Abstract
Transgenerational effects of environmental pollutants on humans and animals are complex. Thus, we used zebrafish to evaluate the effects of parental whole-life cycle exposure to bisphenol A and its analogs (bisphenol S and F) on offspring innate immunity. At adulthood, offspring were examined with/without continued chemicals treatment until 72 h post-fertilization (hpf). To measure offspring immune function, larvae at 72 hpf were expose for 24 h with/without the viral mimic polyinosinic-cytidylic acid (Poly I:C) or the bacterial mimic Pam3Cys-Ser-Lys4 (PAM3CSK4). Data show modified immunity in offspring. Specifically, lysozyme activity was significantly induced in F1 larvae and respiratory burst response and oxidative defense genes were inhibited. Genes of the innate immune system including Toll-like receptors and their downstream molecules and inflammatory cytokines were significantly down-regulated, whereas matrix metalloproteinases were up-regulated in larvae. In addition, recombination-activating genes in the immature adaptive immune system were significantly reduced. Thus, immune defense is diminished by exposing parental generations of zebrafish to environmentally relevant concentration of bisphenols and this suggests that fish chronically exposed to bisphenols in the wild may be vulnerable to pathogens.
Graphical abstract
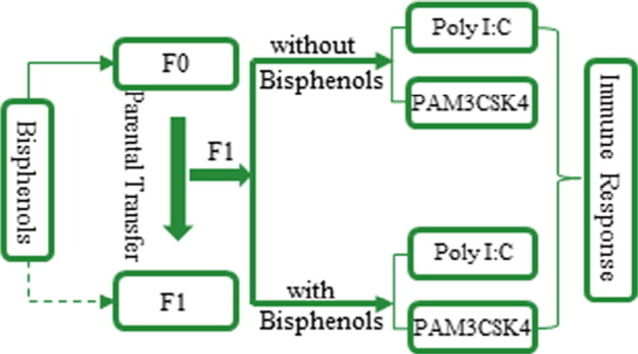
Highlights
-
•
Bisphenol A and its analogs bioaccumulated in zebrafish offspring via the transfer of parental waterborne bisphenols burdens.
-
•
Parental exposure to bisphenol A and its analogs decreased survive of zebrafish offspring.
-
•
Parental exposure to bisphenol A and its analogs influenced zebrafish offspring immunity.
-
•
Parental exposure to bisphenol A and its analogs could result in a decreased healthy status in zebrafish offspring.
1. Introduction
Bisphenol A (BPA) is a purported endocrine-disrupting chemical (EDC), used to make plastics, receipts, food packaging and other products (Vandenberg et al., 2009). Because BPA is a public health concern, restriction and legislation of BPA have been suggested worldwide. Recently, BPA regulation has been tightened due to the potential for continuous exposure and health hazards during fetal and neonatal life (Eladak et al., 2015). For example, BPA was banned in baby bottles in Canada in 2010, in the European Union and in China in 2011 so the industry has sought alternative chemicals. Due to removal of BPA from consumer products, bisphenol analogs such as bisphenol S (BPS) and bisphenol F (BPF) have been used in “BPA-free” products. BPS is used for various industrial applications including polymer production and thermal papers (Ben-Jonathan and Hugo, 2016, Liao et al., 2012) and polyethersulfone plastics used to make baby bottles (National Toxicology Program (NTP) Research Concept, https://ntp.niehs.nih.gov/ntp/about_ntp/bsc/2014/june/bisphenols_concept_508.pdf). BPF is used in epoxy resins used to line food cans and in polymer plastics (Liao and Kannan, 2013). BPS and BPF have been identified everyday household products, environmental compartments, and human specimens (Chen et al., 2016). In natural environments, BPS and BPF appear to have moderate persistence and are more resistant to degradation than BPA (Danzl et al., 2009, Ike et al., 2006). In some Asian rivers and lakes, BPF and BPS can be at the highest level of 2.8 and 7.2 μg/L, respectively (Yamazaki et al., 2015). However, compared to BPA, there is limited information about their toxicological effects in aquatic organisms.
Transgenerational effects are manifested by endocrinological, physiological, or developmental alterations in offspring due to parental exposures. Previous studies suggest multiple physiological alterations occur in offspring exposed to environmental toxicants such as EDCs through the blood or germ line of parents (Schwindt, 2015), so there may be generational consequences of these toxicants. Although reported adverse effects of bisphenol analogs on multiple biological processes including growth, metabolism, and endocrine system function in aquatic organisms have been documented (Kang et al., 2007), transgenerational effects in aquatic wildlife are poorly understood. Transgenerational inheritance of BPA effects have been studied in human and mammals (Schonfelder et al., 2002, Takahashi and Oishi, 2000). Therefore, transgenerational consequences of parental bisphenol analog exposure may exist for aquatic organisms.
Due to external fertilization and release of fish embryos, larvae are exposed to an aquatic environment potentially containing toxicants and pathogens. Because the innate immune system is an initial defense against invading pathogens and is a prerequisite for potentiating the adaptive immune response (Segner et al., 2011), disturbance of this system by environmental toxicants may negatively influence host defense against infections and affect individual or population health. Previous studies suggest that EDCs including BPA can disrupt immune systems of aquatic animals (Milla et al., 2011). Additionally, growth and development, and disease and survival of organisms are partly determined by immune capacity. Therefore, whether exposure to environmental toxicants by parents alters offspring immunity is of interest. In mammals, transmaternal BPA exposure affected the development of immune organs in mice offspring (Yang et al., 2008), decreasing immune defense and increasing the risk of disease (Bodin et al., 2014). However, whether exposure to parental BPA and its analogs induces immunotoxicological changes in aquatic offspring is not clear.
Because zebrafish share orthologous genes with the mouse and human, they may be used to model immunotoxicological effects of environmental toxicants (Sullivan and Kim, 2008). Thus, we evaluated immunological effects of low concentrations of BPA and its analogs—BPS and BPF—on zebrafish offspring exposed over their whole life cycle. Then, we used a disease challenge to assess the integrated immune system response and measured gene expression for those genes responsible for eliminating invading pathogens. We selected the following genes that are expressed exclusively in the immune system: toll-like receptors (TLRs) and downstream signaling molecule mRNA such as myeloid differentiation factor 88 (MyD88), Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF), inflammatory cytokines including type I interferon (IFN), myxovirus-resistance gene (Mx), tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β), and matrix metalloproteinases (MMP) (Li et al., 2017, Liao et al., 2016, Medzhitov, 2001). Oxidative defense is involved in immune response (Biller-Takahashi et al., 2015), so myeloperoxidase (MPX), and superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) mRNA expression was measured. Recombination-activating genes (RAGs) in immature T and B lymphocytes were also measured. Lysozyme activity and respiratory burst are common used as sensitive biomarkers for immunotoxicity (Nayak et al., 2007).
2. Materials and methods
2.1. Test chemicals
BPA, BPS and BPF were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals were dissolved in dimethyl sulfoxide (DMSO) and the final DMSO concentration in the exposure water was 0.01% (v/v).
2.2. Fish maintenance and experimental set-up
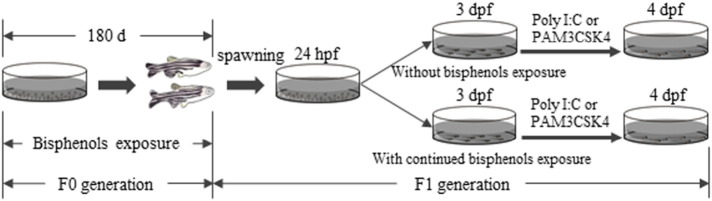
All zebrafish (AB strain) used in experiments were originally obtained from China Zebrafish Resource Center (Wuhan, China). Brood stock was maintained in our laboratory for > 5 generations. In this study, newly hatched eggs were collected after brood adult spawning and this was the F0 generation. F0 generation fish were exposed to BPA, BPS and BPF throughout their whole life span, and their offspring (F1 generation) were used for subsequent experiments. Waterborne BPA, BPS and BPF exposure of F0 fish began at 24 h post-fertilization (hpf) (Fig. 1 ). Briefly, 24 hpf embryos were randomly distributed into 90 mm petri dishes and exposed to BPA (0.1, 1, 10 μg/L), BPS (10 μg/L), and BPF (10 μg/L) in triplicate at each nominal concentration. Controls received 0.01% DMSO (v/v). Each dish contained 100 embryos. Bisphenol concentrations were selected according to previous studies (Qiu et al., 2016b, Wu et al., 2011, Xu et al., 2013) and environmental abundance in aquatic environments (Yamazaki et al., 2015). After 5 days post-fertilization (dpf), larvae were transferred to 2 L glass containers. As fish grew, culture containers were adjusted from a 15 L sized tank (15–30 dpf) at a density of 5 fish/L, to a 30 L sized tank (31–90 dpf) at a density of 2 fish/L, to a 30 L sized tank (91–180 dpf) at a density of 1 fish/L. After 180 days of exposure, fish were paired (2 females for every 1 male) and a clutch of embryos was obtained from each treatment group. F1 embryos received continued treatment with BPA, BPS or BPF at concentrations used to treat parents or they were not treated at all until 72 hpf.
Fig. 1.
A schematic diagram showing parental exposure to BPA, BPF and BPS in F0 zebrafish and experimental timeline for dosing and pathogenic interventions in F1 offspring.
To measure innate immune defense of F1 generation zebrafish, larvae at 72 hpf were expose for another 24 h in the presence or absence of bacterial mimic synthetic bacterial lipoprotein Pam3Cys-Ser-Lys4 (PAM3CSK4, 5 μg/mL), or viral mimic dsRNA mimetic polyinosinic-cytidylic acid (Poly I:C, 10 μg/mL). In preliminary trials, this concentration of bacterial/viral mimic induced immune response but did not cause microscopic damage in healthy zebrafish larvae, and this was a concentration used in previous immune defense studies (Heffelfinger, 2010). PAM3CSK4 and Poly I:C are well-known toll-like receptor1/2 (TLR1/2) heterodimers and TLR3 agonists, respectively. All tested fish were cultured at 28 ± 0.5 °C with a 14 light:10 h dark cycle.
During the exposure period, exposure medium was performed semi-statically, and fresh solutions were replaced daily. In addition, actual concentrations of exposure media were measured once weekly using LC-MS/MS according to published methods (Yamazaki et al., 2015). The actual concentrations of exposure media are listed in Supporting information (Table S1, Supporting information).
2.3. Analysis of BPA, BPS and BPF contents in F1 eggs
After F0 zebrafish spawning, F1 eggs were immediately collected for bisphenol analog analysis. Methods for extraction, clean up, analysis, and quality assurance and quality control (QA/QC) are described in detail in Supporting information (Text S1, Supporting information).
2.4. Lysozyme activity assay
F1 generation embryos were washed with PBS (100 mM, pH 7.2) and prepared for lysozyme activity assay. Briefly, 150 larvae were homogenized with 250 μL of PBS on ice and centrifuged at 3000 × g for 15 min at 4 °C. Lysozyme activity was measured using a commercial ELISA kit according to the manufacturer's instructions (Cusabio, Wuhan, China).
2.5. Respiratory burst assay
Live F1 generation embryos were used to measure respiratory burst using published methods with minor modifications (Xu et al., 2015) (Text S2, Supporting information).
2.6. RNA Isolation, reverse transcriptase, and quantitative real-time PCR assay
F1 generation larvae from each group were pooled as one replicate (n = 3). RNA isolation, purification, quantification of total RNA, and reverse transcriptase reactions were performed as described previously (Xu et al., 2013) (Text S3, Supporting information). Primer sequences of selected genes were designed using Primer Premier 5.0 (Table S2, Supporting information).
2.7. Data analysis
All experimental data were confirmed for normality and homogeneity of variance using a Kolmogorov-Smirnov one-sample test and Levene's test. Intergroup differences were assessed using ANOVA followed by Duncan's test, using SPSS Statistics 18 (SPSS Inc., Chicago, IL). Statistical significance was set at p < 0.05. All data are means ± standard error (SEM).
3. Results
3.1. Bisphenol analogs content in F1 eggs
In F1 eggs, the total body burden showed a dose-dependent relationship between parental exposure concentrations of 0.1, 1, and 10 μg BPA/L with the values of 0.84 ± 0.32, 4.6 ± 0.7, and 16.1 ± 2.5 ng/g wet weight, respectively. The detected BPS and BPF content in the F1 eggs which parental exposure to 10 μg BPF/L and 10 μg BPS/L was 18.7 ± 2.1 ng/g and 19.4 ± 4.8 ng/g wet weight, respectively.
3.2. Hatching and survival rates in F1 generation
Consistent with our previous observations (Wu et al., 2011, Xu et al., 2013), there was no significant difference in hatching and survival among F0 embryos treated with any dose of BPA. Similarly, hatching and survival of F0 embryos were also not significantly altered in response to 10 μg/L BPS or 10 μg/L BPF (data not shown). However, for F1 generations with parental exposure to bisphenol, with/without continued bisphenol treatment, hatching and survival were significantly decreased (p < 0.05) (Table S3, Supporting information). In addition, F1 generations not treated with bisphenol treatment had greater hatching and survival compared to the F1 generation with continued bisphenol treatment (Table S3, Supporting information).
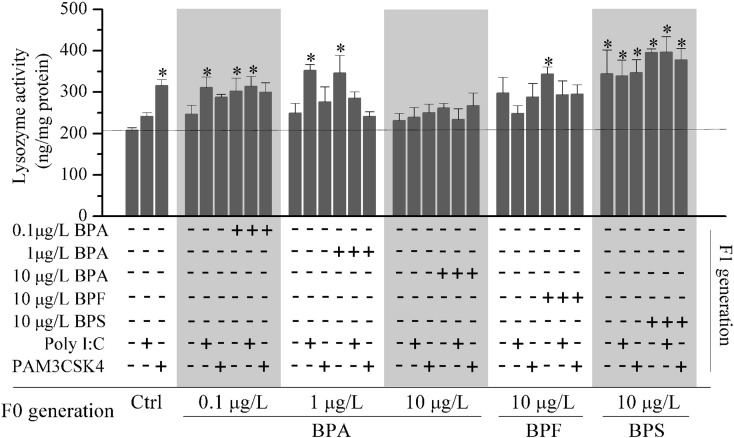
3.3. Lysozyme activity
Lysozyme activity was measured in F1 larvae with/without continued bisphenol treatment with or without bacterial or viral mimic infection (Fig. 2 ). In F1 larvae not treated with bisphenol, lysozyme activity was increased with parental exposure to 10 μg/L BPS compared with controls. In F1 larvae with continued bisphenol treatment, lysozyme activity was significantly increased with parental exposure as shown in Fig. 1. After infection with viral or bacterial mimics, lysozyme activity increased in F1 control larvae infected with PAM3CSK4 and in F1 larvae with/without 0.1 and 1 μg/L BPA, and 10 μg/L BPS exposure.
Fig. 2.
Lysozyme activity in F1 zebrafish larvae with/without continued bisphenol exposure and with Poly I:C or PAM3CSK4 infection. Data are means ± SEM of triplicate samples. *Significant difference at p < 0.05.
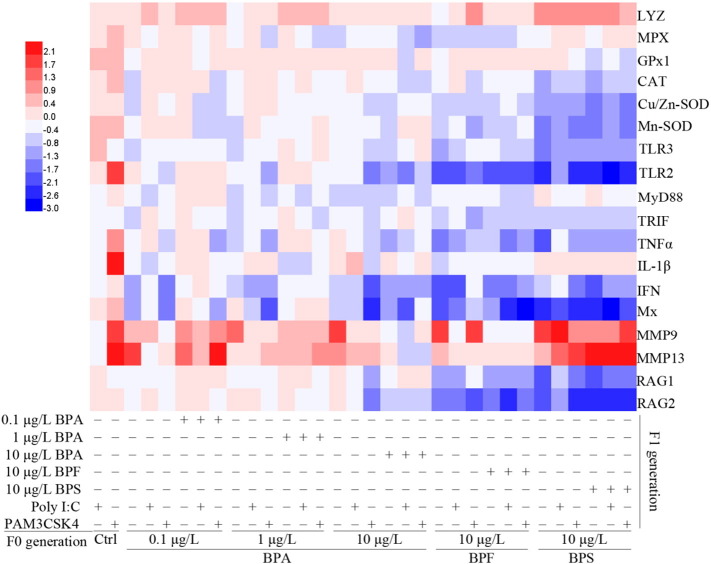
As shown in Fig. 3 and Table S4 (Supporting information), Lysozyme (LYZ) gene expression was upregulated in F1 larvae with/without continued 0.1 and 10 μg/L BPA, and 10 μg/L BPS exposure and after infection with viral or bacterial mimics, LYZ expression was up-regulated in F1 larvae with/without continued 0.1 and 1 μg/L BPA, 10 μg/L BPF, and 10 μg/L BPS exposure. Compared with F1 larvae with/without continued bisphenol exposure, LYZ expression was not different from those infected with Poly I:C or PAM3CSK4.
Fig. 3.
Heat map of gene expression after immunomodified parental exposure to bisphenol in zebrafish offspring. Gene transcripts were assayed in 120 hpf F1 zebrafish larvae with/without continued bisphenol exposure and with Poly I:C or PAM3CSK4 infection relative to F1 control. Expression values are log2(fold change) are means ± SEM.
3.4. Respiratory burst
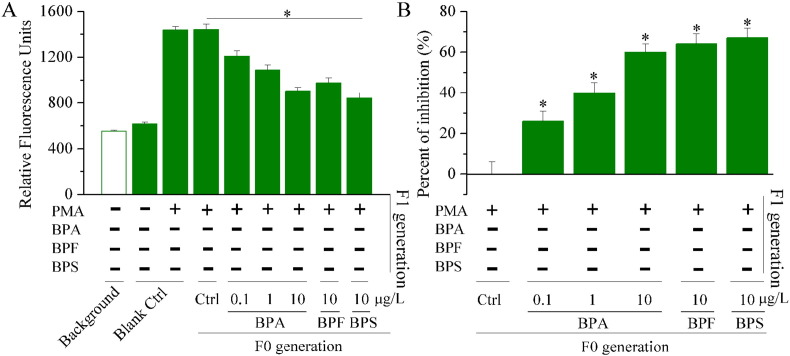
After stimulation with PMA, ROS production was induced via respiratory burst (Fig. 4 ). Compared with PMA-induced F1 controls, respiratory burst activity decreased in PMA-induced F1 larvae after parental exposure to BPA. Similarly, respiratory burst activity was reduced in PMA-induced F1 larvae which had parental exposures to BPF and BPS.
Fig. 4.
Respiratory burst activity of PMA-stimulated and F1 zebrafish embryos, measured by oxidation of H2DCFDA to DCF. (A) Relative fluorescent units in F1 zebrafish embryos. (B) Fold-induction of respiratory burst activity of F1 embryos.
3.5. Expression of oxidative defense genes in F1 generation
Compared with the F1 controls, MPX gene expression was significantly down-regulated in groups with/without continued 10 μg/L BPA, and 10 μg/L BPF exposures (p < 0.05). GPx1 was not changed with/without bisphenol exposure, but CAT was significantly down-regulated in groups with/without 0.1 μg/L BPA and 10 μg/L BPS exposures. Cu/Zn-SOD and Mn-SOD were also significantly down-regulated in groups with/without continued bisphenol exposure compared with controls (Fig. 3, Table S4, Supporting information).
In F1 controls infected with Poly I:C or PAM3CSK4, MPX, GPx1, CAT and Mn-SOD expression increased but Cu/Zn-SOD expression did not. However, after infection with viral or bacterial mimics, MPX expression decreased in F1 larvae with/without continued BPA and BPF exposure compared with the F1 controls. GPx1 did not change in F1 larvae with/without any bisphenol exposure. Compared with F1 controls, after Poly I:C or PAM3CSK4 treatment, CAT, Cu/Zn-SOD and Mn-SOD expression was down-regulated in F1 larvae with/without continued BPA, BPF and BPS exposures (Fig. 3, Table S4, Supporting information).
3.6. Expression of immune-related genes in F1 generation
Compared with F1 controls, TLR3 gene expression was down-regulated in F1 larvae with/without continued all bisphenol treatments, whereas TLR2 expression was only down-regulated in groups with/without continued 10 μg/L BPA, BPF, and BPS exposure. Down-regulation of MyD88 was observed in groups with/without continued 1 and 10 μg/L BPA exposures. Down-regulation of TRIF was observed in F1 larvae with/without continued 0.1 and 1 μg/L BPA, 10 μg/L BPF, and 10 μg/L BPS treatments. TNFα expression was down-regulated in groups with/without continued 10 μg/L BPA, BPF, and BPS exposures. However, IL-1β gene expression was not changed in any group with/without bisphenol treatment. Compared with F1 controls, IFN and Mx were down-regulated in groups with/without continued BPA, BPF, and BPS treatment. Significant up-regulation of MMP9 was observed in F1 larvae with/without continued bisphenol exposure, whereas up-regulation of MMP13 was observed in groups with/without continued BPA and BPS treatment groups compared with F1 controls, respectively. RAG1 and RAG2 were down-regulated after 10 μg/L BPA, BPF and BPS treatments (Fig. 3, Table S4, Supporting information).
Next, TLR3 was up-regulated in F1 controls with Poly I:C infection, whereas TLR2, TNFα, IL-1β, Mx, MMP9 and MMP13 were up-regulated after infection with PAM3CSK4. MyD88, TRIF, IFN, RAG1 and RAG2 were not different in control with viral or bacterial mimic infection (Fig. 2, Table S4, Supporting information). However, compared with the F1 controls, after Poly I:C or PAM3CSK4 infection, TLR2, TLR3, TRIF and TNFα gene expression were down-regulated in F1 larvae with/without continued BPA, BPF, and BPS treatments, whereas MyD88 was only down-regulated in groups with/without continued BPA and BPF exposure. Interestingly, IL-1β gene expression did not change in any group. After Poly I:C or PAM3CSK4 treatment, IFN and Mx were down-regulated in groups with/without continued bisphenol treatment. MMP9 was up-regulated in F1 larvae with/without continued BPA, BPF, and BPS exposures, whereas MMP13 was up-regulated in groups with/without continued BPA, and BPS exposure. After Poly I:C or PAM3CSK4 treatment, down-regulation of RAG1 and RAG2 were observed in groups with 10 μg/L BPA, BPF, and BPS exposure (Fig. 3, Table S4, Supporting information).
4. Discussion
In the present study, zebrafish were used to model and study transgenerational effect of bisphenol analogs on F1 larvae immunity. Data show that effects of bisphenol analog exposure were evident in exposed zebrafish and their offspring, and that this exposure altered immune function in offspring. With and without continued exposure to bisphenol analogs, offspring hatching decreased. Additionally, with viral/bacterial mimic exposure, F1 larvae survival was reduced. Thus, parental exposure to environmentally relevant concentrations of BPA, BPS and BPF may decrease teleost health.
Immunity is an important defense mechanism in animals for protection against infection and preservation of internal homeostasis. In fish, lysozyme is one of the most important maternally-transferred immune factors functioning in the defense of teleost larvae against pathogens (Swain and Nayak, 2009). However, lysozyme activity has been shown to vary depending on changes of environmental factors or stressors, such as aquatic toxicants (Bols et al., 2001, Saurabh and Sahoo, 2008). Fish are the sensitive aquatic organisms and show a wide range of responses even towards minor changes in their environment. Therefore, stimulation or inhibition of lysozyme activity in fish can be used to monitor environmental hazards and fish innate immunity. In adults, cadmium administered to rainbow trout (Sanchez-Dardon et al., 1999) increased serum lysozyme activity and reduced serum lysozyme activity was noted in Korean rockfish after exposure to synthetic pyrethroids (Jee et al., 2005).
Due to maternal-derived immune factors, lysozyme activity in fish embryos partly reflects maternal plasma activity. Although serum lysozyme activity in adult zebrafish was not assayed previous work suggests that low concentrations of BPA increase serum lysozyme activity in common carp (Cyprinus carpio) (Qiu et al., 2016a). For F1 larvae not exposed to bisphenol, slight or significant increases in lysozyme activity and gene expression were noted, indicating maternal transfer of increased lysozymes to offspring perhaps due to maternal induction after exposure. Alternately, bacterial infection can induce lysozyme activity in fish (Saurabh and Sahoo, 2008). Similarly, lysozyme activity and gene expression were significantly up-regulated in F1 control larvae after PAM3CSK4 exposure. However, no significant changes were observed in F1 larvae exposed to PAM3CSK4 compared with those that did not have continued bisphenols exposure. Thus, parental exposure to bisphenols may increase lysozyme activity and disrupt immunity in developing offspring.
Parental exposure to bisphenols increased bisphenols in F1 embryos, and this was associated with some immune disruption. In the present study, PMA induced a respiratory burst response but this response in F1 larvae without bisphenol exposure was inhibited compared to PMA-induced F1 controls. The respiratory burst response can be used to assay immune health and it can be used to measure immunotoxic effects of environmental toxicants (Hermann and Kim, 2005). ROS production after the respiratory burst response can clear pathogens but excessive ROS are damaging (Paiva and Bozza, 2014). In previous work, bisphenol A-exposed zebrafish had more ROS production (Wu et al., 2011, Xu et al., 2013) and here, parental exposure to bisphenol inhibited the respiratory burst response, and reduced the host ability to repel infection. This may be due to delayed induction of antiviral and antibacterial cytokines compared to F1 controls.
In addition, MPX, a mediator of oxidative innate immune defense in zebrafish, is expressed in neutrophils during embryonic development in zebrafish, functioning as an ROS scavenger (van der Vaart et al., 2012). Moreover, SOD, CAT and GPx are antioxidants that repair or prevent ROS damage of macromolecular structures and participate in innate immune defense against immunostimulants (Biller-Takahashi et al., 2015, Liu et al., 2010). Previous study shows that anti-oxidant relevant genes may be induced in fish to fight infection (Liu et al., 2010), and this was confirmed by our results in F1 control larvae with bacterial and viral mimic exposure. However, significant reduction in gene expression was observed in F1 larvae after parental exposure to bisphenols and bacterial and viral mimic treatments, indicating that parental exposure to bisphenol inhibits F1 anti-oxidant defense.
Pathogen detection by the innate immune system depends on recognition of invariant molecules (pathogen-associated molecular patterns, PAMPs) of the microorganism by receptors associated with the cell surface, the best characterized of which are TLRs (Sullivan and Kim, 2008). Generally, TLRs are highly expressed in the skin of zebrafish, which suggests a prominent role in pathogen defense (Li et al., 2017). In the present study, TLR2 was principally responsible for recognition and response to PAM3CSK4 derived from gram-positive or gram-negative bacteria, whereas TLR3 recognize Poly I:C derived from RNA virus. In the F1 control larvae with bacterial and virus mimic treatment, expression of TLR2 and TLR3 were significantly up-regulated, indicating that PAM3SCSK3 and Poly I:C were recognized by the TLRs. However, TLR expression was significantly down-regulated in F1 larvae without bisphenol exposure but with bacterial and virus mimic treatment, indicating that parental exposure to bisphenol inhibited PAMPs recognition in F1 larvae.
Innate immune signaling molecules downstream of TLRs are conserved in zebrafish and include orthologous MyD88, TRIF and other signaling molecules. Because of the complexity of the pathway, the TLR signaling pathway is categorized into MyD88-dependent and TRIF-dependent pathways (Takeuchi and Akira, 2010). The MyD88-dependent pathway is used by all TLRs except TLR3, whereas TLR3 initiate the TRIF-dependent pathway. Previous work showed that knockdown of MyD88 mRNA disrupting clearance of Salmonella enterica serovar Typhimurium Ra bacteria in zebrafish (van der Sar et al., 2006); and another study showed that mice deficient in TLR3 and TRIF were highly susceptible to severe acute respiratory syndrome coronavirus (SARS-CoV) infection, and had more weight loss, more mortality, reduced lung function, increased lung pathology, and higher viral titers (Totura et al., 2015). In the present study, with decreased TLR2 and TLR3 expression, parental exposure to bisphenols also resulted in down-regulating MyD88 and TRIF expression and decreasing survival of F1 larvae. These findings suggest an importance of the TLR signaling pathway for generating a balanced protective innate immune response to bacterial or virus infections, and our results indicate that parental exposure to bisphenols decreased immune defense via inhibiting TLR-associated gene expression in the TLR pathway.
Usually, upon recognition of their cognate ligands, TLRs can induce expression of various host defense genes, including those that induce inflammatory cytokines, chemokines, and other effectors necessary to protect against invading pathogens (Medzhitov, 2001). Important antibacterial cytokines include TNFα and IL-1β, whereas antiviral cytokines include the type I IFNs. TNFα is released by activated macrophages, T lymphocytes and other immune cells in response to various infectious stimuli and can be induced by bacterial acting through TLR2, whereas IL-1β activates neutrophils and macrophages and is reported to be crucial to the clearance of intracellular bacteria (Kawai and Akira, 2011, Nayak et al., 2007). Type I IFNs are produced by many immune cells type in response to viral infection and can be induced by the Poly I:C; Mx is an interferon-inducible gene, enabling indirect measurements of type I IFNs activities in zebrafish (Sullivan and Kim, 2008). Therefore, alterations in expression patterns of these cytokines indicate that parental exposure to bisphenols immunosuppresses F1 larvae. Prenatal exposure to BPA is shown to be associated with TLR-induced cytokine suppression, such as TNFα, in neonates (Liao et al., 2016). High pathogen load, coupled with reductions in important antibacterial and antiviral cytokines, as was modeled here, using bisphenols and zebrafish, may blunt offspring immunity and prevent a competent immune response vital for eradicating infection. In addition, the bioaccumulation and metabolism of environmental chemicals in organisms may be a mechanism of immunotoxicity. For example, endosulfan can accumulate in fish immune organs and induce leucocytopenia, headkidney leucocytes death and alters headkidney somatic index (Kumari et al., 2016). In the present study, BPA and its analogs accumulate slightly in F1 embryos through parent transfer decreasing inflammatory cytokines expression. However, further study is needed to reveal the cell viability affected by bisphenols accumulation in F1 embryos.
MMPs are zinc-dependent endoproteinases members that are primarily expressed in leukocytes. They contribute to inflammation by mediating pro-cytokines, chemokines and other proteins to regulate varied aspects of inflammation and immunity (Parks et al., 2004). Recent studies suggest that MMPs between teleosts and humans in infection have an immune function, and that the MMP9 gene is downstream of the zebrafish homolog of the TLR5 receptor and the MyD88 adaptor, induced by S. typhimurium wt and Ra infection in zebrafish embryos (Stockhammer et al., 2009). Also, MMP9 expression is induced after L. monocytogenes infection and enhances macrophage migration in zebrafish embryos (Shan et al., 2016). Similarly, MMP13 was induced in channel catfish after bacterial infection (Jiang et al., 2010). In addition, MMPs contributes to embryonic development. Previous studies suggest that up-regulation of MMP9 and MMP13 expressions in zebrafish larvae occurs after tetrabromobisphenol A (TBBPA) exposure, suggesting that alteration of MMPs expression may contribute to developmental lesions in zebrafish (McCormick et al., 2010). However, we found no significant malformation in F1 larvae without bisphenol exposure compared with controls so MMPs may act on the innate immune system in F1 larvae, and alterations in expression of MMPs after parental exposure to bisphenols may cause immune disruption in F1 larvae.
To protect the host from infection, innate and adaptive immune responses provide synergistic protection. Although full functionality of the adaptive immune response requires 4–6 weeks to develop, expression of RAGs, RAG1 and RAG2 in T and B lymphocytes are prepared for adaptive immunity in 4 days (Willett et al., 1997). Therefore, RAG1 and RAG2 in immature lymphocytes are useful markers for monitoring lymphoid tissue development (Langenau and Zon, 2005). In the present study, RAG1 and RAG2 expressions did not change in F1 control larvae treated with bacterial or viral mimic compared with those not treated this way, indicating the lymphoid development was not affected by these exposures. However, expression of both RAG genes was significantly inhibited in F1 larvae with parental exposure to bisphenol which appeared to disrupt lymphoid tissue development.
In the real aquatic environment, offspring may continue to be exposed to contaminated water of parents. Therefore, we measured lysozyme activity and immune-related gene expression after continued bisphenol treatment of F1 larvae. Data show that immune function was decreased, likely due to transgenerational effect on immune disruption. Survival was also decreased in F1 larvae after continued bisphenol treatment compared with larvae not treated this way, suggesting diminished health due to bisphenol analogs.
Through these results, we also can find that bacterial and viral mimic exhibit different effects on F1 zebrafish embryos from non-exposed parents. For example, PAM3CSK4 induced TLR2 but Poly I:C induced TLR3 expression in F1 controls. However, there are no significant differences in F1 embryos from bisphenols-exposed parents. This might be parental exposure to bisphenols inhibit immune function that cannot recognize bacterial or viral mimic.
In conclusion, bisphenols increased in zebrafish offspring via transfer of parental waterborne bisphenol burdens. Furthermore, the parental exposure to bisphenol A can decrease pathogen recognition and innate immune dysfunction in zebrafish offspring. More work is required to advance our understanding of the effects of parental exposure to bisphenols on immune system development.
Notes
The authors declare no competing financial interest.
Acknowledgments
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grants Nos. 21407066, 31470554), Natural Science Foundation of Jiangsu Province of China (grants No.·BK20130488).
Editor: D. Barcelo
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2017.08.057.
Appendix A. Supplementary data
Supplementary material
References
- Ben-Jonathan N., Hugo E.R. Bisphenols come in different flavors: is “S” better than “A”? Endocrinology. 2016;157:1321–1323. doi: 10.1210/en.2016-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller-Takahashi J.D., Takahashi L.S., Mingatto F.E., Urbinati E.C. The immune system is limited by oxidative stress: dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu Piaractus mesopotamicus. Fish Shellfish Immunol. 2015;47:360–367. doi: 10.1016/j.fsi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Bodin J., Bolling A.K., Becher R., Kuper F., Lovik M., Nygaard U.C. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol. Sci. 2014;137:311–323. doi: 10.1093/toxsci/kft242. [DOI] [PubMed] [Google Scholar]
- Bols N.C., Brubacher J.L., Ganassin R.C., Lee L.E. Ecotoxicology and innate immunity in fish. Dev. Comp. Immunol. 2001;25:853–873. doi: 10.1016/s0145-305x(01)00040-4. [DOI] [PubMed] [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y.L., Wu Y. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ. Sci. Technol. 2016;50:5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- Danzl E., Sei K., Soda S., Ike M., Fujita M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Environ. Res. Public Health. 2009;6:1472–1484. doi: 10.3390/ijerph6041472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladak S., Grisin T., Moison D., Guerquin M.J., N'Tumba-Byn T., Pozzi-Gaudin S. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015;103:11–21. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Heffelfinger A. North Carolina State University; 2010. Identification and Investigation of Conserved Innate Immune Response Genes Between Zebrafish and Humans. (Master) [Google Scholar]
- Hermann A.C., Kim C.H. Effects of arsenic on zebrafish innate immune system. Mar. Biotechnol. (N.Y.) 2005;7:494–505. doi: 10.1007/s10126-004-4109-7. [DOI] [PubMed] [Google Scholar]
- Ike M., Chen M.Y., Danzl E., Sei K., Fujita M. Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions. Water Sci. Technol. 2006;53:153–159. doi: 10.2166/wst.2006.189. [DOI] [PubMed] [Google Scholar]
- Jee J.H., Masroor F., Kang J.C. Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf) Aquac. Res. 2005;36:898–905. [Google Scholar]
- Jiang Y., Abernathy J.W., Peatman E., Liu H., Wang S., Xu D.H. Identification and characterization of matrix metalloproteinase-13 sequence structure and expression during embryogenesis and infection in channel catfish (Ictalurus punctatus) Dev. Comp. Immunol. 2010;34:590–597. doi: 10.1016/j.dci.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Asai D., Katayama Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit. Rev. Toxicol. 2007;37:607–625. doi: 10.1080/10408440701493103. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kumari U., Srivastava N., Shelly A., Khatri P., Sarat N., Singh D.K., Mazumder S. Inducible headkidney cytochrome P450 contributes to endosulfan immunotoxicity in walking catfish Clarias gariepinus. Aquat. Toxicol. 2016;179:44–54. doi: 10.1016/j.aquatox.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Langenau D.M., Zon L.I. The zebrafish: a new model of T-cell and thymic development. Nat. Rev. Immunol. 2005;5:307–317. doi: 10.1038/nri1590. [DOI] [PubMed] [Google Scholar]
- Li Y., Li Y., Cao X., Jin X., Jin T. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cell. Mol. Immunol. 2017;14:80–89. doi: 10.1038/cmi.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 2012;46:6515–6522. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- Liao S.L., Tsai M.H., Lai S.H., Yao T.C., Hua M.C., Yeh K.W. Prenatal exposure to bisphenol-A is associated with Toll-like receptor-induced cytokine suppression in neonates. Pediatr. Res. 2016;79:438–444. doi: 10.1038/pr.2015.234. [DOI] [PubMed] [Google Scholar]
- Liu H.P., Chen F.Y., Gopalakrishnan S., Qiao K., Bo J., Wang K.J. Antioxidant enzymes from the crab Scylla paramamosain: gene cloning and gene/protein expression profiles against LPS challenge. Fish Shellfish Immunol. 2010;28:862–871. doi: 10.1016/j.fsi.2010.02.008. [DOI] [PubMed] [Google Scholar]
- McCormick J.M., Paiva M.S., Haggblom M.M., Cooper K.R., White L.A. Embryonic exposure to tetrabromobisphenol A and its metabolites, bisphenol A and tetrabromobisphenol A dimethyl ether disrupts normal zebrafish (Danio rerio) development and matrix metalloproteinase expression. Aquat. Toxicol. 2010;100:255–262. doi: 10.1016/j.aquatox.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Milla S., Depiereux S., Kestemont P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: a review. Ecotoxicology. 2011;20:305–319. doi: 10.1007/s10646-010-0588-7. [DOI] [PubMed] [Google Scholar]
- Nayak A.S., Lage C.R., Kim C.H. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio) Toxicol. Sci. 2007;98:118–124. doi: 10.1093/toxsci/kfm072. [DOI] [PubMed] [Google Scholar]
- Paiva C.N., Bozza M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Qiu W., Chen J., Li Y., Chen Z., Jiang L., Yang M. Oxidative stress and immune disturbance after long-term exposure to bisphenol A in juvenile common carp (Cyprinus carpio) Ecotoxicol. Environ. Saf. 2016;130:93–102. doi: 10.1016/j.ecoenv.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Qiu W., Zhao Y., Yang M., Farajzadeh M., Pan C., Wayne N.L. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology. 2016;157:636–647. doi: 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- Sanchez-Dardon J., Voccia I., Hontela A., Chilmonczyk S., Dunier M., Boermans H. Immunomodulation by heavy metals tested individually or in mixtures in rainbow trout (Oncorhynchus mykiss) exposed in vivo. Environ. Toxicol. Chem. 1999;18:1492–1497. [Google Scholar]
- Saurabh S., Sahoo P.K. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 2008;39:223–239. [Google Scholar]
- Schonfelder G., Wittfoht W., Hopp H., Talsness C.E., Paul M., Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt A.R. Parental effects of endocrine disrupting compounds in aquatic wildlife: is there evidence of transgenerational inheritance? Gen. Comp. Endocrinol. 2015;219:152–164. doi: 10.1016/j.ygcen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Segner H., Wenger M., Moller A.M., Kollner B., Casanova-Nakayama A. Immunotoxic effects of environmental toxicants in fish - how to assess them? Environ. Sci. Pollut. Res. Int. 2011;19:2465–2476. doi: 10.1007/s11356-012-0978-x. [DOI] [PubMed] [Google Scholar]
- Shan Y., Zhang Y., Zhuo X., Li X., Peng J., Fang W. Matrix metalloproteinase-9 plays a role in protecting zebrafish from lethal infection with Listeria monocytogenes by enhancing macrophage migration. Fish Shellfish Immunol. 2016;54:179–187. doi: 10.1016/j.fsi.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Stockhammer O.W., Zakrzewska A., Hegedus Z., Spaink H.P., Meijer A.H. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 2009;182:5641–5653. doi: 10.4049/jimmunol.0900082. [DOI] [PubMed] [Google Scholar]
- Sullivan C., Kim C.H. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Swain P., Nayak S.K. Role of maternally derived immunity in fish. Fish Shellfish Immunol. 2009;27:89–99. doi: 10.1016/j.fsi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Takahashi O., Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 2000;108:931–935. doi: 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Totura A.L., Whitmore A., Agnihothram S., Schafer A., Katze M.G., Heise M.T. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6:e00638–15. doi: 10.1128/mBio.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sar A.M., Stockhammer O.W., van der Laan C., Spaink H.P., Bitter W., Meijer A.H. MyD88 innate immune function in a zebrafish embryo infection model. Infect. Immun. 2006;74:2436–2441. doi: 10.1128/IAI.74.4.2436-2441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart M., Spaink H.P., Meijer A.H. Pathogen recognition and activation of the innate immune response in zebrafish. Adv. Hematol. 2012;2012:159807. doi: 10.1155/2012/159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett C.E., Cherry J.J., Steiner L.A. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- Wu M., Xu H., Shen Y., Qiu W., Yang M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011;30:2335–2341. doi: 10.1002/etc.634. [DOI] [PubMed] [Google Scholar]
- Xu H., Yang M., Qiu W., Pan C., Wu M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ. Toxicol. Chem. 2013;32:1793–1799. doi: 10.1002/etc.2245. [DOI] [PubMed] [Google Scholar]
- Xu H., Dong X., Zhang Z., Yang M., Wu X., Liu H. Assessment of immunotoxicity of dibutyl phthalate using live zebrafish embryos. Fish Shellfish Immunol. 2015;45:286–292. doi: 10.1016/j.fsi.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Yamazaki E., Yamashita N., Taniyasu S., Lam J., Lam P.K., Moon H.B. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015;122:565–572. doi: 10.1016/j.ecoenv.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Yang M., Lee H.S., Pyo M.Y. Proteomic biomarkers for prenatal bisphenol A-exposure in mouse immune organs. Environ. Mol. Mutagen. 2008;49:368–373. doi: 10.1002/em.20394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material