the forward primer (5′-GCAGGTCCTATGGGAAGCCTAGACGATTTTTG-3′) and reverse primer (5′-GGGCTAAGCTTACCGGCCATACTTGACGAGG-3′), which included PpuMI and BlpI restriction sites (underlined), respectively. In both cases, plasmid pSL-TRS3a-ORF5-TRS22N-ORF6 (Cruz et al., 2010) was used as a template.
PRRSV sequences, both chemically synthesized or PCR amplified, were digested with restriction endonucleases PpuMI and BlpI and cloned into the same sites of plasmid pSL-TGEV-S7.1-3ab including TGEV genomic sequence from nt 22973 to 25873. PRRSV sequences replaced non-essential genes 3a and 3b, leading to intermediate plasmids pSL-TRS3a-GP3fr, pSL-TRS3a-GP4fr, pSL-TRS3a-GP5fr, pSL-TRS3a-GP3ep-Mloop, pSL-TRS3a-GP3fr-NtermM, pSL-TRS3a-GP5ecto and pSL-TRS3a-M. For the generation of the dicistronic vectors pSL-TRS3a-GP5ecto-TRS22N-M and pSL-TRS3a-GP5fr-TRS22N-M, the sequence of M protein preceded by the optimized synthetic TRS22N (Alonso et al., 2002a) was obtained from pSL-TRS3a-ORF5-TRS22N-ORF6 by digestion with restriction endonuclease BlpI and cloned into the same site of pSL-TRS3a-GP5ecto and pSL-TRS3a-GP5fr.
Finally, all intermediate plasmids containing PRRSV sequences were digested with AvrII. The resulting fragments were cloned into the same sites of plasmid pBAC-TGEV-S7.1 (C.M. Sanchez, M. Becares, S. Zuñiga and L. Enjuanes, unpublished results). This plasmid was derived from the original pBAC-TGEVFL (Almazan et al., 2000), containing restriction sites PacI and MluI flanking S gene (Ortego et al., 2003). Cloning steps led to plasmids pBAC-S7.1-TRS3a-GP3fr, pBAC-S7.1-TRS3a-GP4fr, pBAC-S7.1-TRS3a-M, pBAC-S7.1-TRS3a-GP3ep-NtermM, pBAC-S7.1-TRS3a-GP3ep-Mloop, pBAC-S7.1-TRS3a-GP5ecto-TRS22N-M and pBAC-S7.1-TRS3a-GP5fr-TRS22N-M. All cloning steps were checked by sequencing of the PCR fragments and cloning junctions.
Transfection and recovery of infectious rTGEVs from cDNA clones
BHK-pAPN cells were grown to 90% confluence in 35-mm-diameter plates and transfected with 4 μg of the corresponding pBAC and 12 μl of Lipofectamine 2000 (Invitrogen), according to the manufacturer׳s specifications. After 6 h of incubation at 37 °C, cells were trypsinized and plated over a confluent ST monolayer grown in 35-mm-diameter plate. After a 2-day incubation period, the cell supernatants were harvested (passage 0). rTGEVs were cloned by three plaque purification steps. rTGEVs were grown and titrated as previously described (Jimenez et al., 1986).
Analysis of rTGEVs genomic RNA stability
Two clones of each rTGEV were serially passaged, independently, in ST cells every 24 h. At passage 8 and 16 ten viral clones were plaque purified. RNA from recombinant viruses was purified from infected ST cells grown to overconfluence on 12-well plates. Total intracellular RNA was extracted at 18 hpi using the RNeasy Mini Kit (Qiagen) according to the manufacturer׳s recommendations. Reverse transcription was performed with High Capacity RNA-to-cDNA™ Kit (Life Technologies) according to the manufacturer׳s instructions. PCRs were performed to analyze the size and sequence of viral genomic RNA (gRNA), at the locus where the heterologous genes were inserted, and heterologous mRNA size and sequence synthesis. The primers used and the expected PCR fragment sizes are shown in Table 2.
Table 2.
Analysis of rTGEVs stability by RT-PCR. Expected product size and primers used for the analysis of viral gRNA and heterologous mRNA expressed by rTGEVs.
| Heterologous gene |
Expected size (bp) |
||
|---|---|---|---|
| gRNAa | mRNAb | Reverse primer (5′→3′) | |
| GP3fr | 470 | 301 | TTTGTCGTCGTCGTCCTTGTAATC |
| GP4fr | 455 | 286 | TTTGTCGTCGTCGTCCTTGTAATC |
| GP5 | 1433 | 690 | GTCCTCGTCAAGGGTTGAGCT |
| GP5fr-Mc | 983 | 241 | TTTGTCGTCGTCGTCCTTGTAATC |
| GP5ecto-Mc | 1082 | 305 | GCGCTCAGCTCAGGTCTCGACTGCCCAATCAAAATG |
| M | 793 | 637 | GGGCTAAGCTTACCGGCCATACTTGACGAGG |
| GP3fr-Mloop | 904 | 904 | GGGCTAAGCTTACCGGCCATACTTGACGAGG |
| GP3fr-NtermM | 904 | 904 | GGGCTAAGCTTACCGGCCATACTTGACGAGG |
PCR for gRNA analysis was performed with the forward primer (5′-ATTACGAACCAATTGAAAAAGTGC-3′) and the reverse primer (5′-CCGCCTGAGAAAAGGCTGCATTG-3′) in all cases.
In all cases, forward primer (5′-GTGAGTGTAGCGTGGCTATATCTCTTC-3′), complementary to the viral leader sequence was used.
mRNA size shown in the table corresponds to GP5fr or GP5ecto.
Immunofluorescence analysis
Subconfluent ST cells grown on glass coverslips were mock infected or infected at a multiplicity of infection (moi) of 0.5 with each rTGEV. At 8 hpi cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS and blocked in PBS with 10% FCS. Monoclonal antibodies specific for FLAG (FLAG M2, 1:500, Sigma), PRRSV M protein (EM11E10C7, 1:100, kindly provided by INGENASA), or a polyclonal rabbit serum specific for TGEV (1:1000) were used. Bound primary antibody was detected with a Alexa Fluor 488 or 594-conjugated antibodies specific for mouse or rabbit, respectively (1:500, Invitrogen). Cell nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (1:200, Sigma). Confocal microscopy was performed using a Leica SP5 laser scanning microscope, and images were collected and processed with LAS AF software (Leica, Wetzlar, Germany). The percentage of infected cells expressing PRRSV antigens was estimated by the analysis of 10 independent microscopy fields, which represent an average of more than 400 cells.
Immunization of piglets
Forty-five twelve days-old non-colostrum-deprived piglets, born from PRRSV seronegative sows, were inoculated with rTGEV by three different routes (oral, gastric and intranasal) following standard procedures (Sanchez et al., 1999). Piglets were divided into three 15-animal groups. Piglets of group 1 were inoculated with a mix of 1×108 plaque forming units (pfu)/animal of each of rTGEV-S7.1-TRS3a-GP3fr, rTGEV-S7.1-TRS3a-GP4fr, rTGEV-S7.1-TRS3a-GP5fr-M, rTGEV-S7.1-TRS3a-M and rTGEV-S7.1-TRS3a-GP3ep-NtermM. Piglets of groups 2 and 3 were inoculated with 1×108 pfu/animal of rTGEV-S7.1. Two weeks after the first immunization, all piglets were boosted in the same conditions, and two weeks later piglets of groups 1 and 2 were challenged with 106 TCID50 of PRRSV-Olot91-like per animal by intranasal route. Infected animals were monitored daily to detect symptoms of disease, and body weights were determined every 7 days. Blood samples were taken at days 0, 14, 28, 31, 35, 38, 42, 49 and 56-post-first inoculation. Five and ten animals per group were euthanized and necropsied at days 35 and 56, respectively. Lung macroscopic lesions were evaluated, and lung samples were collected frozen and in 10% buffered-formalin.
Lung damage measurement
Five piglets per group were randomly chosen for histopathological study. Lung representative sections were fixed with 4% paraformaldehyde and stored in 70% ethanol at 4 °C. Paraffin embedding, sectioning and hematoxylin-eosin staining were performed by the histology service in the National Center of Biotechnology (CNB-CSIC, Spain). Samples were examined with a ZEISS Axiophot fluorescence microscope. Determination of the lung damage score was obtained from unbiased observation of 50 microscopy fields per animal, scoring from 0 to 3 attending to interstitial, peribronchiolar, and perivascular inflammation (Page et al., 2012).
Cytokine level measurement
Quantification of porcine IL-1β, IL-10, IFN-α, IFNγ, TNFα, IL-4 and IL-8 in serum samples was carried out using Swine Cytokine Magnetic 7-Plex Panel (Life Technologies, TM), and the Luminex 100 IS analyzer, according to the manufacturer׳s instructions. Three serum samples, corresponding to the same experimental group and the same date of extraction, were randomly pooled and analyzed in a single well. Data were calculated by xPONENT software using a five-parameter model derived from the known reference cytokine concentrations supplied by the manufacturer. The sensitivity of this assay allowed the detection of cytokine concentrations with the following limits of detection: IL-1β (36.808 pg/ml), IL-10 (4.572 pg/ml), IFN-α (2.661 pg/ml), IFNγ (0.342 pg/ml), TNFα (146.86 pg/ml), IL-4 (0.548 pg/ml), IL-8 (0.786 pg/ml).
Viremia measurement
Viral RNA was isolated from 250 μl of serum using MagMAX™ Viral RNA Isolation Kit (Life Technologies) according to the manufacturer׳s instructions. PRRSV RNA quantity was measured by RT-qPCR analysis using a custom TaqMan assay detecting PRRSV N RNA (TaqMan Probe 6-FAM-ACGGCTTTTAATCAAGGC-MGB; forward primer 5′-TTCCCTCTGCTTGCAATCG-3′; reverse primer 5′-GGATGAAAGCGACGCAGTTC-3′), and the AgPath-ID one-step RT-PCR kit (Life Technologies) according to the manufacturer׳s instructions. The data were acquired with an ABI Prism 7500 sequence detection system and analyzed with ABI Prism 7000 SDS version 1.2.3 software (Applied Biosystems). Viremia levels were expressed as the RT-qPCR cycle threshold (Ct) values.
Enzyme-linked immunosorbent assay (ELISA)
Antibodies induced against TGEV and PRRSV viruses or PRRSV purified proteins were detected by ELISA as described before (Sambrook and Russell, 2001). PRRSV GP3, GP4, GP5, M and N proteins were expressed using the baculovirus-insect cell system. Recombinant proteins were purified to near homogeneity by metal chelate affinity chromatography using Ni-NTA agarose (Sigma-Aldrich, Madrid, Spain) as previously described (Nogales et al., 2011). ELISAs were performed using partially purified TGEV (0.2 μg per well) and PRRSV (0.05 μg per well) viruses, or PRRSV purified proteins GP3 (0.25 μg per well), GP4 (0.5 μg per well), GP5 (0.1 μg per well), M (0.1 μg per well) and N (0.2 μg per well). Antigens were bound to 96-well microplates, saturated with 5% bovine serum albumin (BSA) in PBS for 2 h at 37 °C and incubated with serial dilutions of the serum sample in Wash Buffer (0.1% BSA, 0.05% Tween20 in PBS) for 90 min at 37 °C. Microplates were washed three times with Wash Buffer. Bound antibodies were detected by incubation with peroxidase-conjugated protein A (BioRad) diluted 1:10000 in PBS with 0.1% BSA. ELISA was developed with K-Blue TMB substrate (Neogen, Lexington, KY) for 5 min at room temperature. Reactions were stopped with 1.5 M H2SO4, and the absorbance was read at 450 nm. The ELISA values of the sera were expressed as sample to positive ratio [SP-ratio=(OD of sample−OD of negative control)/(OD of positive control−OD of negative control)].
Neutralization assay
Serial dilutions of heat-inactivated serum were incubated for 1 h at 37 °C in the presence of 100 pfus of PRRSV-Olot91 in DMEM containing 5% FCS. The mixtures were added to confluent MARC-145 cells in 24-well plates. After one hour incubation at 37 °C medium was removed and 1 ml of DMEM containing 2% FCS and 0.5% agar was added. After 72 h, cells were fixed with 10% formaldehyde in PBS, stained with a crystal violet solution, and lysis plaques were counted. A positive control serum, obtained from a PRRSV infected pig at 56 dpi led to 98–100% of virus neutralization at a sera dilution of 1:16. In contrast, a non-immune control serum led to a neutralization of 1 to 5% in the same experimental conditions. The neutralization index of each serum sample was expressed relative to the one obtained with the negative control serum in the experiment [Neutralization Index=100%−(pfus serum sample/pfus negative control)×100].
Statistic analysis
Two-tailed, unpaired Student t tests were used to analyze difference in mean values between groups. All results were expressed as means±the standard deviations of the means. Chi-square test was used to analyze statistical significance of differences in percentages of immunized and non-immunized groups. p Values<0.1 were considered significant (Noymer, 2008).
Acknowledgments
We thank H. Nauwynck and M. Vanhee for sharing information about PRRSV epitopes and INGENASA for kindly providing us with anti-M protein monoclonal anitbodies. We also thank M. González, S. Ros and R. Fernández for technical assistance.
This study was supported by Grants from the Ministry of Science and Innovation of Spain (BIO2010-16705), and the European Community׳s Seventh Framework Programme (FP7/2007–2013) under the project PoRRSCon (EC Grant agreement number 245141). M.B. recieved a JAE fellowship from the CSIC-JAE Program (JAEPre_2010) Program co-funded by the European Social Fund. S.Z. was supported by a contract from the PoRRSCon Project (EC Grant agreement number 245141).
References
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- Alejska M., Malinowska N., Urbanowicz A., Figlerowicz M. Two types of non-homologous RNA recombination in brome mosaic virus. Acta Biochim. Pol. 2005;52:833–844. [PubMed] [Google Scholar]
- Almazan F., DeDiego M.L., Galan C., Escors D., Alvarez E., Ortego J., Sola I., Zuñiga S., Alonso S., Moreno J.L., Nogales A., Capiscol C., Enjuanes L. Construction of a SARS-CoV infectious cDNA clone and a replicon to study coronavirus RNA synthesis. J. Virol. 2006;80:10900–10906. doi: 10.1128/JVI.00385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazan F., DeDiego M.L., Sola I., Zuniga S., Nieto-Torres J.L., Marquez-Jurado S., Andres G., Enjuanes L. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. mBio. 2013;4:e00650–00613. doi: 10.1128/mBio.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazan F., Gonzalez J.M., Penzes Z., Izeta A., Calvo E., Plana-Duran J., Enjuanes L. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA. 2000;97:5516–5521. doi: 10.1073/pnas.97.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazan F., Sola I., Zuñiga S., Marquez-Jurado S., Morales L., Becares M., Enjuanes L. Coronavirus reverse genetic systems: Infectious clones and replicons. Virus Res. 2014;189C:262–270. doi: 10.1016/j.virusres.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S., Izeta A., Sola I., Enjuanes L. Transcription regulatory sequences and mRNA expression levels in the coronavirus transmissible gastroenteritis virus. J. Virol. 2002;76:1293–1308. doi: 10.1128/JVI.76.3.1293-1308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S., Sola I., Teifke J., Reimann I., Izeta A., Balach M., Plana-Durán J., Moormann R.J.M., Enjuanes L. In vitro and in vivo expression of foreign genes by transmissible gastroenteritis coronavirus-derived minigenomes. J. Gen. Virol. 2002;83:567–579. doi: 10.1099/0022-1317-83-3-567. [DOI] [PubMed] [Google Scholar]
- Alvarez E., DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Marcos-Villar L., Enjuanes L. The envelope protein of severe acute respiratory syndrome coronavirus interacts with the non-structural protein 3 and is ubiquitinated. Virology. 2010;402:281–291. doi: 10.1016/j.virol.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista E.M., Suarez P., Molitor T.W. T cell responses to the structural polypeptides of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1999;144:117–134. doi: 10.1007/s007050050489. [DOI] [PubMed] [Google Scholar]
- Bentley K., Armesto M., Britton P. Infectious bronchitis virus as a vector for the expression of heterologous genes. PLoS One. 2013;8:e67875. doi: 10.1371/journal.pone.0067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A., Skiadopoulos M.H., Murphy B.R., Collins P.L. Nonsegmented negative-strand viruses as vaccine vectors. J. Virol. 2006;80:10293–10306. doi: 10.1128/JVI.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada-Nova G., Schnitzlein W., Husmann R., Zuckermann F.A. Characterization of the cytokine and maturation responses of pure populations of porcine plasmacytoid dendritic cells to porcine viruses and toll-like receptor agonists. Vet. Immunol. Immunopathol. 2010;135:20–33. doi: 10.1016/j.vetimm.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Thiel V., Siddell S.G., Cavanagh D., Britton P. Reverse genetics system for the avian coronavirus infectious bronchitis virus. J. Virol. 2001;75:12359–12369. doi: 10.1128/JVI.75.24.12359-12369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Casais R., Armesto M., Hodgson T., Izadkhasti S., Davies M., Lin F., Tarpey I., Britton P. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine. 2007;25:5558–5562. doi: 10.1016/j.vaccine.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charerntantanakul W. Porcine reproductive and respiratory syndrome virus vaccines: immunogenicity, efficacy and safety aspects. World J. Virol. 2012;1:23–30. doi: 10.5501/wjv.v1.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costers S., Lefebvre D.J., Van Doorsselaere J., Vanhee M., Delputte P.L., Nauwynck H.J. GP4 of porcine reproductive and respiratory syndrome virus contains a neutralizing epitope that is susceptible to immunoselection in vitro. Arch. Virol. 2010;155:371–378. doi: 10.1007/s00705-009-0582-7. [DOI] [PubMed] [Google Scholar]
- Cruz J.L.G., Sola I., Becares M., Alberca B., Plana J., Enjuanes L., Zuniga S. Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog. 2011;7:e1002090. doi: 10.1371/journal.ppat.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J.L.G., Zuniga S., Becares M., Sola I., Ceriani J.E., Juanola S., Plana J., Enjuanes L. Vectored vaccines to protect against PRRSV. Virus Res. 2010;154:150–160. doi: 10.1016/j.virusres.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Haijema B.J., Boss D., Heuts F.W., Rottier P.J. Coronaviruses as vectors: stability of foreign gene expression. J. Virol. 2005;79:12742–12751. doi: 10.1128/JVI.79.20.12742-12751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Kut E., Sjostrom H., Noren O., Laude H. Determinants essential for the transmissible gastroenteritis virus-receptor interaction reside within a domain of aminopeptidase-N that is distinct from the enzymatic site. J. Virol. 1994;68:5216–5224. doi: 10.1128/jvi.68.8.5216-5224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Baric R.S. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8:270–279. doi: 10.4161/rna.8.2.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokland T. The structural biology of PRRSV. Virus Res. 2010;154:86–97. doi: 10.1016/j.virusres.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Nauwynck H.J., Pensaert M.B. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Microbiol. 1997;56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- Edwards A.M., Arrowsmith C.H., Christendat D., Dharamsi A., Friesen J.D., Greenblatt J.F., Vedadi M. Protein production: feeding the crystallographers and NMR spectroscopists. Nat. Struct. Biol. 2000;7(Suppl.):970–972. doi: 10.1038/80751. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Carrascosa A.L., Moreno M.A., Vinuela E. Titration of African swine fever (ASF) virus. J. Gen. Virol. 1976;32:471–477. doi: 10.1099/0022-1317-32-3-471. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Gorbalenya A.E., de Groot R.J., Cowley J.A., Ziebuhr J., Snijder E.J. The Nidovirales. In: Mahy B.W.J., Van Regenmortel M., Walker P., Majumder-Russell D., editors. Encyclopedia of Virology. third edition. Elsevier Ltd.; Oxford: 2008. pp. 419–430. [Google Scholar]
- Enjuanes L., Sola I., Alonso S., Escors D., Zuniga S. Coronavirus reverse genetics and development of vectors for gene expression. In: Enjuanes L., editor. Current Topics in Microbiology and Immunology. 2005. pp. 161–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Sola I., Zuñiga S., Ortego J. Expression vectors based on coronavirus genomes. In: Hefferon K.L., editor. Virus Expression Vectors. Tranworld Research Network; Kerala: 2007. pp. 147–182. [Google Scholar]
- Figlerowicz M., Alejska M., Kurzynska-Kokorniak A., Figlerowicz M. Genetic variability: the key problem in the prevention and therapy of RNA-based virus infections. Med. Res. Rev. 2003;23:488–518. doi: 10.1002/med.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.E., Zevenhoven-Dobbe J.C., Wills N.M., Go Y.Y., Balasuriya U.B., Atkins J.F., Snijder E.J., Posthuma C.C. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J. Gen. Virol. 2011;92:1097–1106. doi: 10.1099/vir.0.029264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldhof M.F., Vanhee M., Van Breedam W., Van Doorsselaere J., Karniychuk U.U., Nauwynck H.J. Comparison of the efficacy of autogenous inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) vaccines with that of commercial vaccines against homologous and heterologous challenges. BMC Vet. Res. 2012;8:182. doi: 10.1186/1746-6148-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Stoffel W. TMBASE- A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler. 1993;374:166. [Google Scholar]
- Jeong H.J., Song Y.J., Lee S.W., Lee J.B., Park S.Y., Song C.S., Ha G.W., Oh J.S., Oh Y.K., Choi I.S. Comparative measurement of cell-mediated immune responses of swine to the M and N proteins of porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2010;17:503–512. doi: 10.1128/CVI.00365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G., Correa I., Melgosa M.P., Bullido M.J., Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J. Virol. 1986;60:131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.R., Griggs T.F., Gnanandarajah J., Murtaugh M.P. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J. Gen. Virol. 2011;92:1107–1116. doi: 10.1099/vir.0.030213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur V., Elam M.R., Pawlovich T.M., Murtaugh M.P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 1996;77:1271–1276. doi: 10.1099/0022-1317-77-6-1271. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Kwang J., Yoon I.J., Joo H.S., Frey M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Kim W.I., Yoon K.J. Molecular assessment of the role of envelope-associated structural proteins in cross neutralization among different PRRS viruses. Virus Genes. 2008;37:380–391. doi: 10.1007/s11262-008-0278-1. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., Cornelissen L.A., Moormann R.J., Rebel J.M., Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009;27:3704–3718. doi: 10.1016/j.vaccine.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Lai M.M.C. Recombination in large RNA viruses: coronaviruses. Semin. Virol. 1996;7:381–388. doi: 10.1006/smvy.1996.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez O.J., Osorio F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lunney J.K., Benfield D.A., Rowland R.R. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010;154:1–6. doi: 10.1016/j.virusres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. Reverse genetics of the largest RNA viruses. Adv. Virus Res. 1999;53:245–264. doi: 10.1016/S0065-3527(08)60351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Gomez P.A., Zuniga S., Palacio L., Enjuanes L., Sola I. Gene N proximal and distal RNA motifs regulate coronavirus nucleocapsid mRNA transcription. J. Virol. 2011;85:8968–8980. doi: 10.1128/JVI.00869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu E., Diaz I. The challenge of PRRS immunology. Vet. J. 2008;177:345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin A.W., Norman J.O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can. J. Comp. Med. Vet. Sci. 1966;30:190–198. [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Lum M.A. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch. Virol. 1995;140:745–755. doi: 10.1007/BF01309962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh M.P., Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS) Vaccine. 2011;29:8192–8204. doi: 10.1016/j.vaccine.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Murtaugh M.P., Laber J., Elam M., Faaberg K.S., Kapur V. Genetic variation in the PRRS virus. Adv. Exp. Med. Biol. 1998;440:787–794. doi: 10.1007/978-1-4615-5331-1_102. [DOI] [PubMed] [Google Scholar]
- Nogales A., Galan C., Marquez-Jurado S., Garcia-Gallo M., Kremer L., Enjuanes L., Almazan F. Immunogenic characterization and epitope mapping of transmissible gastroenteritis virus RNA dependent RNA polymerase. J. Virol. Methods. 2011;175:7–13. doi: 10.1016/j.jviromet.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noymer A. Encyclopedia of Survey Research Methods. SAGE Publications, Inc.; Thousand Oaks, CA: 2008. Alpha, significance level of test. [Google Scholar]
- Oleksiewicz M.B., Botner A., Normann P. Pocine B-cells recognize epitopes that are conserved between the structural proteins of American- and European-type porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2002;83:1407–1418. doi: 10.1099/0022-1317-83-6-1407. [DOI] [PubMed] [Google Scholar]
- Ortego J., Escors D., Laude H., Enjuanes L. Generation of a replication-competent, propagation-deficient virus vector based on the transmissible gastroenteritis coronavirus genome. J. Virol. 2002;76:11518–11529. doi: 10.1128/JVI.76.22.11518-11529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J., Sola I., Almazan F., Ceriani J.E., Riquelme C., Balasch M., Plana J., Enjuanes L. Transmissible gastroenteritis coronavirus gene 7 is not essential but influences in vivo virus replication and virulence. Virology. 2003;308:13–22. doi: 10.1016/S0042-6822(02)00096-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M., Galeota J.A., Jar A.M., Platt K.B., Osorio F.A., Lopez O.J. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 2002;76:4241–4250. doi: 10.1128/JVI.76.9.4241-4250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page C., Goicochea L., Matthews K., Zhang Y., Klover P., Holtzman M.J., Hennighausen L., Frieman M. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection. J. Virol. 2012;86:13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry D.B., Lunney J., Boyd P., Kuhar D., Blankenship E., Johnson R.K. Differential immunity in pigs with high and low responses to porcine reproductive and respiratory syndrome virus infection. J. Anim. Sci. 2007;85:2075–2092. doi: 10.2527/jas.2006-721. [DOI] [PubMed] [Google Scholar]
- Plagemann P.G. GP5 ectodomain epitope of porcine reproductive and respiratory syndrome virus, strain Lelystad virus. Virus Res. 2004;102:225–230. doi: 10.1016/j.virusres.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Plana-Durán J., Climent I., Sarraseca J., Urniza A., Cortes E., Vela C., Casal J.I. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91. Involvement of ORF3 and ORF5 protein in protection. Virus Genes. 1997;14:19–29. doi: 10.1023/a:1007931322271. [DOI] [PubMed] [Google Scholar]
- Ribes J.M., Ortego J., Ceriani J., Montava R., Enjuanes L., Buesa J. Transmissible gastroenteritis virus (TGEV)-based vectors with engineered murine tropism express the rotavirus VP7 protein and immunize mice against rotavirus. Virology. 2011;410:107–118. doi: 10.1016/j.virol.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J. Mucosal immunity: an overview and studies of enteric and respiratory coronavirus infections in a swine model of enteric disease. Vet. Immunol. Immunopathol. 1996;54:163–169. doi: 10.1016/S0165-2427(96)05702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Wesley R.D. Transmissible gastroenteritis. In: Leman A.D., Straw B.E., Mengeling W.L., D׳Allaire S., Taylor D.J., editors. Diseases of Swine. 7th ed. Wolfe Publishing Ltd.; Ames, Iowa: 1992. pp. 362–386. [Google Scholar]
- Sambrook J., Russell D.W. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Samuelson J., 2011. Bacterial Systems, in Production of Membrane Proteins: Strategies for Expression and Isolation (ed. A. S. Robinson), Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim, Germany, pp. 11–35
- Sanchez C.M., Gebauer F., Suñe C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992;190:92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C.M., Izeta A., Sanchez-Morgado J.M., Alonso S., Sola I., Balasch M., Plana-Duran J., Enjuanes L. Targeted recombination demonstrates that the spike gene of transmissible gastroenteritis coronavirus is a determinant of its enteric tropism and virulence. J. Virol. 1999;73:7607–7618. doi: 10.1128/jvi.73.9.7607-7618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma J.D., Scheen E., Seo S.H., Koval M., Weiss S.R. Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spread in vitro and in the mouse central nervous system. J. Neurovirol. 2002;8:381–391. doi: 10.1080/13550280260422686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Fang S.G., Chen B., Chen G., Tay F.P., Liu D.X. Towards construction of viral vectors based on avian coronavirus infectious bronchitis virus for gene delivery and vaccine development. J. Virol. Methods. 2009;160:48–56. doi: 10.1016/j.jviromet.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola I., Alonso S., Zuniga S., Balasch M., Plana-Duran J., Enjuanes L. Engineering the transmissible gastroenteritis virus genome as an expression vector inducing lactogenic immunity. J. Virol. 2003;77:4357–4369. doi: 10.1128/JVI.77.7.4357-4369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaa B., Sinhadri B.C., Tielesch C., Krause E., Veit M. Signal peptide cleavage from GP5 of PRRSV: a minor fraction of molecules retains the decoy epitope, a presumed molecular cause for viral persistence. PLoS One. 2013;8:e65548. doi: 10.1371/journal.pone.0065548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel V., Herold J., Schelle B., Siddell S. Infectious RNA transcribed in vitro from a cDNA copy of the human coronavirus genome cloned in vaccinia virus. J. Gen. Virol. 2001;82:1273–1281. doi: 10.1099/0022-1317-82-6-1273. [DOI] [PubMed] [Google Scholar]
- Vanhee M., Van Breedam W., Costers S., Geldhof M., Noppe Y., Nauwynck H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine. 2011;29:4794–4804. doi: 10.1016/j.vaccine.2011.04.071. [DOI] [PubMed] [Google Scholar]
- Verheije M.H., Welting T.J., Jansen H.T., Rottier P.J., Meulenberg J.J. Chimeric arteriviruses generated by swapping of the M protein ectodomain rule out a role of this domain in viral targeting. Virology. 2002;303:364–373. doi: 10.1006/viro.2002.1711. [DOI] [PubMed] [Google Scholar]
- Wang Z.D., Ueda S., Uyeda I., Yagihashi H., Sekiguchi H., Tacahashi Y., Sato M., Ohya K., Sugimoto C., Matsumura T. Positional effect of gene insertion on genetic stability of a clover yellow vein virus-based expression vector. J. Gen. Plant. Pathol. 2003;69:327–334. [Google Scholar]
- Wissink E.H., van Wijk H.A., Kroese M.V., Weiland E., Meulenberg J.J., Rottier P.J., van Rijn P.A. The major envelope protein, GP5, of a European porcine reproductive and respiratory syndrome virus contains a neutralization epitope in its N-terminal ectodomain. J. Gen. Virol. 2003;84:1535–1543. doi: 10.1099/vir.0.18957-0. [DOI] [PubMed] [Google Scholar]
- Yount B., Curtis K.M., Fritz E.A., Hensley L.E., Jahrling P.B., Prentice E., Denison M.R., Geisbert T.W., Baric R.S. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA. 2003;100:12995–13000. doi: 10.1073/pnas.1735582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount B., Denison M.R., Weiss S.R., Baric R.S. Systematic assembly of a full-length infectious cDNA of mouse hepatitis virus strain A59. J. Virol. 2002;76:11065–11078. doi: 10.1128/JVI.76.21.11065-11078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]