Abstract
Three phage-displayed peptides designated H, S and F that recognize porcine aminopeptidase N (pAPN), the cellular receptor of porcine transmissible gastroenteritis virus (TGEV) were able to inhibit cell infection by TGEV. These same peptides had no inhibitory effects on infection of Vero cells by porcine epidemic diarrhea virus (PEDV). However, when PEDV, TGEV and porcine pseudorabies virus were incubated with peptide H (HVTTTFAPPPPR), only infection of Vero cells by PEDV was inhibited. Immunofluoresence assays indicated that inhibition of PEDV infection by peptide H was independent of pAPN. Western blots demonstrated that peptide H interacted with PEDV spike protein and that pre-treatment of PEDV with peptide H led to a higher inhibition than synchronous incubation with cells. These results indicate direct interaction with the virus is necessary to inhibit infectivity. Temperature shift assays demonstrated that peptide H inhibited pre-attachment of the virus to the cells.
Keywords: Peptides, Coronavirus, Virus attachment, PEDV
Highlights
-
•
The pAPN-recognizing peptide H was characterized.
-
•
Inhibition of peptide H was independent of pAPN.
-
•
Peptide H interacted with PEDV spike.
-
•
Peptide H inhibited pre-attachment of the virus to the cells.
Introduction
Coronaviruses belong to the family of Coronaviridae and commonly cause respiratory or gastroenteric diseases (Weiss and Navas-Martin, 2005, Lai et al., 2007). Three groups of coronaviruses have been identified, based on differences in serology and genotyping (Cavanagh, 1997, Spaan et al., 2005). These are enveloped viruses and consist of four major structural proteins: spike (S), membrane (M), nucleocapsid (N) and minor small envelop (E) protein (Lai et al., 2007).
The host range and tissue tropism of coronaviruses depend on interactions between the viral S glycoprotein and receptors on susceptible cells (Bosch et al., 2003, Gallagher and Buchmeier, 2001). Porcine transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV) are swine-specific enteric coronaviruses that are antigenically distinguishable (Lai et al., 2007, Pensaert and Yeo, 2006). However, they replicate in the differentiated enterocytes of the small intestine resulting in similar clinical symptoms including lethal watery diarrhea and dehydration in piglets (Pensaert and Yeo, 2006, Sanchez et al., 1992). Two decades ago, porcine aminopeptidase N (pAPN) was identified as a cellular receptor for TGEV (Delmas et al., 1992). Since that time, several limited reports have showed that addition of exogenous pAPN facilitates cell infection by PEDV (Li et al., 2007, Oh et al., 2003.). Recent evidence also indicates that increased pAPN receptor density on the surface of ST cells contributes to cell infection by PEDV (Nam and Lee, 2010). The available data supports the hypothesis that blockage of pAPN is a good strategy for preventing cell infection by TGEV or PEDV. Using the pAPN as a target protein, we identified three 12-mer peptides (designated as H, S or F) by phage display which bind to pAPN and competitively inhibit cell infection by TGEV (Ren et al., 2011a).
The initial purpose of this study was to investigate the role of pAPN-binding peptides H, S and F on cell infection by PEDV. Interestingly, although there was no surface expression of pAPN on Vero cells, peptide H decreased the infectivity of PEDV in vitro. Western blots indicated that peptide H (HVTTTFAPPPPR) interacted with the S protein of PEDV. Altering incubation temperatures further demonstrated that peptide H affected pre-attachment of PEDV to cells. It is important to identify small molecules such as peptides that prevent infection by PEDV, inasmuch as highly effective PEDV vaccines which are currently not available. The peptide H identified herein may be one such candidate.
Results
Cytotoxicity of peptides H, S and F in Vero cells
The cytotoxicities of peptides H, S and F were evaluated by the MTT assay. The CC50 was calculated and defined as the critical concentration of compound that decreased the percentage of formazan produced in uninfected, peptide-treated cells to 50% of that produced in uninfected, peptide-free cells. The CC50 values were greater than 1000 μg/ml. All subsequent antiviral experiments were performed at peptide concentrations below the experimentally-determined CC50 value.
Inhibitory effect of peptides H, S and F on PEDV infection of Vero cells
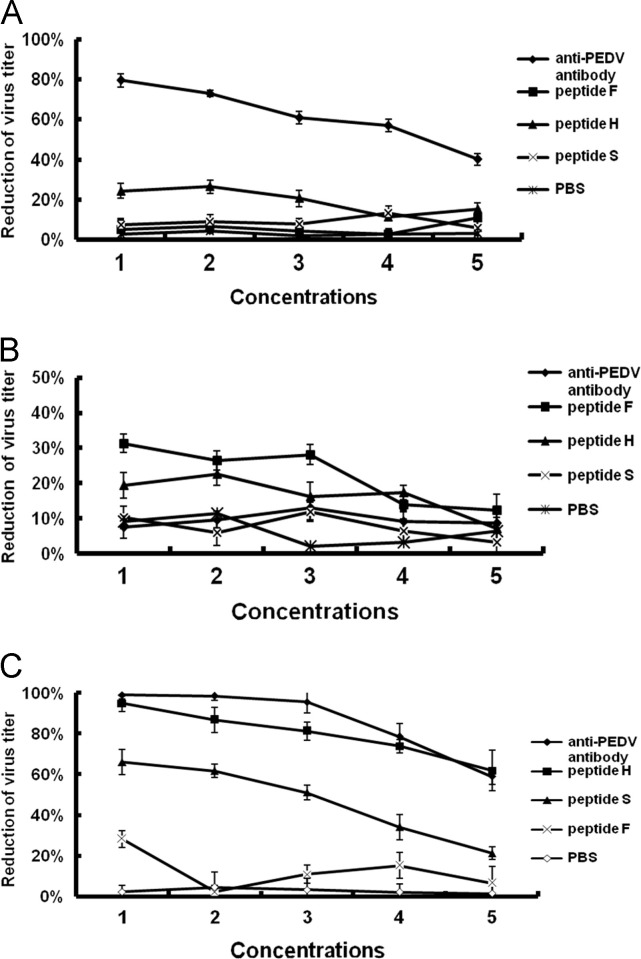
In order to test the abilities of the three peptides to prevent attachment of PEDV to cells, all combinations of peptide, virus and cell treatment were performed. Cell post-treatment assays ( Fig. 1A) were performed to evaluate whether the three peptides were able to inhibit replication of PEDV after infecting Vero cells. Plaque assays indicated that none of the three peptides inhibited PEDV infection; however, rabbit anti-PEDV decreased vial infectivity by more than 50% when the dilutions were reduced from 1:64 to 1:4. When Vero cells were pre-treated with peptide (cell pre-treatment assay) prior to virus infection (Fig. 1B), little changes in virus titers were observed between the control and peptide treatment groups; some small effects were observed with the rabbit anti-PEDV neutralizing antibodies. Finally, in the virus pre-treatment assays where PEDV was incubated with peptides prior to cell infection (Fig. 1C), the results indicated that both peptides H and S inhibited PEDV infectivity where EC50 values were approximately 1 μg/ml and 62.5 μg/ml, respectively. The antiviral activity of peptide H was dose-dependent and at 250 μg/ml it exhibited greater than 95% anti-PEDV activity which is significantly higher than peptides S or F (p<0.01); at 15.6 μg/ml, inhibition was greater than 70%. The Selectivity indices SI of peptides H and S were 1000 and 16, respectively. Peptide F showed little inhibitory activity against PEDV infection even at concentrations≥1000 μg/ml.
Fig. 1.
Inhibition of pAPN-recoginzing peptides on cell infection by PEDV. (A) PEDV (PFU=5×103/ml) was first incubated with Vero cells at 37 °C for 1 h, followed by the addition of various concentrations of peptides H, S or F. (B) Vero cells were incubated with various concentrations of peptides H, S or F at 37 °C for 1 h, then infected with PEDV at an PFU of 5×103/ml. (C) Peptides H, S or F were first incubated with PEDV at 37 °C for 1 h, and then the peptide treated viruses (PFU=5×103/ml) were used to infect Vero cells at 37 °C. Plaque assays were performed at the end of each experiment. Serially-diluted polyclonal antibody against PEDV and PBS were used as positive and negative controls, respectively. Peptide concentrations 1, 2, 3, 4, and 5 are 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, and 15.625 μg/ml, respectively. Anti-PEDV antibody dilutions 1, 2, 3, 4, and 5 are 1:4, 1:8; 1:16; 1:32; and 1:64, respectively. Bars show the standard deviation from three independent assays.
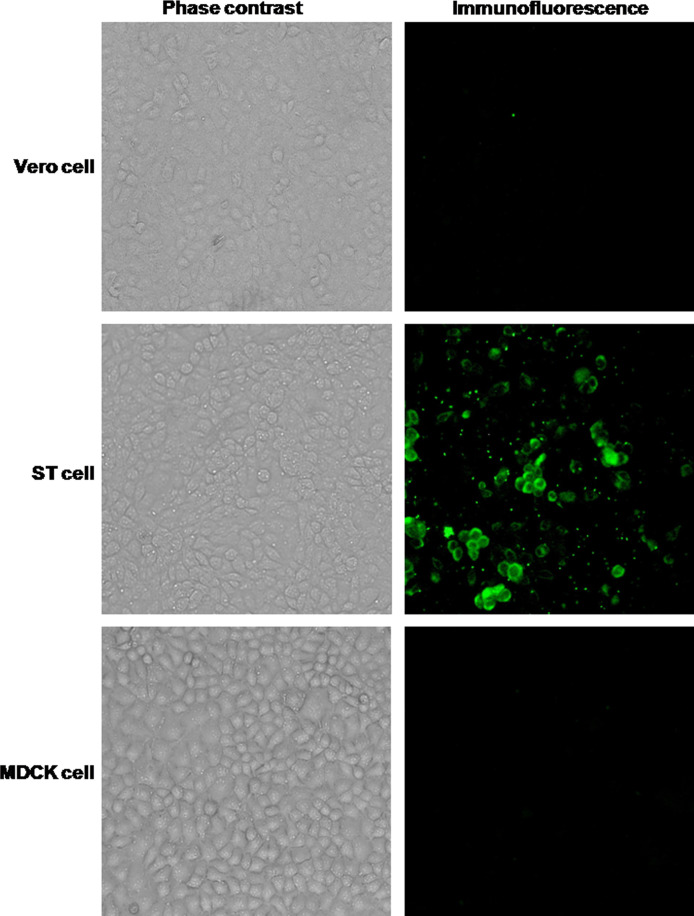
IFA analysis of pAPN expression on Vero cells
Inasmuch as pAPN may be involved in cell infection by PEDV, the existence of pAPN on ST, Vero and MDCK cells was analyzed by IFA. As shown in Fig. 2, the endogenous pAPN expressed only on the surface of ST cells, a porcine cell line. No expression was found on the surfaces of Vero cells or MDCK cells suggesting that the inhibitory activity of peptide H on PEDV infection in vitro did not involve pAPN.
Fig. 2.
Indirect immunofluorescence of pAPN on different cell lines. ST, Vero and MDCK cells were incubated with anti-pAPN antibody followed by FITC-conjugated goat anti-rabbit IgG. Fluorescence images are provided along with representative phase contrast images.
Peptide H specifically inhibits PEDV
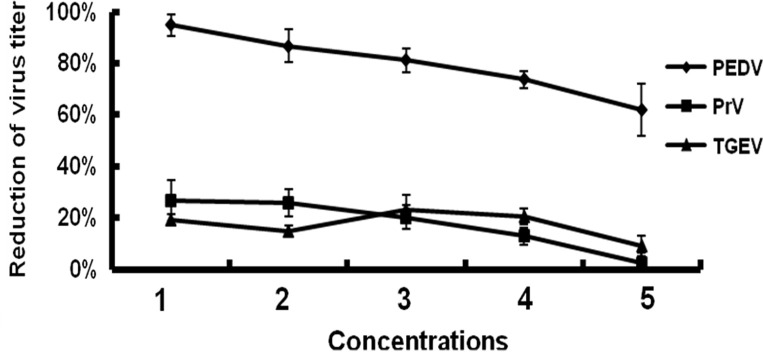
The specificity of the inhibition of peptide H on PEDV infection was assessed by comparing antiviral activities of peptide H on TGEV and PrV. Further, peptide-induced cytotoxicity in ST cells was also evaluated. Results clearly show that peptide H had no demonstrable effects on TGEV or PrV even at very high peptide concentrations (1 mM/ml) ( Fig. 3) suggesting that a non-specific reactivity with virus envelopes is unlikely to be the cause for attenuating PEDV infectivity.
Fig. 3.
Inhibitory specificity of peptide H. Peptide H was incubated with the TGEV, PEDV and PrV at 37 °C for 1 h followed by infection of susceptible cells and plaque assays. The concentrations and reductions in virus titers are indicated. Peptide concentrations 1, 2, 3, 4, and 5 are 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, and 15.625 μg/ml, respectively. Bars show the standard deviation from three independent assays.
Semi-quantitative real-time RT-PCR
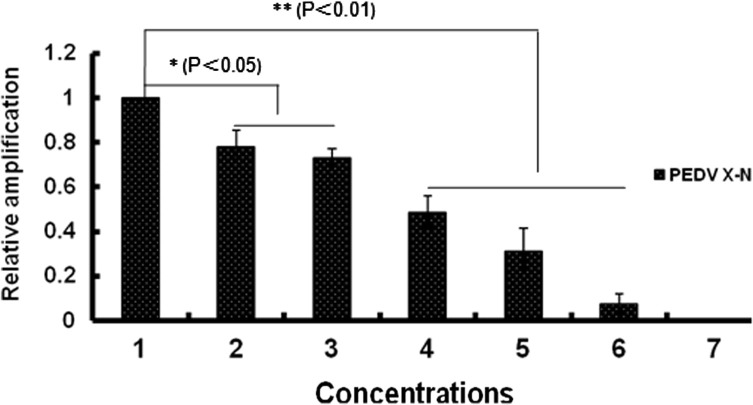
The effect of peptide H on the level of virus RNA was quantified by real-time RT-PCR. The results demonstrated a dose-dependent decrease of viral RNA synthesized in PEDV-infected cells ( Fig. 4). At 31.25 μg/ml and 15.625 μg/ml, peptide H showed reduction of viral RNA synthesis (p<0.05). However, at 250 μg/ml, 125 μg/ml and 62.5 μg/ml it significantly decreased viral RNA synthesis (p<0.01) when compared to the no peptide treatment group. The inhibitory activity of peptide H against PEDV infection was confirmed by conventional RT-PCR. Analysis of the PEDV-RNAs indicated that the density of the amplified sequences decreased with increasing concentrations of peptide H (data not shown).
Fig. 4.
Quantification of PEDV X-N gene. Total RNA was extracted from cell lysates infected with PEDV (PFU=5×103/ml) pretreated with varying concentrations of peptide H. The ΔCt values were measured from triplicate real-time RT-PCR data. Relative amplification of the PEDV X-N gene in the infected cells was normalized to that of beta-actin and calculated using the 2−ΔΔCt method. Peptide concentrations 1, 2, 3, 4, 5, and 6 are 0 μg/ml, 15.625 μg/ml, 31.25 μg/ml, 62.5 μg/ml, 125 μg/ml, 250 μg/ml, respectively; line 7 is cell control group. Displayed results are averages of three independent experiments.
Binding of peptide H to the spike protein of PEDV
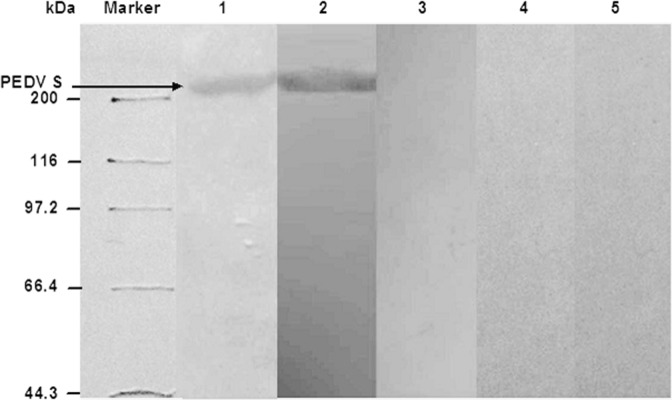
Binding characteristics of the phage H (phage encoding peptide H) to PEDV was analyzed by Western blot. As shown in Fig. 5, the phage H reacted with a protein with an approximate molecular mass of 220 kDa which is coincident with the molecular mass of the PEDV S protein. Antibody against the PEDV S protein was used as a positive control. Other controls including the M13 phage library and two phages bearing non-selected peptides did not react with the PEDV S protein (Fig. 5). These results indicate that the peptide H binds to the S protein of PEDV.
Fig. 5.
Western blot analysis of phage H binding to PEDV. Sedimented PEDV was subjected to SDS-PAGE followed by Western blot on nitrocellulose (NC) membranes. The NC membranes were sliced and incubated with (1) phage H, (2) anti-PEDV S polyclonal antibody, (3) M13 phage library, or control phages bearing the peptides (4) SVSVGMKPSPRP or (5) MSCNDTLCLLPN. The membranes were successively incubated with anti-M13 polyclonal antibody and HRP-conjugated goat anti-rabbit IgG. The protein bands were visualized using 3,3׳-diaminodbenzidine. The sizes of protein markers and location of the PEDV S protein are indicated.
Peptide H inhibits pre-attachment of PEDV
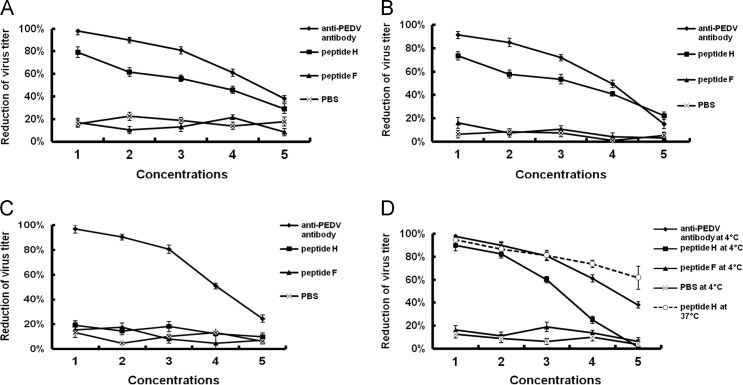
To further examine the mechanism of action of peptide H on cell infection by PEDV, we investigated the effects of incubation temperature on cell infectivity. Plaque assays showed that peptide H exhibited significantly higher inhibitory effects (p<0.01) than peptide F or PBS on the pre-attachment of PEDV to Vero cells ( Fig. 6A) when virus was incubated with peptide H at 4 °C for 1 h prior to incubation with the cells. When peptide H and PEDV were co-incubated with Vero cell at 4 °C for 1 h before shifting to 37 °C (binding only), this allowed us to measure peptide H effects on early- and pre-attachment of the virus. Results showed that PEDV pre-treated with peptide H exhibited slightly higher inhibition (p>0.05) than when peptide H and PEDV were co-incubated with cells absent a pre-incubation step (Fig. 6B) suggesting that there is a direct effect of peptide H on pre-attachment. Little to no inhibition of infection was observed once the virus became attached to the cell surface. Furthermore, when the peptides and PEDV were co-incubated with Vero cells at 37 °C absent any pre-incubation step, no effective inhibition of cell infection was observed (Fig. 6C).
Fig. 6.
Temperature shift and the effect of peptide H on cell infection by PEDV. (A) PEDV was treated with various concentrations of peptide H or with negative control peptide F at 4 °C for 1 h then used to infect Vero cells (PFU=5×103/ml) at 4 °C. (B) PEDV (PFU=5×103/ml), peptides H or F, and Vero cells were co-incubated at 4 °C for 1 h, washed, then shifted to 37 °C. (C) PEDV (PFU=5×103/ml), peptides, and Vero cells were co-incubated at 37 °C for 1 h then washed prior to assaying. (D) PEDV was treated with various concentrations of peptides H or F at 4 °C or 37 °C for 1 h then used to infect Vero cells (PFU=5×103/ml) at 37 °C. Peptide concentrations 1, 2, 3, 4, and 5 are 250 μg/ml, 125 μg/ml, 62.5 μg/ml, 31.25 μg/ml, and 15.625 μg/ml, respectively. Anti-PEDV antibody dilutions 1, 2, 3, 4, and 5 are 1:4, 1:8, 1:16, 1:32 and 1:64, respectively. Plaque assays were further performed at 72 h post-infection. Bars show the standard deviation from three independent assays.
To characterize the temperature effect on interactions between peptide H and PEDV as well as cell infection, various concentrations of the peptides were incubated with PEDV (PFU=5×103/ml) at 4 °C or 37 °C for 1 h, then the peptide-treated viruses were used to infect cells at 37 °C. The results showed that the inhibition rate at 37 °C was significantly higher (p<0.01) than that at 4 °C when the lower concentrations of peptide H were applied. The inhibition ratio reached 61.86% at 37 °C but only 1.25% at 4 °C at the lowest concentration (15.625 μg/ml). In contrast, high concentrations of peptide H gave rise to a similar inhibition of PEDV infectivity (Fig. 6D).
Discussion
Infection with TGEV and PEDV can cause high mortality in piglets and therefore enormous economic loss in the pig industry. The prevalence of PEDV and TGEV in Asian countries such as China and Korea has been documented (Ren et al., 2011b, Li and Ren, 2011). At present, live vaccines against the both viruses are extensively used in China which in turn decreases the occurrence of diseases to some extent. However, small molecule inhibitors to TGEV or PEDV are alternative approaches to controlling swine viral diarrhea diseases.
Using combinatorial phage-display peptide libraries can be a powerful tool for selecting ligands that bind target proteins. Phage display techniques have been used to generate diagnostic and therapeutic peptides for bacteria (Bishop-Hurley et al., 2005, Bishop-Hurley et al., 2010, Carnazza et al., 2008), fungi (Bishop-Hurley et al., 2002, Fang et al., 2006) and viruses (Ren et al., 2011a, Ren et al., 2010, Ferrer and Harrison, 1999, Welch et al., 2007, Wu et al., 2011, Yang et al., 2003).
The pAPN is a member of a membrane-bound metalloprotease family and predominantly expressed on the surface of epithelial cells of the kidney, small intestine, and respiratory tract (Nam and Lee, 2010, Kenny and Maroux, 1982, Lendeckel et al., 2000). It is known that pAPN is a cellular receptor for TGEV and that anti-pAPN antibody efficiently decreases cell infection by this virus (Delmas et al., 1992, Liu et al., 2009). Recently, three pAPN-binding peptides H, S, and F were identified using pAPN as an immobilized target for panning a 12-mer phage display peptide library. These peptides exhibited high affinity binding to pAPN and inhibited cell infection by TGEV completely (Ren et al., 2011). As a member of group I coronaviruses, TGEV and PEDV have similar infection characterizations and as such it is difficult to differentiate these pathogens based only upon clinical symptoms. Recent evidence indicates that PEDV may also bind pAPN, a type II glycoprotein, as a functional receptor (Li et al., 2007, Oh et al., 2003). Interestingly, TGEV can be easily propagated in swine-originated cells such as ST cells (Delmas et al., 1992, Hofmann and Wyler, 1988) whereas PEDV is adapted and cultivated in African green monkey kidney (Vero) cells rather swine cells. Given the stark similarities as well as differences between TGEV and PEDV, we were interested in evaluating the antiviral effects of the H, S and F peptides on cell infection by PEDV.
We first analyzed potential blockage of the pAPN-binding peptides on Vero cell infection by PEDV. Plaque assays indicated no significant decrease in the infectivity of PDEV even though prior studies showed that both anti-pAPN antibody and peptides H, S or F were capable of inhibiting TGEV infection in vitro (Ren et al., 2011a, Liu et al., 2009) if pre-incubated with TGEV permissive cells. There were limited reports indicating that pAPN may serve as a functional receptor of PEDV; however, studies herein clearly demonstrated that pAPN is only expressed on the surface of ST cells and is not present on Vero or MDCK cells. As such, the inhibitory activities of peptide H were not due to binding pAPN. Further, we showed that only pre-treatment with peptide H inhibited infection by PEDV.
The PrV is a swine neurotropic herpesvirus with a DNA genome that can be propagated in many cell lines including Vero cells. Therefore, we used PrV as an additional control to further confirm and evaluate the inhibition specificity of the H peptide. Peptide H did not prevent infection of pretreated TGEV or PrV suggesting its inhibitory activity was specific and not due to virucidal effects of amphipathic peptides. Clearly, peptide H was able to interact with PEDV; however, it was unclear as to the mechanism of its antiviral activity. As such, the binding characteristics between peptide H (using the phage bearing the H peptide) and PEDV were further examined by Western blot. Results clearly showed that phage H reacted with a protein with a molecular mass of 220 kDa closely approximating the molecular mass of the PEDV S protein. This supposition was corroborated using antibody against the PEDV S protein. In contrast, control phages bearing other peptides did not show such reactivity. These results demonstrate that the peptide H abrogates infectivity in part by binding to the PEDV S protein. This is consistent with the function of the coronavirus S protein that mediates cell infection. Further, cell post-treatment assays evaluating the effects of each peptide on the replication of PEDV in vitro clearly demonstrated that the peptides do not interfere with the intracellular replication of PEDV.
Our results showed that only peptide H and not peptides S or F exhibited very high, dose-dependent inhibitory activity against PEDV where as little as 1 μg/ml needed to achieve EC50. This was confirmed by real-time RT-PCR which showed decreasing amounts of viral RNA in PEDV-infected cells. This corroborated the relationship between the antiviral activity of peptide H and either blockage of the viral attachment or entry into Vero cells. The impact of peptide H on the entry of PEDV was first investigated by performing the cell post-treatment and co-incubation assays. When PEDV was incubated with cells prior to treatment with peptide H no significant effects on PEDV infection were observed. Similar results were seen when peptides, PEDV and the cells were combined at the same time and co-incubated at 37 °C suggesting that peptide H did not affect PEDV entry into the cell post-adsorption. However, when PEDV was pre-incubated with peptide H prior to incubation with Vero cells, peptide H blocked the attachment of PEDV as determined from plaque assays and RT-PCR analysis.
It became clear that peptide H did not interact with Vero cells directly. Rather, it exhibits its inhibitory activity via the interplay between the peptide H and PEDV. It is accepted that virus adsorption occurs at 4 °C and internalization does not happen until the temperature is raised to 37 °C (Baldick et al., 2010). Our results clearly showed that the effects of peptide H occurred during the incubation step at 4 °C rather than the 37 °C internalization step again targeting a specific interaction between peptide H and PEDV that affects binding to the cell surface.
Both PEDV and TGEV are group I coronaviruses (Bridgen et al., 1993) and propagate in Vero and ST cells, respectively; PrV is a DNA virus that also propagates in Vero cells. As such, we selected TGEV and PrV as control viruses to examine any specificity in the inhibitory activity of peptide H on cell infection by these viruses. As expected, the results showed no significant inhibitory activity of peptide H on either TGEV or PrV infection. Further, peptide H was not cytotoxic to either ST or Vero cells. These results corroborate our hypothesis that peptide H functions in part, by interacting with the S protein of PEDV and affecting the ability of the virus to bind to the cell surface. Future studies will focus on identifying the specific site of interaction of peptide H and whether or not such a peptide can be used in vivo to abrogate or attenuate PEDV infections.
Materials and methods
Cells and viruses
Swine testis (ST), Monkey kidney cell lines (Vero) and Madin-Darby canine kidney (MDCK) cells were maintained in Dulbecco׳s Modified Eagle Medium (DMEM) (Invitrogen, US) supplemented with 10% fetal bovine serum, (FBS, GIBCO, US), 100 units/ml of penicillin and 100 units/ml of streptomycin. All cells were purchased from American Type Culture Collection (ATCC) and kept in our laboratory. PEDV isolate HLJBY was propagated in Vero cells in the presence of 10 μg/ml trypsin (GIBCO, US) (Ren et al., 2011b). TGEV strain PUR46-MAD was propagated in ST cells (Ren et al., 2008, Yin et al., 2010). Porcine pseudorabies virus (PrV) strain Kaplan was propagated in Vero cells (Ren et al., 2011c).
Cytostatic assay
ST or Vero cells were seeded in 96-well plates at a density of 5×104 cells/well and cultured in DMEM containing 10% FBS at 37 °C under 5% CO2 for 24 h followed by addition of serial dilutions (62.5–1000 μg/ml) of the tested peptides. The cells were allowed to grow for 48 h at 37 °C and proliferation was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Briefly, the medium was removed and 10 μl of MTT solution (0.5 mg/ml, Invitrogen, US) was added and incubated at 37 °C for 4 h. Then 100 μl of dimethyl sulfoxide (DMSO) was added and incubated for 15 min to solubilize the formazan crystals. Cell survival rate was calculated as (OD490treatment)/(OD490control) (Paeshuyse et al., 2006). The 50% cytostatic concentration (CC50) was defined as the concentration inhibiting the proliferation of exponentially growing cells by 50%. Non-cytotoxic concentrations of peptides (≤ CC50) were used for antiviral assays.
Antiviral assays
Three different treatment approaches were used to analyze the antiviral action of the selected peptides H (HVTTTFAPPPPR), S (SVVPSKATWGFA) and F (FKPSSPPSITLW) as previously defined (Kwon et al., 2010).
In the first method, i.e., cell post-treatment assay, Vero cells were grown in 24-well plates at a density of 5×105 cells/well for 24 h. PEDV at a PFU (plaque-forming unit) of 5×103/ml was inoculated onto confluent cells for 1 h followed by removal of the medium and incubation of the infected cells with various non-cytotoxic concentrations (≤ CC50) of peptides for 1 h at 37 °C. The cells were then overlaid with 1% methylcellulose in DMEM and incubated for 72 h at 37 °C followed by crystal violet staining and plaque assays as previously described with modifications (Ren et al., 2011a, Ren et al., 2011d). Briefly, after the medium was removed, the cells were fixed with 4% formaldehyde in PBS for 1 h at room temperature followed by staining with 1% crystal violet solution for 20 min. The staining solution was removed, the cells were washed with PBS and the plaques were counted. Decreases in virus infectivity were calculated from the plaque assay as follows: 100×[1−(treatment wells/control wells)]. Average EC50 values (concentration inducing 50% inhibition of virus replication) were calculated and the effectiveness of each peptide were expressed using the selectivity index (SI) (SI=CC50/EC50) (Paeshuyse et al., 2006, Müller et al., 2007).
In the second method, i.e., cell pre-treatment assay, Vero cells were first grown in 24-well plates at a density of 5×105 cells/well for 24 h then treated with non-cytotoxic concentrations of peptide for 1 h prior to incubation with virus. The peptides were removed and the cells were washed twice with PBS. PEDV at a PFU of 5×103/ml was inoculated onto the pre-treated Vero cells for 1 h. After the virus was removed, the cells were overlaid with 1% methylcellulose in DMEM and incubated for 72 h at 37 °C followed by plaque assays.
In the third experiment i.e., virus pre-treatment, various concentrations of the peptides were mixed with PEDV (PFU=5×103/ml) at 37 °C for 1 h prior to incubation with cells. Vero cells were grown in 24-well plates at 5×105 cells/well for 24 h then the peptide/virus mixture was added to the cultured cells for 1 h. After the mixture was removed and the cells washed with PBS, the cells were overlaid with 1% methylcellulose in DMEM and cultured for an additional 72 h at 37 °C followed by plaque assays as described above.
In parallel, PEDV-neutralizing, rabbit antiserum serially-diluted 1:2 and PBS were used as positive and negative controls, respectively, for the above-mentioned experiments. Each concentration of the peptide and antibody was assayed in triplicate.
Immunofluorescence assays to identify pAPN on cell lines from different species
ST, Vero and MDCK cells were seed into the 24-well plates and incubated at 37 °C for 24 h. Indirect immunofluorescence assays (IFA) were performed (Ren et al., 2011d, Baldick et al., 2010) with modification. The cells were washed twice with pre-chilled PBS then fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. Following two washes with PBS, they were quenched with 0.1 M glycine for 5 min then blocked with 2% BSA (Sigma, US) in PBS for 30 min. Samples were incubated for 1 h with anti-pAPN polyclonal antibody (1:1500 in PBS) (Liu et al., 2009), washed three times with PBS, and incubated with fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit IgG (1:1500 in PBS, Zhongshan, China) for 1 h in the dark. The samples were washed three times with PBS and the fluorescence signals and phase contrast images were detected by fluorescence microscopy (Leica, Wetzlar, Germany).
Virus specificity
Various concentrations of peptide H were incubated with the TGEV, PEDV and PrV (PFU=5×103/ml) at 37 °C for 1 h then added to confluent Vero or ST cell monolayers for 1 h. The mixtures were removed and the cultured cells washed twice with PBS then incubated with 1% methylcellulose in DMEM for 72 h at 37 °C. The cells were then stained with crystal violet staining and plaque assays were performed as described above.
Semi-quantitative real-time reverse transcription-PCR
The effect of peptide H on PEDV infection of Vero cells was confirmed by semi-quantitative real-time reverse transcription (RT-PCR) (Ren et al., 2011d). Vero cells in 6-well plates were infected with PEDV (PFU=5×103/ml) pretreated with different concentrations of peptide H at 37 °C for 1 h. The culture was replaced with DMEM at 37 °C for 24 h then washed 3 times with PBS. The virus-containing cultures were frozen and thawed three times followed by addition of an equal volume of 20% polyethylene glycol (PGE) 8000 at room temperature and incubation for 30 min. The samples were centrifuged at 12,000 rpm for 5 min and the pellets were re-suspended in RNase-free water. Total RNA was extracted with a commercial kit (Fastgene, China) according to the manufacturer׳s instructions. Reverse transcription was performed in a total of 20 μl consisting of 5 μl total RNA (2.5 μg), 1 μl oligo dT, 1 μl dNTP (10 mM), 0.5 μl RNAse inhibitor, 7.5 μl sterile water, 1 μl MLV, and 4 μl 5×RT-PCR buffer. The mixture was incubated at 30 °C for 10 min, 42 °C for 60 min and 95 °C for 5 min. Real-time PCR was performed using ABI PRISM 7500 real-time PCR machine (Applied Biosystems, USA). The information regarding primers and RT-PCR products is provided in Table 1. The real-time PCR mixture included 0.5 μl (0.5 μg) of cDNA template, 10 μl of SYBR Taq polymerase, 0.4 μl of ROX pge 2, 0.5 μl (10 pmol) of each primer, and 8.1 μl of sterile water. The reactions were incubated at 95 °C for 10 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s.
Table 1.
Information on primers and real-time RT-PCR products.
| Primer pairs | PCR product length (bp) |
|---|---|
| Sense 5׳ CACTGGTTGGGCTTTCTATGTC | PEDVX-N |
| Antisense 5׳TGTTAGTGGGTACAGCGTTGTT | 244 |
| Sense 5׳ GGCTCAGAGCAAGAGAGGTATCC | β-actin |
| Antisense 5׳ GGTCTCAAACATGATCTGAGTCATCT | 208 |
We examined virus RNA levels using primers that specifically amplify a 244 bp fragment encompassing the 3׳ end of a small, non-structural gene (X) and the 5׳ end of the PEDV-N gene (Table 1). The expression of PEDV X-N in PEDV-infected Vero cells was normalized to that of beta-actin and taken as 100% compared to expression of the peptide H treatment group. Data analysis is based on the measurement of the cycle threshold (Ct). The difference in ΔCt (Ct sample fragment mean Ct value-beta-actin fragment mean Ct value) was used as a measure of the relative fragment with the 2−ΔΔCt method in correlation to the amplification size of PEDV X-N fragment. For each experimental condition, real-time PCR was conducted in quadruplicate and the resulting average Ct values for the PEDV X-N fragment was used to quantify viral load. The experiment was performed in triplicate.
Western blot analysis of peptide H binding to PEDV
The PEDV (1×106 PFU/ml) was harvested from Vero cells and clarified by centrifugation at 1300g for 15 min followed by ultracentrifugation at 150,000g for 1.5 h to collect the virus. The pellets were suspended in PBS, subjected to 10% SDS-PAGE then blotted to a nitrocellulose (NC) membrane. The NC membranes were blocked overnight at 4 °C with 5% non-fat dry milk in PBS. After three washes with PBS, membranes were sliced and incubated with phage H (1×1011 PFU), anti-PEDV S polyclonal antibody (1:1000 in PBS), M13 phage library (1×1011 PFU), and control phage bearing either the peptides SVSVGMKPSPRP or MSCNDTLCLLPN. The membranes were then washed with PBS and successively incubated with anti-M13 polyclonal antibody (Abcam, 1:600 in PBS) and horseradish peroxidase HRP-conjugated goat anti-rabbit IgG (1:1500 in PBS) at room temperature for 1 h. Protein bands were visualized using 3,3׳-diaminodbenzidine (DAB, The Thermo Scientific)
Temperature shift assays
To examine the effect of temperature on the binding of virus to the cells, four experiments were performed. First, various concentrations of peptide H and control peptide F were incubated with PEDV (PFU=5×103/ml) at 4 °C for 1 h then added to confluent Vero cells seeded in 24-well plates at 4 °C for 1 h followed by infection at 37 °C for 72 h. Second, the peptides, PEDV and Vero cells were co-incubated at 4 °C for 1 h, after which the incubation temperature was raised to 37 °C for 72 h. Third, the peptides, PEDV and Vero cells were co-incubated at 37 °C for 1 h then assayed without prior incubation at 4 °C. Finally, peptides were pre-incubated with PEDV at 4 °C or 37 °C for 1 h followed by cell infection at 37 °C for 72 h. All experiments were terminated by extensive washing of the cells and plaque assays to quantify the infection.
Statistical analysis
Statistical analysis of the data was performed using SPSS 11.5 software; p<0.05 and p<0.01 were defined as statistically significant and highly statistically significant, respectively.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31340003 and 31372438), sponsored by Chang Jiang Scholar Candidates Program for Provincial Universities in Heilongjiang (2013CJHB002), the Program for New Century Excellent Talents in University of Ministry of Education of P.R. China (NCET-10-0144).
References
- Baldick C.J., Wichroski M.J., Pendri A., Walsh A.W., Fang J., Mazzucco C.E., Pokornowski K.A., Rose R.E., Eggers B.J., Hsu M., Zhai W., Zhai G., Gerritz S.W., Poss M.A., Meanwell N.A., Cockett M.I., Tenney D.J. A novel small molecule inhibitor of Hepatitis C virus entry. PLoS Pathog. 2010;6:e1001086. doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Hurley S.L., Mounter S.A., Laskey J., Morris R.O., Elder J., Roop P., Rouse C., Schmidt F.J., English J.T. Phage-displayed peptides as developmental agonists for Phytophthora capsici zoospores. Appl. Environ. Microbiol. 2002;68:3315–3320. doi: 10.1128/AEM.68.7.3315-3320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Hurley S.L., Rea P.J., McSweeney C.S. Phage-displayed peptides selected for binding to Campylobacter jejuni are antimicrobial. Protein Eng. Des. Sel. 2010;23:751–757. doi: 10.1093/protein/gzq050. [DOI] [PubMed] [Google Scholar]
- Bishop-Hurley S.L., Schmidt F.J., Erwin A.L., Smith A.L. Peptides selected for binding to a virulent strain of Haemophilus influenzae by phage display are bactericidal. Antimicrob. Agents Chemother. 2005;49:2972–2978. doi: 10.1128/AAC.49.7.2972-2978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Roitter P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen A., Duarte M., Tobler K., Laude H., Ackermann M. Sequence determination of the nucleocapsid protein gene of the porcine epidemic diarrhoea virus confirms that this virus is a coronavirus related to human coronavirus 229E and porcine transmissible gastroenteritis virus. J.Gen.Virol. 1993;74:1795–1804. doi: 10.1099/0022-1317-74-9-1795. [DOI] [PubMed] [Google Scholar]
- Carnazza S., Foti C., Gioffre G., Felici F., Guglielmino S. Specific and selective probes for Pseudomonas aeruginosa from phage-displayed random peptide libraries. Biosens. Bioelectron. 2008;23:1137–1144. doi: 10.1016/j.bios.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales, a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L׳Haridon R., Vogel L.K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z.D., Laskey J.G., Huang S., Bilyeu K.D., Morris R.O., Schmidt F.J., English J.T. Combinatorially selected defense peptides protect plant roots from pathogen infection. Proc. Natl. Acad. Sci. USA. 2006;103:18444–18449. doi: 10.1073/pnas.0605542103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M., Harrison S.C. Peptide ligands to human immunodeficiency virus type 1 gp120 identified from phage display libraries. J. Virol. 1999;3:5795–5802. doi: 10.1128/jvi.73.7.5795-5802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A.J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol. Rev. 1982;62:91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kwon H.J., Kim H.H., Yoon S.Y., Ryu Y.B., Chang J.S., Cho K.O., Rho M.C., Park S.J., Lee W.S. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol. J. 2010;7:2–9. doi: 10.1186/1743-422X-7-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C., Perlman S., Anderson L.J. Coronaviridae. In: Knipe D.M., Howley P.M., Griffin D.E., Martin M.A., Lamb R.A., Roizman B., Straus S.E., editors. Fields Virology. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 1305–1336. [Google Scholar]
- Lendeckel U., Kähne T., Riemann D., Neubert K., Arndt M., Reinhold D. Review: the role of membrane peptidases in immune functions. Adv. Exp. Med. Biol. 2000;477:1–24. doi: 10.1007/0-306-46826-3_1. [DOI] [PubMed] [Google Scholar]
- Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365:166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Ren X. Reverse transcription loop-mediated isothermal amplification for rapid detection of transmissible gastroenteritis virus. Curr. Microbiol. 2011;62:1074–1080. doi: 10.1007/s00284-010-9825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li G., Sui X., Yin J., Wang H., Ren X. Expression and functional analysis of porcine aminopeptidase N produced in prokaryotic expression system. J. Biotechnol. 2009;141:91–96. doi: 10.1016/j.jbiotec.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V., Chávez J., Reginatto H., Zucolotto F.H., Niero S.M., Navarro R., Yunes D., Schenkel R.A., Barardi E.P., Zanetti C.R.M., Simoes, C.M.O. C.R. Evaluation of antiviral activity of South American plant extracts against Herpes Simplex Virus Type 1 and Rabies Virus. Phytother. Res. 2007;21:970–974. doi: 10.1002/ptr.2198. [DOI] [PubMed] [Google Scholar]
- Nam E., Lee C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet. Microbiol. 2010;144:41–50. doi: 10.1016/j.vetmic.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.S., Song D.S., Park B.H. Identification of a putative cellular receptor 150 kDa polypeptide for porcine epidemic diarrhea virus in porcine enterocytes. J. Vet. Sci. 2003;4:269–275. [PubMed] [Google Scholar]
- Paeshuyse J., Leyssen P., Mabery E., Boddeker N., Vrancken R., Froeyen M., Ansari I.H., Dutartre H., Rozenski J., Gil L.H.V.G., Letellier C., Lanford R., Canard B., Koenen F., Kerkhofs P., Donis R.O., Herdewijn P., Watson J., Clercq E.D., Puerstinger G., Neyts J. A novel, highly selective inhibitor of Pestivirus replication that targets the viral RNA-dependent RNA polymerase. J. Virol. 2006;80:149–160. doi: 10.1128/JVI.80.1.149-160.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., Yeo S.G. Porcine epidemic diarrhea. In: Straw B.E., Zimmerman J.J., D׳Allaire S., Taylor D.J., editors. Diseases of Swine. Wiley-Blackwell; Ames: 2006. pp. 367–372. [Google Scholar]
- Ren X., Glende J., Yin J., Schwegmann-Wessels C., Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Liu B., Yin J., Zhang H., Li G. Phage displayed peptides recognizing porcine aminopeptidase N inhibit transmissible gastroenteritis coronavirus infection in vitro. Virology. 2011;410:299–306. doi: 10.1016/j.virol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Meng F., Yin J., Li G., Li X., Wang C., Herrler G. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis Coronavirus. PloS One. 2011;6:e18669. doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Suo S., Jang Y.S. Development of a porcine epidemic diarrhea virus M protein-based ELISA for virus detection. Biotechnol. Lett. 2011;33:215–220. doi: 10.1007/s10529-010-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Wang M., Yin J., Li G. Phages harboring specific peptides that recognize the N protein of the porcine reproductive and respiratory syndrome virus distinguish the virus from other viruses. J. Clin. Microbiol. 2010;48:1875–1881. doi: 10.1128/JCM.01707-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Yin J., Li G., Herrler G. Cholesterol dependence of pseudorabies herpesvirus entry. Curr. Microbiol. 2011;62:261–266. doi: 10.1007/s00284-010-9700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C., Gebauer M.F., Sune C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis Coronavirus. Virology. 1992;190:92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W.J.M., Brian D., Cavanagh D., Groot R.J., Enjuanes L., Gorbalenya A.E., Holmes K.V., Masters P.S., Rottier P.J., Taguchi F., Talbot P. Family Coronaviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberg U., Ball L.A., editors. Virus Taxonomy, Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; London: 2005. pp. 947–964. [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.D., VanDemark A.P., Heroux A., Hill C.P., Kay M.S. Potent D-peptide inhibitors of HIV-1 entry. Proc. Natl. Acad. Sci. USA. 2007;104:16828–16833. doi: 10.1073/pnas.0708109104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Li G., Qin C., Ren X. Phage displayed peptides to Avian H5N1 virus distinguished the virus from other viruses. PloS One. 2011;6:e23058. doi: 10.1371/journal.pone.0023058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Gao L., Li L., Lu Z., Fan X., Patel C.A., Pomerantz R.J., DuBois G.C., Zhang H. Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency Virus type I (HIV-1) Vif proteins. J. Biol. Chem. 2003;278:6596–6602. doi: 10.1074/jbc.M210164200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Glende J., Schwegmann-Wessels C., Enjuanes L., Herrler G., Ren X. Cholesterol is important for a post-adsorption step in the entry process of transmissible gastroenteritis virus. Antivir Res. 2010;88:311–316. doi: 10.1016/j.antiviral.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]