Abstract
Infection of polarized intestinal epithelial cells by porcine epidemic diarrhea virus (PEDV) was characterized. Indirect immunofluorescence assay, real-time PCR, and transmission electron microscopy confirmed PEDV can be successfully propagated in immortalized swine small intestine epithelial cells (IECs). Infection involved porcine aminpeptidase N (pAPN), a reported cellular receptor for PEDV, transient expression of pAPN and siRNA targeted pAPN increased and decreased the infectivity of PEDV in IECs, respectively. Subsequently, polarized entry into and release from both Vero E6 and IECs was analyzed. PEDV entry into polarized cells and pAPN grown on membrane inserts occurs via apical membrane. The progeny virus released into the medium was also quantified which demonstrated that PEDV is preferentially released from the apical membrane. Collectively, our data demonstrate that pAPN, the cellular receptor for PEDV, mediates polarized PEDV infection. These results imply the possibility that PEDV infection may proceed by lateral spread of virus in intestinal epithelial cells.
Keywords: PEDV, pAPN, Receptor, Epithelial
Highlights
-
•
PEDV infection of polarized intestinal epithelial cells (IECs) was characterized.
-
•
Porcine aminpeptidase N (pAPN) facilitated PEDV infection in IECs.
-
•
PEDV entry into and release from polarized cell via its apical membrane.
-
•
PEDV infection may proceed by lateral spread of virus in IECs.
Introduction
Porcine epidemic diarrhea virus (PEDV), a member of the family Coronaviridae, is an enveloped virus with a positive-stranded RNA genome that causes diarrhea in pigs and is associated with high mortality in newborn piglets (Song and Park, 2012). Other members of the genus Alphacoronavirus include transmissible gastroenteritis virus (TGEV), human coronavirus 229E (HCoV-229E), feline coronavirus (FCoV), canine coronavirus (CCoV) and human coronavirus NL63 (HCoV-NL63) (Adams and Carstens, 2012, Adams and Carstens, 2012).
PEDV encodes four structural proteins: a large spike or peplomer glycoprotein (S), a membrane glycoprotein (M), a small envelope protein (E) and a phosphorylated nucleocapsid protein (N) (Cavanagh and Britton, 2008, Egberink et al., 1988). The spike (S) glycoprotein of PEDV is the dominant surface protein and is responsible for initiating infection and for inducing neutralizing antibodies (Duarte and Laude, 1994, Yeo et al., 2003).
APN (CD13) is one of the type II cell surface metalloproteases the large glycosylated ectodomain of which has a zinc metal ion at the active site (Mina-Osorio, 2008). It is known that APN serves as a cellular receptor for several alphacoronaviruses, such as TGEV, HCoV-229E and FCoV (Delmas et al., 1992, Yeager et al., 1992, Tresnan et al., 1996). Only very limited data are available indicating that porcine APN (pAPN) plays a role for PEDV infection. Previously, it has been reported that rabbit anti-pAPN polyclonal antibody inhibited PEDV binding to pAPN protein and pre-treatment of Vero E6 cells with a soluble pAPN increased the viral infectivity (Oh et al., 2003). Mature pAPN is a 150-kDa glycosylated protein that is highly expressed in small intestinal mucosa (Oh et al., 2003, Delmas et al., 1992). Li and colleagues demonstrated that MDCK cells, a canine kidney cell line, became susceptible to PEDV infection after transient expression of pAPN; infection was inhibited by anti-pAPN polyclonal antibodies (Li et al., 2007). A swine testicular cell line (ST) that expresses only low levels of the enzyme, is resistant to PEDV infection. However, recombinant ST cells constitutively expressing high levels of pAPN could be infected efficiently (Nam and Lee, 2010). The available data indicate an association between pAPN and PEDV infection, although PEDV can be serially propagated in Vero E6 cells, a monkey cell line which does not express pAPN, if a protease is added for release of virions from the cell surface (Hofmann and Wyler, 1988, Shirato et al., 2011).
The primary target of coronaviruses is the respiratory or intestinal epithelium. Epithelial cell layers form a primary barrier to infection by microorganisms entering their host via body cavities such as the respiratory or intestinal tract (Ren et al., 2006, Cong and Ren, 2014). Epithelial cells grow with a polarized topology that involves the separation of the plasma membrane into apical and basolateral domains (Rossen et al., 1994, Cong and Ren, 2014). It has been demonstrated that the entry and release of several coronaviruses in polarized epithelial cells is restricted to the apical plasma membrane, e.g. TGEV, HCoV-229E and severe acute respiratory syndrome associated coronavirus (SARS-CoV) (Ren et al., 2006, Rossen et al., 1994, Wang et al., 2000, Jia et al., 2005, Tseng et al., 2005). Feline coronavirus (FCoV) and mouse hepatitis coronavirus (MHV) mediated apical entry and basolateral release in polarized epithelial cells (Rossen et al., 2001, Rossen et al., 1995, Rossen et al., 1996). The recently identified coronavirus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and also the canine coronavirus (CCoV) enter and exit at both sites of polarized epithelial cells (Pratelli, 2011, Tao et al., 2013). These examples show that coronaviruses have evolved different ways to interact with polarized cells. As this knowledge is important to understand how a virus gets across the epithelial barrier, the polarity of virus infection has to be determined for each virus. Though PEDV has become of increasing epidemiological importance in recent years, the polarized entry and release of PEDV in epithelial cells has not been documented.
In this study, we analyzed PEDV with respect to polarized entry into and release from intestinal epithelial celIs (IEC) that are derived from the target tissue of this virus. These data will help to understand the course of infection in the natural host.
Results
PEDV can be propagated in IECs
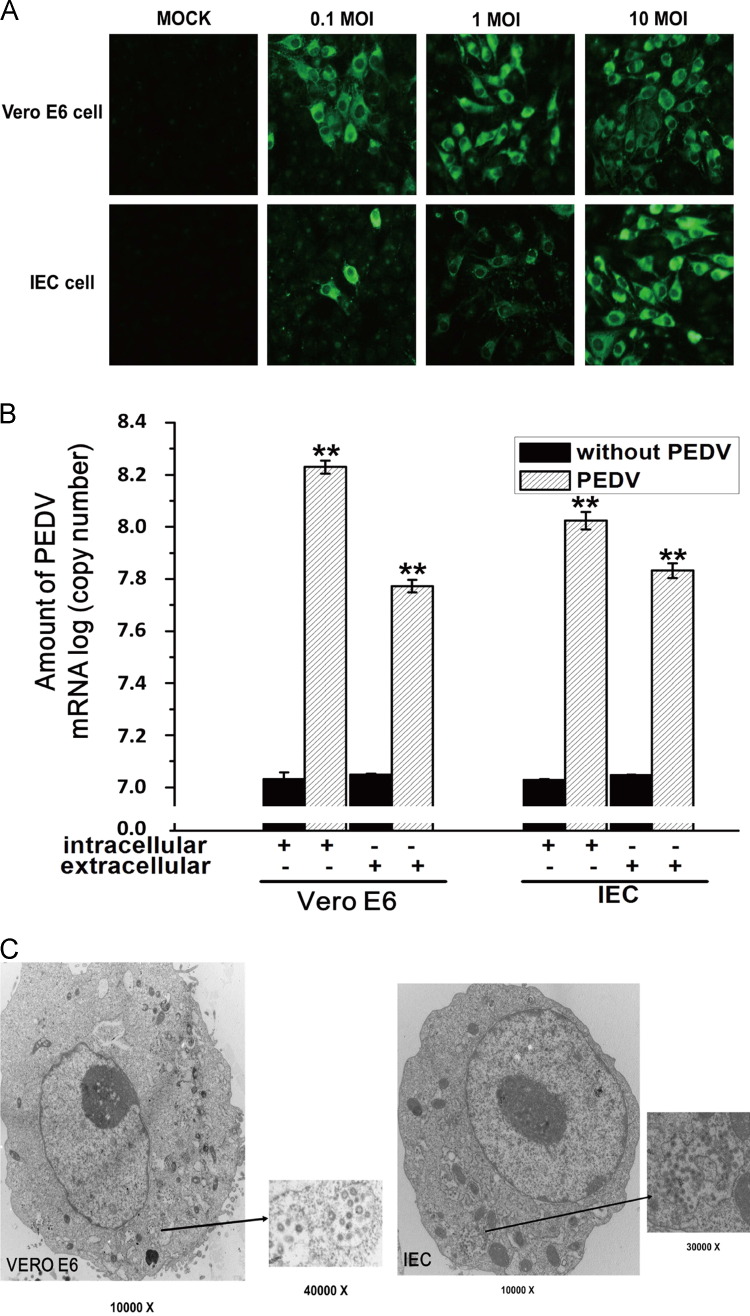
To determine whether IECs can be infected by PEDV, the growth of PEDV was determined with IECs infected at an MOI of 0.1, 1 or 10. At 48 h.p.i., the IFA results showed that IECs are somewhat less susceptible to infection by PEDV than are Vero E6 cells. However, at an MOI of 10, the majority of cells was infected ( Fig. 1A). The intracellular and extracellular distributions of PEDV was determined by real-time PCR. As shown in Fig. 1B, viral RNA was detected both in the intracellular fraction and in the extracellular medium. To further confirm the susceptibility of the IEC cell line to infection by PEDV, infection was analyzed by transmission electron microscopy. As shown in Fig. 1C, numerous round-to-pleomorphic virus particles with diameters from 90 to 160 nm were observed in PEDV-infected IEC and Vero E6 cells, whereas these particles were not observed in mock-inoculated control cultures.
Fig. 1.
PEDV can be successfully propagated in IECs. (A) Indirect immunofluorescence analysis of Vero E6 and IECs inoculated with PEDV at different MOI (0.1, 1 and 10) and incubated for 48 h. Cells were fixed in 4% formaldehyde for 15 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min at room temperature and processed for indirect immunofluorescence using anti-PEDV polyclonal antibody (1:100) and FITC-labeled goat anti-rabbit IgG (1:200). (B) Real-time PCR of the copy numbers of PEDV mRNA in Vero E6 cells infected at an MOI of 1.0 and in IECs infected at an MOI of 10 at 72 h post-infection. All data are expressed as mean±SD. ⁎p<0.05; ⁎⁎p<0.01. (C) Vero E6 and IECs were infected by PEDV at an MOI of 1 or 10 and incubated for 48 h. The cells were fixed with glutaraldehyde followed by 4% osmic acid. Sections were visualized by transmission electron microscopy. The PEDV particles are shown (black arrows indicate virus particles).
We have passaged PEDV in IECs more than 10 times, and at each passage the presence of virus was confirmed by RT-PCR and IFA (data not shown). Our results indicate that IECs, which are derived from a target tissue for porcine coronaviruses, can be successfully infected by PEDV and the virus titer on IECs is about 8×105 TCID50/ml.
Expression of pAPN in IECs enhances susceptibility to infection by PEDV
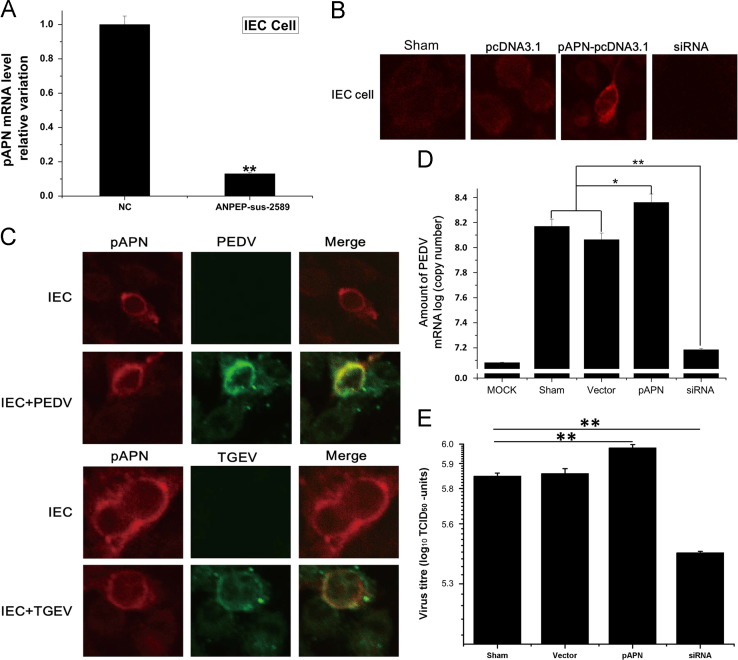
To determine the importance of pAPN for PEDV infection, we analyzed the expression of APN in IECs in more detail. The level of pAPN-mRNA was reduced in siRNA-transfected IECs by about 90%, as determined by real-time PCR ( Fig. 2A). The effect of siAPN was also evaluated in IECs by immunofluorescence analysis. As shown in Fig. 2B, mock-treated IECs and cells transfected with pcDNA3.1 showed weak expression of endogenous pAPN. Bright fluorescence was observed in cells transfected with pcDNA3.1-pAPN and almost no fluorescence signals were detectable in siRNA-transfected cells. Then we evaluated the infection of IECs by PEDV. Cells transfected with pcDNA3.1-pAPN were infected by either PEDV or TGEV. As shown in Fig. 2C, the staining of PEDV antigen co-localized with the staining of pAPN. Co-staining was also observed when the transfected cells were analyzed for TGEV antigen. This result was confirmed by real-time PCR analysis and virus titration. Fig. 2D and E shows the increase in the copy numbers of PEDV-mRNA and virus titre in pcDNA3.1-pAPN transfected IECs and the decline in siRNA-transfected cells.
Fig. 2.
Expression of pAPN in IECs provided susceptibility to PEDV. (A) Silencing efficiency of siRNA targeting pAPN. IECs were transfected with APN-siRNA (ANPEP-sus-2589), 24 h later, the total RNA was analyzed by real-time PCR. RNA expression levels were normalized to â-actin. NC (negative control) represents IECs transfected with an unrelated siRNA control. All data are expressed as mean±SD. *p<0.05; **p<0.01. (B) IECs were transfected with plasmid pcDNA3.1-pAPN.Cells were fixed at 24 h pi and processed for indirect immunofluorescence using anti-pAPN polyclonal antibody (1:200) and TRITC-labeled goat anti-rabbit IgG (1:200). (C) IECs were transfected with plasmid pcDNA3.1-pAPN and 24 h later infected with TGEV (MOI=1) and PEDV (MOI=10). Cells were fixed at 48 h pi and processed for indirect immunofluorescence using anti-TGEV polyclonal antibody (1:200) or anti-PEDV polyclonal antibody (1:100) and FITC-labeled goat anti-rabbit IgG (1:200). Top row: IECs are not infected PEDV. Second row: IECs are infected PEDV. Third row: IECs are not infected TGEV. Bottom row: IECs are infected TGEV. (D) Real-time PCR of the copies of PEDV-RNA in IECs expressing pAPN after infection by PEDV at an MOI of 10. IECs transfected control siRNA and pcDNA3.1 are as controls. All data are expressed as mean±SD. ⁎p<0.05; ⁎⁎p<0.01. (E) Virus titre of PEDV in IECs expressing pAPN after infection by PEDV at an MOI of 10. IECs transfected control siRNA and pcDNA3.1 are as controls. All data are expressed as mean±SD. ⁎p<0.05; ⁎⁎p<0.01.
Our results indicate that pAPN plays a significative role in PEDV infection.
Entry of PEDV into polarized epithelial cells
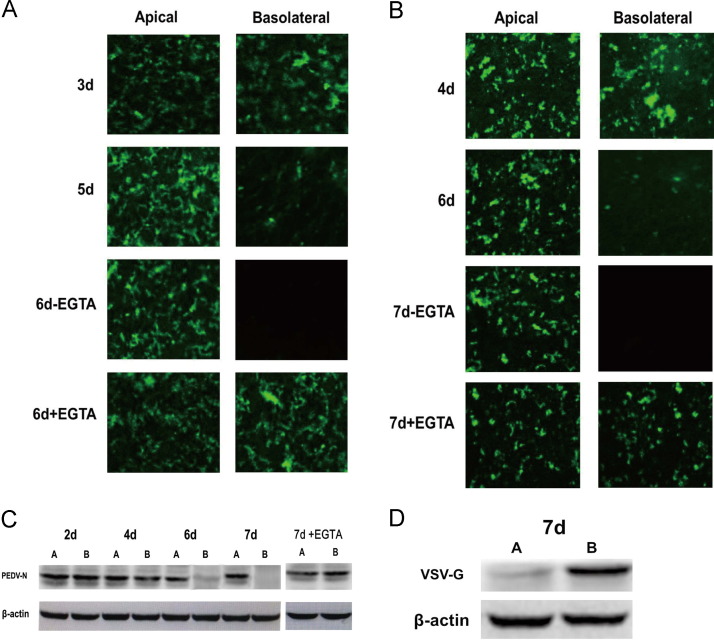
The entry of PEDV into susceptible Vero E6 and IECs was investigated. After several days of culture, the filter-grown cells had formed tight monolayers. For Vero E6 cells we have shown previously that cell develop a transepithelial electrical resistance (Ren et al., 2006). For convenience, the tightness of the monolayers was determined by adding medium (phenol red) to the apical compartment to a slightly higher level than in the basolateral compartment (Cerneus et al., 1993). No leakage of culture medium from the apical to the basolateral compartment occurred from 4 to 8 day post-seeding (dps), indicating that Vero E6 and IEC cell monolayers were already completely tight by 4–8 dps (Rossen et al., 1994). To determine the polarity of PEDV entry into Vero E6 and IECs, cell monolayers were inoculated with PEDV from either site of the cell layer, i.e. virus was added to the medium in the upper or lower chambers facing the apical or basolateral site of the epithelial cell layer, respectively. The efficiency of infection was monitored by IFA in cells infected at the indicated times post-seeding. As shown in Fig. 3A (Vero E6 cells) and B (IECs), PEDV infected cells from the apical site at all times points analyzed. By constrast, basolateral infection was successful only up to three (Vero) or four dps (IEC), respectively. Two days later, the susceptibility of both cell lines to basolateral infection was greatly reduced and by day six (Vero) and seven (IEC), both cell lines were resistant to infection, if the inoculum was applied to the basolateral chamber (Fig. 3A and B). This result illustrates the progress of polarization of these cells and shows that PEDV is able to infect polarized cells via the apical membrane domain. This conclusion is supported by the finding that treatment with ethylene glycol tetraacetic acid (EGTA) prior to inoculation rendered the cells susceptible to infection from the basolateral site (Figs. 3A and B). Depletion of calcium by EGTA treatment abolishes the functional integrity of the tight junctions between the cells and thus the polarized distribution of virus receptors (Rossen et al., 1994).
Fig. 3.
Polarized entry of PEDV in polarized epithelial cells. (A) Vero E6 cells were seeded on polycarbonate filters. At 3 days, 5 days and 6 days post-seeding, cells were infected from either the apical or the basolateral site with PEDV at an MOI of 0.5. At 6 days, the Vero E6 cells were treated with 30 mM EGTA for 15 min prior to infection by PEDV. Cells were fixed and processed for indirect immunofluorescence analysis using anti-PEDV polyclonal antibodies (1:100) and FITC-labeled goat anti-rabbit IgG (1:200). The left column shows the apical and the right column the basolateral infection. (B) IECs were seeded on polycarbonate filters. At 4 days, 6 days and 7 days post-seeding, cells were infected from either the apical or the basolateral site with PEDV at an MOI of 5. At 7 days post-seeding, the IECs were treated with 30 mM EGTA for 15 min prior to infection by PEDV. Cells were fixed and processed for indirect immunofluorescence analysis using anti-PEDV polyclonal antibodies (1:100) and FITC-labeled goat anti-rabbit IgG (1:200). The left column shows the apical and the right column the basolateral infection. (C) The nucleocapsid protein synthesis in IECs infected with PEDV from the apical or basolateral site. Cells were infected from the apical (lanes A) or basolateral site (lanes B) at the indicated times after 24 h. At 7 days post-seeding, the IECs were treated with EGTA for 15 min prior to infection by PEDV. Proteins were separated in an SDS-10% polyacrylamide gel. Viral proteins were precipitated from the cell lysates with a monoclonal antibody PEDV-N (1:500) and β-actin served as a protein loading control (1:1000). (D) The envelope glycoprotein synthesis in IECs infected with VSV from the apical or basolateral site. Cells were infected from the apical (lanes A) or basolateral site (lanes B) at 7 days post-seeding. Proteins were separated in an SDS-10% polyacrylamide gel. Viral proteins were precipitated from the cell lysates with polyclonal antiserum VSV-G (1:500).
To further analyze the entry of PEDV into polarized IECs, virus-specific protein synthesis within filter-grown cells infected from the apical or basolateral sites, at different times post-seeding, was analyzed by Western blot. In cells infected at 2 or 4 dps, viral N protein was detectable irrespective of the site of inoculation, via the apical or basolateral plasma membrane (Fig. 3C). At 6 dps, PEDV infection via the basolateral compartment was decreased and infection by PEDV at 7 dps was restricted to the apical site. Again, cells grown for 7 days on filters and treated with EGTA prior to infection showed similar amounts of viral N protein by Western blot analysis, irrespective of apical or basolateral application of the virus inoculum (Fig. 3C). As a control, VSV G protein was analyzed in a similar way by Western blot (Fig. 3D). IECs were infected by the VSV preferentially from the basolateral domain, which is a characteristic feature of the VSV infection of polarized cells.
Release of PEDV from polarized epithelial cells
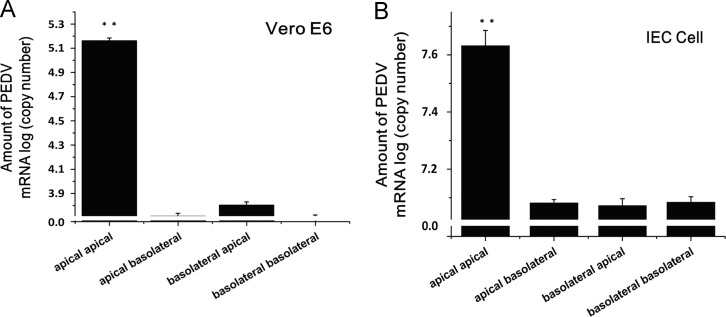
To investigate the release of PEDV from the polarized cells, the copy numbers of PEDV genomic RNA in the apical and basolateral media were determined by real-time PCR. PEDV was released from Vero E6 cells mainly into the apical medium ( Fig. 4A). The same result was obtained when IECs were analyzed (Fig. 4B).
Fig. 4.
Release of PEDV in polarized epithelial cells. PEDV was used to infect either polarized Vero E6 at an MOI of 0.5 or IECs at an MOI of 5, and 24 h later, the apical and the basolateral medium were collected for extracting RNA. Real-time PCR was performed to determine the copies of PEDV genomic RNA. All data are expressed as mean±SD. ⁎p<0.05; ⁎⁎p<0.01.
pAPN mediated the infection of PEDV in polarized IECs
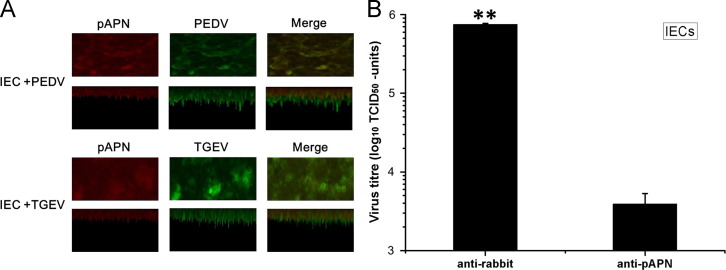
To confirm the pAPN distribution in plasma membrane, the polarized IEC cells were examined in confocal microscopy, which showed the fluorescence intensity distribution of pAPN and virus from the basolateral to apical (Second row and forth row, from the bottom up). As shown in Fig. 5A, the scanning of PEDV antigen co-localized with pAPN in polarized IECs (first row). As a control, the co-staining was also observed in TGEV-infected IECs (third row) (Rossen et al., 1994). For the z-scan of polarized IECs, the pAPN was restricted to the apical site which agreed with PEDV and TGEV.
Fig. 5.
pAPN mediated the infection of PEDV in polarized IECs (A) IECs were seeded on polycarbonate filters for 7 days and cells were infected with PEDV (5 MOI) or TGEV (2 MOI). Cells were fixed and processed for laser confocal microscopy using anti-PEDV mouse polyclonal antibody (1:100) or anti-TGEV mouse polyclonal antibody (1:200) and anti-pAPN rabbit polyclonal antibody (1:100). Top row: The polarized IECs are infected by PEDV. Second row: The z-scan of PEDV infection in polarized IECs, basolaterial to apical (from the bottom up). Third row: The polarized IECs are infected TGEV. Bottom row: The z-scan of TGEV infection in polarized IECs, basolaterial to apical (From the bottom up). (B) The working rabbit anti-pAPN antibody was heat inactivated at 55 °C for 1 h and incubated from the apical side of polarized IECs for 1 h. Then the IECs were infected with PEDV (5 MOI). At 24 hpi and the apical medium was collected for virus titration. The virus titers were determined with Vero E6 cells. A serum from a non-immunized rabbit served as a control. All data are expressed as mean±SD. ⁎p<0.05; ⁎⁎p<0.01.
To further determine whether pAPN serves as a receptor for PEDV in polarized IECs, an inhibition experiment was performed. Prior to inoculation with heat inactivated rabbit anti-pAPN antibody or negative serum for 1 h from the apical site. As shown in Fig. 5B, the release of progeny virus into the culture medium in IECs were calculated and it was significantly decrease in polarized IECs using anti-pAPN antibody, but almost no decrease in using rabbit negative serum. Thus, the entry of PEDV into polarized IECs is mediated by pAPN.
Discussion
The in vitro adaptation of PEDV to growth in Vero cells by serial passage in this culture system was first reported in 1988 (Hofmann and Wyler, 1988). Recently, Zhao et al. showed IPEC-J2 cells (a model of normal intestinal epithelial cells) were susceptible to TGEV and PEDV infection through destroying the epithelial barrier (Zhao et al., 2014). Since then, little information has been reported regarding the polarized infection characteristics of this virus. To further determine whether a cell line derived from the target tissue was susceptible to PEDV infection, immortalized epithelial cells of the swine small intestine (IEC) were investigated in this study. A porcine intestinal epithelial cell (IECs) line was used by Xu et al., 2013a, Xu et al., 2013b to investigate the subcellular localization and function of PEDV N protein. The analysis of IECs for susceptibility to infection was of interest since enterocytes are the target cells of PEDV. In contrast to other group I coronaviruses like TGEV, a distinct characteristic of PEDV infection is the requirement of trypsin in the medium for in vitro infection. Interestingly, we found that PEDV can be serially propagated in IECs in the presence of trypsin. PEDV was detected by real-time PCR in intracellular and extracellular spaces in IECs (Fig. 1B). We were able to serially propagate PEDV on IECs for 10 passages and the virus titer was 8×105 TCID50/ml. We assume that PEDV can be adapted to IECs, as these cells are derived from the natural target organ of PEDV, the porcine intestine. It remains to be shown in the future that the failed isolates of PEDV can be grown on IEC or whether they also need adaptation like CV777 which has to be adapted to be growth in Vero E6 cells as several blind passages.
Previous reports suggested that pAPN may play a role in mediating PEDV infection (Oh et al., 2003, Li et al., 2007, Nam and Lee, 2010); our data confirm the importance of pAPN for PEDV infection. APN is abundantly expressed in the brush border membrane of the small intestine and the kidney, whereas its expression is detected to a lesser extent in the liver, lung and colon. In ST cells, pAPN is expressed in minor amounts (Delmas et al., 1992, Nam and Lee, 2010, Kenny and Maroux, 1982). A recent report showed that over-expression of pAPN in ST cells could rescue PEDV replication in the ST cells (Oh et al., 2003). We found that pAPN is expressed in higher amounts in IECs than it is in ST cells (data not shown). To examine whether pAPN serves as an essential receptor for PEDV, we analyzed whether an increase or decrease of pAPN expression affects infection by PEDV. High expression levels of pAPN were found to increase the susceptibility of the cells to infection by PEDV (Fig. 2C). Interference with pAPN expression in IECs by siRNA inhibited PEDV infection. The immune electron microscopy (IEM) results are also consistent with PEDV entry into IECs via pAPN. Taken together, these data indicate that pAPN plays a crucial role in PEDV infection. Like several other coronaviruses, such as FCoV, TGEV, CCoV, and HCoV-229E that use APN as a receptor (Tusell et al., 2007), PEDV also enters cells by a pAPN-dependent mechanism. There is, however, also a difference between PEDV and TGEV as far as the involvement of APN in infection is concerned. PEDV requires larger amounts of this protease. ST cells which express a low amount of APN are susceptible to TGEV infection but refractory to PEDV infection (Nam and Lee, 2010). On the other hand, Vero cells are susceptible to infection by PEDV but resistant to infection by TGEV. These differences suggest that recognition of APN by PEDV is characterized by a lower affinity and by a less stringent species specificity when compared to the interaction of TGEV with APN. Ongoing research tries to find out whether APN of Vero E6 cells can interact with PEDV. To explain the differences between PEDV and TGEV, one should also consider the possibility that a factor different from APN may also contribute to the entry process of PEDV. Cooperation with this factor and with APN may be required for efficient infection of the target cells of PEDV, the intestinal epithelial cells.
As epithelial cells are primary target cells for coronaviruses such as PEDV and also essential entities required for virus pathogenesis (Tashiro et al., 1990), we explored the interaction of PEDV with polarized Vero E6 cells and IECs. Our data demonstrate that PEDV enters Vero E6 and IECs preferentially via the apical plasma membrane and is also released from this site of polarized epithelial cells. This finding is consistent with a localized infection restricted in the intestine. Several studies have investigated the interaction of coronaviruses and other viruses with epithelial cells using a similar approach (Ren et al., 2006, Rossen et al., 1994, Wang et al., 2000, Jia et al., 2005, Tseng et al., 2005, Rossen et al., 2001, Rossen et al., 1995, Rossen et al., 1996, Pratelli, 2011, Tao et al., 2013, Fuller et al., 1984). Though entry into and egress from polarized cells for several coronaviruses is restricted to the apical plasma membrane, other coronaviruses show a different pattern (see Introduction). Therefore, entry and release of PEDV could not be predicted. Our results show that both the early and late phase of the replication cycle of PEDV is associated with the apical site of polarized cells. The co-localization of pAPN and PEDV in polarized IECs as well as the observations confocal microscopy (Fig. 5 A) highlight the importance of pAPN for the polarized infection by PEDV (Fig. 5B).
In summary, a target cell line (IECs) has been identified that is permissive for PEDV and allows serial virus propagation. pAPN facilitates infection and, therefore, may function as a receptor for PEDV in IECs; this conclusion is consistent with the colocalization of APN and PEDV antigen in infected cells. In addition, we provide the first report regarding the polarized infection of Vero E6 and IECs by PEDV. The entry and release of PEDV occurred at the apical surface in polarized Vero E6 and IECs consistent with the lateral spread of PEDV in the intestinal epithelium.
Materials and methods
Cells, virus, antisera and reagents
Vero E6 and immortalized epithelial cells from the swine small intestine (IECs) were available from the cell collection of our institute which was isolated from the ileum of a new-born piglet (Wang et al., 2010, Xu et al., 2013a, Xu et al., 2013b). Vero E6 cells were maintained with Dulbecco׳s modified Eagle׳s medium (DMEM; Gibco) containing 10% fetal calf serum (FCS). Intestinal epithelial cells (IECs) were maintained with Dulbecco׳s modified Eagle׳s F12 Ham medium (DMEM-F12) containing 10% FCS. PEDV strain CV777, TGEV strain PUR46-MAD and vesicular stomatitis virus (VSV) strain Indiana were grown as previously described (Hofmann and Wyler, 1988, Ren et al., 2008, Yin et al., 2010)
The following rabbit polyclonal antibodies were generated in our laboratory and used in this study: anti-pAPN, anti-PEDV and anti-TGEV antibodies (Meng et al., 2014, Lv et al., 2014). A monoclonal antibody against the PEDV N protein was also generated in our laboratory. An anti-VSV G-protein antibody was purchased from Abcam. A monoclonal anti-â-actin antibody was purchased from Sigma. The secondary antibodies were purchased from BD Biosciences. Porcine aminopeptidase N small interfering RNA (siRNA) was purchased from GenePharma Co., Ltd. (Shanghai, China).
Infections
For analysis of the susceptibility of cells to PEDV infection, Vero E6 and IECs were inoculated with PEDV at a multiplicity of infection (MOI) of 0.1, 1 or 10 in the presence of 2.5 ìg/ml of trypsin (Sigma, USA). After the cells had been incubated at 37 °C for 1 h, unadsorbed virus was washed away with phosphate-buffered saline (PBS), pH 7.4, and DMEM or DMEM-F12 was added to the cells. At 12 h, 24 h, 36 h, 48 h and 72 h post-infection, the Vero E6 and IECs, as well as individual supernatants were collected and used for titration assays, real-time PCR assays, indirect immunofluorescence assays, Western blot and transmission electron microscopy.
For infection of polarized cells by PEDV in Vero E6 and IECs, polycarbonate membrane filters attached to the bottom of plastic cups (Transwell inserts, 0.4 μm; Costar Corp., Cambridge, Mass) were placed into 24-well tissue culture plates. Subsequently, 100 μl of cell suspension containing 1×104 cells were added to each filter, while 600 μl/well of cell growth medium was added to each well of the 24-well plate. The tightness of the monolayers was checked by adding medium to the upper chambers up to a slightly higher level than in the lower chamber (Rossen et al., 1994, Cerneus et al., 1993). Routinely every other day, the growth medium was replaced to achieve optimal cell growth. Filter-grown Vero E6 cells and IECs were infected with PEDV at an MOI of 0.5 or 5 from either the apical or the basolateral site at different time points after the cells had become highly polarized as determined by adding medium to the apical compartment up to a slightly higher level than in the basolateral compartment. Infection was allowed to take place for 1 h at 37 °C, after which the inoculum was removed. The filter-grown cells were washed with PBS and culture medium was added. IECs infected by VSV at an MOI of 0.5 were used as control. The infected cells and supernatants were used for titration assays, real-time PCR assays, indirect immunofluorescence assays and Western blot.
Virus titration
The amounts of infectious PEDV particles released into the media of infected cells were determined as tissue culture infectious doses (TCID50) on Vero E6 cells. PEDV was collected from the Vero E6 cells when the cytopathic effect (CPE) was observed and cells revealed visible lesions. Monolayers of cells were inoculated with serial dilutions of the PEDV in DMEM containing 2.5 μg/ml trypsin. After 1 h, the inoculum was replaced by culture medium. Cells were incubated at 37 °C, and after 3 days, CPE was observed.
Real-time PCR
Quantification of virus and pAPN was determined by the real-time PCR assay. Briefly, the total RNA was extracted from cells and supernatants to quantify the copies of PEDV, and the cell RNA was for quantification of pAPN with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer׳s instructions. First-strand cDNA was synthesized by using Moloney murine leukemia virus (M-MLV) reverse transcriptase and oligo (dT) (both reagents were from Takara Bio Inc., Shiga, Japan). Real-time PCR was performed using an ABIPRISM 7500 RT-PCR machine (Applied Biosystems, Carlsbad, CA), and under the following conditions: 10 s at 95 °C, followed by 40 cycles of 5 s at 95 °C, 34 s at 60 °C. The copy numbers of viral RNA were calculated with reference to the standard curve obtained from a serially diluted DNA eukaryotic expression plasmid bearing the full-length PEDV S gene (pcDNA3.1-PEDV-S) that contained a target region sequence in the S gene (up: 5′-GTGCTGTTTATTCTGTCACGC; low: 5′-CTCTGTACAATTGGAGCCGTC; position: 2066–2229 in S gene.). The expression level of pAPN (sense primer: 5′-GGGAACTTGCCGACGAC; antisense primer: 5′-TTGGACAGGGCCGTGAG; position: 536–728 in pAPN gene) was normalized to that of â-actin (sense primer: 5′-GGCTCAGAGCAAGAGAGGTATCC, antisense primer: 5′-GGTCTCAAACATGATCTGAGTCATCT) according to the comparative cycle threshold (CT) method used for quantification as recommended by the manufacture׳s protocol.
Confocal microscopy
To determine the optimal virus titers, the Vero E6 and IECs were infected by PEDV at an MOI of 0.1 or 10. The cells were incubated with PEDV and TGEV for 48 h at 37 °C.
IFA was then used to determine the expression level of pAPN in IECs and ST cells using Vero E6 cells as a control group. At 24 h after transfection of IECs and ST cells with plasmid-expressing pAPN (pcDNA3.1-pAPN) or empty vector pcDNA3.1 alone using Lipofectamine Plus according to the manufacturer׳s instructions (Invitrogen, Carlsbad, CA), the cells were incubated with PEDV or TGEV at an MOI of 1 for 48 h at 37 °C; cells infected with TGEV served as a control.
For immunofluorescence analysis of the PEDV entry into polarized Vero E6 cells and IECs, the cells grown on permeable supports were inoculated with PEDV at an MOI of 0.5 or 5 for 24 h from the apical or the basolateral site. At different time points, the cells were rinsed once with PBS and fixed in 4% formaldehyde for 15 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min at room temperature and incubated with the anti-pAPN, anti-PEDV or anti-TGEV antibody (1:100 or 1:200 dilution) for 2 h followed by fluoresceine isothiocyanate (FITC)-labeled goat anti-rabbit or anti-mouse IgG (H+L) secondary antibody (1:200 dilution) for 1 h. Images were all taken using TCS SP2 AOBS confocal microscope (Leica, Wetzlar, Germany).
Small interfering RNA transfection
ST and IECs were grown to approximately 80% confluence and then transiently transfected with pAPN siRNA at a concentration of 40 nM, 80 nM, or 160 nM using Lipofectamine Plus. A scrambled siRNA was used as a negative control. The silencing efficiency was determined by real-time PCR. The sequence of the primers used for the amplification of pAPN is provided above. Twenty-four hours after transfection, the cells were infected with PEDV or TGEV as described above prior to IFA and real-time PCR.
Western blot
Cells were lysed in 0.1% Triton X-100 in phosphate-buffered saline (PBS), and the total protein was separated on a 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (SDS-PAGE) and transferred to nitrocellulose membrane. Mouse anti-PEDV-N monoclonal primary antibody or rabbit anti-VSV-G polyclonal primary antibody (1:500 dilution, room temperature, 4 h), Horseradish peroxidase (HRP)-labeled goat anti-mouse or anti-rabbit IgG (H+L) secondary antibody (1:2500 dilution, room temperature, 1 h) was used.
Transmission electron microscopy
For ultrastructural analysis, Vero E6 and IECs were mock-infected or infected with PEDV at an MOI of 1 or 10 for 48 h. The cells were fixed in 2.5% glutaraldehyde in PBS, pH 7.2, for 10 min at room temperature. After three washes in PBS, the cells were post-fixed in 1% osmium tetroxide, 1% potassium ferrocyanide, 100 mM sodium cacodylate buffer, pH 7.4, for 1 h at room temperature. After several rinses in distilled water, the cells were dehydrated through a graded series of acetone washes and embedded in Eponate 12 resin (Ted Pella Inc., Altadena, CA). Sections of 70–80 nm were viewed using a Tecnai 12 transmission electron microscope.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31340003 and 31372438), and in part supported by Grants from the Ministry of Science and Innovation of Spain (BIO2010-16705).
References
- Adams M.J., Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 2012;157(7):1411–1422. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Britton P. Coronaviruses: general features. In: B.W.J. Mahy, M.H. van Regenmortel., editors. Encyclopedia of Virology. third edition. Academic Press; New York: 2008. pp. 549–554. [Google Scholar]
- Cerneus D.P., Strous G.J., van der Ende A. Bidirectional transcytosis determines the steady state distribution of the transferrin receptor at opposite plasma membrane domains of BeWo cells. J. Cell Biol. 1993;122:1223–1230. doi: 10.1083/jcb.122.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Ren X. Coronavirus entry and release in polarized epithelial cells: a review. Rev. Med. Virol. 2014;24(5):308–315. doi: 10.1002/rmv.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L’Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature. 1992;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M., Laude H. Sequence of the spike protein of the porcine epidemic diarrhea virus. J. Gen. Virol. 1994;75(Pt 5):1195–1200. doi: 10.1099/0022-1317-75-5-1195. [DOI] [PubMed] [Google Scholar]
- Egberink H.F., Ederveen J., Callebaut P., Horzinek M.C. Characterization of the structural proteins of porcine epizootic diarrhea virus, strain CV777. Am. J. Vet. Res. 1988;49:1320–1324. [PubMed] [Google Scholar]
- Fuller S.D., von Bonsdorff C.H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984;38:65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26(11):2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford C., Perlman S., McCray P.B. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A.J., Maroux S. Topology of microvillar membrane hydrolases of kidney and intestine. Physiol. Rev. 1982;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365:166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X., Wang P., Bai R., Cong Y., Suo S., Ren X., Chen C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 2014;35(13):4195–4203. doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Suo S., Zarlenga D.S., Cong Y., Ma X., Zhao Q., Ren X. A phage-displayed peptide recognizing porcine aminopeptidase N is a potent small molecule inhibitor of PEDV entry. Virology. 2014;456–457:20–27. doi: 10.1016/j.virol.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol. Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam E., Lee C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet. Microbiol. 2010;144(1-2):41–50. doi: 10.1016/j.vetmic.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S., Song D.S., Park B.K. Identification of a putative cellular receptor 150 kDa polypeptide for porcine epidemic diarrhea virus in porcine enterocytes. J. Vet. Sci. 2003;4(3):269–275. [PubMed] [Google Scholar]
- Pratelli A. Basic science track. Entry and release of canine coronavirus from polarized epithelial cells. New Microbiol. 2011;34(1):25–32. [PubMed] [Google Scholar]
- Ren X., Glende J., Al-Falah M., de Vries V., Schwegmann Wessels C, Qu X., Tan L., Tschernig T., Deng H., Naim H.Y., Herrler G. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006;87:1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- Ren X., Glende J., Yin J., Schwegmann-Wessels C., Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137(2):220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W., Bekker C.P., Voorhout W.F., Strous G.J., van der Ende A., Rottier P.J. Entry and release of transmissible gastroenteritis coronavirus are restricted to apical surfaces of polarized epithelial cells. J. Virol. 1994;68(12):7966–7973. doi: 10.1128/jvi.68.12.7966-7973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W., Voorhout W.F., Horzinek M.C., van der Ende A., Strous G.J., Rottier P.J. MHV-A59 enters polarized murine epithelial cells through the apical surface but is released basolaterally. Virology. 1995;210(1):54–66. doi: 10.1006/viro.1995.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W., Bekker C.P., Strous G.J., Horzinek M.C., Dveksler G.S., Holmes K.V., Rottier P.J. A murine and a porcine coronavirus are released from opposite surfaces of the same epithelial cells. Virology. 1996;224:345–351. doi: 10.1006/viro.1996.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen J.W., Kouame J., Goedheer A.J., Vennema H., Rottier P.J. Feline and canine coronavirus are released from the basolateral side of polarized epithelial LLC-PK1 cells expressing the recombinant feline aminopeptidase-N cDNA. Arch. Virol. 2001;146(4):791–799. doi: 10.1007/s007050170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Masuyama S., Ujike M., Taguchi F. Role of proteases in the release of porcine epidemic diarrhea virus from infected cells. J. Virol. 2011;85(15):7872–7880. doi: 10.1128/JVI.00464-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Hill T.E., Morimoto C., Peters C.J., Ksiazek T.G., Tseng C.T. Bilateral entry and release of Middle East respiratory syndrome-coronavirus induces profound apoptosis of human bronchial epithelial cells. J. Virol. 2013;87(17):9953–9958. doi: 10.1128/JVI.01562-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Yamakawa M., Tobita K., Seto J.T., Klenk H.D., Rott R. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J. Virol. 1990;64:4672–4677. doi: 10.1128/jvi.64.10.4672-4677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996;70(12):8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusell S.M., Schittone S.A., Holmes K.V. Mutational analysis of aminopeptidase N, a receptor for several group 1 coronaviruses, identifies key determinants of viral host range. J. Virol. 2007;81(3):1261–1273. doi: 10.1128/JVI.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Tseng J., Perrone L., Worthy M., Popov V., Peters C.J. Apical entry and release of severe acute respiratory syndrome-associated Coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 2005;79(15):9470–9479. doi: 10.1128/JVI.79.15.9470-9479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Deering C., Macke M., Shao J., Burns R., Blau D.M., Holmes K.V., Davidson B.L., Perlman S., McCray P.B., Jr. Human coronavirus 229E infects polarized airway epithelial from the apical surface. J. Virol. 2000;74(19):9234–9239. doi: 10.1128/jvi.74.19.9234-9239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang Y., Tong G., Liu F., Zhou H., He L., Yang X., Xu Y., Hong H. The isolation and identification of neonatal swine intestinal epithelial cells. Acta Vet. Zootech. Sin. 2010;41(1):92–98. [Google Scholar]
- Xu X., Zhang H., Zhang Q., Dong J., Liang Y., Huang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013;10:26. doi: 10.1186/1743-422X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang H., Zhang Q., Huang Y., Dong J., Liang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 2013;164(3–4):212–221. doi: 10.1016/j.vetmic.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.G., Hernandez M., Krell P.J., Nagy E.E. Cloning and sequence analysis of the spike gene of porcine epidemic diarrhea virus Chinju99. Virus Genes. 2003;26:239–246. doi: 10.1023/A:1024443112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Glende J., Schwegmann-Wessels C., Enjuanes L., Herrler G., Ren X. Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus. Antiviral Res. 2010;88(3):311–316. doi: 10.1016/j.antiviral.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Gao J., Zhu L., Yang Q. Transmissible gastroenteritis virus and porcine epidemic diarrhoea virus infection induces dramatic changes in the tight junctions and microfilaments of polarized IPEC-J2 cells. Virus Res. 2014;192:34–45. doi: 10.1016/j.virusres.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]