Abstract
To ensure the safety of wastewater reuse for irrigation of food crops and drinking water pathogenic viruses must be reduced to levels that pose no significant risk. To achieve this goal minimum reduction of viruses by treatment trains have been suggested. For use of edible crops a 6-log reduction and for production of potable drinking water a 12-log reduction has been suggested. These reductions were based on assuming infective virus concentrations of 105 to 106 per liter. Recent application of molecular methods suggests that some pathogenic viruses may be occurring in concentrations of 107 to 109 per liter. Factors influencing these levels include the development of molecular methods for virus detection, emergence of newly recognized viruses, decrease in per capita water use due to conservation measures, and outbreaks. Since neither cell culture nor molecular methods can assess all the potentially infectious virus in wastewater conservative estimates should be used to assess the virus load in untreated wastewater. This review indicates that an additional 2- to 3-log reduction of viruses above current recommendations may be needed to ensure the safety of recycled water. Information is needed on peak loading of viruses. In addition, more virus groups need to be quantified using better methods of virus quantification, including more accurate methods for measuring viral infectivity in order to better quantify risks from viruses in recycled water.
Keywords: Viruses, Reduction, Wastewater, Recycle, Reuse
Graphical abstract
Highlights
-
•
Guidelines are needed for reduction of viruses from wastewater for water reuse.
-
•
Recycled wastewater for direct potable reuse requires a 12-log virus reduction.
-
•
New methods of virus quantification suggest an additional 2–3 log-virus reduction.
1. Introduction
Reuse of wastewater is practiced for augmenting water supplies that are subsequently used for both irrigation and potable purposes (NRC, 2012). Since domestic wastewater always contains pathogens capable of infecting humans they must be reduced to levels that do not pose a threat to populations that may be exposed. Various guidelines have been suggested for the needed reductions of pathogens by the treatment process to ensure minimal risk to the exposed population. In the case of recycled water used for irrigation a 6- to 7-log reduction of viruses by the treatment process has been suggested (WHO, 2006, Sano et al., 2016). In the Groundwater Replenishments Reuse Project of the state of California a 12-log reduction of virus is required when treated wastewater is used for groundwater recharge intended for indirect potable reuse (Title 22 and 17 California Code of Regulations State Board, 2015). The Texas Commission on Environmental Quality (Texas, 2015) has established a minimum baseline target for virus reduction of 8 logs although this is subject to collection of additional data (Sano et al., 2016). These reductions are designed to produce recycled water that results in a yearly risk of infection of 1:10,000 or less to persons who may ingest the irrigated food or water. These reductions were based on observations that infectious virus concentrations in untreated domestic wastewater were no greater than 5- to 6-log per liter. The data used for these assumptions were based on studies conducted before 1996 when virus quantification was based on animal cell culture assays (Asano et al., 2007). In the last 25 years major advances in molecular methods have greatly expanded our knowledge on both the quantity and types of viruses present in domestic wastewater and we believe a reassessment of the types and numbers of viruses present in wastewater and the factors which may influence this in the future is needed.
2. Factors which influence the concentration of viruses in wastewater
Many factors may influence the concentration and types of viruses in wastewater (Table 1 ). The incidence of infection within the population is the major factor. Basically, all viruses which infect humans are likely to end up in domestic wastewater. Incidence includes persons with both symptomatic and asymptomatic infections. Persons with symptomatic infections may excrete viruses for many weeks after a clinically observable infection. Other viruses may largely cause asymptomatic infections and be excreted throughout the lifetime of the individual (e.g. polyomavirus). Greater concentrations are usually released from infected individuals during clinical infections and then decrease over time. As shown in Table 2 , the concentration of enteric viruses during diarrhea may be as high as 1010–1012 viral particles per gram of feces (Haas et al., 2014). Thus, an infected person with even a normal bowel movement of 100–300 g per day may excrete 1012 to 1014 per day (Feachem et al., 1983). Children tend to excrete greater numbers of viruses during an infection (Ayukekbong et al., 2011). New types and strains of viruses have evolved over time and the levels of these viruses will increase when they enter a new population that has not been previously exposed (La Rosa et al., 2012). Viral infections can also vary greatly seasonally. For example, norovirus infections in North America peak in the winter months resulting in greater concentrations in the wastewater during this time of year (Kitajima et al., 2014). Other viruses appear to remain at high levels throughout the year i.e. adenoviruses, Aichi viruses (Kitajima et al., 2014). The socioeconomic status of the community is also a factor because of supposed greater spread of a virus through a population with poor hygiene.
Table 1.
Factors which influence the concentration of viruses in wastewater.
| Incidence of infection within a community |
| Social economic status of the community |
| Season |
| Per capita water use |
| Time of day |
| Age distribution within the community |
| Chronic infections |
Table 2.
Concentration of enteric viruses in feces.
| Enteric viruses | Per gram of fecesa |
|---|---|
| Enteroviruses | 103–107 |
| Rotavirus | 1010 |
| Adenovirus | 1011 |
| Norovirus | 1011 |
Enteroviruses are based on infectivity assay in cell culture and data for the other viruses are from electron micrographs.
Adapted from Haas et al., 2014.
Water use per capita will result in greater concentrations of pathogens in the wastewater. Usually domestic water usage is greatest in the morning and the evening and this will also influence the concentration of virus that enters a wastewater treatment facility (Almeida et al., 1999). Water use in the United States has been decreasing significantly since the requirement for use of low-flush toilets and of more efficient washing machines which use less water. Usage per single family homes has decreased 22% from 1999 to 2016 (DeOreo et al., 2016). Water use for cloth washing machines has decreased in the United States by 46% and toilet flushing by 37%. These two home devices have had the biggest impact on the decrease in per capita water use. Since both of these devices will carry the largest pathogen load to sewers the virus concentration can be expected to continue to increase as these new devices come into widespread use. In addition, since these devices are used more commonly during certain times of the day or days (i.e. morning or evening) this can also be expected to influence maximum concentrations (Butler, 1993). Finally, the increasing use of cold water laundry washes can be expected to increase the number of infectious viruses to sewers as water temperature is a major factor in inactivating viruses in laundry (Gerba and Kennedy, 2007). Decreasing use of chlorine in washer loads would also be expected to have the same effect.
3. Estimate of infective virus levels in wastewater
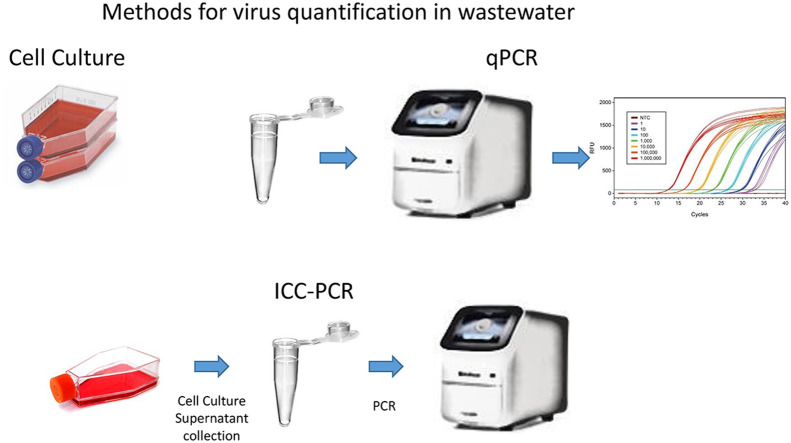
Estimates on the concentration of viruses in wastewater before the development of molecular methods were based on detection of growth of the viruses in cell culture (Fig. 1 ). These are infectivity assays and indicate that the virus is capable of reproducing in susceptible hosts. Infectivity is measured by the production of plaques (areas of cell destruction by the virus) in cell monolayers (plaque forming units or PFU) or a dilution assay in which cell destruction is observed and is referred to as tissue culture infectious dose which infects 50% of the inoculated cultures (TCID50). It is important to recognize that these methods are not capable of detecting all the viruses in wastewater and always underestimate the true number of infectious viruses present (Table 3 ). Many factors influence the ability to detect viruses in cell culture. Different cell lines will give a wide range of values for the same type of virus. Most cell lines used for virus detection in wastewater have only been capable of detecting enteroviruses, reoviruses or adenoviruses. Primary kidney monkey or human primary cell culture are more sensitive for virus detection and have generally yielded greater numbers in wastewater than continuous cell lines (immortal cell lines) (Table 4 ). Primary cell lines are no longer used for virus testing of environmental samples and probably explain why lower numbers of virus have been reported since their use was discontinued. Also, cell culture is fairly insensitive in detecting all potentially infectious viruses, especially by those excreted in the feces. Generally enteric viruses grown in the laboratory will produce one plaque of cytopathogenic effect for every 10 to 100 observed under an electron microscope (Mahalanabis et al., 2010). In the case of rotaviruses from a human stool this ratio was observed to be 1:46,000 (Ward et al., 1984). The difference is that laboratory-grown viruses have been selected for those that grow well in cell culture. Ward et al. (1984) observed that by passing human rotavirus from a stool specimen and this ratio of cell culture infective to infective virus decreased from 1:46,000 to 1: 6000. The assay method requires the virus to come in contact with the cell monolayer and the receptor on the cell must be exposed for virus attachment. In addition, virus present in a stool may be in the form of aggregates, which will only produce one plaque or one TCID50 in cell culture, yet may represent many infectious virions (Wallis and Melnick, 1967, Kahler et al., 2016, Galasso et al., 1964, Langlet et al., 2007). It has also been found that the number of infective viruses observed in cell culture is related to how long samples are exposed to the cells, the presence of enzymes, polyelectrolytes, incubation temperatures, inoculum volume, and passage number of the cell line (Spendlove and Schaffer, 1965, Benton and Ward, 1982, Benton and Hurst, 1986, Dahling, 1991). The ratio of viral particles to infective virions in cell culture for reovirus has been found to range from 1 infective virion per every 2 to 2.5 viral particle (Wallis et al., 1964, McClain and Spendlove, 1966). Blackmer et al. (2000) found that poliovirus 1 could be detected by integrated cell culture PCR for up to 6 min after chlorine exposure, but only 1 min by observation for cytopathogenic effects if only one passage was performed. This indicates that without additional passage of viruses in cell culture infective virus levels would be underestimated.
Fig. 1.
Methods of virus quantification and detection in wastewater.
Table 3.
Factors influencing infectivity assays for viruses.
| Primary vs. continuous cell lines | Generally primary cell lines are more sensitive |
|---|---|
| Specific cell line | Numbers of viruses detected and types of viruses are dependent on the specific cell line; how many passages of the cell line have been performed |
| Assay conditions | Rocking cells, addition of certain enzymes and polyelectrolytes (e.g., trypsin), PFU vs TCID50; monolayer vs. suspended cells. |
| Ratio of viral particles to infectivity | Much lower for viruses directly isolated from human feces vs. laboratory grown viruses |
| Number of passages of the sample in cell culture | Passage of negative samples on first passage onto a second passage results in greater numbers of virus detected in a sample |
PFU = plaque forming units; TCID50 = Tissue culture infectious dose.
Table 4.
Concentrations of viruses in untreated wastewater as determined by cell culture assay (greatest values known).
| Maximum Concentration of virus per liter | Method of assay | Cell line | Location | Reference |
|---|---|---|---|---|
| 276,000 | PFU; 80–100% efficiency | PMK; human amnion | San Diego, California, United States of America | England et al., 1964 |
| 210,000 | TCID50 | PMK | Johannesburg, South Africa | Malherbe and Strickland-Cholmley, 1967 |
| 1,106,000 | PFU; direct inoculation | BGM | Haifa, Israel | Buras, 1976 |
| 95,000 | TCID50 | PMK | Worcester and Pietermaritzburg, South Africa | Grabow and Nupen, 1972 |
| 210,000 | IF; reovirus only | Mouse L929 | Dugway, UT, United States of America | Adams et al., 1982 |
| 463,500 | TCID50 | PMK | Windhoek, Namibia | Nupen et al., 1974 |
IF = infectious foci; PMK = primary monkey kidney; BGM = Buffalo green monkey.
Few studies have ever looked at cell culture assays and quantitative polymerase chain reaction (qPCR) on the same samples in untreated wastewater. Using several cell lines Francy et al. (2011) found a ratio of enterovirus detected by qPCR vs. infectively in cell culture of 10.7:1 in untreated wastewater. Using only one cell line each for the detection of enteroviruses and adenoviruses with a concentration method with less than a 10% efficiency for infectious virus, Hewitt et al. (2011) found a maximum ratio of virus detected by qPCR to infectivity that ranged from 25:1 to 794:1 in wastewater. Since untreated wastewater represents recently excreted feces it can be expected that most of the viruses detected by qPCR are infectious. In a study of norovirus outbreaks related to consumption of contaminated shellfish it was found that one norovirus detected by qPCR in shellfish had an average probability of infection of 29–40% in persons consuming the oysters (Thebault et al., 2013). These oysters were contaminated by waste discharges into the ocean, requiring the virus to be transported via water currents to the shellfish and then harvested and brought to market. This indicates that even one norovirus detected by qPCR in an environmental sample has a significant probability of causing infection.
Thus, estimating ratios of infectious virus to genome copies detected by qPCR will probably never be known with certainty in the foreseeable future.
In summary determination of virus infectivity by cell culture can underestimate virus infectivity levels in wastewater by at least 2 to 3 orders of magnitude.
4. Concentration of viruses in wastewater as determined by cell culture
The greatest levels observed for viruses in wastewater detected by cell culture are shown in Table 4. Most of the viruses detected were either enteroviruses or reoviruses. The greatest value reported was by Buras (1976), who inoculated the wastewater directly into cell culture, without first attempting to concentrate the virus. Most of the other studies first concentrated the virus from a few 100 mL to a few liters. A review by Irving (1982) of the concentration of viruses in wastewater indicated that most studies have found a range of a few hundred viruses to 10,000 viruses per liter by cell culture assays. However, we feel that for a risk assessment for water recycling the greatest value should be considered rather than an average because of uncertainty in the estimates can be significant.
5. Concentration of viruses determined by qPCR
The advantage of qPCR is that it is capable of detecting viruses which will not grow in cell lines and is more efficient in detection of the virus (Fig. 1). Viral nucleic acids degrade rapidly (within a few minutes) in wastewater and detection is likely limited to intact virions (Limsawat and Ohgaki, 1997). In addition, the concentration of naked virus RNA is much less efficient by membrane filters, which are often employed for primary concentration of viruses (Haramoto et al., 2007). However, both infectious and non-infections viruses can be detected by qPCR. While different techniques have been developed for differentiating infectious vs. non-infections virions, these techniques tend to be dependent upon the method of inactivation (protein capsids vs. nucleic acid) and are virus-type specific (Rodriguez et al., 2009).
Table 5 is a selection of studies showing the greatest levels of viruses detected by PCR. Only virus groups/virus with the greatest levels reported for a virus are shown. Studies usually involved a selection of enteric viruses or only one group of viruses (e.g. noroviruses). Not all studies used the same methods for the concentration of viruses, nucleic extraction, or primers and hydrolysis probes. Still, overall adenoviruses appear to occur in the greatest concentrations in most studies in which they were included. The same could be said for noroviruses. In most studies enteroviruses occur at levels 100 to 1000-fold less. Few studies have included reoviruses, but at least one study reported significant numbers (Table 5). Several studies have reported finding peak levels of adenoviruses and noroviruses at or above 109/liter. Peak levels of virus groups/types at 108/liter were reported by several studies. If one considers that the methods for concentrating the virus are less than 100% then values of 1010/liter can occur (La Rosa et al., 2010). It should be pointed out these are only for groups of viruses or individual virus types. If all the enteric viruses which could be assayed in an individual sample are included, then levels of virus would be greater. Thus, these values should be considered conservative estimates of the virus load in untreated wastewater. The median values for adenoviruses, noroviruses and Aichi viruses are in the 106 genome copies per liter range.
Table 5.
Concentration of viruses detected in untreated wastewater by qPCR.
| Genome copies per liter | Virus | Location | Remarks | Reference |
|---|---|---|---|---|
| 51,000,000 | Norovirus GI, GII | Tucson, Arizona, United States of America | Sample collected the same day; composite sample; 24% efficiency | Schmitz et al., 2016 |
| 15,500,000 | Adenovirus | |||
| 1,100,800 | Norovirus GI, GII | Tucson, AZ, USA | 105.3% efficiency | Kitajima et al., 2014 |
| 5,191,200 | Adenovirus | 73.8% efficiency | ||
| 31,000,000 | Rotavirus | Yungas region, Bolivia | >10% efficiency | Symonds et al., 2014. |
| 158,000 | Norovirus GI | Kyoto, Shiga, Saitama, Osaka, Tokyo, Ibaraki, Japan | 19% efficiency | Katayama et al., 2008 |
| 316,227 | Adenovirus | |||
| 5,700,000,000 | Norovirus GII | Central Italy | 35% efficiency | La Rosa et al., 2010 |
| 1,600,000,000 | Norovirus GI | |||
| 9,800,000,000 | Adenovirus | |||
| 1,258,925,412 | Adenovirus | Traverse City, Michigan, USA | 1 MDS method for conc. 30–50% efficiency | Simmons et al., 2011 |
| 398,107,170 | Norovirus GII | |||
| 1,000,000,000 | Norovirus GI | Northwestern France | Composite; efficiency >10% | Da Silva et al., 2007 |
| 60,000,000 | Norovirus GII | |||
| 1,000,000,000 | Norovirus GI | Northwest France | Grab; >10% efficiency | Da Silva et al., 2008 |
| 39,810,717 | Norovirus GII | |||
| 2,200,000 | Adenovirus | Japan | Not given | Hata et al., 2012 |
| 510,000 | Sapovirus | |||
| 416,686,938 | Adenovirus | Several treatment plants across New Zealand | Beef extract flocculation –eff. Not given | Hewitt et al., 2011 |
| 4,677,351 | Enterovirus | |||
| 63,095,734 | Adenovirus | Edmonton, Canada | 30 to 50% efficiency | Qiu et al., 2015 |
| 19,952,623 | Reovirus | |||
| 10,000,000 | Norovirus | |||
| 10,000,000 | Sapovirus | |||
| 12,589,254 | Norovirus GII | New Orleans, Louisiana, USA | Composite; eff. Not provided; ultracentrifugation | Montazeri et al., 2015 |
| 12,589,254 | Norovirus GI |
6. Impact of time of and type of sampling i.e. composite vs. grab
Another factor to consider is sample collection. Most studies have involved grab samples, likely often collected at the convenience of the laboratory performing the analysis (Table 4, Table 5). The volume of wastewater received by a treatment plant varies throughout the day depending on when bathing, toilet usage and laundry washing takes place (Asano et al., 2007). These activities can influence the peak load of viruses into the sewer system. Using composite samples collected over a 24-h period is designed to catch the peak flows, but only represents an average concentration.
7. Impact of outbreaks
The level of a given virus in wastewater is dependent upon the incidence of infection within the community (Sinclair et al., 2009). Seasonal peaks of noroviruses and enteroviruses are clearly seen in studies in temperate climates indicating differences in the number of infected individuals (Sinclair et al., 2009) Introduction of a new virus type or one without a significant amount of herd immunity could result in dramatic spikes or peaks of the virus in community sewage.
8. Emerging viruses
Within the last two decades' new viruses have been identified in fecal specimens and in sewage using conventional and highly-sensitive genome sequencing technologies (Ng et al., 2015, Kapoor et al., 2008, Kapoor et al., 2009 2010). Novel viruses may also occur/appear as a result of virus mutations and genetic recombinations among virus types of the same or different species within the same genus (i.e., inter- and intra-typic recombination events), which play an important role on the evolution and epidemiology (e.g., spread, emergence and disappearance) of these viruses (Robinson et al., 2013, Tapparel et al., 2013). Frequent recombinations and mutations in enteroviruses have been recognized as the main mechanisms for the observed high rate of evolution, thus enabling them to rapidly respond and adapt to new environmental challenges (Kyriakopoulou et al., 2015). Table 6 lists new viruses recently identified in sewage, feces or urine.
Table 6.
New viruses and new virus types excreted in feces or urine identified since 1996.
| Virus | Reference |
|---|---|
| EV73 | Oberste et al., 2001 |
| EV 76, 89, 90, 91 | Oberste et al., 2005 |
| EV 74-75 | Oberste et al., 2004 |
| EV 77-78 | Norder et al., 2003, Bailly et al., 2004 |
| EV 79–88, 97, and 100-101 | Oberste et al., 2007 |
| EV 105 and EV 116 | Grard et al., 2010, Lukashev et al., 2012 |
| HAdV-G52 | Jones et al., 2007 |
| HAdV-D53 | Engelmann et al., 2006 |
| HAdV-D54 | Ishiko et al., 2008 |
| Human Astrovirus AstV-MLB1 | Finkbeiner et al., 2008 |
| Human Polyomavirus-9 (HPyV9) | Scuda et al., 2011 |
| MW Polyomavirus (MWPyV) HPyV10 | Siebrasse et al., 2012, Buck et al., 2012 |
| Merkel cell polyomavirus (MCPyV) | Feng et al., 2008 |
| Severe acute respiratory syndrome-related coronavirus SARS coronavirus (SARS-CoV) | Peiris et al., 2003, Drosten et al., 2003, Ksiazek et al., 2003 |
| Small circular, Rep-encoding, ssDNA (CRESS-DNA) genomes (CRESS-DNA viruses) characterized in fecal or environmental samples | Ng et al., 2015 |
| Human cyclovirus 1, 2 and 3 Human feces associated circovirus (HufaCV) |
Li et al., 2010, Biagini et al., 2012 |
| Human Bocavirus (HBoV) [HBoV-1, HBoV-2, HBoV-3, HBoV-4] | Allander et al., 2005, Arthur et al., 2009, Kapoor et al., 2009, Kapoor et al., 2010 |
| Human Cosavirus (HCosV-A) | Kapoor et al., 2008 |
| Human Salivirus | Greninger et al., 2009, Li et al., 2010 |
| Human Cardiovirus [Saffold cardiovirus] | Jones et al., 2007 |
| Human picobirnavirus D strain CDC23 (HuPBV-D-CDC23) and Human picobirnavirus E strain CDC16 (HuPBV-E-CDC16) | Ng et al., 2014 |
9. Discussion
Because of difficulties in assessing the levels of human pathogenic viruses in water, wastewater treatment technology has been relied upon to prevent waterborne transmission. This approach depends upon accurate knowledge of the concentration of infectious virus in the water to be treated. In the United States, it is assumed that a 4-log reduction of viruses is needed for drinking water treatment plants which obtain their untreated water from surface water sources (Regli et al., 1991). This is based on the assumption of a likely level of virus in surface waters in the United States to result in a risk of infection of less than 1:10,000 per year. This same approach has been suggested for treatment designed for wastewater intended for potable reuse. Each treatment process in the treatment train is given a value or credit for a log removal of virus (Sano et al., 2016). A 12-log removal requirement has been suggested and used in California for this purpose (Title 22 and 17 California Code of Regulations State Board, 2015). This level of removal was based upon an assumption of the presence of 105 to 106 infectious viruses per liter. This level of virus was also based upon levels detected in untreated wastewater determined with cell culture. The cell lines used would largely only detect enteroviruses and, if additional effort were made, reoviruses. In addition, the methods have not always been optimized to detect all of the potentially infectious viruses. Generally, enteroviruses and reoviruses have been detected in untreated wastewater by cell culture at levels from 103 to 104 per liter, although 105 and 106 have been documented (Table 5). Given the known limitation in cell culture detection for enteric viruses greater levels of infectious virus are likely present. The ratio of virion to infectious virus in cells has been reported as low as 1 to 1.2 depending on assay and pretreatment conditions (McClain and Spendlove, 1966).
10. Conclusions
Determining the numbers of viruses in untreated wastewater will be a moving target for the near future as many factors interact to influence our assessment. Changing water-use patterns in households, emergence of new viral pathogens, better technology for the concentration of viruses and detection by PCR will influence our knowledge on the presence of viruses in wastewater. Our assessment at present indicates that the 12-log removal goal required when treated wastewater is used for groundwater recharge should probably be reassessed given the significant increase of our knowledge on viruses present in untreated wastewater. This review indicates that an additional 2- to 3-log reduction of viruses above current recommendations may be needed to ensure the safety of recycled water.
To better understand the significance of enteric virus levels in wastewater we recommend:
-
•
the concentration efficiency of every sample be documented by use of a model virus
-
•
collection of samples at peak flows into the wastewater treatment facility
-
•
use of methods which could assess infectivity by qPCR or other methods
-
•
peak values of viruses should be considered rather than average values of virus if untreated wastewater is to be used when determining viral removal requirements
-
•
the ratio of infective virus to virions (as detected by qPCR) should be considered to be less than 1:10 unless proven otherwise.
Acknowledgements
We wish to thank Mr. Jeffrey R. Bliznick for providing the graphic in Fig. 1. This review was supported in part by the United States Department of Agriculture-National Institute of Food and Agriculture. Grant number 20166800725064, that established CONSERVE: A Center of Excellence at the Nexus of Sustainable Water Reuse, Water and Health.
References
- Adams D.J., Ridinger D.N., Spendlove R.S., Barnett B.B. Protamine precipitation of two particle types from polluted water. Appl. Environ. Microbiol. 1982;44(3):589–596. doi: 10.1128/aem.44.3.589-596.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. Erratum Proc. Natl. Acad. Sci. USA. 2005; 102:15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M.C., Butler D., Friedler E. At-source domestic wastewater quality. Urban Water. 1999;1(1):49–55. [Google Scholar]
- Arthur J.L., Higgins G.D., Davidson G.P., Givney R.C., Ratcliff R.M. A Novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Franklin Burton H., Leverenz L., Tsuchihashi R., Tchobanoglous G. McGraw-Hill; New York: 2007. Water Reuse: Issues, Technologies, and Applications. [Google Scholar]
- Ayukekbong J., Lindh M., Nenonen N., Tah F., Nkuo-Akenji T., Bergström T. Enteric virus in healthy children in Cameroon: viral load and genotyping of norovirus strains. J. Med. Virol. 2011;83(12):2135–2142. doi: 10.1002/jmv.22243. [DOI] [PubMed] [Google Scholar]
- Bailly J.L., Cardoso M.C., Labbé A., Peigue-Lafeuille H. Isolation and identification of an enterovirus 77 recovered from a refugee child from Kosovo, and characterization of the complete virus genome. Virus Res. 2004;99(2):147–155. doi: 10.1016/j.virusres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Benton W.H., Hurst C.J. Evaluation of mixed cell types and 5-Iodo-2’-deoxyuridine treatment upon plaque assay titers of human enteric viruses. Appl. Environ. Microbiol. 1986;51(5):1036–1040. doi: 10.1128/aem.51.5.1036-1040.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W.H., Ward R.L. Induction of cytopathogenicity in mammalian cell lines challenged with culturable enteric viruses and its enhancement by 5-Iododeoxyuridiene. Appl. Environ. Microbiol. 1982;43(4):861–868. doi: 10.1128/aem.43.4.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini P., Bendinelli M., Hino S., Kakkola L., Mankertz A., Niel C., Okamoto H., Raidal S., Teo C.G., Todd D. Circoviridae. In: King A.M.Q., Adams M.J., Cartens E.B., Lefkowitz E.J., editors. Virus Taxonomy, IXth Report of the International Committee for the Taxonomy of Viruses. Elsevier/Academic Press; London: 2012. pp. 343–349. [Google Scholar]
- Blackmer F., Reynolds K.A., Gerba C.P., Pepper I.L. Use of integrated cell culture-PCR to evaluate the effectiveness of poliovirus inactivation by chlorine. Appl. Environ. Microbiol. 2000;66(5):2267–2268. doi: 10.1128/aem.66.5.2267-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C.B., Phan G.Q., Raiji M.T., Murphy P.M., McDermott D.H., McBride A.A. Complete genome sequence of a tenth human polyomavirus. J. Virol. 2012;86(19):10887. doi: 10.1128/JVI.01690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buras N. Concentration of enteric viruses from wastewater and effluent: a two-year survey. Water Res. 1976;10(4):295–298. [Google Scholar]
- Butler D. The influence of dwelling occupancy and day of the week on domestic appliance wastewater discharges. Build. Environ. 1993;28(1):73–79. [Google Scholar]
- Da Silva A.K., Le Guyader F.S., Le Saux, Pommepuy M., Montgomery M.A., Elemelech M. Norovirus removal and particle association in a waste stabilization pond. Environ. Sci. Technol. 2008;42(24):9151–9157. doi: 10.1021/es802787v. [DOI] [PubMed] [Google Scholar]
- Da Silva A.K., Le Saux J.C., Parnaudeau S., Pommepuy M., Montgomery M.A., Elemelech M., Le Guyader F.S. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl. Environ. Microbiol. 2007;73(24):7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahling D.R. Detection and enumeration of enteric viruses in cell-culture. Crit. Rev. Environ. Contrib. Sci. 1991;21(3–4):237–263. [Google Scholar]
- DeOreo W.B., Mayer P.W., Dziegielwski B., Kiefer J.C. Water Research Foundation; Denver, CO: 2016. Residential Uses of Water 2016. [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Engelmann I., Madisch I., Pommer H., Heim A. An outbreak of epidemic Keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin. Infect. Dis. 2006;43(7):64–66. doi: 10.1086/507533. [DOI] [PubMed] [Google Scholar]
- England B., Leach R.E., Adams B., Shiosaki R. Virological assessment of sewage treatment at Santee, California. In: Berg G., editor. Transmission of Viruses by the Water Route. Interscience; NY: 1964. pp. 401–417. [Google Scholar]
- Feachem R.G., Bradley D.J., Garelick H., Mara D.D. John Wiley and Sons; NY: 1983. Sanitation and Disease. Health Aspects of Excreta and Wastewater Management. [Google Scholar]
- Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Allred A.F., Tarr P.I., Klein E.J., Kirkwood C.D., Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008;4:e1000011. doi: 10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy D.S., Stelzer E.A., Bushon R.N., Brady A.M.G., Mailot B.E., Spencer S.K., Borchardt M.A., Elber A.G., Riddell K.R., Gellner T.M. 2011. Quantifying Viruses and Bacteria in Wastewater—Results, Interpretation Methods, and Quality Control: U.S. Geological Survey Scientific Investigations Report 2011–5150. Raston, Va. [Google Scholar]
- Galasso G.J., Sharp J., Sharp D.G. The influence of degree of aggregation and virus quality on the plaque titer of aggregated vaccina virus. J. Immunol. 1964;92:870–878. [PubMed] [Google Scholar]
- Gerba C.P., Kennedy D. Enteric virus survival during household laundering and impact of disinfection with sodium hypochlorite. Appl. Environ. Microbiol. 2007;73(14):4425–4428. doi: 10.1128/AEM.00688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow W.O.K., Nupen E.M. The load of infectious micro-organisms in the wastewater of two south African hospitals. Water Res. 1972;6(12):1557–1563. [Google Scholar]
- Grard G., Drexler J.F., Lekana-Douki S., Caron M., Lukashev A., Nkoghe D., Gonzalez J.P., Drosten C., Leroy E. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo, October–November 2010. Euro Surveill. 2010;15:19723. doi: 10.2807/ese.15.47.19723-en. [DOI] [PubMed] [Google Scholar]
- Greninger A.L., Runckel C., Chiu C.Y., Haggerty T., Parsonnet J., Ganem D., DeRisi J.L. The complete genome of klassevirus – a novel picornavirus in pediatric stool. Virol. J. 2009;6(82):1–9. doi: 10.1186/1743-422X-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C.H., Rose J.B., Gerba C.P. Microbial agents and transmission. In: Haas C., editor. Quantitative Microbial Risk Assessment. John Wiley & Sons, Inc.; Hoboken, NJ: 2014. pp. 15–62. [Google Scholar]
- Haramoto E., Katayama H., Oguma K., Ohgaki S. Recovery of naked viral genomes in water in Japan. Water Res. 2007;142(1–2):169–173. doi: 10.1016/j.jviromet.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Hata A., Kitajima M., Katayama H. Occurrence and reduction of human viruses, F-specific RNA coliphage genogroups and microbial indicators at a full-scale wastewater treatment plant in Japan. J. Appl. Microbiol. 2012;114(2):545–554. doi: 10.1111/jam.12051. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Leonard M., Greening G.E., Lewis G.D. Influence of wastewater treatment process and the population size on human profiles in wastewater. Water Res. 2011;45(18):6267–6276. doi: 10.1016/j.watres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Irving L.G. Viruses in wastewater effluents. In: BulterButler M., Medlen A.R., Morris R., editors. Viruses and Disinfection of Water and Wastewater. University of Surrey; United Kingdom: 1982. pp. 11–32. [Google Scholar]
- Ishiko H., Shimada Y., Konno T., Hayashi A., Ohguchi T., Tagawa Y., Aoki K., Ohno S., Yamazaki S. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J. Clin. Microbiol. 2008;46(6):2002–2008. doi: 10.1128/JCM.01835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.S., Lukashov V.V., Ganac R.D., Schnurr D.P. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J. Clin. Microbiol. 2007;45(7):2144–2150. doi: 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler A.M., Cromeans T.L., Metcalfee M.G., Humphrey C.D., Hill V.R. Aggregation of adenovirus 2 in source water and impacts on disinfection by chlorine. Food Environ. Virol. 2016;8(2):148–155. doi: 10.1007/s12560-016-9232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Slikas E., Li L., Bodhidatta L., Sethabutr Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Victoria J., Simmonds P., Slikas E., Chieochansin T., Naeem A., Shaukat S., Sharif S., Alam M.M., Angez M., Wang C., Shafer R.W., Zaidi S., Delwart E. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20482–20487. doi: 10.1073/pnas.0807979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Slikas E., Simmonds P., Chieochansin T., Naeem A. A newly identified bocavirus species in human stool. J. Infect. Dis. 2009;199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Haromoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42(6–7):1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes – identification of potential viral indicators. Sci. Total Environ. 2014;488–489:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulou Z., Pliaka V., Amoutzias G.D., Markoulatos P. Recombination among human non-polio enteroviruses: implications for epidemiology and evolution. Virus Gene. 2015;50(2):177–188. doi: 10.1007/s11262-014-1152-y. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Fratini M., Libera S.D., Iaconell M.I., Muscillo M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super. Sanità. 2012;48(4):397–406. doi: 10.4415/ANN_12_04_07. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Pourshaban M., Iaconelli M., Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from treatment plants in Italy. Ann. Ist. Super. Sanità. 2010;46(3):266–273. doi: 10.4415/ANN_10_03_07. [DOI] [PubMed] [Google Scholar]
- Langlet J., Gaboriaud F., Gantzer C. Effects of pH on plaque forming unit counts and aggregation of MS2 bacteriophages. J. Appl. Microbiol. 2007;103(5):1632–1638. doi: 10.1111/j.1365-2672.2007.03396.x. [DOI] [PubMed] [Google Scholar]
- Li L., Kapoor A., Slikas B., Bamidele O.S., Wang C., Shaukat S., Masroor M.A., Wilson M.L., Ndjango J.B., Peeters M., Gross-Camp N.D., Muller M.N., Hahn B.H., Wolfe N.D., Triki H., Bartkus J., Zaidi S.Z., Delwart E. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 2010;84(4):1674–1682. doi: 10.1128/JVI.02109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limsawat S., Ohgaki S. Fate of liberated viral RNA in wastewater determined by PCR. Appl. Environ. Microbiol. 1997;63(7):932–2933. doi: 10.1128/aem.63.7.2932-2933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev A.N., Drexler J.F., Kotova V.O., Amjaga E.N., Reznik V.I., Gmyl A.P. Novel serotypes 105 and 116 are members of distinct subgroups of human enterovirus C. J. Gen. Virol. 2012;93:2357–2362. doi: 10.1099/vir.0.043216-0. [DOI] [PubMed] [Google Scholar]
- Mahalanabis M., Reynolds K.A., Pepper I.L., Gerba C.P. Comparison of multiple passage integrated cell culture-PCR and cytopathogenic effects in cell culture for the assessment of poliovirus survival in wastewater. Food Environ. Virol. 2010;2(4):225–230. [Google Scholar]
- Malherbe H.H., Strickland-Cholmley M. Quantitative studies on viral survival in sewage purification processes. In: Berg G., editor. Transmission of Viruses by the Water Route. Interscience Publishers; New York: 1967. pp. 379–387. [Google Scholar]
- McClain M.E., Spendlove R.S. Multiplicity of reovirus particles after exposure to ultraviolet light. J. Bacteriol. 1966;92(5):1422–1429. doi: 10.1128/jb.92.5.1422-1429.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri N., Goettert D., Achberger E.C., Johnson C.N., Prinyawiwatkul W., Janes M.E. Pathogenic enteric viruses and microbial indicators during secondary treatment of municipal wastewater. Appl. Environ. Microbiol. 2015;81(18):6436–6645. doi: 10.1128/AEM.01218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.F.F., Vega E., Kondov N.O., Markey C., Deng X., Gregoricus N., Vinjé J., Delwart E. Divergent picobirnaviruses in human feces. Genome Announc. 2014;2(3) doi: 10.1128/genomeA.00415-14. e00415–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.F.F., Zhang W., Sachsenroder J., Kondov N.O., da Costa A.C., Vega E., Holtz L.R., Wu G., Wang D., Stine C.O., Antonio M., Mulvaney U.S., Muench M.O., Deng X., Ambert-Balay K., Pothier P., Vinje J., Delwart E. A diverse group of small circular ssDNA viral genomes in human and non-human primate stools. Virus Evol. 2015;1(1):1–12. doi: 10.1093/ve/vev017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norder H., Bjerregaard L., Magnius L., Lina B., Aymard M., Chomel J.-J. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. J. Gen. Virol. 2003;84(Pt 4):827–836. doi: 10.1099/vir.0.18647-0. [DOI] [PubMed] [Google Scholar]
- NRC. National Research Council . The National Academies Press; Washington, DC: 2012. Water Reuse. [Google Scholar]
- Nupen E.M., Bateman B.W., McKenny N.C. Virus Survival in Water and Wastewater Systems. Center for Research in Water Resources, University of Texas; Austin: 1974. The reduction of virus by the various unit processes used in the reclamation of sewage to potable waters; pp. 106–114. [Google Scholar]
- Oberste M.S., Schnurr D., Maher K., Al-Busaidy S., Pallansch M.A. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. J. Gen. Virol. 2001;82(Pt 2):409–416. doi: 10.1099/0022-1317-82-2-409. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Michele S.M., Belliot G., Uddin M., Pallansch M.A. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A. J. Gen. Virol. 2005;86(Pt 2):445–451. doi: 10.1099/vir.0.80475-0. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Nix W.A., Michele S.M., Uddin M., Schnurr D., Al-Busaidy S., Akoua-Koffi C., Pallansch M.A. Molecular identification of 13 new enterovirus types, EV79-88, EV97, and EV100-101, members of the species Human Enterovirus B. Virus Res. 2007;128(1–2):34–42. doi: 10.1016/j.virusres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Michele S.M., Maher K., Schnurr D., Cisterna D., Junttila N., Uddin M., Chomel J.J., Lau C.S., Ridha W., Al-Busaidy S., Norder H., Magnius L.O., Pallansch M.A. Molecular identification and characterization of two proposed new enterovirus serotypes, EV74 and EV75. J. Gen. Virol. 2004;85(11):3205–3212. doi: 10.1099/vir.0.80148-0. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Lee B.E., Neuman N., Ashbolt N., Craik N., Craik S., Maal-Bared R., Pang X. Assessment of human virus removal during municipal wastewater treatment in Edmonton, Canada. J. Appl. Microbiol. 2015;119(6):1729–1739. doi: 10.1111/jam.12971. [DOI] [PubMed] [Google Scholar]
- Regli S., Rose J.B., Haas C.H., Gerba C.P. Modeling the risk from Giardia and viruses in drinking water. J. Am. Water Works Assoc. 1991;84(11):76–84. [Google Scholar]
- Robinson C.M., Singh G., Lee J.Y., Dehghan S., Rajaiya J., Liu E.B., Yousuf M.A., Betensky R.A., Jones M.S., Dyer D.W., Seto D., Chodosh J. Molecular evolution of human adenoviruses. Sci. Rep. 2013;3(1812):1–7. doi: 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.A., Pepper I.L., Gerba C.P. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental simples. Appl. Environ. Microbiol. 2009;75(2):297–307. doi: 10.1128/AEM.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano D., Amarasiri M., Hata A., Watanabe T., Katayama H. Risk management of viral infectious diseases in wastewater and reuse: a review. Environ. Int. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B.W., Kitajima M., Campillo M.E., Gerba C.P., Pepper I.L. Virus reduction during advanced bardenpho and conventional wastewater treatment processes. Environ. Sci. Technol. 2016;50(17):9524–9532. doi: 10.1021/acs.est.6b01384. [DOI] [PubMed] [Google Scholar]
- Scuda N., Hofmann J., Calvignac-Spencer S., Ruprecht K., Liman P., Kühn J., Hengel H., Ehlers B. A novel human polyomavirus closely related to the African green monkey derived lymphotropic polyomavirus (LPV) J. Virol. 2011;85(9):4586–4590. doi: 10.1128/JVI.02602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebrasse E.A., Reyes A., Lim E.S., Zhao G., Mkakosya R.S. Identification of MW polyomavirus, a novel polyomavirus in human stool. J. Virol. 2012;86(19):10321–10326. doi: 10.1128/JVI.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons P.J., Kuo D.H., Xagoraraki I. Removal of human enteric viruses by full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45(9):2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Sinclair R.G., Choi C.Y., Riley M.R., Gerba C.P. Pathogen surveillance through monitoring of sewer systems. Adv. Appl. Microbiol. 2009;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R.S., Schaffer F.L. Enzymatic enhancement of infectivity of reovirus. J. Bacteriol. 1965;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds E.M., Verbyla M.E., Kafle R.C., Breibart M., Mihelcic J.R. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res. 2014;65:257–270. doi: 10.1016/j.watres.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Tapparel C., Siegrist F., Petty T.J., Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013;14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Texas Water Development Board . 2015. Final Report. Direct Potable Reuse Document.http://www.twdb.texas.gov/publications/reports/contracted.reports/doc/1248321508_Vol1.pdf Available at: Accessed June 12, 2016. [Google Scholar]
- Thebault A., Tenuis P.F., LePendu J., LeGuyader F.D., Enis J.B. Infectivity of GI and GII noroviruses established from oyster related outbreaks. Epidemics. 2013;5(2):98–110. doi: 10.1016/j.epidem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Title 22 and 17 California Code of Regulations . State Water Resources Control Board, Division of Drinking Water; July 16, 2015. Regulations Related to Recycled Water. [Google Scholar]
- Wallis C., Melnick J.L. Virus aggregation as the cause of the non-neutralizable persistant fraction. J. Virol. 1967;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Smith K.O.O., Melnick J.L. Reovirus activation by heating and inactivation by cooling in MgCl2 solutions. Virology. 1964;22:608–669. doi: 10.1016/0042-6822(64)90083-2. [DOI] [PubMed] [Google Scholar]
- Ward R.L., Knowton D.R., Pierce M.J. Efficiency of human rotavirus propagation in cell culture. J. Clin. Microbiol. 1984;19(6):748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . vol. 4. WHO; Geneva: 2006. http://www.susana.org/en/resources/library/details/1004 (WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater). Last Accessed July 2015. [Google Scholar]