Abstract
Asthma is a growing epidemic worldwide. Exacerbations of asthma have been associated with bacterial and viral respiratory tract infections and air pollution. We correlated the asthma admission rates with fluctuations in respiratory virus activity and traffic-related air pollution, namely particulate matter with an aerodynamic diameter ≤ 10 μm (PM10), nitrogen dioxide (NO2), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3). A probabilistic risk assessment framework was developed based on a detrended fluctuation analysis to predict future respiratory virus and air pollutant associated asthma incidence. Results indicated a strong association between asthma admission rate and influenza (r = 0.80, p < 0.05) and SO2 level (r = 0.73, p < 0.05) in Taiwan in the period 2001–2008. No significant correlation was found for asthma admission and PM10, O3, NO2, and CO. The proposed fluctuation analysis provides a simple correlation exponent describing the complex interactions of respiratory viruses and air pollutants with asthma. This study revealed that there was a 95% probability of having exceeded 2987 asthma admissions per 100,000 population. It was unlikely (30% probability) that the asthma admission rate exceeded 3492 per 100,000 population. The probability of asthma admission risk can be limited to below 50% by keeping the correlation exponent of influenza to below 0.9. We concluded that fluctuation analysis based risk assessment provides a novel predictor of asthma incidence.
Keywords: Asthma, Air pollution, Respiratory virus activity, Detrended fluctuation analysis, Risk assessment
Research highlights
► Respiratory virus and air pollutant associated asthma incidence was assessed. ► A strong association was found between asthma admission and influenza and SO2. ► Fluctuation analysis provides a risk predictor of asthma incidence.
1. Introduction
Asthma is a growing epidemic worldwide and is the most common chronic respiratory disease in developed countries affecting millions of children and adults (Global Initiative for asthma (GINA) (2009)). Asthma is primarily characterized by airflow obstruction resulting from inflammation and remodeling of small airways. Asthma is a multidimensional disease, with several systemic manifestations, and it is associated with a number of co-morbid diseases (Pearce and Douwes, 2006). It is clear that much more clinical and basic research is needed to understand the complexity of asthma, so that more effective management of asthma and its various co-morbidities are possible in the future.
Asthma has been associated with exposure to traffic-related air pollution and tobacco smoke (Pekkanen et al., 1999, Guo et al., 1999, Lee et al., 2003, King et al., 2004, Huang et al., 2005, Barnett et al., 2005, Chen et al., 2006, Tsai et al., 2006, Gilliland et al., 2006, Suglia et al., 2008, Ko et al., 2007c, McConnell, 2007, Shankardass et al., 2009, Weinmayr et al., 2010). Collectively, these studies found that exposure to traffic-related outdoor air pollutants such as particulate matter (PM) with an aerodynamic diameter ≤ 10 μm (PM10), nitrogen dioxide (NO2), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3) increases the risk of asthma or asthma-like symptoms.
It is generally recognized that air pollution exacerbates asthma in children (Schildcrout et al., 2006). Recent studies have shown that both PM10 and NO2 have been associated with increases in the frequency of asthma symptoms and with lung function decrements in children (Ostro et al., 2001, Schildcrout et al., 2006, Jerrett et al., 2008, Weinmayr et al., 2010). Current evidence indicates that PM10 increases cough, lower respiratory symptoms, and lower peak expiratory flow (PEF) (Ward and Ayres, 2004, Nel, 2005). Recently, Weinmayr et al. (2010), using meta-analysis, provided strong evidence that PM10 may be an aggravating factor of asthma in children.
Papi et al. (2006) indicated that chronic obstructive pulmonary disease (COPD) was significantly exacerbated by respiratory viral infections that caused reduction of forced expiratory volume in 1 s (FEV1) and airway inflammation. On assessing the effects of a winter influenza season on patients with COPD, Gorse et al. (2006) found that laboratory-documented influenza-caused illness was associated significantly with lower FEV1. Ko et al. (2007a) reported that the most prevalent viruses detected during acute exacerbations of COPD in Hong Kong were the influenza A virus and coronavirus. They indicated that among 196 patients with a mean age of 76 years, mean FEV1 was 40% of predicted normal and the FEV1/FVC (forced vital capacity) ratio was reduced to 58% of normal. Singh and Busse, 2006, De Serres et al., 2009 also suggested that the influenza virus frequently causes acute exacerbations of asthma and COPD.
Furthermore, exacerbations of asthma have been associated with bacterial and viral respiratory tract infections as well as exposure to airborne pollutants (Holt et al., 1999, Frey and Suki, 2008). Current studies also indicated that asthma symptoms are exacerbated by air pollutants such as diesel exhaust, PM10, NO2, SO2, and O3 and respiratory virus such as adenovirus, influenza, parainfluenza, and respiratory syncytial virus (RSV) (Jaspers et al., 2005, Murdoch and Jennings, 2009, Wong et al., 2009). Murphy et al. (2000) reported that influenza in patients with asthma can cause acute exacerbations. The strength of the association is still not well defined because of the small number of studies of hospital admissions, and the complexity of time series modeling.
There are few studies that have been able to examine a range of pollutants. When multiple pollutants have been examined, the independent effect of each pollutant is usually addressed in multipollutant models. However, these are sensitive to the assumptions inherent in the time series modeling. This suggests that an approach less sensitive to model assumptions is desirable.
Recently, a method used in statistical physics called detrended fluctuation analysis (DFA) was used to predict the risk of severe asthma exacerbations based on temporal fluctuations in lung airway function (Peng et al., 1993, Peng et al., 2002, Frey et al., 2005, Frey, 2007, Frey and Suki, 2008). Frey et al. (2005) indicated that chronic asthma could be treated as a dynamic disease of the respiratory system.
Frey et al. (2005) employed DFA to predict the risk of airflow obstruction by calculating a conditional probability. They also revealed that DFA could characterize long-range temporal patterns of lung-function. Thus, Frey, 2007, Frey and Suki, 2008 suggested that correlations can be used to assess the risk of future asthma episodes and to improve the assessment of asthma severity for children and adults. On the other hand, DFA has also been applied to investigate the time-scaling properties of air pollution time series including NO2, SO2, O3, and PM10 (Varotsos et al., 2005, Shi et al., 2008).
DFA has been applied to physiology, air pollution, and atmospherics (Varotsos et al., 2005, Shi et al., 2008). The novel methodology of a fluctuation analysis such as DFA can detect the intrinsic self-similarity and unnoticed trends embedded in a seemingly non-stationary time series. Frey and Suki (2008) suggested that a fluctuation analysis-based risk assessment approach can improve predictions for chronic diseases. Thus, we attempted to link disease incidence and risk factors.
The risk of exacerbations risk of asthma by respiratory virus activity and environmental stimuli is challenging to calculate. Uncertainties can be quantified by constraining risk-based predictive model parameters to reproduce a temporal history of lung function fluctuations, asthma severity and stability, and fluctuating environmental stimuli (e.g., allergens, infections, and pollutants) (Frey and Suki, 2008).
Little research has been done to link lung function to exacerbations risk of chronic asthma by associating respiratory virus activity and environmental stimuli. From the point of view of the health surveillance of asthma, we hope that the proposed fluctuation analysis-based risk assessment scheme will enable early identification of risk factors, and that it will complement environmental monitoring of hazards and risk assessment.
The purpose of this study was twofold: (1) to correlate the asthma admission rate with fluctuations in respiratory virus activity and traffic-related air pollution factors and (2) to provide a probabilistic risk assessment framework from a DFA-based predictive model to predict future respiratory virus and air pollutant associated asthma incidence. This study used DFA to quantify virus activity and environmental pollution data and to correlate these with incidence of asthma. We conducted a risk-based study to assess whether respiratory viruses and air pollution caused exacerbations of asthma cases.
2. Materials and methods
2.1. Study data
Annual virological surveillance data in Taiwan were obtained from the Epidemiologic Bulletin reported by the Center for Disease Control, Taiwan during the period 2001–2008 (CDC, Taiwan). The daily-based positive rates of respiratory viral isolations were obtained from a laboratory-based surveillance network, consisting of 10 clinical virology contract laboratories distributed around Taiwan. The number of laboratory-confirmed respiratory infections was obtained using positive viral culture or direct immunofluorescence. A rate was derived from the percentage of respiratory virus positives divided by the total number of respiratory infection specimens.
Air pollution data in Taiwan were obtained from the Taiwan Air Quality Monitoring Network in the period 2001–2008. More than seventy monitoring stations have been established by the Taiwan Environmental Protection Administration (EPA, Taiwan). We selected major air monitoring stations in two urban cities: Taipei (five stations) and Kaohsiung (four stations). Daily readings of the air pollutants PM10, NO2, SO2, CO, and O3 were gathered. We also used general monitoring stations located at local schools and government organizations, representing common air pollutant variations and densely populated areas.
The National Health Insurance (NHI) Program, which provides a compulsory universal health insurance database, covers most of the population. From the database, we selected patients on the basis of the International Classification of Disease, Clinical Modification (ICD-9-CM) code (DOH, Taiwan). Therefore, inpatient claims data for all patients admitted in Taiwan during January 2001 to December 2008 with a principal diagnosis of asthma or asthmatic bronchitis (ICD-9-CM code 493) were extracted from the NHI Research Database. Admissions were categorized into five age groups: 0–4, 5–14, 15–44, 45–64, and ≥ 65 years (Chen et al., 2006). The data were recorded as number of outpatients and hospitalizations of asthma per year, and then converted into total asthma admission. The annual number of cases was divided by the year-end population to obtain an asthma admission rate per 100,000 population. Annual population data for the period 2001–2008 was released by the Population Affairs Administration, Ministry of Interior, Taiwan.
2.2. Fluctuation analysis
To detect the long-range correlations embedded in a nonstationary time series of respiratory virus activity and environmental stimuli, this study applied a DFA for minimizing the effect of nonstationary trends. DFA has been applied successfully to detect long-range correlations in highly complex heart beat time series and other physiological signals (Peng et al., 1993, Peng et al., 1995, Peng et al., 2002). The detailed computational algorithm of DFA can be found elsewhere (Peng et al., 1993, Peng et al., 1995, Peng et al., 2002).
Briefly, the time series was first integrated and then divided into nonoverlapping windows of size n. The local trend in each window was removed by fitting and subtracting a regression line from the integrated data. The root-mean-square values of the detrended signal were calculated for a given window length n to yield the detrended fluctuation function (DFF, F(n)). This calculation was then repeated for increasing n, and logF(n) was plotted against logn. Typically, a measure of the fluctuations of F(n) increases with an increase in n. A linear relationship between logF(n) and logn indicates the presence of scaling, which can be characterized by the slope α of the fitted regression line.
Mathematically, the DFF F(n) can be described by a power law functional form as:
| (1) |
in that DFF F(n) can be characterized by a root-mean-square fluctuation of the integrated and detrended time series as:
| (2) |
Where y(k) = ∑ i = 1 k[v(i) − v ave] is the integrated time series data, v(i) is the data value of respiratory virus activity and environmental stimuli at time i, v ave is the average data value, n is the window size of the integrated time series, y n(k) is the fitted least-squared line in each window size n representing the trend in that window, N is the number of measurements, and y(k) − y n(k) represents the detrended integrated time series. The exponent α characterizes the correlation properties of the entire range of the time series of data. The properties of α indicate that there is no correlation in the time series at α = 0.5, whereas for increasingly higher values of α it shows increasingly stronger long-range correlations (Peng et al., 2002).
2.3. Probabilistic risk model
In 1910, Hill developed a model to describe the general dose–response relationship (Hill, 1910). Thus, this study employed the Hill model commonly used in pharmacodynamic modeling to describe the effects of environmental stimuli on influenza virus activity. Thus, in this study, a biologically based empirical four-parameter Hill equation was used to associate the relationship between respiratory virus activity and environmental stimuli represented by the DFA-derived exponent α,
| (3) |
where α v is the DFA-derived exponent based on the time series data of respiratory virus activity, y min and y max are the minimum and maximum values of α v, α e is the DFA-derived exponent based on the time series data of environmental stimuli, a is the fitted coefficient, and the exponent n H is the fitted Hill coefficient. Alternatively, Eq. (3) can also be used to represent a dose–response model. Therefore, the cumulative distribution function (cdf) of the predicted dose–response model in Eq. (3) describing the relationship of respiratory virus activity (α v) with given environmental stimuli (α e) can be expressed as the conditional cdf of P(α v|α e).
Risk characterization is the phase of risk assessment where the results of the virus and environmental stimuli are associated with a quantitative effect on asthma severity. This provides the risk estimates of asthma episodes measured by fluctuations of respiratory virus activity and environmental factors. The risk at a specific levels of environmental stimuli can be calculated as the probability density functions (pdfs) of DFA-derived α e multiplied by the conditional probability P(α v|α e).
Therefore, a joint probability function (JPF) can be used to calculate the risk probability and can be expressed as,
| (4) |
Where P(R av) represents the respiratory virus exponent α v-based risk estimate based on the association of environmental stimuli and respiratory virus activity given a known correlation property of day-to-day air pollutant data. A risk profile was generated from the cumulative distribution of simulation outcomes. Each point on the risk curve represents both the probability that the chronic respiratory disease will exacerbate and also the frequency by which that level of effect would be exceeded. The x-axis of the risk curve can be interpreted as a magnitude of effect (i.e., respiratory virus activity-associated respiratory disease), and the y-axis can be interpreted as the probability that an exacerbation effect of at least that magnitude will occur. To assess the risk of asthma incidence, we corrected DFA-derived α v from respiratory virus activity and asthma admission rate to construct a mechanistic relationship: asthma admission rate = f(α v). Finally, the risk probability of respiratory virus and environmental stimuli on asthma admission rate can then be established.
2.4. Uncertainty and data analysis
Optimal statistical models were selected on the basis of least squared criteria from a set of generalized linear and nonlinear autoregression models provided by TableCurve 2D package (AISN Software Inc., Mapleton, OR, USA) fitted to the study data. A value of p < 0.05 was judged significant. To quantify the uncertainty and its impact on the estimation of expected risk, a Monte Carlo (MC) technique was implemented. The MC simulation algorithm depends on the statistics of input parameter and all possible distributions of parameters can be simulated considering random sampling of probability outcomes. The Monte Carlo simulation was performed with 10,000 iterations to generate 2.5- and 97.5-percentiles as the 95% CI for all fitted models. The Crystal Ball® software (Version 2000.2, Decisionerring, Inc., Denver, Colorado, USA) was employed to implement the MC simulation. Fig. 1 illustrates the computational algorithm implemented in this study.
Fig. 1.
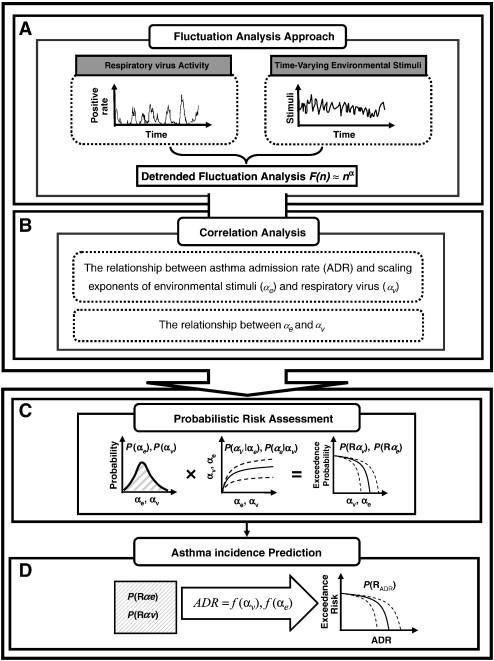
Schematic showing the overall framework for respiratory virus and air pollution associated asthma incidence.
3. Results
3.1. DFA of time series data
Fig. 2 demonstrates the time series of daily air pollution data for PM10, CO, NO2, SO2, and O3 in Taipei and Kaohsiung located in northern and southern Taiwan, respectively, from 2001 to 2008. Fig. 3 illustrates the daily positive rate data of total respiratory viruses (including adenovirus, parainfluenza, influenza, and RSV) and influenza in the Taiwan region from 2001 to 2008. We found that Kaohsiung had relative higher annual mean values (8 years) of PM10 (~ 76 μg m− 3), SO2 (~ 8 ppb), and O3 (~ 31 ppb) levels than Taipei (~ 50 μg m− 3 for PM10, ~ 4 ppb for SO2, and ~ 24 ppb for O3), whereas there was no significant difference in CO (~ 0.7 ppm) and NO2 (~ 24 ppb) levels (Table 1 , Fig. 2). The 8-year annual-average positive rates of total virus and influenza were estimated to be 13.55 ± 8.73% (mean ± sd) and 6.50 ± 7.22%, respectively (Table 1, Fig. 3). Generally, males had higher annual-average asthma admissions (ranging from 3406 to 3608 per 100,000) than females (ranging from 3186 to 3506 per 100,000) (Fig. 4A). Children, particularly children aged 0–4 years had the highest asthma admissions during the study period (ranging from 9928 to 11,600 per 100,000) (Fig. 4B).
Fig. 2.
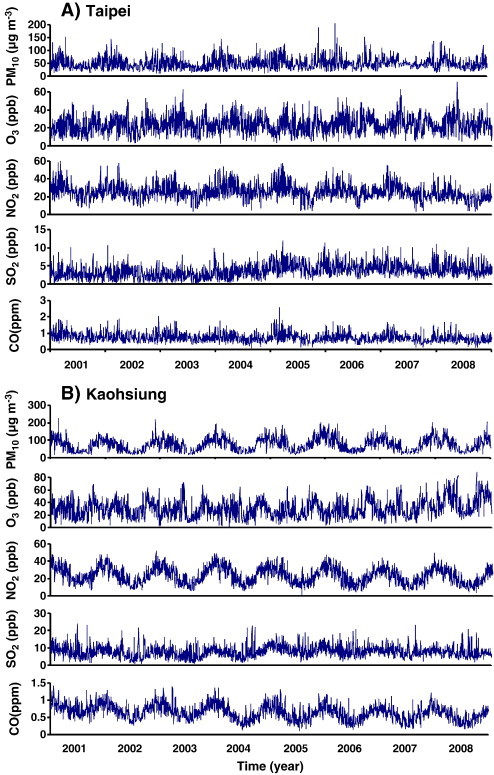
Time series of daily air pollution data including PM10, O3, NO2, SO2, and CO in cities of (A) Taipei and (B) Kaohsiung during 2001–2008.
Fig. 3.
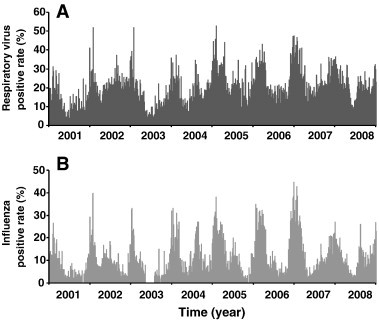
Daily positive rate data of (A) total respiratory viruses (including adenovirus, parainfluenza, influenza, and RSV) and (B) influenza in Taiwan region during 2001–2008.
Table 1.
Annual-average air pollution and respiratory virus (positive rate, %) data (mean ± sd) and DFA-derived exponents of environmental stimuli (αe) and total virus (including adenovirus, parainfluenza, influenza, and RSV) (αv) and influenza (αi) during 2001–2008.
| 2000–2001 | 2001–2002 | 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | Average | COVa | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Air pollution | |||||||||||
| PM10 | Tb | 46.47 ± 21.15 | 47.48 ± 21.87 | 43.50 ± 20.14 | 53.21 ± 20.79 | 54.36 ± 2.77 | 51.59 ± 23.41 | 52.57 ± 14.49 | 50.36 ± 21.38 | 49.95 ± 21.46 | 0.43 |
| (μg m− 3) | Kc | 73.95 ± 36.45 | 64.06 ± 30.17 | 72.19 ± 33.49 | 79.52 ± 36.81 | 83.89 ± 38.82 | 78.53 ± 36.56 | 75.60 ± 36.85 | 77.26 ± 36.30 | 75.63 ± 36.15 | 0.48 |
| CO | T | 0.86 ± 0.08 | 0.78 ± 0.27 | 0.77 ± 0.25 | 0.70 ± 0.24 | 0.77 ± 0.32 | 0.72 ± 0.24 | 0.72 ± 0.25 | 0.66 ± 0.23 | 0.75 ± 0.27 | 0.39 |
| (ppm) | K | 0.79 ± 0.18 | 0.70 ± 0.20 | 0.72 ± 0.21 | 0.62 ± 0.24 | 0.61 ± 0.22 | 0.59 ± 0.21 | 0.59 ± 0.20 | 0.54 ± 0.18 | 0.64 ± 0.22 | 0.46 |
| NO2 | T | 26.63 ± 69.70 | 26.09 ± 8.13 | 24.47 ± 7.77 | 27.56 ± 8.30 | 23.37 ± 9.63 | 25.98 ± 7.44 | 25.90 ± 8.23 | 20.19 ± 6.38 | 25.40 ± 8.34 | 0.33 |
| (ppb) | K | 26.67 ± 9.18 | 23.57 ± 9.48 | 24.12 ± 9.93 | 25.68 ± 9.62 | 23.55 ± 9.13 | 22.57 ± 9.10 | 22.47 ± 8.77 | 21.28 ± 8.47 | 23.74 ± 9.36 | 0.39 |
| SO2 | T | 2.87 ± 2.29 | 2.85 ± 1.59 | 2.62 ± 1.46 | 3.46 ± 1.61 | 4.83 ± 1.81 | 4.57 ± 1.56 | 4.34 ± 1.76 | 4.07 ± 1.62 | 3.70 ± 1.81 | 0.49 |
| (ppb) | K | 8.41 ± 3.40 | 6.97 ± 3.34 | 6.69 ± 3.16 | 7.66 ± 3.15 | 9.55 ± 3.10 | 8.53 ± 2.96 | 8.10 ± 2.75 | 7.44 ± 2.74 | 7.92 ± 3.20 | 0.40 |
| O3 | T | 21.80 ± 8.71 | 23.02 ± 9.14 | 24.40 ± 9.59 | 25.28 ± 8.70 | 22.15 ± 7.72 | 25.08 ± 8.80 | 24.80 ± 9.14 | 24.62 ± 9.21 | 23.89 ± 9.21 | 0.36 |
| (ppb) | K | 24.49 ± 12.67 | 29.72 ± 12.62 | 27.74 ± 12.97 | 28.29 ± 12.32 | 26.90 ± 12.42 | 28.60 ± 12.89 | 30.73 ± 12.96 | 42.98 ± 16.39 | 30.56 ± 14.06 | 0.34 |
| αe | 0.77 ± 0.12 | 0.74 ± 0.10 | 0.77 ± 0.11 | 0.76 ± 0.12 | 0.81 ± 0.11 | 0.74 ± 0.17 | 0.79 ± 0.07 | 0.74 ± 0.15 | |||
| Respiratory virus (positive rate,%) | |||||||||||
| Total viruses | 7.21 ± 6.15 | 14.82 ± 6.72 | 8.50 ± 7.94 | 11.72 ± 7.03 | 16.75 ± 9.02 | 16.38 ± 9.94 | 18.44 ± 8.74 | 14.62 ± 6.72 | 13.55 ± 8.73 | 0.64 | |
| Influenza | 3.22 ± 4.72 | 5.70 ± 5.31 | 3.78 ± 5.83 | 7.05 ± 6.33 | 7.94 ± 7.95 | 9.25 ± 9.80 | 8.77 ± 8.57 | 6.24 ± 5.12 | 6.50 ± 7.22 | 1.11 | |
| αv | 0.91 | 0.94 | 0.84 | 0.92 | 0.91 | 0.94 | 0.83 | 0.94 | |||
| αi | 0.95 | 0.91 | 0.88 | 0.92 | 0.97 | 0.86 | 0.95 | 0.86 | |||
Coefficient of variation (COV) = standard deviation/mean.
T = Taipei.
K = Kaohsiung.
Fig. 4.
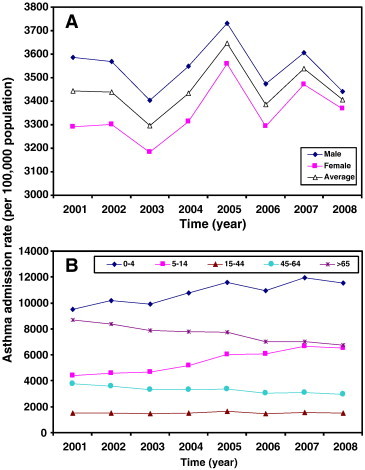
Time-course of 8-year annual mean values of (A) gender- and (B) age-specific asthma admission rate during 2001–2008.
The variability (coefficient of variation, COV) of the time series of air pollution data ranged from 0.33 to 0.49, indicating that air pollution data dispersion existed during the period 2001–2008 (Table 1). Variability was also found in respiratory viruses data with higher dispersion than the time-series of air pollution (Table 1). To assess the variability of respiratory viruses and environmental stimuli, the detrended fluctuation function F(n) was calculated from time series data as shown in Fig. 2, Fig. 3. The fitted power law function exponents for environmental stimuli (α e) were distributed among air pollution data and ranged between 0.4 and 0.9 from 2001 to 2008 (Fig. 5A).
Fig. 5.
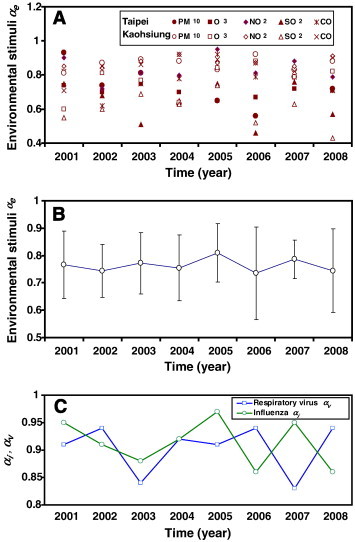
(A, B) Annual distribution of correlation exponents for environmental stimuli αe with the mean and standard deviation. (C) Time courses of DFA-derived exponents for respiratory virus αv and influenza αi, respectively.
Our results show that the annual-average DFA-derived exponents for environmental stimuli (α e), total viruses (α v), and influenza (α i) ranged from 0.74 to 0.81, 0.83 to 0.94, and 0.86 to 0.95, respectively (Table 1). The results show that there are strong correlations between past and future air pollution and respiratory virus data, indicating that past data have significant effects on the current and future data. To demonstrate the potential variability of air pollution, the time course of air pollution-specific α e was assembled into an annual-average value for the period 2001–2008 (Fig. 5B). The dimensionless exponents can be considered a normalizing process that provides us a unique chance to combine all pollutants into one correlation parameter. Similarly, the time courses of DFA-derived respiratory virus α v and influenza α i are also shown for the period 2001–2008 (Fig. 5C).
3.2. Risk estimate of asthma admission rate
To investigate the potential impact of environmental stimuli and respiratory virus fluctuations on the asthma admission rate (Fig. 6A), a linear regression model was used to correlate DFA-derived exponents and asthma admission rates during the period 2001–2008 (Fig. 6B, C and D). Note, however, that the lowest and highest asthma admission rates occurred in 2003 and 2005 with values of 3298 and 3648 per 100,000 population, respectively. Other years averaged 3430 per 100,000 population (Fig. 6A). Our results indicate that influenza α i correlates more significantly with asthma admission rates (y = 1960x + 1661.5, r 2 = 0.64, p < 0.05) than α e (y = 2930.3x + 1211.9, r 2 = 0.49, p = 0.05) and α v (y = 985.9x + 2562.7, r 2 = 0.18, p = 0.05) (Fig. 6B, C and D).
Fig. 6.
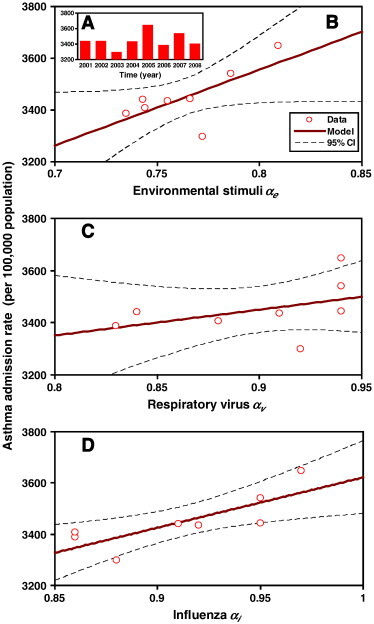
(A) Annual-average asthma admission rate during 2001–2008. The correlations between asthma admission rate and (B) environmental stimuli αe, (C) respiratory virus αv and (D) influenza αi, respectively.
Furthermore, to investigate the contribution of specific air pollutants and influenza to asthma admission rate, a sensitivity analysis based on the Pearson correlation was performed. Our results indicate that SO2 (r = 0.73, p < 0.05) and influenza (r = 0.799, p < 0.05) contributed significantly to asthma incidence (Table 2 ). NO2 had a high degree of correlation yet was not significantly correlated with asthma admission (r = 0.56, p > 0.05). There were strong correlations of DFA-derived α values between SO2 and influenza (r = 0.95, p < 0.01) and CO and NO2 (r = 0.74, p < 0.05), respectively (Table 2). The O3 level was negatively correlated with the mainly traffic-related pollutants and influenza virus but positively correlated with CO.
Table 2.
Sensitivity analysis showing the relative contributions (represented as Pearson correlation coefficient) of influenza and air pollutants to asthma incidence.
| Influenza | PM10 | O3 | NO2 | SO2 | CO | Asthma admission rate | |
|---|---|---|---|---|---|---|---|
| Influenza | 1.000 | 0.050 | −0.487 | 0.535 | 0.947⁎⁎ | 0.223 | 0.799⁎ |
| PM10 | 1.000 | −0.210 | −0.164 | 0.045 | −0.706 | −0.408 | |
| O3 | 1.000 | −0.308 | −0.360 | 0.011 | −0.086 | ||
| NO2 | 1.000 | 0.420 | 0.744⁎ | 0.557 | |||
| SO2 | 1.000 | 0.236 | 0.730⁎ | ||||
| CO | 1.000 | 0.488 | |||||
| Asthma | 1.000 |
p < 0.05.
p < 0.01.
To establish a conditional probability by which the DFA-derived correlation exponent (α) can be used as a predictor of the potential impact of influenza- and environmental stimuli on asthma, the Hill model was used to construct the relationships between environmental stimuli α e and influenza α i. Our results indicate that the α I and α e relationship is well described by a Hill-based regression equation y = a/(1 + (b/x)nH) with fitted parameters a = 1.0 and b = 0.5 and a fitted Hill coefficient n H = 5.56 (r 2 = 0.95, p < 0.001) (Fig. 7A). Given the constructed Hill-based dose–response profile (Fig. 7A) and a fitted ensemble distribution of environmental stimuli α e (the distribution can be optimally fitted by a lognormal distribution with a geometric mean of 0.73 and a geometric standard deviation of 1.19, Fig. 7B), influenza α i-based risk estimates can then be determined by the proposed risk model shown in Eq. (4) (Fig. 7C).
Fig. 7.
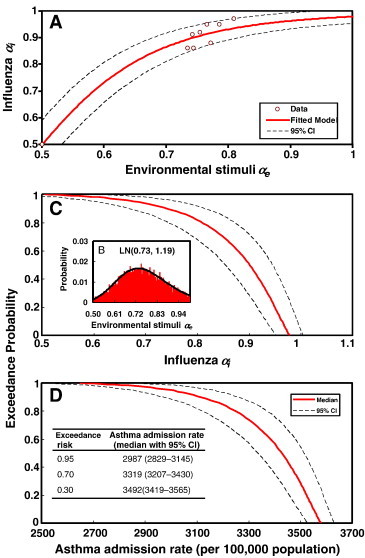
(A) Hill-based regression model to construct the relationship between influenza αi and environmental stimuli αe. (B) The lognormal (LN) fitted ensemble distribution of environmental stimuli αe with a geometric mean 0.73 and a geometric standard deviation 1.19. (C) The exceedance probability of influenza αi based on representative probability density function of environmental stimuli αe. (D) The risk profile of asthma admission rate converting from exceedance probability profile of influenza αi. Inset shows the most likely (95%), likely (70%), and unlikely (30%) probabilities of asthma admission risks.
To demonstrate the potential impact of influenza and environmental stimulus triggers on risk estimates of asthma admission rate, we converted the influenza α i-based risk profile (Fig. 7C) into an asthma admission rate-based risk profile (Fig. 7D). This was based on a linear regression model describing the relationship between asthma admission and influenza α i (Fig. 6D). We found that a 95% probability of asthma admission rate was exceeded 2987 (95% CI 2829–3145) per 100,000 population. It was likely (70% probability) to have exceeded 3319 (95% CI 3207–3430) per 100,000 population, yet it was unlikely (30% probability) to have exceeded 3492 (95% CI 3419–3565) per 100,000 population (Fig. 7D).
4. Discussion
4.1. Association between air pollutants, influenza virus and asthma
Our study found a strong association among influenza (r = 0.80, p < 0.05) and SO2 level (r = 0.73, p < 0.05) and asthma admission rate based on the data from 2001 to 2008. SO2 is a respiratory tract irritant that has been shown to cause acute respiratory health effects including coughing, decreased lung function in controlled human exposure, and significant airway injury at high concentration (Witek et al., 1985, Chen et al., 2007). Kim et al. (1996) indicated that influenza virus and SO2 had significant impacts on the occurrence of pneumococcal disease in Houston. Chen et al. (2006) found that seasonality in adult asthma admissions in Taiwan from 1998 to 2001 were significantly correlated with high levels of O3, CO, SO2, NO2, and PM10. Ko et al. (2007b) reported that SO2, NO2, and O3 had a greater effect than PM10 and PM2.5 on COPD in Hong Kong.
This study indicated that the correlation exponents of influenza and SO2 have high correlation (r = 0.947). There may be collinearity between the two datasets. Compared with other air pollutants, the embedded trends in the time series of daily records have low correlations with influenza. This reveals that intrinsic variations among time-varying risk factors are completely different.
In this study, no significant correlation was found between asthma admission and PM10, O3, NO2, and CO. Generally, NO2 effects were often the strongest and appeared to be generally independent of the impact of other pollutants on asthma or asthma-like symptoms (Guo et al., 1999, Barnett et al., 2005, Gauderman et al., 2005, Tsai et al., 2006, Jerrett et al., 2008, Weinmayr et al., 2010). However, differences have been observed among cities with different climates during short- or long-term exposures (Bouhuys et al., 1978, Oftedal et al., 2009). Bouhuys et al. (1978) found that, in US towns, high concentrations of SO2, NO2, and total suspended particulates were not associated with lung function loss when sex, race, age, height, and weight were adequately taken into account. Chen et al. (2007) observed no clear dose–response fashion relationships between health risk response and increasing amounts of air pollutants such as NO2, SO2, and CO. Oftedal et al. (2009) reported that no positive associations were found between long-term NO2 exposures and asthma onset or respiratory symptoms in 9–10-year old children in Oslo.
It has been suggested that airborne viral infections are a major risk factor for exacerbations of chronic asthma and COPD (Mallia and Johnston, 2006, Varkey and Varkey, 2008). In addition, airborne virus infections cause virus-induced damage and innate inflammation (Cameron et al., 2006, Proud and Chow, 2006). Mallia and Johnston (2006) indicated exacerbations were associated with virus-induced airway diseases. In asthmatic subjects, virus isolation studies have found that influenza infections have been detected in most cases of hospitalization, resulting in near-fatal and acute exacerbations (Teichtahl et al., 1997, Tan et al., 2003).
4.2. DFA-based risk model
Our study showed that fluctuation analysis can provide a simple parameter, α, that measures the complex correlation of day-to-day data of respiratory viruses and air pollutants. The present study provided further evidence that influenza virus is related to asthma admission rate. Currently, most epidemiological studies use statistical analyses such as the auto-regressive integrated moving average (ARIMA), to correlate environmental triggers and allergic asthma (Chen et al. 2006). This general methodology can only point to the trigger-specific risk factors associated with disease incidence. In this study, we constructed a fluctuation analysis-based probabilistic risk assessment framework that can completely describe the multiple triggers related to asthma incidence.
By focusing on long-range correlations in time-series fluctuations of air pollution and respiratory virus data, and their influence on asthma incidence, this study built an integrated risk assessment approach. Using a DFA-based risk assessment approach, we constructed the conditional probability describing a relationship between influenza and environmental stimuli. Thereafter, the environmental stimuli-associated and influenza-associated asthma incidence risks could be estimated. We used the pharmacodynamic Hill equation to construct the relationship between influenza virus and air pollutants because it is a biologically based dose–response model. Compared with linear regression, the nonlinear model can describe the relationship between α e and α i. We showed that the conditional probability functions of P(asthma admission rate|α) are robust indicators of the probability that asthma incidence will occur for environmental stimuli-associated and influenza-associated asthmatics.
A multidimensional approach that includes a combination of several clinical and physiological parameters such as symptoms, behavioral factors, lung function, and inflammatory markers is useful for describing future asthma events (Frey and Suki, 2008). Frey and Suki (2008) suggested that the fluctuation analysis approach can be used to identify the dynamic patterns of clinical symptoms of complex chronic diseases. To improve risk assessment of asthma severity, the fluctuation analysis approach can be applied to analyze the long-term temporal fluctuations of clinical and physiological markers.
4.3. Limitations of this study
In asthma admission, this study used all patient admission data in the year. The study data included all possible reason for asthma admission. Although most asthma cases are caused by air pollution and virus infection, they are also major risk factors for asthma exacerbation and incidence (Chen et al., 2006, Xirsagar et al., 2006). Secondly, the reported data that we used were combined arbitrarily. They were used here to analyze observational data which have not been analyzed before. Finally, determining the probabilistic of future asthma episodes caused by influenza virus infection and air pollutants is challenging. It requires a synthesis of uncertainties along the cause-effect chain from viral infection and specific air pollutants to lung function variations. Velthove et al. (2010) further indicated that using hospital admissions as a measurement to estimate asthma incidence might lead to an underestimation of disease exacerbations because of a trend towards outpatient care. Therefore, they suggested that other markers of exacerbations should be taken into account.
In view of the current knowledge of multiple domains of asthma and asthma control, no single measurement can adequately assess asthma control (Reddel et al., 2009). Therefore, uncertainties in future predictions of environmental stimuli-associated and influenza-associated asthma incidence can be quantified by constraining the present risk-based predictive model parameters to reproduce the temporal history of lung function fluctuations, asthma severity and stability, and fluctuating environmental stimuli (e.g., allergens, infections, and pollutants) (Frey and Suki, 2008). In addition, the result shows that asthma incidences were most likely in children aged 0–4 years. Therefore, age-specific asthma incidence should be considered in future studies. We think there is room for further improvement, especially by including experimental analysis of airborne virus infection on respiratory symptoms (Holt et al., 1999), the effect of respiratory virus infection on lung function in asthmatics (Frey and Suki, 2008), a DFA of airborne pollutants with climatic factors (Chen et al., 2006, Wong et al., 2009, Murdoch and Jennings, 2009), and socioeconomic status (Shankardass et al., 2009).
In conclusion, we emulated the detrended fluctuation approach by combining respiratory virus activity with air pollution data, and compared these to asthma admission data for the period 2001–2008. We also found that the probability of asthma admission can be limited to below 50% by keeping the correlation exponent of influenza (α i) below ~ 0.90. If the acceptable probability of exceedance were 50%, this would limit the annual asthma admission rate to 3425 per 100,000 populations or lower. We concluded that fluctuation analysis-based risk assessment provides a novel predictor for assessing the potential incidence of asthma.
References
- Barnett A.G., Williams G.M., Schwartz J., Neller A.H., Best T.L., Petroeschevsky A.L. Air pollution and child respiratory health — a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171:1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- Bouhuys A., Beck G.J., Schoenberg J.B. Do present levels of air-pollution outdoors affect respiratory health? Nature. 1978;276:466–471. doi: 10.1038/276466a0. [DOI] [PubMed] [Google Scholar]
- Cameron R.J., de Wit D., Welsh T.N., Ferguson J., Grissell T.V., Rye P.J. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32:1022–1029. doi: 10.1007/s00134-006-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.H., Xirasagar S., Lin H.C. Seasonality in adult asthma admissions, air pollutant levels, and climate: a population-based study. J Asthma. 2006;43:287–292. doi: 10.1080/02770900600622935. [DOI] [PubMed] [Google Scholar]
- Chen T.M., Gokhale J., Shofer S., Kuschner W.G. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci. 2007;333:249–256. doi: 10.1097/MAJ.0b013e31803b900f. [DOI] [PubMed] [Google Scholar]
- De Serres G., Lampron N., La Forge J., Rouleau I., Bourbeau J., Weiss K. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46:129–133. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U. Predicting asthma control and exacerbations: chronic asthma as a complex dynamic model. Curr Opin Allergy Clin Immunol. 2007;7:223–230. doi: 10.1097/ACI.0b013e32810fd771. [DOI] [PubMed] [Google Scholar]
- Frey U., Brodbeck T., Majumdar A., Taylor D.R., Town G.I., Silverman M. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nature. 2005;438:667–670. doi: 10.1038/nature04176. [DOI] [PubMed] [Google Scholar]
- Frey U., Suki B. Complexity of chronic asthma and chronic obstructive pulmonary disease: implications for risk assessment, and disease progression and control. Lancet. 2008;372:1088–1099. doi: 10.1016/S0140-6736(08)61450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman W.J., Avol E., Lurmann F., Kuenzli N., Gilliland F., Peters J. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology. 2005;16:737–743. doi: 10.1097/01.ede.0000181308.51440.75. [DOI] [PubMed] [Google Scholar]
- Gilliland F.D., Islam T., Berhane K., Gauderman W.J., McConnell R., Avol E. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174:1092–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for asthma (GINA) available from: http://www.ginasthma.com (2009).
- Gorse G.J., O'Connor T.Z., Young S.L., Habib M.P., Wittes J., Neuzil K.M. Impact of a winter respiratory virus season on patients with COPD and association with influenza vaccination. Chest. 2006;130:1109–1116. doi: 10.1378/chest.130.4.1109. [DOI] [PubMed] [Google Scholar]
- Guo Y.L.L., Lin Y.C., Sung F.C., Huang S.L., Ko Y.C., Lai J.S. Climate, traffic-related air pollutants, and asthma prevalence in middle-school children in Taiwan. Environ Health Perspect. 1999;107:1001–1006. doi: 10.1289/ehp.991071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 1910;40:i–vii. [Google Scholar]
- Holt P.G., Macaubas C., Stumbles P.A., Sly P.D. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- Huang Y., Dominici F., Bell M.L. Bayesian hierarchical distributed lag models for summer ozone exposure and cardio-respiratory mortality. Environmetrics. 2005;16:547–562. doi: 10.1002/env.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers I., Ciencewicki J.M., Zhang W.L., Brighton L.E., Carson J.L., Beck M.A. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85:990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- Jerrett M., Shankardas K., Berhane K., Gauderman W.J., Kunzli N., Avol E. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–1438. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.E., Musher D.M., Glezen W.P., Rodriguez-Barradas M.C., Nahm W.K. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clin Infect Dis. 1996;22:100–106. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- King M.E., Mannino D.M., Holguin F. Risk factors for asthma incidence - a review of recent prospective evidence. Panminerva Med. 2004;46:97–110. [PubMed] [Google Scholar]
- Ko F.W.S., Chan P.K.S., Chan M.C.H., To K.W., Ng S.S.S., Chau S.S.L. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132:900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F.W.S., Tam W., Wong T.W., Chan D.P.S., Tung A.H., Lai C.K.W. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62:780–785. doi: 10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F.W.S., Tam W., Wong T.W., Lai C.K.W., Wong G.W.K., Leung T.F. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin Exp Allergy. 2007;37:1312–1319. doi: 10.1111/j.1365-2222.2007.02791.x. [DOI] [PubMed] [Google Scholar]
- Lee Y.L., Lin Y.C., Hsiue T.R., Hwang B.F., Guo Y.L.L. Indoor and outdoor environmental exposures, parental atopy, and physician-diagnosed asthma in Taiwanese schoolchildren. Pediatrics. 2003;112:E389–E395. doi: 10.1542/peds.112.5.e389. [DOI] [PubMed] [Google Scholar]
- Mallia P., Johnston S.L. How virus infections cause exacerbation of airway diseases. Chest. 2006;130:1203–1210. doi: 10.1378/chest.130.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. Childhood incident asthma and traffic-related air pollution in a longitudinal cohort study. Epidemiology. 2007;5:S187. [Google Scholar]
- Murdoch D.R., Jennings L.C. Association of respiratory virus activity and environmental factors with the incidence of invasive pneumococcal disease. J Infect. 2009;58:37–46. doi: 10.1016/j.jinf.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Murphy K.R., Eivindson A., Pauksens K., Stein W.J., Tellier G., Watts R. Efficacy and safety of inhaled zanamivir for the treatment of influenza in patients with asthma or chronic obstructive pulmonary disease — a double-blind, randomised, placebo-controlled, multicentre study. Clin Drug Invest. 2000;20:337–349. [Google Scholar]
- Nel A. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- Oftedal B., Nystad W., Brunekreef B., Nafstad P. Long-term traffic-related exposures and asthma onset in schoolchildren in Oslo, Norway. Environ Health Perspect. 2009;117:839–844. doi: 10.1289/ehp.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B., Lipsett M., Mann J., Braxton-Owens H., White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12:200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Papi A., Bellettato C.M., Braccioni F., Romagnoli M., Casolari P., Caramori G. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- Pearce N., Douwes J. The global epidemiology of asthma in children. Int J Tuberc Lung Dis. 2006;10:125–132. [PubMed] [Google Scholar]
- Pekkanen J., Remes S., Kajosaari M., Husman T., Soininen L. Infections in early childhood and risk of atopic disease. Acta Pediatr. 1999;88:710–714. doi: 10.1080/08035259950168964. [DOI] [PubMed] [Google Scholar]
- Peng C.K., Buldtrev S.V., Goldberger A.L., Havins S., Mantegna R.N., Simons M. Fractal mechanisms and heart rate dynamics — long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28:59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- Peng C.K., Mietus J., Hausdorff J.M., Havlin S., Stanley H.E., Goldberger A.L. Long-range anticorrelations and non-Gaussian behaviour of the heartbeat. Phys Rev Lett. 1993;70:1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- Peng C.K., Mietus J.E., Liu Y.H., Lee C., Hausdorff J.M., Stanley H.E. Quantifying fractal dynamics of human respiration: age and gender effects. Ann Biomed Eng. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- Proud D., Chow C.W. Role of viral infections in asthma and chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;35:513–518. doi: 10.1165/rcmb.2006-0199TR. [DOI] [PubMed] [Google Scholar]
- Reddel H.K., Taylor D.R., Bateman E.D., Boulet L.P., Boushey H.A., Busse W.W. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations standardising endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Schildcrout J.S., Sheppard L., Lumley T., Slaughter J.C., Koenig J.Q., Shapiro G.G. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- Shankardass K., McConnell R., Jerrett M., Milam J., Richardson J., Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci USA. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K., Liu C.Q., Ai N.S., Zhang X.H. Using three methods to investigate time-scaling properties in air pollution indexes time series. Nonlin Anal: Real World Appl. 2008;9:693–707. [Google Scholar]
- Singh A.M., Busse W.W. Asthma exacerbations 2: aetiology. Thorax. 2006;61:809–816. doi: 10.1136/thx.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S.F., Gryparis A., Schwartz J., Wright R.J. Association between traffic-related black carbon exposure and lung function among urban women. Environ Health Perspect. 2008;116:1333–1337. doi: 10.1289/ehp.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.C., Xiang X.Y., Qiu D.W., Ng T.P., Kam S.F., Hegele R.G. Epidemiology of respiratory viruses in patients hospitalized with near-fetal asthma, acute exacerbation's of asthma, or chronic obstructive pulmonary disease. Am J Med. 2003;115:272–277. doi: 10.1016/s0002-9343(03)00353-x. [DOI] [PubMed] [Google Scholar]
- Teichtahl H., Buckmaster N., Pertinikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112:591–596. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.S., Cheng M.H., Chiu H.F., Wu T.N., Yang C.Y. Air pollution and hospital admissions for asthma in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2006;18:549–554. doi: 10.1080/08958370600686176. [DOI] [PubMed] [Google Scholar]
- Varkey J.B., Varkey B. Viral infections in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:89–94. doi: 10.1097/MCP.0b013e3282f4a99f. [DOI] [PubMed] [Google Scholar]
- Varotsos C., Ondov J., Efstathiou Scaling properties of air pollution in Athens, Greece and Baltimore, Maryland. Atmos Environ. 2005;39:4041–4047. [Google Scholar]
- Velthove K.J., Leufkens H.G.M., Schweizer R.C., van Solinge W.W., Souverein P.C. Medication changes prior to hospitalization for obstructive lung disease: a case-crossover study. Ann Pharmacother. 2010;44:267–273. doi: 10.1345/aph.1M513. [DOI] [PubMed] [Google Scholar]
- Ward D.J., Ayres J.G. Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med. 2004;61:e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmayr G., Romeo E., De Sario M., Weiland S.K., Forastiere F. Short term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:449–457. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek T.J., Jr., Schachter E.N., Beck G.J., Cain W.S., Colice G., Leaderer B.P. Respiratory symptoms associated with sulfur dioxide exposure. Int Arch Occup Environ Health. 1985;55:179–183. doi: 10.1007/BF00378381. [DOI] [PubMed] [Google Scholar]
- Wong C.M., Yang L., Thach T.Q., Chau P.Y.K., Chan K.P., Thomas G.N. Modification by influenza on health effects of air pollution in Hong Kong. Environ Health Perspect. 2009;117:248–253. doi: 10.1289/ehp.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xirsagar S., Lin H.C., Liu T.C. Seasonality in pediatric asthma admissions: the role of climate and environmental factors. Eur J Pediatr. 2006;165:747–752. doi: 10.1007/s00431-006-0164-6. [DOI] [PubMed] [Google Scholar]


