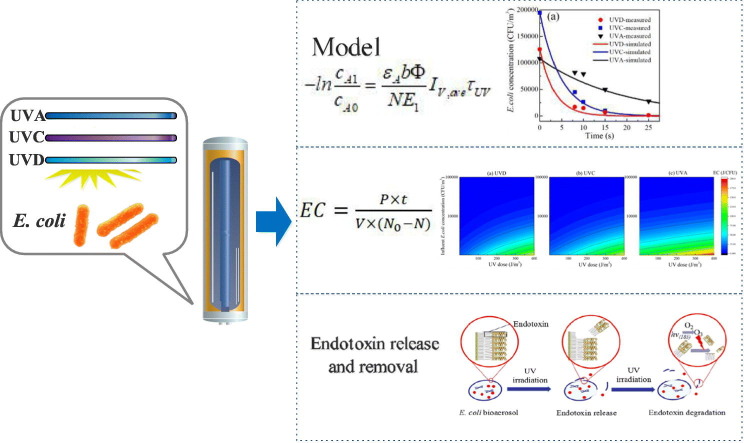
Abstract
Airborne bacteria-containing bioaerosols have attracted increased research attention on account of their adverse effects on human health. Ultraviolet germicidal irradiation (UVGI) is an effective method to inactivate airborne microorganisms. The present study models and compares the inactivation performance of three UV sources in the UVGI for aerosolized Escherichia coli. Inactivation efficiency of 0.5, 2.2 and 3.1 logarithmic order was obtained at an exposure UV dose of 370 J/m3 under UVA (365 nm), UVC (254 nm) and UVD (185 nm) sources, respectively. A Beer–Lambert law-based model was developed and validated to compare the inactivation performances of the UV sources, and modeling enabled prediction of inactivation efficiency and analysis of the sensitivity of several parameters. Low influent E. coli concentrations and high UV doses resulted in high energy consumption (EC). The change in airborne endotoxin concentration during UV inactivation was analyzed, and UVC and UVA irradiation showed no marked effect on endotoxin degradation. By contrast, both free and bound endotoxins could be removed by UVD treatment, which is attributed to the ozone generated by the UVD source. The results of this study can provide a better understanding of the air disinfection and airborne endotoxin removal processes.
Keywords: Bioaerosol, UV germicidal irradiation (UVGI), Model, Energy utilization, Airborne endotoxin
Graphical abstract
1. Introduction
Bioaerosols are airborne microbial cells with fragments and particulate matter of biological origin (Liang et al., 2012). These small particles affect human health by causing infectious diseases, acute toxic reactions, and allergies (Gergen, 2011). Prevention and control measures for bioaerosols have attracted worldwide attention as a result of the recent pandemics of severe acute respiratory syndrome (SARS) and influenza H1N1 (Zhang et al., 2010). Ultraviolet germicidal irradiation (UVGI) systems are used as commercial engineering controls to prevent the transmission of infectious diseases (Jensen et al., 1994). UVGI is an effective method of inactivating airborne microorganisms (Xu et al., 2005). Germicidal lamps containing mercury vapors under low pressure are a commonly used UV source for UVGI (Ryan et al., 2010).
Microorganisms present different UV sensitivities, which is often measured by the UV fluence required for decreasing one logarithmic order of bacterial number (Lytle and Sagripanti, 2005; Sagripanti et al., 2009), and this value of 70.3, 73.3, and 18.3 J·m−2 have been observed for vegetative cells of B. atrophaeus, P. agglomerans, and Y. ruckeri, respectively (King et al., 2011). Higher UV sensitivity was observed at higher relative humidity according to Walker's study on MS2 Bacteriophage (Walker and Ko, 2007). Other than the species of microorganism and relative humidity (Xu et al., 2005), UV wavelength is also an important parameter determining the UV inactivation efficiency of bacterial aerosol. Maximum effectiveness is usually observed at around 254 nm (UVC) (Koller, 1952) and many studies have been conducted on the inactivation of airborne microorganisms by using UVC radiation. Inactivation performance under other UV wavelengths has also been explored. Kim and Jang (2018), for example, investigated several photocatalytic reactions by UVD (185 nm) with short irradiation times to inactivate airborne MS2 viruses. Araud et al. (Araud et al., 2018) also investigated the inactivation mechanisms of viruses via solar UVA and visible light. However, the inactivation performance of different UV sources, especially UVD, which can damage microorganisms by producing ozone in addition to UV irradiation, has rarely been compared. In addition, few studies have reported the energy efficiency of UVGI devices, which is also a factor to consider when choosing a UV source with which to conduct inactivation.
The effectiveness of UVGI devices has been modeled in previous studies, as summarized in Table 1 . Inactivation kinetics has been being studied since twentieth century (Crittenden et al., 2011). Reaction rates vary by one and one-half orders of magnitude for UV disinfection (Zhao et al., 2014). Utilization of the Chick-Watson model (Ryan et al., 2010; Xu et al., 2003; Kowalski and William, 2000) is the standard method for modeling the response of microorganisms to UVGI. In some cases, the two-stage curve (Noakes et al., 2015), such as Rennecker-Marinasis model (Crittenden et al., 2011) and Collins-Selleck model (Collins and Selleck, 1971), were used to describe the unusually high resistance of microorganisms to irradiation because many microbial decay curves exhibit a decay curve shoulder (time-delayed response) when exposed to UVGI. Most of the previous models were kinetic fitting of inactivation efficiency, while the present work takes mechanism of photoreaction into account, providing more insight into the process of UV inactivating bacteria.
Table 1.
Summary of models on bacteria inactivation using UV irradiation.
| Model | Equation | Reference |
|---|---|---|
| Chick-Watson |
where I = UV intensity, W/m2 t = exposure time, s k = inactivation rate, m2/J. |
Ryan et al., 2010 |
| Scheible |
where D = UV dose, J/m2 k = inactivation rate constant, s−1. |
Gibson et al., 2017 |
| Rennecker-Marinas | For It < b, For It > b, where ˄cw = coefficient of specific lethality, 1/(J/m2), b = lag coefficient, J/m2. |
Crittenden et al., 2011 |
| Collins-Selleck | For It < b, For It > b, where ˄cw = log-based coefficient of specific lethality, 1/(J/m2), b = lag coefficient, J/m2. |
Collins and Selleck, 1971 |
Microbial inactivation through UVGI results from denaturation of enzymes, proteins, and membranes and disruption of cellular metabolic activities (Takashima et al., 2007). However, intracellular substances may be released when the cells in bioaerosols are damaged or destroyed (Mattsbybaltzer et al., 1991). Endotoxins are complexes of lipopolysaccharides (LPS), proteins, and hazardous biological substances (Fig. S1 in Supporting Information) (Liu et al., 2018; Wen et al., 2017). They are widely distributed in the outer cell wall membranes of Gram-negative bacteria and other microorganisms (Nilsson et al., 2011).
Several studies have observed waterborne endotoxin release during inactivation of Gram-negative bacteria (Dufour et al., 2017; Zhang et al., 2016; Sreeja and Shetty, 2016). The results of these works verify that embedded endotoxins can be released into the environment after the death of bacteria or cells. Airborne endotoxins may exist as shed membrane complexes (free endotoxins) or bound endotoxins when combined with other biological and nonbiological particles (Oldenburg et al., 2007). Chronic exposure to endotoxins induces and exacerbates airway symptoms (Mendy et al., 2016), such as asthma, coughing, chronic obstructive pulmonary diseases, and organic dust lung diseases (Kong et al., 2013; Reiman and Uitti, 2000). Endotoxins released from airborne bacteria under UV irradiation have not been reported.
In this study, the inactivation performances of three different UV sources toward aerosolized Escherichia coli (E. coli) were investigated and compared, and the inactivation efficiency and rate of these sources were analyzed. A Beer–Lambert law-based model was developed and validated to compare the inactivation performances of the UV sources, and their of energy utilization efficiencies during UV irradiation were analyzed. The distribution and fate of airborne endotoxins were also investigated. The results of this study may provide important insights into the control of airborne microbes by using UV irradiation and help mitigate the environmental impacts of bioaerosols.
2. Materials and methods
2.1. Preparation and generation of airborne E. coli
E. coli (CMCC1.3373) inoculated into nutrient broth medium was cultured in a constant-temperature oscillation incubator (IS-RSDA, Crystal Technology & Industries, Inc., USA) at 37 °C and 170 r/min for 24 h. The suspension was centrifuged at 4000 r/min for 10 min, washed thrice with 0.85% NaCl solution to remove the medium, and then diluted. The bacteria-containing aqueous suspension was aerosolized by an aerosol generator (Topas, ATM226, Germany) to particle size of 1–5 μm (Fig. S2 in Support Information).
2.2. Experimental set-up for UVGI system
A schematic representation of the experimental setup for inactivating airborne microorganisms is shown in Fig.1 . The UV unit is cylinder-shaped with its dimension of Φ7cm × 47 cm, including a vertically placed ultraviolet lamp in it. UV irradiation of UVA (365 nm), UVC (254 nm) and UVD (185 nm) was obtained using three kind of commercial low pressure mercury lamp with two types of output power (GPH550T5L, 28W and GPH287T5L, 14W, Hanovia Ltd., USA). The bacterial bioaerosol is generated by nebulizing the bacteria-containing aqueous suspension, diluted with sterile air. The air stream containing airborne bacteria passed through UV unit from bottom to top, and was subsequently collected and measured.
Fig. 1.
Experimental setup for bioaerosol exposure to the UVGI.
The airflow rate varied through the UV unit to change the bacteria's exposure time to UV irradiation, ranging from 0.26 to 0.65 m3/h. UV dose is obtained by multiplying I v,ave by exposure time, where I v,ave is calculated using LSSE model as discussed before, and exposure time is determined by the reactor volume(m3) divided by airflow rate (m3/h). The bacteria concentration was calculated as CFUs obtained by plate counting method divided by the volume of collected bioaerosol (m3). Besides, each of experiments was conducted in duplication.
2.3. Sampling and analysis of airborne E. coli
A six-stage Andersen sampler (ZLKZR-B01, Beijing Jolyc Technology Co., Ltd., China) was used to obtain bioaerosol samples. A glass Petri dish containing 25 mL of nutrient agar medium was placed on each stage of the device, and samples were obtained at a flow rate of 28.3 L/min for 5 min. After sampling, the nutrient agar plates were incubated for 48 h in a constant-temperature incubator at 37 °C. The number of colony-forming units (CFUs) on each nutrient agar plate was manually counted.
2.4. Airborne endotoxin analysis
Airborne endotoxin samples (Liu et al., 2018) were collected with an AGI-30 impinger (Qingdao Junray Intelligent Instrument Co., Ltd., China). Samples were stored in a sealed pyrogen-free flask at 4 °C for no more than 2 h. The airborne endotoxin concentrations of the samples were determined using a limulus amebocyte lysate (LAL) assay reagent with a chromogenic endpoint. The kinetic chromogenic LAL assay is widely applied to airborne endotoxin tests (Liu et al., 2018; Wen et al., 2017).
Airborne endotoxins may exist in a bound or free state (Demonty and Grawe, 1982). Bound endotoxins remain bound to the bacterial outer membrane, whereas free endotoxins are shed from membrane complexes containing phospholipids, proteins, and LPS. Water samples were filtered through a 0.22 μm PTFE membrane prior to the LAL assay. The endotoxin that remained in the filtrate was referred to as “free-endotoxin”. The amount of endotoxin determined prior to filtration was referred to as “total-endotoxin”. The amount of “bound-endotoxin” was measured by subtracting the “free-endotoxin” from the “total-endotoxin”. A schematic of LAL method and detailed information on the detection of airborne endotoxins is provided in Fig. S3 in the Supporting Information.
2.5. Modeling of airborne E. coli inactivation by UV irradiation
2.5.1. Photochemical process
In the gas phase, photons are absorbed to inactivate bacteria in bioaerosols. Herein, the quantum yield (Φ) is defined as follows:
| (1) |
where N A is the number of inactivated airborne bacteria and x is the number of absorbed photons.
The equation can be further expanded as follows:
| (2) |
where c A is the concentration of airborne bacteria, N (mol−1) is the Avogadro constant of 6.023 × 1023 (Becker, 2001), E 1 (J) is the energy of one photon, I V (W·m−3) is the absorbed energy density, and t (s) is the exposure time.
Based on the Beer–Lambert law (Mortensen and Xiao, 2007), the kinetic model for bacterial inactivation can be written as follows:
| (3) |
where ε A (dm3·mol−1·cm−1) is the molar extinction coefficient, b (cm) is the optical length, and I and I 0 (W·m−3) are the absorbed and incident energy densities, respectively.
| (4) |
UV light absorption is a weak process in the gas phase; hence,
| (5) |
Therefore,
| (6) |
where –r A (mg·m−3·hr−1) is the bacterial inactivation rate.
2.5.2. Photochemical inactivation in the UV reactor
The flow pattern of the UV reactor used in this study can be considered as an ideal plug flow. Thus, the following equation is given as follows:
| (7) |
where Q (m3·h−1) is the gas flow rate, V (m3) is the reactor volume, and t (sec) is the time. From Eqs. (6), (7):
| (8) |
where N UV and N 0 are the respective bacterial concentrations in the bioaerosol at the outlet and inlet (CFU/m3), I V, ave (W·m−3) is the average absorbed energy density inside the reactor. τ (sec) is exposure time inside the reactor, which is calculated as reactor volume(m3) divided by airflow rate (m3/s).
2.5.3. Determination of the average absorbed energy density
The average absorbed energy density inside the reactor was calculated by the line source with spherical emission (LSSE) model. A summary of models and detailed information for solving I V,ave can be found in Table 2 and Fig. S4 in the Supporting Information.
| (9) |
where S L (W·m−1) is the light source strength per unit length (lamp power divided by lamp length), λ (nm) is the wavelength, P i is the emitting energy fraction at wavelength λ i, V is the reactor volume, H is the reactor height, r 1 is the lamp radius, R is the reactor internal radius, L is the lamp length, μi is the absorbency per unit optical thickness, r (m) is the radial coordinate, z (m) is the rectangular coordinate, and l (m) is the point source height.
Table 2.
Summary of models on determination of the average absorbed energy density inside various reactors.
| Model | Reactor type | Reaction phase | Abstract | Classification | Reference |
|---|---|---|---|---|---|
| Two-fluid model | Cube | Liquid-gas | Prediction of energy flow density | Incidence model | Akehata et al., 1976 |
| Tube | Bubbling dispersion system, Gas-solid-liquid phase | Prediction of energy flow density | Radial incidence model | Inokawa, 1980 | |
| Model based on distribution function | Tube | Air phase | Estimation of absorption rate of radiation energy | Incidence model | Yokota et al., 1981 |
| Water or NIS solution system | Estimation of filter effect of dispersed phase | Incidence model | |||
| Tubular bubble tower | Nitrogen radiation measurement system | Estimation of absorption rate of radiation energy on account of air/water ratio | LSDE | Yokota et al., 1981 | |
| Nitrogen-chorine-toluene system | Estimation of radiation energy absorption rate in half-batch reactor | LSDE | Yokota et al., 1981 | ||
| Model based on Monte-carlo method | Tube | Absorption-diffusion medium | Estimation of energy absorption in a continuous reactor | LSSE | Jacob and Dranoff, 1968 |
| Parallel plate | Fiber bundle suspension system | Prediction of the absorption rate of light energy in light synthesis reaction, on account of cell concentration | Point lights model | Koizumi et al., 1980 | |
| Model based on effective absorption rate | Cubic bubble tower | Nitrogen‑potassium nitrate aqueous solution | Estimation of radiation field in gas-liquid dispersed phase | LSPP, LSDE |
Otake et al., 1983 |
| Tubular bubbling tower | Nitrogen-aqueous solution | Estimating the absorption rate of Light Energy in Gas-liquid system on account of modified attenuation coefficient | LSDE | Yokota et al., 1981 | |
| Annular stirring tower | Chlorine‑nitrogen- chloroform solution | Prediction of the energy absorption rate of chlorination Reaction | ESVE | Alfano et al., 1986 |
LSDE: Line Source Diffuse Emission; LSSE: Line Source Spherical Emission.
LSPP: Line Source Parallel Plane; ESVE: Extense Source Volumetric Emission.
Using the LSSE model, the UV average absorbed energy densities of two lamps in this study (with different powers and lengths) were calculated to be 15.2 and 36.1 W/m3, respectively.
3. Results and discussion
3.1. Model fitting and simulation of inactivation performance by UVGI
3.1.1. Model fitting
The experimental data obtained from the three UV sources with a UV average absorbed energy density of 15.2 W/m3 were fitted to a model to obtain the related parameters. The inactivation efficiency (E) is defined as the logarithmic order of bacteria concentration decreasing after UV inactivation (Ishiguro et al., 2013; Wang et al., 2018):
| (10) |
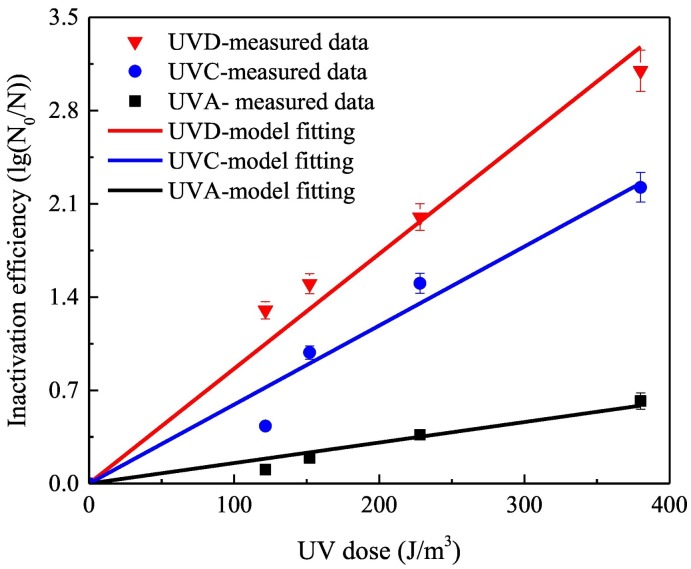
As shown in Fig. 2 and Table 3 , inactivation efficiency showed pseudo-first-order reaction kinetics, which also indicates that it corresponds to the theoretical model where the inactivation rate constant k (s−1) under the three UV sources is related to ε A, b, and Φ.
Fig. 2.
Model fitting of airborne E.coli inactivation under different UV sources (UV density = 15.2 W/m3).
Table 3.
Model fitting results.
| UV source | Reaction rate constant k (s−1) | Fitting formula | R2 |
|---|---|---|---|
| UVD | 0.131 | 0.9925 | |
| UVC | 0.090 | 0.9863 | |
| UVA | 0.023 | 0.9822 |
, where inactivation rate constant k (s−1) was defined as the log order of bacteria number being inactivated per unit time.
In Fig. 2, the inactivation efficiency of the three UV sources gradually increased at different rates with increasing exposure time or UV dose. The inactivation rate increased under different UV wavelengths, following the order UVD > UVC > UVA at the same exposure time. The UV doses required to decrease 1 logarithmic (lg) order of airborne E. coli by UVD, UVC, and UVA irradiation were 116, 169, and 649 J/m3, respectively. At an exposure UV dose of 370 J/m3, the inactivation efficiency of UVD was 3.1 lg orders, which is slightly higher than that of UVC, which was 2.2 lg orders. The inactivation efficiency of UVA was much lower than those of the two other UV irradiation sources, and its efficiency was only 0.5 lg orders at the same UV dose.
The effective performance of UVD could be attributed to the generation of reactive oxygen species (Ono et al., 2016) by vacuum ultraviolet photolysis of water vapor. UVC irradiation is generally utilized to inactivate aerosolized microorganisms because bacterial cells strongly absorb UV radiation (peak absorption, 250–270 nm). The generally accepted mechanism for this process is that UV irradiation inactivates bacteria by damaging their DNA, thereby preventing microorganisms from replicating and multiplying. While the damage to DNA molecules brought about by UVA irradiation (Tang and Guo, 2005) is far less than that brought about by UVC, UVA still confers slight damage to the B-form backbone of bacterial DNA by cutting off phosphodiester bonds and disrupting the main spatial structure of DNA as well as affecting the thymine groups.
3.1.2. Model stimulation of inactivation performance by UVGI
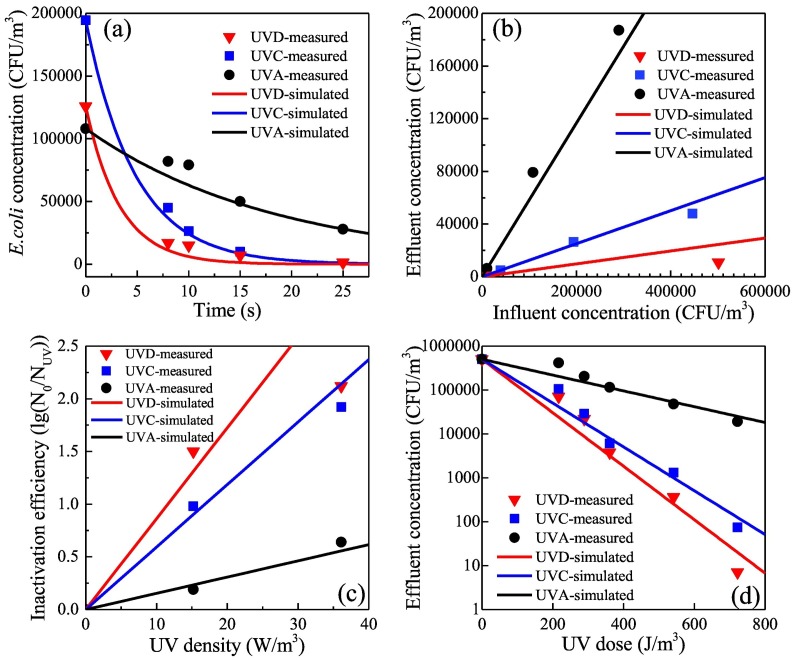
Experimental data on inactivation efficiency were obtained under two UV average absorbed energy densities (15.2 and 36.1 W/m3). The linear relation of inactivation efficiency versus UV dose was fitted according to the former set of data obtained at 15.2 W/m3, and the inactivation rate constant k obtained in this step was used to predict the relation under the latter UV average absorbed energy density condition (36.1 W/m3). Results are shown in Fig. 3 .
Fig. 3.
Model simulation and verification in terms of (a) retention time (b) influent E.coli concentration (c) UV average absorbed energy density (d) UV dose.
Fig. 3(a)–(d) illustrate the effects of exposure time, initial bacteria concentration, UV average absorbed energy density, and UV dose on effluent bacteria concentration, which are four key parameters for reactor design and performance. Given a certain influent bacteria concentration, the curve of predicted effluent concentration after exposure to the three UV sources based on the inactivation rate constant k (Fig. 2) is shown in Fig. 3(a); measured values are also marked individually.
Comparison of the predicted and measured values reveals that the model is reasonable. Experiments on three influent bacteria concentrations (generated by nebulizing bacterial suspensions of 106, 107, and 108 CFU/mL) and two UV average absorbed energy densities were conducted to explore their influence on k. The prediction results of the curve in Eq. (9) and the values measured at an exposure time of 10 s are shown in Fig. 3(b) and (c). Straight lines with different slopes indicate that the influent concentration and UV average absorbed energy density have no effect on k, which coincides with the model. This finding confirms that inactivation efficiency remains relatively unchanged despite variations in initial bacteria concentration but increases with increasing UV average absorbed energy density.
3.2. Parameter sensitivity of model
To evaluate the sensitivity of the objective function to changes in different parameters in the model, the relative sensitive index (RSI) is defined as follows:
| (11) |
where p 0 and p are the values of an operating parameter before and after adjustment, respectively, y i0 is the original value of the objective function, and y i is the value of this function after adjustment. In the model, the objective function is the E. coli concentration in the effluent after UV inactivation; an influent E. coli concentration of 1.94 × 105 CFU/m3, exposure time of 10 s, UV average absorbed energy density of 15.2 W/m3, and k of 0.00593 under UVC irradiation (fitted from Section 3.1) were also adopted. Moreover, the value of p is 1.2 times of p 0. The sensitivity of the parameters of the model was analyzed, and the RSIs of t, Q, r, and S L are listed in Table 4 .
Table 4.
RSI of main operating parameters.
| Parameter | cA0 | τ | SL | r | Q |
|---|---|---|---|---|---|
| RSI | 1 | −1.703 | −1.703 | −1.767 | 2.072 |
Effluent E.coli concentration N UV was directly proportional to influent concentration N 0 with an RSI of 1. By contrast, negative relations existed between N UV and exposure time t, light source strength per unit length S L, and reactor radius r. Among the parameters tested, N UV was the most sensitive to flow rate Q (RSI, 2.072), thereby indicating that high flow rate results in significantly low inactivation efficiency.
3.3. Energy utilization during inactivation
Energy consumption (EC), a common index in the electrochemical oxidation field (Gurung et al., 2018; Wang et al., 2014), was used to measure the energy utilization efficiency of a process. In this study, only the energy consumed by UV lamps was taken into account while other consumption was not included. As discussed above, our model showed good agreement with the experimental data. To optimize airborne bacteria inactivation and improve economic efficiency, EC (Zhou et al., 2018) was adjusted and applied to evaluate energy utilization during UV inactivation process. Here, EC is defined as the average electrical energy consumed (power multiplied by time) required to inactivate 1 CFU of bacterium in 1 m3 of air (Eq. 12). Thus a lower EC corresponds to a higher electrical energy utilization efficiency when inactivating 1 CFU of bacterium.
| (12) |
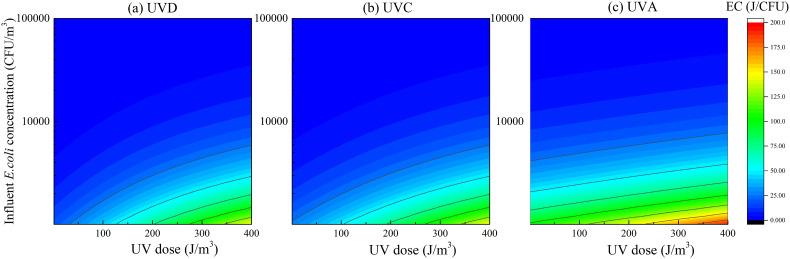
Fig. 4 illustrates changes in EC (log scale) as a function of UV dose and influent E. coli concentration after exposure to different UV sources. Cool colors (such as purple and blue) represent low EC values, while warm colors (such as red and orange) represent high values. A significant difference in EC between UVD and UVC was not found, although the latter was slightly higher than the former. The EC values obtained from the UVA treatment were remarkably higher than those obtained from the UVD and UVC treatments, corresponding to the different inactivation efficiencies observed in Section 3.1. Furthermore, low influent E. coli concentrations and high UV doses resulted in relatively higher EC values, thus suggesting that higher EC is required to achieve higher inactivation efficiency.
Fig. 4.
EC calculation under (a) UVD (b) UVC (c) UVA source.
3.4. Changes in airborne endotoxins during inactivation
3.4.1. Endotoxin degradation and the corresponding mechanism
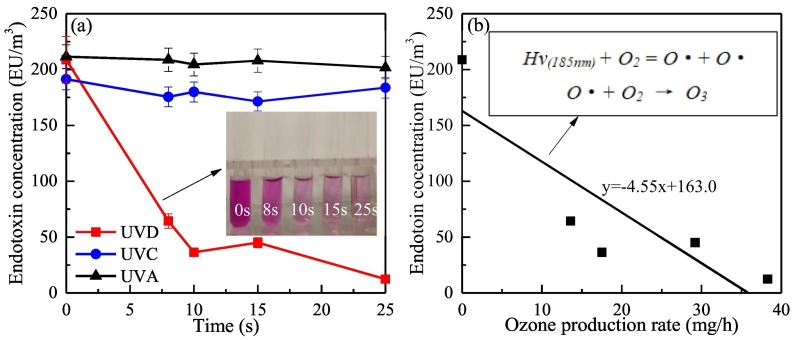
As a constituent of the Gram-negative bacterial cell membrane, endotoxins may be released during bacterial inactivation and bursting. Thus, measuring the change in endotoxin concentration from airborne microorganisms during UV treatment is necessary. The concentrations of airborne endotoxins were measured before and after UV treatment with different UV sources, and the results are shown in Fig. 5(a).
Fig. 5.
(a) Removal of airborne endotoxin under different UV source.
(b) Endotoxin concentration vs. ozone production rate under UVD.
The total endotoxin concentration did not change significantly after treatment by UVA or UVC because the photo energy of UVA and UVC irradiation is too weak to break the molecular structure of endotoxins. UVA and UVC irradiation exerted no significant effect on the removal of endotoxins. However, the total-endotoxin concentration showed an obvious decrease with increasing exposure time under UVD treatment, possibly due to the generation of ozone. The change in endotoxin concentration with ozone production rate is shown in Fig. 5(b).
UVD irradiation is capable of sterilizing bioaerosols by generating ozone from oxygen in the air, as well as by damaging the DNA of microorganisms through irradiation (Eischeid et al., 2009). The ozone generation path is as follows:
Ozone inactivates airborne bacteria by oxidizing their cell membrane. Besides DNA, the cellular structure of the microbes is destroyed, which increases the possibility of degrading endotoxins attached to the cell membrane. As ozone can oxidize endotoxins and decompose them into smaller molecules, removal of airborne endotoxins and the pathways of endotoxin degradation by ozone should be studied through further experiments.
3.4.2. Changes in free and bound endotoxins
The change in different types of endotoxins was further explored during UVD irradiation. Airborne endotoxins are present in the air in two main forms. The first corresponds to pure LPS molecules (free endotoxins). The second form corresponds to LPSs associated with other cell wall components or an intact bacterial cell (bound endotoxins) (Duquenne et al., 2013). Fig. 6 shows the change in the concentrations of total, free, and bound endotoxins with exposure time. Free endotoxins initially made up about 20% of the total endotoxin concentration. The concentrations of both free and bound endotoxins decreased gradually as exposure time increased, thereby indicating that UVD treatment has good ability to remove endotoxins regardless of type. The ratio of free endotoxins first increased and then decreased with time, indicating a possible release or transformation from bound to free endotoxins at the beginning of the UV irradiation process and subsequent degradation. A proposed degradation mechanism is shown in Fig. 7 .
Fig. 6.
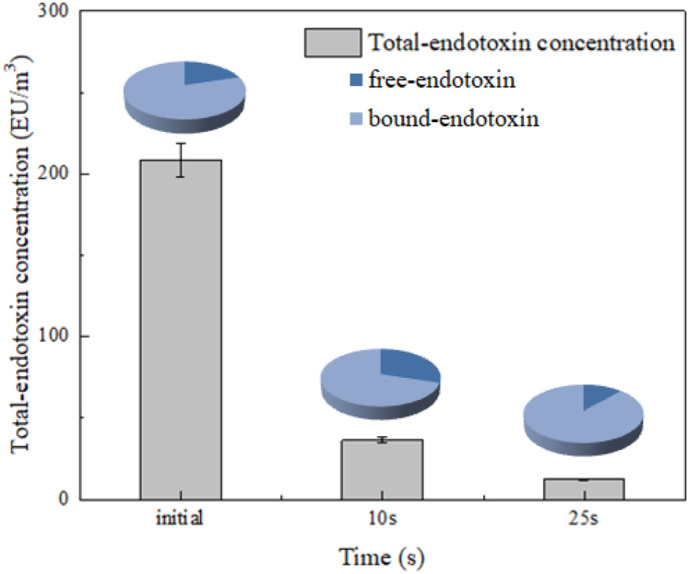
Degradation of different type of endotoxin during UVD treatment.
Fig. 7.
Proposed degradation mechanism of endotoxin under UVD treatment.
To the best of our knowledge, the degradation of airborne endotoxins has never been reported, and few scholars have conducted research on the degradation of waterborne endotoxins (Oh et al., 2014). Some oxidative processes employing free chlorine, ozone, plasma, and advanced oxidation processes (O3/H2O2 and UV/H2O2) (Oh et al., 2014; Zhang et al., 2016) have been reported for waterborne endotoxin degradation. The present study is the first to report endotoxin degradation in an atmospheric environment. The results of this study may help provide new strategies to control airborne endotoxins and mitigate the environmental impacts of bioaerosols.
4. Conclusion
This study investigated and compared the inactivation performance of three UV sources (UVD, UVC, and UVA) toward aerosolized E. coli by modeling their inactivation efficiency, energy utilization, and endotoxin removal ability. Inactivation efficiency of 0.5, 2.2 and 3.1 logarithmic order was obtained at an exposure UV dose of 370 J/m3 under UVA, UVC and UVD treatment, respectively. UVD and UVC were significantly more effective than UVA for inactivating bacteria. Modeling enabled prediction of inactivation efficiencies and analysis of the parameters that may affect inactivation constant k. Energy utilization efficiency was also analyzed by measuring E values. High EC was generally required in the case of relatively low influent E. coli concentrations and high UV doses, especially for the UVA inactivation process.
Changes in airborne endotoxin concentration during UV inactivation were measured in this work. Whereas UVC and UVA showed no effect on endotoxin degradation, both free and bound endotoxins could be removed by UVD treatment, which was attributed to the ozone generated by the UVD source during irradiation. The ratio of free endotoxins first increased then decreased with time, indicating a possible release or transformation from bound to free endotoxins during UV irradiation.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (Grant No. 51678402).
Editor: Ching-Hua Huang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2018.11.266.
Appendix A. Supplementary data
Supplementary material
References
- Akehata T., Ito K., Inokwa A. Applications of the second law of thermodynamics to cryogenics - a review/energy. Trans. Jpn. Soc. Mech. Eng., B. 1976;34:583–588. [Google Scholar]
- Alfano O.M., Romero R.L., Cassano A.E. Radiation field modeling in photoreactors-I. Homogeneous media. Chem. Eng. Sci. 1986;41:421–444. [Google Scholar]
- Araud E., Shisler J.L., Nguyen T.H. Inactivation mechanisms of human and animal rotaviruses by solar UVA and visible light. Environ. Sci. Technol. 2018;52:5682–5690. doi: 10.1021/acs.est.7b06562. [DOI] [PubMed] [Google Scholar]
- Becker P. History and progress in the accurate determination of the Avogadro constant. Rep. Prog. Phys. 2001;64:1945–2008. [Google Scholar]
- Collins H., Selleck R. JSAE. Vol. 97. ASCE; 1971. Problem in obtaining adequate sewage disinfection; pp. 549–562. [Google Scholar]
- Crittenden J.C., Trussell R.R., Hand D.W., Howe K.J. third edition. John Wiley & Sons, Inc; 2011. MWH's water treatment: principles and design; pp. 912–924. [Google Scholar]
- Demonty J., Grawe J.D. Release of endotoxic lipopolysaccharide by sensitive strains of Escherichia coli submitted to the bactericidal action of human serum. Med. Microbiol. Immunol. 1982;170:265–277. doi: 10.1007/BF02123317. [DOI] [PubMed] [Google Scholar]
- Dufour N., Delattre R., Ricard J.D., Debarbieux L. The lysis of pathogenic Escherichia coli by bacteriophages releases less endotoxin than by β-lactams. Clin. Infect. Dis. 2017;64:1582–1588. doi: 10.1093/cid/cix184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquenne P., Marchand G., Duchaine C. Measurement of endotoxins in bioaerosols at workplace: a critical review of literature and a standardization issue. Ann. Occup. Hyg. 2013;57:137–172. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- Eischeid A.C., Meyer J.N., Linden K.G. UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl. Environ. Microbiol. 2009;75:23–28. doi: 10.1128/AEM.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen P.J. Understanding the economic burden of asthma. J. Allergy Clin. Immunol. 2011;107:S445–S448. doi: 10.1067/mai.2001.114992. [DOI] [PubMed] [Google Scholar]
- Gibson J., Drake J., Karney B. UV disinfection of wastewater and combined sewer overflows. Adv. Exp. Med. Biol. 2017;996:267. doi: 10.1007/978-3-319-56017-5_22. [DOI] [PubMed] [Google Scholar]
- Gurung K., Ncibi M.C., Shestakova M., Sillanpaa M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B Environ. 2018;221:329–338. [Google Scholar]
- Inokawa A. The exergy method of energy systems analysis. Trans. Jpn. Soc. Mech. Eng., B. 1980;38:178–183. [Google Scholar]
- Ishiguro H., Yao Y., Nakano R. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl. Catal. B Environ. 2013;129:56–61. doi: 10.1016/j.apcatb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S.M., Dranoff J.S. Design and analysis of perfectly mixed photochemical reactors. Chem. Eng. Prog. Symp. Ser. 1968;64:54–63. [Google Scholar]
- Jensen P.A., Lambert L.A., Iademarco M.F., Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. MMWR. 1994;43.RR-17:1–141. [PubMed] [Google Scholar]
- Kim J., Jang J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci. Technol. 2018;52:557–566. [Google Scholar]
- King B., Kesavan J., Sagripanti J. Germicidal UV sensitivity of bacteria in aerosols and on contaminated surfaces. Aerosol Sci. Technol. 2011;45:645–653. [Google Scholar]
- Koizumi J., Yabe I., Aiba S. The exergy method of energy systems analysis. Trans. Jpn. Soc. Mech. Eng., B. 1980;38:178–183. [Google Scholar]
- Koller L.R. John Wiley & Sons; New York: 1952. Ultraviolet Radiation; p. 8. [Google Scholar]
- Kong X., Wang C., Ji Analysis of microbial metabolic characteristics in mesophilic and thermophilic biofilters using Biolog plate technique. Chem. Eng. J. 2013;230:415–421. [Google Scholar]
- Kowalski W.J., William P.B. Effective UVGI system design through improved modeling. ASHRAE Trans. 2000;106:4–15. [Google Scholar]
- Liang Y.D., Wu Y., Sun K., Chen Q., Shen F.X., Zhang J., Yao M.S., Zhu T., Fang J. Rapid inactivation of biological species in the air using atmospheric pressure nonthermal plasma. Environ. Sci. Technol. 2012;46:3360–3368. doi: 10.1021/es203770q. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang Z., Wen N., Wang C. Determination and risk assessment of airborne endotoxin concentrations in a university campus. J. Aerosol Sci. 2018;115:146–157. [Google Scholar]
- Lytle C.D., Sagripanti J.L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005;79:14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsbybaltzer I., Lindgren K., Lindholm B., Edebo L. Endotoxin shedding by enterobacteria: free and cell-bound endotoxin differ in limulus activity. Infect. Immun. 1991;59:689–695. doi: 10.1128/iai.59.2.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Cohn R.D., Thorne P.S. Endotoxin exposure, serum vitamin D, asthma and wheeze outcomes. Respir. Med. 2016;114:61–68. doi: 10.1016/j.rmed.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen N.A., Xiao S. Slow-light enhancement of Beer-Lambert-Bouguer absorption. Appl. Phys. Lett. 2007;90:374. [Google Scholar]
- Nilsson S., Merritt A.S., Bellander T. Endotoxins in urban air in Stockholm, Sweden. Atmos. Environ. 2011;45:266–270. [Google Scholar]
- Noakes C.J., Khan M.A.I., Gilkeson C.A. Modeling infection risk and energy use of upper-room ultraviolet germicidal irradiation systems in multi-room environments. Sci. Technol. Built Environ. 2015;21:99–111. [Google Scholar]
- Oh B.T., Seo Y.S., Sudhakar D., Choe J.H., Lee S.M., Park Y.J., Cho M. Oxidative degradation of endotoxin by advanced oxidation process (O3/H2O2 & UV/H2O2) J. Hazard. Mater. 2014;279:105–110. doi: 10.1016/j.jhazmat.2014.06.065. [DOI] [PubMed] [Google Scholar]
- Oldenburg M., Latza U., Baur X. Exposure–response relationship between endotoxin exposure and lung function impairment in cotton textile workers. Int. Arch. Occup. Environ. Health. 2007;80:388–395. doi: 10.1007/s00420-006-0145-0. [DOI] [PubMed] [Google Scholar]
- Ono R., Yonetamari K., Tokumitsu Y., Yonemori S., Yasuda H., Mizuno A. Inactivation of Bacillus atrophaeus by OH radicals. J. Phys. D. Appl. Phys. 2016;49 [Google Scholar]
- Otake T., Tone S., Higuchi K., Nakao K. Applicability of effective absorption coefficient. Int. Chem. Eng. 1983;23:288–297. [Google Scholar]
- Reiman M., Uitti J. Exposure to microbes, endotoxins and total dust in cigarette and cigar manufacturing: an evaluation of health hazards. Ann. Occup. Hyg. 2000;44:467–473. [PubMed] [Google Scholar]
- Ryan K., McCabe K., Clements N., Hernandez M., Miller S.L. Inactivation of airborne microorganisms using novel ultraviolet radiation sources in reflective flow-through control devices. Aerosol Sci. Technol. 2010;44:541–550. [Google Scholar]
- Sagripanti J.L., Levy A., Robertson J., Merritt A., Inglis T.J.J. Inactivation of virulent Burkholderia pseudomallei by sunlight. Photochem. Photobiol. 2009;85:978–986. doi: 10.1111/j.1751-1097.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- Sreeja S., Shetty V.K. Microbial disinfection of water with endotoxin degradation by photocatalysis using Ag@TiO2 core shell nanoparticles. Environ. Sci. Pollut. Res. Int. 2016;23 doi: 10.1007/s11356-016-6841-8. (181541-18154) [DOI] [PubMed] [Google Scholar]
- Takashima H., Miyakawa Y., Kanno Y. Microwave sterilization with metal thin film coated catalyst in liquid phase. Mater. Sci. Eng. C Mater. Biol. Appl. 2007;27:898–903. [Google Scholar]
- Tang W.L., Guo Z.Y. Influence of ultraviolet radiation on calf thymus DNA studied by Raman spectroscopy. In: Chance B., Chen M., Chiou A., Luo Q., editors. Proceedings of the Society of Photo-optical Instrumentation Ginerrs. SPIE; 2005. pp. 384–395. [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ. Sci. Technol. 2007;41:5460. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Wang C., Huang Y.K., Zhao Q., Ji M. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014;243:1–6. [Google Scholar]
- Wang D., Zhu B., He X. Iron oxide nanowires based filter for inactivation of airborne bacteria. Environ. Sci. Nano. 2018;5:1096–1106. doi: 10.1039/C8EN00133B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen N., Liu H., Fu Y., Wang Optimization and influence mechanism of sampling and analysis of airborne endotoxin based on limulus amebocyte lysate assay. Aerosol Air Qual. Res. 2017;17:1000–1010. [Google Scholar]
- Xu P., Peccia J., Fabian P. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmos. Environ. 2003;37:405–419. [Google Scholar]
- Xu P., Kujundzic E., Peccia J., Schafer M.P., Moss G., Hernandez M., Miller S.L. Impact of environmental factors on efficacy of upper-room air ultraviolet germicidal irradiation for inactivating airborne mycobacteria. Environ. Sci. Technol. 2005;39:9656–9664. doi: 10.1021/es0504892. [DOI] [PubMed] [Google Scholar]
- Yokota T., Iwano T., Saito A.T., Tadaki T. Photochlorination of toluene in a bubble column photochemical reactor. Int. Chem. Eng. 1981;7:164–170. [Google Scholar]
- Zhang Q., Damit B., Welch J., Park H. Microwave assisted nanofibrous air filtration for disinfection of bioaerosols. J. Aerosol Sci. 2010;41:880–888. doi: 10.1016/j.jaerosci.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Fang Z., Liu W., Tian F., Bai M. Rapid removal of bacterial endotoxin and natural organic matter in water by dielectric barrier discharge plasma: efficiency and toxicity assessment. J. Hazard. Mater. 2016;318:15–23. doi: 10.1016/j.jhazmat.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A.J., Xin H. Inactivation of airborne Enterococcus faecalis and infectious bursal disease virus using a pilot-scale ultraviolet photocatalytic oxidation scrubber. J. Air Waste Manage. Assoc. 2014;64:38–46. doi: 10.1080/10962247.2013.831800. [DOI] [PubMed] [Google Scholar]
- Zhou S., Yu Y., Zhang W., Meng X., Luo J., Deng L., Shi Z., Crittenden J. Oxidation of microcystin-LR via activation of peroxymonosulfate using ascorbic acid: kinetic modeling and toxicity assessment. Environ. Sci. Technol. 2018;52:4305–4312. doi: 10.1021/acs.est.7b06560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material